1. Introduction
Uric acid (UA) is a waste product of purine metabolism and a mediator of several pathological processes including oxidative stress, inflammation, and endothelial dysfunction [
1,
2]. Decreased excretion and increased synthesis of UA both contribute to higher circulating UA concentrations. Hyperuricemia is a risk factor for, and marker of, cardiovascular disease (CVD) [
3,
4,
5]. It is also associated with the incidence and prognosis of heart failure (HF) [
6]. Epidemiologic, experimental, and clinical data show that patients with hyperuricemia are at increased risk of cardiac, renal, and vascular damage, and cardiovascular (CV) events [
7,
8,
9].
The association between UA and CV events was first reported by Kannel et al. in 1967 [
10], who showed that subjects with hyperuricemia had an increased risk of myocardial infarction. More recently, there is now evidence of an association between hyperuricemia and chronic metabolic syndrome [
1], kidney disease [
11], hypertension [
12], diabetes mellitus [
13], and acute coronary syndrome [
14]. More detailed information regarding the predisposition of individuals with hyperuricemia to oxidative stress and its effects need to be elucidated in order to initiate the appropriate treatment for optimal maintenance of UA levels, thereby improving the long-term prognosis and quality of life of patients [
1].
CV risk factors and outcomes differ between men and women [
15]. Gender differences are also apparent in HF patients, both with regard to etiology, left ventricular ejection fraction (LVEF) and prognosis [
16,
17]. The association between sUA and CV disease outcomes appears to be more pronounced in female than in male [
18,
19] but the role of gender in the relationship between sUA and survival in HF patients has not been clearly established.
The pathophysiology of older adults with CVD is complex and involves multiple dysregulated pathways. Potentially, general laboratory parameters such as sUA and exercise capacity may be useful as biomarkers for assessing prognosis in older adults with CVD. However, although the association between elevated sUA, CVD and mortality is well recognized [
1,
2], yet it is still undecided whether the association reflects a causal inference or whether sUA is a risk marker reflecting the burden of the underlying disease. In addition, there was little information on the association between sUA and exercise capacity in older adults with CVD. Therefore, here, we examined the prognostic significance of sUA and exercise capacity in older Japanese adults with CVD.
2. Materials and Methods
2.1. Study Population
A total of 411 of patients aged ≥65 years who were hospitalized for CVD in the Department of Cardiology at the National Center for Geriatrics and Gerontology, Obu, Japan, between August 2018 and March 2023 were enrolled in the study. All of the patients were enrolled once they were stable and on optimal pharmacological therapy according to current guidelines for the treatment of CVD. The inclusion criteria were structural heart disease consisting of coronary artery disease (having experienced angina pectoris or myocardial infarction, with or without a history of revascularization procedures), symptomatic HF (including conditions such as non-ischemic cardiomyopathy, ischemia, tachycardia, bradycardia, valvular disease, and hypertension), and aortic disease, peripheral artery disease, and other vascular diseases. Non-ischemic cardiomyopathies were considered as ventricular myocardial abnormalities in the absence of coronary artery disease or valvular, pericardial, or congenital heart disease. Tachycardia and bradycardia included atrial, supraventricular, and ventricular arrhythmias; sick sinus syndrome; and atrioventricular block in the absence of structural heart disease. Valvular heart disease was diagnosed on the basis of hemodynamic or echocardiographic findings or a history of valvular or congenital cardiac surgery. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mm Hg, or a history of treatment for hypertension. HF was defined as pulmonary venous congestion or edema on chest X-ray plus any indicative symptoms (e.g., dyspnea, ankle swelling, peripheral edema, or fatigue).
The exclusion criteria were severe respiratory dysfunction (receipt of long-term oxygen therapy for respiratory disease), liver dysfunction (Child–Pugh score class C), stroke, renal dysfunction (albuminuria and glomerular filtration rate category G5), malignant tumors carrying a prognosis of less than 1 year, difficulty walking 10 m even with a walking aid, Mini-Mental State Examination score less than 18 points, and living in a nursing care facility before admission.
2.2. Study Overview
Within 3 day of study enrollment, a standard physical examination was conducted and standard laboratory, echocardiography, physical function, and cardiopulmonary exercise test (CPX) parameters were evaluated in the patients. All of the patients were in a stable condition at the time of testing. We then retrospectively examined the influence of sUA on prognosis using a composite endpoint of rehospitalization due to worsening HF and all-cause mortality. The study protocol complied with the Declaration of Helsinki, and written informed consent was obtained from each subject. The ethics review board of the National Center for Geriatric and Gerontology approved the study (approval no. 1272).
2.3. CPX Procedure
Each patient underwent CPX on a cycle ergometer at a progressively increasing work rate up to maximum tolerance. The test protocol was conducted in accordance with the recommendations of the American Thoracic Society and American College of Chest Physicians [
20]. The oxygen and carbon dioxide sensors were calibrated before each test with known oxygen, nitrogen, and carbon dioxide concentrations. Test termination criteria were patient request, volitional fatigue, ventricular tachycardia, or ≥2 mm horizontal or downsloping ST-segment depression during exercise. A qualified exercise physiologist conducted each test under a physician’s supervision. A 12-lead electrocardiogram was monitored continuously, and blood pressure was measured every minute during exercise and throughout the 5-min recovery period. Respiratory gas exchange variables, including VO
2, VCO
2, and minute ventilation (VE), were acquired continuously throughout the test with an Oxycon Pro ergospirometer (CareFusion; San Diego, CA, USA); percent gas-exchange data were obtained breath-by-breath. Peak VO
2 and peak respiratory exchange ratio were determined as the highest 30-s average values obtained during the final stage of the test. The ratio of the increase in VO
2 to the increase in work rate (WR) [ΔO
2/ΔWR] was calculated by least-squares linear regression from the data recorded between 30 s after the start of the test and 30 s before the end of the test.
2.3. Statistical Analysis
Data are presented as mean ± standard deviation, unless otherwise stated. Variables were compared between CVD patients with and without sUA-lowering agent by using Student’s t-test for unpaired data. The chi-squared test was used to assess the significance of differences between dichotomous variables. Baseline characteristics and hemodynamic variables were compared among groups by one-way factorial analysis of variance; if a significant difference was detected, intergroup comparisons were performed with Scheffe’s multiple comparison test. Cox proportional hazard regression analysis was performed to identify independent predictors of cardiac events. Cumulative cardiac event estimates were calculated by the Kaplan–Meier method; differences between the survival curves were assessed by the log-rank test. All analyses were performed with the SPSS 27.0 software package (SPSS Inc., Chicago, Illinois). A P-value of ˂0.05 was considered statistically significant.
