Submitted:
01 April 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
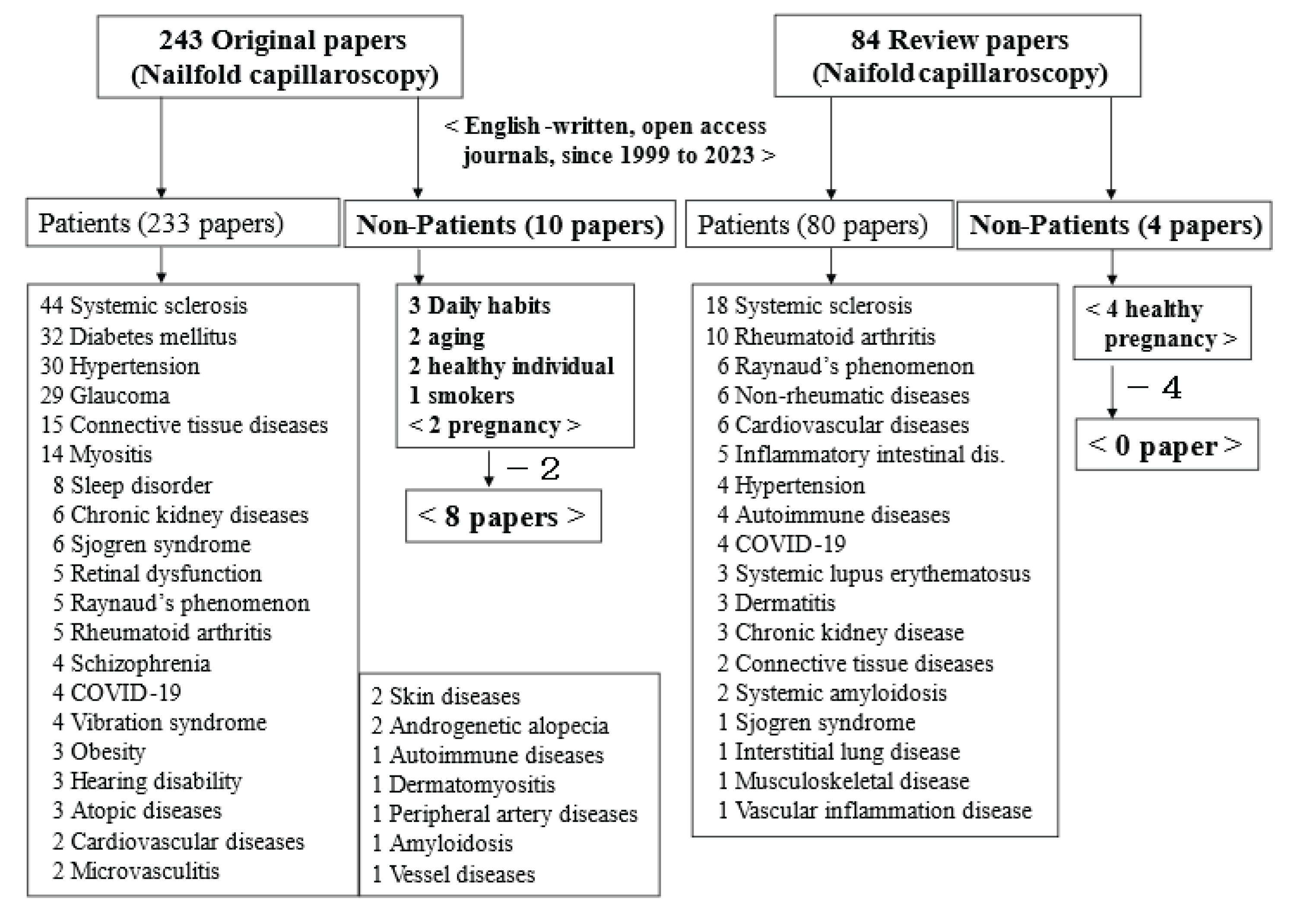
1. Introduction
2. Normal Capillaroscopy Pattern in Healthy Subjects
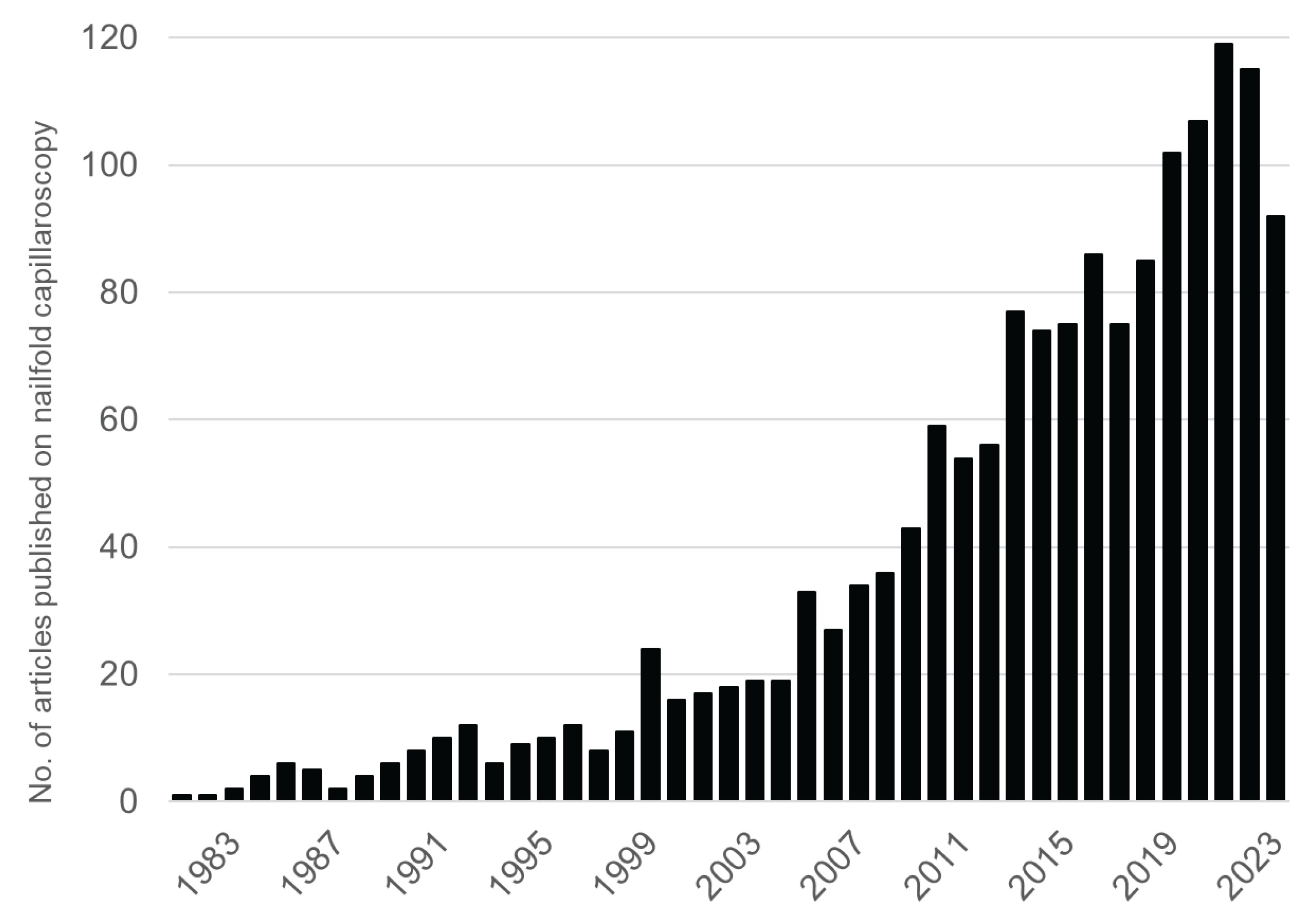
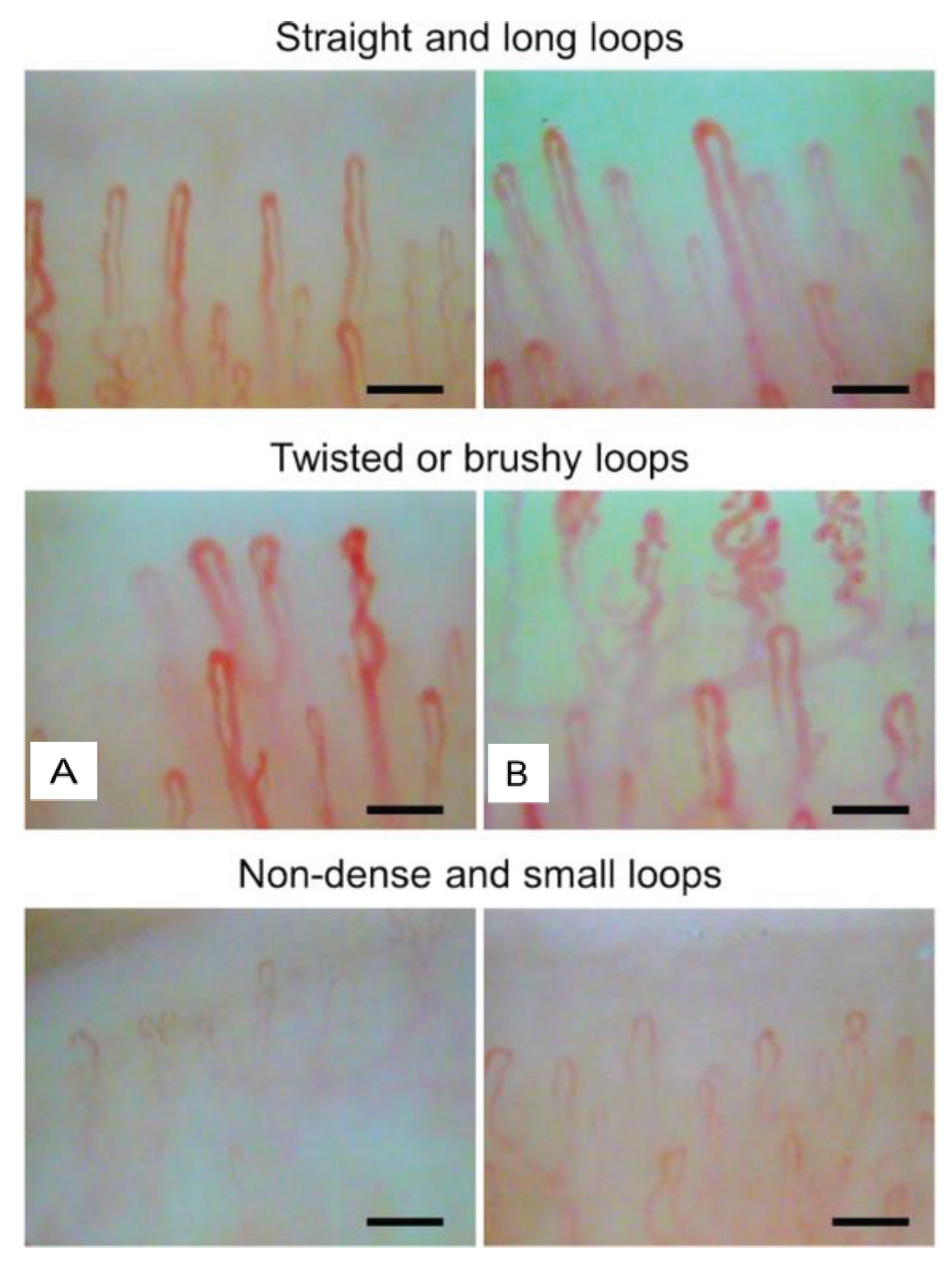
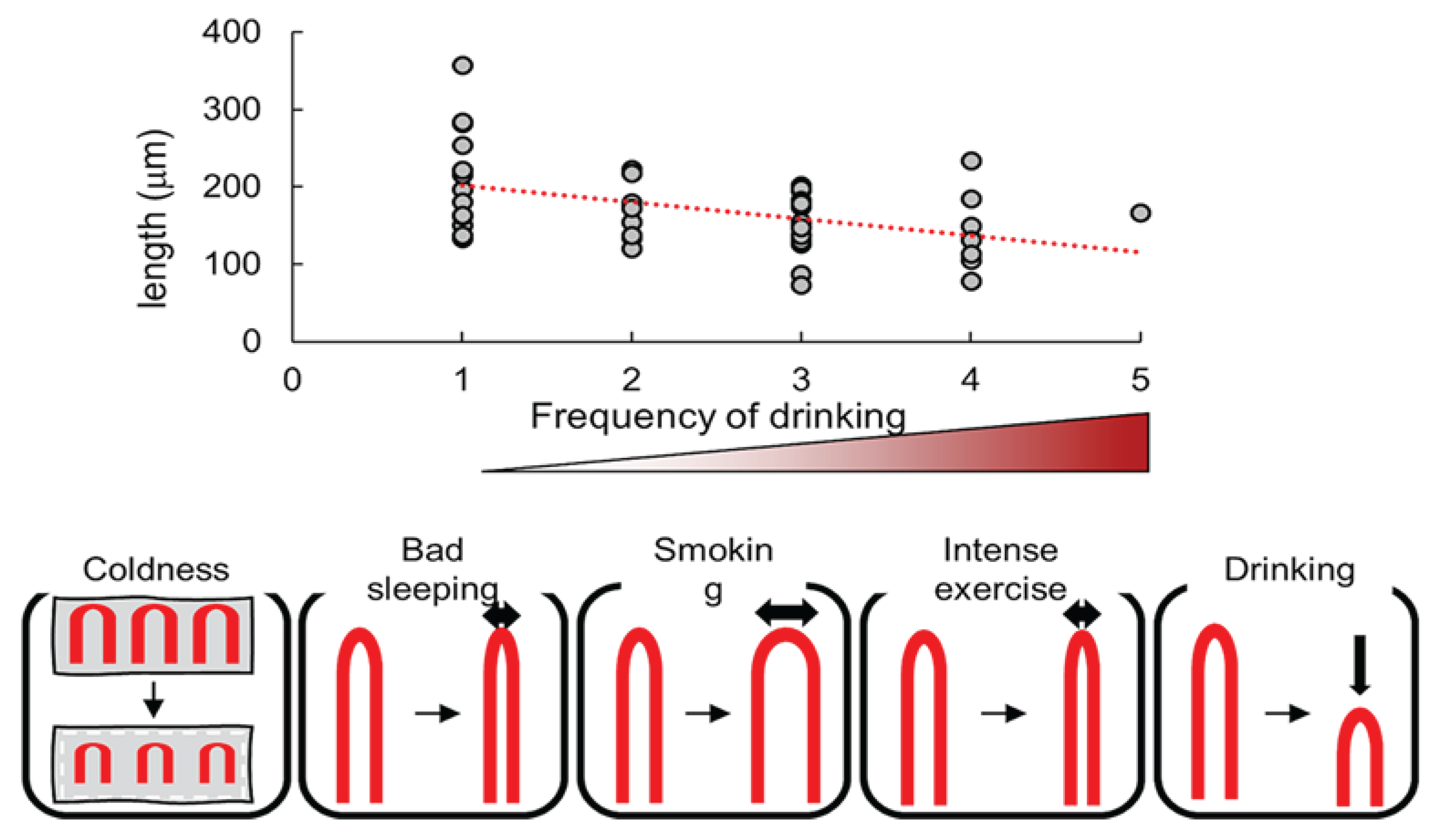
3. Nailfold Capillary Patterns Correlate with Age, Gender, Lifestyle Habits
4. Nailfold Capillaroscopy (NFC) and Its Application for Peripheral Artery Diagnosis
5. The Role of Dietary or Nutritional Supplementation in Microcirculation
6. The Effects of Dietary Flavonoids on Microvascular Health
7. Conclusion and Prospects
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardization of nailfold capillaroscopy for the assessment of patients with Raynaud's phenomenon and systemic sclerosis. Autoimm. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, F.; Khanna, D.; Franzen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger Jr., T. A.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.; Berza, I.; Popescu, D.N.; Parvu, M.I. Nailfold capillaroscopy in systemic diseases: short overview for internal medicine. Rom. J. Intern. Med. 2021, 59, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M. Atlas of capillaroscopy in rheumatic diseases. Elsevier 2010, 25–43. [Google Scholar]
- Cutolo, M.; Sullia, A.; Smith, V. How to perform and interpret capillaroscopy. Best Pract. Res. Clin. 2013, 27, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, M.M.; Felis-Giemza, A. ; Olesinska Capillaroscopy – A role in modern rheumatology. Reumatologia 2016, 54, 67–72. [Google Scholar] [CrossRef] [PubMed]
- [12] Mansueto, N.; Rotondo, C.; Corrado, A.; Cantatore, F.P. Nailfold capillaroscopy: a comprehensive review on common findings and clinical usefulness in non-rheumatic disease. J. Med. Invest. 2021, 68, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Grassi, W.; De Angelis, R. Capillaroscopy: questions and answers. Clin. Rheumatol. 2009, 26, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Nerenxa Ajasllari, N.; Mancarella, L.; Brus, V.; Riccardo Meliconi, R.; Ursini, F. Nailfold capillaroscopy in common non-rheumatic conditions. Microvascular Res. 2020, 131, 104036. [Google Scholar] [CrossRef]
- Maldonado, G.; Rios, C. Nailfold capillaroscopy in diabetes mellitus Potential technique for the microvasculature evaluation. Endocrinol. & Metabol. Syndrome 2017, 6, e125. [Google Scholar]
- Maldonado, G.; Guerrero, R.; Paredes, C.; Ríos, C. Nailfold capillaroscopy in diabetes mellitus. Microvasc. Res. 2017, 112, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kayser, C.; Bredemeir, M.; Caleiro, M.T.; Capobianco, K.; Fernandes, T.M.; De Araujo Fontenele, S.M.; et al. Position article and guidelines 2018 recommendations of the Brazilian Society of Rheumatology for the indication, interpretation and performance of nailfold capillaroscopy. Adv. Rheumatol., 2019, 59, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Emrani, Z.; Karbalaie, A.; Fatemi, A.; Etehadtavakol, M.; Erlandsson, B.E. Capillary density: An important parameter in nailfold capillaroscopy. Microvasc. Res. 2017, 109, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, M.E.; Fatemi, A.; Karbalaie, A.; Emrani, Z.; Erlandsson, B.E. Nailfold capillaroscopy in rheumatic diseases: which parameters should be evaluated? Res. Int. 2015, 974530. [Google Scholar]
