1. Introduction
To the best of the available knowledge, no interventional research has explored the potential benefits of a plant-based diet (PBD) for patients with COVID-19, particularly for high-risk elderly individuals with comorbidities. Recent studies suggest that PBD may reduce the occurrence and severity of COVID-19, potentially leading to lower mortality rates [
1,
2,
3]. However, these studies have utilized mainly retrospective questionnaires to survey a diverse population, and their ability to establish causal relationships between PBD and COVID-19 outcomes has been questioned [
4]. Additionally, concerns have been raised regarding the potential lack of vitamins and essential minerals in PBD, which may not be suitable for COVID-19 patients [
5].
According to the World Health Organization (WHO), older individuals aged 65 or above are most likely to require hospitalization. This correlation between age and health conditions suggests that the severity of COVID-19 is directly linked to the affected individuals' age and pre-existing health issues [
6,
7]. Elderly patients have a higher case fatality rate, estimated at 15-17%, due to their aging bodies and increased likelihood of having pre-existing health conditions such as heart disease, diabetes, hypertension, hyperlipidemia, autoimmune disease, and respiratory disorders [
8]. These underlying conditions can exacerbate COVID-19 symptoms, leading to a higher mortality risk. Intensive care unit (ICU) admission is a critical factor in the prognosis of coronavirus patients, particularly when mechanical ventilation is employed, as this significantly elevates the mortality rate [
9]. At the outset of the pandemic, a shortage of ICU facilities and human resources led to the inability to admit numerous critical coronavirus patients, ultimately resulting in numerous fatalities.
In the Asian region, Indonesia ranks second in COVID-19 deaths [
10] and has the highest mortality rate [
11]. It's alarming that most Indonesians consume an unhealthy diet (omnivorous) similar to the American standard diet. 25% of the Indonesian population is categorized as obese, and more than 90% of the patients residing in big cities lack vitamin D. It is important to note that an unhealthy lifestyle and poor dietary habits can lead to chronic inflammation [
12,
13], leaving one's body vulnerable to severe inflammation caused by COVID-19. Therefore, lifestyle change by adopting healthy habits is crucial to reduce the risk of developing a severe form of the disease or succumbing to it.
Table 1.
COVID-19 Cases and Death: comparison of Indonesia with other countries [
10,
11].
Table 1.
COVID-19 Cases and Death: comparison of Indonesia with other countries [
10,
11].
| COUNTRY |
TOTAL COVID CASES |
TOTAL COVID DEATHS |
MORTALITY RATE |
POPULATION |
| USA |
110.486.719 |
1.191.840 |
1% |
334.805.269 |
| India |
45.021.383 |
533.412 (1st in Asia) |
1.2% |
1.406.631.776 |
| Japan |
33.803.572 |
74.694 (5th in Asia) |
0.2% |
125.584.838 |
| Australia |
11.768.389 |
23.910 |
0.2% |
26.068.792 |
| Indonesia |
6.823.536 |
161.954 (2nd in Asia) |
2.4% |
279.134.505 |
| Malaysia |
5.244.578 |
37.315 (8th in Asia) |
0.7% |
33.181.072 |
| Singapore |
2.945.715 |
1.933 |
0.07% |
5.943.546 |
| China |
503.302 |
5.272 |
1% |
1.448.471.400 |
| Bhutan |
62.697 |
21 |
0.03% |
787.941 |
Three years prior to our COVID-19 study, lifestyle modifications (whole food PBD, regular physical activity, stress management, restorative sleep, stopping smoking, and supplement consumption) were introduced to our cardiology patients at Bethsaida Hospital in Indonesia. The outcomes of these modifications were astonishingly satisfactory. Patients suffering from hypertension achieved remission without resorting to medication. Those with hyperlipidemia were able to reach their optimal lipid levels. Many patients have their kidney function improved, and many patients with type 2 diabetes or glucose intolerance were able to manage their sugar levels without the need for insulin or extensive medication. Moreover, coronary obstructions in numerous cardiac patients regressed, and there was a low occurrence of in-stent restenosis (ISR). These results align with previous research that has demonstrated the effectiveness of PBD interventions in treating patients with chronic inflammatory diseases [
14,
15,
16]. The researchers believe our previous PBD's experience in managing chronic inflammatory conditions will also be beneficial in managing COVID-19.
Our investigation started by considering how PBD will help coronavirus patients. The researchers hypothesized several mechanisms that may provide insight into their concepts. These mechanisms include enhancing nitric oxide (NO) availability, modifying the microbiota, repairing endothelial dysfunction, reducing inflammation, protecting against oxidative stress, fortifying mitochondria, extending telomeres, and restricting caloric intake [
17]. As researchers delved further into the severe symptoms, multi-organ damage, and fatal consequences of SARS-CoV-2, they proposed that acute, severe inflammation was the underlying cause [18-23}. The investigators have thoroughly assessed that the form of PBD dietary selection with properties that suppress inflammation, combat oxidative stress, modulate the immune system, are anti-thrombotic, and have the ability to eliminate viruses will be implemented throughout the study. The quantity of these foods should be determined with precision, and the food processing method should be carefully considered. The ideas have been expanded to include supplements that can enhance the immune system, reduce inflammation, enhance antioxidants, have antithrombotic properties, and support the anti-viral properties of PBD to overcome coronavirus. The researchers commenced an examination of various supplementations, such as vitamins, minerals, and natural substances, with the objective of improving the efficiency of PBD, as previously demonstrated in research conducted during previous viral epidemics [
17]. It was hypothesized that by combining PBD intervention with strategic supplementation, the severity and fatality rates of COVID-19 could be reduced to as low as possible.