3. Results
3.1. Comparison of Patients Using an sUA-Lowering Agent or Not
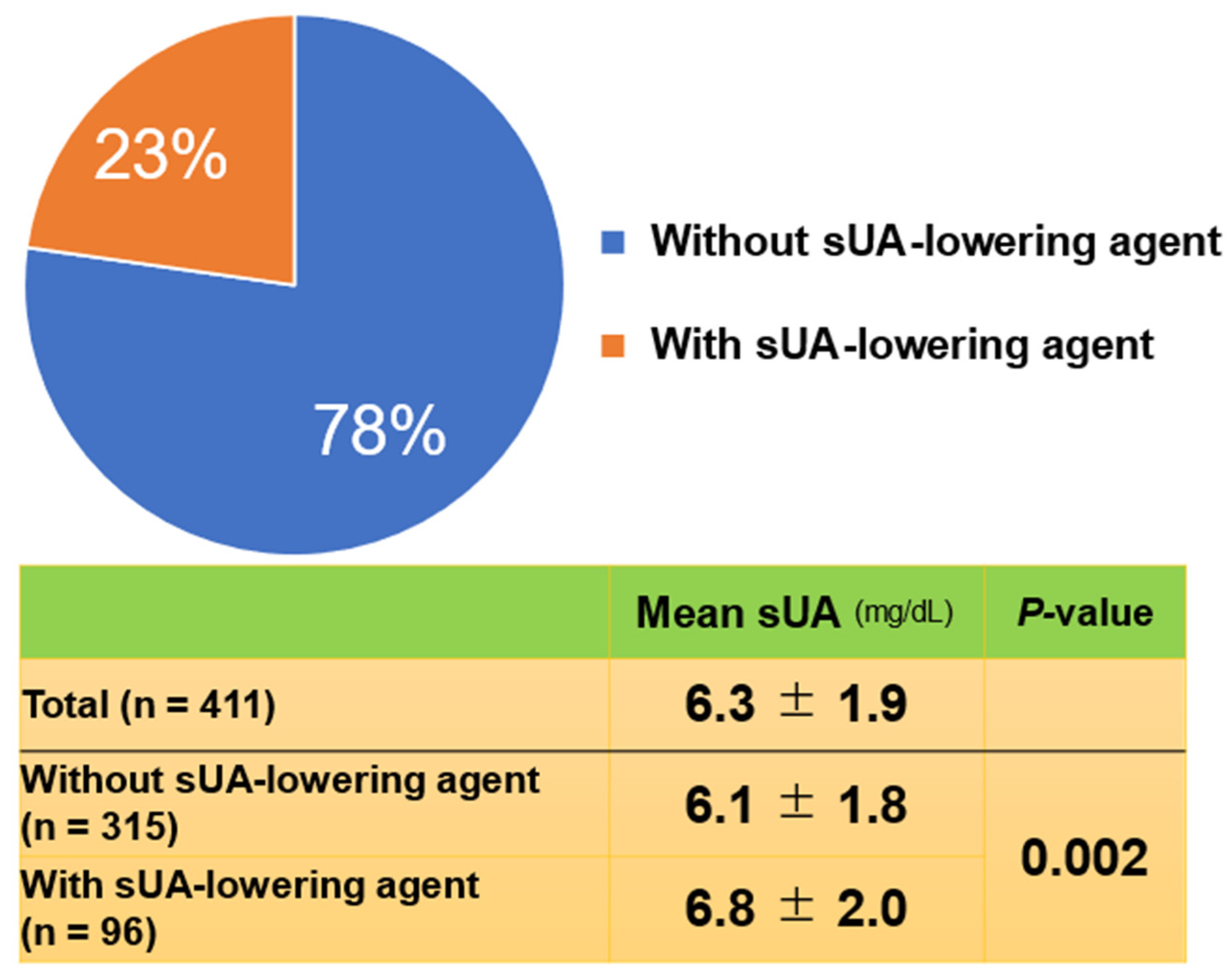
A total of 411 patients (mean age ± SD, 81 ± 7 years) were enrolled. Of the 411 patients enrolled, 217 were male (53%). In the total cohort, the mean sUA level was 6.3 mg/dL. When the patients were stratified by those on a sUA-lowering agent or not, 96 (23%) were on a sUA-lowering therapy and 315 (77%) were not (
Figure 1). Unexpectedly, mean serum sUA level in the patients on an sUA-lowering agent (6.8 mg/dL) was significantly higher (
P = 0.002) than in those not (6.1 mg/dL). The sUA-lowering agents prescribed were febuxostat (n = 77; 80%), allopurinol (n = 18; 19%), and benzbromarone (n = 1; 1%).
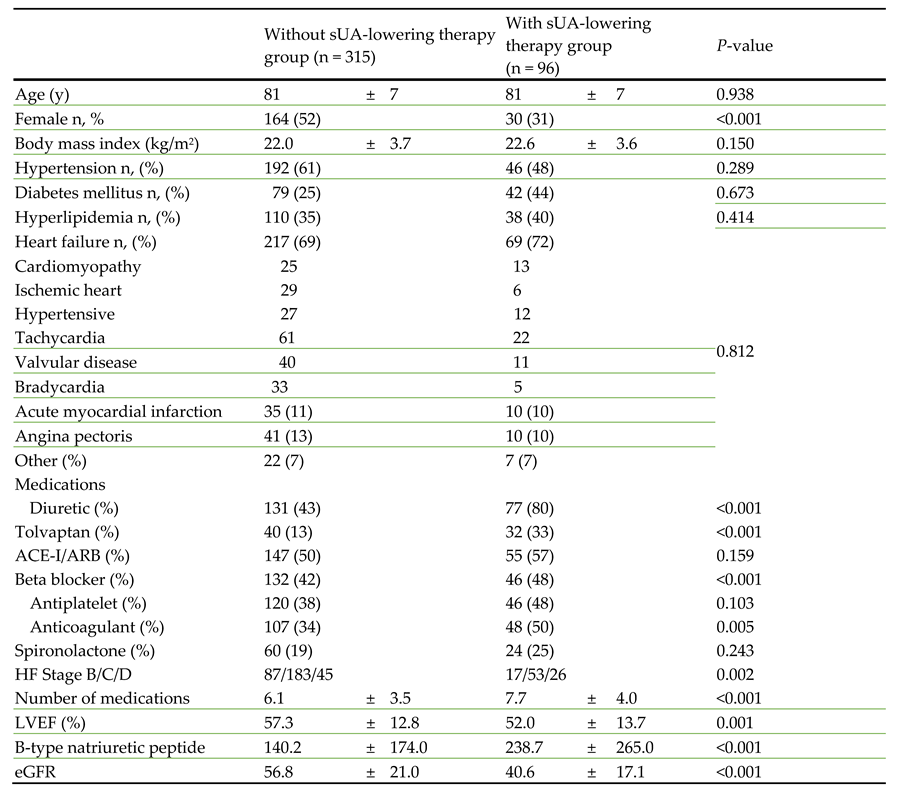
Comparing the two groups, there were no significant differences in age, body mass index, hypertension, diabetes mellitus, or hyperlipidemia. Use of diuretics, tolvaptan, beta blockers, and anticoagulants were significantly more frequent in the patients on an sUA-lowering agent than in those not, but there were no significant differences between the two groups for the use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers, antiplatelets, or spironolactone (
Table 1). HF stage B/C/D, number of medications, and, BNP were significantly higher, LVEF and eGFR were significantly lower in the patients using an sUA-lowering agent that in those not.
ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction sUA, serum uric acid.
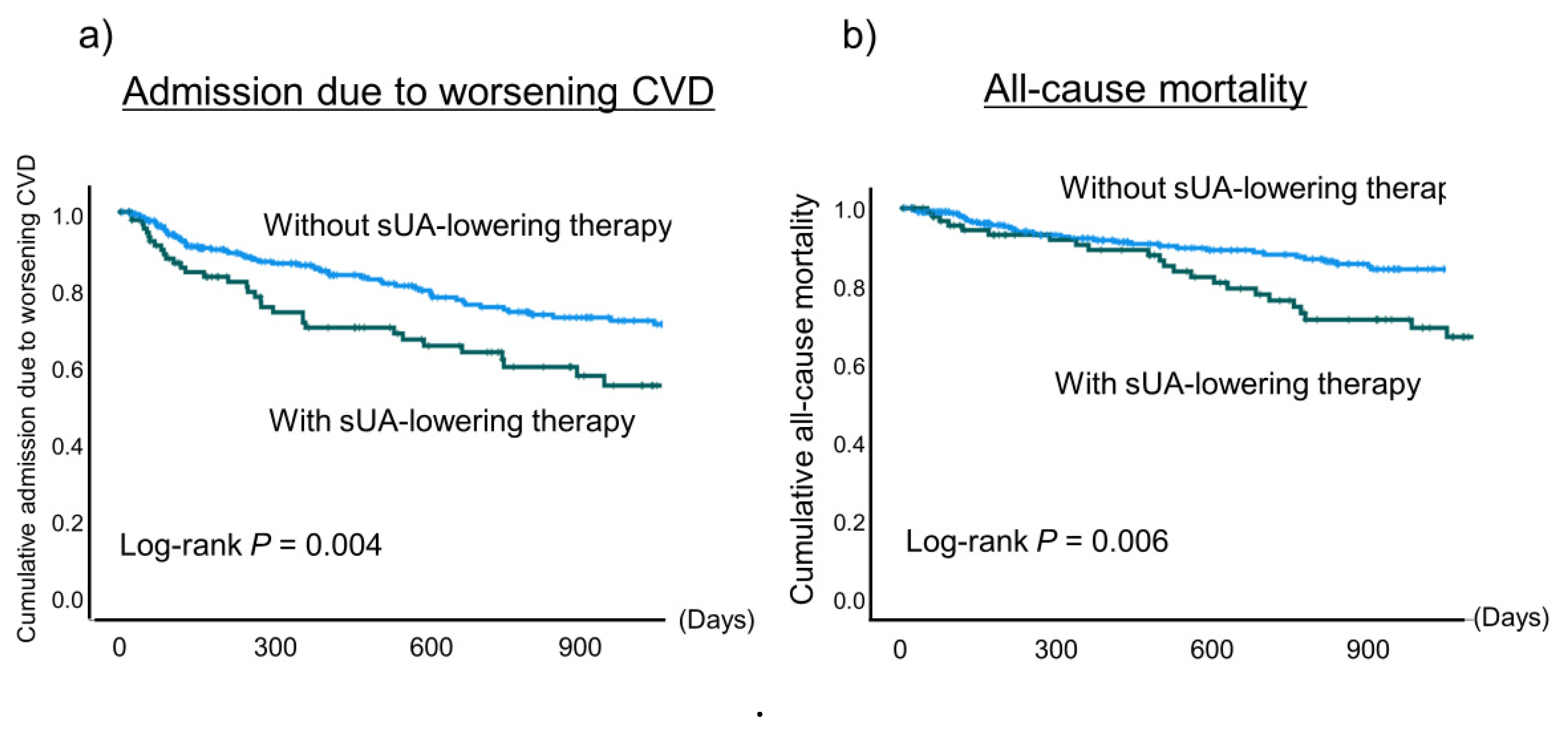
In addition, the incidence of admission due to worsening CVD and all-cause mortality were significantly higher in the patients using a sUA-lowering agent than in those not (
P = 0.004 and
P = 0.006, respectively) (
Figure 2).