- Roldán, L.M.C.; Franco, C.J.V.; Navas, M.A.M. Capillaroscopy in systemic sclerosis: A narrative literature review. Rev. Colomb. Reumatol. 2016, 23, 250–258. [Google Scholar]
- Ingegnoli, F.; Gualtierotti, R.; Lubatti, C.; Bertolazzi, C.; Gutierrez, M.; Boracchi, P. , et al. Nailfold capillary patterns in healthy subjects: A real issue in capillaroscopy. Microvasc. Res. 2013, 90, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, P.; Tamburello, A.; Sciascera, A.; Gilardi, A.G.; Mazzone, A. Nailfold videocapillaroscopy in internal medicine. Ital. J. Med. 2015, 9, 234–242. [Google Scholar] [CrossRef]
- Kayser, C.; Sekiyama, J.Y.; Próspero, L.C.; Camargo, C.Z.; Andrade, L.E.C. Nailfold capillaroscopy abnormalities as predictors of mortality in patients with systemic sclerosis. Clin Exp Rheumatol. 2013, 31 (SUPPL.76), S103–S108. [Google Scholar]
- Cutolo, M.; Melsens, K.; Wijnant, S.; Ingegnoli, F.; Thevissen, K.; Keyser, F.D.; et al. Nailfold capillaroscopy in systemic lupus erythematosus: A systematic review and critical appraisal. Autoimmun. Rev. 2018, 17, 344–352. [Google Scholar] [CrossRef]
- Doughty, K.N.; Del Pilar, N.X.; Audette, A.; Katz, D.L. Lifestyle medicine and the management of cardiovascular disease. Curr. Cardiol. Rep. 2017, 19, 116. [Google Scholar] [CrossRef]
- Kerschbaum, E.; Nu¨ssler, V. Cancer Prevention with Nutrition and Lifestyle. Visc. Med. 2019, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Rev. Endocrinol. 2018, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, E.P.; Yuksel, S.; Soylu, K.; Aydin, F. Microvascular abnormalities in asymptomatic chronic smokers: A video capillaroscopic study. Microvasc Res. 2019, 124, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Harada, Y.; Masuda, N.; Matsuki, Y.; Urakami, S.; Kobayashi, K.; et al. Research on the Relationship Between Blood Flow by Microscope and the Lifestyle Mainly in Female University Students. Proceeding Jissen Women’s Univ. Fac. Hum. Life Sci. 2012, 49, 183–189. [Google Scholar]
- Gorasiya, A.R.; Mehta, H.H.; Prakashey, A.; Dave, M. Nailfold capillaroscopy of healthy individuals--An observational study. Indian Dermatol. Online J. 2022, 13, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Nakano, S.; Kikuchi, A.; Matsunaga, Y. Nailfold capillary patterns correlate with age, gender, lifestyle habits, and fingertip temperature. PLoS ONE 2022, 17, e0269661. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Kohchi, C.; Ogawa, K.; Nakamoto, T.; Yoshimura, H.; Soma, G. Effects of 3 months Continuous intake of supplement containing Pantoea agglomerans LPS to maintain normal bloodstream in adults: Parallel double-blind randomized controlled study. Food Sci. Nutr. 2017, 6, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Akazawa-Kudoh, S.; Fujimoto, Y.; Sawada, M.; Takeno, D.; Yamaguchi, N. Fermented Herbal Decoction Improves a Performance Status of Skin Conditions by Reconstituting Peripheral Capillary. E-Cronicon Gynaecol. 2018, 7, 284–292. [Google Scholar]
