Submitted:
01 April 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Compositional Analysis of Protein Peptides Extracted from Three Sites
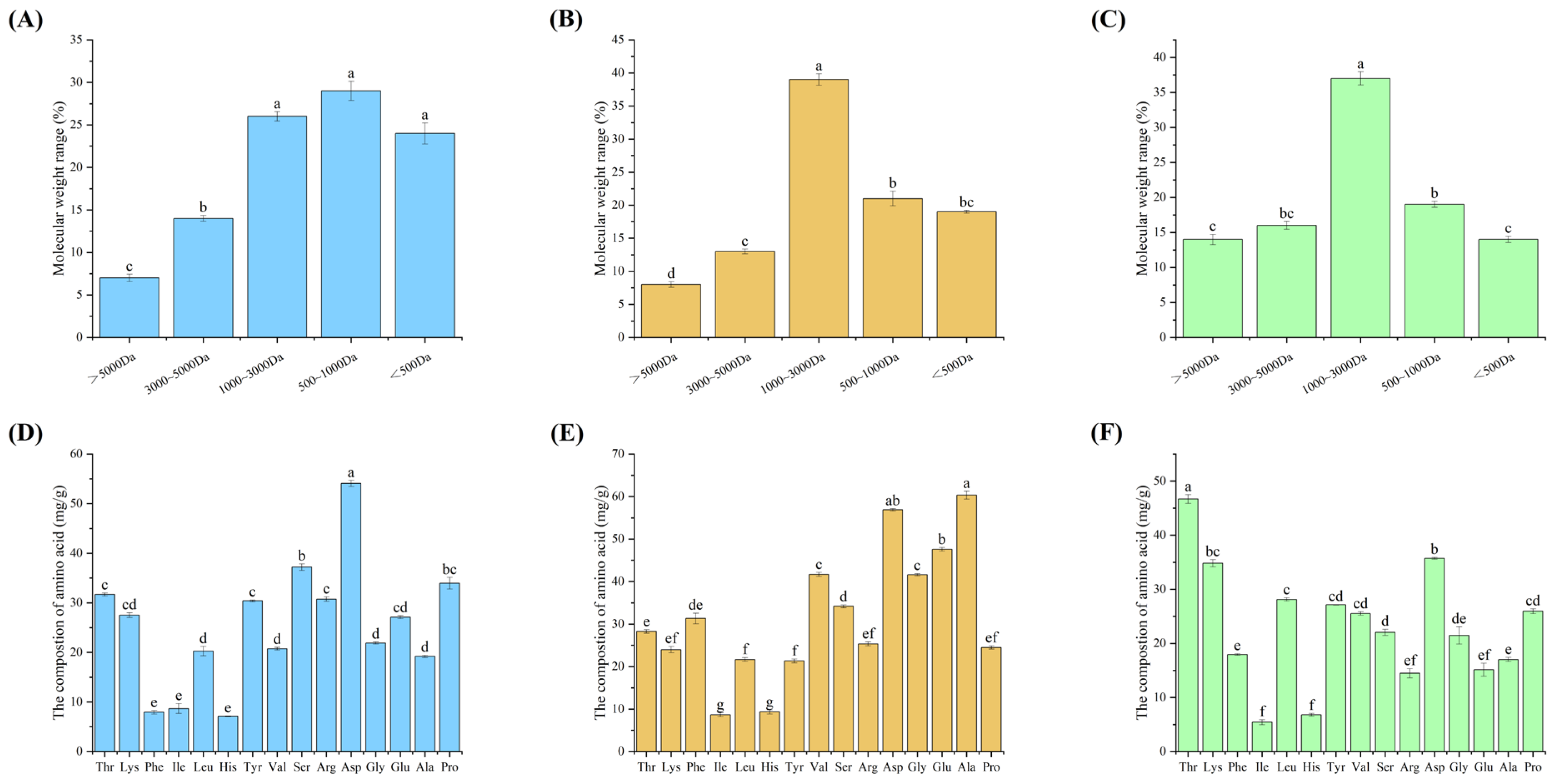
2.2. Differential Value-Added Profile of the Three Proteolytic Peptides for Osteoblasts
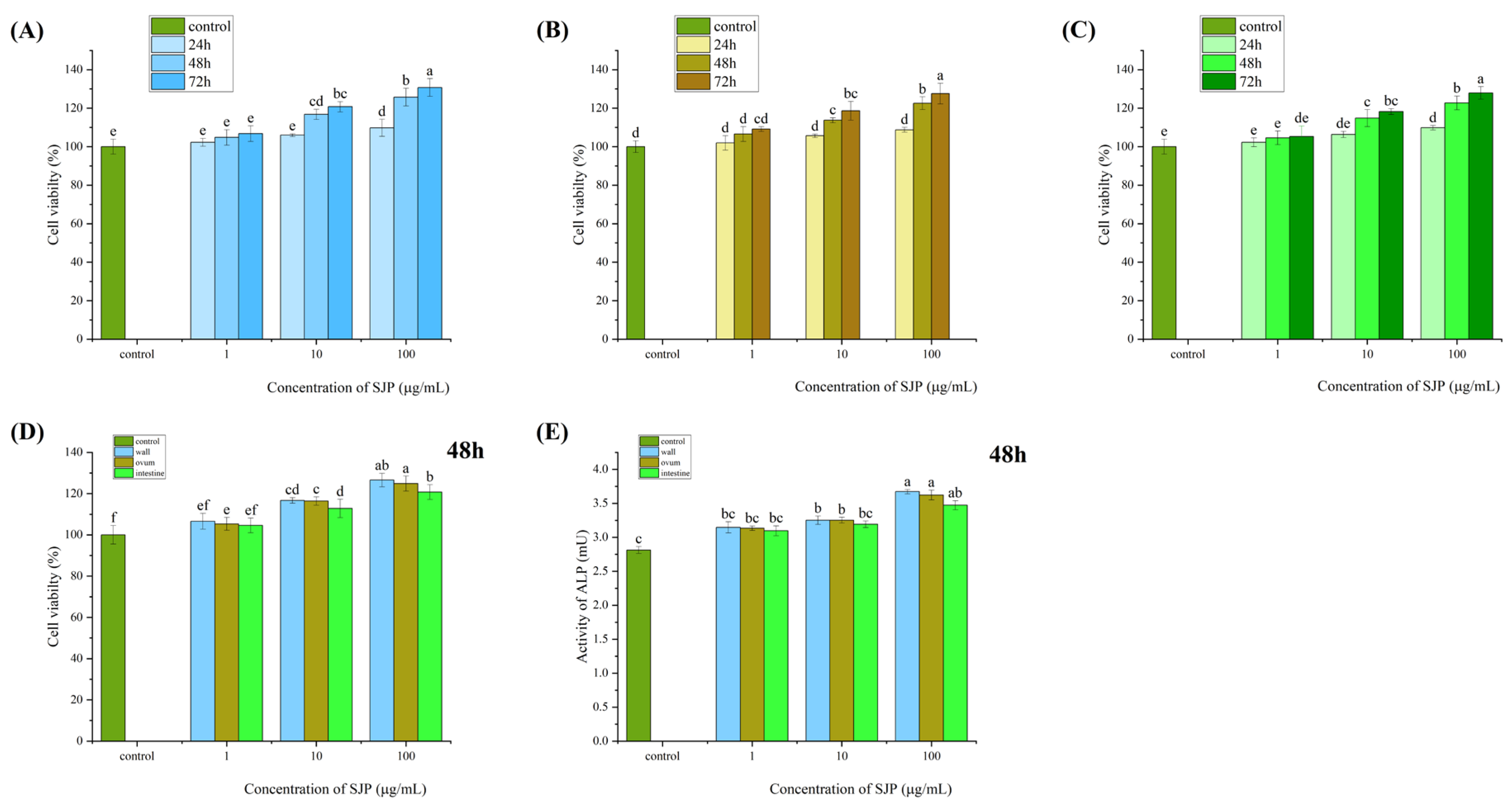
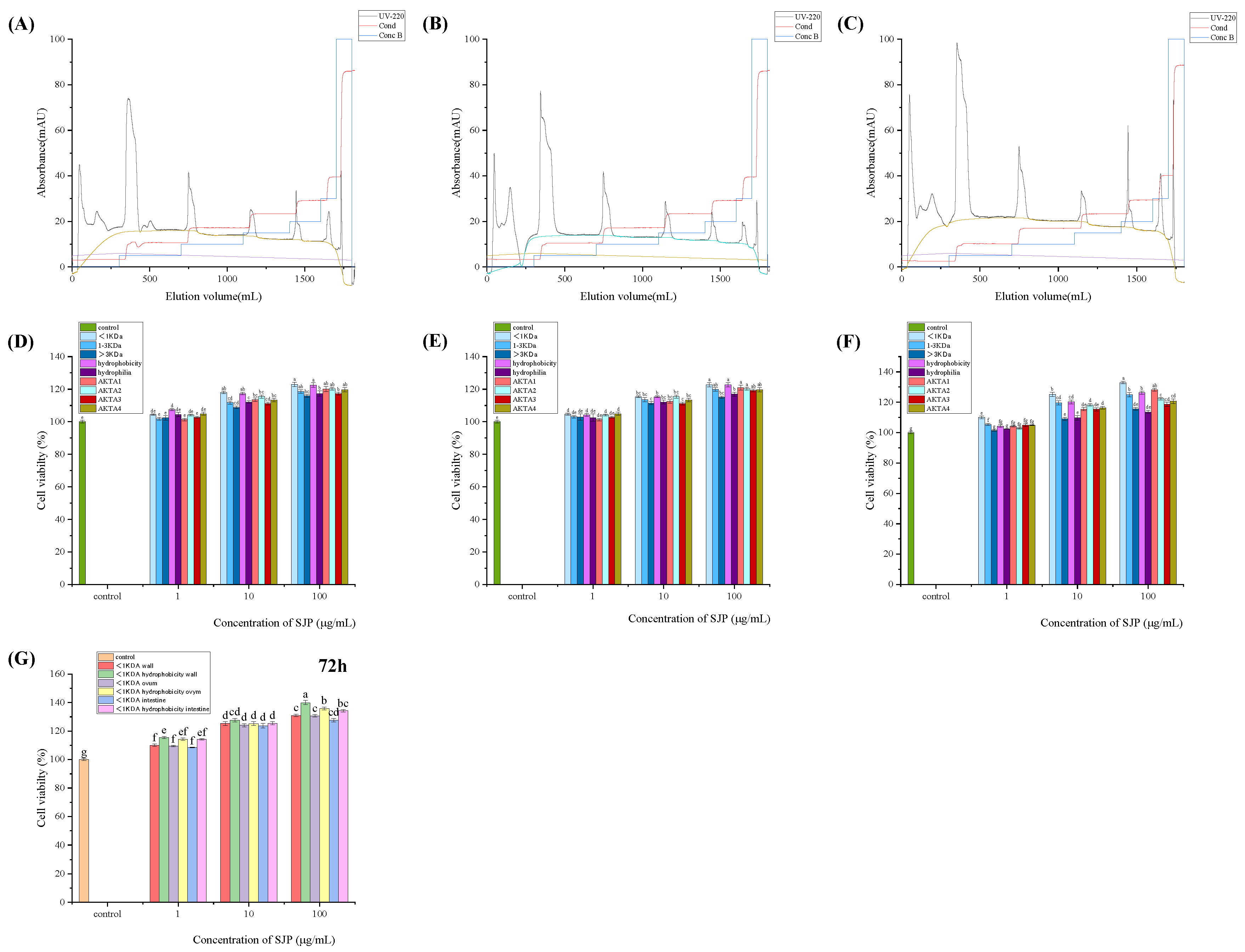
2.3. Three Purifications of Sea Cucumber Protein Peptides for Osteoblasts Value-Added Situation
2.4. The Enhancement of Each Index after Efficient Purification of Sea Cucumber Protein Peptides
2.5. Prediction and Screening of Sea Cucumber Peptides' Bone-Enhancing Function Activity
2.6. Interaction between Bone-Enhaning Sea Cucumber Peptides and Osteogenic Receptor Protein
| Component | Cell Viability Enhancement Rate | Protein Content Increase Rate | Yield |
|---|---|---|---|