3.2. Comparison of Patients with Low, Moderate, and High sUA
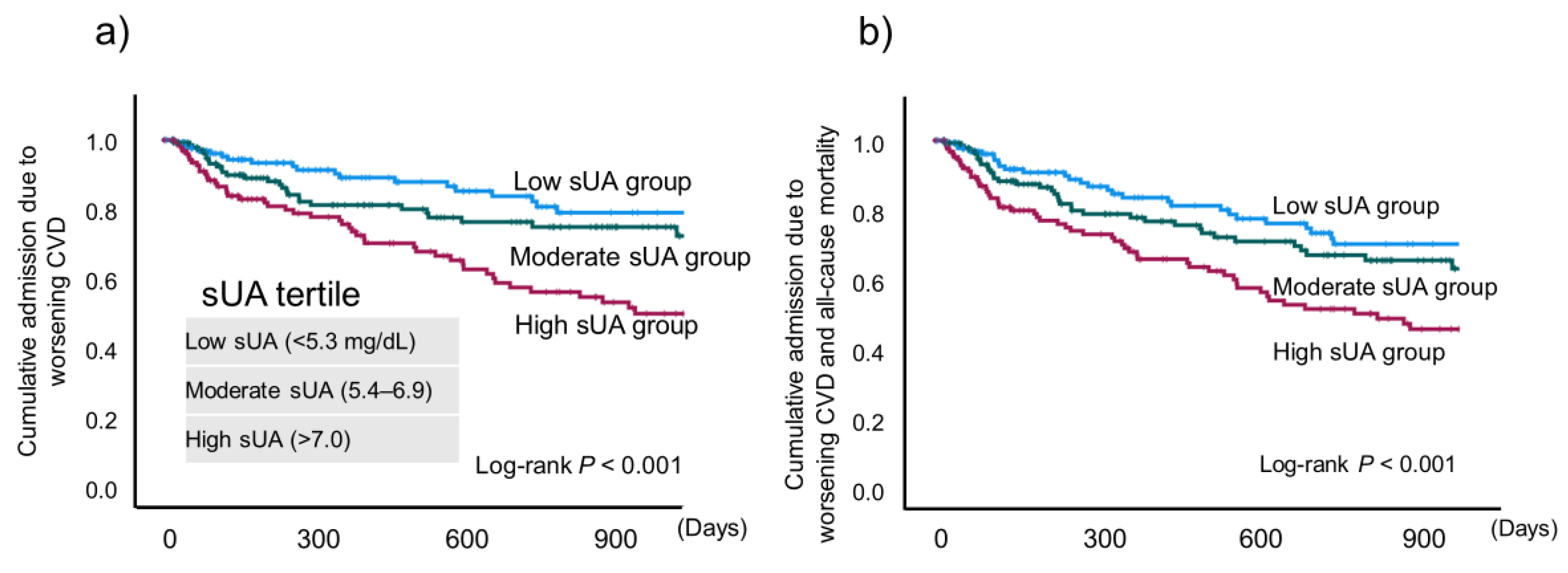
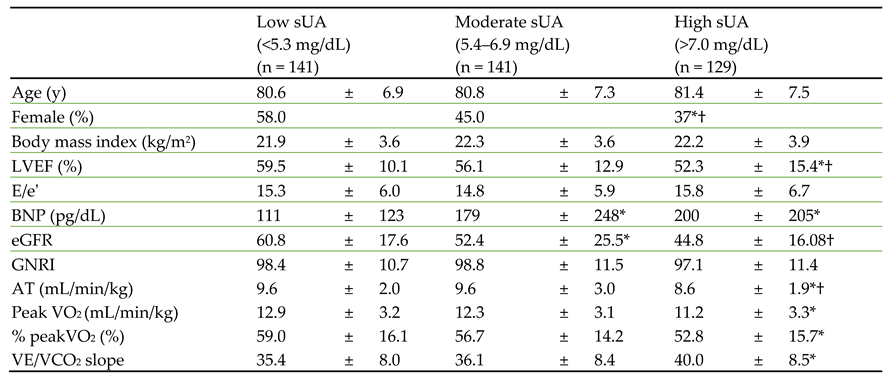
Next, we stratified the patients into low (<5.3 mg/dL), moderate (5.4–6.9 mg/dL), and high (>7.0 mg/dL) sUA groups and compared their baseline characteristics (
Table 2). There were no significant differences in age or body mass index among the three groups, but there were significantly more male patients in the high sUA group than in the other groups. LVEF, plasma BNP, and estimated glomerular filtration rate were significantly worse in the high UA group than in the other groups.
3.3. Comparison of Exercise Capacity and Prognosis in Patients with Low, Moderate, or High sUA Level
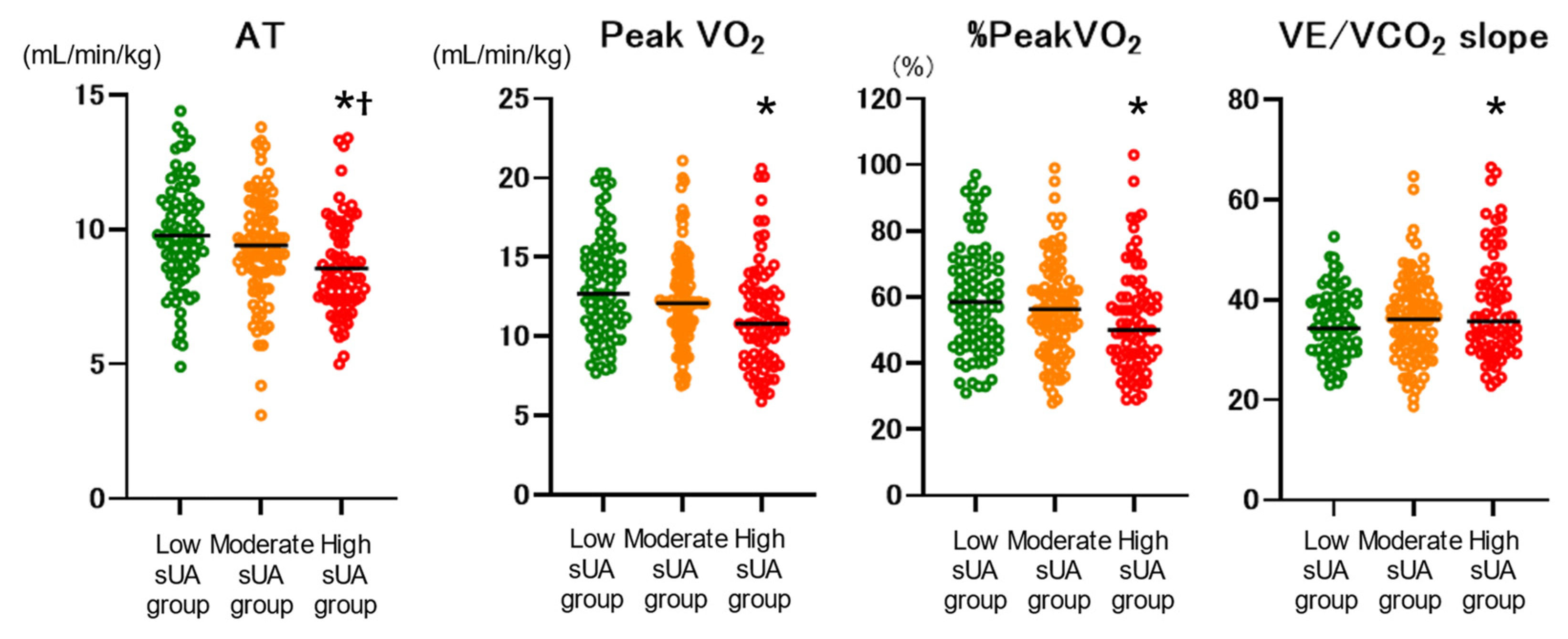
Exercise capacity parameters were compared among the low, moderate, and high sUA groups (
Figure 3). Anaerobic threshold (AT), peak VO
2, %peakVO
2, and VE/VCO
2 slope were significantly worse in the high sUA group compared with the low sUA group. AT was also significantly worse than the moderate sUA group. In addition, the high sUA group showed a significantly higher incidence of the composite endpoint (
P < 0.001) (
Figure 4).