- Inagawa, H.; Kohchi, C.; Soma, G. Oral administration of lipopolysaccharides for the prevention of various disease: Benefit and usefulness. Anticancer Res. 2011, 31, 2431–2436. [Google Scholar]
- Braun-Fahrländer, C.; Riedler, J.; Herz, U.; Eder, W.; Waser, M.; Grize, L. Allergy and Endotoxin Study Team: Environmental exposure to endotoxin and its relation to asthma in school-age children. New England J. of Med. 2002, 347, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, H.; Nishizawa, T.; Tsukioka, D.; Suda, T.; Chiba, Y.; Okutomi, T.; Morikawa, A.; Soma, G.I.; Mizuno, D. Homeostasis as regulated by activated macrophage. II. LPS of plant origin other than wheat flour and their concomitant bacteria. Chem. Pharm. Bull. (Tokyo) 1992, 40, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Henrique Silva, H. The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Henrique Silva, H. Correction: Raposo et al. The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology 2021, 10, 616. Biology 2023, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- McCarron, J.G.; Lee, M.D.; Wilson, C. The Endothelium Solves Problems That Endothelial Cells Do Not Know Exist. Trends Pharmacol. Sci. 2017, 38, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Lundwall, K.; Jörneskog, G.; Jacobson, S.H.; Spaak, J. Paricalcitol, Microvascular and Endothelial Function in Non-Diabetic Chronic Kidney Disease: A Randomized Trial. Am. J. Nephrol. 2015, 42, 265–273. [Google Scholar] [CrossRef]
- Maranhão; P. A., Coelho de Souza, M. das G.; Kraemer-Aguiar, L. G.; Bouskela. E. Dynamic nailfold videocapillaroscopy may be used for early microvascular dysfunction in obesity. Microvasc. Res., 2016, 106, 31–35. [Google Scholar] [CrossRef]
- Tian, J.; Xie, Y.; Li, M.; Oatts, J.; Han, Y.; Yang, Y.; Shi, Y.; Sun, Y.; Sang, J.; Cao, K.; Xin, C.; Labisi Siloka, L.; Wang, H.; and Wang, N. The Relationship Between Nailfold Microcirculation and Retinal Microcirculation in Healthy Subjects. Front. Physiol., 2020, 11, article 880. [Google Scholar] [CrossRef]
- Wijnand, J.G.J.; van Rhijn-Brouwer, F.C.C.; Spierings, J.; Teraa, M.; de Borst, G.J.; Verhaar, M.C. Naiflold capillaroscopy in patients with peripheral artery disease of the lower limb (CAPAD Study). Eur. J. Vasc. Endovasc. Surg. 2022, 63, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Kunikata, H.; Yasuda, M.; Kodama, S.; Maeda, Y.; Nakano, J.; Takeno, D.; Fuse, N.; Nakazawa, T. Relationship between nailfold capillaroscopy parameters and the severity of diabetic retinopathy. Graefe's Archive for Clinic. and Experiment. Ophthalmol. 2024, 262, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Tucker, A.T.; Harwood, S.M.; Pearse, R.M.; Raftery, M.J.; Yaqoob, M.M. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin D deficiency: an exploratory, double blind, randomised controlled trial. PLoS One 2014, 9, e99461. [Google Scholar]
