1. Introduction
Sensory integration is an important function that supports quality of human life [
1]. Even trivial daily movements, such as reaching an object, are based on the integration of multiple sources of sensory information, including visual and proprioceptive information, and a decline in function affects the quality of life, especially in older adults [
2]. In recent years, intensive studies have been conducted worldwide to elucidate its mechanisms and age-related decline [
2,
3].
Most studies have used performance [
4,
5], biological signals [
6], and brain activity measurements [
7,
8,
9] during motor tasks as indices to investigate the relationship between function and aging. A representative example of a motor task is one that combines arm reaching (extending the arm from a fixed position toward a target) and a visual rotation paradigm; using such a task, it has been shown that the ability to detect a gap (mismatch) between visual and proprioceptive feedback declines with age [
4,
5]. In addition, muscle and brain activity signals measured during other motor tasks such as walking and tool manipulation have shown age-related decreases in reaction time and changes in activity in the visual, parietal, and motor cortical areas [
6,
7,
8,
9].
However, such indices during motor tasks are task-dependent [
10]; therefore, the use of resting-state functional magnetic resonance imaging (rsfMRI) [
5,
11,
12,
13] to investigate the brain functional networks underlying sensory integration has attracted increasing interest in recent years. In particular, functional connectivity between brain regions has been extensively studied, including the relationship between age-related decline in motor performance and resting-state functional connectivity (rsFC) across various age groups [
11,
13] and between motor performance and rsFC in children [
12]. We also analyzed the performance during a motor task combining arm reaching and visual rotation paradigms using KINARM, a precision musculoskeletal robot, and rsfMRI data to show differences in rsFC among motor-related areas related to the ability to detect a gap in participants in their 20’s and 60’s, with and without visual rotation [
5]. These results suggest that rsFC is significantly associated with sensory integration and can be used as an index to detect and monitor function on a regular basis, without performing motor tasks.
In this study, we examined the possibility of using a commercially available virtual reality (VR) system to perform an arm-reaching exercise task with the aim of detecting the decline in sensory integration function due to aging using off-the-shelf hardware, thus supporting future large-scale deployment. We used a conventional reaching task with visual rotation but employed a different performance index considering the nature of the VR-HMD and its intended use in home settings. The gap-discrimination performance index used in conventional tasks is effective only for experiments performed by fixing the face and hand positions in a known manner with a precise exoskeleton robot such as KINARM. Accordingly, considering that we freely move our head and hands when using a VR-HMD, we instead used the accuracies of the reaching position under various disturbances as a performance index. This idea is based on the fact that older adults show a significant decline in sensory integration function in complex motor tasks [
10,
13,
14] and that motor accuracy is widely known to be valid as a proxy for measuring sensory integration function [
15].
First, we tested for a significant difference in motor accuracy during the proposed VR-HMD reaching task between the older and middle-aged adult groups. Next, we determined whether rsFC was significantly correlated with motor accuracy in older adults. To use the motor index from this task as an indicator of declining sensory integration function in old age, not only comparing middle-aged and older adults but also more specifically assessing the correlates of the differences in ability that occur in older age, we also conducted an rsFC analysis within the older adult group, especially further expanding the target brain areas to the whole brain.
2. Materials and Methods
2.1. Participants
Ninety-four healthy participants (53 in the middle-aged group, 43 ± 11 years, 43 males; 41 in the older adult group, 77 ± 6 years, 15 males) participated in the VR-HMD reaching task. Inclusion and exclusion criteria were based on the absence of disease at the time of measurement. The age range and dominant hand were 23-59 years for the middle-aged group (three left-handed and one ambidextrous) and 67-97 years for the older adult group (two left-handed). The participants were instructed to avoid caffeine, alcohol, and strenuous exercise prior to the experiment. The daily physical activity level of the older adult group was determined using a questionnaire and classified as follows: no daily physical activity (level 0); light physical activity, such as walking at least once a week (level 1); strenuous physical activity, such as sports at least once a week (level 2); and strenuous physical activity, such as sports at least three times a week (level 3). level 2), and three or more times a week (level 3) for sports and other strenuous exercises. (See
Tables S1 and S2.)
The rsfMRI measurements were performed on the group of 41 older adults who performed the VR reaching task, 19 of whom (76 ± 4 years old, 12 males) had no contraindications and agreed to undergo MRI scanning. The time of the visits varied across participants according to their availability, but there were no correlations between the MRI acquisition time and the rsFCs or between the VR testing time and the mean gap angles indicated in the study. Therefore, this parameter was not included as a covariate in the general linear model analysis used in this study. The time gap between the behavioral test and MRI scan varied according to the availability of the participants, ranging from 0 to 60 days (18 ± 20 days). For participants who performed the tasks on the same day, an MRI scan was first conducted to avoid the effect of the VR task on resting-state brain activity. This research protocol was approved by the Ethical Review Committee for Research Involving Human Subjects of the Tokyo Institute of Technology (approval no. 2021172, November 29, 2021) and conducted in accordance with the Declaration of Helsinki.
