Submitted:
31 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Intervention
2.2. Study Participants
2.3. Outcomes
2.4. Data Collection
2.5. Growth
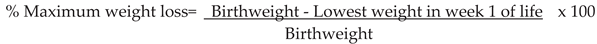
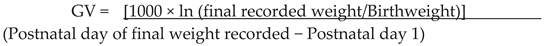
2.6. Morbidities
2.7. Neurodevelopment
2.8. Statistical Analysis
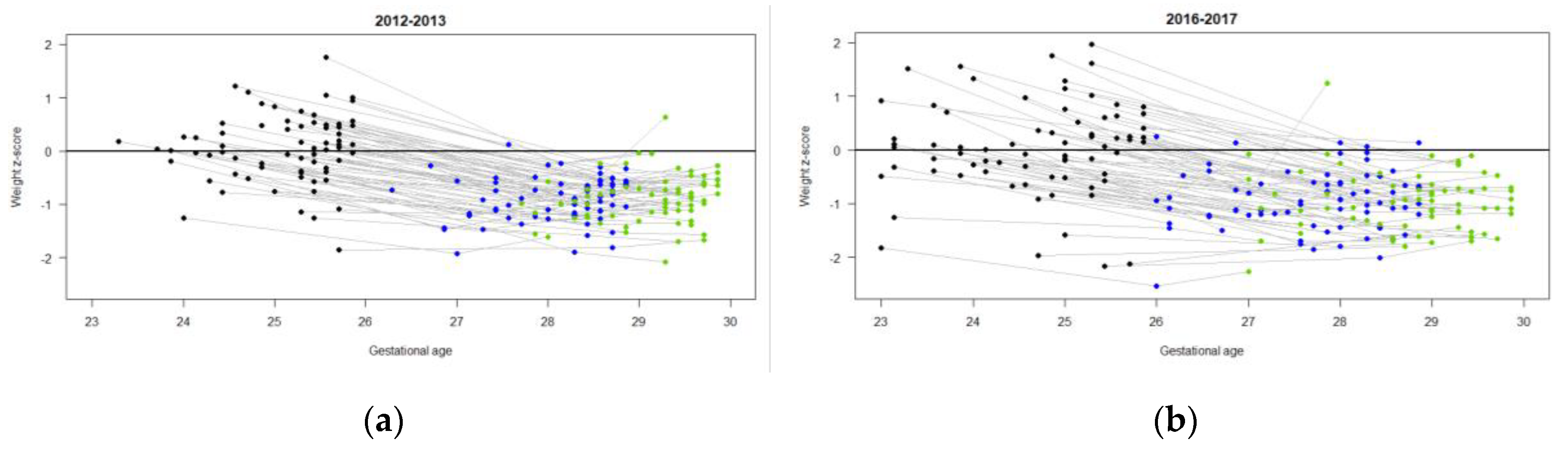
3. Results
3.1. Maternal-Newborn Characteristics
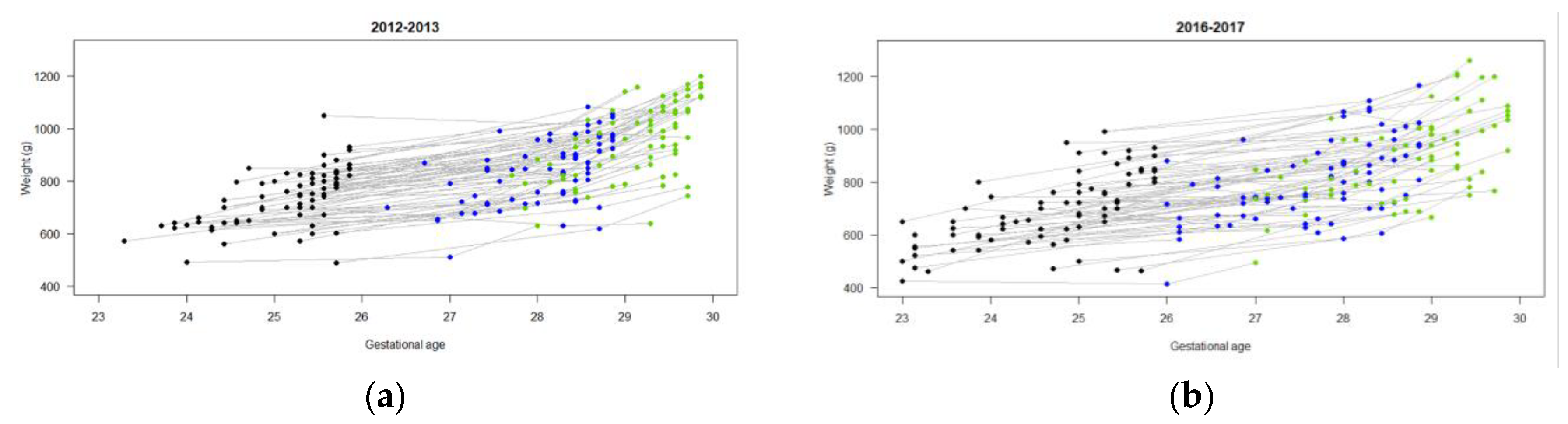
3.2. Nutrition and Growth Outcomes
3.3. Morbidities
3.4. Neurodevelopment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koletzko, B.; Wieczorek, S.; Domellöf, M.; Poindexter, B.B. Defining Nutritional Needs of Preterm Infants. World Rev. Nutr. Diet. 2021, 122, 5–11. [Google Scholar] [CrossRef]
- Fenton, T.R.; Elmrayed, S.; Alshaikh, B. Nutrition, Growth and Long-Term Outcomes. World Rev. Nutr. Diet. 2021, 122, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; Van Den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; Van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef]
- Rochow, N.; Raja, P.; Liu, K.; Fenton, T.; Landau-Crangle, E.; Göttler, S.; Jahn, A.; Lee, S.; Seigel, S.; Campbell, D.; et al. Physiological Adjustment to Postnatal Growth Trajectories in Healthy Preterm Infants. Pediatr. Res. 2016, 79, 870–879. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Cormack, B.E.; Bloomfield, F.H. Approaches to Growth Faltering. World Rev. Nutr. Diet. 2021, 122, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Dai, S.; Lalari, V.; Alshaikh, B. Neonatal and Preterm Infant Growth Assessment. Clin. Perinatol. 2022, 49, 295–311. [Google Scholar] [CrossRef]
- Dunn, M.; Gibbins, S.; Morris, M.; Ursprung, R.; Network Reviewers on behalf of Vermont Oxford. NICQ Next: Innovation in NICQ. Quality Improvement Toolkit: Micro-Premature Infant Care; 2014. [Google Scholar]
- Taylor, G.L.; O’Shea, T.M. Extreme Prematurity: Risk and Resiliency. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101132. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.V.Y.; Unger, S.; Asbury, M.; Kiss, A.; Bishara, R.; Bando, N.; Tomlinson, C.; Gibbins, S.; O’Connor, D.L. Neonatal Morbidity Count Is Associated With a Reduced Likelihood of Achieving Recommendations for Protein, Lipid, and Energy in Very Low Birth Weight Infants: A Prospective Cohort Study. J. Parenter. Enter. Nutr. 2018, 42, 623–632. [Google Scholar] [CrossRef]
