Submitted:
29 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussions
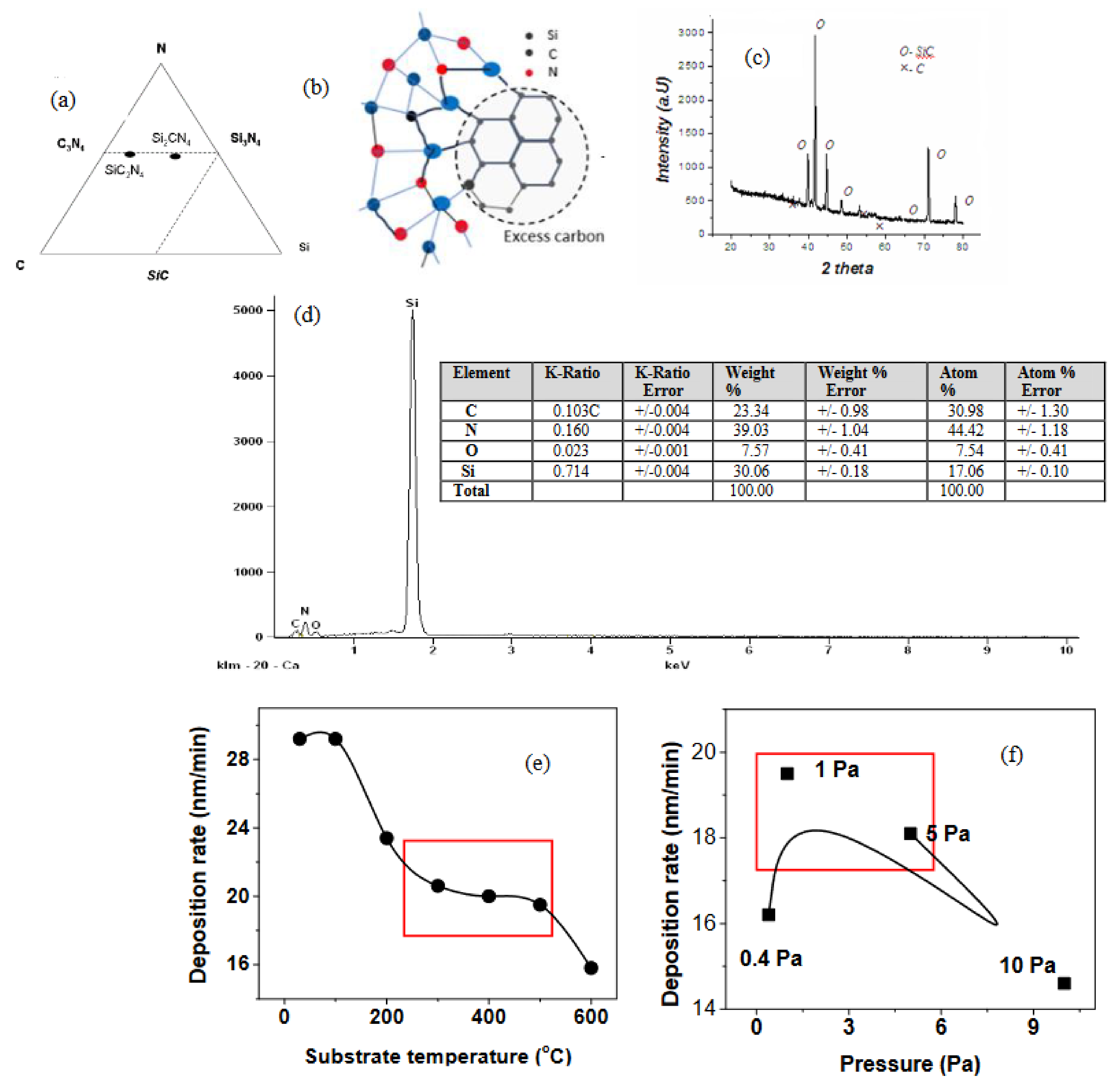
3.1. X-Ray Analysis
3.2. Sputtering and Deposition Rates
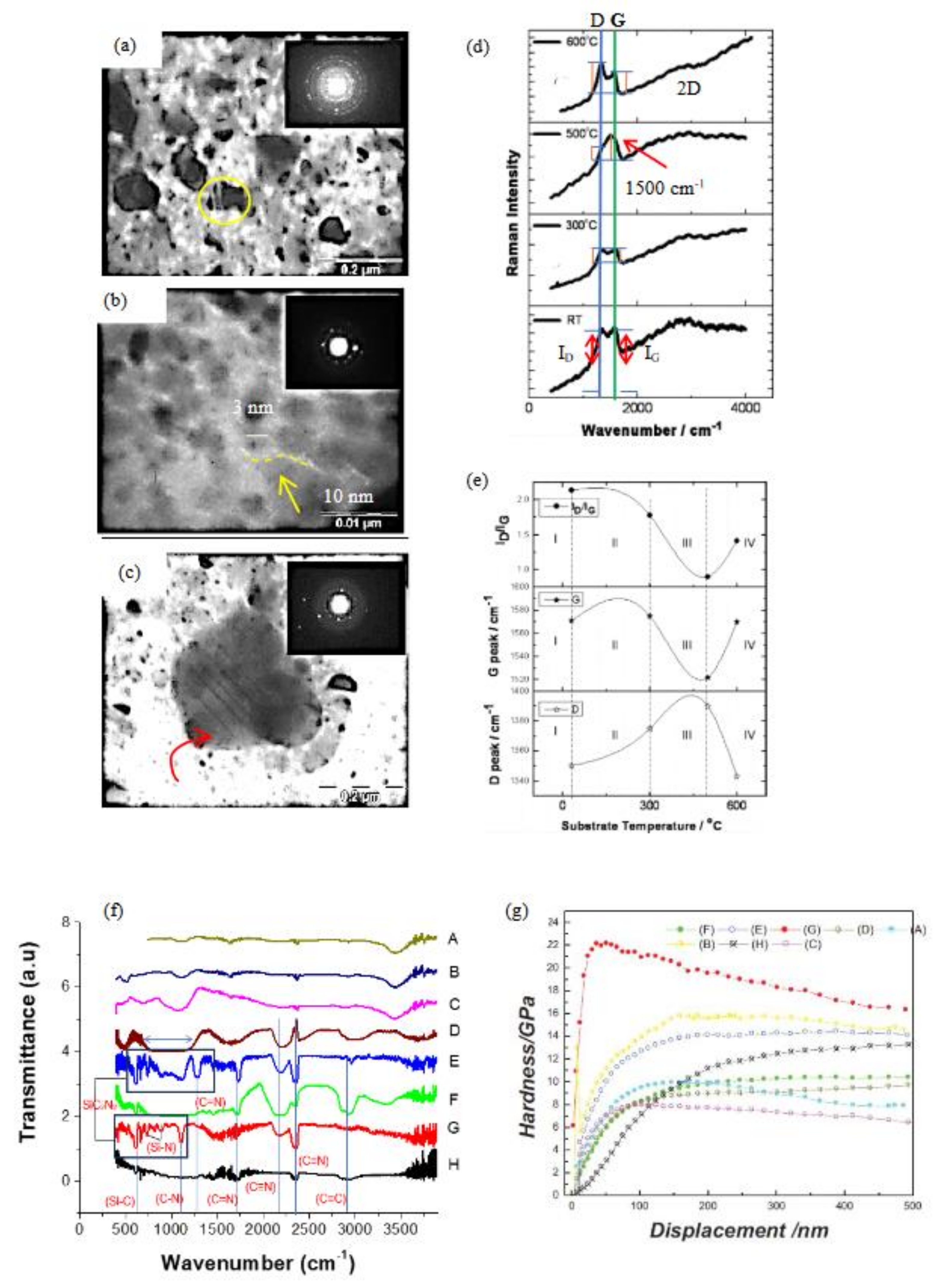
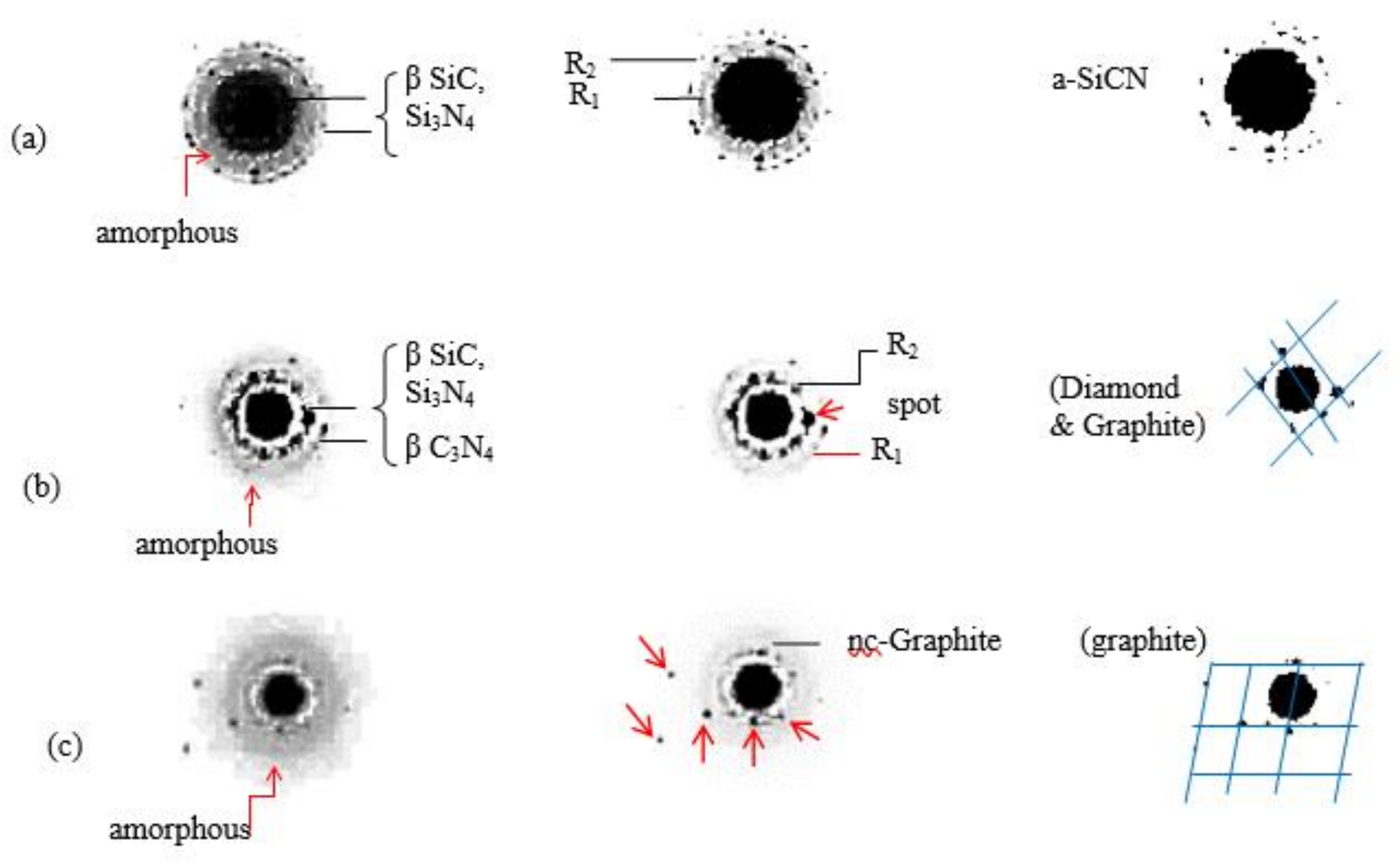
3.3. Nucleation & Growth
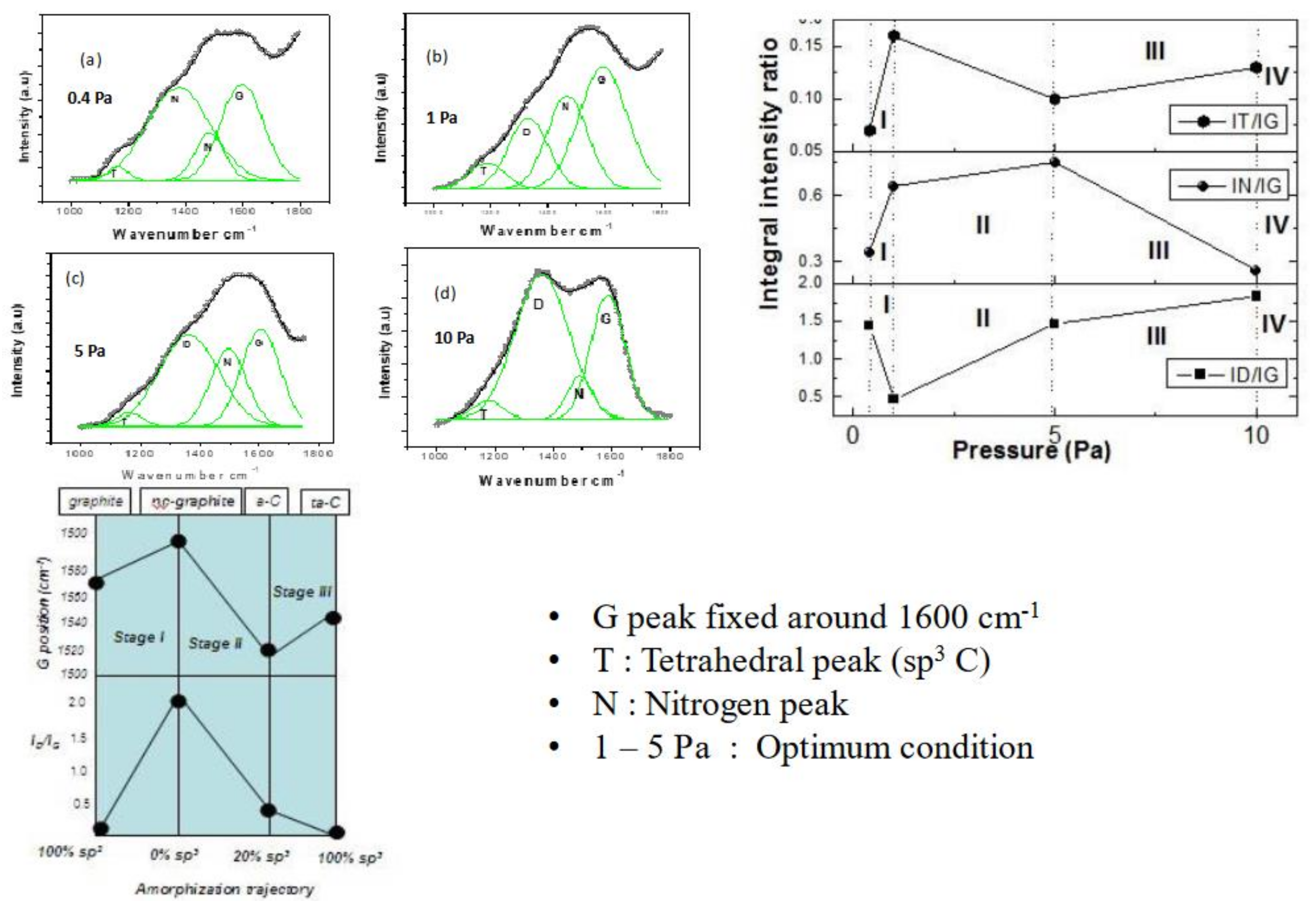
3.4. The 4-Stage Model Based on Deposition Pressure
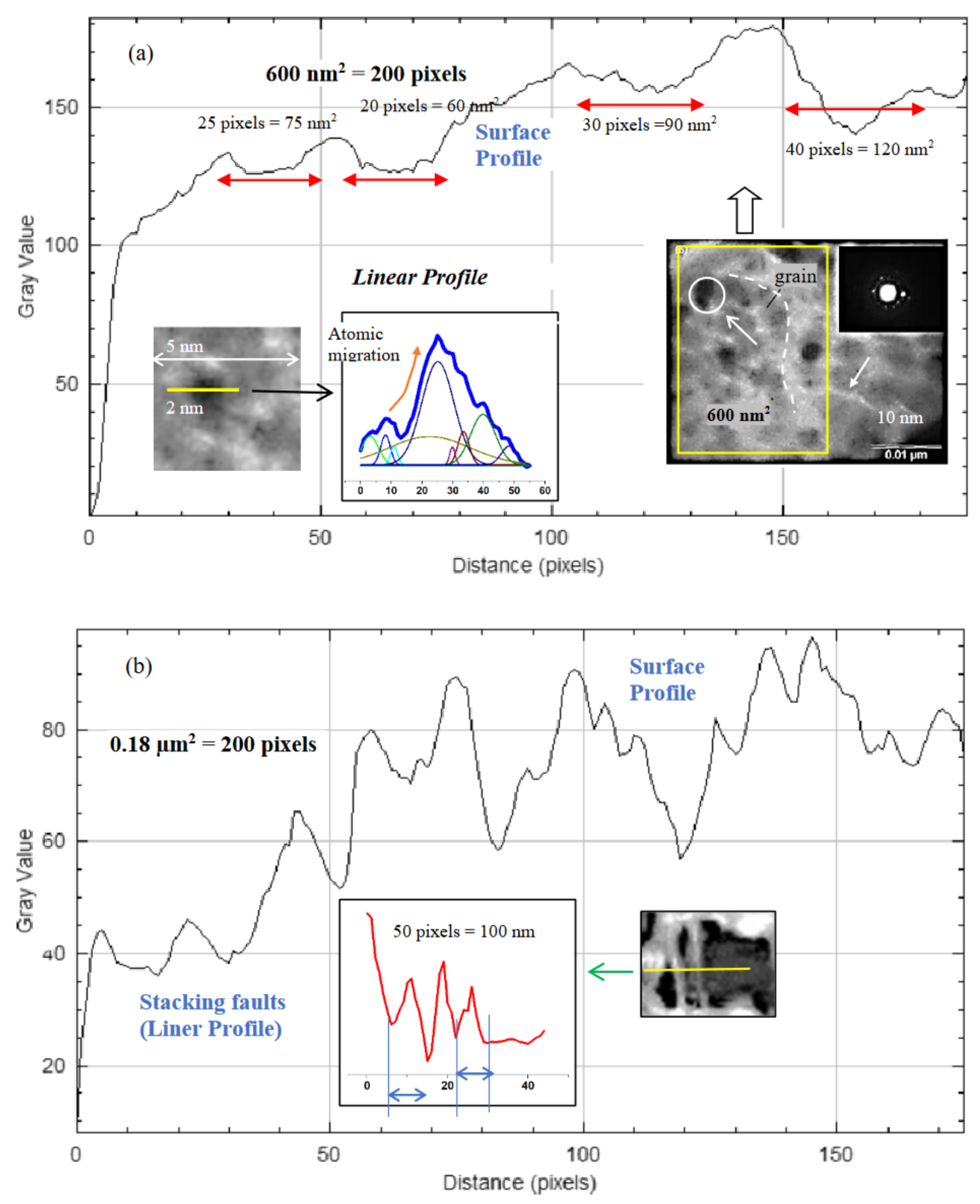
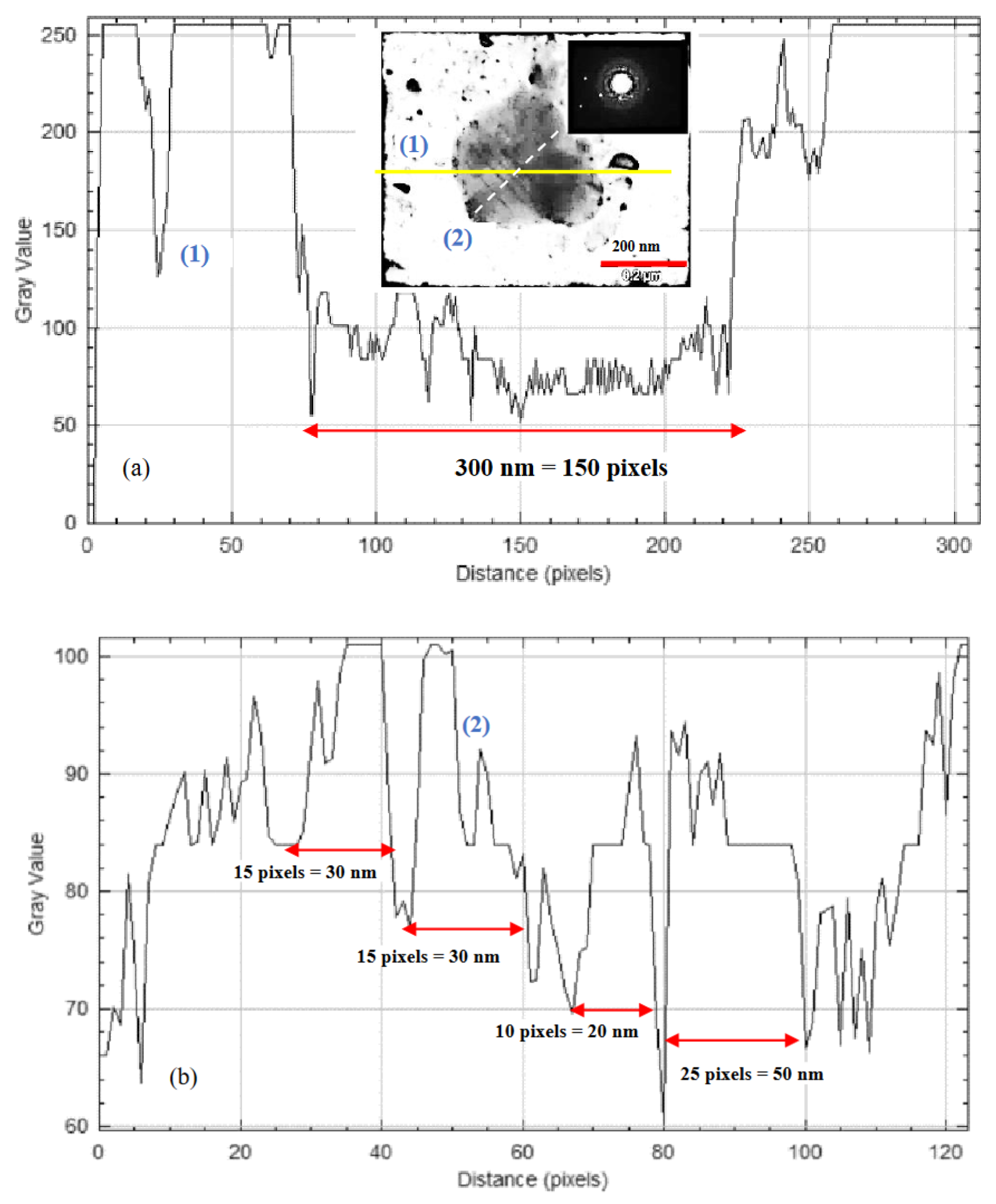
3.5. Microstructure
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, S.R.; Raj, R. Mechanical Properties of a Fully Dense Polymer Derived Ceramic Made by a Novel Pressure Casting Process. Acta Mater. 2002, 50, 4093–4103. [Google Scholar] [CrossRef]