Undertaking an intervention study on the disease with uncertain treatment and vaccination at the outset of the COVID-19 pandemic, given its high mortality rate among the elderly, would have been impossible unless these elderly researchers were confident that their interventions would protect them from severe illness and death upon contracting the virus.
2. The study aims
The primary objective is to assess the effectiveness of whole foods PBD, employing suitable food quantification and preparation methods, as well as supplements, in diminishing the severity, hospitalization, and mortality rates associated with COVID-19 in high-risk elderly cardiology patients with multiple comorbidities who have had their health issues managed and have adhered to a PBD program for at least three months before contracting the coronavirus.
Our second objective is to assess the efficacy of these interventions in an acute care setting for patients who present with at least one comorbidity and have not had their health issues addressed before presenting to our clinic with COVID-19 (represented the high-risk general population). In essence, we wish to determine whether our interventions are effective for any patients.
The third objective is to evaluate the recovery time for high-risk elderly patients in comparison to the general population, specifically by determining whether they recover sooner or later.
To assess the severity and mortality of our study population, we will use a general population with a comparable age demographic, prevalent comorbidities, and unhealthy dietary habits (omnivorous) as a reference. According to the available literature [
24,
25,
26,
27,
28,
29], this group exhibits specific hospitalization and mortality rates when contracting COVID-19. We aim to employ this reference group as a basis for evaluating the severity and mortality of our study population.
3. Material and methods
This interventional study employs the general population's severity and mortality rates as a point of comparison. The study's participants were recruited from the cardiology clinic at Bethsaida Hospital, Indonesia. Information was collected from medical records and through direct communication with patients or their families to obtain all data. The data collected was limited to individuals who met specific criteria, and the study was not randomized.
The research participants were categorized into two groups: those with PBD and those without PBD or non-PBD (NPBD). PBD group consisted of 1750 participants, most of whom are over 60 years old and have at least the following comorbidities, including coronary artery disease, hypertension, hyperlipidemia, and glucose intolerance or type 2 diabetes mellitus. All participants in this group have had their health conditions optimized through coronary intervention and medications by researchers in the cardiology clinic at Bethsaida Hospital, as seen in the result (
Table 2). Furthermore, they have been following PBD and taking vitamin B12, vitamin D, and multivitamin supplements for at least three months before contracting COVID-19 and are eligible to enroll in the trial.
In the NPBD group, 1720 participants were registered at the clinic, comprising patients who had not previously been diagnosed or treated for their comorbidities, such as hyperlipidemia, hypertension, hyperglycemia, obesity, and atherosclerosis. Researchers assessed these participants at the cardiology clinic upon their first presentation with COVID-19 (
Table 2). The majority of participants in this group are afflicted with at least one comorbidity. Most of these participants were not on any regular medications or supplementations. These participants followed the Indonesian standard diet (omnivorous). This group is more representative of the Indonesian population in general.
As guided by the International literature, we diagnosed our COVID-19 patients through clinical symptoms and signs, blood tests, X-ray/CT scans, and positive PCR tests [
30,
31]. The study was conducted between April 2020 and June 2023.
4. Inclusion and Exclusion Criteria
The study enrolled participants aged 18 or older who tested positive for SARS-CoV-2 using PCR. They could be a) asymptomatic with no clinical symptoms, or with b) mild illness with COVID-19 symptoms (such as fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, anosmia, or dysgeusia) but without shortness of breath or abnormal chest imaging. They could also have c) moderate illness with clinical symptoms or radiologic evidence of lower respiratory tract disease and oxygen saturation or SpO2 ≥ 94% on room air, or d) severe illness with SpO2 ≤ 94% but still could be increased to ≥ 90% with oxygen 2-6 liters per minute. Patients who experience shortness of breath have to be able to take PBD nutrition and supplementation orally. Their lung infiltrates should no greater than 50% of the total lung volume. [
30,
31].
Participants who refused to adhere to the recommended PBD plan or supplements regimen and those who could not consistently follow through with our diet intervention and supplementation during their illness were excluded from the study. Individuals who were severely ill and required hospitalization during their initial presentation to the clinic with COVID-19 were excluded from the study.
5. Definition of Intervention
5.1. Dietary Intervention
All participants will be given a strictly guided PBD as part of the intervention. For breakfast, they will be served a one-liter blender containing 400 grams of raw vegetables such as spinach, arugula, celery, parsley, or any vegetables rich in fiber, nitrates, carotenoids, and phytochemicals. This will be mixed with 400 grams of fresh fruits such as pomegranates, berries, grapes, or fruits full of antioxidants and phytonutrients. Two tablespoons of flaxseeds or chia seeds were added to the blender and mixed. For lunch, they will consume 500-750 ml of porridge made from legumes, including 50g of soybeans, 100g of green beans, and 50g of barley, mixed with two dates for flavor. For dinner, they will have a colorful fruit salad consisting of apples, kiwis, oranges, mangos, and berries paired with 100-200 grams of whole grains mixed with 200-300 ml of soy or almond milk. The researchers would allow reasonable modifications for food preparations as long as the vegetables and fruits are raw and contain the recommended nutrients. We provided all participants with educational reading and visual materials and monitored them closely by video-calling them by telephone or computer. Patients were advised to incorporate Indonesian spices like ginger, turmeric, clove, cinnamon, lemongrass, and fermented foods into their diet, which are rich in antioxidants and act as anti-inflammatories. Fruits and vegetables should be procured from supermarkets that offer good quality products. Drinking coffee and green tea are also encouraged. Food amount restriction is no longer necessary due to loss of appetite in most COVID-19 patients.