- Bartholomew, J.; Martin, C.R.; Allred, E.; Chen, M.L.; Ehrenkranz, R.A.; Dammann, O.; Leviton, A. Risk Factors and Correlates of Neonatal Growth Velocity in Extremely Low Gestational Age Newborns. The ELGAN Study. Neonatology 2013, 104, 298–304. [Google Scholar] [CrossRef]
- Stoltz Sjostrom, E.; Lundgren, P.; Ohlund, I.; Holmstrom, G.; Hellstrom, A.; Domellof, M. Low Energy Intake during the First 4 Weeks of Life Increases the Risk for Severe Retinopathy of Prematurity in Extremely Preterm Infants. Arch Dis Child Fetal Neonatal Ed 2016, 101, F108–113. [Google Scholar] [CrossRef]
- Horbar, J.D.; Ehrenkranz, R.A.; Badger, G.J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Buzas, J.S.; Bertino, E.; Gagliardi, L.; Bellù, R. Weight Growth Velocity and Postnatal Growth Failure in Infants 501 to 1500 Grams: 2000-2013. Pediatrics 2015, 136, e84–92. [Google Scholar] [CrossRef] [PubMed]
- Weisglas-Kuperus, N.; Hille, E.T.M.; Duivenvoorden, H.J.; Finken, M.J.J.; Wit, J.M.; van Buuren, S.; van Goudoever, J.B.; Verloove-Vanhorick, S.P.; * for the D.P.-19 C.S.G. Intelligence of Very Preterm or Very Low Birthweight Infants in Young Adulthood. Arch. Dis. Child. - Fetal Neonatal Ed. 2009, 94, F196–F200. [Google Scholar] [CrossRef]
- Biniwale, M.A.; Ehrenkranz, R.A. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia. Semin. Perinatol. 2006, 30, 200–208. [Google Scholar] [CrossRef]
- Hendson, L.; Church, P.T.; Banihani, R. Follow-up Care of the Extremely Preterm Infant after Discharge from the Neonatal Intensive Care Unit. Paediatr. Child Heal. 2022, 27, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Church, P.T.; Banihani, R.; Luther, M.; Maddalena, P.; Asztalos, E. Premature Infants: The Behavioral Phenotype of the Preterm Survivor. In Follow-Up for NICU Graduates: Promoting Positive Developmental and Behavioral Outcomes for At-Risk Infants; Needelman, H., Jackson, B., Eds.; Springer: Cham, 2018. [Google Scholar]
- Burnett, A.C.; Youssef, G.; Anderson, P.J.; Duff, J.; Doyle, L.W.; Cheong, J.L.Y. Exploring the “Preterm Behavioral Phenotype” in Children Born Extremely Preterm. J. Dev. Behav. Pediatr. 2019, 40, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Hanna, S.; Sharma, A. Neurodevelopmental Outcomes of Extremely Preterm Infants: Theoretical and Epidemiological Perspectives to Guide Shared-Care Decision-Making. Paediatr. Child Heal. (United Kingdom) 2022, 32, 18–27. [Google Scholar] [CrossRef]
