1. Introduction
The concept of seeing what you treat is extremely strong [
1]. Image guided superficial radiation therapy (IGSRT) combines superficial radiation therapy (SRT) with full dermal visualization (FDV) via high resolution dermal ultrasound (HRDUS). IGSRT utilizes the SRT-100 Vision, a medical device that was FDA cleared for the treatment of nonmelanoma skin cancer (NMSC) and keloids in 2013.
The gold standard for delivery of IGSRT includes a comprehensive cancer care model that supports dermatology practices with an organized multidisciplinary team. This team is composed of radiation therapists, medical physicists, radiation oncologists and derma-tologists, all of whom have extensive experience in the safe and effective delivery of IGSRT. A multidisciplinary team of experts, combined with the ability to visualize the entire dermis offers a multitude of benefits before, during and after IGSRT.
These improvements have allowed dermatologists using IGSRT to achieve NMSC cure rates that exceed 99% across multiple retrospective studies [2-5] One meta-analysis showed a statistically significant improvement in NMSC 2-year recurrence probability, when NMSC lesions were treated with IGSRT compared to Mohs micrographic surgery (MMS) [
6].
The focus of this discussion will be limited to the benefits of IGSRT during therapy:
1. Visual confirmation of changing tumor depth before each radiation dose is delivered.
2. Adaptive Radiotherapy: adapting to tumor depth changes by adjusting treatment parameters like energy(kV), TDF (time, dose, fractionation) and dose/boost.
3. Visual confirmation of radiobiologic response: replacement of cancerous cells (solid black, hypoechoic) with healthy normal tissue (speckled green/hyperechoic)
4. Patient visualizes tumor response, improving compliance & patient outcomes
5. Ability to treat NMSC tumors classified as high risk per NCCN guidelines
Differences between IGSRT and IGRT
To understand IGSRT, we first need to understand how it evolved from its predecessor IGRT (
Table 1). IGSRT stands for Image Guided Superficial Radiation Therapy, which is primarily used by dermatologists for treating skin cancer. IGSRT uses low energy su-perficial radiation (SRT) because the skin is the most superficial organ in the human body. IGSRT radiation is primarily absorbed by the dermis, which is where skin cancers grow. It may be easier to think of IGSRT as Image Guided Skin Radiation Therapy.
Table 1.
A comparison of image guided superficial radiation therapy (IGSRT) and image-guided radiation therapy (IGRT).
Table 1.
A comparison of image guided superficial radiation therapy (IGSRT) and image-guided radiation therapy (IGRT).
IGRT stands for Image Guided Radiation Therapy. IGRT utilizes a more penetrating form of radiation called external beam (XRT), which requires a linear accelerator. In terms of tissue penetration IGRT is excellent. That’s why IGRT is mainly used by radiation on-cologists to treat cancers deep inside the body, ie. breast, lung, colon or prostate cancer.
Imaging and Radiation Exposure in IGSRT and IGRT
IGSRT utilizes ultrasound imaging, which has the advantage of quickly and easily gathering images without exposing the patient to any additional radiation. Unfortunately, the imaging used in IGRT may expose the patient to a higher dose of radiation via CT or PET scans. Finally, both IGSRT and IGRT are both associated with an improved thera-peutic index, which means less toxicity and improved tumor control.
2. Materials and Methods
Medical records from 7 dermatology clinics of 883 patients with 1,507 cases of NMSC treated with IGSRT between 2017 and 2018 were retrospectively reviewed. The data was analyzed to determine the following endpoints:
1. Percentage of HRDUS images that measured a change in the NMSC’s depth of invasion (DOI) compared to that of the previous image.
2. How often does HRDUS identify the need for compensatory changes in treatment parameters like kV, TDF, dose & boost doses. All compensatory changes were docu-mented as a percentage of the total number of cases.
3. Percentage of HRDUS images that confirmed a biologically effective dose (BED) was delivered as evidenced by a consistently uniform pattern of the tumor kill/repopulation cycle. A grading system of 1+ (mild), 2+ (moderate), 3+ (complete) indicates the degree of repopulation seen on HRDUS imaging.
4. Ability to treat NMSC tumors classified as high risk per 2024 NCCN guidelines
3. Results
In this study, 1,507 NMSC lesions were reviewed, with 26,975 HRDUS images collected. On average, 17.9 HRDUS images were taken per NMSC lesion.
Of the NMSC lesions, 92% (1,386 out of 1,507) displayed daily depth fluctuations. This is significant because when these fluctuations increase tumor depth, adaptive replanning can be used to improve efficacy. Conversely, when these fluctuations decrease tumor depth, adaptive replanning can be used to reduce toxicity according to the ALARA principle of radiation safety.
Of the NMSC lesions, 40% (598 out of 1,507) required at least 1 adaptive replanning during therapy, while 60% (909 out of 1,507) of lesions required no changes during therapy. This is important because it demonstrates the need for constant imaging so that the high frequency of changes can be detected so that adaptive replanning can be successful.