- Riedel, R.; Kleebe, H.-J.; Schönfelder, H.; Aldinger, F. A Covalent Micro/Nano-Composite Resistant to High-Temperature Oxidation. Nature 1995, 374, 526–528. [Google Scholar] [CrossRef]
- Ren, Z.; Mujib, S.B.; Singh, G. High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review. Materials 2021, 14, 614. [Google Scholar] [CrossRef] [PubMed]
- Barrios, E.; Zhai, L. A Review of the Evolution of the Nanostructure of SiCN and SiOC Polymer Derived Ceramics and the Impact on Mechanical Properties. Mol. Syst. Des. Eng. 2020, 5, 1606–1641. [Google Scholar] [CrossRef]
- Lin, X.; Gong, H.; Chen, Z. Fabrication of SiCN (Fe)/Al2O3 wave-absorbing ceramics with enhanced electromagnetic performance. Ceram Int. 2023, 49, 23851–23863. [Google Scholar] [CrossRef]
- Geng, T.B.; Yu, G.Y.; Shao, G.F.; Huang, X.G. Enhanced electromagnetic wave absorption properties of ZIF-67 modified polymer-derived SiCN ceramics by in situ construction of multiple heterointerfaces. Rare Metals 2023, 42, 1635–1644. [Google Scholar] [CrossRef]
- Xue, J.; Hu, S.; Li, X.; Li, F.; Liu, Y.; Wei, H. Enhanced microwave absorbing properties of Y2O3 modified PDC SiCN ceramics with heterogeneous amorphous interface. Journal of Alloys and Compounds 2023, 931, 167499. [Google Scholar] [CrossRef]
- Han, D., You, G., Zhang, Y., Tian, H., He, J., Liang, J.,… & Shao, G. Increased microwave absorption property of porous Si3N4 ceramics by loading polymer-derived SiCN for a multifunctional design. Journal of Materials Chemistry C 2023, 11, 6130–6137.