5.2. Supplementation Intervention
Participants are expected to continue taking their prescribed medications and supplements as instructed during their regular visits to our clinic. However, they should stop taking medicines prescribed by other doctors for the purpose of treating COVID-19 (antibiotics, antivirals, antiparasitics, for example, Azithromycin, Doxycycline, any types of Cephalosporin, Lopinavir/ Ritonavir, Oseltamivir, Favipiravir, Chloroquine, and Ivermectin). All participants received only symptomatic treatments (e.g., oxygen, antipyretics for high temperature, anti-cough, anti-nausea meds, anti-diarrheal meds, painkillers, anxiolytics, and sleeping tablets). Participants who experience complications from COVID-19 will be treated in accordance with either the International guidelines or the consensus of experts in the relevant field. The research interventions plan will not be altered in any manner. For instance, in the case of high D-dimer levels, anticoagulation therapy (non-vitamin K antagonist oral anticoagulants / NOACs) will be administered.
The researchers offer the patients a variety of supplements for this study. These include Vitamin C, Vitamin D, B3/ Nicotinamide Riboside Chloride (Truniagen), Zinc, Copper, Selenium, CoQ10, Astaxanthin, Quercetin, Curcumin and Taurine. The daily dosage of Vitamin C is 1 gram, and the dosage of Vitamin D varies between 5000 IU and 20000 IU based on the patient's laboratory level of Vitamin D (target 60-80 ng/mL). Two doses of 300 mg of Truniagen are also provided. Additionally, the participants were provided with daily 60 mg of Zinc, 3 mg of Copper, 150 mcg of Selenium, 300 mg of CoQ10, 12 mg of Astaxanthin, 2.4 grams of Quercetin, 100 mg of Curcumin, and 1 gram of Taurine. All supplements the clinic provides are standardized to ensure consistency in quantity and quality.
6. Reference Group
The reference group for comparison in our study on COVID-19 severity and mortality was derived from the literature on the general population of Indonesia who contracted the virus between March 2020 and June 30, 2023. The mortality rate for this group was as follows: for those aged 40-49, it was 2-3%; for those aged 50-59, it was 5-8%; for those aged 60-69, it was 15-17%; and for those above 70 years of age, it was 18-20% [
24,
25,
26,
27]. For the severity rate of elderly individuals with comorbidities, according to the literature, it is estimated that approximately 5-20% of the elderly with multiple comorbidities developed severe illness. It is important to note that these figures are based on the available data and may vary depending on the specific population being studied [
28,
29]. Recovery from COVID-19 has been defined in various ways. In this study, we have chosen to define recovery based on the clinical symptoms, along with the presence or absence of a negative PCR result. According to the literature, the general recovery time for the elderly population is typically between 14 and 21 days [
24,
25,
26,
27,
28,
29].
7. Variables measured
Anthropometric and metabolic parameters, such as age, body weight, height, Body Mass Index (BMI), systolic and diastolic blood pressures, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), white blood cell count (WBC), high-sensitivity C-reactive protein (hs-CRP), random glucose, hemoglobin A1C (HbA1C), NO reading, and platelet aggregation profile were obtained. Vital signs parameters, including breathing rate, oxygen saturation, pulse rate, and temperature, were recorded [
30,
31], and any signs of consolidation in the X-ray or CT scan were checked and calculated to determine the severity of the disease. All parameters were recorded during the initial visit of the participants before embarking on our intervention trial.
8. Statistical analysis
The study utilized various statistical methods to analyze the data. The mean ± standard deviation (x ± s) was employed to assess continuous baseline variables in both groups. Numerical values and percentages were used to present count data. The severity of the patients was categorized as mild, moderate, or severe based on their symptoms, signs, laboratory findings, and radiological results (X-ray and CT thorax) following the guidelines provided in [
30,
31]. The chi-square test was used for gender, and the independent t-test for all other variables in the statistical analysis. Additionally, a multivariate analysis with Cox regression was conducted to identify the factors associated with COVID-19 recovery and severity.