Of the NMSC lesions, 83% (1,250 out of 1,507) were considered high-risk per the 2024 NCCN guidelines. This is significant because SRT without imaging was generally limited to low-risk NMSC due to lack of visualization. The 100% visualization possible with IGSRT allows this new technology to treat both low and high risk NMSC
Full Dermal Visualization (FDV)
The high cure rates of IGSRT are also attributed to its ability to visualize the entire dermis before, during and after therapy. FDV allows dermatologists and radiation therapists to accurately measure and document frequent fluctuations in depth and monitor tumor response prior to each fraction of radiation delivered. Full dermal visualization of the constantly changing NMSC tumor depth throughout therapy allows the dermatologist and radiation therapist to make compensatory adjustments (in kV, TDF and dose) in real time prior to every dose delivered. This marks the first time in history that dermatologists and radiation therapists have been able to visualize the reactive changes that basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) undergo during radiation therapy. The two most significant patterns of reactive change that have emerged from FDV analysis obtained prior to each fraction are fluctuating tumor depth (aka “the moving target”) and progressive tumor replacement (aka “repopulation”). Both of these visual patterns are dynamic and unpredictable, therefore difficult to accurately assess without serial imaging.
NMSC Measured Depth Fluctuations
While the reasons why NMSC tumors expand and contract repeatedly during IGSRT are not fully understood, this means that NMSCs treated with radiation are moving targets. This “moving target” or “constantly changing depth” is significant because of percent-age depth dose (PDD) which is the percentage of the original radiation dose that is de-livered at a given measured depth. Thus, as radiation goes deeper it becomes weaker. PDD relays what percentage of the prescribed radiation dose actually reaches a partic-ular depth in the dermis, most notably the depth at the bottom of the tumor.
Moving Target and Percentage Depth Dose (PDD)
As radiation passes into the skin, there is a reduction in effective dose for deeper layers. For thicker tumors, this translates into possible undertreatment of the tumor base. Thus, performing HRDUS prior to each fraction allows for continuous measurement of the effect dose at the depth most at risk for recurrence
Multidisciplinary Team Approach
IGSRT has an excellent safety profile and significantly higher NMSC cure rates than any other noninvasive therapy for NMSC, including SRT without imaging, topical 5-FU, topical imiquimod and photodynamic therapy (PDT) [
7]. HRDUS is the imaging tech-nology that has allowed IGSRT to become the gold standard for the non-invasive treatment of NMSC. Optimized delivery of IGSRT requires a radiation therapist, medical physicist and radiation oncologist. Because IGSRT primarily treats NMSC, a treating dermatologist in a dermatology clinic is the optimal setting. The gold standard for de-livery of IGSRT is in a dermatology clinic where the dermatologist and radiation therapist are supported by a multidisciplinary team (MDT) including radiation oncologists and medical physicists. Traditionally SRT (without imaging) was operated by dermatologists in isolation, without the support and benefits of an MDT. It is widely known that MDTs significantly improve the quality of cancer care. The integration of all the professionals involved in the treatment of a specific cancer guarantees full and continued support to patients during diagnosis, treatment and follow-up periods and it is perceived positively by most patients [
8].
Clinical Example
An 83-year-old male presents with a NMSC being treated with a 5 cm cone at an energy of 70 kV. The PDD at the epidermal surface (depth = 0.0 mm) is 100%. This means that 100% of the dose is delivered at the skin surface of the NMSC. The dose is 100% because it has not yet entered the skin. The deepest depth of the NMSC is measured at a thickness (depth) of 2.0 mm. PDD tables show that at the deepest tumor depth of 2.0 mm the dose has been diminished to 88%. Based on this, adaptive changes can be made to compensate for the decrease in dose to the bottom of the tumor.
Additional reasons to perform HRDUS prior to each fraction are to confirm the correct anatomic location of the tumor prior to delivering each radiation dose (patient safety to avoid geographic miss) and to monitor the effectiveness by observing the tumor’s re-sponse to therapy, known as repopulation (decreased tumor cell density and uniformity of replacement of cancer cells with healthy normal tissue).
However, once IGSRT has begun, the depth of the BCC is constantly changing, like a moving target, up and down, in an unpredictable fashion. Thus, our BCC, which was previously only 2.0 mm deep has now expanded to a deepest depth measurement of 3.0 mm, at which point the PDD has dropped to 83%. This means that the increase in tu-mor depth caused the cancer cells at the base of the tumor to receive only 83% of the originally prescribed tumoricidal dose.
When the tumor depth reaches 3.0 mm for any reason, we can make adaptive changes, such as switching the energy from 70kV to 100kV in mid treatment. This temporarily boosts the dose delivered to the base of the tumor from a PDD of 83% to 86% until the tumor depth changes again, which it will because the depth is constantly changing. This change in energy doesn’t change the total dose. However, if the increase in PDD from 83% to 86% (that we achieved by increasing the energy from 70kV to 100KV) isn’t enough to adequately dose the tumor, we can always increase the TDF, which stands for time dose fractionation.