- Chitalia, N.; Ismail, T.; Tooth, L.; Boa, F.; Hampson, G.; Goldsmith, D.; Kaski, J.C.; Banerjee, D. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS One 2014, 9, e91363. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Curatola, G.; Panuccio, V.; Tripepi, R.; Pizzini, P.; Versace, M.; Bolignano, D.; Cutrupi, S.; Politi, R.; Tripepi, G.; et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension 2014, 64, 1005–1011. [Google Scholar] [CrossRef]
- Tavakol, M.; Fatemi, A.; Karbalaie, A.; Emrani, Z.; Erlandsson, B. Nailfold capillaroscopy in rheumatic diseases: which parameters should be evaluated? Biomed. Res. Int. 2015, 2015, 974530. [Google Scholar]
- Teraa, M.; Sprengers, R.W.; Westerweel, P.E.; Gremmels, H.; Goumans, M.J.; Teerlink, T.; et al. JUVENTAS study group. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS One 2013, 8e55592. [Google Scholar]
- Avouac, J.; Vallucci, M.; Smith, V. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res. Ther. 2013, 15, R55. [Google Scholar] [CrossRef]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Amina Ouchene, A.; et al. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients, 2023, 15, 3320. [Google Scholar] [CrossRef]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Bronzato, S.; Durante, A. Dietary Supplements and Cardiovascular Diseases. Int. J. Prev. Med. 2018, 9, 80. [Google Scholar]
- Deaton, C. , Froelicher E.S., Wu L.H., Ho C., Shishani K., Jaarsma T. The Global Burden of Cardiovascular Disease. Eur. J. Cardiovasc. Nurs. 2011, 10, S5–S13. [Google Scholar] [CrossRef]
- Lozano, R. , Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [PubMed]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third Who Report on Neglected Tropical Diseases 2015. WHO; Geneva, Switzerland: 2015.
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Pereira, T.M.C.; Peotta, V.A.; Baldo, M.P.; Campos-Toimil, M. Review Article Probiotics as Beneficial Dietary Supplements to Prevent and Treat Cardiovascular Diseases: Uncovering Their Impact on Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 3086270. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L. Annals of Internal Medicine Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef] [PubMed]
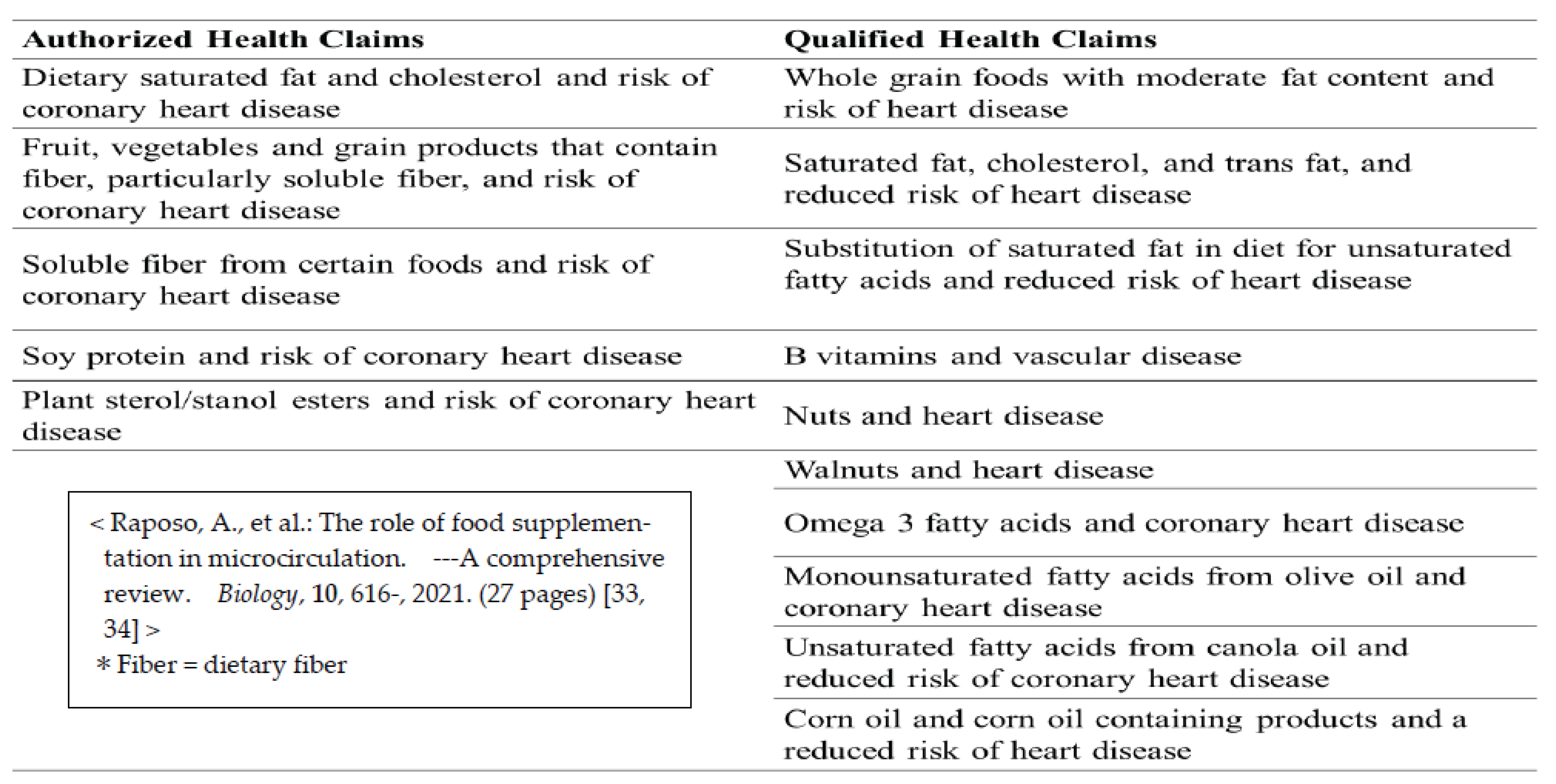
- FDA. Label Claims for Conventional Foods and Dietary Supplements 2019. Available online: https://www.fda.gov/food/food-labeling-nutrition/label-claims-conventional-foods-and-dietary-supplements.
- The European Parliament and the Council of the European Union Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, L136, 1–40.
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef] [PubMed]
- 61. The European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union, 16 May.
- Nutrition and Health Claims. EU Register of Nutrition and Health Claims. 2018. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home.