- Ramel, S.E.; Belfort, M.B. Preterm Nutrition and the Brain. World Rev. Nutr. Diet. 2021, 122, 46–59. [Google Scholar] [CrossRef]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and Criteria Changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and Reliability of a System to Classify Gross Motor Function in Children with Cerebral Palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content Validity of the Expanded and Revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A Systematic Review and Meta-Analysis to Revise the Fenton Growth Chart for Preterm Infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Patel, A.L.; Engstrom, J.L.; Meier, P.P.; Jegier, B.J.; Kimura, R.E. Calculating Postnatal Growth Velocity in Very Low Birth Weight (VLBW) Premature Infants. J. Perinatol. 2009, 29, 618–622. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal Necrotizing Enterocolitis. Therapeutic Decisions Based upon Clinical Staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Paul Chan, R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Peter Campbell, J.; Capone, A.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef]
- Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; Yoder, B.A.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants An Evidence-Based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; Harcourt Assessment: San Antonio, TX, 2006. [Google Scholar]
- Rochow, N.; Fusch, G.; Mühlinghaus, A.; Niesytto, C.; Straube, S.; Utzig, N.; Fusch, C. A Nutritional Program to Improve Outcome of Very Low Birth Weight Infants. Clin. Nutr. 2012, 31, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.E.; Walden, R. V.; Gargus, R.A.; Tucker, R.; Mance, M.; Nye, J.; Vohr, B.R.; McKinley, L.; Mance, M.; Nye, J.; et al. First-Week Protein and Energy Intakes Are Associated With 18-Month Developmental Outcomes in Extremely Low Birth Weight Infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Assad, M.; Jerome, M.; Olyaei, A.; Nizich, S.; Hedges, M.; Gosselin, K.; Scottoline, B. Dilemmas in Establishing Preterm Enteral Feeding: Where Do We Start and How Fast Do We Go? J. Perinatol. 2023, 43, 1194–1199. [Google Scholar] [CrossRef]
- Patel, A.L.; Taylor, S.N. Dilemmas in Initiation of Very Preterm Infant Enteral Feeds—When, What, How? J. Perinatol. 2023, 43, 108–113. [Google Scholar] [CrossRef]
