Submitted:
01 April 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fabrication of conductive hydrogels
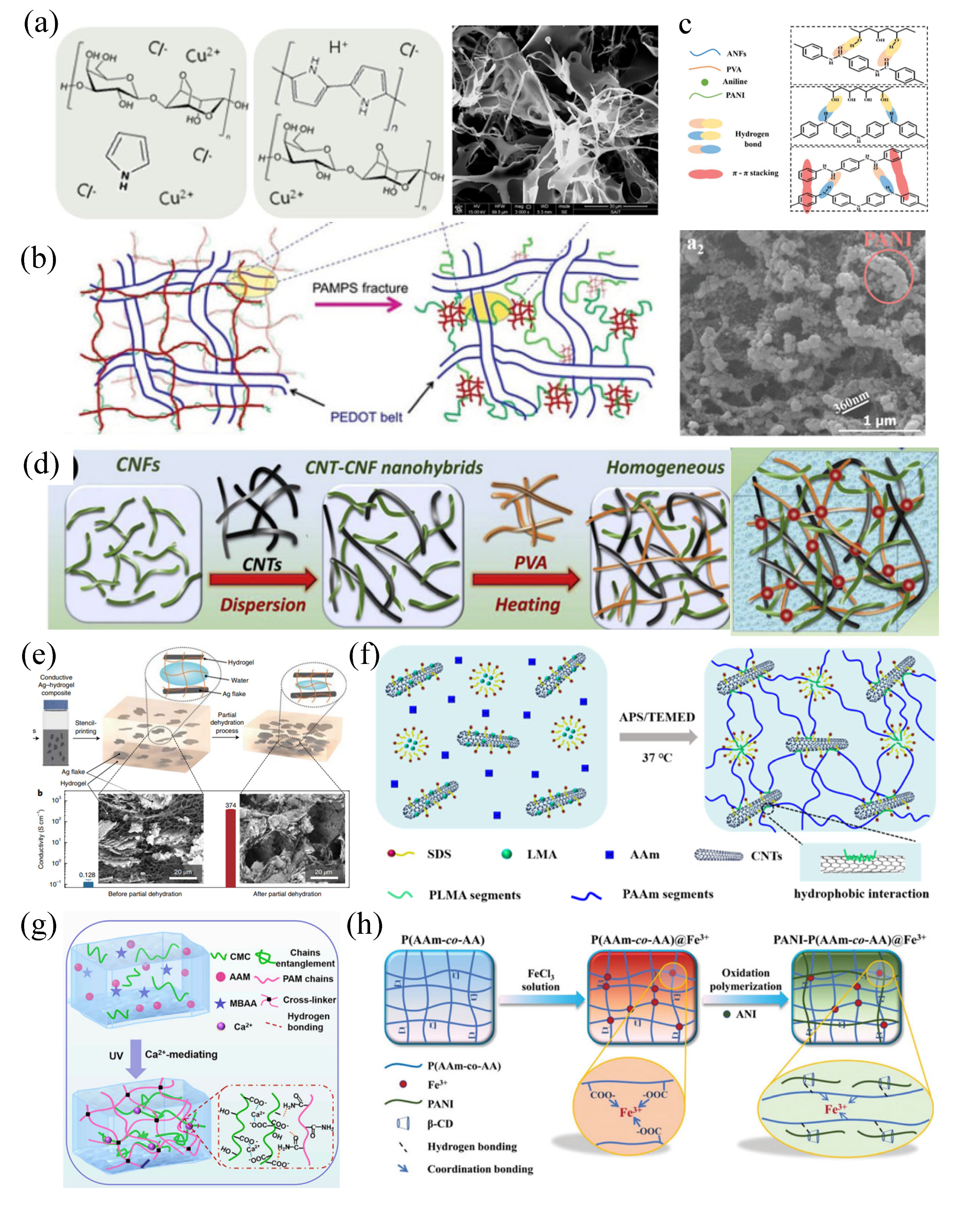
2.1 Electronic conductive hydrogels
2.2. Nanoparticle conductive hydrogels
2.3. Ion conductive hydrogels
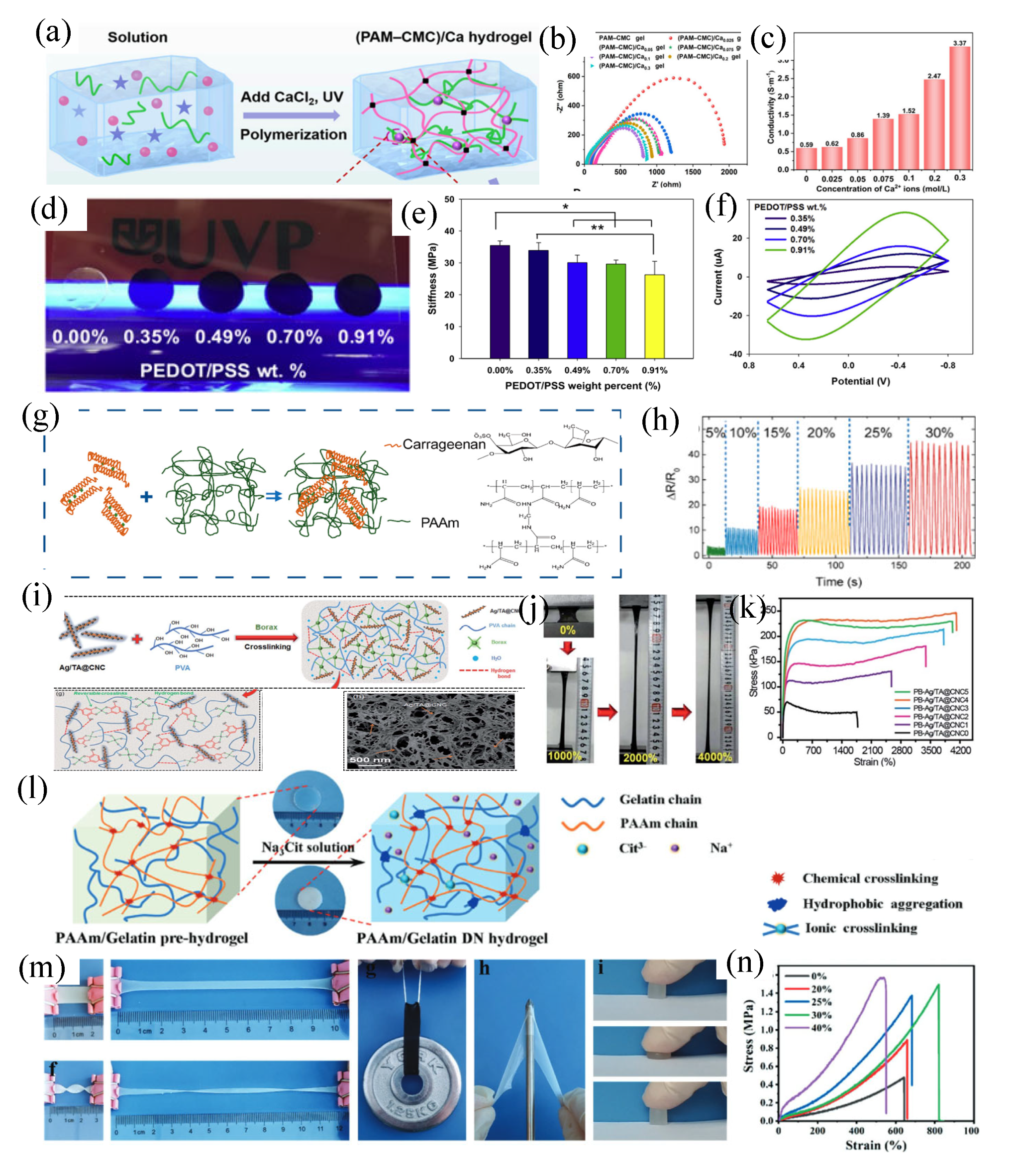
3. Key properties and enhancement strategies of conductive hydrogels
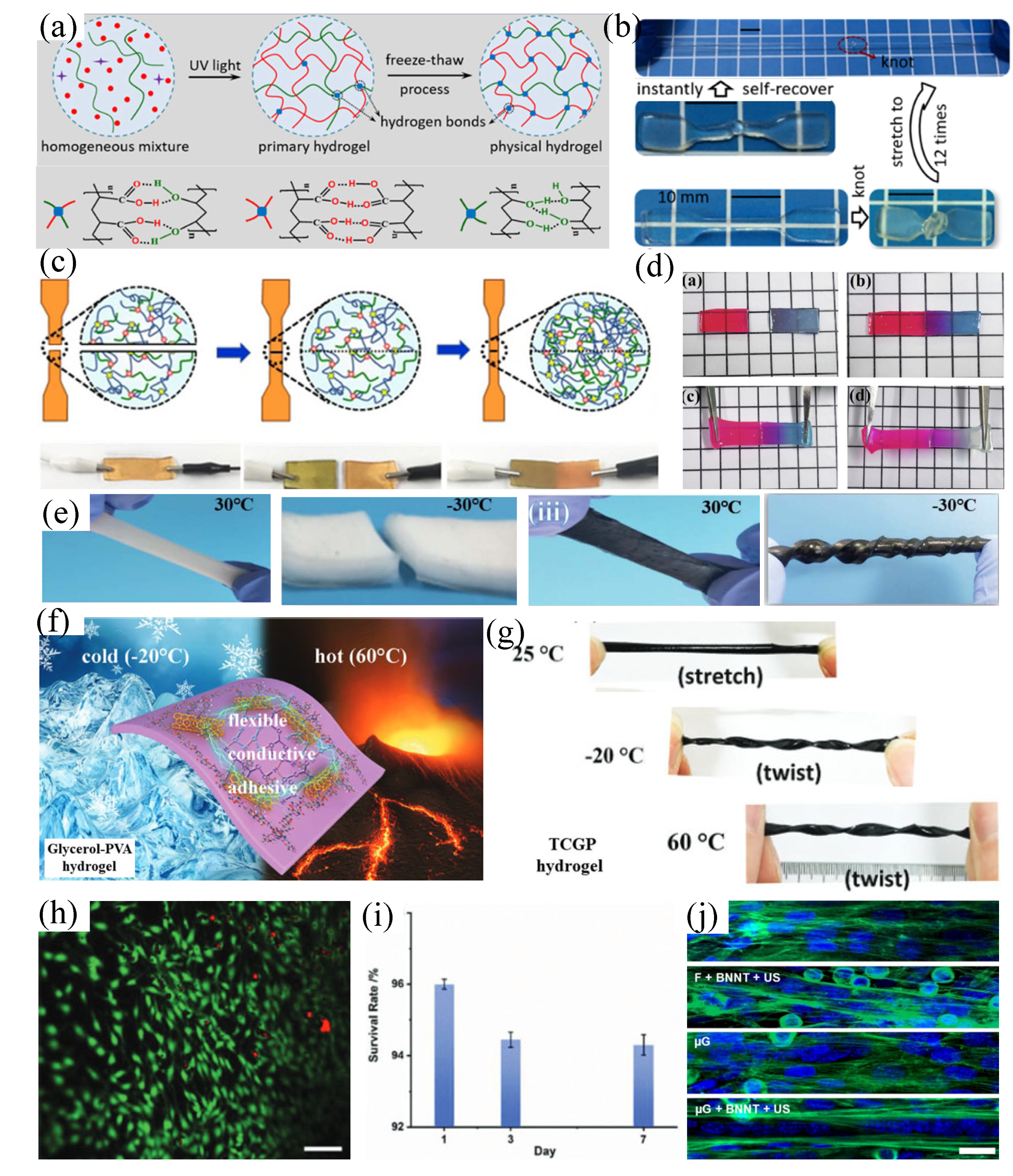
3.2 Mechanical strength and flexibility
3.3 Long-term stability