TDF is a mathematical expression used in radiotherapy to calculate the biological effect of a given radiation dose. By increasing the TDF we can increase the biological effectiveness of a radiation treatment. Increasing the TDF increases the total dose. The primary goal of IGSRT is to reach a high enough TDF to eradicate the NMSC (biologic effect), while the secondary goal is to keep the total dose low enough to avoid too many side effects. Performing HRDUS prior to every fraction allows this delicate balance between efficacy and tolerability to be optimized based on objective measurements.
The delivery of SRT without full dermal visualization via HDRUS imaging prior to each fraction (treatment, dose given) prevents clinicians from monitoring NMSC tu-mor depth changes. Tumor depth is constantly changing. This pattern of constant change is not predictable, so depth measurements must be repeated prior to each fraction of radiation given. In the absence of the ability to monitor depth changes prior to each fraction (treatment, dose given), the clinician cannot gauge when the tumor has reached a maximal depth that requires a compensatory adjustment in energy (kV) or TDF (or both), which could put the patient at risk for incomplete tumor clearance and recurrence. Without IGSRT, the ability to achieve a 99% cure rate for the BCC or SCC is lost.
Rapidly Growing Tumors
Per fraction HRDUS is also beneficial for the early detection of rapidly growing well differentiated SCC tumors such as keratoacanthoma. In these scenarios the danger is that these tumors can reach depths that are inappropriate for IGSRT much more quickly than IGSRT can shrink these tumors. Such tumors would require electrodessication and cu-rettage before continuing IGSRT, or conversion of the treatment modality to surgery.
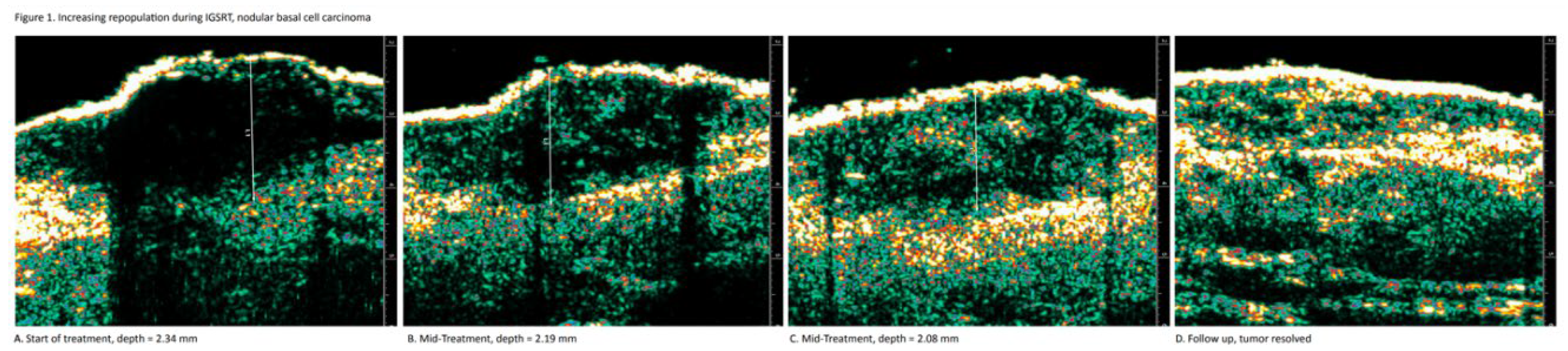
Repopulation
Repopulation is a visual pattern that confirms biologically effective dosing (BED) during IGSRT as well as monitoring treatment response. BED is confirmed by a gradual and uniform reduction in (hypoechoic or black) tumor cell density, while treatment response is confirmed by a gradual and uniform infiltration of healthy dermal tissue (green speckling) within the tumor. It is theorized that healthy, normal tissue infiltrates into areas where the tumor has undergone damage/destruction. This monitoring is achieved by viewing serial HRDUS images of the NMSC tumor and the surrounding normal dermis during therapy. Prior to radiation, the clearly defined solid tumor, regardless of mor-phology, is predominantly hypoechoic (black). The dense green speckled pattern that surrounds the tumor indicates the infiltration of healthy dermis (
Figure 1).
Monitoring NMSC tumor repopulation during IGSRT is necessary because of the PDD concept, which dictates that the radiation dose will always be weakest at the deepest margin of the tumor. Each dose of radiation causes the necrosis of a percentage of the cancer cells, so it is important that this process occurs uniformly throughout the tumor, from top to bottom. A uniformly distributed pattern of cancer cell necrosis within the NMSC tumor indicates that the biologic effect of the radiation is optimized for complete tumor clearance.
Repopulation can confirm the biologic effectiveness of IGSRT by confirming the pro-gressive emergence of healthy dermis (green speckling) within the tumor over the course of therapy. Green speckling is associated with healthy normal dermis, and healthy cells cannot migrate into a solid malignant tumor unless the tumor itself is damaged enough to present an opportunity, such as wounds or cavities formed via cancer cell necrosis.