- Valverde, R.; Dinerstein, N.A.; Vain, N. Mother’s Own Milk and Donor Milk. World Rev. Nutr. Diet. 2021, 122, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Bergner, E.M.; Taylor, S.N.; Gollins, L.A.; Hair, A.B. Human Milk Fortification: A Practical Analysis of Current Evidence. Clin. Perinatol. 2022, 49, 447–460. [Google Scholar] [CrossRef]
- Hair, A.B.; Scottoline, B.; Good, M. Dilemmas in Human Milk Fortification. J. Perinatol. 2023, 43, 103–107. [Google Scholar] [CrossRef]
- Fenton, T.R.; Griffin, I.J.; Hoyos, A.; Groh-Wargo, S.; Anderson, D.; Ehrenkranz, R.A.; Senterre, T. Accuracy of Preterm Infant Weight Gain Velocity Calculations Vary Depending on Method Used and Infant Age at Time of Measurement. Pediatr. Res. 2019, 85, 650–654. [Google Scholar] [CrossRef]
- McKinley, L.T.; Przystac, L.; Tucker, R.; Trail-Burns, E.; Vohr, B.R.; Laptook, A.R.; Bliss, J.M. Implementation of a Nutrition Care Bundle and Improved Weight Gain of Extremely Preterm Infants to 36 Weeks Postmenstrual Age. J. Pediatr. 2022, 241, 42–47. [Google Scholar] [CrossRef]
- Westin, V.; Klevebro, S.; Domellöf, M.; Vanpée, M.; Hallberg, B.; Stoltz Sjöström, E. Improved Nutrition for Extremely Preterm Infants – A Population Based Observational Study. Clin. Nutr. ESPEN 2018, 23, 245–251. [Google Scholar] [CrossRef]
- Roggero, P.; Giannì, M.L.; Orsi, A.; Amato, O.; Piemontese, P.; Liotto, N.; Morlacchi, L.; Taroni, F.; Garavaglia, E.; Bracco, B.; et al. Implementation of Nutritional Strategies Decreases Postnatal Growth Restriction in Preterm Infants. PLoS One 2012, 7, e51166. [Google Scholar] [CrossRef]
- Stefanescu, B.M.; Gillam-Krakauer, M.; Stefanescu, A.R.; Markham, M.; Kosinski, J.L. Very Low Birth Weight Infant Care: Adherence to a New Nutrition Protocol Improves Growth Outcomes and Reduces Infectious Risk. Early Hum. Dev. 2016, 94, 25–30. [Google Scholar] [CrossRef]
- Graziano, P.D.; Tauber, K.A.; Cummings, J.; Graffunder, E.; Horgan, M.J. Prevention of Postnatal Growth Restriction by the Implementation of an Evidence-Based Premature Infant Feeding Bundle. J. Perinatol. 2015, 35, 642–649. [Google Scholar] [CrossRef]
- Khanam, S.; Khan, J.; Sharma, D.; Chawla, D.; Murki, S. Nutritional Bundle to Improve Growth Outcomes among Very Low Birth Weight Infants. J. Matern. Neonatal Med. 2015, 28, 1851–1855. [Google Scholar] [CrossRef]
- Vermont Oxford Network Database Summary for Infants 501 to 1500 Grams Born in Year 2012. Burlington, VT: Vermont Oxford Network; 2012.