- Wu, C., Fu, Y., Zeng, Y., Chen, G., Pan, X., Lin, F.,… & Hai, Z. Ultrafast high-temperature sintering of polymer-derived ceramic nanocomposites for high-temperature thin-film sensors. Chemical Engineering Journal 2023, 463, 142518.
- Chen, G., Zeng, Y., Zhao, F., Wu, C., Pan, X., Lin, F.,… & Hai, Z. Conformal fabrication of functional polymer-derived ceramics thin films. Surf Coat Technol 2023, 464, 129536.
- Li, L., He, Y., Xu, L., Shao, C., He, G., Sun, D., & Hai, Z. Oxidation and Ablation Behavior of Particle-Filled SiCN Precursor Coatings for Thin-Film Sensors. Polymers 2023, 15, 3319.
- Cui, Z., Chen, X., Li, X., & Sui, G. Thin-film temperature sensor made from polymer-derived ceramics based on laser pyrolysis. Sensors and Actuators A: Physical 2023, 350, 114144.
- Ma, K., Bekiaris, N., Ramaswami, S., Ding, T., Probst, G., Burggraf, J., & Uhrmann, T. 0.5 ¼m Pitch Wafer-to-wafer Hybrid Bonding with SiCN Bonding Interface for Advanced Memory. In Proceedings of the IEEE 73rd Electronic Components and Technology Conference (ECTC); 2023; pp. 1110–1114.
- Stiharu, I., Andronenko, S., Zinnatullin, A., & Vagizov, F. SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study. Micromachines 2023, 14, 925.
- Xia, X., Chiang, C.C., Gopalakrishnan, S.K., Kulkarni, A.V., Ren, F., Ziegler, K.J., & Esquivel-Upshaw, J.F. Properties of SiCN Films Relevant to Dental Implant Applications. Materials 2023, 16, 5318.