| Intestine separation | 129.30% | 112.00% | 31.20% |
| Ovum separation | 131.20% | 109.10% | 39.30% |
| Wall separation | 133.70% | 111.50% | 37.80% |
| Specimen | Ligand | Receptor | Arithmetic | Score |
|---|---|---|---|---|
| Intestine extraction | SGEGGQGSLTR | 1L5G | CDOCKER | 274.357 |
| 3C4M | CDOCKER | 236.992 | ||
| 3VI4 | CDOCKER | 232.065 | ||
| 4GIQ | CDOCKER | 148.756 | ||
| AYLGKDVY | 1L5G | CDOCKER | 175.322 | |
| 3C4M | CDOCKER | 135.32 | ||
| 3VI4 | CDOCKER | 143.67 | ||
| 4GIQ | CDOCKER | 139.043 | ||
| TGSKLVVS | 1L5G | CDOCKER | 146.116 | |
| 3C4M | CDOCKER | 100.055 | ||
| 3VI4 | CDOCKER | 153.105 | ||
| 4GIQ | CDOCKER | 127.719 | ||
| KRSIEGGNLVY | 1L5G | CDOCKER | 205.241 | |
| 3C4M | CDOCKER | 160.821 | ||
| 3VI4 | CDOCKER | 147.637 | ||
| 4GIQ | CDOCKER | 160.906 | ||
| GEGGQGSLTR | 1L5G | CDOCKER | 171.331 | |