Radiation causes necrosis and death of cancer cells via a combination of traumatic breaks in double stranded DNA and a complex immune response [
9]. Macrophages then remove necrotic cancer cells via phagocytosis and this cycle repeats with each dose of radiation creating small defects or “wounds” within the tumor. Those wounds then activate fi-broblasts, which migrate into the wound cavity and begin creating new extracellular matrix and collagen (healthy dermis, green speckling) until the wound or cavity is completely repopulated with healthy skin.
Dermatologists and radiation therapists can monitor the gradual repopulation of a BCC or SCC to gauge the biologic effectiveness of IGSRT therapy for any given tumor, which is important. This visual data creates a valuable feedback loop for the clinician. Repopu-lation patterns in which HRDUS images reveal a uniform and evenly spaced pattern of healthy normal tissue (green speckling) within the tumor indicates a uniform and evenly spaced pattern of cancer cell death, and the absence of radioresistance.
Absence or dimunition of the expected repopulation pattern on HRDUS alerts the der-matologist of the possibility of a tumor with greater radioresistance and the subsequent possibility of residual pockets of tumor at the end of therapy. Within the NMSC tumor the uniformity of the green speckling pattern in repopulating areas within the cancer mass confirms that the radiation is eradicating cancer cells effectively at all tumor depths.
In cases where HRDUS identifies residual tumor 3-6 weeks after the final fraction, the radiation oncologist can assist in determining the most appropriate boost doses, known as salvage therapy. In addition, tiny foci of radioresistant tumor can be accurately localized using HRDUS so that targeted injections with 5-FU can be delivered to assist in tumor clearance.
Dermatologists and radiation therapists can also share HRDUS images of repopulation with patients so that they can visually confirm that the radiation therapy is working effectively. Theoretically, when patients can see their cancer being steadily killed off and replaced by healthy normal tissue at each visit, patient compliance with therapy may increase and they may be less likely to miss appointments. In this manner, repopulation images can theoretically be utilized to improve patient outcomes.
4. Discussion
Image Guided Radiation Therapy (IGRT)
Evolutionary changes in radiotherapy are intuitively linked to medical imaging. Emerging imaging technologies have enabled substantial improvements in planning, dose delivery and verification. Image guidance is a necessary and natural corollary to high precision radiotherapy [
10].
A fully optimized IGRT system requires 3 essential elements:
1. 3D volumetric measurements of soft tissues including tumors
2. Efficient acquisition and comparison of the 3D measurements
3. An efficacious process for clinically meaningful intervention [
11]
Such an ideal IGRT system would allow for systematic and random set up errors to be corrected on a daily basis [
11].
Furthermore, the broadest and most appropriate context of IGRT includes:
1. Detection and diagnosis
2. Delineation of target
3. Determining biologic attributes
4. Dose distribution design
5. Dose delivery assurance
6. Deciphering treatment response [
11]
IGRT has also been described as adaptive radiotherapy, which involves changing the original treatment plan to address changes in tumor size, biology or function. The goal is to adapt to the change in tumor volume by modification of the dose prescription, target volumes and/or treatment plan. In other words, IGRT measures changing tumor volumes, which in turn allows adaptation to daily variations and deviations resulting in improved dose delivery. Adaptive radiotherapy is most beneficial in tumors that exhibit motion or variation in tumor volume, ie there are daily variations that, if properly addressed, result in improved dose delivery. It is well known that anatomic changes can occur during a course of radiotherapy including changes in tumor size and shape [
10].
Described another way, the goal of IGRT is to quickly acquire images in the treatment room to guide radiation delivery based on instant knowledge of the target location and changes in tumor volume during treatment. IGRT accurately measures variations in tumor position, size and shape combined with adjustments made to maximize the ge-ometric accuracy and precision of radiation therapy [
12]. Changes in tumor position, size and shape that take place during radiation therapy are measured and compared to previous measurements to boost geometric accuracy, optimize radiation delivery and enhance uniformity in doses delivered [
13]. More than 90% of radiation oncologists surveyed are utilizing at least one type of image guidance technology in their clinical practices [
14].
It is anticipated that IGRT will remain a driving force for research and development, specifically to create novel types of radiation treatments as described in these pages. Furthermore, IGRT as a core technology will facilitate the current shift of paradigm toward personalized radiotherapy [
1].
It will be clear that this real-time visualization also allows immediate dose accumulation and optimization. Ideally, the tumor anatomy is being followed in real time while the dose delivery is being optimized continuously, making robotic self-navigating treatment optimizations [
1].