9. Results
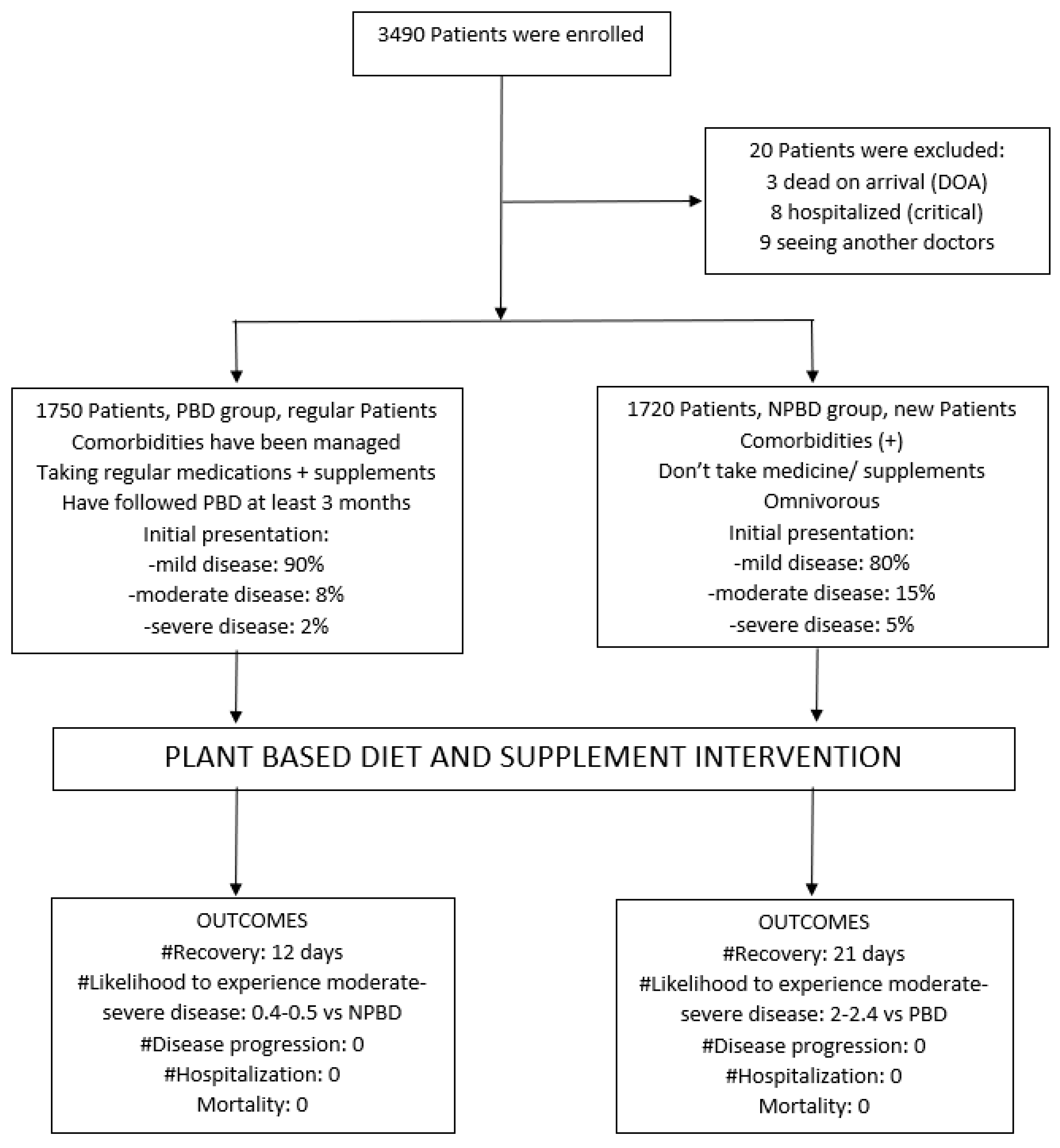
3490 individuals were screened for the study between April 2020 and June 2023. However, we could not include 20 individuals in our final analysis. These 20 individuals comprised three who were declared dead upon arrival, eight who were found to have critical illness and were immediately hospitalized, and nine who opted out of our diet intervention program and sought treatment elsewhere.
The PBD group, comprising 934 males and 816 females with an average age of 64, primarily consists of older patients with multiple comorbidities or concurrent chronic medical conditions. These individuals regularly attend cardiology appointments and adhere to a healthy lifestyle, including consuming a whole food PBD and taking their prescribed medications and supplements. It is evident from their metabolic parameters (BMI, blood pressure, cholesterol, triglyceride, glucose, WCC, hs-CRP, platelet function, and NO reading [
32]) that their health is satisfactorily optimized. Despite having a history of comorbidities, upon their initial presentation with COVID-19 to our clinic, their comorbidities were effectively managed and under control, as shown in
Table 2.
In contrast, the NPBD group comprises 889 males and 831 females, with an average age of 50.5, as illustrated in
Table 2. These participants are typically younger than those in the PBD group and exhibit fewer comorbidities. They are all new patients who visited our clinic for the first time to receive COVID-19 treatments. The participants in Group NPBD had unhealthy lifestyle habits, consumed poor dietary choices (omnivorous), and had unsatisfactory metabolic parameters, as shown in
Table 2. Most of them did not take any medications or supplements. Many participants in the PBD group exhibited a decrease in platelet aggregation due to their use of antiplatelet medications, such as acetylsalicylic acid, clopidogrel, or ticagrelor. Consequently, we determined that the metabolic parameters of the PBD group were superior to those of the NPBD group. The sole determinant of the PBD group's susceptibility to severe illness and death from COVID-19 appears to be their advanced age.
Comparing the vital signs between the PBD and NPBD groups during the initial presentation: although the average age of participants in the PBD group was 14 years older than that of the NPBD group, this does not necessarily mean that the PBD group presented with a worse respiratory presentation. It is well known that as individuals age, their respiratory rate increases. This is particularly relevant when dealing with geriatric patients suffering from respiratory illness. Moreover, the oxygen saturation of the geriatric population tends to decrease with age [
33,
34]. The heart rate in the PBD group was significantly lower compared to the other groups, which can be attributed to the fact that most participants in this PBD group were cardiac patients who typically received medication such as beta-blockers, calcium channel blockers, or Ivabradine. Temperature in the elderly is generally lower than in younger individuals, which is an important consideration when assessing the severity of respiratory infections in the geriatric population [
35]. Additionally, it is essential to recognize that elderly individuals may exhibit low temperatures even during sepsis [
36]. When evaluating clinical symptoms and signs in the geriatric population, it is crucial to consider other relevant clinical parameters. It has been observed that D-dimer levels ≥ 1 µg/ml are associated with worse outcomes in COVID-19 infection [
37]. However, all of our participants had much lower levels of D-dimer, possibly because we measured it early in the development of their disease. Our approach was to administer oral anticoagulation to all patients with elevated D-dimer levels, provided there were no contraindications. The anticoagulation dosage was varied based on the patient's laboratory results and the level of their D-Dimer.