| 3C4M | CDOCKER | 131.638 | ||
| 3VI4 | CDOCKER | 146.215 | ||
| 4GIQ | CDOCKER | 136.137 | ||
| Ovum extraction | KSYELPDGQVI | 1L5G | CDOCKER | 202.511 |
| 3C4M | CDOCKER | 152.484 | ||
| 3VI4 | CDOCKER | 192.802 | ||
| 4GIQ | CDOCKER | 169.882 | ||
| AGRDLTDYLM | 1L5G | CDOCKER | 195.779 | |
| 3C4M | CDOCKER | 146.028 | ||
| 3VI4 | CDOCKER | 177.333 | ||
| 4GIQ | CDOCKER | 106.331 | ||
| NAPAMYVAIQ | 1L5G | CDOCKER | 166.736 | |
| 3C4M | CDOCKER | 115.185 | ||
| 3VI4 | CDOCKER | 134.672 | ||
| 4GIQ | CDOCKER | 111.114 | ||
| DLAGRDLTDYLMK | 1L5G | CDOCKER | 233.578 | |
| 3C4M | CDOCKER | 167.606 | ||
| 3VI4 | CDOCKER | 198.733 | ||
| 4GIQ | CDOCKER | 184.698 | ||
| VPIYEGY | 1L5G | CDOCKER | 141.316 | |
| 3C4M | CDOCKER | 120.023 | ||
| 3VI4 | CDOCKER | 105.006 | ||
| 4GIQ | CDOCKER | 84.0958 | ||