2.2 VR-HMD Arm-Reaching Task
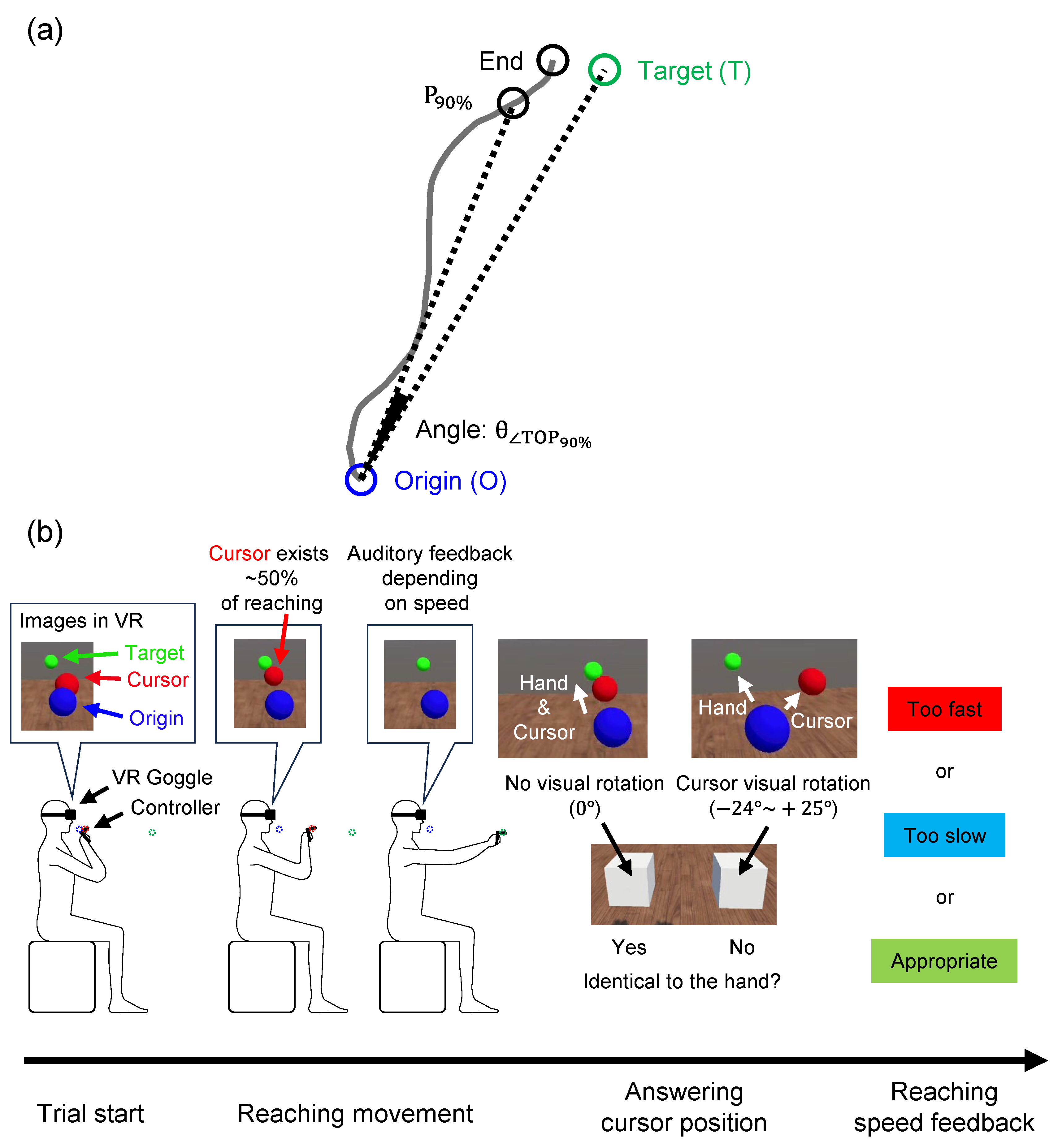
The sensory integration function was examined using the gap angle between the hand reaching and target positions (
Figure 1(a),
) while performing a VR-HMD reaching task under multiple cognitive loads. The participants wore a VR-HMD (Meta Quest 2, Meta Platforms, Inc., California) on their faces and held the two controllers with their hands. In the VR space, they moved a red sphere (i.e., a cursor) representing their right-hand position from a blue sphere (i.e., the origin) to a green sphere (i.e., the target) by moving their right arm (see
Figure 1(b)). Because the VR-HMD covered the entire field of view, the hands and controllers could not be seen directly. However, the positions of the right hand, origin, and target could be recognized through the VR image. The invisibility of their hands from the VR-HMD wearers provided the advantage of being able to perform motor tasks exclusively by relying on proprioceptive feedback. The positions of the origin/target were determined based on arm length through a calibration process performed by each participant before starting the experiment. The origin position was fixed anterior to the chin, and the target position was randomly determined per trial on an arc centered anteriorly at 90% of the maximum distance from the origin.