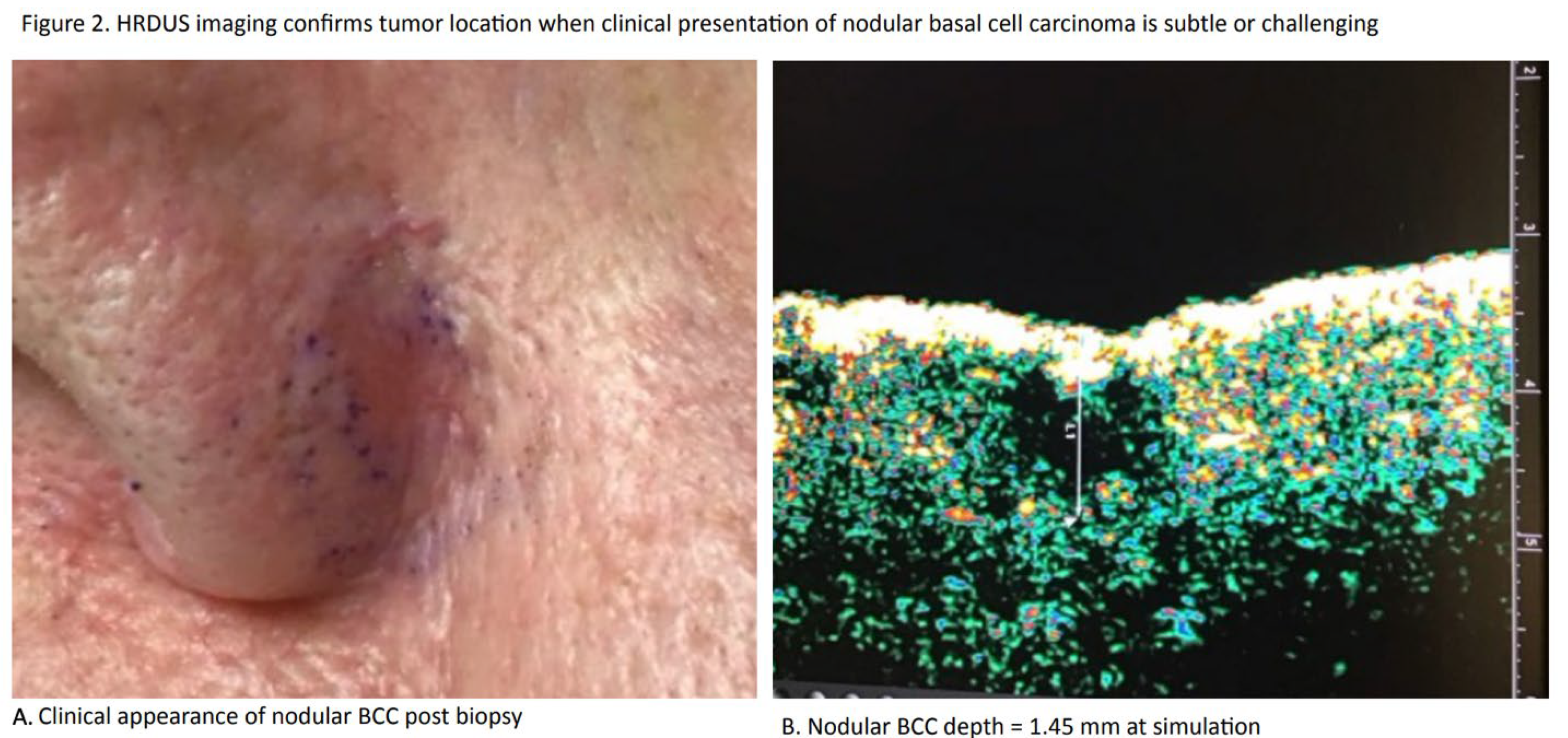
Tumor Localization
Both IGSRT and IGRT use imaging to precisely define tumor location (
Figure 2) but for different reasons. A dermatologist using IGSRT imaging to clearly define the location of the tumor is doing so to confirm the correct anatomic location of the tumor to avoid ge-ographic miss in the absence of surface erythema. A radiation oncologist using IGRT imaging to clearly define the location of the tumor might be doing so to allow conformal dosing to spare the surrounding tissues.
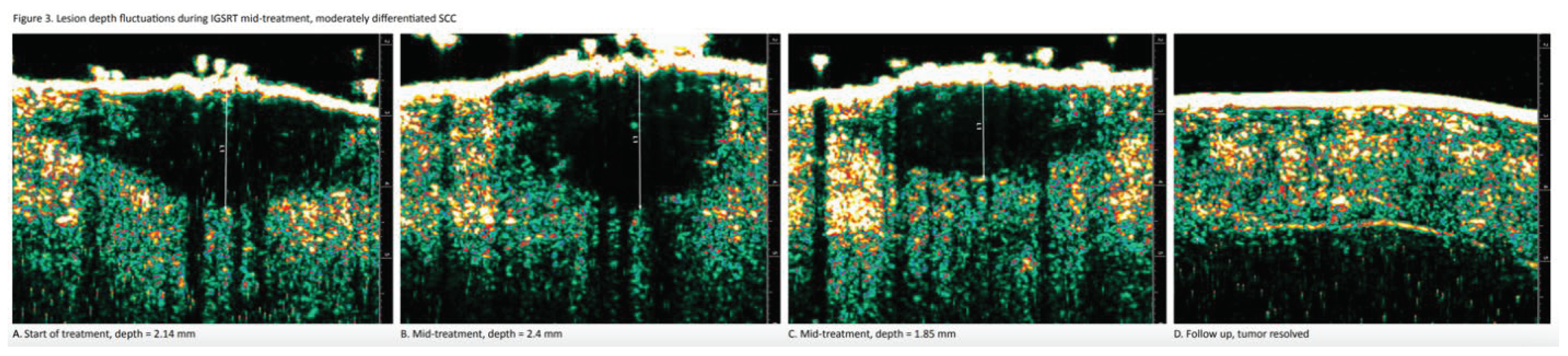
Moving Target Concept
Both IGSRT and IGRT require frequent imaging to better define uncertainties commonly encountered when treating the tumor or target. In IGSRT, the dermatologist is treating skin tumors in which the depth of invasion is constantly changing (
Figure 3). In IGRT, radiation oncologists are more focused on tumor location in relation to changes in patient position due to weight loss, tumor shrinkage or internal organ motion.