3.4 Biocompatibility
4. Application demonstration in flexible electronics
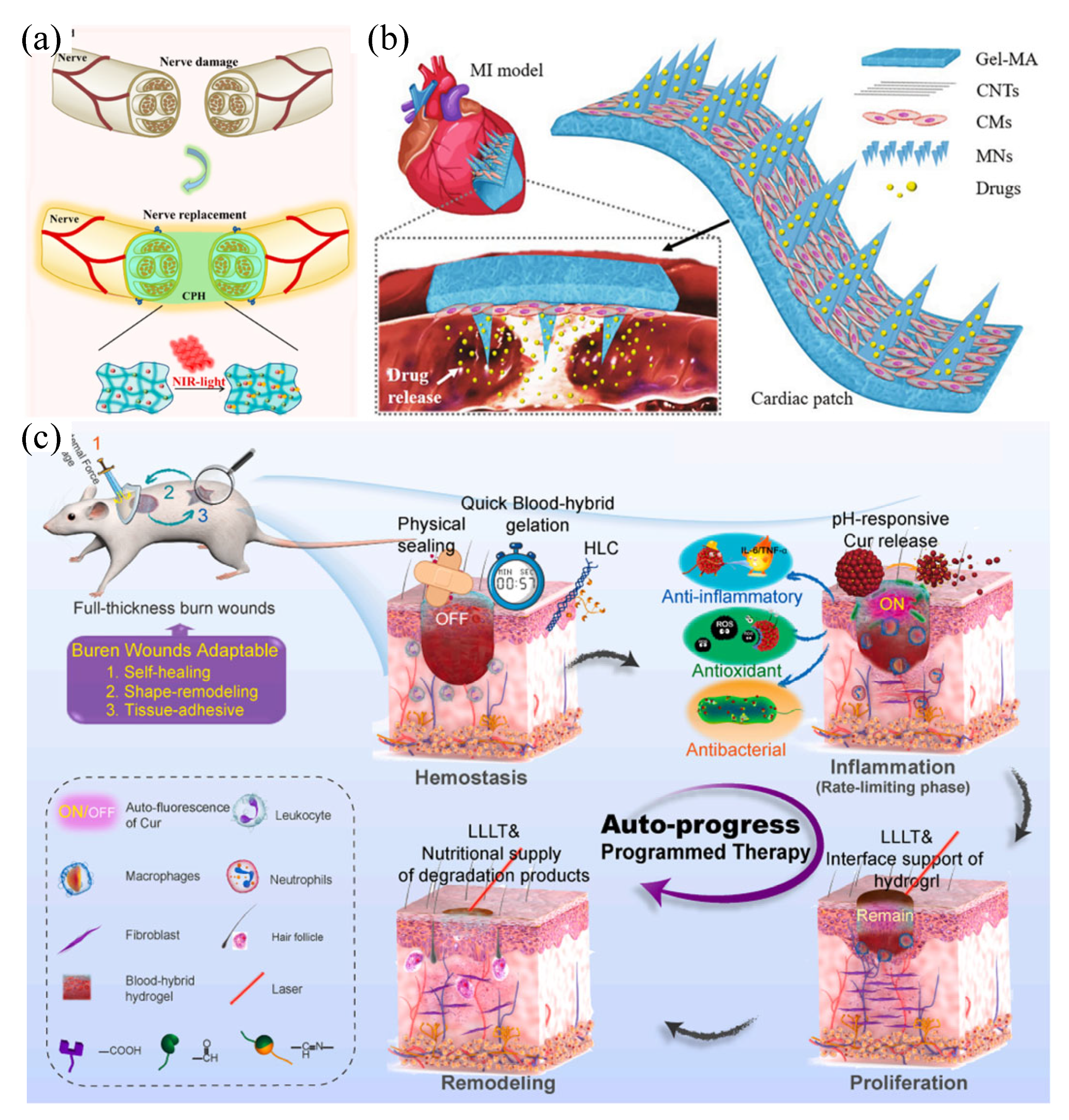
4.1 Applications in tissue engineering
4.2 Applications in soft wearable devices
5. Conclusion and prospects
Author Contributions
Funding
Conflicts of Interest
References
- Ma, X. , Yang, Z., Wang, Y., Zhang, G., Shao, Y., Jia, H., Cao, T., Wang, R., Liu, D., Remote Controlling DNA Hydrogel by Magnetic Field, ACS Applied Materials & Interfaces (2017) 9(3) 1995-2000.