The older adults show a marked decline in sensory integration during complex motor tasks [
10,
13,
14]. To investigate the reaching performance in situations that mimic everyday life during which participants pay attention to a variety of distractors, we created four specifications that would interfere with reaching. First, the red sphere representing the participant’s right-hand position was visually rotated from its actual position every two or three trials. The rotation angle was randomly chosen from 1° to 24° clockwise or from 1° to 25° counterclockwise around the vertical upward axis. After each rotated trial, one or two non-rotated trials were performed to wash out the after-effects of the rotation. Second, in order to prevent reaching by paying attention only to the proprioceptive senses, participants were asked to respond whether the rotation was applied or not after each trial through a question “Identical to the hand?” Third, the red sphere disappeared when the arm was extended to approximately 50% of its full reach, to prevent answering the question based only on the difference between the end position of reaching and the target position. Fourth, to draw attention toward maintaining a constant reaching speed, auditory feedback was provided at the end of trial depending on the speed deviated from the specified value, and a visual message of “too fast” or “too slow” was displayed.
The participants were instructed to reach the target by relying on their own kinesthesia, not on the VR images, and they performed 108 task trials after 10 practice trials to familiarize themselves with the task. We asked the participants to perform a practice session consisting of 10 trials. Because we explained the basic guidelines of VR technology prior to the practice session, most participants learned to perform the task in a single session. Another session was conducted with a few participants who could not correctly perform the task after a single practice session. In all trials, hand positions were recorded at a sampling rate of 72 Hz, and answers to the questions and target positions were recorded once for each trial.
) was formed by three points projected on the horizontal plane; 90% position of the reach (), origin (O), and target (T); (b) Overview of the VR-HMD reaching task. A trial started by positioning the hand controller (red sphere) at the origin (blue sphere) in front of the chin. A target (green sphere) was displayed on an arc centered anteriorly, and the participants reached the target. In some trials, the red sphere was randomly rotated from the actual hand position in the range of −25° to +24°. In addition, the red sphere disappeared when the hand was extended by 50% of the distance between the origin and the target. At the end of the reach, sound feedback was provided according to the reaching speed. To answer whether the rotation was applied during the trial, two white boxes appeared at the end of the trial. Visual feedback on the reaching speed was displayed after the answering time.
2.3. Behavioral Data Analyses
2.3.1. Mean Gap Angle Calculation
To examine movement accuracy in the reaching task, the mean of the angle (
) was calculated across all trials for each participant (mean gap angle). The reason for using the 90% position rather than the end of the reach was to minimize the effect of a certain number of participants who stopped reaching before the target because of the disappearance of the red sphere in the second half of the reach, which caused an increase in the gap angle and did not reflect their reaching accuracy. The mean gap angle was calculated as follows:
where
represents taking the average of 108 trials. Additionally,
represents the vector from the origin (O) to 90% of the reach (
), and
represents the vector from the origin (O) to the target (T).
and
are the absolute values (vector lengths) of
and
, respectively.
We considered the mean gap angle as an index reflecting sensory integration function and used it as an explanatory variable for the rsFC analysis using rsfMRI measurement data (see Section 3.5.5).