Participants in the PBD group exhibited a more rapid recovery due to their metabolic advantage over those in the NPBD group. Despite the PBD group having older patients, they recovered more quickly than those in the NPBD group, with this difference being statistically significant (p<0.0005).
In the PBD group, 90% of the participants had mild disease, 8% had moderate disease, and 2% experienced severe disease. Patients with severe disease require only 2-4 liters of oxygen per minute to maintain oxygen saturation above 90%. They all responded well to the intervention, and none progressed to worse conditions since their initial presentation. On the other hand, in the NPBD group, 80% of the participants had mild diseases, 15% had moderate diseases, and 5% had severe cases. As shown in
Table 3 and
Table 4, participants with severe symptoms in the NPBD group remained stable during the diet intervention and low oxygen supplementation (2-4 L/minute). No deterioration was observed in any of the individuals in the NPBD group either. Participants in the PBD group exhibit a lower prevalence of moderate and severe diseases compared to participants in the NPBD group. Although they are older and have more comorbidities, they still responded well to the intervention, highlighting the PBD's effectiveness.
The study grouped the subjects into three categories based on the severity of their disease: severe, moderate, and mild. The results showed that the subjects in the NPBD group were twice as likely to have experienced moderate disease (with 95% confidence limit: 1.42-3.12) and 2.4 times more likely to have experienced severe disease (with 95% confidence limit: 1.2-5.0) with a significant p-value of less than 0.0005 and 0.016 respectively, compared to those in the PBD group.
Both participants responded positively to our intervention; neither worsened nor required hospitalization. No deaths occurred in either group.
11. Study limitation
Despite the numerous factors that needed to be considered, we employed the general population as a reference in our study. From the outset, we recognized that it was not feasible to create a control group. None of the patients who came to our clinic were willing to serve as a control subject. They all anticipated receiving treatment since our clinic was renowned among COVID-19 patients. Furthermore, we believed it would be unethical to form a control group by withholding treatment, as we had accumulated significant experience demonstrating the effectiveness of our intervention during the earlier phases of the COVID-19 pandemic.
Our inability to analyze data based on whether the participants had received vaccines or not stems from our trial conducted well before the vaccines were made available.
Figure 1.
Summary of the PBD and Supplementations Study.
Figure 1.
Summary of the PBD and Supplementations Study.
12. Discussions
In the PBD group, with an average age of 64 years, their comorbidity was properly managed and demonstrated in their optimized metabolic variables from their initial presentation (
Table 2). Despite being younger and having fewer comorbidities, participants in the NPBD group (with an average age of 50.5 years) displayed unsatisfactory metabolic parameters. These included high blood pressure, overweight/obesity, hyperlipidemia, elevated WCC (compared with the PBD group), elevated hs-CRP, elevated sugar/HbA1C, increased platelet aggregation, and poor NO readings, similar to those seen in most of the general population. Please refer to
Table 2 for further details. According to our previous explanation, despite the statistical significance demonstrated by the large sample size, we maintain that the differences in vital signs between the two groups were not clinically significant and do not warrant caution for participants in the PBD group. In contrast to the NPBD group, the recovery time for the PBD group was shorter.
It was observed that more patients in the PBD group had a milder form of the disease when they first sought medical attention, which is evident from
Table 3 and
Table 4. Both groups responded well to our interventions; none of the participants' conditions worsened during their illness, and none needed to be hospitalized. The prevalence of severe COVID-19 in the PBD group was significantly lower at 2% compared to the general population, particularly among individuals aged 60-65, where the incidence ranges from 10% to 20% [
24,
25,
26,
27,
28,
29]. Comparable to the general population aged 50-55, the study found that the incidence of severe disease in the NPBD group was 5%. In contrast to the general population of Indonesia, which has a fatality rate of 5-17%, our study has a zero fatality rate [
24,
25,
26,
27].
Table 3.
Association between PBD and supplement interventions and severity of COVID-19 (comparing severe versus mild cases).
Table 3.
Association between PBD and supplement interventions and severity of COVID-19 (comparing severe versus mild cases).
| Group |
Severity |
RR (95 % CI) |
Adj. RR (95 % CI) * |
p-Value
(of Adj. RR) |
| Severe |
Mild |
|
| Plant-Based Diet |
35 (2%) |
1575 (90%) |
0.37 (0.25-0.54) |
0.41 (0.20-0.84) |
0.016 |
| Non-Plant-Based Diet |
86 (5%) |
1376 (80%) |
1.0 |
1.0 |
|
Table 4.
Association between PBD and supplement interventions and severity of COVID-19 (comparing moderate versus mild cases).
Table 4.