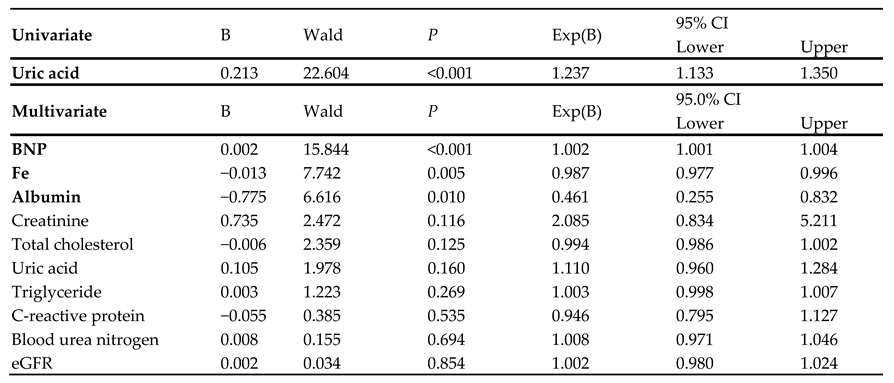
3.4. Cox Proportion Hazard Ratio Analysis
A univariate Cox regression analysis, but not a multivariate analysis, indicated that sUA was significantly associated with the composite endpoint (hazard ratio: 1.237;
P < 0.001) (
Table 3). Plasma BNP, serum iron, and albumin levels were significant independent predictors of cardiac event (B = 0.002, −0.013, and −0.075, respectively).
3.5. Comparison of the Patients Stratified by Gender
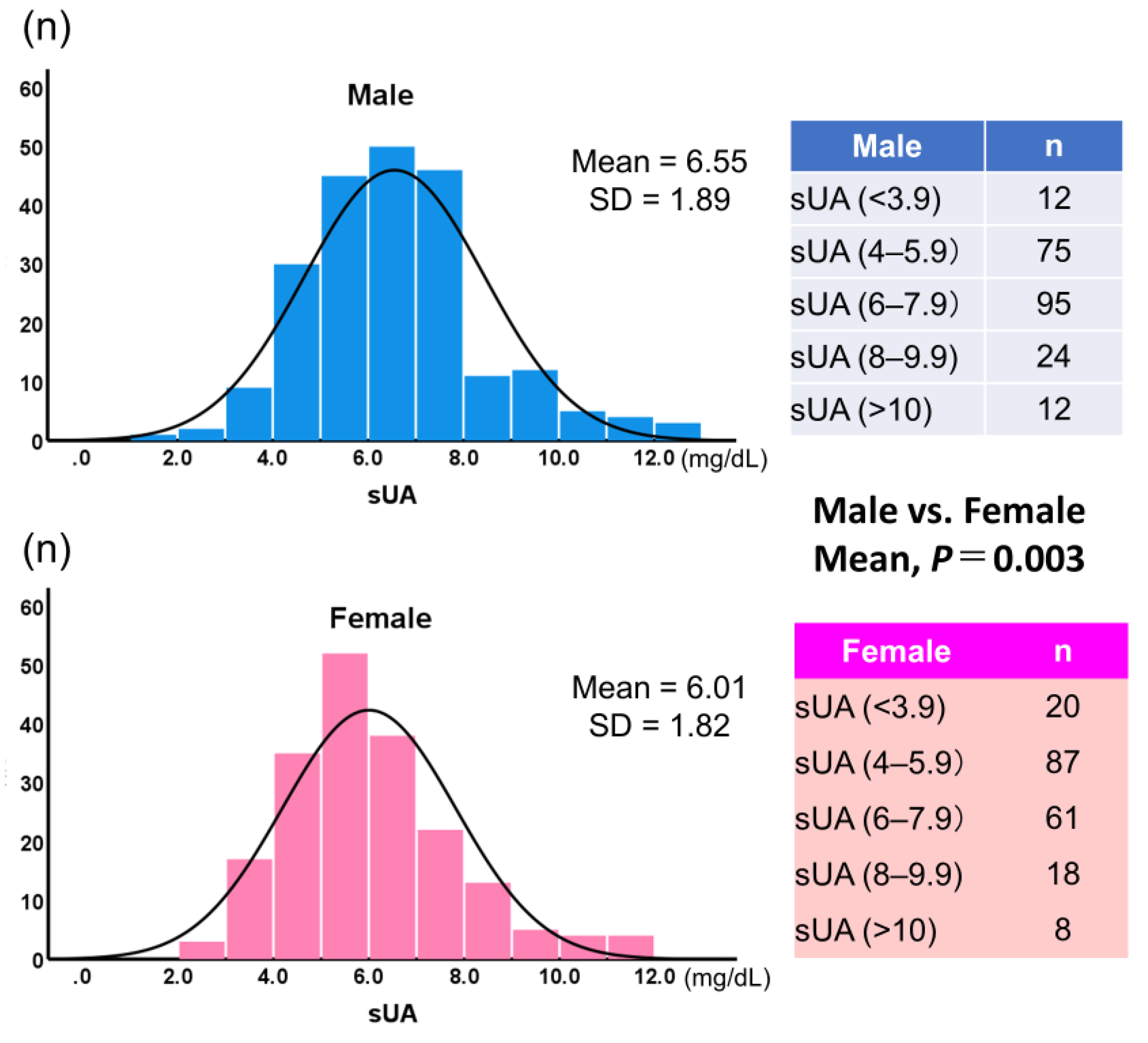
Figure 5 shows histograms of sUA by gender. Mean sUA was significantly higher in male than in female (6.55 ± 1.89, 6.00 ± 1.82, respectively;
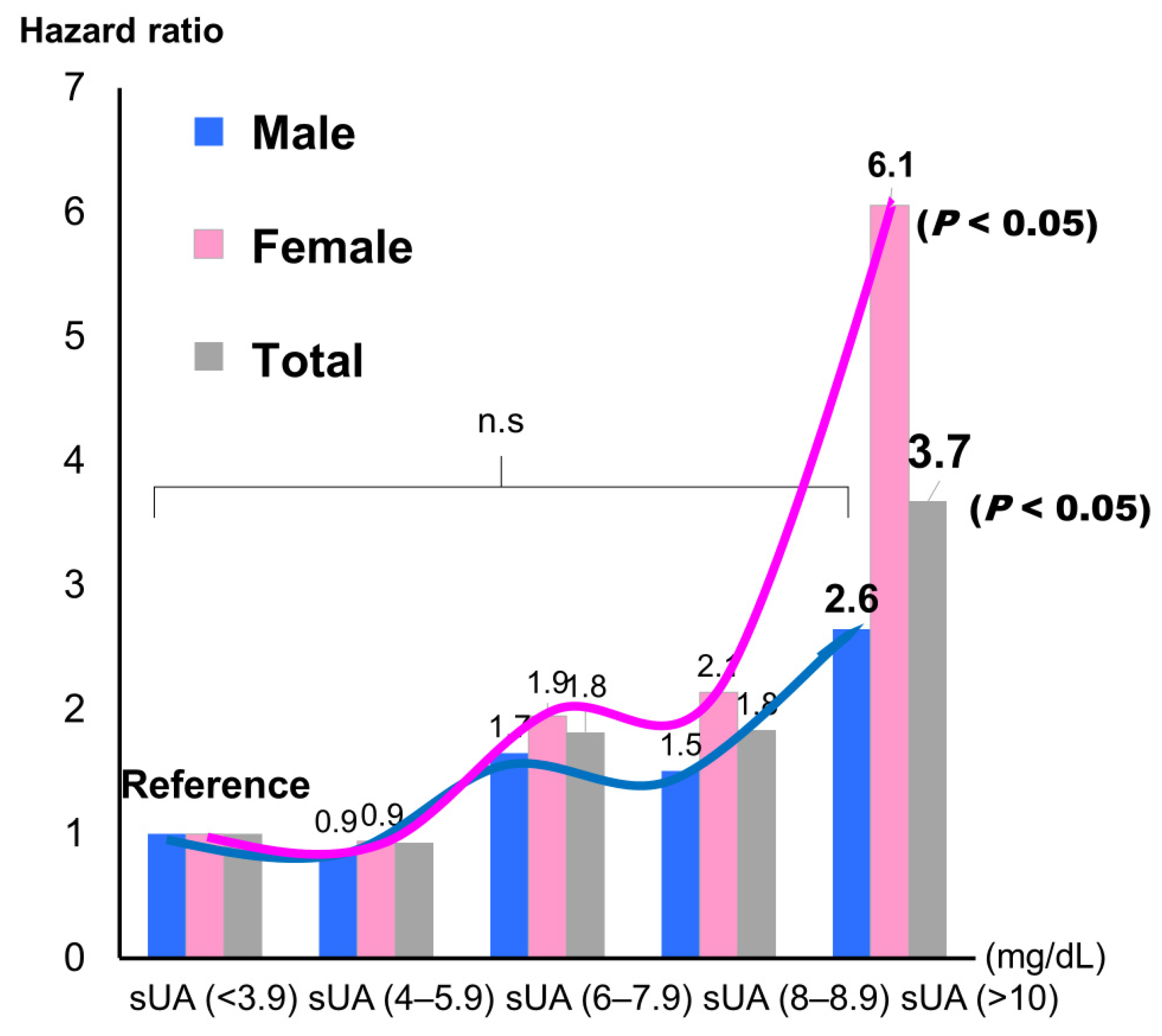
P = 0.003). When sUA was classified into five groups and the lowest group was used as the reference, the highest group showed a significantly higher incidence for the composite endpoint, but only in female (hazard ratio: 6.12;
P < 0.05) (
Figure 6).
Discussion
There are four main findings from the present study:
- 1)
Mean serum sUA level and incidence of hospitalization for worsening CVD were significantly higher in the patients on sUA-lowering therapy compared with those not.
- 2)
When the patients were divided into three groups by sUA level (<5.3, 5.4–6.9, and >7.0 mg/dL), the highest sUA group showed a significantly worse exercise capacity and composite endpoint (rehospitalization due to worsening CVD and all-cause mortality; P < 0.001) by Kaplan Meire method.
- 3)
Female (hazard ratio: 6.12, P < 0.05), but not male, showed a significantly higher incidence of the composite endpoint by Cox regression analysis.
- 4)
A univariate Cox regression analysis, but not a multivariate analysis, revealed that sUA was significantly associated with the composite endpoint (hazard ratio: 1.237; P < 0.001).
Together, these results suggest that higher sUA is associated with worse exercise capacity and prognosis in older adults with CVD, but the interpretation likely needs to be considered in light of potential gender-associated differences.
4.1. Comparison of Patients with or without sUA-Lowering Therapy
Mean serum sUA level and the incidences of admission due to worsening CVD and of all-cause mortality were significantly higher in the patients on sUA-lowering therapy than in those not. One possible explanation for the significantly higher sUA in the treated group is that the dose of the sUA-lowering agent was insufficient to afford control. Another explanation is that the patients in the treated group were more sick, as indicated by a significantly lower LVEF and higher BNP. A randomized, prospective study is needed to examine this finding in more detail. It could also be possible that co-administration of diuretics somehow altered some of the clinical parameters. Indeed, a mechanistically plausible explanation of our findings is that unfavorable effects of diuretics are mediated through activation of the neuroendocrine system, in particular, the renin–angiotensin–aldosterone system [