2.3.2. Statistical Analyses
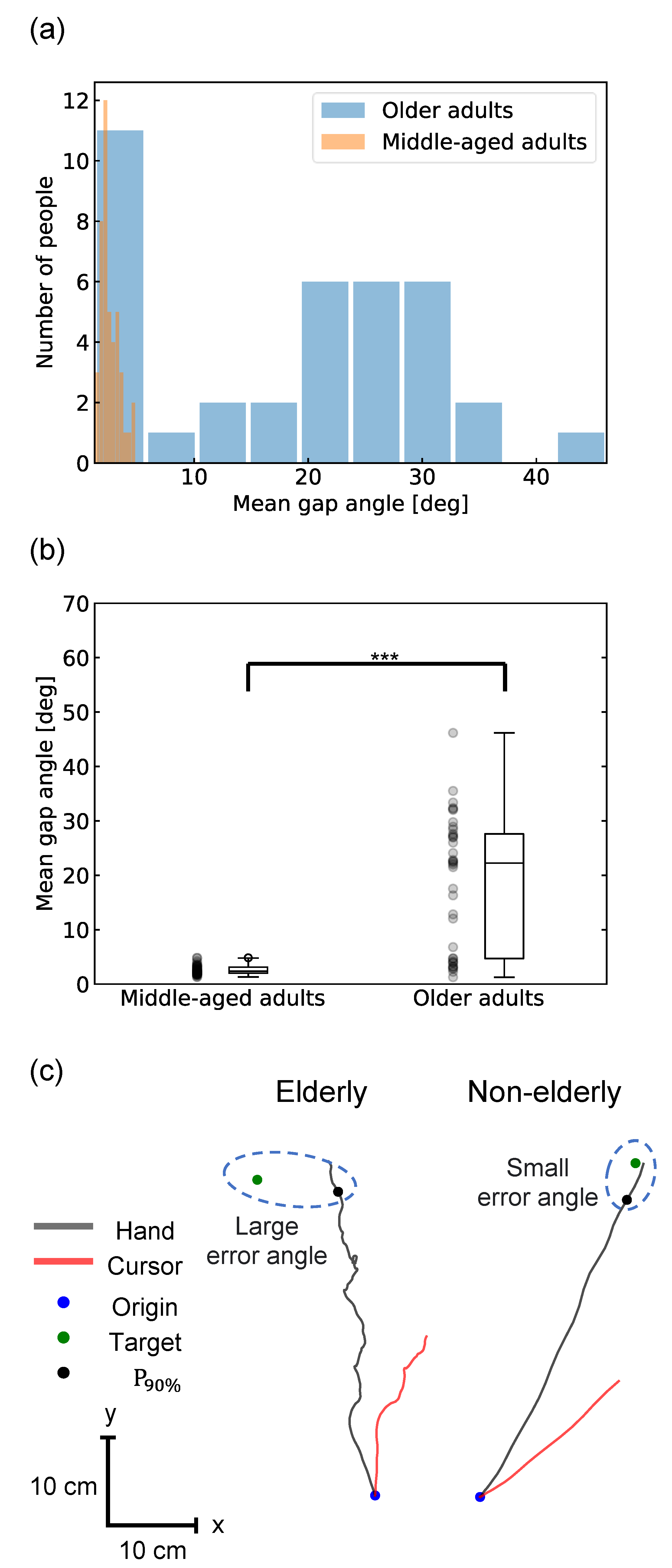
For behavioral analyses, histograms of the individual mean gap angles were created for both groups and a normal distribution test was performed (
Figure 2(A)), and it was confirmed that they all deviated from the normal distribution. Therefore, a Mann-Whitney U test was used in addition to a two-sample t-test to assess the differences in the mean gap angle between the middle-aged and older adult groups (
Figure 2(B)). The correlations between age and the mean gap angle and between the daily physical activity level and the mean gap angle within the older adult group were computed using Spearman’s rank-order correlation and Pearson's correlation coefficient.
2.4 MRI Acquisition
We used a 3 T Magnetom Prisma scanner (Siemens AG, Munich, Germany) to acquire rsfMRI series and structural volumes. The participants were instructed to look at the displayed crosshairs without thinking about specific matters, and two sessions of 6-min resting-state brain activity (functional images) and structural images (approximately 5 min) were acquired.
Functional images were acquired, using a T2*-weighted gradient-echo echoplanar imaging sequence (AP and PA phase encode directions) with the following parameters: repetition time (TR) = 800 ms, echo time (TE) = 34.4 ms, flip angle (FA) = 52◦, field of view (FOV) = 206.4 × 206.4 mm, matrix size = 86 × 86, 60 slices, slice thickness = 2.4 mm, 450 volumes. For the anatomical MRI acquisition, T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence was used with the following parameters (TR = 1.9 ms, TE = 2.52 ms, FA = 9◦, FOV = 256 × 256 mm; matrix size = 256 × 256, 224 slices, slice thickness = 1.0 mm).
2.5 MRI MRI Data Analyses
We calculated rsFC using the CONN toolbox [
16] (RRID:SCR_009550) release 22.a [
17] and SPM [18] (RRID:SCR_007037) release 12.7771.
2.5.1. Preprocessing
Functional and anatomical data were preprocessed using a flexible preprocessing pipeline [
19], including realignment with the correction of susceptibility distortion interactions, slice timing correction, outlier detection, direct segmentation, Montreal Neurological Institute (MNI) space normalization, and smoothing. Functional data were realigned using SPM [
20] realign and unwarp procedures [
21], where all scans were co-registered to a reference image (first scan of the first session) using a least-squares approach and a six-parameter (rigid body) transformation [
22] and resampled using b-spline interpolation to correct for motion and magnetic susceptibility interactions. Temporal misalignment between different slices of the functional data (acquired in interleaved order) was corrected following the SPM slice-timing correction (STC) procedure [
23,
24] using sinc temporal interpolation to resample each BOLD time series slice to a common mid-acquisition time. Potential outlier scans were identified using artifact detection tools (ART) [
25] as acquisitions with framewise displacement above 0.5 mm or global BOLD signal changes above three standard deviations [
26,
27]. A reference BOLD image was computed for each participant by averaging all scans and excluding outliers. Functional and anatomical data were normalized to standard MNI space, segmented into gray matter, white matter, and cerebrospinal fluid (CSF) tissue classes, and resampled to 2-mm isotropic voxels following a direct normalization procedure [
26,
28] using the SPM unified segmentation and normalization algorithm [
29,
30] with the default IXI-549 tissue probability map template. Finally, functional data were smoothed using spatial convolution with a Gaussian kernel of 8-mm full width at half maximum (FWHM).