| Wall extraction | KSYELPDGQVITIG | 1L5G | CDOCKER | 285.931 |
| 3C4M | CDOCKER | 272.861 | ||
| 3VI4 | CDOCKER | 199.712 | ||
| 4GIQ | CDOCKER | 199.459 | ||
| VPIYEGYALPHAILRL | 1L5G | CDOCKER | 177.91 | |
| 3C4M | CDOCKER | 145.818 | ||
| 3VI4 | CDOCKER | 115.815 | ||
| 4GIQ | CDOCKER | 127.528 | ||
| DLAGRDLTDY | 1L5G | CDOCKER | 214.347 | |
| 3C4M | CDOCKER | 164.821 | ||
| 3VI4 | CDOCKER | 203.027 | ||
| 4GIQ | CDOCKER | 189.014 | ||
| IVRDIKEKLNYVAL | 1L5G | CDOCKER | 223.277 | |
| 3C4M | CDOCKER | 214.109 | ||
| 3VI4 | CDOCKER | 204.98 | ||
| 4GIQ | CDOCKER | 241.457 | ||
| AVLSLYASGRTTGIVLDSGDGVTH | 1L5G | CDOCKER | 256.585 | |
| 3C4M | CDOCKER | 214.712 | ||
| 3VI4 | CDOCKER | 151.5 | ||
| 4GIQ | CDOCKER | 189.974 |
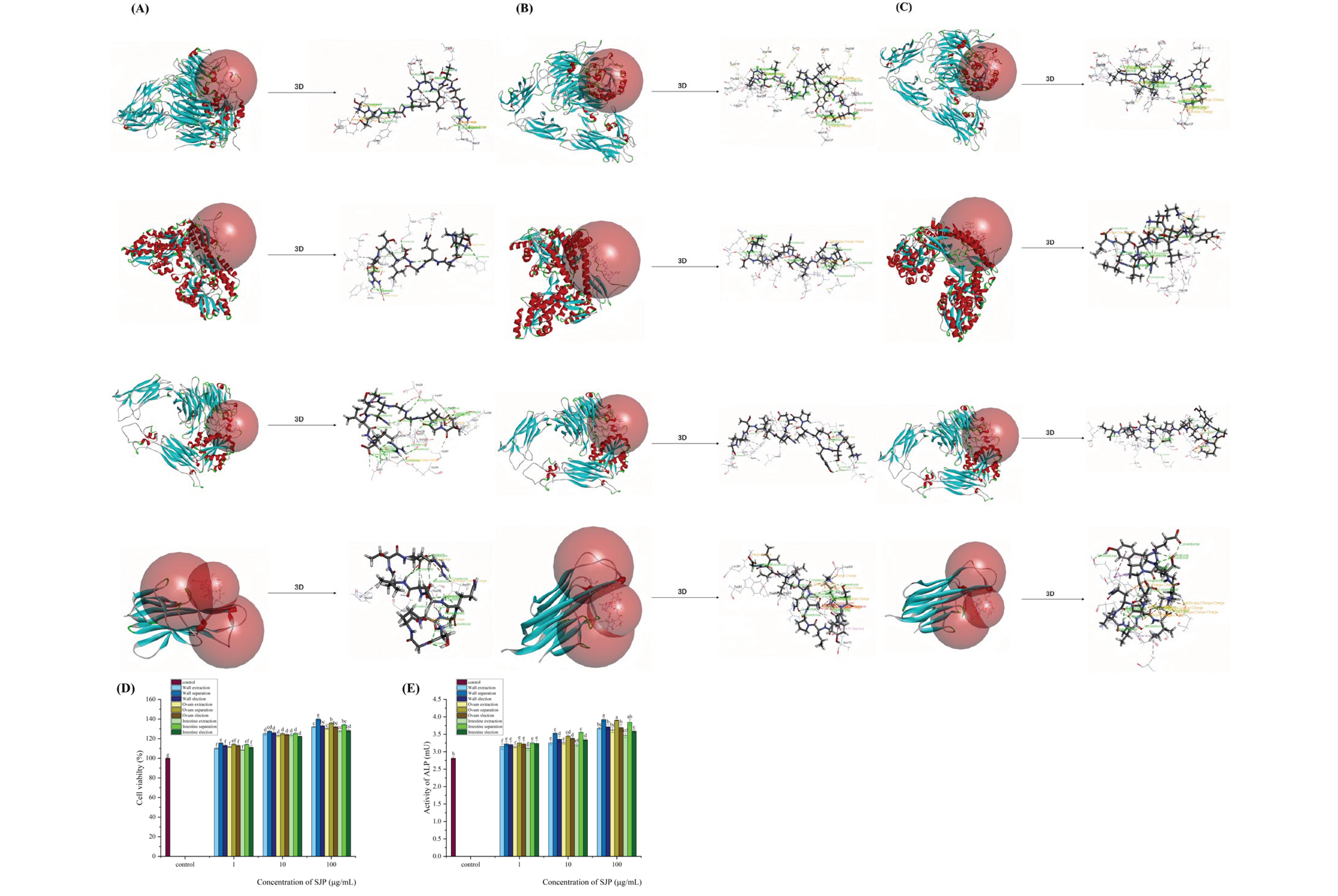
3. Discussion
4. Materials and Chemicals
4.1. Preparation of Sea Cucumber Enzymatic Powder
4.2. Amino Acid Composition and Molecular Weight Distribution of Sea Cucumber Enzyme Powder Extracted from Three Sites
4.3. Identification of Enzymatic Peptide Sequences of Sea Cucumber Extracted from Three Sites by UPLC-Q-TOF Coupled with CESI-Q-TOF
4.4. Purification and Separation of Sea Cucumber Peptides based on Differences in Molecular Weight Distribution, Hydrophilicity and Ionization
4.5. Molecular Docking Predicts the Analysis of Osteoactive Peptides in Sea Cucumbers
4.6. Cell Culture and MTT Assay
4.7. Alkaline Phosphatase (ALP) Activity Assay
Acknowledgments
References
- Jingwei J ,Shan G ,Zelong Z , et al.A novel short-type peptidoglycan recognition protein with unique polysaccharide recognition specificity in sea cucumber, Apostichopus japonicus.J].Fish shellfish immunology,2023,144109263-109263.