- Jiang, Y. , Wang, Y., Li, Q., Yu, C., Chu, W., Natural Polymer-based Stimuli-responsive Hydrogels, Current Medicinal Chemistry (2020) 27(16) 2631-2657.
- Wu, L. , Li, L., Qu, M., Wang, H., Bin, Y., Mussel-Inspired Self-Adhesive, Antidrying, and Antifreezing Poly (acrylic acid)/Bentonite/Polydopamine Hybrid Glycerol-Hydrogel and the Sensing Application, ACS Applied Polymer Materials (2020) 2(8) 3094-3106.
- Liu, Z. , Zhang, T., Yang, M., Gao, W., Wu, S., Wang, K., Dong, F., Dang, J., Zhou, D., Zhang, J., Hydrogel Pressure Distribution Sensors Based on an Imaging Strategy and Machine Learning, ACS Applied Electronic Materials (2021) 3(8) 3599-3609.
- Su, X. , Hao, D., Xu, X., Guo, X., Li, Z., Jiang, L., Hydrophilic/Hydrophobic Heterogeneity Anti-Biofouling Hydrogels with Well-Regulated Rehydration, ACS APPLIED MATERIALS & INTERFACES (2020) 12(22) 25316-25323.
- Tang, Y. , Zhang, X., Li, X., Ma, C., Chu, X., Wang, L., Xu, W., A review on recent advances of Protein-Polymer hydrogels, EUROPEAN POLYMER JOURNAL (2022) 162 110881.
- Le Goff, G.C. , Srinivas, R.L., Hill, W.A., Doyle, P.S., Hydrogel microparticles for biosensing, European Polymer Journal (2015) 72 386-412.
- Cui, L. , Yao, Y., Yim, E.K.F., The effects of surface topography modification on hydrogel properties, APL Bioengineering (2021) 5(3) 031509.
- Volpi, M. , Paradiso, A., Costantini, M., Swie, W., Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering, ACS BIOMATERIALS SCIENCE & ENGINEERING (2022) 8(2) 379-405.
- Wu, X. , Li, H., Incorporation of Bioglass Improved the Mechanical Stability and Bioactivity of Alginate/Carboxymethyl Chitosan Hydrogel Wound Dressing, ACS applied bio materials (2021) 4(2) 1677-1692.
- Hu, Y. , Du, Z., Deng, X., Wang, T., Yang, Z., Zhou, W., Wang, C., Dual Physically Cross-Linked Hydrogels with High Stretchability, Toughness, and Good Self-Recoverability, MACROMOLECULES (2016) 49(15) 5660-5668.
- Hu, J. , Hiwatashi, K., Kurokawa, T., Liang, S.M., Wu, Z.L., Gong, J.P., Microgel-Reinforced Hydrogel Films with High Mechanical Strength and Their Visible Mesoscale Fracture Structure, MACROMOLECULES (2011) 44(19) 7775-7781.
- Samadi, N. , Sabzi, M., Babaahmadi, M., Self-healing and tough hydrogels with physically cross-linked triple networks based on Agar/PVA/Graphene, INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES (2018) 107 2291-2297.
- Jiang, X. , Xiang, N., Zhang, H., Sun, Y., Lin, Z., Hou, L., Preparation and characterization of poly(vinyl alcohol)/sodium alginate hydrogel with high toughness and electric conductivity, CARBOHYDRATE POLYMERS (2018) 186 377-383.
- Kim, C.-C. , Lee, H.-H., Oh, K.H., Sun, J.-Y., Highly stretchable, transparent ionic touch panel, SCIENCE (2016) 353(6300) 682-687.
- Wei, J. , Xie, J., Zhang, P., Zou, Z., Ping, H., Wang, W., Xie, H., Shen, J.Z., Lei, L., Fu, Z., Bioinspired 3D Printable, Self-Healable, and Stretchable Hydrogels with Multiple Conductivities for Skin-like Wearable Strain Sensors, ACS Applied Materials & Interfaces (2021) 13(2) 2952-2960.
- Su, G. , Yin, S., Guo, Y., Zhao, F., Guo, Q., Zhang, X., Zhou, T., Yu, G., Balancing the mechanical, electronic, and self-healing properties in conductive self-healing hydrogel for wearable sensor applications, Materials Horizons (2021) 8(6) 1795-1804.
- Liu, Z. , Wang, Y., Ren, Y., Jin, G., Zhang, C., Chen, W., Yan, F., Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper, Materials Horizons (2020) 7(3) 919-927.
- Bai, J. , Wang, R., Wang, X., Liu, S., Wang, X., Ma, J., Qin, Z., Jiao, T., Biomineral calcium-ion-mediated conductive hydrogels with high stretchability and self-adhesiveness for sensitive iontronic sensors, Cell Reports Physical Science (2021) 2(11) 100623.
- Sun, L. , Zhu, X., Zhang, X., Chen, G., Bian, F., Wang, J., Zhou, Q., Wang, D., Zhao, Y., Induced cardiomyocytes-integrated conductive microneedle patch for treating myocardial infarction, CHEMICAL ENGINEERING JOURNAL (2021) 414.
- Korupalli, C. , Li, H., Nguyen, N., Mi, F.-L., Chang, Y., Lin, Y.-J., Sung, H.-W., Conductive Materials for Healing Wounds: Their Incorporation in Electroactive Wound Dressings, Characterization, and Perspectives, ADVANCED HEALTHCARE MATERIALS (2021) 10(6) 2001384.
- Zhou, N. , Wang, T., Chen, S., Hu, Q., Cheng, X., Sun, D., Vupputuri, S., Qiu, B., Liu, H., Guo, Z., Conductive polyaniline hydrogel enhanced methane production from anaerobic wastewater treatment, JOURNAL OF COLLOID AND INTERFACE SCIENCE (2021) 581 314-322.
- Sun, X. , Wang, H., Ding, Y., Yao, Y., Liu, Y., Tang, J., Fe3+-Coordination mediated synergistic dual-network conductive hydrogel as a sensitive and highly-stretchable strain sensor with adjustable mechanical properties, JOURNAL OF MATERIALS CHEMISTRY B (2022) 10(9) 1442-1452.
- Hur, J. , Im, K., Kim, S.W., Kim, J., Chung, D.-Y., Kim, T.-H., Jo, K.H., Hahn, J.H., Bao, Z., Hwang, S., Park, N., Polypyrrole/Agarose-Based Electronically Conductive and Reversibly Restorable Hydrogel, ACS NANO (2014) 8(10) 10066-10076.
- Wang, Z. , Cong, Y., Fu, J., Stretchable and tough conductive hydrogels for flexible pressure and strain sensors, Journal of Materials Chemistry B (2020) 8(16) 3437-3459.
- Rivero, R.E. , Molina, M.A., Rivarola, C.R., Barbero, C.A., Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite, Sensors and Actuators B: Chemical (2014) 190 270-278.
- Li, T. , Liang, B., Ye, Z., Zhang, L., Xu, S., Tu, T., Zhang, Y., Cai, Y., Zhang, B., Fang, L., Mao, X., Zhang, S., Wu, G., Yang, Q., Zhou, C., Cai, X., Ye, X., An integrated and conductive hydrogel-paper patch for simultaneous sensing of Chemical-Electrophysiological signals, BIOSENSORS & BIOELECTRONICS (2022) 198 113855.
- Yang, Z. , Ma, J., Bai, B., Qiu, A., Losic, D., Shi, D., Chen, M., Free-standing PEDOT/polyaniline conductive polymer hydrogel for flexible solid-state supercapacitors, ELECTROCHIMICA ACTA (2019) 322 134769.
- Li, L. , Zhang, Y., Lu, H., Wang, Y., Xu, J., Zhu, J., Zhang, C., Liu, T., Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage, NATURE COMMUNICATIONS (2020) 11(1) 62.