2.5.2. Denoising
In addition, functional data were denoised using a standard denoising pipeline [
31], including the regression of potential confounding effects characterized by white matter timeseries (5 CompCor noise components), CSF timeseries (5 CompCor noise components), motion parameters and their first-order derivatives (12 factors) [
32], outlier scans (below 41 factors) [
27], session and task effects and their first-order derivatives (two factors), and linear trends (two factors) within each functional run, followed by bandpass frequency filtering of the BOLD timeseries [
33] between 0.008 Hz and 0.09 Hz. CompCor [
34,
35] noise components within the white matter and CSF were estimated by computing the average BOLD signal and largest principal components orthogonal to the BOLD average, motion parameters, and outlier scans within each participant’s eroded segmentation mask. From the number of noise terms included in this denoising strategy, the effective degrees of freedom of the BOLD signal after denoising were estimated to range from 103.4 to 110.1 (average 108.4) across all participants [
26].
2.5.3. Regions of Interest (ROIs)
The atlas provided by CONN [
16] (RRID:SCR_009550) release 22.a [
17] was used. In addition, for a more detailed analysis of the motor and sensory areas, the precentral and postcentral gyri were replaced with six left and right sensory-motor area templates defined in the Human Motor Area Template (HMAT [
36]).
2.5.4. First-Level Analysis
Seed-based connectivity maps (SBC) and ROI-to-ROI connectivity matrices (RRCs) were used to characterize the patterns of functional connectivity with 140 ROIs. Functional connectivity strength was represented by Fisher-transformed bivariate correlation coefficients from a weighted general linear model (weighted-GLM; [
37]), defined separately for each pair of seed and target areas, modeling the association between their BOLD signal time series. To compensate for possible transient magnetization effects at the beginning of each run, individual scans were weighted using a step function convolved with an SPM canonical hemodynamic response function and then rectified.
2.5.5. Group-Level Analyses
Group-level analyses were performed using general linear models (GLM). The RRC obtained in the first-level analysis was used as the objective variable. Age, sex, physical activity levels, and the mean gap angle calculated in the behavioral data analyses were used as explanatory variables to estimate the functional connectivity between ROIs with a high mean gap angle and correlation. The results had an ROI-level threshold of p < 0.05 (FDR corrected).
3. Results
3.1 Behavioral Difference between Older Adults and Middle-Aged Adults
None of the participants had any illness at the time of measurement. The daily physical activity levels for the older adults were six at level 0, 24 at level 1, eighy at level 2, and three at level 3. (See
Tables S1 and S2.) Of the participants in the VR-HMD reaching task experiment, 12 (nine in the middle-aged adult group and three in the older adult group) gave the same answer to the question about rotation all the time, or 107 out of 108 times. Therefore, data from 12 participants were excluded from the analysis because they may not have fully understood the task and/or were reaching without paying proper attention. In addition, one older adult participant with a large signal dropout in the orbitofrontal region on fMRI was excluded from analysis. Data from 81 participants (44 middle-aged adults, 43 ± 11 years, 34 men; 37 older adults, 77 ± 6 years, 12 men) were analyzed. Detailed information is summarized in
Tables S1 and S2.
Movement accuracy, that is, the mean gap angle between the reached hand and target positions, was calculated for each participant. The comparison between the middle-aged and older adult groups showed a significant difference: the middle-aged group had 2.6 ± 0.8° and the older adult group had 18.8 ± 12.2° (t-score=8.8, p < 0.001, two-sample t-test; U-statistic=1501, p<0.001) (see
Figure 2(B)). While all the participants in the middle-aged group showed less than 5 °, the older adult group varied widely, with 12 participants having < 10° and 21 having > 20°. Participants with a large mean gap angle were unable to reach the target straight and tended to reach it by meandering away from the target (
Figure 2(C)). The correlation coefficient between age and the mean gap angle did not show significant correlation within each group (Pearson’s r=0.01, p=0.9 and Spearman’s r=0.007, p=1.0 for the middle-aged adult group, and Pearson’s r=0.19, p=0.3 and Spearman’s r=0.09, p=0.6 for the older adult group.) For the older adult group, the Pearson's correlation coefficient between physical activity level and the mean gap angle was Pearson’s r = –0.22, p=0.2, and Spearman’s r = –0.13, p=0.5, showing no significant correlation. The individual mean map angles are summarized in
Table S3.