21,
22]. Clinicians might consider adding other medications, instead of increasing the dose of diuretics, to relieve HF symptoms.
Despite improvements in pharmacotherapy, morbidity and mortality rates in community-based populations with chronic HF still remain high [
23,
24]. In the present study, 70% of the patients had HF, and the average age of our CVD patients was 81 years. We believe that these associations sUA, exercise capacity and prognosis obtained from this study are shown as real-world data in older adults with CVD, who needed hospitalization due to worsening CVD. However, further investigations are required to clarify the relationship between sUA and exercise capacity, and their prognostic significance, especially in older patients with CVD, including in those with refractory HF.
4.2. Exercise Capacity
In our population, the patients with high sUA (according to tertile) had significantly lower AT, peak VO
2, and significantly higher VE/VCO
2 slope. In addition, AT had statistically stronger tendency in accordance with previous study [
25]. The impact of hyperuricemia on exercise tolerance has been little investigated. Leyver et al. [
26] evaluated the relationship between sUA concentration and measures of functional capacity obtained through CPX, and they reported an inverse relationship between sUA concentration and peak VO
2 in heart failure patients. It has also been reported that in patients with congestive HF, sUA concentration is inversely related to ventilatory AT, independent of the hyperuricemic effects of renal impairment and diuretic therapy [
25]. This relationship may be explained by the early switch to anaerobic metabolism and consequent accumulation of lactate in the cells of heart failure patients. Reduced intracellular oxygen availability, by depletion of adenosine triphosphate and accumulation of hypoxanthine and UA, may also account for the observed association between AT and sUA. Thus, the relationship between increased sUA concentration and impairment of exercise tolerance in heart failure could be consequent to dysfunction of oxidative metabolism. However, a direct role of UA cannot be excluded.
High sUA levels can cause injury to the endothelium by increasing platelet aggregation and through direct induction of inflammation [
13]. UA may also stimulate vascular smooth cell proliferation, but reducing nitric oxide availability [
2]. Moreover, high UA levels have been shown to significantly increase angiotensin II expression in cultured endothelial cells, which induce endothelial cell senescence and apoptosis [
27]. Finally, elevated sUA levels may lead to endothelial dysfunction, a key feature of chronic HF, which contributes to increased peripheral vasoconstriction and impaired exercise capacity [
28].