- Wang, J. , Lin, Y., Mohamed, A., Ji, Q., Jia, H., High strength and flexible aramid nanofiber conductive hydrogels for wearable strain sensors, Journal of Materials Chemistry C (2021) 9(2) 575-583.
- Lin, F. , Wang, Z., Shen, Y., Tang, L., Zhang, P., Wang, Y., Chen, Y., Huang, B., Lu, B., Natural skin-inspired versatile cellulose biomimetic hydrogels, JOURNAL OF MATERIALS CHEMISTRY A (2019) 7(46) 26442-26455.
- Kim, Y.S. , Cho, K., Lee, H.J., Chang, S., Lee, H., Kim, J.H., Koh, W.-G., Highly conductive and hydrated PEG-based hydrogels for the potential application of a tissue engineering scaffold, REACTIVE & FUNCTIONAL POLYMERS (2016) 109 15-22.
- Xia, S. , Song, S., Jia, F., Gao, G., A flexible, adhesive and self-healable hydrogel-based wearable strain sensor for human motion and physiological signal monitoring, JOURNAL OF MATERIALS CHEMISTRY B (2019) 7(30) 4638-4648.
- Han, L. , Lu, X., Wang, M., Gan, D., Deng, W., Wang, K., Fang, L., Liu, K., Chan, C.W., Tang, Y., Weng, L.-T., Yuan, H., A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics, SMALL (2017) 13(2) 1601916.
- Park, J. , Jeon, J., Kim, B., Lee, M.S., Park, S., Lim, J., Yi, J., Lee, H., Yang, H.S., Lee, J.Y., Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration, Advanced Functional Materials (2020) 30(39) 2003759.
- Qin, Z. , Sun, X., Yu, Q., Zhang, H., Wu, X., Yao, M., Liu, W., Yao, F., Li, J., Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors, ACS Applied Materials & Interfaces (2020) 12(4) 4944-4953.
- Han, J. , Wang, H., Yue, Y., Mei, C., Chen, J., Huang, C., Wu, Q., Xu, X., A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network, CARBON (2019) 149 1-18.
- Wu, N. , Wang, X., Das, C.M., Ma, M., Qiao, N., Fan, T., Zhang, H., Xu, G., Yong, K.-T., Bioengineering applications of black phosphorus and their toxicity assessment, ENVIRONMENTAL SCIENCE-NANO (2021) 8(12) 3452-3477.
- An, D. , Fu, J., Xie, Z., Xing, C., Zhang, B., Wang, B., Qiu, M., Progress in the therapeutic applications of polymer-decorated black phosphorus and black phosphorus analog nanomaterials in biomedicine, JOURNAL OF MATERIALS CHEMISTRY B (2020) 8(32) 7076-7120.
- Lin, J.-h. , Du, X.-s., Self-healable and redox active hydrogel obtained via incorporation of ferric ion for supercapacitor applications, CHEMICAL ENGINEERING JOURNAL (2022) 446 137244.
- Homaeigohar, S. , Tsai, T.-Y., Young, T.-H., Yang, H.J., Ji, Y.-R., An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering, CARBOHYDRATE POLYMERS (2019) 224 115112.
- Rastin, H. , Zhang, B., Mazinani, A., Hassan, K., Bi, J., Tran Thanh, T., Losic, D., 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks, NANOSCALE (2020) 12(30) 16069-16080.
- Liu, Y.-J. , Cao, W.-T., Ma, M.-G., Wan, P., Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic "Soft and Hard" Hybrid Networks, ACS APPLIED MATERIALS & INTERFACES (2017) 9(30) 25559-25570.
- Yu, J. , Dang, C., Liu, H., Wang, M., Feng, X., Zhang, C., Kang, J., Qi, H., Highly Strong and Transparent Ionic Conductive Hydrogel as Multifunctional Sensors, MACROMOLECULAR MATERIALS AND ENGINEERING (2020) 305(12) 2000475.
- Wang, S. , Zhang, Y., A functional triboelectric nanogenerator based on the LiCl/PVA hydrogel for cheerleading training, Materials Technology (2022) 37(13) 2752-2757.
- Liu, X. , Wu, Z., Jiang, D., Guo, N., Wang, Y., Ding, T., Weng, L., A highly stretchable, sensing durability, transparent, and environmentally stable ion conducting hydrogel strain sensor built by interpenetrating Ca2+-SA and glycerol-PVA double physically cross-linked networks, ADVANCED COMPOSITES AND HYBRID MATERIALS (2022) 5(3) 1712-1729.
- Zhao, S. , Tseng, P., Grasman, J., Wang, Y., Li, W., Napier, B., Yavuz, B., Chen, Y., Howell, L., Rincon, J., Omenetto, F.G., Kaplan, D.L., Programmable Hydrogel Ionic Circuits for Biologically Matched Electronic Interfaces, ADVANCED MATERIALS (2018) 30(25) 1800598.
- Bai, J. , Wang, R., Wang, X., Liu, S., Wang, X., Ma, J., Qin, Z., Jiao, T., Biomineral calcium-ion-mediated conductive hydrogels with high stretchability and self-adhesiveness for sensitive iontronic sensors, CELL REPORTS PHYSICAL SCIENCE (2021) 2(11) 100623.
- Ohm, Y. , Pan, C., Ford, M.J., Huang, X., Liao, J., Majidi, C., An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics, Nature Electronics (2021) 4(3) 185-192.
- Qin, Z. , Sun, X., Yu, Q., Zhang, H., Wu, X., Yao, M., Liu, W., Yao, F., Li, J., Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors, ACS APPLIED MATERIALS & INTERFACES (2020) 12(4) 4944-4953.
- Li, Z. , Cui, C., Zhang, Z., Meng, X., Yan, Q., Ouyang, J., Xu, W., Niu, Y., Zhang, S., The Investigation of a Multi-Functional Peptide as Gelator, Dyes Separation Agent and Metal Ions Adsorbent, CHEMISTRYSELECT (2019) 4(27) 7838-7843.
- Ji, S. , Wan, C., Wang, T., Li, Q., Chen, G., Wang, J., Liu, Z., Yang, H., Liu, X., Chen, X., Water-Resistant Conformal Hybrid Electrodes for Aquatic Endurable Electrocardiographic Monitoring, ADVANCED MATERIALS (2020) 32(26) 2001496.
- Dong, R. , Ma, P.X., Guo, B., Conductive biomaterials for muscle tissue engineering, BIOMATERIALS (2020) 229.
- Guo, B. , Ma, P.X., Conducting Polymers for Tissue Engineering, BIOMACROMOLECULES (2018) 19(6) 1764-1782.
- Radisic, M. , Park, H., Shing, H., Consi, T., Schoen, F.J., Langer, R., Freed, L.E., Vunjak-Novakovic, G., Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds, PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA (2004) 101(52) 18129-18134.
- Thompson, B.C. , Richardson, R.T., Moulton, S.E., Evans, A.J., O'Leary, S., Clark, G.M., Wallace, G.G., Conducting polymers, dual neurotrophins and pulsed electrical stimulation - Dramatic effects on neurite outgrowth, JOURNAL OF CONTROLLED RELEASE (2010) 141(2) 161-167.
- Lee, H.-R. , Kim, C.-C., Sun, J.-Y., Stretchable Ionics - A Promising Candidate for Upcoming Wearable Devices, ADVANCED MATERIALS (2018) 30(42) 1704403.
- Yu, X. , Yang, H., Meng, H., Sun, Y., Zheng, J., Ma, D., Xu, X., Three-Dimensional Conductive Gel Network as an Effective Binder for High-Performance Si Electrodes in Lithium-Ion Batteries, ACS APPLIED MATERIALS & INTERFACES (2015) 7(29) 15961-15967.
- Zhou, Y. , Wan, C., Yang, Y., Yang, H., Wang, S., Dai, Z., Ji, K., Jiang, H., Chen, X., Long, Y., Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics, ADVANCED FUNCTIONAL MATERIALS (2019) 29(1) 1806220.
