1. Introduction
Nuclear magnetic resonance (NMR) spectroscopy and magnetic
resonance imaging (MRI) are indispensable tools in science and medicine, offering
insights into functions of biological processes. Traditional analytical methods,
such as mass spectroscopy and chromatography are significantly more sensitive, but
they require destruction of samples during analysis. [
1,
2,
3].
In contrast, NMR and MRI rely on the detection of signals from nuclear spins without
altering samples, making these modalities truly non-invasive and ideal for studying
biological systems.
The non-invasiveness of NMR spectroscopy and imaging
(NMR/MRI) stems from the low energy of spin-field interaction quanta (
) compared to thermal energy of the environment (
). Here,
is a frequency of spin precession (
is a gyromagnetic ratio of the spins,
is a static magnetic field used in NMR/MRI, typically
several tesla),
is temperature,
and
k
are Plank’s and Boltzmann’s constants, respectively. In magnetic resonance,
polarization
(
P) is defined as a dimensionless quantity directly proportional to the ratio
of the above-mentioned energies [
4]:
. At room temperature,
is about
joules while
for protons is on the order of
joules even at magnetic fields of modern high-field
NMR spectrometers. It is this low interaction energy between spins and the external
field that is a cornerstone to noninvasiveness of NMR/MRI: spins report on their
environment without disturbing it.[
5,
6]. At the
same time, low spin-field interaction energy dictates overall poor sensitivity of
magnetic resonance techniques. Given typical
P values of 0.001-0.0001%, only
relatively large concentrations of spins (>1 mM) can be measured with sufficient
signal-to-noise ratio (SNR) in a conceivable amount of time.[
7,
8,
9] This sets stringent limits on applications of NMR/MRI
and constrains studies to large sample volumes as compared to other analytical methods.
Hyperpolarization refers to situations
in which
P can be much higher than a thermal equilibrium
value, for example, >10%.[
10,
11]. By enhancing polarization levels through hyperpolarization
techniques (see below) NMR/MRI can achieve sensitivity to enable detection of low-concentration
samples (e.g., <1 μM) with high SNR.
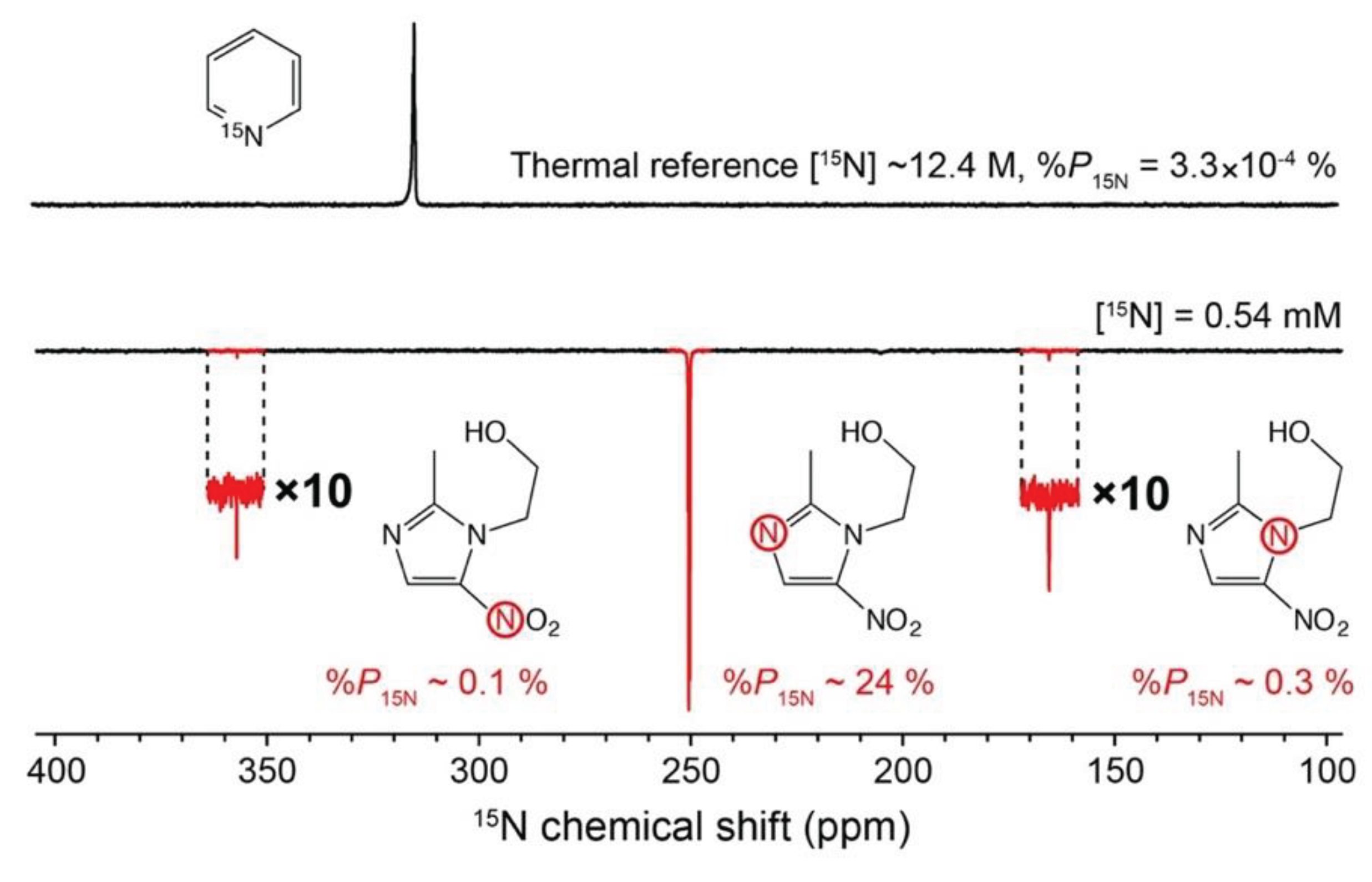
As an example of hyperpolarization, see
Figure 1.