4.3. Prognostic Significance
Of the laboratory parameters examined in the present study, only BNP, serum iron, and albumin were independent predictors of CV event. These findings are consistent with previous reports of the associations of these independent predictors with the pathophysiology of CVD in older adults [
29,
30,
31]. Furthermore, sUA did not show a significant association with CV events in a multivariate analysis. In addition, in both men and women, when the patients were stratified into five sUA groups and the lowest sUA group (<3.9 mg/dL) was set as the reference group, the hazard ratio slightly decreased for the second-lowest group, although not significantly so, and it then increased with each subsequent group (
Figure 6).
The pathophysiologic role of UA has been studied in a wide variety of disease processes and debated for decades, yet a complete understanding is still not at hand. Most previous studies have focused on the pathophysiologic effects of high levels of UA. More recently, research has also shifted to the impact of hypouricemia, with multiple studies showing the potentially damaging effects that can be caused by abnormally low levels of sUA. Thus, both high and low sUA concentrations may be risk factors for renal, CV, and pulmonary comorbidities and U-shaped association with CV mortality [
32,
33]. To confirm the issues, further research is needed.
4.4. Gender Differences
sUA concentrations are usually higher in males than in females [
34]. In addition, vascular parameters are usually higher in males than in females [
34]. Due to a gender difference in the relationship between sUA and Framingham Risk Score, sUA can be used as marker of risk of future CVD events in males only [
35]. Gender differences in CVD pathophysiology, clinical presentation, prevention, and management have also been observed [
36,
37]. Lifestyle may play an important role in the gender difference in the development of CVDs [
36]. In accordance with previous studies [
34,
38], we found similar gender differences in the older adults with CVD examined in the present study. However, the hazard ratio for CV events was larger in female than in male. An sUA concentration of over 10 mg/dL, especially in female, indicated a high risk of experiencing a CV event. Recently, a growing body of evidence has suggested that elevated sUA levels may increase the risk of cardiovascular morbidity and mortality in CVD [
39,
40]. Aggressive sUA-lowering therapy may be beneficial to CV outcomes [
41], but more research is needed. However, optimal management of all comorbidities is of the utmost importance in older adults with CVD, irrespective of aggressive sUA-lowering therapy.
4.5. Clinical Implications
Laboratory parameters are commonly evaluated in daily practice because they can be determined by inexpensive, repeatable, and noninvasive testing. Here, we did not include specialized parameters relevant to frailty, such as plasma interleukin 6 and tumor necrosis factor alpha concentrations, as we wished to test only those biomarkers used in general practice. To our knowledge, this is the first study to have investigated the prognostic significance of sUA and exercise capacity in older adults hospitalized for worsening CVD, and to find a gender difference among these patients. The primary goals of CVD therapy are to improve quality of life and extend survival. The assessment of sUA in daily clinical practice might be useful for diagnostic tests to determine exercise capacity or prognosis even in older adults with CVD.
4.6. Study Limitations
This was a single-center study with a small sample size. Moreover, we did not assess repeated measures over time in the enrolled patients. We did not check alcohol consumption or assess changes in the trajectory of exercise capacity or frailty due to medical intervention or cardiac rehabilitation. Finally, the findings of our study, based on a non-randomized design, are largely hypothesis generating and call for similar analyses of larger and more recent databases, prospective follow-up studies, and for confirmation through randomized clinical trials.
5. Conclusion
High sUA weas significantly associated with worse exercise capacity by CPX in older adults with CVD. Although sUA might have some degree of prognostic significance in this population, this significance may differ between men and women.
Author Contributions
Conceptualization: A.H., A.S., T.K., M.K., K.H., I.U., and T.M; Methodology: A.H., A.S., K.H., and I.U.; Formal analysis: A.H.; Investigation: A.H. K.T. K.H., and I.U.; Resources: A.H., T.K., M.K., and A.S.; Data curation: A.H.; Writing—original draft preparation: A.H.; Writing—review and editing: A.H. and A.S.; Visualization: A.H.; Supervision: A.S. and T.M.; Project administration: A.H. and A.S. Funding acquisition: A.S.All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed with the support of 2022–2024 geriatrics and gerontology research funds from the Ministry of Health, Labour and Welfare, Japan.
Institutional Review Board Statement
The study protocol complied with the Declaration of Helsinki, and written informed consent was obtained from each subject. The ethics review board of the National Center for Geriatric and Gerontology, Japan, approved the study (approval no. 1272).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the staff members of the National Center for Geriatrics and Gerontology (Obu, Japan), particularly Chinatsu Makita, Chieko Hokao, and Kaori Inaguma (technical assistants).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int J Mol Sci 2022, 23. [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J Adv Res 2017, 8, 487-493. [CrossRef]
- Zhang, S.; Wang, Y.; Cheng, J.; Huangfu, N.; Zhao, R.; Xu, Z.; Zhang, F.; Zheng, W.; Zhang, D. Hyperuricemia and Cardiovascular Disease. Curr Pharm Des 2019, 25, 700-709. [CrossRef]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503-1507. [CrossRef]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep 2016, 6, 19520. [CrossRef]
- Huang, H.; Huang, B.; Li, Y.; Huang, Y.; Li, J.; Yao, H.; Jing, X.; Chen, J.; Wang, J. Uric acid and risk of heart failure: A systematic review and meta-analysis. Eur J Heart Fail 2014, 16, 15-24. [CrossRef]
- Muiesan, M.L.; Agabiti-Rosei, C.; Paini, A.; Salvetti, M. Uric Acid and Cardiovascular Disease: An Update. Eur Cardiol 2016, 11, 54-59. [CrossRef]
- Kuwabara, M.; Kodama, T.; Ae, R.; Kanbay, M.; Andres-Hernando, A.; Borghi, C.; Hisatome, I.; Lanaspa, M.A. Update in uric acid, hypertension, and cardiovascular diseases. Hypertens Res 2023, 46, 1714-1726. [CrossRef]
- Rahimi-Sakak, F.; Maroofi, M.; Rahmani, J.; Bellissimo, N.; Hekmatdoost, A. Serum uric acid and risk of cardiovascular mortality: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc Disord 2019, 19, 218. [CrossRef]
- Kannel, W.B.; Castelli, W.P.; McNamara, P.M. The coronary profile: 12-year follow-up in the Framingham study. J Occup Med 1967, 9, 611-619.