Association between PBD and supplement interventions and severity of COVID-19 (comparing moderate versus mild cases).
| Group |
Severity |
RR (95 % CI) |
Adj. RR (95 % CI) * |
p-Value
(of Adj. RR) |
| Moderate |
Mild |
| Plant-Based Diet |
140 (8%) |
1575 (90%) |
0.52 (0.43-0.63) |
0.48 (0.32-0.70) |
<0.0005 |
| Non-Plant-Based Diet |
258 (15%) |
1376 (80%) |
1.0 |
1.0 |
|
Our research findings demonstrate that the PBD and supplementation intervention is effective for elderly individuals with multiple comorbidities. Additionally, the intervention has proven effective in the acute setting, such as administering PBD and supplements to any patients with COVID-19.
Our research findings indicate that the positive outcomes observed, such as shorter recovery time, reduced severity, and zero mortality in patients who followed our interventions, may be attributed to the quality, selection, and quantity of foods provided, as well as the preparation of the food (raw vegetables rather than cooked vegetables), in comparison to previous studies [
1,
2,
3]. Our study's findings also addressed concerns about offering PBD to COVID-19 patients, particularly in relation to the scarcity of vitamins and minerals [
4,
5]. Recent research has acknowledged the significance of micronutrient supplements, including vitamin C, vitamin D, vitamin B3/NAD+, zinc, copper, selenium, anti-inflammatory natural products such as astaxanthin, curcumin, quercetin, CoQ10, and taurine, in COVID-19 [
17]. To the best of our knowledge, our study was unique in that it involved the administration of supplements, which has not been included in any previous PBD studies on COVID-19. Our initial hypothesis that the supplements would enhance the anti-inflammatory, antioxidant, immune regulatory, antithrombotic, and anti-viral properties of the PBD may also explain the outstanding results of the study.
The administration of antiplatelet medication to participants in the PBD group was another important aspect of our research, which could enhance the antithrombotic effect of PBD [
38,
39] and possibly help prevent the development of thrombosis in COVID-19 patients. Additionally, we were among the innovators in employing non-vitamin K antagonist oral anticoagulants (NOACs) for COVID-19 treatment, providing these medications even during the initial stages of the disease, when D-Dimer levels were only slightly elevated (with adjusted dose). Postmortem examinations have revealed that a substantial proportion of COVID-19 fatalities displayed thromboembolism, further underscoring the crucial role that these medications play in managing the disease [
40,
41].
Acknowledging the significant bond between physicians and patients demonstrated in our research is essential. This relationship is characterized by medical professionals who also act as researchers, being accessible to patients by phone to address their concerns and alleviate their anxiety, particularly during the peak of the COVID-19 pandemic when many doctors have taken leave. This connection involves providing quality care and support to patients during our research. The provision of quality care and support is crucial because negative emotions such as bad mood, resentment, anxiety, and lack of sleep can lead to severe inflammation and cytokine storms, which can worsen a patient's prognosis [
42,
43].
During times of crisis, panic can hinder a practitioner’s effectiveness. Unfortunately, the COVID-19 pandemic led to widespread panic, causing physicians to prescribe medications such as antivirals, antibiotics, and antiparasitics without evidence-based support [
30,
31], which can harm patients by damaging their microbiota [
17] and exacerbating their condition. Therefore, in our study, we discontinued all unproven treatments provided to our participants by other doctors. Panic among healthcare professionals can negatively impact the emotional state of patients and worsen their illness. Research has shown that patients' conditions can deteriorate during hospitalization due to inadequate management, a lack of emotional support, and the provision of an unhealthy omnivorous diet in hospitals.
The principle of "first, do no harm" was upheld in this study despite its limitations. Nonetheless, the intervention prevented 3,470 hospitalizations, saved numerous lives, and resulted in substantial cost savings for the government. It is essential to consider safe and affordable interventions, such as dietary changes and supplementation, before resorting to vaccinations or new antiviral medications. The researchers implemented the intervention long before the availability of the COVID-19 vaccine, and over half of our participants were recruited before vaccinations were available. Our researchers are aware of the limitations of vaccine efficacy in specific populations, including elderly individuals with comorbidities [
44,
45]. Our findings indicate that the exceptional results of our studies are unlikely to be attributable to vaccinations, as many individuals in the general population still experienced severe COVID-19 symptoms and mortality despite being vaccinated.
Achieving optimal health goals, such as maintaining a BMI of 20-21, normal blood pressure, an LDL level of less than 55 mg/dL, optimal Hb A1C, high reading salivary NO strip, and low hs-CRP can be challenging without making dietary modifications to control the risk factors. Both doctors and patients are responsible for ensuring their optimal health. The COVID-19 pandemic has highlighted the limitations of relying solely on medications to tackle pandemics. The more the researchers learn about the advantages of consuming PBD and a healthy diet, the more reasons were discovered to support it. On the other hand, as the negative consequences of an omnivorous diet become increasingly apparent, it becomes more difficult to justify this choice. While these diets may provide temporary satisfaction, the long-term health risks they entail are challenging to overlook. Therefore, it is essential to carefully consider the potential costs and benefits of one's dietary choices.
Due to the substantial mortality rate connected to COVID-19, patients are frequently inclined to comply with PBD interventions. Furthermore, numerous individuals have observed an elevated number of fatalities among patients who were treated at top hospitals in Indonesia. Maintaining such healthy eating habits is of utmost importance. Regrettably, it is with great sorrow that we report the unfortunate occurrence of more than five of our previous study participants' fatalities (all had received complete vaccinations) as a result of subsequent COVID-19 infections outside the course of our study. Despite our previous outstanding findings, these individuals appeared to disregard our intervention for their subsequent COVID-19 infection. It seems that they continued to follow an omnivorous diet and relied on various COVID-19 medications, which ultimately proved to be detrimental to their lives.