At the top, an
15N NMR spectrum of a fully labeled
15N-pyridine
as a neat liquid was measured at the magnetic field of 9.4 tesla. Since molecules
are isotopically enriched with
15N nuclei (>99%) and present at high
concentration (~12.4 M), a sufficient SNR is obtained in a single acquisition. For
comparison, a sample of 50 mM metronidazole—a well-known antibiotic and hypoxia
probe—at natural isotopic abundance of
15N (0.35%) gives a strong
15N
NMR signal after being flushed with parahydrogen (
pH
2) gas for
~30 seconds (
Figure 1, bottom). For the same
sample at thermal equilibrium to give an NMR signal with SNR comparable to the neat,
[
15N]-labeled pyridine, about 30 years of continuous signal averaging
would be necessary. This example demonstrates the power of hyperpolarization, in
this case, SABRE technique (SABRE = signal amplification by reversible exchange):
molecules at low concentration and natural isotopic abundance can be detected with
sufficient SNR on a time scale of seconds.[
12]
This Editorial Essay briefly explores applications
of hyperpolarization techniques in both medical diagnostics and emerging spin technologies.
Current landscape of methods is examined with a particular focus on medical applications.
Hyperpolarization-enhanced MRI is compared to positron-emission tomography (PET)
and the promises in molecular imaging and disease monitoring are critically assessed.
I also explore novel quantum sensing modalities empowered by spin hyperpolarization
in biomedical research and beyond. Through this analysis, I hope to highlight promising
research directions related to spin technologies which may refine our understanding
of key (bio)molecular processes.
2. Hyperpolarization Techniques
Given the wealth of existing literature on the topic
of nuclear and electron hyperpolarization, here I refrain from delving into the
foundational principles of these techniques. However, it is important to note that
hyperpolarization technologies broadly fall into two categories, although some methods
may formally qualify to both groups:
-
(1)
Techniques Utilizing Electromagnetic Fields
A significant fraction of hyperpolarization methods
uses electromagnetic fields. Notably, in dynamic nuclear polarization (DNP) polarization
transfer from unpaired electrons to spin-active nuclei is facilitated by the application
of microwaves.[
14,
15,
16,
17,
18,
19] DNP allows generating strong
NMR signals for molecules increased by orders of magnitude compared to thermal equilibrium,
resulting in dramatically decreased signal averaging times. DNP encompasses various
methodologies such as Overhauser-DNP, dissolution-DNP, as well as emerging approaches
like bullet-DNP.[
19,
20,
21] Optical pumping techniques,
involving visible or infrared radiation, play a major role in hyperpolarization
of both electron and nuclear spins. Spin-exchange optical pumping (SEOP) and metastability-exchange
optical pumping (MEOP) of noble gases[
22] exemplify
this group of methods along with optical pumping of defects in solids like NV-centers
in diamond.[
23,
24,
25] Visible light applied for generating
hyperpolarization is a signature of chemically induced dynamic nuclear polarization
(CIDNP) and related approaches[
5,
26,
27].
In principle, pumping with electromagnetic radiation
allows polarizing molecules in all phases of ordinary matter (gas, liquid, solid,
and even plasma[
28]). Direct pumping of nuclear
magnetization with light seems to be possible in the gas or solid via optical pumping
while hyperpolarization of molecules in solution necessitates more complex interactions.
-
(2)
Techniques Utilizing Chemistry and Spin Statistics
Another subset of hyperpolarization techniques relies
on intricate spin statistics facilitating the generation of hyperpolarized states.
Chemical reactions and chemical exchange, notably in parahydrogen-induced polarization
(PHIP), underpin these methodologies. PHIP variants like PASADENA, ALTADENA and
SABRE demonstrate remarkable polarization levels (up to 50%) on various nuclei.[
14,
29] While challenges remain in clinical translation,
specifically, the ability to control all stages of chemical transformations and
fields at each moment of sample transfer, recent advancements have showcased reproducible
polarization levels on biologically relevant nuclei, fostering optimism for future
developments.[
30,
31,
32]
For a comprehensive exploration of hyperpolarization,
readers are encouraged to consult recent reviews that delve into the physicochemical
principles of these techniques.[
33] The semantic
breadth of “hyperpolarization” terminology highlights its diverse manifestations
which extend beyond simple magnetization to encompass complex spin orders with broad
implications for both fundamental research and technological innovation.[
10,
34,
35]
3. Medical Applications of Hyperpolarization
As of 2024, biomedical science remains a major driver
for the hyperpolarization research if accounted by the number of peer-reviewed publication
devoted to this subject in recent years[
36,
37,
38].