- Liu, T. , Zhang, R., Liu, J., Zhao, L., Yu, Y., High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide, e-Polymers (2021) 21(1) 391-397.
- Qi, J. , Wang, A.C., Yang, W., Zhang, M., Hou, C., Zhang, Q., Li, Y., Wang, H., Hydrogel-based hierarchically wrinkled stretchable nanofibrous membrane for high performance wearable triboelectric nanogenerator, Nano Energy (2020) 67 104206.
- Dong Nyoung, H. , Lee, S.-J., Timsina, R., Qiu, X., Castro, N.J., Zhang, L.G., Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering, MATERIALS SCIENCE AND ENGINEERING C-MATERIALS FOR BIOLOGICAL APPLICATIONS (2019) 99 582-590.
- Wang, S. , Yu, L., Wang, S., Zhang, L., Chen, L., Xu, X., Song, Z., Liu, H., Chen, C., Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions, Nature Communications (2022) 13(1) 3408.
- Yao, X. , Zhang, S., Qian, L., Wei, N., Nica, V., Coseri, S., Han, F., Super Stretchable, Self-Healing, Adhesive Ionic Conductive Hydrogels Based on Tailor-Made Ionic Liquid for High-Performance Strain Sensors, Advanced Functional Materials (2022) 32(33) 2204565.
- Cai, G. , Wang, J., Qian, K., Chen, J., Li, S., Lee, P.S., Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection, ADVANCED SCIENCE (2017) 4(2).
- Shao, C. , Wang, M., Meng, L., Chang, H., Wang, B., Xu, F., Yang, J., Wan, P., Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties, Chemistry of Materials (2018) 30(9) 3110-3121.
- Han, S. , Liu, C., Lin, X., Zheng, J., Wu, J., Liu, C., Dual Conductive Network Hydrogel for a Highly Conductive, Self-Healing, Anti-Freezing, and Non-Drying Strain Sensor, ACS APPLIED POLYMER MATERIALS (2020) 2(2) 996-1005.
- Guo, B. , Ma, Z., Pan, L., Shi, Y., Properties of conductive polymer hydrogels and their application in sensors, JOURNAL OF POLYMER SCIENCE PART B-POLYMER PHYSICS (2019) 57(23) 1606-1621.
- Shi, H. , Liu, C., Jiang, Q., Xu, J., Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review, ADVANCED ELECTRONIC MATERIALS (2015) 1(4) 1500017.
- Kayser, L.V. , Lipomi, D.J., Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS, ADVANCED MATERIALS (2019) 31(10) 1806133.
- Fonner, J.M. , Schmidt, C.E., Ren, P., A combined molecular dynamics and experimental study of doped polypyrrole, POLYMER (2010) 51(21) 4985-4993.
- Li, X.F. , Kolega, J., Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells, JOURNAL OF VASCULAR RESEARCH (2002) 39(5) 391-404.
- Li, X.-G. , Wei, F., Huang, M.-R., Xie, Y.-B., Facile synthesis and intrinsic conductivity of novel pyrrole copolymer nanoparticles with inherent self-stability, JOURNAL OF PHYSICAL CHEMISTRY B (2007) 111(21) 5829-5836.
- Golabi, M. , Padiolleau, L., Chen, X., Jafari, M.J., Sheikhzadeh, E., Turner, A.P.F., Jager, E.W.H., Beni, V., Doping Polypyrrole Films with 4-N-Pentylphenylboronic Acid to Enhance Affinity towards Bacteria and Dopamine, PLOS ONE (2016) 11(11) 1-20.
- Song, R.-B. , Wu, Y., Lin, Z.-Q., Xie, J., Tan, C.H., Loo, J.S.C., Cao, B., Zhang, J.-R., Zhu, J.-J., Zhang, Q., Living and Conducting: Coating Individual Bacterial Cells with In Situ Formed Polypyrrole, ANGEWANDTE CHEMIE-INTERNATIONAL EDITION (2017) 56(35) 10516-10520.
- Xu, D. , Fan, L., Gao, L., Xiong, Y., Wang, Y., Ye, Q., Yu, A., Dai, H., Yin, Y., Cai, J., Zhang, L., Micro-Nanostructured Polyaniline Assembled in Cellulose Matrix via Interfacial Polymerization for Applications in Nerve Regeneration, ACS APPLIED MATERIALS & INTERFACES (2016) 8(27) 17090-17097.
- Lee, I. , Luo, X., Cui, X.T., Yun, M., Highly sensitive single polyaniline nanowire biosensor for the detection of immunoglobulin G and myoglobin, BIOSENSORS & BIOELECTRONICS (2011) 26(7) 3297-3302.
- Lai, J. , Yi, Y., Zhu, P., Shen, J., Wu, K., Zhang, L., Liu, J., Polyaniline-based glucose biosensor: A review, JOURNAL OF ELECTROANALYTICAL CHEMISTRY (2016) 782 138-153.
- Guan, L. , Yan, S., Liu, X., Li, X., Gao, G., Wearable strain sensors based on casein-driven tough, adhesive and anti-freezing hydrogels for monitoring human-motion, J Mater Chem B (2019) 7(34) 5230-5236.
- Zhang, H. , Niu, W., Zhang, S., Extremely stretchable, sticky and conductive double-network ionic hydrogel for ultra-stretchable and compressible supercapacitors, Chemical Engineering Journal (2020) 387 124105.
- Zhang, X. , Cai, J., Liu, W., Liu, W., Qiu, X., Synthesis of strong and highly stretchable, electrically conductive hydrogel with multiple stimuli responsive shape memory behavior, Polymer (2020) 188 122147.
- Han, L. , Liu, K., Wang, M., Wang, K., Fang, L., Chen, H., Zhou, J., Lu, X., Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance, ADVANCED FUNCTIONAL MATERIALS (2018) 28(3) 1704195.
- Pi, M. , Jiang, L., Wang, Z., Cui, W., Shi, L., Ran, R., Robust and ultrasensitive hydrogel sensors enhanced by MXene/cellulose nanocrystals, Journal of Materials Science (2021) 56(14) 8871-8886.
- Chen, G. , Matsuhisa, N., Liu, Z., Qi, D., Cai, P., Jiang, Y., Wan, C., Cui, Y., Leow, W.R., Liu, Z., Gong, S., Zhang, K.-Q., Cheng, Y., Chen, X., Plasticizing Silk Protein for On-Skin Stretchable Electrodes, ADVANCED MATERIALS (2018) 30(21) 1800129.
- Li, Z. , Xu, W., Wang, X., Jiang, W., Ma, X., Wang, F., Zhang, C., Ren, C., Fabrication of PVA/PAAm IPN hydrogel with high adhesion and enhanced mechanical properties for body sensors and antibacterial activity, EUROPEAN POLYMER JOURNAL (2021) 146 110253.
- Naficy, S. , Razal, J.M., Spinks, G.M., Wallace, G.G., Whitten, P.G., Electrically Conductive, Tough Hydrogels with pH Sensitivity, CHEMISTRY OF MATERIALS (2012) 24(17) 3425-3433.
- Zhan, J. , Wu, Y., Wang, H., Liu, J., Ma, Q., Xiao, K., Li, Z., Li, J., Luo, F., Tan, H., An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma, INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES (2020) 163 1208-1222.
- Li, Y. , Wang, H., Niu, Y., Ma, S., Xue, Z., Song, A., Zhang, S., Xu, W., Ren, C., Fabrication of CS/SA Double-Network Hydrogel and Application in pH-Controllable Drug Release, CHEMISTRYSELECT (2019) 4(48) 14036-14042.
- Czakkel, O. , Berke, B., Laszlo, K., Effect of graphene-derivatives on the responsivity of PNIPAM-based thermosensitive nanocomposites - A review, EUROPEAN POLYMER JOURNAL (2019) 116 106-116.
- Wang, Z. , Chen, J., Gong, Y., Zhang, H., Xu, T., Nie, L., Fu, J., Ultrastretchable Strain Sensors and Arrays with High Sensitivity and Linearity Based on Super Tough Conductive Hydrogels, CHEMISTRY OF MATERIALS (2018) 30(21) 8062-8069.