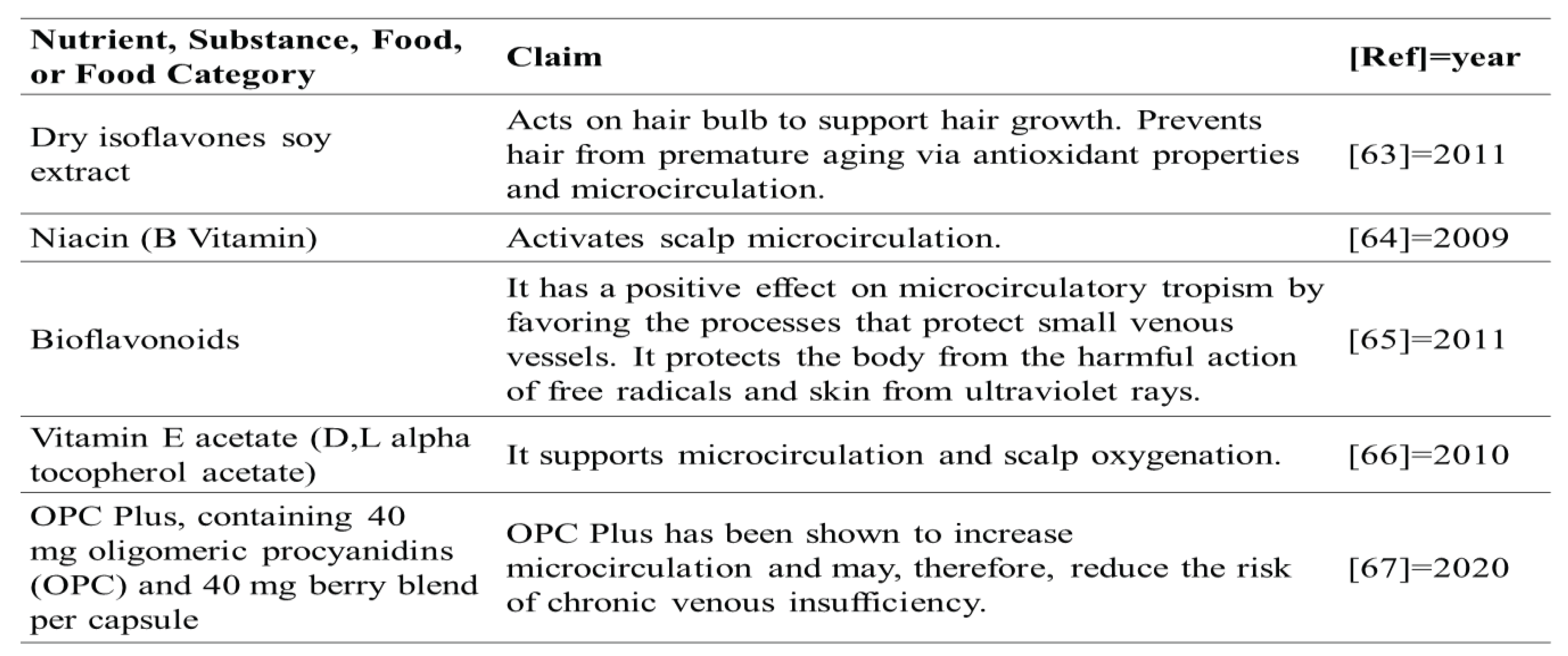
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to soy isoflavones and protection of DNA, proteins and lipids from oxidative damage (ID 1286, 4245), -------- to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J., 2011, 9, 2264.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to niacin and energy-yielding metabolism (ID 43, 49, 54), -------- to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J., 2009, 7, 1224.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to: Flavonoids and ascorbic acid in fruit juices, including berry juices (ID 1186); flavonoids from citrus (ID 1471); -------- to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J., 2011, 9, 2082.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin E and protection of DNA, proteins and lipids from oxidative damage (ID 160, 162, 1947), -------- to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1816. [Google Scholar] [CrossRef]
- Globe Newswire Dietary Supplements Market Size, Share & Trends Analysis Report by Ingredient (Vitamins, Minerals), By Form, By Application, By End User, By Distribution Channel, By Region, and Segment Forecasts, 2020–2027. 2020.
- Sagris, M.; Kokkinidis, D.G.; Lempesis, I.G.; Giannopoulos, S.; Rallidis, L.; Mena-Hurtado, C.; Bakoyiannis, C. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev. Cardiovasc. Med. 2020, 21, 565–575. [Google Scholar] [PubMed]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circulation Res. 2015, 116, 1509–1526. [Google Scholar] [PubMed]
- Hiatt, W.R.; Goldstone, J.; Smith Jr., S. C.; McDermott, M.; Moneta, G.; Roberta Oka, R.; Anne B Newman, A.B.; William H Pearce, W.H. American Heart Association Writing Group 1.: Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008, 118, 2826–2829. [Google Scholar] [CrossRef] [PubMed]
- Nosova, E.V.; Conte, M.S.; Grenon, S.M. Advancing beyond the “heart-healthy diet” for peripheral arterial disease. J. of Vascul. Surg. 2015, 61, 265–274. [Google Scholar] [CrossRef]
- Thomas, J.; Delaney, C.; Suen, J.; Miller, M. Nutritional status of patients admitted to a metropolitan tertiary care vascular surgery unit. Asia Pacific J. of Clin. Nutr. 2019, 28, 64–71. [Google Scholar]
- Gardner, A.W.; Bright, B.C.; Ort, K.A.; Montgomery, P.S. Dietary intake of participants with peripheral artery disease and claudication. Angiology 2011, 62, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S. H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; McDermott, M.M.; Hiatt, W.R. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; Hubbard, V.S.; de Jesus, J.M.; Lee, I.M.; et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–99. [Google Scholar] [CrossRef]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C. , Henkin, Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Georgina, F.; Dodd, G.F; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Croce, G.; Tiberti, S.; Aggio, A.; Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Des. 2009, 15, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: an overview. Scientific World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Alkerwi, A. Cardiovascular health and cognitive function: The Maine-Syracuse longitudinal study. PLoS ONE 2014, 9, e89317. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Wright, C.B.; Dong, C.; Cheung, K.; DeRosa, J.; Nannery, M.; Stern, Y.; Elkind, M.S.; Sacco, R.L. Ideal cardiovascular health and cognitive aging in the northern Manhattan study. J. Am. Heart Assoc. 2016, 5, e002731. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Perier, M.C.; Gaye, B.; Proust-Lima, C.; Helmer, C.; Dartigues, J.F.; Berr, C.; Tzourio, C.; Empana, J.P. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 2018, 320, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.; Boland, L.L.; Mosley, T.; Howard, G.; Liao, D.; Szklo, M.; McGovern, P.; Folsom, A.R. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001, 56, 42–48. [Google Scholar] [CrossRef]
- Vicario, A.; Cerezo, G.H. At the heart of brain disorders—Preventing cognitive decline and dementia. Eur. Cardiol. Rev. 2015, 10, 60–63. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of us adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of cvd: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Tuomainen, T.P.; Kurl, S.; Salonen, J.T. Flavonoid intake and the risk of ischaemic stroke and cvd mortality in middle-aged Finnish men: The kuopio ischaemic heart disease risk factor study. Br. J. Nutr. 2008, 100, 890–895. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and us populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.; Spencer, J.P.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and framingham risk score in healthy men and women: A randomised, controlled, double-masked trial: The flaviola health study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nut. 2008, 88, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–120. [Google Scholar] [CrossRef] [PubMed]
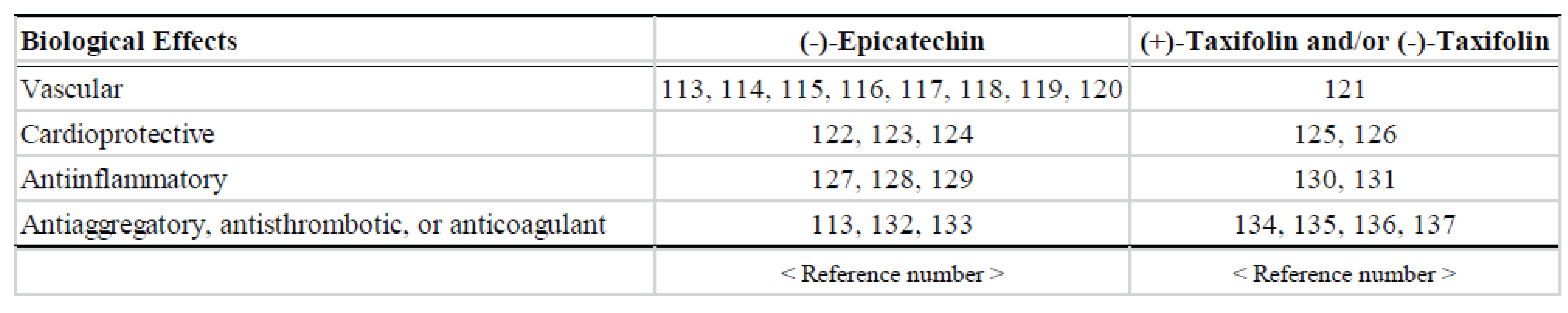
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef]
- Fisher, N.D.; Hollenberg, N.K. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens. 2006, 24, 1575–1580. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodman, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: A randomized controlled clinical trial. Mol. Nutr. Food Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Mulder, T.P.; Draijer, R.; Desideri, G.; Molhuizen, H.O.; Ferri, C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J. Hypertens. 2009, 27, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, T.H.; Eijsvogels, T.M.; Greyling, A.; Draijer, R.; Hopman, M.T.; Thijssen, D.H. Effect of black tea consumption on brachial artery flow-mediated dilation and ischaemia-reperfusion in humans. Appl. Physiol. Nutr. Metab. 2014, 39, 145–151. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and cerebrovascular functions are enhanced in postmenopausal women after ingestion of chocolate with a high concentration of cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef]