The interest is not surprising due to immense applicability of MRI in medical diagnostics
even without using hyperpolarization. While there are no yet clear avenues for generating
hyperpolarization inside a living object without bringing hyperpolarized molecules
from the outside (exogenous injections do not make hyperpolarization-enhanced MRI
fully non-invasive), the existing alternative clinical approaches to monitor metabolism
involve radioactive samples and, thus, MRI is freed from this complication. Coupled
with the ability of selecting specific regions in the object under study and harnessing
information from heteronuclei (e.g.,
13C and
15N) hyperpolarization-enhanced
MRI provides a novel toolkit for understanding chemical composition and functions
of tissue, disease progression, and treatment[
39,
40,
41].
Conceptually, in the context of molecular imaging
(i.e., imaging of specific molecules and their transformations rather than imaging
of bulk medium), hyperpolarization-enhanced MRI share similarities with positron-emission
tomography (PET) and it is worth delving deeper into the comparative analysis of
these two modalities. Both technologies in its current implementation require injection
of exogenous contrast agents bearing a signal-generating nuclear isotope.
Comparison of PET and Hyperpolarization-Enhanced MRI
In PET, a radioactive agent is injected into the patient
(ideally) immediately after its production. The radioactive decay typically happens
on a timescale of minutes (
τ1/2~110
mins for
18F nuclei) generating positrons that annihilate with the nearby
matter; measured signals are derived from the detection of
-photons emitted upon this annihilation.[
42] While PET offers high sensitivity for observing
metabolic activity, its resolution is limited to 3-5 mm.[
43] In hyperpolarization-enhanced MRI, a hyperpolarized
exogenous contrast agent (with polarization typically “stored” in the magnetization
of heteronuclei such as
13C) has a short in vivo lifetime providing a
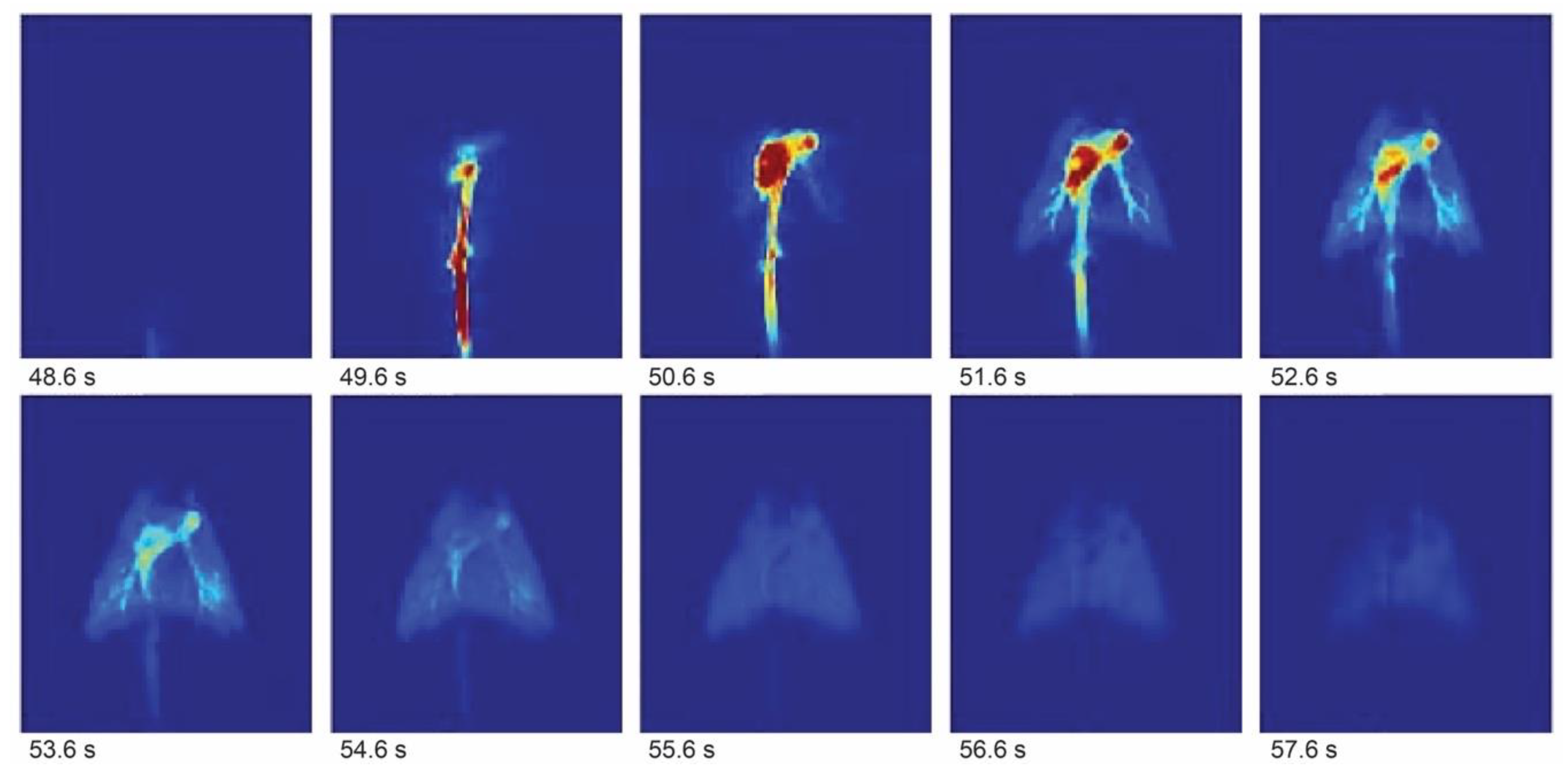
time-window of, at best, up to 5 min after injection. This can be used for angiography
and perfusion (
Figure 2) but is often not
sufficient for monitoring metabolic processes of interest.[
44] A time-window of at least a few hours would be more
appropriate for studying unknown details of the Krebs cycle (such as its reversibility)
and other metabolic transformations.[
45] However,
unlike PET, MRI faces no fundamental resolution limitations, with bottlenecks being
practical, e.g., available SNR per voxel, ability to provide large field gradients
in short time intervals etc. Typical resolution of conventional proton MRI is about
1 mm and sub-100-μm-resolution microimaging
has been demonstrated with hyperpolarization.[
46]
Despite these differences, both hyperpolarization-enhanced