- Wei, J. , Wang, Q., Hofmeister Effect-Aided Assembly of Enhanced Hydrogel Supercapacitor with Excellent Interfacial Contact and Reliability, Small Methods (2019) 3(11) 1900558.
- Wang, G. , Zhang, Q., Wang, Q., Zhou, L., Gao, G., Bio-Based Hydrogel Transducer for Measuring Human Motion with Stable Adhesion and Ultrahigh Toughness, ACS Applied Materials & Interfaces (2021) 13(20) 24173-24182.
- Sun, X. , Yao, F., Wang, C., Qin, Z., Zhang, H., Yu, Q., Zhang, H., Dong, X., Wei, Y., Li, J., Ionically Conductive Hydrogel with Fast Self-Recovery and Low Residual Strain as Strain and Pressure Sensors, MACROMOLECULAR RAPID COMMUNICATIONS (2020) 41(13) 2000185.
- Wang, Y. , Zhang, L., Lu, A., Transparent, Antifreezing, Ionic Conductive Cellulose Hydrogel with Stable Sensitivity at Subzero Temperature, ACS APPLIED MATERIALS & INTERFACES (2019) 11(44) 41710-41716.
- Wang, L. , Daoud, W.A., Hybrid conductive hydrogels for washable human motion energy harvester and self-powered temperature-stress dual sensor, NANO ENERGY (2019) 66 104080.
- Mo, J. , Dai, Y., Zhang, C., Zhou, Y., Li, W., Song, Y., Wu, C., Wang, Z., Design of ultra-stretchable, highly adhesive and self-healable hydrogels via tannic acid-enabled dynamic interactions, Materials Horizons (2021) 8(12) 3409-3416.
- Zhang, Y. , Li, T., Miao, L., Kaur, P., Men, S., Wang, Q., Gong, X., Fang, Y., Zhai, C., Zhang, S., Zhang, L., Ye, L., A highly sensitive and ultra-stretchable zwitterionic liquid hydrogel-based sensor as anti-freezing ionic skin, Journal of Materials Chemistry A (2022) 10(8) 3970-3988.
- Lu, X. , Li, Y., Feng, W., Guan, S., Guo, P., Self-healing hydroxypropyl guar gum/poly (acrylamide-co-3-acrylamidophenyl boronic acid) composite hydrogels with yield phenomenon based on dynamic PBA ester bonds and H-bond, COLLOIDS AND SURFACES A-PHYSICOCHEMICAL AND ENGINEERING ASPECTS (2019) 561 325-331.
- Zhang, Y.S. , Khademhosseini, A., Advances in engineering hydrogels, SCIENCE (2017) 356(6337).
- Sasaki, M. , Karikkineth, B.C., Nagamine, K., Kaji, H., Torimitsu, K., Nishizawa, M., Highly Conductive Stretchable and Biocompatible Electrode-Hydrogel Hybrids for Advanced Tissue Engineering, ADVANCED HEALTHCARE MATERIALS (2014) 3(11) 1919-1927.
- Sontyana, A.G. , Mathew, A.P., Cho, K.-H., Uthaman, S., Park, I.-K., Biopolymeric In Situ Hydrogels for Tissue Engineering and Bioimaging Applications, TISSUE ENGINEERING AND REGENERATIVE MEDICINE (2018) 15(5) 575-590.
- Cui, J. , Chen, J., Ni, Z., Dong, W., Chen, M., Shi, D., High-Sensitivity Flexible Sensor Based on Biomimetic Strain-Stiffening Hydrogel, ACS Applied Materials & Interfaces (2022) 14(41) 47148-47156.
- Hua, D. , Gao, S., Zhang, M., Ma, W., Huang, C., A novel xanthan gum-based conductive hydrogel with excellent mechanical, biocompatible, and self-healing performances, CARBOHYDRATE POLYMERS (2020) 247 116743.
- Fang, Y. , Xu, J., Gao, F., Du, X., Du, Z., Cheng, X., Wang, H., Self-healable and recyclable polyurethane-polyaniline hydrogel toward flexible strain sensor, COMPOSITES PART B-ENGINEERING (2021) 219 108965.
- Chen, W. , Bu, Y., Li, D., Liu, C., Chen, G., Wan, X., Li, N., High-strength, tough, and self-healing hydrogel based on carboxymethyl cellulose, Cellulose (2019) 27(2) 853-865.
- Chen, J. , Peng, Q., Thundat, T., Zeng, H., Stretchable, Injectable, and Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics, CHEMISTRY OF MATERIALS (2019) 31(12) 4553-4563.
- Chen, H. , Song, Y., Guo, H., Miao, L., Chen, X., Su, Z., Zhang, H., Hybrid porous micro structured finger skin inspired self-powered electronic skin system for pressure sensing and sliding detection, Nano Energy (2018) 51 496-503.
- Ren, Z. , Ke, T., Ling, Q., Zhao, L., Gu, H., Rapid self-healing and self-adhesive chitosan-based hydrogels by host-guest interaction and dynamic covalent bond as flexible sensor, CARBOHYDRATE POLYMERS (2021) 273.
- Wang, Z. , Zhang, Y., Yin, Y., Liu, J., Li, P., Zhao, Y., Bai, D., Zhao, H., Han, X., Chen, Q., High-Strength and Injectable Supramolecular Hydrogel Self-Assembled by Monomeric Nucleoside for Tooth-Extraction Wound Healing, Advanced Materials (2022) 34(13) 2108300.
- Yang, C. , Suo, Z., Hydrogel ionotronics, NATURE REVIEWS MATERIALS (2018) 3(6) 125-142.
- Chen, F. , Zhou, D., Wang, J., Li, T., Zhou, X., Gan, T., Handschuh-Wang, S., Zhou, X., Rational Fabrication of Anti-Freezing, Non-Drying Tough Organohydrogels by One-Pot Solvent Displacement, ANGEWANDTE CHEMIE-INTERNATIONAL EDITION (2018) 57(22) 6568-6571.
- Xie, Z. , Li, H., Mi, H.-Y., Feng, P.-Y., Liu, Y., Jing, X., Freezing-tolerant, widely detectable and ultra-sensitive composite organohydrogel for multiple sensing applications, Journal of Materials Chemistry C (2021) 9(31) 10127-10137.
- Wang, M.X. , Chen, Y.M., Gao, Y., Hu, C., Hu, J., Tan, L., Yang, Z., Rapid Self-Recoverable Hydrogels with High Toughness and Excellent Conductivity, ACS Applied Materials & Interfaces (2018) 10(31) 26610-26617.
- Wu, Z. , Shi, W., Ding, H., Zhong, B., Huang, W., Zhou, Y., Gui, X., Xie, X., Wu, J., Ultrastable, stretchable, highly conductive and transparent hydrogels enabled by salt-percolation for high-performance temperature and strain sensing, Journal of Materials Chemistry C (2021) 9(39) 13668-13679.
- Yang, N. , Qi, P., Ren, J., Yu, H., Liu, S., Li, J., Chen, W., Kaplan, D.L., Ling, S., Polyvinyl Alcohol/Silk Fibroin/Borax Hydrogel Ionotronics: A Highly Stretchable, Self-Healable, and Biocompatible Sensing Platform, ACS Applied Materials & Interfaces (2019) 11(26) 23632-23638.
- Morelle, X.P. , Illeperuma, W.R., Tian, K., Bai, R., Suo, Z., Vlassak, J.J., Highly Stretchable and Tough Hydrogels below Water Freezing Temperature, Advanced Materials (2018) 30(35) 1801541.
- Shuai, L. , Guo, Z.H., Zhang, P., Wan, J., Pu, X., Wang, Z.L., Stretchable, self-healing, conductive hydrogel fibers for strain sensing and triboelectric energy-harvesting smart textiles, NANO ENERGY (2020) 78 105389.