- Jochmann, N.; Lorenz, M.; Krosigk, A.; Martus, P.; Bohm, V.; Baumann, G.; Stangl, K.; Stangl, V. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br. J. Nutr. 2008, 99, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.J.; Keaney, J.F., Jr.; Holbrook, M.; Gokce, N.; Swerdloff, P.L.; Frei, B.; Vita, J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001, 104, 151–156. [Google Scholar] [CrossRef]
- Grassi, D.; Draijer, R.; Schalkwijk, C.; Desideri, G.; D’Angeli, A.; Francavilla, S.; Mulder, T.; Ferri, C. Black tea increases circulating endothelial progenitor cells and improves flow mediated dilatation counteracting deleterious effects from a fat load in hypertensive patients: A randomized controlled study. Nutrients 2016, 8, 727. [Google Scholar] [CrossRef]
- Bernatova, I. and Liskova, S. Mechanisms modified by (-)-epicatechin and taxifolin relevant for the treatment of hypertension and viral infection: Knowledge from preclinical studies. Antioxidants (Basel). 2021, 10, 467. [Google Scholar] [CrossRef]
- Miller, K.B.; Hurst, W.J.; Flannigan, N.; Ou, B.; Lee, C.Y.; Smith, N.; Stuart, D.A. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J. Agric. Food Chem. 2009, 57, 9169–9180. [Google Scholar] [CrossRef]
- Alanon, M.E.; Castle, S.M.; Siswanto, P.J.; Cifuentes-Gomez, T.; Spencer, J.P. Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates. Food Chem. 2016, 208, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef] [PubMed]
- Vega-Villa, K.R.; Remsberg, C.M.; Takemoto, J.K.; Ohgami, Y.; Yáñez, J.A.; Andrews, P.K.; Davies, N.M. Stereospecific pharmacokinetics of racemic homoeriodictyol, isosakuranetin, and taxifolin in rats and their disposition in fruit. Chirality 2011, 23, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, F.; He, J.; Duan, C. HPLC-DAD-ESI-MS/MS analysis and antioxidant activities of nonanthocyanin phenolics in mulberry (Morus alba L.). J. Food Sci. 2008, 73, C512–C518. [Google Scholar] [CrossRef] [PubMed]
- Lantto, T.A.; Dorman, H.J.D.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R.; Raasmaja, A. Chemical composition, antioxidative activity and cell viability effects of a Siberian pine (Pinus sibirica Du Tour) extract. Food Chem. 2009, 112, 936–943. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Kluknavsky, M.; Balis, P.; Puzserova, A.; Radosinska, J.; Berenyiova, A.; Drobna, M.; Lukac, S.; Muchova, J.; Bernatova, I. (−)-Epicatechin prevents blood pressure increase and reduces locomotor hyperactivity in young spontaneously hypertensive rats. Oxid. Med. Cell. Longev. 2016, 2016, 6949020. [Google Scholar] [CrossRef]
- Garate-Carrillo, A.; Navarrete-Yañez, V.; Ortiz-Vilchis, P.; Guevara, G.; Castillo, C.; Mendoza-Lorenzo, P.; Ceballos, G.; Ortiz-Flores, M.; Najera, N.; Bustamante-Pozo, M.M.; et al. Arginase inhibition by (−)-epicatechin reverses endothelial cell aging. Eur. J. Pharmacol. 2020, 885, 173442. [Google Scholar] [CrossRef]
- Galleano, M.; Bernatova, I.; Puzserova, A.; Balis, P.; Sestakova, N.; Pechanova, O.; Fraga, C.G. (−)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life 2013, 65, 710–715. [Google Scholar] [CrossRef]
- Aggio, A.; Grassi, D.; Onori, E.; D’Alessandro, A.; Masedu, F.; Valenti, M.; Ferri, C. : Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur. J. Nutr. 2013, 52, 263–272. [Google Scholar] [CrossRef] [PubMed]
- MacRae, K.; Connolly, K.; Vella, R.; Fenning, A. Epicatechin’s cardiovascular protective effects are mediated via opioid receptors and nitric oxide. Eur. J. Nutr. 2019, 58, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, A.; Marinko, M.; Vranic, A.; Jankovic, G.; Milojevic, P.; Stojanovic, I.; Nenezic, D.; Ugresic, N.; Kanjuh, V.; Yang, Q.; et al. Mechanisms underlying the vasorelaxation of human internal mammary artery induced by (−)-epicatechin. Eur. J. Pharmacol. 2015, 762, 306–312. [Google Scholar] [CrossRef]
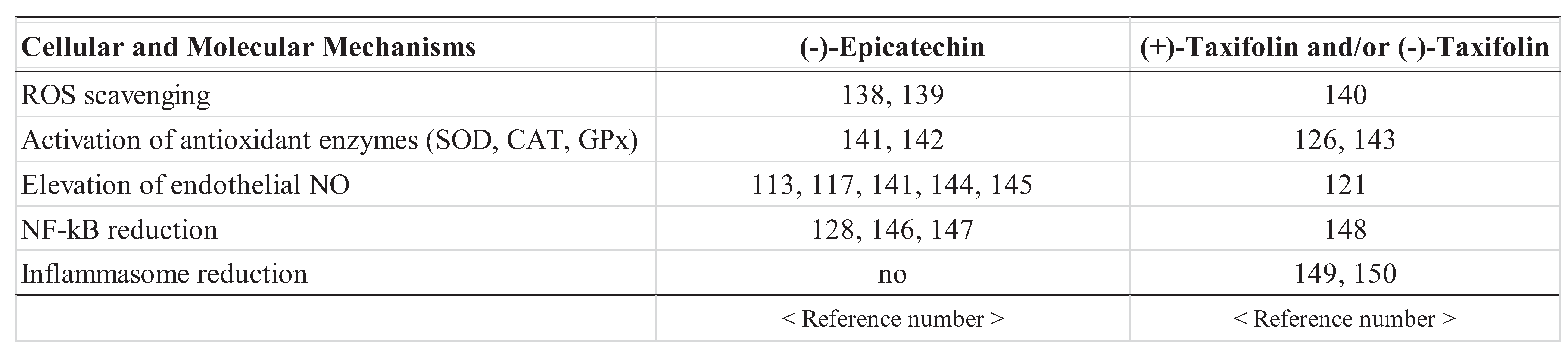
- Marinko, M.; Jankovic, G.; Nenezic, D.; Milojevic, P.; Stojanovic, I.; Kanjuh, V.; Novakovic, A. (−)-Epicatechin-induced relaxation of isolated human saphenous vein: Roles of K+ and Ca2+ channels. Phytother. Res. 2018, 32, 267–275. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Aliev, O.I.; Sidekhmenova, A.V.; Shamanaev, A.Y.; Anishchenko, A.M.; Fomina, T.I.; Chernysheva, G.A.; Smol’yakova, V.I.; Arkhipov, A.M. Dihydroquercetin improves microvascularization and microcirculation in the brain cortex of SHR rats during the development of arterial hypertension. Bull. Exp. Biol. Med. 2017, 163, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.J.; Kubo, E.; Fujii, K.; Nishimura, Y.; Kobuchi, S.; Ohkita, M.; Yoshimura, M.; Kiso, Y.; Matsumura, Y. Antihypertensive effect of French maritime pine bark extract (Flavangenol): Possible involvement of endothelial nitric oxide-dependent vasorelaxation. J. Hypertens. 2009, 27, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef]
- Calabró, V.; Piotrkowski, B.; Fischerman, L.; Vazquez Prieto, M.A.; Galleano, M.; Fraga, C.G. Modifications in nitric oxide and superoxide anion metabolism induced by fructose overload in rat heart are prevented by (−)-epicatechin. Food Funct. 2016, 7, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.G.; Taub, P.R.; Barraza-Hidalgo, M.; Rivas, M.M.; Zambon, A.C.; Ceballos, G.; Villarreal, F.J. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J. Am. Coll. Cardiol. 2010, 55, 2869–2876. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Fu, F.F.; El-Kassaby, Y.A.; Wang, G. Temporospatial flavonoids metabolism variation in Ginkgo biloba leaves. Front. Genet. 2020, 11, 1503. [Google Scholar] [CrossRef]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019, 10, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.C.D.P.; Seito, L.N.; Di Stasi, L.C.; Akiko Hiruma-Lima, C.; Pellizzon, C.H. Epicatechin used in the treatment of intestinal inflammatory disease: An analysis by experimental models. Evid. Based Complement. Alternat. Med. 2012, 2012, 508902. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Fischerman, L.; Toblli, J.E.; Fraga, C.G.; Galleano, M. LPS-induced renal inflammation is prevented by (−)-epicatechin in rats. Redox Biol. 2017, 11, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, Z.; Oteiza, P.I. (−)-Epicatechin mitigates high fat diet-induced neuroinflammation and altered behavior in mice. Food Funct. 2020, 11, 5065–5076. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Saito, S.; Tanaka, M.; Yamakage, H.; Kusakabe, T.; Shimatsu, A.; Ihara, M.; Satoh-Asahara, N. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 10031–10038. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Sinegre, T.; Teissandier, D.; Milenkovic, D.; Morand, C.; Lebreton, A. Epicatechin influences primary hemostasis, coagulation and fibrinolysis. Food Funct., 2019, 10, 7291–7298. [Google Scholar] [CrossRef] [PubMed]
