Submitted:
31 March 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
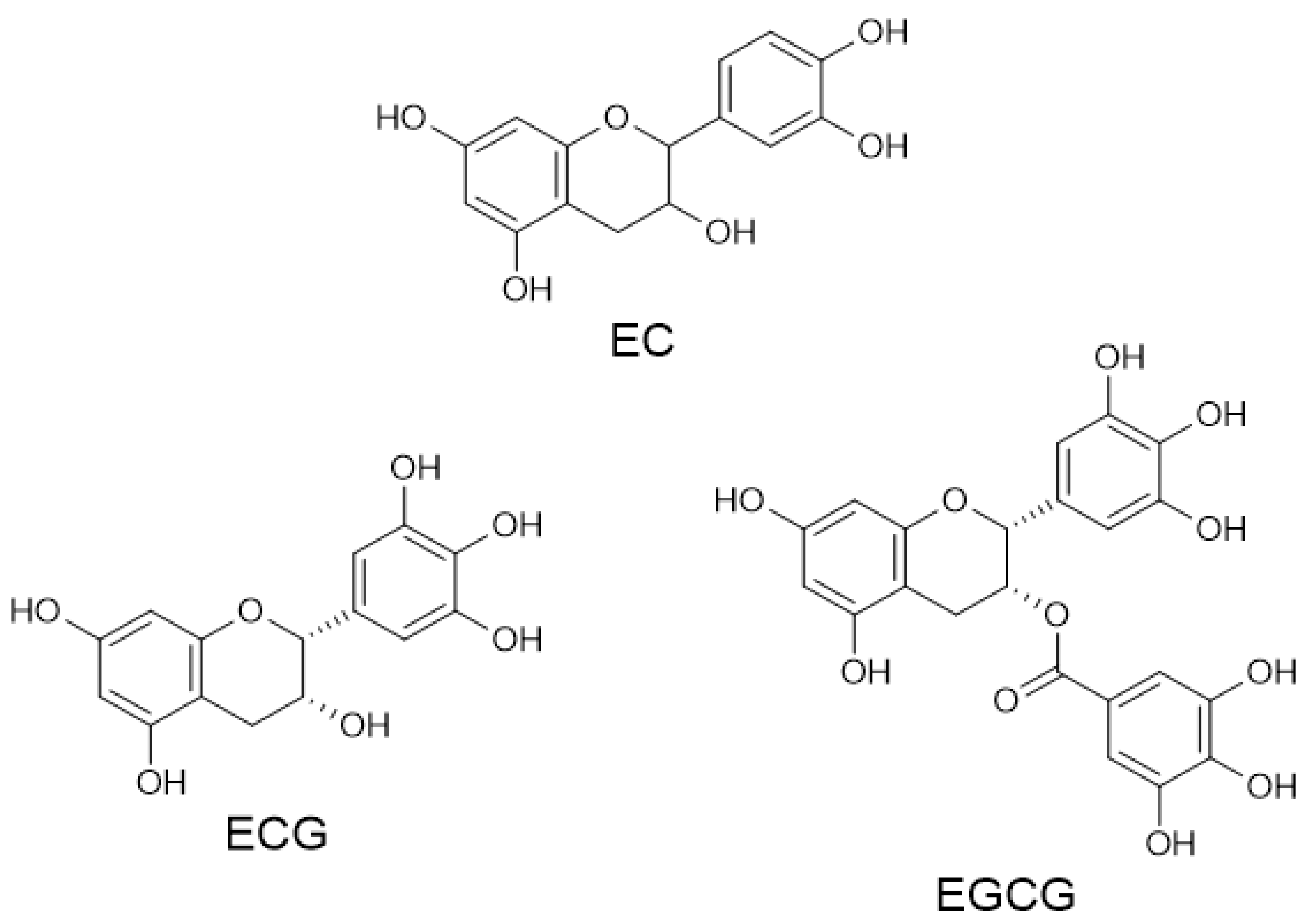