- Vermont Oxford Network Database Summary for Infants 501 to 1500 Grams Born in Year 2013. Burlington, VT: Vermont Oxford Network; 2013.
- Vermont Oxford Network Database Summary for Infants 501 to 1500 Grams Born in Year 2016. Burlington, VT: Vermont Oxford Network; 2016.
- Vermont Oxford Network Database Summary for Infants 501 to 1500 Grams Born in Year 2017. Burlington, VT: Vermont Oxford Network; 2017.
- Horbar, J.D.; Greenberg, L.T.; Buzas, J.S.; Ehret, D.E.Y.; Soll, R.F.; Edwards, E.M. Trends in Mortality and Morbidities for Infants Born 24 to 28 Weeks in the US: 1997–2021. Pediatrics 2024, 153, e2023064153. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional Influences on Brain Development. Acta Paediatr. Int. J. Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
| Before Nutrition Bundle | After Nutrition Bundle | |
|---|---|---|
| TFI ordered at birth (mL/kg/d) | 80 | 100 |
| PN ordered at birth | 10% dextrose 2.5 % amino acids 1.5 mmol/100mL calcium gluconate |
10% dextrose 5 % amino acids 2 mmol/100mL calcium gluconate 2 mmol/100mL sodium acetate |
| Volume of PN ordered at birth (mL/kg/d) | 80 | 50 |
| Weight used to order fluids DOL 1-5 | Daily weight | Birthweight |
| Use of complex fluid sheet to consistently include all fluids in actual intakes | No | Yes |
| Umbilical arterial line solution | Na chloride (7.7 mmol/100mL) | Na acetate (7.7 mmol/100mL) |
| Age enteral feeds initiated after birth | variable | By 6h |
| Initiation of probiotics | No | Yes |
| Feed volume when PN discontinued (mL/kg/d) | 120 | Beyond 120 |
| Initiation of H.M.F. (mL/kg/d of enteral feeds) | variable | At 120 |
| Maternal-newborn Outcomes |
2012-2013 n = 72 |
2016-2017 n = 73 |
||
|---|---|---|---|---|
| % (n) | % (n) | |||
| Male sex | 40.3 (29) | 53.4 (39) | ||
| Singleton | 26.4 (19) | 11.0 (8) | ||
| Maternal PIH | 1.4 (1) | 9.6 (7) | ||
| Magnesium Sulphate | 34.7 (25) | 67.1 (49) | ||
| Caesarean delivery | 63.9 (46) | 65.8 (48) | ||
| GDM | ||||
| Insulin | 1.4 (1) | 2.7 (2) | ||
| Diet | 4.2 (3) | 2.7 (2) | ||
| Antenatal Steroids | ||||
| Partial Course | 6.9 (5) | 15.1 (11) | ||
| Full Course | 73.6 (53) | 72.6 (53) | ||
| Size for Gestational Age | ||||
| Small | 8.3 (6) | 9.6 (7) | ||
| Appropriate | 90.3 (65) | 82.2 (60) | ||
| Large | 1.4 (1) | 8.2 (6) | ||
| median (Q1, Q3) | median (Q1, Q3) | |||