- Sinegre, T.; Milenkovic, D.; Bourgne, C.; Teissandier, D.; Nasri, Y.; Dannus, L.T.; Morand, C.; Lebreton, A. Impact of epicatechin on the procoagulant activities of microparticles. Nutrients, 2020, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Aliev, O.I.; Sidekhmenova, A.V.; Shamanaev, A.Y.; Anishchenko, A.M.; Nosarev, A.V.; Pushkina, E.A. Modes of hypotensive action of dihydroquercetin in arterial hypertension. Bull. Exp. Biol. Med., 2017, 162, 353–356. [Google Scholar] [CrossRef]
- Kubatiev, A.A.; Yadigarova, Z.T.; Rud’ko, I.A.; Tyukavkina, N.A.; Bykov, V.A. Diquertin suppresses ADP- and thrombin-induced accumulation of cytoplasmic calcium in human thrombocytes. Pharm. Chem. J., 1999, 33, 629–630. [Google Scholar] [CrossRef]
- Ivanov, I.S.; Sidehmenova, A.V.; Smol’yakova, V.I.; Chernysheva, G.A.; Plotnikov, M.B. Inhibition of adenosine diphosphate-induced platelet aggregation by alpha-lipoic acid and dihydroquercetin in vitro. Indian J. Pharmacol., 2014, 46, 430–432. [Google Scholar] [PubMed]
- Chen, Y.; Deuster, P. Comparison of quercetin and dihydroquercetin: Antioxidant-independent actions on erythrocyte and platelet membrane. Chem. Biol. Interact. 2009, 182, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ruijters, E.J.; Weseler, A.R.; Kicken, C.; Haenen, G.R.; Bast, A. The flavanol (−)-epicatechin and its metabolites protect against oxidative stress in primary endothelial cells via a direct antioxidant effect. Eur. J. Pharmacol. 2013, 715, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Strigunova, E.N.; Kostyuk, T.V.; Afanas’ev, I.B. Experimental evidence that flavonoid metal complexes may act as mimics of superoxide dismutase. Arch. Biochem. Biophys. 2004, 428, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Shubina, V.S.; Shatalin, Y.V. Antioxidant and iron-chelating properties of taxifolin and its condensation product with glyoxylic acid. J. Food Sci. Technol. 2017, 54, 1467–1475. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Romero, M.; O’Valle, F.; Lopez-Sepulveda, R.; Quintela, A.M.; Galindo, P.; Zarzuelo, M.J.; Bailón, E.; et al. Chronic (−)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitric oxide-deficient rats. Br. J. Nutr. 2011, 106, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Fraga, C.G.; Galleano, M. (−)-Epicatechin administration protects kidneys against modifications induced by short-term l-NAME treatment in rats. Food Funct. 2020, 11, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Kishiwaki, Y.; Matsumura, M.; Sawada, H.; Hashimoto, R.; Gotoh, K.; Umemoto, K.; Fujimoto, Y. Taxifolin Potently Diminishes Levels of Reactive Oxygen Species in Living Cells Possibly by Scavenging Peroxyl Radicals. Am. J. Pharmacol. Toxicol. 2018, 13, 1–6. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Zarzuelo, M.J.; Galindo, P.; Quintela, A.M.; López-Sepulveda, R.; Romero, M.; Tamargo, J.; Vargas, F.; et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 2012, 52, 70–79. [Google Scholar] [CrossRef]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (−)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2015, 90, 35–46. [Google Scholar] [CrossRef]
- Morrison, M.; Van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NF-kB in vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Rodríguez Lanzi, C.; Fraga, C.G.; Galleano, M. Dietary (−)-epicatechin affects NF-κB activation and NADPH oxidases in the kidney cortex of high-fructose-fed rats. Food Funct. 2019, 10, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Wang, W.-Y.; Chang, C.-C.; Liou, K.-T.; Sung, Y.-J.; Liao, J.-F.; Chen, C.-F.; Chang, S.; Hou, Y.-C.; Chou, Y.-C.; et al. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-oxidative effect and modulation of NF-kB activation. J. Biomed. Sci. 2006, 13, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Wang, S.; Zhang, X.; Zai, W.; Fan, J.; Chen, W.; Bian, Q.; Luan, J.; Shen, Y.; Zhang, Y.; et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine 2018, 41, 45–53. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, X.; Cai, Q.; Zhuang, J.; Tan, X.; He, W.; Zhao, M. Protective effect of taxifolin on H2O2-induced H9C2 cell pyroptosis. Zhong Nan Da Xue Xue Bao. Yi Xue Ban J. Cent. South Univ. Med. Sci. 2017, 42, 1367–1374. [Google Scholar]
| Parameter | Description |
|---|---|
| Skin transparency | Allows a good visualization of the capillaries |
| Subpapillary venous plexus | Visible in up to 30% of healthy individuals |
| General view | Homogeneously sized, regularly arranged |
| Capillary orientation | Straight, parallel fashion, usually perpendicular to the nailfold |
| Capillary density | Number of capillaries over 1 mm nailfold, more than 7 capillaries/mm |
| Capillary morphology | Inverted "U", hairpin shape, but also tortuous and/ or crossing capillaries (nonspecific variations) |
| Capillary length | less than 300 mm |
| Capillary diameter | less than 20 mm for each loop (afferent, apical, efferent) |
| Pericapillary edema | Absent |
| Hemorrhages | Absent (occasionally observed after microtrauma) |
| Giant capillary | Absent |
| Neoangiogenesis | Absent |