MRI and PET can visualize specific metabolic pathways by using tracer molecules
and appropriate image reconstruction techniques. In MRI, [
13C]-pyruvate
is one of the most promising and well-developed hyperpolarized contrast agents for
observing metabolism within the Krebs cycle[
38]
while PET-agents [
18F]-FDG (fluorodeoxyglucose) and [
13N]-ammonia
are routinely used clinically (and numerous other agents have been tried in research[
47]).
Figure 2.
Hyperpolarization-enhanced
13C magnetic resonance images showing lungs of a pig after injection of a hyperpolarized [
13C]-2-hydroxyethylacrylate with 1 s time resolution. Adapted with permission from Ref. [
48].
Figure 2.
Hyperpolarization-enhanced
13C magnetic resonance images showing lungs of a pig after injection of a hyperpolarized [
13C]-2-hydroxyethylacrylate with 1 s time resolution. Adapted with permission from Ref. [
48].
It is interesting to note that both PET and hyperpolarization-enhanced
heteronuclear MRI exploit the lack of intrinsic background signal to observe molecular
processes without interference. PET has no background signal owing to the absence
radioactive positron-emitting nuclei in the body as well as quiet gamma-ray background
in the environment. Similarly, signals from naturally abundant thermally polarized
heteronuclei such as 13C or 15N are virtually absent in MRI.
Monitoring metabolic changes by conventional 1H MRI, on the other hand,
is challenging due to the large background signal originating from thermally polarized
protons in H2O and lipids. Stargazing provides good analogy: observation
of stars from inside a megapolis is challenging because of optical pollution; one
would need to go to the mountains or far in the wilderness (where the background
light is absent) to notice myriads of stars with a bear eye.
Developing endogenous hyperpolarized contrast agents
generated on demand (or naturally produced) inside the object that is being investigated
seems highly desirable. Green fluorescent protein (GFP) in combination with optical
detection serves as an inspiration: generation of GFP is possible in various environments
via genetic manipulations.[
49] In the case of MRI,
approaches of generating genetically encoded signal contrast in vivo have been proposed
based on the use of hyperpolarized
129Xe gas.[
50,
51] Parahydrogen is another option since it offers
a unique possibility of bringing latent nuclear spin order inside the object to
be studied in such a way that magnetization is generated only in vivo and on demand
(
Figure 3).[
52]
While typically information encoding and signal detection are inseparable parts
of the measurement, MRI fundamentally permits separating these two steps in time
and/or space. Further interdisciplinary innovation is likely necessary to unlock
opportunities provided by genetically-encoded hyperpolarized MRI sensors.[
53]
Challenges of Hyperpolarized Molecular MRI.