- Kumagai, T.; Ota, T.; Tamura, Y.; Chang, W.X.; Shibata, S.; Uchida, S. Time to target uric acid to retard CKD progression. Clin Exp Nephrol 2017, 21, 182-192. [CrossRef]
- Piani, F.; Cicero, A.F.G.; Borghi, C. Uric Acid and Hypertension: Prognostic Role and Guide for Treatment. J Clin Med 2021, 10. [CrossRef]
- Verdoia, M.; Barbieri, L.; Schaffer, A.; Cassetti, E.; Nardin, M.; Bellomo, G.; Aimaretti, G.; Marino, P.; Sinigaglia, F.; De Luca, G.; et al. Impact of diabetes on uric acid and its relationship with the extent of coronary artery disease and platelet aggregation: A single-centre cohort study. Metabolism 2014, 63, 640-646. [CrossRef]
- Nakahashi, T.; Tada, H.; Sakata, K.; Yoshida, T.; Tanaka, Y.; Nomura, A.; Terai, H.; Horita, Y.; Ikeda, M.; Namura, M.; et al. The Association Between Serum Uric Acid and Mortality in Patients with Acute Coronary Syndrome After Percutaneous Coronary Intervention. Int Heart J 2022, 63, 447-453. [CrossRef]
- Maas, A.H.; van der Schouw, Y.T.; Regitz-Zagrosek, V.; Swahn, E.; Appelman, Y.E.; Pasterkamp, G.; Ten Cate, H.; Nilsson, P.M.; Huisman, M.V.; Stam, H.C.; et al. Red alert for women's heart: The urgent need for more research and knowledge on cardiovascular disease in women: Proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. European heart journal 2011, 32, 1362-1368. [CrossRef]
- Regitz-Zagrosek, V.; Seeland, U. Sex and gender differences in myocardial hypertrophy and heart failure. Wien Med Wochenschr 2011, 161, 109-116. [CrossRef]
- Lenzen, M.J.; Rosengren, A.; Scholte op Reimer, W.J.; Follath, F.; Boersma, E.; Simoons, M.L.; Cleland, J.G.; Komajda, M. Management of patients with heart failure in clinical practice: Differences between men and women. Heart 2008, 94, e10. [CrossRef]
- Holme, I.; Aastveit, A.H.; Hammar, N.; Jungner, I.; Walldius, G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med 2009, 266, 558-570. [CrossRef]
- Hoieggen, A.; Alderman, M.H.; Kjeldsen, S.E.; Julius, S.; Devereux, R.B.; De Faire, U.; Fyhrquist, F.; Ibsen, H.; Kristianson, K.; Lederballe-Pedersen, O.; et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 2004, 65, 1041-1049. [CrossRef]
- American Thoracic, S.; American College of Chest, P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003, 167, 211-277. [CrossRef]
- Knight, R.K.; Miall, P.A.; Hawkins, L.A.; Dacombe, J.; Edwards, C.R.; Hamer, J. Relation of plasma aldosterone concentration to diuretic treatment in patients with severe heart disease. Br Heart J 1979, 42, 316-325. [CrossRef]
- Bayliss, J.; Norell, M.; Canepa-Anson, R.; Sutton, G.; Poole-Wilson, P. Untreated heart failure: Clinical and neuroendocrine effects of introducing diuretics. Br Heart J 1987, 57, 17-22. [CrossRef]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card Fail Rev 2023, 9, e11. [CrossRef]
- Tedeschi, A.; Agostoni, P.; Pezzuto, B.; Corra, U.; Scrutinio, D.; La Gioia, R.; Raimondo, R.; Passantino, A.; Piepoli, M.F. Role of comorbidities in heart failure prognosis Part 2: Chronic kidney disease, elevated serum uric acid. Eur J Prev Cardiol 2020, 27, 35-45. [CrossRef]
- Leyva, F.; Chua, T.P.; Anker, S.D.; Coats, A.J. Uric acid in chronic heart failure: A measure of the anaerobic threshold. Metabolism 1998, 47, 1156-1159. [CrossRef]
- Leyva, F.; Anker, S.; Swan, J.W.; Godsland, I.F.; Wingrove, C.S.; Chua, T.P.; Stevenson, J.C.; Coats, A.J. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. European heart journal 1997, 18, 858-865. [CrossRef]
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective. Front Pharmacol 2020, 11, 582680. [CrossRef]
- Freilich, M.; Arredondo, A.; Zonnoor, S.L.; McFarlane, I.M. Elevated Serum Uric Acid and Cardiovascular Disease: A Review and Potential Therapeutic Interventions. Cureus 2022, 14, e23582. [CrossRef]
- Jensen, J.; Ma, L.P.; Bjurman, C.; Hammarsten, O.; Fu, M.L. Prognostic values of NTpro BNP/BNP ratio in comparison with NTpro BNP or BNP alone in elderly patients with chronic heart failure in a 2-year follow up. Int J Cardiol 2012, 155, 1-5. [CrossRef]
- von Haehling, S.; Jankowska, E.A.; van Veldhuisen, D.J.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015, 12, 659-669. [CrossRef]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. J Clin Med 2023, 12. [CrossRef]
- Park, J.H.; Jo, Y.I.; Lee, J.H. Renal effects of uric acid: Hyperuricemia and hypouricemia. Korean J Intern Med 2020, 35, 1291-1304. [CrossRef]
- Crawley, W.T.; Jungels, C.G.; Stenmark, K.R.; Fini, M.A. U-shaped association of uric acid to overall-cause mortality and its impact on clinical management of hyperuricemia. Redox Biol 2022, 51, 102271. [CrossRef]
- Liu, H.; Liu, J.; Zhao, H.; Zhou, Y.; Li, L.; Wang, H.; Group, B.R. Relationship between Serum Uric Acid and Vascular Function and Structure Markers and Gender Difference in a Real-World Population of China-From Beijing Vascular Disease Patients Evaluation Study (BEST) Study. J Atheroscler Thromb 2018, 25, 254-261. [CrossRef]
- Huang, J.H.; Li, R.H.; Huang, S.L.; Sia, H.K.; Yu, C.H.; Tang, F.C. Gender Difference in the Relationships between Inflammatory Markers, Serum Uric Acid and Framingham Risk Score. Int J Environ Res Public Health 2021, 18. [CrossRef]
- Group, E.U.C.C.S.; Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. European heart journal 2016, 37, 24-34. [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145-2154. [CrossRef]
- Perticone, M.; Maio, R.; Shehaj, E.; Gigliotti, S.; Caroleo, B.; Suraci, E.; Sciacqua, A.; Andreozzi, F.; Perticone, F. Sex-related differences for uric acid in the prediction of cardiovascular events in essential hypertension. A population prospective study. Cardiovasc Diabetol 2023, 22, 298. [CrossRef]
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759-766. [CrossRef]
- Sharaf El Din, U.A.A.; Salem, M.M.; Abdulazim, D.O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J Adv Res 2017, 8, 537-548. [CrossRef]
- Ying, H.; Yuan, H.; Tang, X.; Guo, W.; Jiang, R.; Jiang, C. Impact of Serum Uric Acid Lowering and Contemporary Uric Acid-Lowering Therapies on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2021, 8, 641062. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).