- He, P. , Wu, J., Pan, X., Chen, L., Liu, K., Gao, H., Wu, H., Cao, S., Huang, L., Ni, Y., Anti-freezing and moisturizing conductive hydrogels for strain sensing and moist-electric generation applications, JOURNAL OF MATERIALS CHEMISTRY A (2020) 8(6) 3109-3118.
- Kim, M. , Ahn, Y., Lee, K., Jung, W., Cha, C., In situ facile-forming chitosan hydrogels with tunable physicomechanical and tissue adhesive properties by polymer graft architecture, CARBOHYDRATE POLYMERS (2020) 229 115538.
- Hurtado, A. , Cano-Vicent, A., Tuñón-Molina, A., Aparicio-Collado, J.L., Salesa, B., i Serra, R.S., Serrano-Aroca, Á., Engineering alginate hydrogel films with poly(3-hydroxybutyrate-co-3-valerate) and graphene nanoplatelets: Enhancement of antiviral activity, cell adhesion and electroactive properties, International Journal of Biological Macromolecules (2022) 219 694-708.
- Wang, M. , Rojas, O.J., Ning, L., Li, Y., Niu, X., Shi, X., Qi, H., Liquid metal and Mxene enable self-healing soft electronics based on double networks of bacterial cellulose hydrogels, CARBOHYDRATE POLYMERS (2023) 301 120330.
- Guo, Y. , Wang, Y., Chen, H., Jiang, W., Zhu, C., Toufouki, S., Yao, S., A new deep eutectic solvent-agarose gel with hydroxylated fullerene as electrical "switch" system for drug release, CARBOHYDRATE POLYMERS (2022) 296 1-15.
- Wang, X. , Xue, J., Ma, B., Wu, J., Chang, J., Gelinsky, M., Wu, C., Black Bioceramics: Combining Regeneration with Therapy, ADVANCED MATERIALS (2020) 32(48) 2005140.
- Hao, Y.-L. , Li, S.-J., Yang, R., Biomedical titanium alloys and their additive manufacturing, RARE METALS (2016) 35(9) 661-671.
- Ma, Z. , Sameoto, D., A Review of Electrically Driven Soft Actuators for Soft Robotics, MICROMACHINES (2022) 13(11) 1881.
- Ricotti, L. , Fujie, T., Vazao, H., Ciofani, G., Marotta, R., Brescia, R., Filippeschi, C., Corradini, I., Matteoli, M., Mattoli, V., Ferreira, L., Menciassi, A., Boron Nitride Nanotube-Mediated Stimulation of Cell Co-Culture on Micro-Engineered Hydrogels, PLOS ONE (2013) 8(8) 1-15.
- Wang, C. , Rubakhin, S.S., Enright, M.J., Sweedler, J.V., Nuzzo, R.G., 3D Particle-Free Printing of Biocompatible Conductive Hydrogel Platforms for Neuron Growth and Electrophysiological Recording, ADVANCED FUNCTIONAL MATERIALS (2021) 31(14) 2010246.
- Li, G. , Li, C., Li, G., Yu, D., Song, Z., Wang, H., Liu, X., Liu, H., Liu, W., Development of Conductive Hydrogels for Fabricating Flexible Strain Sensors, SMALL (2022) 18(5) 2101518.
- Qin, C. , Lu, A., Flexible, anti-freezing self-charging power system composed of cellulose based supercapacitor and triboelectric nanogenerator, Carbohydrate Polymers (2021) 274 118667.
- Long, Y. , Bai, M., Liu, X., Lu, W., Zhong, C., Tian, S., Xu, S., Ma, Y., Tian, Y., Zhang, H., Zhang, L., Yang, J., A zwitterionic cellulose-based skin sensor for the real-time monitoring and antibacterial sensing wound dressing, Carbohydrate Polymers (2022) 297 119974.
- Xu, Z. , Zhou, F., Yan, H., Gao, G., Li, H., Li, R., Chen, T., Anti-freezing organohydrogel triboelectric nanogenerator toward highly efficient and flexible human-machine interaction at − 30 °C, Nano Energy (2021) 90 106614.
- Wu, Y. , Wang, L., Guo, B., Shao, Y., Ma, P.X., Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering, BIOMATERIALS (2016) 87 18-31.
- Kharkar, P.M. , Kiick, K.L., Kloxin, A.M., Designing degradable hydrogels for orthogonal control of cell microenvironments, CHEMICAL SOCIETY REVIEWS (2013) 42(17) 7335-7372.
- Yuk, H. , Lu, B., Zhao, X., Hydrogel bioelectronics, CHEMICAL SOCIETY REVIEWS (2019) 48(6) 1642-1667.
- Cooke, M.E. , Jones, S.W., ter Horst, B., Moiemen, N., Snow, M., Chouhan, G., Hill, L.J., Esmaeli, M., Moakes, R.J.A., Holton, J., Nandra, R., Williams, R.L., Smith, A.M., Grover, L.M., Structuring of Hydrogels across Multiple Length Scales for Biomedical Applications, ADVANCED MATERIALS (2018) 30(14) 1705013.
- He, L. , Xiao, Q., Zhao, Y., Li, J., Reddy, S., Shi, X., Su, X., Chiu, K., Ramakrishna, S., Engineering an Injectable Electroactive Nanohybrid Hydrogel for Boosting Peripheral Nerve Growth and Myelination in Combination with Electrical Stimulation, ACS APPLIED MATERIALS & INTERFACES (2020) 12(47) 53150-53163.
- Saracino, G.A.A. , Cigognini, D., Silva, D., Caprini, A., Gelain, F., Nanomaterials design and tests for neural tissue engineering, CHEMICAL SOCIETY REVIEWS (2013) 42(1) 225-262.
- Thompson, T. , Steffert, T., Ros, T., Leach, J., Gruzelier, J., EEG applications for sport and performance, METHODS (2008) 45(4) 279-288.
- George, J. , Hsu, C.-C., Nguyen, L.T.B., Ye, H., Cui, Z., Neural tissue engineering with structured hydrogels in CNS models and therapies, BIOTECHNOLOGY ADVANCES (2020) 42 1-37.
- Zhao, Y. , Song, S., Ren, X., Zhang, J., Lin, Q., Zhao, Y., Supramolecular Adhesive Hydrogels for Tissue Engineering Applications, CHEMICAL REVIEWS (2022) 122(6) 5604-5640.
- Liu, X. , Miller, A.L., II, Park, S., Waletzki, B.E., Zhou, Z., Terzic, A., Lu, L., Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation, ACS APPLIED MATERIALS & INTERFACES (2017) 9(17) 14677-14690.
- Roshanbinfar, K. , Vogt, L., Greber, B., Diecke, S., Boccaccini, A.R., Scheibel, T., Engel, F.B., Electroconductive Biohybrid Hydrogel for Enhanced Maturation and Beating Properties of Engineered Cardiac Tissues, ADVANCED FUNCTIONAL MATERIALS (2018) 28(42) 1803951.
- Spencer, A.R. , Sani, E.S., Soucy, J.R., Corbet, C.C., Primbetova, A., Koppes, R.A., Annabi, N., Bioprinting of a Cell-Laden Conductive Hydrogel Composite, ACS APPLIED MATERIALS & INTERFACES (2019) 11(34) 30518-30533.
- Sheng, F. , Zhang, B., Zhang, Y., Li, Y., Cheng, R., Wei, C., Ning, C., Dong, K., Wang, Z.L., Ultrastretchable Organogel/Silicone Fiber-Helical Sensors for Self-Powered Implantable Ligament Strain Monitoring, ACS Nano (2022) 16(7) 10958-10967.
- Dong, M. , Shi, B., Liu, D., Liu, J.-H., Zhao, D., Yu, Z.-H., Shen, X.-Q., Gan, J.-M., Shi, B.-L., Qiu, Y., Wang, C.-C., Zhu, Z.-Z., Shen, Q.-D., Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve, ACS NANO (2020) 14(12) 16565-16575.