Despite its immense potential, the widespread clinical
adoption of hyperpolarization-enhanced MRI faces significant constraints. These
limitations primarily stem from the prevalence of hardware optimized for detecting
protons (
1H) and the absence of refined pulse sequences for effective
polarization transfer, crucial for improving the signal-to-noise ratio (SNR) of
heteronuclear signals. Additionally, hyperpolarization-enhanced MRI necessitate
interdisciplinary working group and requires advanced infrastructure.[
54]
The first proposals for using hyperpolarized contrast
agents emerged in the early 1990s, but the steady stream of research publications
has (up to date) not been sufficient to convince practicing physicians in their
utility. As of 2024, the number of hospitals in the world equipped with the necessary
devices and expertise to observe metabolic transformations using hyperpolarization-enhanced
13C MRI remains fewer than 20.[
54] While
the principles of
dDNP methodology are known since 2003,[
19] the anticipated widespread clinical application
of this method has not been materialized, despite advancements in other research
areas driven by the Moore's Law.[
55] Polarization
levels are not universally high even for
dDNP and can vary depending on the
specific preparation method employed and are extremely technically challenging to
maintain. But potentially the biggest drawback of the existing modality is short
lifetime of hyperpolarized molecules in vivo—particularly concerning the most interesting
molecules like pyruvate (
T1 of carbon-13 at 3 T is only ~30 s
in vivo [
56])—limiting applications to tissues
with high cellularity and rapid transfer through cell membranes. It is essential
for the research community to maintain a balanced perspective on this emerging technology
since even niche applications without revolutionary clinical impact can still be
valuable.
In summary, hyperpolarization-enhanced MRI is a molecular
imaging modality offering sensitivity and resolution comparable to PET. However,
resolving challenges related to polarization lifetime is critical for successful
clinical adaptation. As hyperpolarization technology matures, the prospect of MRI
with heteronuclear detection becoming commonplace holds promise for advancing our
understanding of metabolic changes in both research and clinical contexts. Moreover,
the development of joint modalities combining the sensitivity of PET with the resolution
of MRI could further enhance diagnostic capabilities signaling exciting prospects
toward future developments.
4. Emerging Spin Technologies
In today's world, appreciation of the technology may
often outweigh the appreciation for the research that underpins it. Yet, it is crucial
to recognize that without ongoing scientific exploration technological innovation
would likely stagnate. This is particularly evident in the realm of spin technologies,
where the fundamental quantum nature of spins opens doors to a plethora of applications
across diverse fields.[
57] From enhancing chemical
reactions dependent on nuclear spins to the development of quantum sensors utilizing
single defects in crystal lattices, the breadth of potential applications is vast.[
58]
In the context of MRI, quantum phenomena such as entanglement
and long-lived spin states offer avenues for extending polarization lifetimes, thus
enhancing imaging capabilities.[
59] PHIP, SABRE,
and, in general, magnetization transfer catalysis (MTC) demonstrate how transient
molecular interactions can be leveraged to amplify spin signals, enabling novel
detection schemes. In addition to hyperpolarization, principles of quantum metrology
hold potential for enabling precise differentiation of chemicals and their transformations
through high-resolution analysis of spectral frequencies and phases.[
57] By leveraging key quantum concepts like squeezing
and entanglement, spin techniques could improve MRI by achieving unprecedented resolution.
5. Conclusion
In the landscape of hyperpolarization-enhanced NMR/MRI,
challenges and opportunities lie ahead. Key questions persist: will nuclear hyperpolarization
unveil novel, previously unknown, dimensions of metabolism? How can spin order be
efficiently preserved in molecules within biochemical processes over extended timeframes
beyond a few minutes? Will innovative NMR detection methods, bolstered by hyperpolarization,
transition to practical clinical applications? Could portable point-of-care NMR
devices revolutionize healthcare diagnostics?
These questions not only underscore the ongoing evolution
of hyperpolarization techniques but also point to potential avenues for future research
and technological advancement. It is already evident that hyperpolarization represents
a promising trajectory—one that complements established high-field NMR/MRI modalities—in
our journey of improving magnetic resonance methods. The rallying cry remains 'Molecules,
up your spins!' and with each discovery we shape the future of truly quantum
molecular imaging.
Funding
The work is supported by Alexander von Humboldt Foundation in the framework of the Sofja Kovalevskaja Award.
Acknowledgments
The author thanks Prof. Dmitry Budker, Dr. Andrey Pravdivtsev,
Dr. Sheng Chi Fan, Prof. Alexander Pines, Prof. Thomas Budinger, and Prof. Kev Salikhov
for stimulating discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds ...... are available from the
authors.
References
- Nagornov, K.O.; Gorshkov, M.V.; Kozhinov, A.N.; Tsybin, Y.O. , High-resolution Fourier transform ion cyclotron resonance mass spectrometry with increased throughput for biomolecular analysis. Anal. Chem. 2014, 86, 9020–9028. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos Soares Maciel, E.; de Toffoli, A.L.; Sobieski, E.; Domingues Nazário, C.E.; Lanças, F.M. , Miniaturized liquid chromatography focusing on analytical columns and mass spectrometry: A review. Anal. Chim. Acta 2020, 1103, 11–31. [Google Scholar] [CrossRef]
- Huffman, R.G.; Leduc, A.; Wichmann, C.; Di Gioia, M.; Borriello, F.; Specht, H.; Derks, J.; Khan, S.; Khoury, L.; Emmott, E.; Petelski, A.A.; Perlman, D.H.; Cox, J.; Zanoni, I.; Slavov, N. , Prioritized mass spectrometry increases the depth, sensitivity and data completeness of single-cell proteomics. Nat. Methods 2023, 20, 714–722. [Google Scholar] [CrossRef]
- Abragam, A., The principles of nuclear magnetism. Clarendon Press: Oxford, 1961.