- Yingqiu Z ,Huachen L ,Xiao C , et al.A potential feeding regulation strategy during aestivation: Relaxation of intestine mediated by pedal peptide/orcokinin-type neuropeptides in sea cucumber Apostichopus japonicusJ].Aquaculture,2024,579.
- Cheng, X.; Zhao, K.; Zha, X.; Du, X.; Li, Y.; Chen, S.; Wu, Y.; Li, S.; Lu, Y.;Zhang, Y. Opportunistic Screening Using Low-Dose CT and the Prevalence of Osteoporosis in China: A Nationwide, Multicenter Study. J. Bone Miner. Res.2021, 36 (3), 427−435Wei Q ,Holle A ,Li J , et al.BMP-2 Signaling and Mechanotransduction Synergize to Drive Osteogenic Differentiation via YAP/TAZ.J].Advanced science (Weinheim, Baden-Wurttemberg,Germany),2020,7(15):1902931-1902931.
- Tianming W ,Zheng C ,Zhangfei S , et al.Existence and functions of a kisspeptin neuropeptide signaling system in a non-chordate deuterostome species.J].eLife,2020,9.
- Qiufang B ,Ming Z ,Jizhi Z , et al.NGF mediates protection of mesenchymal stem cells-conditioned medium against 2,5-hexanedione-induced apoptosis of VSC4.1 cells via Akt/Bad pathway.J].Molecular and cellular biochemistry,2020,469(1-2):53-64.
- Li X ,Dong Y ,Yin H , et al.Mesenchymal stem cells induced regulatory dendritic cells from hemopoietic progenitor cells through Notch pathway and TGF-β synergisticallyJ].Immunology Letters,2020,222(prepublish):49-57.
- Xingwei S ,Weiwei Z ,Chen Q , et al.Focal adhesion kinase promotes BMP2-induced osteogenic differentiation of human urinary stem cells via AMPK and Wnt signaling pathways.J].Journal of cellular physiology,2020,235(5):4954-4964.
- Bowen M ,Dongle W ,Yangfan C , et al.Interleukin-20 differentially regulates bone mesenchymal stem cell activities in RANKL-induced osteoclastogenesis through the OPG/RANKL/RANK axis and the NF-κB, MAPK and AKT signalling pathways.J].Scandinavian journal of immunology,2020,91(5):e12874.
- Hu Z ,Cao X ,Guo M , et al.Identification and characterization of a novel short-type peptidoglycan recognition protein in Apostichopus japonicusJ].Fish and Shellfish Immunology,2020,99257-266.
- Chaiyamoon A ,Tinikul Y ,Chaichotranunt S , et al.Existence of two mature sequences of cubifrin neuropeptide and their effects on spawning in the sea cucumber, Holothuria scabraJ].Aquaculture,2020,519734753-734753.
- Chaiyamoon A ,Tinikul R ,Nontunha N , et al.Characterization of TRH/GnRH-like peptides in the sea cucumber, Holothuria scabra , and their effects on oocyte maturationJ].Aquaculture,2020,518734814-734814.
- Chieu D H ,Suwansa-ard S ,Wang T , et al.Identification of neuropeptides in the sea cucumber Holothuria leucospilotaJ].General and Comparative Endocrinology,2019,283113229.
- Dinh H C ,Luke T ,K M S , et al.Aquaculture Breeding Enhancement: Maturation and Spawning in Sea Cucumbers Using a Recombinant Relaxin-Like Gonad-Stimulating Peptide.J].Frontiers in genetics,2019,1077.
- Chen M ,Talarovicova A ,Zheng Y , et al.Neuropeptide precursors and neuropeptides in the sea cucumber Apostichopus japonicus: a genomic, transcriptomic and proteomic analysisJ].Scientific Reports,2019,9(1):1-22.
- Cusimano G M ,Spinello A ,Barone G , et al.A Synthetic Derivative of Antimicrobial Peptide Holothuroidin 2 from Mediterranean Sea Cucumber (Holothuria tubulosa) in the Control of Listeria monocytogenesJ].Marine Drugs,2019,17(3):159.
- Xianliang L ,Wangxin L ,Baodong Z , et al.Sea cucumber peptides positively regulate sexual hormones in male mice with acute exhaustive swimming: possibly through the Casup2+/sup/PKA signaling pathway.J].Food function,2023,.