- Choi, H.J.; Lu, K. A high compatibility SiOCN coating on stainless steel. Journal of Materials Science 2023, 58, 3790–3801. [Google Scholar] [CrossRef]
- Liu, K., Peng, W., Han, D., Liang, Y., Fan, B., Lu, H.,… & Shao, G. Enhanced piezoresistivity of polymer-derived SiCN ceramics by regulating divinylbenzene-induced free carbon. Ceramics International 2023, 49, 2296–2301.
- Sulyaeva, V.S., Kolodin, A.N., Khomyakov, M.N., Kozhevnikov, A.K., & Kosinova, M.L. Enhanced Wettability, Hardness, and Tunable Optical Properties of SiCxNy Coatings Formed by Reactive Magnetron Sputtering. Materials 2023, 16, 1467.
- Tomastik, J.; Ctvrtlik, R.; Ingr, T.; Manak, J.; Opletalova, A. Effect of Nitrogen Doping and Temperature on Mechanical Durability of Silicon Carbide Thin Films. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, Jan & Petry, Nils-Christian & Thor, Nathalie & Kredel, Samuel & Teppala, Dharma & Galetz, Mathias & Lepple, Maren & Pundt, Astrid & Ionescu, Emanuel & Riedel, Ralf. Exceptional Hardness and Thermal Properties of SiC/(Hf,Ta)C(N)/(B)C Ceramic Composites Derived from Single-Source Precursor. Advanced Engineering Materials 2024. [CrossRef]
- Bernauer, Jan & Kredel, Samuel & Ionescu, Emanuel & Riedel, Ralf. Polymer-derived ceramic Coatings with Excellent Thermal Cycling Stability. Adv Eng Mater 2024. [CrossRef]
- Thor, Nathalie & Bernauer, Jan & Petry, Nils-Christian & Ionescu, Emanuel & Riedel, Ralf & Pundt, A. & Kleebe, Hans-Joachim. Microstructural Evolution of Si(HfxTa1-x)(C)N Polymer-Derived Ceramics upon High-Temperature Anneal. J Eur Ceram Soc 2022, 43, 1417–1431.
- Zhu, Runqiu & Jin, Shuo & Xing, Ruizhe & Song, Yan & Yu, Zhen & Liu, Ziyu & Kong, Jie. Nanoparticles-doped polymer-derived SiCN ceramic with enhanced mechanical properties and electromagnetic wave absorption. Journal of the American Ceramic Society 2024. [CrossRef]
- Wang, Beibei & Liu, Xingmin & Liu, Yue & Fu, Qiangang & Riedel, Ralf. Single-source-precursor synthesized SiCN/MWCNT nanocomposites with improved microwave absorbing performance. Journal of Materials Science: Materials in Electronics 2024, 35. [CrossRef]
- Ye, Xiao-Juan & Wang, Xiao-Han & Cao, Hong-Bao & Lu, Zheng & Liu, Chun-Sheng. Penta-SiCN monolayer is a well-balanced performance anode material for Li-ion batteries. Physical Chemistry Chemical Physics 2023, 25. [CrossRef]
- Chiang, Chao-Ching & Xia, Xinyi & Craciun, Valentin & Rocha, Mateus & Camargo, Samira & Rocha, Fernanda & Gopalakrishnan, Sarathy Kannan & Ziegler, Kirk & Ren, Fan & Esquivel-Upshaw, Josephine. Enhancing the Hydrophobicity and Antibacterial Properties of SiCN-Coated Surfaces with Quaternization to Address Peri-Implantitis. Materials 2023, 16, 5751. [CrossRef]
- Qiang Yan, Siyao Chen, Haofan Shi, Xiaofei Wang, Songhe Meng, Jinping Li, Fabrication of polymer-derived SiBCN ceramic temperature sensor with excellent sensing performance. ournal of the European Ceramic Society 2023, 43, 7373–7380. [CrossRef]
- Yan, Yuangao & Fei, Xuan & Huang, Liuying & Yu, Yuxi & Wen, Yichen & Zhao, Gang. Effect of BN content on the structural, mechanical, and dielectric properties of PDCs-SiCN(BN) composite ceramics. J Am Ceram Soc. 2023, 106, 6951–6961.
- Anand, R & Lu, Kathy & Nayak, Bibhuti & Behera, Shantanu. Structural evolution and oxidation resistance of polysilazane-derived SiCN-HfO 2 ceramics. J Am Ceram Soc. 2023, 107, 1657–1668.
- Chery, Emmanuel & Fohn, Corinna & De Messemaeker, Joke & Beyne, Eric. Reliability Challenges in Advanced 3D Technologies: The Case of Through Silicon Vias and SiCN–SiCN Wafer-to-Wafer Hybrid-Bonding Technologies. IEEE Tran on Device and Mater Rel 2023, 23, 615–622.
- Ramlow, Heloisa & Marangoni, Cintia & Motz, Günter & Singh, Gurpreet & Machado, Ricardo. Electrochemical performance of SiCN embedded carbon (SiCN–C) fiber mat electrodes for lithium-ion battery: Electrospinning polysilazane in air or protective atmosphere. Open Ceramics 2023, 14, 100351.
- Ramlow, Heloisa & Souza, Graciano & Fonseca, Mikael & Raizer, Adroaldo & Rambo, Carlos & Machado, Ricardo. Lightweight and flexible nanostructured C/SiCN nanofiber nonwoven for electromagnetic reflection shielding of 5G C-Band frequencies. Materials in Electronics 2023, 34, 1631.