Acknowledging the noteworthy outcome of PBD intervention, it is essential to recognize the substantial dedication required from patients and healthcare professionals. Despite evidence of its effectiveness, there has been reluctance among the medical community to embrace this approach fully. Undertaking extensive PBD intervention trials is practically unfeasible due to the necessity for substantial funding and the difficulty in recruiting willing researchers to participate in such studies. As a result, researchers can only hope for more favorable circumstances to encourage the broader acceptance and application of PBD in the future.
13. Conclusion
Elderly individuals who suffer from multiple comorbidities do not necessarily experience an increase in the severity or mortality of COVID-19 upon contracting the virus. Rather, it depends on how effectively their comorbidities are managed prior to their infection. The use of well-designed PBD, particularly when combined with carefully selected supplements, has significantly reduced COVID-19 severity and mortality rates, including for elderly patients with comorbidities. Additionally, implementing PBD and supplementation during the acute phase of the illness can still provide benefits in decreasing the severity and mortality of the disease.
Implementing PBD does not constitute the sole use of PBD; rather, it is essential to consider the concurrent use of medications and supplements to optimize their efficacy in combating the disease.
Author Contributions
Conceptualization, D.M.; methodology, D.M., and M.K.S.; validation, D.M., A.M.H., I.N.E.L., and M.K.S.; formal analysis, M.K.S., and H.U.; investigation, D.M.; resources, D.M.; data curation, D.M., and M.K.S.; writing-original draft preparation, D.M.; writing-review and editing, D.M., A.M.H., I.N.E.L. and M.K.S.; visualization, D.M.; supervision, A.M.H. and M.K.S.; project administration, D.M.; funding, D.M., and I.N.E.L All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethics Committee of Bethsaida Hospital reviewed and approved this study on 23 March 2020 under decision letter number 214/BH/PE/ETIK/03.2020 concerning the protection of human rights and welfare in medical research.
Informed Consent Statement
Patient consent was waived due to an institutional and ethical committee review that determined this study does not require informed consent.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. Due to privacy concerns, they are not publicly available.
Acknowledgments
The authors acknowledge Rustiwahyuni, Nuraini Puspita Lestari, Dega Ary Wiyoga, Tulus Sitepu, Cecilia Saraswati, Saefulloh, Prestis Pramudji, Kartika Sari, Rita Maya, Sutrisno Mulijono, andJayadi Sutanto for their contributions to data gathering. .
Conflicts of Interest
The authors declare no conflict of interest.
References
- Acosta-Navarro JC, Dias LF, Gomes de Gouveia LA, et al. Vegetarian and plant-based diets associated with lower incidence of COVID-19. BMJ Nutrition, Prevention & Health. 2024; O:e000629. [CrossRef]
- Soltanieh S, Salavatizadeh M, Ghazanfari T, et al. A plant-based diet and COVID-19 severity: results from a cross-sectional study. BMJ Nutrition, Prevention & Health. 2023; O:e000688. [CrossRef]
- Kim H, Rebholz CM, Hedge S, et al. Plant-based diets, pescatarian diets and COVID-19 severity: a population-based case-control study in six countries. BMJ Nutrition, Prevention & Health. 2021; 4:e000272.
- Kuhnle G, Piernas C, McConway K, Johnson I and Balloux F. Expert reaction to study looking at plant-based, fish and other diets and COVID-19 severity. Science Media Centre.June 7, 2021. https://www.sciencemediacentre.
- Rayman M, Stewart G, Mellor D and McCoway K. Expert reaction to observational study on types of diet and COVID-19 infection. https://www.sciencemediacentre.
- World Health Organization. Coronavirus (COVID-19) dashboard. https://covid19.who.int/ [accessed 15 January 2024].
- Gkoufa A, Maneta E, Ntoumas GN, et al. Elderly adults with COVID-19 admitted to intensive care unit: A narrative review. World J Crit Care Med. 2021; 10(5):278-289. [CrossRef]
- Wang X, Fang X, Cai Z, et al. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Res Wash DC. 2020:2402961. [CrossRef]
- Schultz MJ, van Oosten PJ, Hol L. Mortality among elderly patients with COVID-19 ARDS-age still does matter. Pulmonology. 2023; 29:353-355. [CrossRef]
- Covid-19: Indonesia becomes Asia’s new pandemic epicenter as delta variant spreads. BMJ. 2021; 374:n1815.
- Worldometer COVID-19 CORONAVIRUS PANDEMIC. https://www.worldometers.info/coronavirus/. (accessed January 16, 2024).
- Ruiz-Núñez, Pruimboom L, Dijck-Brouwer DAJ, et al. Lifestyle and nutritional imbalances associated with Western diseases: causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. The Journal of Nutritional Biochemistry. 2013; 24(7):1183-1201.
- Margina D, Ungurianu A, Purdel C, et al. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int J Environ Res Public Health. 2020; 17(11): 4135. [CrossRef]
- Craig WJ, Mangels AR, Fresán U, et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients. 2021 Nov 19;13(11):4144. [CrossRef]
- Peña-Jorquera H, Cid-Jofré V, Landaeta-Díaz L, et al. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome-A Comprehensive Review. Nutrients. 2023 Jul 21;15(14):3244. [CrossRef]
- Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. The British Medical Journal. 2014; 349:g4490.