Real Time Adaptive Radiotherapy
Both IGSRT and IGRT precisely and quickly measure and compare “moving target” images so that changes and variations can be accounted for. A dermatologist using IGSRT may compensate for a measured increase in tumor depth by increasing energy (kV), TDF or dose. A radiation oncologist using IGRT may compensate for a measured change in expected tumor location by changing the patient’s position, dose, field size or target volume. Adaptive replanning is frequently utilized in the treatment of head and neck cancers.
Biologic Effectiveness
Both IGSRT and IGRT use imaging to confirm dose delivery and decipher treatment response. A dermatologist using IGSRT imaging can confirm that a biologically effective dose is being delivered by identifying a uniform decrease in tumor cell density and an increase of healthy dermal tissue infiltrating into the tumor. A radiation oncologist using IGRT imaging can use a biomarker like fluorodeoxyglucose (FDG) and a PET scan to identify areas of hypoxia or radioresistance within the tumor.
The reasons for tumor depth fluctuations in BCC and SCC during IGSRT are not fully understood but are most likely related to the inflammatory and biologic effects of radiation therapy. Radiation creates traumatic DNA double-strand breaks causing cell death in highly replicating tumor cells. The death of those cancer cells triggers an immunological reaction that contributes to eradicating the tumor via antigen presentation and subsequent T-cell activation [
15]. Cancer cell death and the immunological reaction it triggers both manifest histologically as acute radiation dermatitis (ARD) during IGSRT.
Histologically ARD is a vacuolar interface dermatitis characterized by epidermal edema, vacuolization of the basal cell layer and a lymphocytic infiltrate in the papillary dermis. Individual keratinocytes, predominantly in the basal layer are necrotic, manifesting as colloid or Civatte bodies. Dermal changes include dermal and endothelial cell edema, vasodilation, erythrocyte extravasation, and fibrin thrombi in vessels. The papillary dermis may include an accumulation of melanophages. A lymphocytic inflammatory infiltrate is noted throughout the dermis [
16,
17,
18,
19].
Histologically BCC and SCC tumors always originate in the epidermis and frequently extend into the papillary dermis. Prior to IGSRT, BCC and SCC are both easily visible hypoechoic (black) masses on HRDUS. Additional studies are needed to clarify the re-lationship between the complex inflammatory pathways caused by radiation and the visible expansion and contraction of BCC and SCC tumors during IGSRT.
As the number of radiation doses increase over time small green speckles (healthy dermis) begins to increase uniformly across the hypoechoic area of damaged BCC or SCC. Because each dose of radiation only kills a percentage of a tumor’s cancer cells, these green speckles may theoretically represent the migration of healthy tissue into vacant spaces left behind by clusters of cancer cells that have died. This progressive pattern theoretically depicts repopulation or the replacement of dead cancer cells with healthy normal cells. More studies are necessary to better understand ARD, as well as the precise mechanisms of NMSC tumor depth fluctuations and repopulation. This is challenging because ARD is rarely biopsied, mostly due to concerns about wound healing during RT (radiation therapy) [
20].
Rationale for Daily Imaging During IGSRT
1. Tumor Expansion: Adaptive Changes Optimize Tumor Control
Nonmelanoma skin cancer (NMSC) tumors expand multiple times during IGSRT therapy [
21]. On these occasions the increased tumor depth triggers an adaptive increase in kV or dose, especially with regard to the cancer cells at the bottom of the tumor. The mathematical model that quantifies the increase in radiation dose as tumor depth increases is known as percentage depth dose. Without HRDUS imaging prior to delivery of every dose, radiation therapists and dermatologists would not know when it is appropriate to compensate for deeper tumor depths by increasing energy (kV), time dose fractionation (TDF) or dose.
Image-driven, real-time adaptations to tumor expansion explain why IGSRT con-sistently achieves 99% cure rates in multiple retrospective studies [2-5]. This is because HRDUS imaging collects precise tumor measurements prior to each radiation dose delivery. These measurements inform the provider when to increase energy, TDF and dose to ensure better tumor coverage. Without HRDUS imaging prior to delivery of every dose, these tumor depth increases could never be known or addressed, resulting in reduced treatment efficacy.