- Ramlow, Heloisa & Ribeiro, Luiz & Schafföner, Stefan & Motz, Günter & Machado, Ricardo. (2024). Thermo-oxidative resistance of C-rich SiCN(O) nonwovens influenced by the pretreatment of the silazane. J. Europ Ceram Soc 2024. [CrossRef]
- d’Eril, Marco & Graczyk-Zajac, Magdalena & Riedel, Ralf. On the Reversible Sodium Plating/stripping Reaction in Porous SiCN(O) Ceramic: A Feasibility Study. Batteries & Supercaps 2023, 6, e202200491.
- Li, Qiang & Chen, Cheng & Wang, Mingge & Lv, Yaohui & Mao, Yulu & Xu, Manzhang & Wang, Yingnan & Wang, Xuewen & Zhang, Zhiyong & Wang, Shouguo & Zhao, Wu & Stiens, J. Study on Photoelectricity Properties of SiCN Thin Films Prepared by Magnetron Sputtering. J Mater Res Technol. 2021, 15, 460–467.
- Jiang, Minming & Xu, Ke & Liao, Ningbo & Zheng, Beirong. Effect of sputtering power on piezoresistivity and interfacial strength of SiCN thin films prepared by magnetic sputtering. Ceram Int. 2021, 48, 2112–2117.
- Das, S., Kumar, D., Borah, R. et al. Impact of elevated temperature over different properties of CVD SiCN coating developed in Nitrogen gas atmosphere. Silicon 2022, 14, 9643–9657. [CrossRef]
- Haofan, Shi & Li, Jinping & Zhang, Gaoming & Meng, Songhe. Effect of free carbon content changed by divinylbenzene on the conductivity of SiCN ceramic. J. Mater Sci: Materials in Electronics 2023, 34, 1401.
- Matar, Samir & Etourneau, Jean & Solozhenko, Vladimir. Novel (Super)Hard SiCN from Crystal Chemistry and First Principles. Silicon 2022, 15, 511–520.
- Bhattacharyya, AS, Growth of stable SiC2N2-Si2CN4 phases during nitrogen incorporated sputter deposition of silicon carbide. Chemical Physics Impact 2024, 8, 100454. [CrossRef]
- Bhattacharyya, A.S.; Mishra S.K.; Mukherjee, S.; Hot Properties. Eur. Coat. J. 2009, 3, 108–114.
- Bhattacharyya, A.S.; Mishra, S.K. Raman Studies on Nanocomposite Silicon Carbonitride Thin Film Deposited by r.f. Magnetron Sputtering at Different Substrate Temperatures. J. Raman Spectrosc. 2010, 41, 1234–1239. [CrossRef]
- Bhattacharyya, AS Mishra, SK Mukherjee, S. Correlation of structure and hardness of rf magnetron sputtered silicon carbonitride films. J. Vac. Sci. Technol A 2010, 28, 505–509. [CrossRef]
- R. Dash, K. Bhattacharyya, A.S. Bhattacharyya. Fracture associated with static and sliding indentation of multicomponent hard coatings on silicon substrates. Fatigue & Fracture of Engineering Materials & Structures 2023, 46, 1641–1645.
- Arnab Sankar Bhattacharyya, Kushal Bhattacharyya, Effect of loading rate and coating thickness on wear and adhesion during sliding indents of Si–C–N/glass coatings useful for automotive applications. Results in Surfaces and Interfaces 2024, 100196. [CrossRef]
- Choi, H.J., & Lu, K. A high compatibility SiOCN coating on stainless steel. Journal of Materials Science 2023, 58, 3790–3801.
- Mishra, SK, Bhattacharyya, AS, Effect of substrate temperature on the adhesion properties of magnetron sputtered nano-composite Si–C–N hard thin films. Materials Letters 2008, 62, 398–402. [CrossRef]
- Bhattacharyya, A S, Modeling of sputter-based atomic layer deposition with altered parameters. Materials Today Comm 2024, 38, 107697. [CrossRef]
- Wu, C., Lin, F., Pan, X., Chen, G., Zeng, Y., Xu, L., … & Hai, Z. Abnormal Graphitization Behavior in Near-Surface/Interface Region of Polymer-Derived Ceramics. Small 2023, 19, 2206628.
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys 1970, 53, 1126. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Physical Review B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Santra, TS, Liu CH, Bhattacharyya, TK, Patel, P, Barik TK, Characterization of diamond-like nanocomposite thin films grown by plasma enhanced chemical vapor deposition. Appl. Phys. 2010, 107, 124320. [CrossRef]
- Sharma, R Chadha, N. Saini, P. Determination of defect density, crystallite size and number of graphene layers in graphene analogs using X-ray diffraction and Raman spectroscopy. Ind J Pure & Appl Phy 2017, 55, 625–629.
- Varsanyi, G. Vibrational Spectra of Benzene Derivatives, Academic Press: New York, NY, USA, 1969.
- Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Graphene Domain Signature of Raman Spectra of sp2 Amorphous Carbons. Nanomaterials 2020, 10, 2021. [Google Scholar] [CrossRef] [PubMed]
- Franz Tavella, Hauke Höppner, Victor Tkachenko, Nikita Medvedev, Flavio Capotondi, Torsten Golz, Yun Kai, Michele Manfredda, Emanuele Pedersoli, Mark J. Prandolini, Nikola Stojanovic, Takanori Tanikawa, Ulrich Teubner, Sven Toleikis, Beata Ziaja, Soft x-ray induced femtosecond solid-to-solid phase transition. High Energy Density Physics 2017, 24, 22–27.