- Salikhov, K.M.; Molin, Y.N.; Sagdeev, R.Z.; Buchachenko, A.L., Spin polarization and magnetic effects in chemical reactions. Elsevier: Amsterdam, 1984.
- Fisher, M.P.A.; Radzihovsky, L. , Quantum indistinguishability in chemical reactions. Proceedings of the National Academy of Sciences 2018, 115, E4551–E4558. [Google Scholar] [CrossRef]
- Roose, B.W.; Zemerov, S.D.; Dmochowski, I.J. , Nanomolar small-molecule detection using a genetically encoded (129)Xe NMR contrast agent. Chem Sci 2017, 8, 7631–7636. [Google Scholar] [CrossRef]
- Wen, L.; Meng, H.; Gu, S.; Wu, J.; Zhao, Y. , Toward Nanomolar Multi-Component Analysis by 19F NMR. Anal. Chem. 2022, 94, 8024–8032. [Google Scholar] [CrossRef] [PubMed]
- Eshuis, N.; Hermkens, N.; van Weerdenburg, B.J.A.; Feiters, M.C.; Rutjes, F.P.J.T.; Wijmenga, S.S.; Tessari, M. , Toward Nanomolar Detection by NMR Through SABRE Hyperpolarization. J. Am. Chem. Soc. 2014, 136, 2695–2698. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.H.; Bengs, C. , Hyperpolarization and the physical boundary of Liouville space. Magn. Reson. 2021, 2, 395–407. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Kovtunov, K.V.; Koptyug, I.V.; He, P.; Groome, K.A.; Best, Q.A.; Shi, F.; Goodson, B.M.; Shchepin, R.V.; Truong, M.L.; Coffey, A.M.; Waddell, K.W.; Chekmenev, E.Y. , In Situ and Ex Situ Low-Field NMR Spectroscopy and MRI Endowed by SABRE Hyperpolarization. ChemPhysChem 2014, 15, 4100–4107. [Google Scholar] [CrossRef]
- Kircher, R.; Xu, J.; Barskiy, D.A. , In Situ Hyperpolarization Enables 15N and 13C Benchtop NMR at Natural Isotopic Abundance. J. Am. Chem. Soc. 2024, 146, 514–520. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Shchepin, R.V.; Coffey, A.M.; Theis, T.; Warren, W.S.; Goodson, B.M.; Chekmenev, E.Y. , Over 20% N-15 Hyperpolarization in Under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J. Am. Chem. Soc. 2016, 138, 8080–8083. [Google Scholar] [CrossRef] [PubMed]
- Kirill, K.; Ekaterina, P.; Oleg, S.; Samuel, C.; Dennis, K.; Basile, V.; Sami, J.; Eduard, C.; Boyd, G.; Danila, B.; Igor, K., Hyperpolarized NMR: d-DNP, PHIP, and SABRE. Chemistry – An Asian Journal 2018.
- Ardenkjaer-Larsen, J.H. , On the present and future of dissolution-DNP. J. Magn. Reson. 2016, 264, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jähnig, F.; Kwiatkowski, G.; Ernst, M. , Conceptual and instrumental progress in dissolution DNP. J. Magn. Reson. 2016, 264, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Buratto, R.; Bornet, A.; Milani, J.; Mammoli, D.; Vuichoud, B.; Salvi, N.; Singh, M.; Laguerre, A.; Passemard, S.; Gerber-Lemaire, S.; Jannin, S.; Bodenhausen, G. , Drug Screening Boosted by Hyperpolarized Long-Lived States in NMR. ChemMedChem 2014, 9, 2509–2515. [Google Scholar] [CrossRef]
- Comment, A.; Rentsch, J.; Kurdzesau, F.; Jannin, S.; Uffmann, K.; van Heeswijk, R.B.; Hautle, P.; Konter, J.A.; van den Brandt, B.; van der Klink, J.J. , Producing over 100ml of highly concentrated hyperpolarized solution by means of dissolution DNP. J. Magn. Reson. 2008, 194, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. , Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Kouřil, K.; Kouřilová, H.; Bartram, S.; Levitt, M.H.; Meier, B. , Scalable dissolution-dynamic nuclear polarization with rapid transfer of a polarized solid. Nat. Commun. 2019, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Abragam, A., The principles of nuclear magnetism. Oxford university press: 1961.