3.2 rsFC of Older Adults Reflecting the Mean Gap Angle
We analyzed rsFC using 16 participants in the older adult group (76 ± 4 years, 10 males) by excluding two participants who were judged to have insufficient understanding of the VR-HMD reaching task and one participant whose fMRI imaging showed a large signal dropout in the orbitofrontal region.
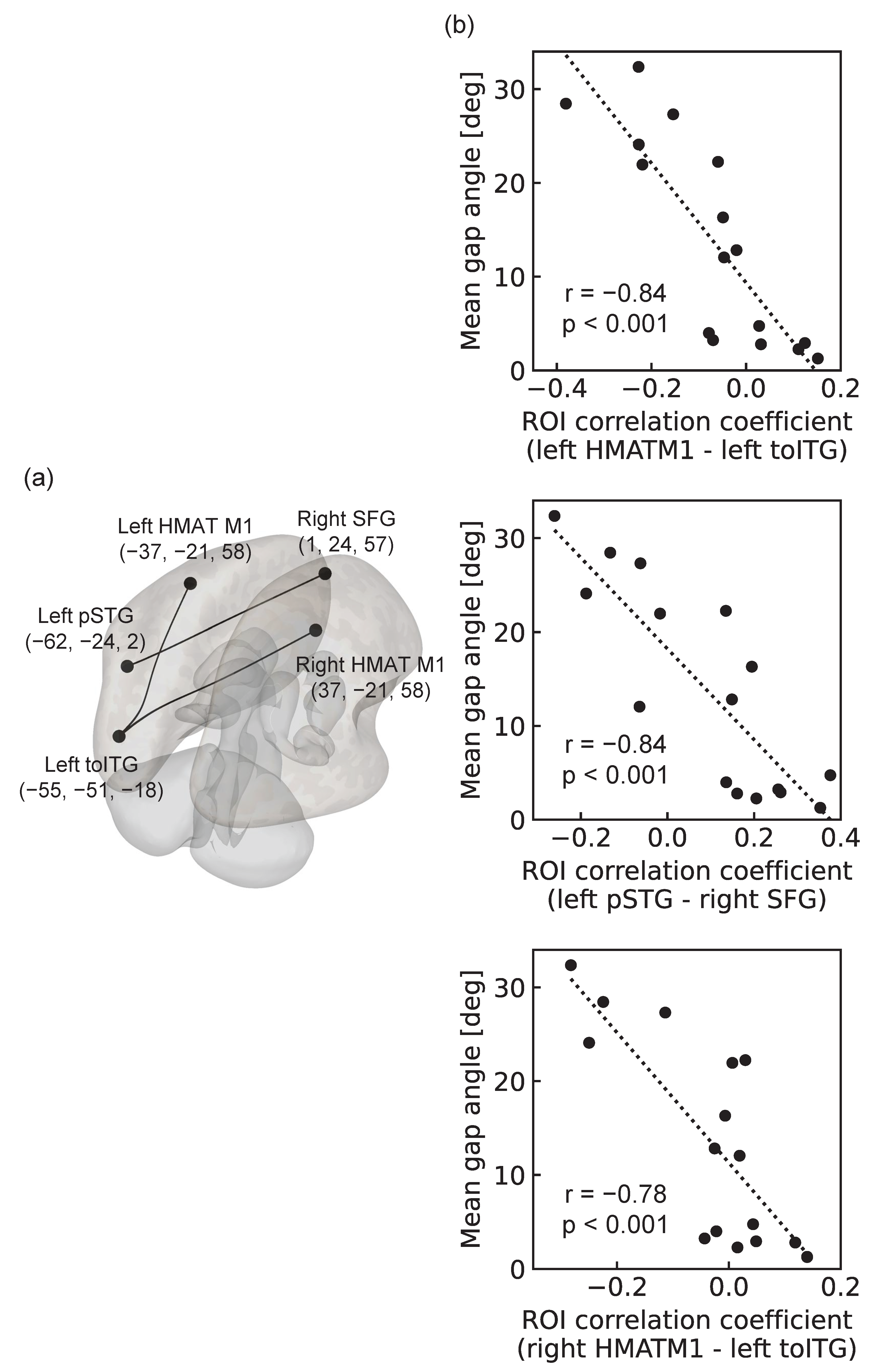
Three pairs of rsFCs were significantly correlated with the mean gap angle. The first was the association of the left HMAT M1 with the left inferior temporal gyrus, temporooccipital part (toITG) (T(12)= − 5.60, p-FDR=0.02); the second was the association of the left superior temporal gyrus, posterior division (pSTG), and right superior frontal gyrus (SFG) binding (T(12) = –5.69, p-FDR=0.02); and the third was the right HMAT M1 and binding of the left inferior temporal gyrus and temporooccipital part (toITG) (T(12) = –4.98, p-FDR=0.03) (see
Figure 3). All were negatively correlated, showing greater functional connectivity strength in participants with higher motor accuracy, that is, smaller mean gap angles.