- Nagano, F., Inoue, F., Phommahaxay, A., Peng, L., Chancerel, F., Naser, H.,… & Iacovo, S. Origin of Voids at the SiO2/SiO2 and SiCN/SiCN Bonding Interface Using Positron Annihilation Spectroscopy and Electron Spin Resonance. ECS Journal of Solid-State Science and Technology 2023, 12, 033002.
- AS Bhattacharyya, Nanocomposite Si-C-N coatings, arXiv:1608.05667 [cond-mat.mtrl-sci].
- A.S. Bhattacharyya, Nanocomposite Si-C-N coatings, 2018 ISBN 978-613-8-38960-6 Lap-Lambert Academic.
| S. No | Composites/Coatings | Properties | Reference |
|---|---|---|---|
| 1. | SiC/(Hf,Ta)C(N)/(B)C; SiHfTa(B)CN coatings; |
Exceptional high hardness and thermal cycle stability; crack-free; amorphous, good adhesion | [20,21] |
| 2. | Si(HfxTa1-x)(C)N | residual carbon triggered decomposition reaction, resulting in the formation of Transition Metal Carbides, gaseous nitrogen and SiC, tetragonal hafnium oxide (t-HfO2) within the SiCN matrix-improved phase stability and oxidation resistance | [22,29] |
| 3. | Ni–Fe–C/HfO2/SiCN; SiCN/MWCNT |
metal-organic framework (Ni–Fe–MOF) and hafnium dioxide (HfO2) nanoparticles; improved brittleness and EM wave absorption; MWCNT-COOH increased the interfacial polarization loss and the conduction loss, improving microwave absorbing performance. | [23,24] |
| 4. | Penta-SiCN monolayer; SiCN embedded carbon (SiCN–C); Porous SiCN(O) | low diffusion energy barrier and robust wettability useful in anode material for Li-ion batteries; stable cycling with a charge capacity, Reversible Na Plating/stripping Reaction for sodium metal batteries | [25,31,34] |
| 5. | SiCN-coated implants | Hydrophobicity and Antibacterial Properties, replacing Ti implants to avoid Peri-implantitis | [26], |
| 6. | PDC SiCN | Free carbon enriched interface, high conductivity, strain gauge | [49] |
| 7. | polymer-derived SiBCN; PDCs-SiCN(BN) |
High-temperature sensing due to good thermomechanical properties, hexagonal boron nitride (h-BN) with weakened polarization relaxation behavior, reduced porosity, and resistance to crack expansion causes low dielectric loss and high-temperature resistance | [27,28] |
| 8. | SiCN–SiCN wafer-to-wafer hybrid-bonding | next generation of 3D Systems-on-Chip with interconnect densities above 10 mm−2 | [30] |
| 9. | C/SiCN and SiCN(O) nanofiber, nonwoven | tunable electrical conductivity, EM reflection shielding in the 5G C-band, and thermo-oxidation resistance |
[32,33] |
| 10. | Sputtered SiCN coatings | Photoelectric properties, SiCN-based MEMS pressure sensors. | [35,36] |
| 11. | CVD-grown SiCN coatings | Increased intensity of Si-N-Si, decreased crystallite size with temperature along with increased hardness and modulus | [37], |
| Deposition Conditions |
Temp(°C) | Time (min) | t(μm) | Rate (nm/min) |
Hardness (GPa) |
|---|---|---|---|---|---|
| 1 Pa 400 W |
RT | 120 | 3.5 | 29.2 | 10 |
| 100 | 120 | 3.5 | 29.2 | - | |
| 200 | 150 | 3.5 | 23.4 | - | |
| 300 | 180 | 3.7 | 20.6 | 14 | |
| 400 | 210 | 4.2 | 20.0 | - | |
| 500 | 210 | 4.1 | 19.5 | 22 | |
| 600 | 240 | 3.8 | 15.8 | 8 | |
| 400 W 500 °C |
Pressure (Pa) |
Time (min) | t(μm) | Rate (nm/min) |
Hardness (GPa) |
| 0.4 | 240 | 3.9 | 16.2 | 13 | |
| 1 | 210 | 4.1 | 19.5 | 22 | |
| 5 | 210 | 3.8 | 18.1 | - | |
| 10 | 240 | 3.5 | 14.6 | - | |
| 500 °C | Power (W) |
Time (min) | t(μm) | Rate (nm/min) |
Hardness (GPa) |
| 1 Pa | 200 | 240 | 3.5 | 14.6 | 9 |
| 300 | 210 | 3.9 | 18.6 | 15 | |
| 10 Pa | 400 | 210 | 3.5 | 16.7 | 7 |
| Temperature (°C) |
ID/IG (Integral) |
ID/IG (Height) |
(nm) |
(nm) |
× 1011 (cm−2) |
|---|---|---|---|---|---|
| 30 | 2.25 | 1.0 | 8.5 | 6 | 6.7 |
| 300 | 1.75 | 1.0 | 11 | 8 | 5.2 |
| 500 | 0.5 | 3/4 | 38 | 28 | 1.5 |
| 600 | 1.3 | 4/3 | 15 | 11 | 3.9 |
| Pressure (Pa) |
ID/IG (integral) |
ID/IG (height) |
(nm) |
(nm) |
× 1011 (cm−2) |
| 0.4 | 1.5 | - | 13 | 9 | 4.5 |
| 1.0 | 0.5 | - | 38 | 28 | 1.5 |
| 5.0 | 1.5 | - | 13 | 9 | 4.5 |
| 10 | 1.85 | - | 7 | 7.5 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