A meta-analysis compared the cure rates of radiotherapy modalities with (IGSRT) and without (XRT and SRT) image guidance. IGSRT’s real-time adaptive use imaging prior to every dose led to superior cure rates (local control) when compared to XRT and SRT without imaging [
20]. This study demonstrates, the connection between image guidance and high rates of efficacy. Using IGSRT without adaptive radiotherapy could pose a higher risk of NMSC recurrence.
Furthermore, one of the IGSRT cure rate studies demonstrated that 29% of NMSC tumors treated with IGSRT required an adaptive energy change during therapy based on image-driven measurements [
4].
2. Tumor Shrinkage: Adaptive Changes Minimize Toxicity
NMSC tumors shrink multiple times during IGSRT therapy [
21]. On these occasions the decreased tumor depth causes an unnecessary increase in radiation dose and toxicity. The mathematical model that quantifies the increase in radiation dose as tumor depth decreases is known as percentage depth dose. Without pre-fraction HRDUS images, radiation therapists and dermatologists would not know when surges in radiation toxicity could be avoided by decreasing energy (kV), time dose fractionation (TDF) or dose.
Image-driven, real-time adaptations to tumor shrinkage explain why IGSRT con-sistently reduces toxicity as evidenced by RTOG scores in multiple retrospective studies [2-5]. This is because HRDUS imaging collects precise tumor measurements prior to each radiation delivery. These measurements inform the provider when to decrease energy, TDF and dose to ensure lower toxicity scores.
Minimizing radiation toxicity is highly significant and medically necessary according to the ALARA principle. In an effort to maximize radiation protection, the Interna-tional Commission on Radiological Protection (ICRP) introduced the “as low as rea-sonably achievable” (ALARA) principle in the 1970s [
22]. Assuming that an increase in radiation increases the cancer risk at any dose, the goal of ALARA is to achieve the lowest radiation dose possible to the population, taking into account societal factors and costs [
22]. ALARA is particularly important in radiation therapy for cancer treatment, as the physician must balance the beneficial effects of radiation while simultaneously working to minimize the harmful radiation effects [
23]. This plays into the physician’s duty to uphold the principle of primum non nocere, as many of the major cancers and diseases in the Western world are thought to be preventable by reducing exposure to disease-causing carcinogens [22, 23].
3. IGSRT’s Imaging/Adaptive Changes: Optimizing the Fundamentals of Radiotherapy
The main point is that the imaging component of IGSRT allows better coverage of tumor and minimizes unnecessary normal tissue dose/complications, in a real-time adaptive manner. Achieving an optimal balance between tumor control and mini-mizing normal tissue toxicity is a fundamental goal in radiotherapy planning. Ide-ally, we’d want 100% tumor control with 0% normal tissue complications, but achieving this balance is challenging. The decision to perform image guidance prior to every radiation dose delivered during IGSRT provides real-time data that provides dermatologists and radiation therapists with a clear pathway to achieving the optimal balance between tumor control and minimizing toxicity, as evidenced by IGSRT’s high cure rates and low toxicity [2-5]. The importance of achieving this optimal balance is even more relevant and significant in a vulnerable patient population of frail elderly patients with co-morbidities.
5. Conclusions
In summary, this study clearly demonstrates that 92% of NMSC tumors undergoing IGSRT exhibit measurable changes in the depth of invasion compared to that of the previous image. Because these measurements are collected immediately prior to treatment, they allow the opportunity for adaptive changes in treatment parameters, such as kV, TDF, dose and boost. These adaptive changes are necessary in nearly 40% of cases, directly benefitting patients by maximizing efficacy and minimizing toxicity.
Any measured increase in NMSC depth is clinically significant during IGSRT be-cause it lowers the percent of the prescription dose received by the tumor cells as a function of their depth (PDD). A lower PDD means significantly less radiation is delivered to the deepest tumor cells, diminishing therapeutic efficacy, potentially allowing the deepest cells to receive significantly less than required for cure. Thus, by performing HRDUS depth measurements prior to every fraction, dermatologists and RTTs know exactly when to make adaptive increases in kV, TDF and dose/boost. This ensures that every NMSC tumor is adequately and uniformly radiated to achieve 99% cure rates [2-5].
Any measured decrease in NMSC depth is clinically significant during IGSRT be-cause it is an opportunity to decrease unnecessary radiation, minimizing toxicity. The guiding principle of radiation safety is ALARA, which stands for “as low as reasonably achievable”. ALARA means avoiding exposure to radiation that does not have a direct benefit to the patient, even if the dose is small. Thus, by performing HRDUS depth measurements prior to every fraction, dermatologists and RTTs know exactly when to make adaptive decreases in kV, TDF and dose/boost. This ensures that the safety of every NMSC patient is optimized by reducing radiation toxicity whenever possible.
This study also discussed the vital nature of repopulation, a visual pattern that confirms biologically effective dosing (BED) during IGSRT and allows for monitoring of treatment response. HRDUS imaging before each treatment session allows dermatolo-gists and radiation therapists to monitor the gradual repopulation of a tumor and gauge the biologic effectiveness of IGSRT therapy. Additionally, this visualization allows cli-nicians to monitor for radioresistance within a tumor, which is a possibility in the absence of the normal, expected repopulation pattern on HRDUS. Further studies are needed to better understand the precise nature of depth fluctuations and repopulation.