- Zhang, Y. , Chen, S., Xiao, Z., Liu, X., Wu, C., Wu, K., Liu, A., Wei, D., Sun, J., Zhou, L., Fan, H., Magnetoelectric Nanoparticles Incorporated Biomimetic Matrix for Wireless Electrical Stimulation and Nerve Regeneration, ADVANCED HEALTHCARE MATERIALS (2021) 10(16) 2100695.
- Bagheri, B. , Zarrintaj, P., Surwase, S.S., Baheiraei, N., Saeb, M.R., Mozafari, M., Kim, Y.C., Park, O.O., Self-gelling electroactive hydrogels based on chitosan-aniline oligomers/agarose for neural tissue engineering with on-demand drug release, COLLOIDS AND SURFACES B-BIOINTERFACES (2019) 184 110549.
- Martin-Granados, C. , McCaig, C.D., Harnessing the Electric Spark of Life to Cure Skin Wounds, Advances in wound care (2014) 3(2) 127-138.
- Guo, A. , Song, B., Reid, B., Gu, Y., Forrester, J.V., Jahoda, C.A.B., Zhao, M., Effects of Physiological Electric Fields on Migration of Human Dermal Fibroblasts, JOURNAL OF INVESTIGATIVE DERMATOLOGY (2010) 130(9) 2320-2327.
- Fang, X. , Jin, Q., Jing, F., Zhang, H., Zhang, F., Mao, H., Xu, B., Zhao, J., Integrated biochip for label-free and real-time detection of DNA amplification by contactless impedance measurements based on interdigitated electrodes, BIOSENSORS & BIOELECTRONICS (2013) 44 241-247.
- Yao, S. , Myers, A., Malhotra, A., Lin, F., Bozkurt, A., Muth, J.F., Zhu, Y., A Wearable Hydration Sensor with Conformal Nanowire Electrodes, ADVANCED HEALTHCARE MATERIALS (2017) 6(6) 1601159.
- Yu, R. , Zhang, H.L., Guo, B.L., Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering, NANO-MICRO LETTERS (2022) 14(1) 1-46.
- Wang, J. , Wang, L., Wu, C., Pei, X., Cong, Y., Zhang, R., Fu, J., Antibacterial Zwitterionic Polyelectrolyte Hydrogel Adhesives with Adhesion Strength Mediated by Electrostatic Mismatch, ACS Applied Materials & Interfaces (2020) 12(41) 46816-46826.
- Shen, S. , Fan, D., Yuan, Y., Ma, X., Zhao, J., Yang, J., An ultrasmall infinite coordination polymer nanomedicine-composited biomimetic hydrogel for programmed dressing-chemo-low level laser combination therapy of burn wounds, Chemical Engineering Journal (2021) 426 130610.
- Venkatalaxmi, A. , Padmavathi, B.S., Amaranath, T., A general solution of unsteady Stokes equations, FLUID DYNAMICS RESEARCH (2004) 35(3) 229-236.
- Manouchehri, S. , Bagheri, B., Rad, S.H., Nezhad, M.N., Kim, Y.C., Park, O.O., Farokhi, M., Jouyandeh, M., Ganjali, M.R., Yazdi, M.K., Zarrintaj, P., Saeb, M.R., Electroactive bio-epoxy incorporated chitosan-oligoaniline as an advanced hydrogel coating for neural interfaces, PROGRESS IN ORGANIC COATINGS (2019) 131 389-396.
- Wang, P. , Pu, Y., Ren, Y., Yang, R., Zhang, W., Tan, X., Xue, W., Liu, S., Li, S., Chi, B., Dynamic regulable sodium alginate/poly(γ-glutamic acid) hybrid hydrogels promoted chondrogenic differentiation of stem cells, CARBOHYDRATE POLYMERS (2022) 275 118692.
- Huang, H. , Han, L., Li, J., Fu, X., Wang, Y., Yang, Z., Xu, X., Pan, L., Xu, M., Super-stretchable, elastic and recoverable ionic conductive hydrogel for wireless wearable, stretchable sensor, Journal of Materials Chemistry A (2020) 8(20) 10291-10300.
- Gao, W. , Ota, H., Kiriya, D., Takei, K., Javey, A., Flexible Electronics toward Wearable Sensing, Accounts of Chemical Research (2019) 52(3) 523-533.
- Shen, Z. , Zhu, X., Majidi, C., Gu, G., Cutaneous Ionogel Mechanoreceptors for Soft Machines, Physiological Sensing, and Amputee Prostheses, Advanced Materials (2021) 33(38) 2102069.
- Peng, K. , Zheng, L., Zhou, T., Zhang, C., Li, H., Light manipulation for fabrication of hydrogels and their biological applications, ACTA BIOMATERIALIA (2022) 137 20-43.
- Lin, Y.-L. , Zheng, S., Chang, C.-W., Lee, M.-J., Chen, Y.-F., Chen, J.-T., Photoresponsive Single-Ion Nanocomposite Hydrogels: Competition of Host-Guest Interactions, MACROMOLECULES (2022) 55(19) 8940-8949.
- Pang, Y. , Wei, C., Li, R., Wu, Y., Liu, W., Wang, F., Zhang, X., Wang, X., Photothermal conversion hydrogel based mini-eye patch for relieving dry eye with long-term use of the light-emitting screen, INTERNATIONAL JOURNAL OF NANOMEDICINE (2019) 14 5125-5133.
- Shao, C. , Meng, L., Cui, C., Yang, J., An integrated self-healable and robust conductive hydrogel for dynamically self-adhesive and highly conformable electronic skin, Journal of Materials Chemistry C (2019) 7(48) 15208-15218.
- Wang, Q. , Pan, X., Guo, J., Huang, L., Chen, L., Ma, X., Cao, S., Ni, Y., Lignin and cellulose derivatives-induced hydrogel with asymmetrical adhesion, strength, and electriferous properties for wearable bioelectrodes and self-powered sensors, CHEMICAL ENGINEERING JOURNAL (2021) 414 128903.
- Pang, Q. , Hu, H., Zhang, H., Qiao, B., Ma, L., Temperature-Responsive Ionic Conductive Hydrogel for Strain and Temperature Sensors, ACS Applied Materials & Interfaces (2022) 14(23) 26536-26547.
- Lei, H. , Zhao, J., Ma, X., Li, H., Fan, D., Antibacterial Dual Network Hydrogels for Sensing and Human Health Monitoring, ADVANCED HEALTHCARE MATERIALS (2021) 10(21) 2101089.
- Li, G. , Zhang, X., Sang, M., Wang, X., Zuo, D., Xu, J., Zhang, H., A supramolecular hydrogel electrolyte for high-performance supercapacitors, Journal of Energy Storage (2021) 33 101931.
- Guan, Q. , Lin, G., Gong, Y., Wang, J., Tan, W., Bao, D., Liu, Y., You, Z., Sun, X., Wen, Z., Pan, Y., Highly efficient self-healable and dual responsive hydrogel-based deformable triboelectric nanogenerators for wearable electronics, Journal of Materials Chemistry A (2019) 7(23) 13948-13955.
- Zhou, G. , Yang, L., Li, W., Chen, C., Liu, Q., A Regenerable Hydrogel Electrolyte for Flexible Supercapacitors, iScience (2020) 23(9) 101502.
- Pu, X. , Liu, M., Chen, X., Sun, J., Du, C., Zhang, Y., Zhai, J., Hu, W., Wang, Z.L., Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing, Science Advances (2017) 3(5) e1700015.


| Conductive components | Conductivity (S cm-1) | REF | |
| Electronic conductive hydrogels | PEDOT: PSS | 10-2-4.38×103 | [70,71] |
| PPY | 10-2-7.5×103 | [72,73,74,75,76] | |
| PANI | 10-2-2×102 | [77,78,79] | |
| Nanoparticle conductive hydrogels | K+, Li+ | 0.02-0.0736 | [80,81] |
| Ca2+ | 0.0337 | [19] | |
| Fe3+ | 0.00216 | [82] | |
| Ion conductive hydrogels | CNTs | 0.082 | [83] |
| MXene | 0.01092 | [84] | |
| Silver | >350 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