- Barskiy, D.A.; Coffey, A.M.; Nikolaou, P.; Mikhaylov, D.M.; Goodson, B.M.; Branca, R.T.; Lu, G.J.; Shapiro, M.G.; Telkki, V.V.; Zhivonitko, V.V.; Koptyug, I.V.; Salnikov, O.G.; Kovtunov, K.V.; Bukhtiyarov, V.I.; Rosen, M.S.; Barlow, M.J.; Safavi, S.; Hall, I.P.; Schroder, L.; Chekmenev, E.Y. , NMR Hyperpolarization Techniques of Gases. Chem. Eur. J. 2017, 23. [Google Scholar]
- Kehayias, P.; Jarmola, A.; Mosavian, N.; Fescenko, I.; Benito, F.M.; Laraoui, A.; Smits, J.; Bougas, L.; Budker, D.; Neumann, A.; Brueck, S.R.J.; Acosta, V.M. , Solution nuclear magnetic resonance spectroscopy on a nanostructured diamond chip. Nat. Commun. 2017, 8, 188. [Google Scholar] [CrossRef]
- Budker, D.; Barskiy, D.; Lenz, T. , Kernspinresonanz ohne Magnetfeld. Physik in unserer Zeit 2023, 54, 294–301. [Google Scholar] [CrossRef]
- King, J.P.; Jeong, K.; Vassiliou, C.C.; Shin, C.S.; Page, R.H.; Avalos, C.E.; Wang, H.-J.; Pines, A. , Room-temperature in situ nuclear spin hyperpolarization from optically pumped nitrogen vacancy centres in diamond. Nat. Commun. 2015, 6, 8965. [Google Scholar] [CrossRef] [PubMed]
- Goez, M. , Photo-CIDNP spectroscopy. Annu. Rep. NMR Spectrosc. 2009, 66, 77–147. [Google Scholar]
- Morozova, O.B.; Ivanov, K.L. , Time-Resolved Chemically Induced Dynamic Nuclear Polarization of Biologically Important Molecules. ChemPhysChem 2019, 20, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Maul, A.; Blümler, P.; Nacher, P.J.; Otten, E.; Tastevin, G.; Heil, W. , Nuclear hyperpolarization of $^{3}\mathrm{He}$ by magnetized plasmas. Phys. Rev. A 2018, 98, 063405. [Google Scholar] [CrossRef]
- Rayner, P.J.; Burns, M.J.; Olaru, A.M.; Norcott, P.; Fekete, M.; Green, G.G.R.; Highton, L.A.R.; Mewis, R.E.; Duckett, S.B. , Delivering strong 1H nuclear hyperpolarization levels and long magnetic lifetimes through signal amplification by reversible exchange. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E3188–E3194. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Blanchard, J.W.; Barskiy, D.; Cavallari, E.; Dagys, L.; Van Dyke, E.; Tsukanov, M.; Bliemel, B.; Münnemann, K.; Aime, S.; Reineri, F.; Levitt, M.H.; Buntkowsky, G.; Pines, A.; Blümler, P.; Budker, D.; Eills, J. , Rapid hyperpolarization and purification of the metabolite fumarate in aqueous solution. Proceedings of the National Academy of Sciences 2021, 118, e2025383118. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, F.; Sirbu, A.; Brahms, A.; Assaf, C.; Herges, R.; Hövener, J.-B.; Pravdivtsev, A.N. , Spying on parahydrogen-induced polarization transfer using a half-tesla benchtop MRI and hyperpolarized imaging enabled by automation. Nat. Commun. 2023, 14, 4774. [Google Scholar] [CrossRef] [PubMed]
- de Maissin, H.; Groß, P.R.; Mohiuddin, O.; Weigt, M.; Nagel, L.; Herzog, M.; Wang, Z.; Willing, R.; Reichardt, W.; Pichotka, M.; Heß, L.; Reinheckel, T.; Jessen, H.J.; Zeiser, R.; Bock, M.; von Elverfeldt, D.; Zaitsev, M.; Korchak, S.; Glöggler, S.; Hövener, J.-B.; Chekmenev, E.Y.; Schilling, F.; Knecht, S.; Schmidt, A.B. , In Vivo Metabolic Imaging of [1-13C]Pyruvate-d3 Hyperpolarized By Reversible Exchange With Parahydrogen**. Angewandte Chemie International Edition 2023, 62, e202306654. [Google Scholar] [CrossRef] [PubMed]
- Eills, J.; Budker, D.; Cavagnero, S.; Chekmenev, E.Y.; Elliott, S.J.; Jannin, S.; Lesage, A.; Matysik, J. r.; Meersmann, T.; Prisner, T. , Spin hyperpolarization in modern magnetic resonance. Chem. Rev. 2023, 123, 1417–1551. [Google Scholar] [CrossRef]
- Xu, J.; Budker, D.; Barskiy, D.A. , Visualization of dynamics in coupled multi-spin systems. Magn. Reson. 2022, 3, 145–160. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Pravdivtsev, A. Magnetization and Polarization of Coupled Nuclear Spin Ensembles. arXiv arXiv:2308.15837 2023.