- Cong X ,Liu H ,Zheng Y , et al.A Putative Role of Vasopressin/Oxytocin-Type Neuropeptide in Osmoregulation and Feeding Inhibition ofApostichopus japonicusJ].International Journal of Molecular Sciences,2023,24(18):.
- Xueying G ,Kui D ,Libin Z .The effects of kisspeptin-type neuropeptides on feeding behavior and intestinal metabolism in the sea cucumber, Apostichopus japonicusJ].Aquaculture,2023,571.
- Xueying G ,Libin Z ,Kang X .Effect of Kisspeptin-Type Neuropeptide on Locomotor Behavior and Muscle Physiology in the Sea Cucumber Apostichopus japonicusJ].Animals,2023,13(4):705-705.
- Jiahui X ,G S V .Critical role of the mTOR pathway in poultry skeletal muscle physiology and meat quality: an opinion paper.J].Frontiers in physiology,2023,141228318-1228318.
- Ziqian W ,Xudong W ,Jingcun S , et al.Identification of Functional Modules and Key Pathways Associated with Innervation in Graft Bone-CGRP Regulates the Differentiation of Bone Marrow Mesenchymal Stem Cells via p38 MAPK and Wnt6/β-Catenin.J].Stem cells international,2023,20231154808-1154808.
- Retraction: MNK1-Induced eIF-4E Phosphorylation in Myeloma Cells: A Pathway Mediating IL-6-Induced Expansion and Expression of Genes Involved in Metabolic and Proteotoxic Responses.J].PloS one,2023,18(9):e0291491-e0291491.
- Hongxu L ,Zhaoxia Z ,Zhaoying W , et al.Human Mesenchymal Stem Cells-Derived Exosome Mimetic Vesicles Regulation of the MAPK Pathway and ROS Levels Inhibits Glucocorticoid-Induced Apoptosis in Osteoblasts.J].Stem cells international,2023,20235537610-5537610.
- W F F ,R R M ,B P M , et al.Effects of HSP70 chaperones Ssa1 and Ssa2 on Ste5 scaffold and the mating mitogen-activated protein kinase (MAPK) pathway in Saccharomyces cerevisiae.J].PloS one,2023,18(10):e0289339-e0289339.
- Mathematical A C M I M .Retracted: miR-138 Reduces the Dysfunction of T Follicular Helper Cells in Osteosarcoma via the PI3K/Akt/mTOR Pathway by Targeting PDK1.J].Computational and mathematical methods in medicine,2023,20239765985-9765985.
- Bo C ,Weidong S ,Song C , et al.Bone Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Ameliorated Lipopolysaccharide-Induced Lung Injury Via the miR-21-5p/PCSK6 Pathway.J].Journal of immunology research,2023,20233291137-3291137.
- Yuko K ,Miho K ,Christella W , et al.Regulation of Syk activity by antiviral adaptor MAVS in FcεRI signaling pathway.J].Frontiers in allergy,2023,41098474-1098474.
- Kyungsoo K ,Gyeom M K ,Min G L .Improving bone morphogenetic protein (BMP) production in CHO cells through understanding of BMP synthesis, signaling and endocytosisJ].Biotechnology Advances,2023,62108080-108080.
- Xi T ,Danning W ,Pei L , et al.Bone marrow mesenchymal stem cells alleviate stress-induced hyperalgesia via restoring gut microbiota and inhibiting neuroinflammation in the spinal cord by targeting the AMPK/NF-κB signaling pathway.J].Life sciences,2022,314121318-121318.
- Yingqiu Z ,Xiao C ,Huachen L , et al.Nervous System Development and Neuropeptides Characterization in Embryo and Larva: Insights from a Non-Chordate Deuterostome, the Sea Cucumber Apostichopus japonicusJ].Biology,2022,11(10):1538-1538.
- Hao Y ,Yingying T ,Xiaoxuan F , et al.Novel Peptides Derived from Sea Cucumber Intestine Promotes Osteogenesis by Upregulating Integrin-Mediated Transdifferentiation of Growth Plate Chondrocytes to Osteoblasts.J].Journal of agricultural and food chemistry,2022,70(41):.
- Y. C Z ,Qi Z ,Q. Q P , et al.Analysis of lncRNA in the skeletal muscle of rabbits at different developmental stages#13;J].Frontiers in Veterinary Science,2022,9948929-948929.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