- Mulijono D, Hutapea AM, Lister INE, et al. Plant-Based Diet and Supplements Reduced COVID-19 Severity and Mortality in Elderly Patients with Multiple Comorbidities (Part2: Exploring the Underlying Mechanisms of Successful Intervention). Preprints 2024. 2024030100. [CrossRef]
- Wong RSY, Inflammation in COVID-19: from pathogenesis to treatment. Int J Clin Exp Pathol.2021; 14(7):831-844.
- Clemente-Suárez VJ, Bustamante-Sanchez A, Tornero-Aquilera JF, et al. Inflammation in COVID-19 and the Effects of Non-Pharmacological Interventions during the Pandemic: A Review. Int J Mol Sci. 2022; 23(24):15584.
- Sefik E, Qu R, Junqueira C, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature, 2022; 606:585-593.
- Buicu A-L, Cernea S, Benedek I, et al. Systemic Inflammation and COVID-19 Mortality in Patients with Major Noncommunicable Diseases: Chronic Coronary Syndromes, Diabetes and Obesity. J Clin Med. 2021; 10(8):1545.
- Weber AdAP, Viero FT, Pillat MM, et al. Changes in markers of inflammation and their correlation with death in patients with COVID-19 in the intensive care unit. Cytokine. 2024; 175:156509.
- Manjili RH, Zarei M, Habibi M, et al. COVID-19 as an Acute Inflammatory Disease. J Immunol. 2020; 205(1):12-19.
- Karyono DR, Wicaksana AL. Current prevalence, characteristics, and comorbidities of patients with COVID-19 in Indonesia. Journal of Community Empowerment for Health. 2020; 3(2):77-84.
- Surendra H, Praptiningsih CY, Ersanti AM, et al. Clinical characteristics and factors associated with COVID-19-related mortality and hospital admission during the first two epidemic waves in 5 rural provinces in Indonesia: A retrospective cohort study. PLoS ONE. 2023; 18(3):e0283805.
- Data Pemantauan COVID-19. 2023. corona.jakarta.go.
- Sumiati, Aini N, Tama TD. Sex and age differences in the cOVID-19 mortality in East Jakarta, Indonesia: Analysis of COVID-19 surveillance system. Journal of Public Health in Africa. 2022; 13(s2):2420.
- Zhang L, Fan T, Yang S, et al. Comparison of clinical characteristics of COVID-19 between elderly patients and young patients: a study based on a 28-day follow-up. Aging (Albany NY). 2020; 12(20):19898-19910.
- Perrotta F, Corbi G, Mazzeo G, et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clinical and Experimental Reserch. 2020; 32:1599-1608.
- Cascella M, Rajnik M, Aleem A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19). NCBI bookshelf. August 18, 2023.
- Clinical management of COVID-19: Living guideline, 18 August 2023. World Health Organization 2023.
- Babateen A, Shannon O, Mathers JC, et al. Validity and reliability of test strips for the measurement of salivary nitrite concentration with and without the use of mouthwash in healthy adults. Nitric Oxide. 2019; 91(5).
- Ogburn-Russell L, Johnson JE. Oxygen saturation levels in the well elderly: altitude makes a difference. J Gerontol Nurs. 1990 Oct;16(10):26-30. [CrossRef]
- Takayama A, Nagamine T, Kotani K. Aging is independently associated with an increasing normal respiratory rate among an older adult population in a clinical setting: A cross-sectional study. Geriatr Gerontol Int. 2019 Nov;19(11):1179-1183. [CrossRef]
- Blatteis, CM. Age-dependent changes in temperature regulation - a mini review. Gerontology. 2012;58(4):289-95. [CrossRef]
- Shimazui T, Nakada TA, Walley KR, et al. JAAM FORECAST Group. Significance of body temperature in elderly patients with sepsis. Crit Care. 2020 Jun 30;24(1):387. [CrossRef]
- Zhan H, Chen H, Liu C, et al. Diagnostic Value of D-Dimer in COVID-19: A Meta-Analysis and Meta-Regression. Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211010976. [CrossRef]
- Pieters M, Swanepoel AC. The effect of plant-based diets on thrombotic risk factors. Pol Arch Intern Med. 2021 Oct 27;131(10):16123. [CrossRef]
- Kubatka, P., Mazurakova, A., Koklesova, L. et al. Antithrombotic and antiplatelet effects of plant-derived compounds: a great utility potential for primary, secondary, and tertiary care in the framework of 3P medicine. EPMA Journal 13, 407–431 (2022). [CrossRef]
- Wichman D, Sperhake J-P, Lutgehetmann M. et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020; 173:268-277.
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020; 383:120-128.
- Shayestefar M, Memari A-H, Nakhostin-Ansari A, et al. COVID-19 AND FEAR, WHICH COMES FIRST? Psychiatria Danubina. 2021; 33(13):S335-340.
- Boucas AP, Rheinheimer J, Lagopoulos J. Why Severe COVID-19 Patients Are at Greater Risk of Developing Depression: A Molecular Perspective. The Neuroscientist. 2022; 28(1):11-19.
- Choi WS, Cheong HJ. COVID-19 Vaccination for people with comorbidities. Infect Chemother. 2021; 53(1):155-158.
- Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012; 22(4):307-18.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).