4. Discussion
In this study, to propose a task capable of assessing sensory integration function in daily life at home, we examined whether VR-HMD-based reaching task performance was associated with rsFC between the brain regions involved in sensory integration function. The task performance was assessed using the gap angle between the hand-reaching and target positions (i.e., the mean gap angle) during the VR-HMD reaching task with multiple cognitive loads. As expected, the gap angle was significantly smaller in the middle-aged group than that in the older adult group. The mean gap angle was 2.6 ± 0.8° and 18.8 ± 12.2° for the middle-aged and older adult groups, respectively (p < 0.001). Furthermore, in the older adult group, a significant correlation was observed between the gap angle and rsFC between the left/right HMAT M1 and left ITG, suggesting that the integrated function of motor control and visual information processing is involved in the decline in sensory integration in old age. In particular, since the rsFC between the left HMAT M1 and the left ITG survived even without including daily physical activity level as a covariate of GLM (T(12)= − 5.55, p-FDR=0.02), this rsFC would be the best index for detecting a decline in sensory integration function in old age. Based on these results, arm-reaching movements could be used as an indicator of declining sensory integration function in old age on a daily basis. For routine detection, it is necessary to construct a system using an off-the-shelf, economical device that can be used at home, rather than KINARM, which is a large, research-grade, and prohibitively expensive device. This study demonstrated that it is possible to construct a system that satisfies these requirements, detecting functional decline without requiring large space for experiments, expensive equipment, or specialized knowledge.
The two-sample t-test and Mann-Whitney U test for the middle-aged and older adult groups confirmed that the movement accuracy (mean gap angle) of the VR reaching task reflected the decline in sensory integration function during old age (2.6 ± 0.8°, middle-aged group vs. 18.8 ± 12.2°, older adult group; p < 0.001). As expected, little correlation was observed between age and mean gap angle in the middle-aged group (Pearson’s r=0.01, p=0.9; Spearman’s r = 0.007, p = 1.0). Because the decline in sensory integration function in old age is unique to older adults and does not appear in middle-aged participants, the fact that the mean gap angle is generally small for middle-aged people and that there is no correlation between age and the mean gap angle supports the idea that the mean gap angle represents the decline in sensory integration function in old age. In contrast, a positive but non-significant trend was observed in the older adult group (Pearson’s r = 0.19, p = 0.3; Spearman’s r = 0.09, p = 0.6). There was also a non-significant negative trend between physical activity level and mean gap angle (Pearson’s r = –0.22, p = 0.2; Spearman’s r = –0.13, p = 0.5). This may be due to individual differences in daily physical activity levels and other factors besides aging [
8,
10,
14], which may contribute to a decline in sensory integration in older adults. In other words, it may be determined by the superposition of the progression or prevention of functional decline due to a variety of factors, including aging, physical activity level, and other individual characteristics. These results suggest that the mean gap angle detects a decline in sensory integration, including individual differences other than aging. Although various indices that indicate differences between middle-aged and older adult groups have been studied [
5,
6,
7,
9,
11,
13], to the best of our knowledge, no studies have identified indices for individual differences in the decline of sensory integration in healthy older adults, and we consider the mean gap angle to be very useful.
It should be noted that the participants included in the analysis of this study were 34 males and 10 females (77% male proportion) in the middle-aged group and 12 males and 25 females (32%) in the older adult group, which is a different gender ratio for each group. Regarding sex differences in fine motor movements, studies have shown differences in motor tendencies in computer-pointing tasks [38]. In contrast, a study examining sex differences in gross and fine motor movements and the effects of aging showed no significant sex differences in any of the movements, indicating a strong effect of aging [39]. For the mean gap angles in this study, the mean gap angle between men and women in each group was 2.6 ± 0.8° for men in the middle-aged group (42 ± 11 years), 2.4 ± 0.9° for women in the middle-aged group (45 ± 10 years), 12.7 ± 11.5° for men in the older adult group (77 ± 5 years), and 21.7 ± 11.2°, and the results of the two-sample t-test for each group were significantly different only for the different combinations of age groups (see
Table S4). Based on these results, we believe that the mean gap angle is useful, even when sex bias is considered.