| Gestational age at birth (weeks) | 25.3 (24.7, 25.6) | 24.9 (24.0, 25.3) | ||
| mean (sd) | n | mean (sd) | n | |
| Birthweight (g) | 733 (107) | 72 | 694 (141) | 73 |
| Weight z-score | 0.0 (0.6) | 72 | 0.0 (0.9) | 73 |
| Birth length (cm) | 31.9 (1.9) | 62 | 31.5 (2.4) | 49 |
| Length z-score | 0.0 (0.8) | 62 | 0.0 (1.0) | 49 |
| Birth head circumference (cm) | 22.7 (1.2) | 72 | 22.0 (1.5) | 68 |
| Head circumference z-score | 0.1 (0.8) | 71 | -0.1 (0.9) | 68 |
| Maternal age (years) | 30.5 (5.6) | 72 | 32.2 (5.8) | 71 |
| Apgar Score | ||||
| 1 min | 4.7 (2.5) | 71 | 4.4 (2.3) | 71 |
| 5 min | 6.8 (2.0) | 71 | 6.7 (1.8) | 70 |
| Neonatal Morbidities & use of Corticosteroids | % (n) | n | % (n) | n |
| Free from any neonatal morbidity | 19.4(14) | 72 | 28.8(21) | 73 |
| Systemic Corticosteroids | 11.1(8) | 72 | 27.8(20) | 72 |
| Brain Injury or IVH or PVHD | 14.1(10) | 71 | 16.4(12) | 73 |
| CLD | 41.4(29) | 70 | 42(29) | 69 |
| PDA | 46.4(33) | 71 | 24.7(18) | 73 |
| LOS | 45.7(32) | 70 | 46.5(38) | 71 |
| NEC | 7.1(5) | 70 | 4.2(3) | 71 |
| ROP | 5.7(4) | 70 | 14.9(10) | 67 |
|
2012-2013 n = 72 |
2016-2017 n = 73 |
|||
| First feeds type | % (n) | % (n) | ||
| EBM | 31.9 (23) | 5.5 (4) | ||
| DBM | 54.2 (39) | 83.6 (61) | ||
| EBM/DBM | 13.9 (10) | 11.0 (8) | ||
| median (Q1,Q3) | n | median (Q1, Q3) | n | |
| DOL 120 feeds | 14 (11, 18) | 72 | 11 (10, 13) | 70 |
| DOL HMF 1:50 | 17 (15, 20.5) | 71 | 11 (10, 13.5) | 71 |
| DOL HMF 1:25 | 20 (18, 25) | 71 | 13 (12, 16.2) | 72 |
| DOL lowest weight (days) | 4 (4,5) | 72 | 5 (4,7) | 65 |
| mean (sd) | n | mean (sd) | n | |
| Time first PN provided (min) | 54.0 (22.9) | 63 | 59.5 (25.1) | 53 |
| Time first enteral feed provided (h) | 29.8 (20.6) | 65 | 13.1 (11.8) | 67 |
| % max weight loss | 10.9 (5.6) | 72 | 7.5 (5.8) | 66 |
| DOL weight regained (days) | 11.8 (5.8) | 72 | 10.2 (6.) | 65 |
| Weight (g) | ||||
| Birth | 733 (107) | 72 | 695 (141) | 73 |
| DOL 7 | 703 (106) | 72 | 693 (137) | 67 |
| DOL 14 | 781 (112) | 72 | 734 (129) | 71 |
| DOL 21 | 844 (123) | 70 | 806 (151) | 72 |
| DOL 28 | 956 (144) | 71 | 893 (162) | 69 |
| 4-8 weeks CA | ||||
| Weight (kg) | 4.4 (0.6) | 65 | 4.5 (0.6) | 60 |
| Weight z-score | -0.5 (0.9) | 65 | -0.6 (1.1) | 60 |
| Length (cm) | 53.3 (3.3) | 45 | 54.0 (1.9) | 40 |
| Length z-score | -1.0 (1.1) | 37 | -1.3 (1.2) | 28 |
| HC (cm) | 37.8 (1.1) | 61 | 37.5 (1.2) | 59 |