| Blood flow characteristics | Dynamic, no stasis |
| Authors and Published Year | NFC morphology |
|---|---|
| Ingegnoli, F., et al. 2013, Microvasc. Res., 90, 90–95. [16] | Based on the cluster analysis three major “normal” morphologic capillaroscopic patterns were depicted: 1) the “normal” pattern mainly with 2 to 5 U-shaped loops/mm and ≤2 tortuous loops/mm; 2) the “perfect normal” pattern with ≥5 U-shaped loops/mm; and 3) the “unusual normal” with at least 1 meandering or bushy loop, or at least 1 microhemorrhage, or with N4 crossed loops/mm. Regarding the loop measurements, the majority of subjects had a median of 7 capillaries/mm with a median length of 198 μm. |
| Faggioli, P., et al. 2015, Italian J. of Med., 9, 234-242. [17] |
Under physiological conditions the normal pattern is characterized by: 1) the orderly arrangement of the capillaries to comb; 2) density of 9-13 mm (maximum 3 per dermal papilla); 3) 6-9µm diameter afferent branch, efferent branch 8-21µm (>50 micron: megacapillaries); 4) length 200-500 µm. |
| Tavakol, M.E., et al. 2015, Biomed. Res. Int., 2015, 974530. [14] | Nailfold capillary density appears to be similar in healthy adults and healthy children across Europe. European authors found the mean capillary density in healthy children to be in the range of 5–7.3 compared to 7.3–10.3 in healthy adults. Brazilian authors showed slightly higher capillary counts, ranging from 6–7.3 capillaries per millimeter in children to 9.11–10.1 capillaries per millimeter in adults. |
| Emrani, Z., et al. 2017, Microvasc. Res., 109, 7–18. [13] | Density of finger capillaries in healthy control subjects were summarized by collecting 17 articles published from 1990 to 2016 as follows. The average capillary density was 8.45 ± 1.32/mm for individuals aged 40 or less and 8.71 ± 1.40 for individuals older than 40 years of age in healthy subjects*. Moreover, the average capillary densities in healthy males and females were found to be 8.83 ± 1.50 and 8.60 ± 1.26/mm, respectively.** < *Ingegnoli, F., Herrick, A.L., 2013. Nailfold capillaroscopy in pediatrics. Arthritis Care Res. 65, 1393–1400. **Hoerth, C., Kundi, M., Katzeschlager, R., Hirschl, M., 2012. Qualitative and quantitative assessment of nailfold capillaries by capillaroscopy in healthy volunteers. Vasa, 41, 19–26. > |
| Nailfold capillary | Dilated | Neoangio-genesis | Meandering | Tortuous | Ramified | Plexus Visibility | Micro hemorrhage | Receding | Angulated |
|---|---|---|---|---|---|---|---|---|---|
| Male (72) | 25 | 29 | 34 | 29 | 4 | 29 | 4 | 23 | 9 |
| % | 34.72 | 40.27 | 47.22 | 40.27 | 5.55 | 40.27 | 5.55 | 31.94 | 12.5 |
| Female (78) | 25 | 29 | 33 | 39 | 6 | 26 | 4 | 24 | 13 |
| % | 32.05 | 37.17 | 42.3 | 50 | 7.69 | 33.33 | 5.12 | 30.76 | 16.66 |
| P | 0.8624 | 0.8247 | 0.549 | 0.3026 | 0.8442 | 0.3797 | 0.9074 | 0.8768 | 0.6244 |
| BMI*<24.9 (84) | 30 | 38 | 42 | 36 | 5 | 38 | 4 | 24 | 15 |
| % | 35.71 | 45.23 | 50 | 42.85 | 5.95 | 45.23 | 4.76 | 28.571 | 17.85 |
| BMI* >25 (66) | 20 | 20 | 25 | 32 | 5 | 17 | 4 | 23 | 7 |
| % | 30.3 | 30.3 | 37.87 | 48.48 | 7.57 | 25.75 | 6.06 | 34.84 | 10.6 |
| P | 0.6007 | 0.0909 | 0.1879 | 0.6016 | 0.9474 | 0.0222** | 0.7253 | 0.5187 | 0.3108 |
| 20-40 years (78) | 22 | 31 | 34 | 23 | 3 | 32 | 5 | 17 | 11 |
| % | 28.2 | 39.74 | 43.58 | 29.48 | 3.84 | 41.02 | 6.41 | 21.79 | 14.1 |
| 41-60 years (72) | 28 | 27 | 33 | 45 | 7 | 23 | 3 | 30 | 11 |
| % | 38.88 | 37.5 | 45.83 | 62.5 | 9.72 | 31.94 | 4.61 | 41.66 | 15.27 |
| P | 0.225 | 0.9092 | 0.911 | 0.0002** | 0.2654 | 0.3254 | 0.8074 | 0.0229** | 0.9699 |
| Control (n=26) | LPSp supplement (n=26) | |||
|---|---|---|---|---|
| Months (m) | 0 m | +3 m | 0 m | +3 m |
| Per field | 4.92 ± 0.30 | 4.42 ± 0.25 | 4.65 ± 0.25 | 5.12 ± 0.271 |
| Relative value | 1.0 ± 0.0 | 1.057 ± 0.17 | 1.0 ± 0.0 | 1.201 ± 0.10 |
| Authors and Published Year | Title and Description |
|---|---|
|
<Example 1 > Lundwall, K., et al., 2015 |
Paricalcitol, Microvascular and Endothelial Function in Non-Diabetic Chronic Kidney Disease: A Randomized Trial |
| < Am. J. Nephrol., 42, 265-273, 2015 > [38] | Endothelial function declined significantly over 3 months in patients with moderate CKD, and this decline could be ameliorated by VDRA (vitamin D receptor activator) treatment, possibly through increased capillary blood flow. |
|
<Example 2 > Maranhao, P.A., et al., 2016 |
Dynamic Nailfold Videocapillaroscopy may be Used for Early Microvascular Dysfunction in Obesity |
| < Microvasc. Res., 106, 31-35, 2016 > [39] | The authors could speculate that the derangement on microvascular hemodynamics occurs before the diagnosis of hypertension, diabetes and other metabolic syndromes. Therefore, NFC is the most appropriate technique to precociously assess microvascular dysfunction in obesity. |
|
< Example 3 > Tian, J., et al., 2020 |
The Relationship Between Nailfold Microcirculation and Retinal Microcirculation in Healthy Subjects |
| < Front. Physiol., 11, article 880; Sec. Vascular Physiology, 2020 > [40] | In healthy subjects, there was direct relationship between nailfold capillary and retinal microcirculation. Therefore, abnormalities seen in the NFC are associated with reduced retinal nerve fiber layer (RNFL) thickness and retinal vessel density (VD). |
|
< Example 4 > Wijnand, J.G.J., et al., 2022 |
Naiflold Capillaroscopy in Patients with Peripheral Artery Disease of the Lower Limb (CAPAD Study) |
| < Eur. J. Endovascl Surg., 63, 900-901, 2022 > [41] | NFC abnormalities can be used as markers for inflammation and endothelial dysfunction in PAD. |
|
<Example 5> Okabe, T., et al., 2023 |
Relationship between Nailfold Capillaroscopy Parameters and the Severity of Diabetic Retinopathy |
| < Graefe's Archive Clin. & Exp. Ophthalmol., doi:10.1007/s00417- 023-06220-z., 2023 > [42] | Alterations in NFC morphology, such as capillary shortening, may be closely correlated with the presence of DR (diabetic retinopathy) and PDR (proliferative DR). |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