- Nikolaou, P.; Goodson, B.M.; Chekmenev, E.Y. , NMR Hyperpolarization Techniques for Biomedicine. Chem. Eur. J. 2015, 21, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Jensen, P.R.; Karlsson, M.; Lerche, M.H. , Hyperpolarized NMR Probes for Biological Assays. Sensors 2014, 14, 1576–1597. [Google Scholar] [CrossRef] [PubMed]
- Kurhanewicz, J.; Vigneron, D.B.; Brindle, K.; Chekmenev, E.Y.; Comment, A.; Cunningham, C.H.; DeBerardinis, R.J.; Green, G.G.; Leach, M.O.; Rajan, S.S.; Rizi, R.R.; Ross, B.D.; Warren, W.S.; Malloy, C.R. , Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 2011, 13, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Nagana Gowda, G.A.; Raftery, D. , NMR Metabolomics Methods for Investigating Disease. Anal. Chem. 2023, 95, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Shchepin, R.V.; Birchall, J.R.; Chukanov, N.V.; Kovtunov, K.V.; Koptyug, I.V.; Theis, T.; Warren, W.S.; Gelovani, J.G.; Goodson, B.M.; Shokouhi, S.; Rosen, M.S.; Yen, Y.-F.; Pham, W.; Chekmenev, E.Y. , Hyperpolarizing Concentrated Metronidazole 15NO2 Group over Six Chemical Bonds with More than 15 % Polarization and a 20 Minute Lifetime. Chemistry – A European Journal 2019, 25, 8829–8836. [Google Scholar] [CrossRef] [PubMed]
- Svyatova, A.; Skovpin, I.V.; Chukanov, N.V.; Kovtunov, K.V.; Chekmenev, E.Y.; Pravdivtsev, A.N.; Hövener, J.B.; Koptyug, I.V. , 15N MRI of SLIC-SABRE hyperpolarized 15N-labelled pyridine and nicotinamide. Chemistry–A European Journal 2019, 25, 8465–8470. [Google Scholar] [CrossRef] [PubMed]
- Budinger, T.F.; Van Brocklin, H.F. Positron emission tomography. CRC Press inc.–1995: 1985.
- Moses, W.W. , Fundamental Limits of Spatial Resolution in PET. Nucl Instrum Methods Phys Res A 2011, 648, S236–s240. [Google Scholar] [CrossRef] [PubMed]
- Angelovski, G. What We Can Really Do with Bioresponsive MRI Contrast Agents. Angewandte Chemie International Edition 2016, 55, 7038–7046. [Google Scholar] [CrossRef]
- Lane, N. Transformer: The Deep Chemistry of Life and Death. Profile: 2022.
- Coffey, A.M.; Kovtunov, K.V.; Barskiy, D.A.; Koptyug, I.V.; Shchepin, R.V.; Waddell, K.W.; He, P.; Groome, K.A.; Best, Q.A.; Shi, F.; Goodson, B.M.; Chekmenev, E.Y. High-Resolution Low-Field Molecular Magnetic Resonance Imaging of Hyperpolarized Liquids. Anal. Chem. 2014, 86, 9042–9049. [Google Scholar] [CrossRef]
- Zhu, L.; Ploessl, K.; Kung, H.F. PET/SPECT imaging agents for neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Olsson, L.E.; Axelsson, O.; Mansson, S.; Karlsson, M.; Petersson, J.S. Molecular imaging using hyperpolarized 13C. British Journal of Radiology 2003, 76, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M. Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem. Rev. 2002, 102, 759–782. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Ramirez, R.M.; Sperling, L. .; Sun, G.; Sun, J.; Pines, A.; Schaffer, D.V.; Bajaj, V.S. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nature Chem. 2014, 6, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Jayapaul, J.; Schröder, L. Molecular Sensing with Host Systems for Hyperpolarized 129Xe. Molecules 2020, 25, 4627. [Google Scholar] [CrossRef] [PubMed]
- Hövener, J.B.; Schwaderlapp, N.; Lickert, T.; Duckett, S.B.; Mewis, R.E.; Highton, L.A.R.; Kenny, S.M.; Green, G.G. R.; Leibfritz, D.; Korvink, J.G.; Hennig, J.; von Elverfeldt, D. A hyperpolarized equilibrium for magnetic resonance. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Bricco, A.R.; Miralavy, I.; Bo, S.; Perlman, O.; Korenchan, D.E.; Farrar, C.T.; McMahon, M.T.; Banzhaf, W.; Gilad, A.A. A Genetic Programming Approach to Engineering MRI Reporter Genes. ACS Synthetic Biology 2023, 12, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Chaumeil, M.M.; Bankson, J.A.; Brindle, K.M.; Epstein, S.; Gallagher, F.A.; Grashei, M.; Guglielmetti, C.; Kaggie, J.D.; Keshari, K.R.; Knecht, S.; Laustsen, C.; Schmidt, A.B.; Vigneron, D.; Yen, Y.-F.; Schilling, F. , New Horizons in Hyperpolarized 13C MRI. Molecular Imaging and Biology 2023. [Google Scholar] [CrossRef]
- Pearl, R. Breaking The Rules Of Healthcare: Selecting The Best Technology. Forbes 2022.
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I. , Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Science translational medicine 2013, 5, 198ra108. [Google Scholar] [CrossRef]
- Pezze, L.; Smerzi, A.; Oberthaler, M.K.; Schmied, R.; Treutlein, P. Quantum metrology with nonclassical states of atomic ensembles. Rev. Mod. Phys. 2018, 90, 035005. [Google Scholar] [CrossRef]
- Budker, D. Extreme nuclear magnetic resonance: Zero field, single spins, dark matter…. J. Magn. Reson. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sonnefeld, A.; Razanahoera, A.; Pelupessy, P.; Bodenhausen, G.; Sheberstov, K. Long-lived states of methylene protons in achiral molecules. Science Advances 2022, 8, eade2113. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).