| HC z-score | 0.3 (0.8) | 61 | -0.1 (0.9) | 59 |
| Adjusted Estimates* | ||||
| 2012-2013 | 2016-2017 | Difference | ||
| Estimate (95%CI) | Estimate (95%CI) | Estimate (95%CI) | p | |
| Weight z-score change | ||||
| DOL 7 | -0.7 (-0.8, -0.6) | -0.6 (-0.7, -0.5) | 0.1 (-0.1, 0.2) | .072 |
| DOL 14 | -0.7 (-0.8, -0.7) | -0.8 (-0.9, -0.8) | -0.1 (-0.2, 0.0) | .057 |
| DOL 21 | -0.9 (-1.0, -0.9) | -1.0 (-1.1, -0.9) | 0.0 (-0.2, 0.1) | .48 |
| DOL 28 | -0.9 (-1.0, -0.9) | -1.0 (-1.1, -0.9) | -0.1 (-0.2, 0.0) | .15 |
| Growth velocity | ||||
| DOL 7 | -6.2 (-8.2, -4.3) | -0.7 (-2.8, 1.4) | 5.6 (2.7, 8.4) | .0002 |
| DOL 14 | 5.6 (3.7, 7.6) | 4.5 (2.4, 6.6) | -1.1 (-4.0, 1.7) | .45 |
| DOL 21 | 7.4 (5.5, 9.4) | 7.5 (5.4, 9.6) | 0.1 (-2.8, 2.9) | >.9 |
| DOL 28 | 10.0 (8.1, 12.0) | 9.5 (7.4, 11.6) | -0.6 (-3.4, 2.3) | .71 |
| 4-8 weeks CA | ||||
| Weight z-score change | -0.6 (-0.9, -0.3) | -0.6 (-0.9, -0.3) | 0.0 (-0.5, 0.4) | .89 |
| HC z-score change | 0.2 (-0.1, 0.4) | -0.1 (-0.3, 0.2) | -0.2 (-0.6, 0.2) | .19 |
| Gestational age at birth | Baseline* | |||
|---|---|---|---|---|
| Estimate (95%CI) | p | Estimate (95%CI) | p | |
| Weight z-score change | GA x DOL F(3,3 92) 8.56, p<.0001 | BW x DOL F(3,392) 10.22, p<.0001 | ||
| DOL 7 | 0.2 (0.1, 0.2) | <.0001 | -0.37 (-0.44, -0.30) | <.0001 |
| DOL 14 | 0.0 (-0.1, 0.1) | >.9 | -0.48 (-0.55, -0.40) | <.0001 |
| DOL 21 | 0.0 (-0.1, 0.1) | >.9 | -0.52 (-0.59, -0.45) | <.0001 |
| DOL 28 | 0.1 (0.0, 0.1) | .17 | -0.59 (-0.66, -0.52) | <.0001 |
| Growth velocity | GA x DOL F(3,392) 7.89, p<.0001 | BW x DOL F(3,392) 5.40, p=.001 | ||
| DOL 7 | 5.4 (3.1, 7.7) | <.0001 | -0.05 (-0.06, -0.03) | <.0001 |
| DOL 14 | 0.8 (-1.5, 3.0) | .50 | -0.03 (-0.04, -0.01) | <.0001 |
| DOL 21 | 07 (-1.6, 3.0) | .55 | -0.02 (-0.03, 0.00) | 0.009 |
| DOL 28 | 1.5 (-0.8, 3.8) | .21 | -0.02 (-0.03, 0.00) | 0.016 |
| 4-8 weeks CA | ||||
| Weight z-score change | 0.0 (-0.3, 0.2) | .72 | -0.5 (-0.8, -0.2) | .004 |
| HC z-score change | 0.3 (0.0, 0.5) | .026 | -0.6 (-0.8, -0.4) | .0005 |
| 2012-2013 | 2016-2017 | Difference2 | ||
|---|---|---|---|---|
| Estimate (95%CI) | Estimate (95%CI) | Estimate (95%CI) | p | |
| Motor | 96.3 (92.2, 100.4) | 95.0 (90.8, 99.3) | ||
| Language | 90.4 (86.1, 94.6) | 89.1 (84.7, 93.5) | -1.3 (-5.3, 2.7) | .53 |
| Cognitive | 97.0 (92.9, 101.0) | 95.7 (91.5, 99.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