Next, assuming that the decline in sensory integration in old age is related to resting-state brain activity, three rsFCs that were significantly correlated with motor accuracy were identified in the older adult group. The first was the connection between the left HMAT M1 and left toITG. Left HMAT M1 is the left primary motor cortex [36], and is thought to play a major role in the motor control of the right arm [
40]. The left toITG is thought to play an important role in visual information processing. For example, the hemodynamics of the left ITG changes during active work and passive observation in a visual information task, suggesting that the left ITG may be involved in visual information processing [
41]. The functional connectivity between these two regions tended to be stronger in older adults with higher motor accuracy, suggesting that the integration of motor control and visual information processing through this connection is related to a decline in sensory integration function in older adults. Visual processes such as visual acuity and depth perception have been found to decline with age [
3]. Age-related declines in motor skills requiring vision, such as catching a ball [
10] and reaction time to visual stimuli [
14], have also been reported. Therefore, we considered this connection to increase the reliability of the mean gap angle as a measure of sensory integration. In addition, this connection was obtained from individual differences in the visuomotor abilities of healthy older adult people, unlike the comparison between middle-aged and older adult groups in previous studies, and the mean gap angle may serve as a new index for individual differences in the decline of sensory integration in healthy older adults [
3,
10,
14].
The second connectivity was the connection between the left pSTG and right SFG. The left pSTG plays an important role in phonological processing [
42,
43], and the right SFG controls motor inhibition [
44]. The functional connectivity between these two areas also tends to be stronger in older adults with higher motor accuracy, suggesting that the connection between phonological processing and motor inhibitory control is related to a decline in sensory integration in older adults. However, previous studies have not implicated phonological processing in the decline of sensory integration related to movement. Therefore, we considered two interpretations of this connection. The first is the effect of age-related brain dedifferentiation and compensation. Dedifferentiation is the loss of functional brain localization and diffusion of activity throughout the brain due to age-related changes in neurotransmission [
45]. Compensation means compensating for the loss of function by activating more brain regions as we age [
46]. These phenomena raise the possibility that activities other than those in brain regions generally thought to be involved in motor control may also be involved in sensory integration functions in old age. Several studies have suggested dedifferentiation and compensation of the motor system [
15,
47,
48]. This dedifferentiation and compensation may improve motor control by increasing the left and right pSTG activity and compensating for reduced motor system function. The second factor was the effect of task dependence. In the motor task in this study, the procedures for each step of the task were explained by visual messages in the text in the VR space and auditory messages in the audio. Further research is required to determine whether this connection is derived from understanding the task explanation in the motor task without using these messages.
The third was between the right HMAT M1 and left toITG. The right HMAT M1 is the right primary motor cortex [
36] and is thought to play a major role in motor control, mainly in the left arm. However, it has also been found to be involved in motor control of the right arm, and it has been noted that the involvement of the ipsilateral arm of the primary motor cortex in control increases with age [
49,
50]. A study using fMRI measurements during a tapping task with the fingers of the right hand in various age groups confirmed that the amount of bilateral M1 activation increases with age, with a significant increase in ipsilateral M1 activation [49]. In addition, a study using transcranial magnetic stimulation (TMS) showed that stimulation of the ipsilateral primary motor cortex had no effect on the younger group but increased the reaction time in the older group [
50]. These studies suggest that primary motor cortex dedifferentiation and compensation intensify with age. This third connectivity tends to be stronger in older adults with higher motor accuracy, suggesting that it functions as dedifferentiation and compensation of the right primary motor cortex. This integration of motor control and visual information processing plays an important role in enhancing sensory integration function during old age. We believe that this connectivity supports the idea that the mean gap angle is a useful indicator of declining sensory integration in old age.
In this study, a VR-HMD task was proposed as a system for detecting the decline in sensory integration functions in old age. It was suggested that the mean gap angle during the task could serve as an effective index of functional decline. For the practical application of this index to detect sensory integration function in old age, it is necessary not only to examine hand-reaching movements using the VR tasks in this study, but also to verify the index’s relevance to the decline in motor functions, such as walking and stair climbing, that older adults experience in daily life. Future research should investigate the extent of the relationship between mean gap angle and motor control abilities in real space. Because the physical activity levels in this study were based on participants' self-reports, they may be biased by factors such as measurement error [
51], social desirability, or social approval [
52]. In addition, the time period between the measurement of the VR reaching task and MRI measurements in this study ranged from 0 to 60 days, depending on the participant. Given that older adults undergo significant brain changes within a short period of time, even in healthy subjects [
53], differences in the measurement period for each participant may have affected the results.
Author Contributions
Conceptualization, N.Y.; methodology, S.I., H.M. and N.Y.; software, S.I., H.M., K.S. and L.M.; validation, S.I. and H.M.; formal analysis, S.I., H.M. and L.M.; investigation, H.M., K.S. and N.Y.; resources, N.Y.; data curation, S.I. and N.Y.; writing—original draft preparation, S.I; writing—review and editing, L.M. and N.Y.; visualization, S.I.; supervision, N.Y.; project administration, N.Y.; funding acquisition, N.Y. All authors read and agreed to the published version of the manuscript.