Author Contributions
Conceptualization, J.S.; writing—original draft preparation, J.S., P.H..; writing—review and editing, J.S., J.H., A.F., P.H..; All authors have read and agreed to the pub-lished version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
Dr. Jeffrey Stricker and Dr. Janine Hopkins have no conflicts of interest to disclose. Dr. Aaron Farberg is an advisor for Castle Biosciences, Inc., Novartis, Sun Pharma, Regeneron. Peyton Harris has no conflicts of interest to disclose.
References
- Gregoire V, Guckenberger M, Haustermans K, Lagendijk JJW, Menard C, Potter R, et al. Image guidance in radiation therapy for better cure of cancer. Mol Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef] [PubMed]
- Moloney M, Kaczmarksi P, Zheng S, Malik A, Ladd D, Serure D, Yu L. Updated Results of 3,050 Non-melanoma Skin Cancer (NMSC) Lesions in 1725 Patients Treated with High Resolution Dermal Ultrasound-Guided Superficial Radiotherapy, A Multi-institutional Study. Journal of Investigative Dermatology. 2022, 142.
- Tran A, Moloney M, Kaczmarski P, Zheng S, Desai A, Desai T, Yu L. Analysis of image-guided superficial radiation therapy (IGSRT) on the treatment of early-stage non-melanoma skin cancer (NMSC) in the outpatient dermatology setting. Journal of Cancer Research and Clinical Oncology. 2023, 149, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Yu L, Oh C, Shea CR. The Treatment of Non-Melanoma Skin Cancer with Image-Guided Superficial Radiation Therapy: An Analysis of 2917 Invasive and In Situ Keratinocytic Carcinoma Lesions. Oncology and Therapy. 2021, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yu LM, Mairead; Beers, Raymond; Serure, Donna. Enhancing Cosmesis While Acheiving High Cure-Rates for Early-Stage Non-Melanoma Skin Cancer In The Outpatient Dermatology Clinic Using Novel Non-Invasive Modality. American Journal of Biomedical Science & Research. 2021, 12, 525–532. [Google Scholar]
- McClure EM, Sedor G, Jin Y, Kattan MW. Image-guided superficial radiation therapy has superior 2-year recurrence probability to Mohs micrographic surgery. Clin Transl Radiat Oncol. 2023, 43, 100678. [Google Scholar]
- Network NCC. Squamous Cell Skin Cancer (Version 1.2024) 2024 [Available from: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf.
- Taberna M, Gil Moncayo F, Jane-Salas E, Antonio M, Arribas L, Vilajosana E, et al. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front Oncol. 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Jeong H, Bok S, Hong BJ, Choi HS, Ahn GO. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 2016, 51, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gupta T, Narayan CA. Image-guided radiation therapy: Physician's perspectives. J Med Phys. 2012, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Greco C, Clifton Ling C. Broadening the scope of image-guided radiotherapy (IGRT). Acta Oncol. 2008, 47, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Verellen D, De Ridder M, Storme G. A (short) history of image-guided radiotherapy. Radiother Oncol. 2008, 86, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Dawson LA, Sharpe MB. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol. 2006, 7, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey of image-guided radiation therapy use in the United States. Cancer. 2010, 116, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Gomez V, Mustapha R, Ng K, Ng T. Radiation therapy and the innate immune response: Clinical implications for immunotherapy approaches. Br J Clin Pharmacol. 2020, 86, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, M. Vacuolar Interface Dermatitis: Patholines Pathology Guidelines; [Available from: https://patholines.org/Vacuolar_interface_dermatitis.
- Horn TD J-H, JM. Interface Dermatitis. Barnhill’s Dermatopathology, 4th Edition: McGraw Hill; 2019.
- Jean L Bolognia JLJ, Ronald P Rapini. Dermatology: Elsevier; 2007.
- Junkins-Hopkin, J. Disorders associated with physical agents: Heat, cold, radiation, and trauma. Lever’s Histopathology of the Skin, 10th Edition: Wolters Kluwer-Lippincott Williams & Wilkins; 2009.
- Kisonas J, Venius J, Grybauskas M, Dabkeviciene D, Burneckis A, Rotomskis R. Acute Radiation Dermatitis Evaluation with Reflectance Confocal Microscopy: A Prospective Study. Diagnostics (Basel). 2021, 11.
- Jeffery, S. Understanding Tumor Depth Fluctuations, Repopulation and Geographic Miss in Non-Melanoma Skin Cancer During Image Guided Superficial Radiation Therapy. 2024.
- Samet JM, Goldman L. Regulation. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, editors. Cancer Epidemiology and Prevention: Oxford University Press; 2017. p. 0.
- Siegel, E. Primum non nocere: A call for a re-evaluation of radiation doses used in CT. Applied Radiology. 2006. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).