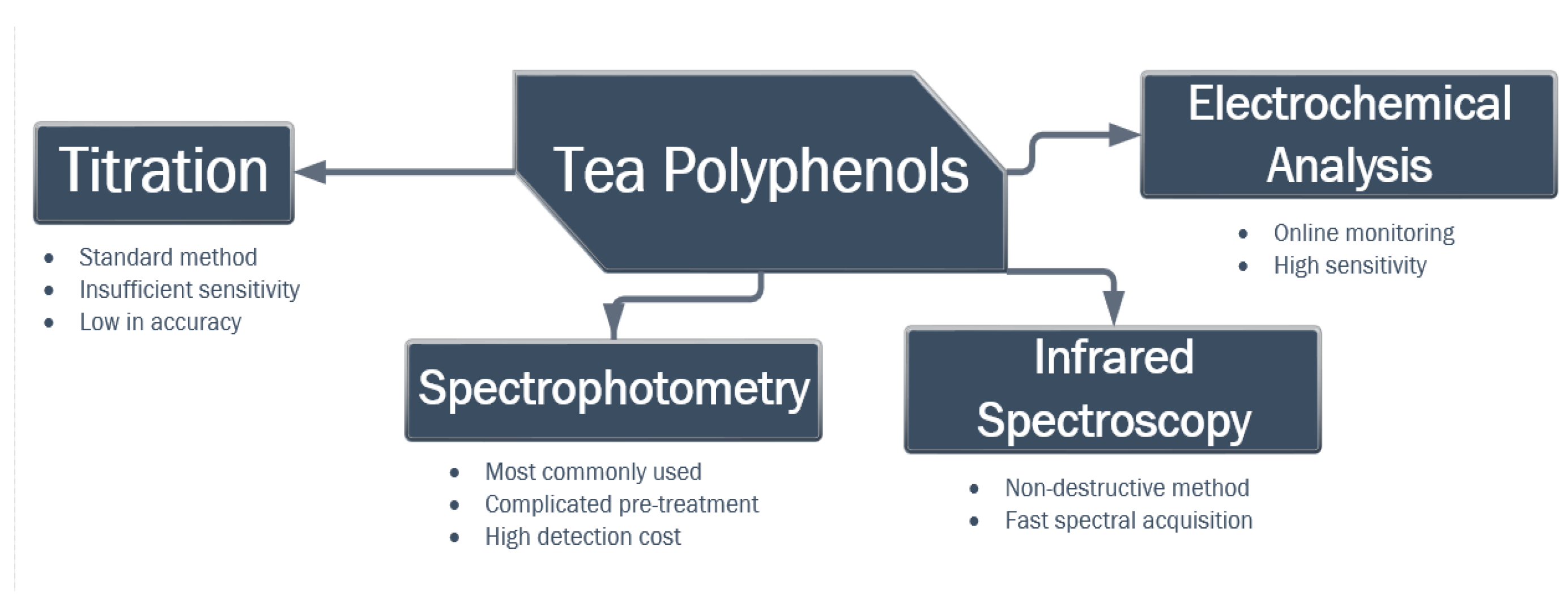
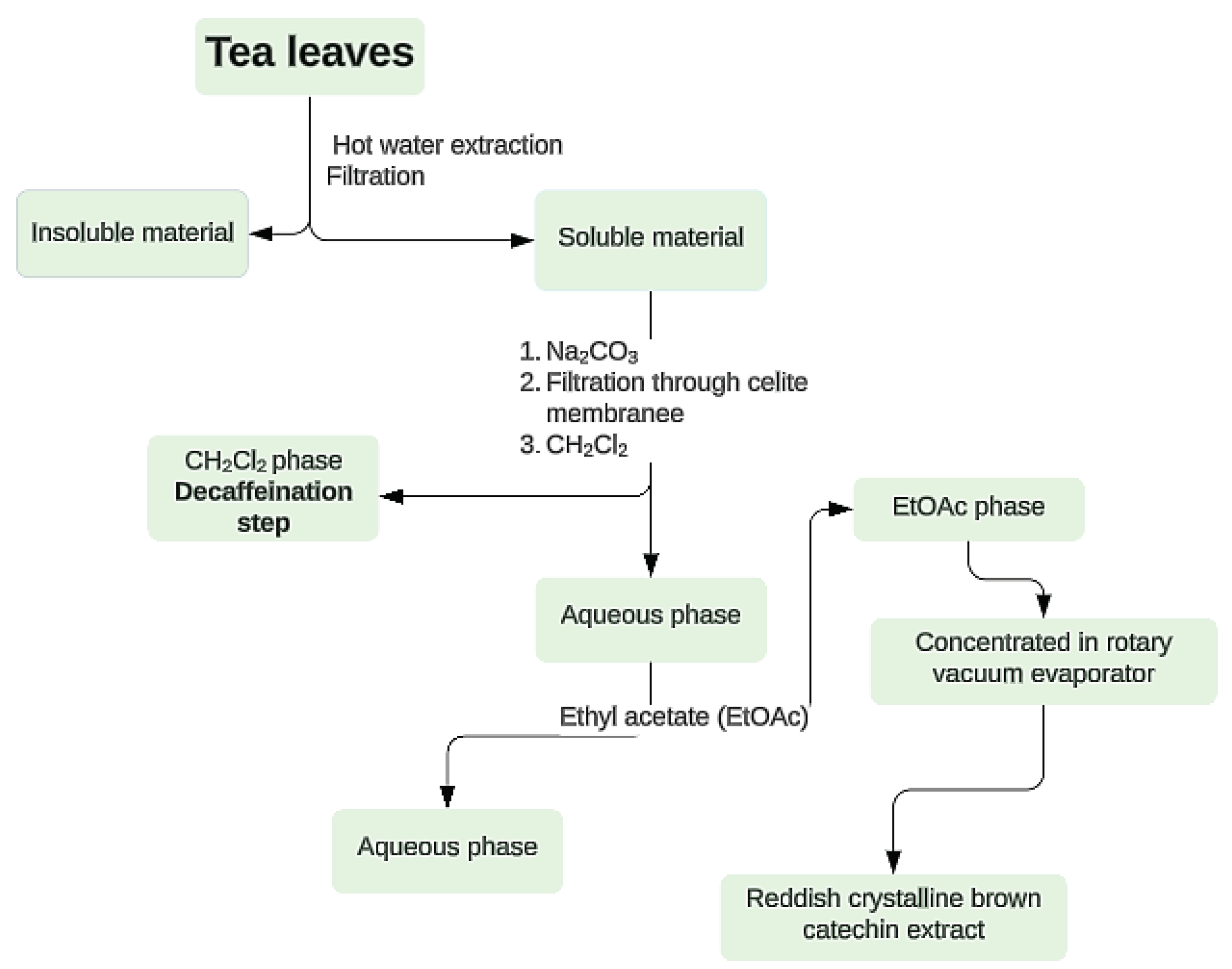
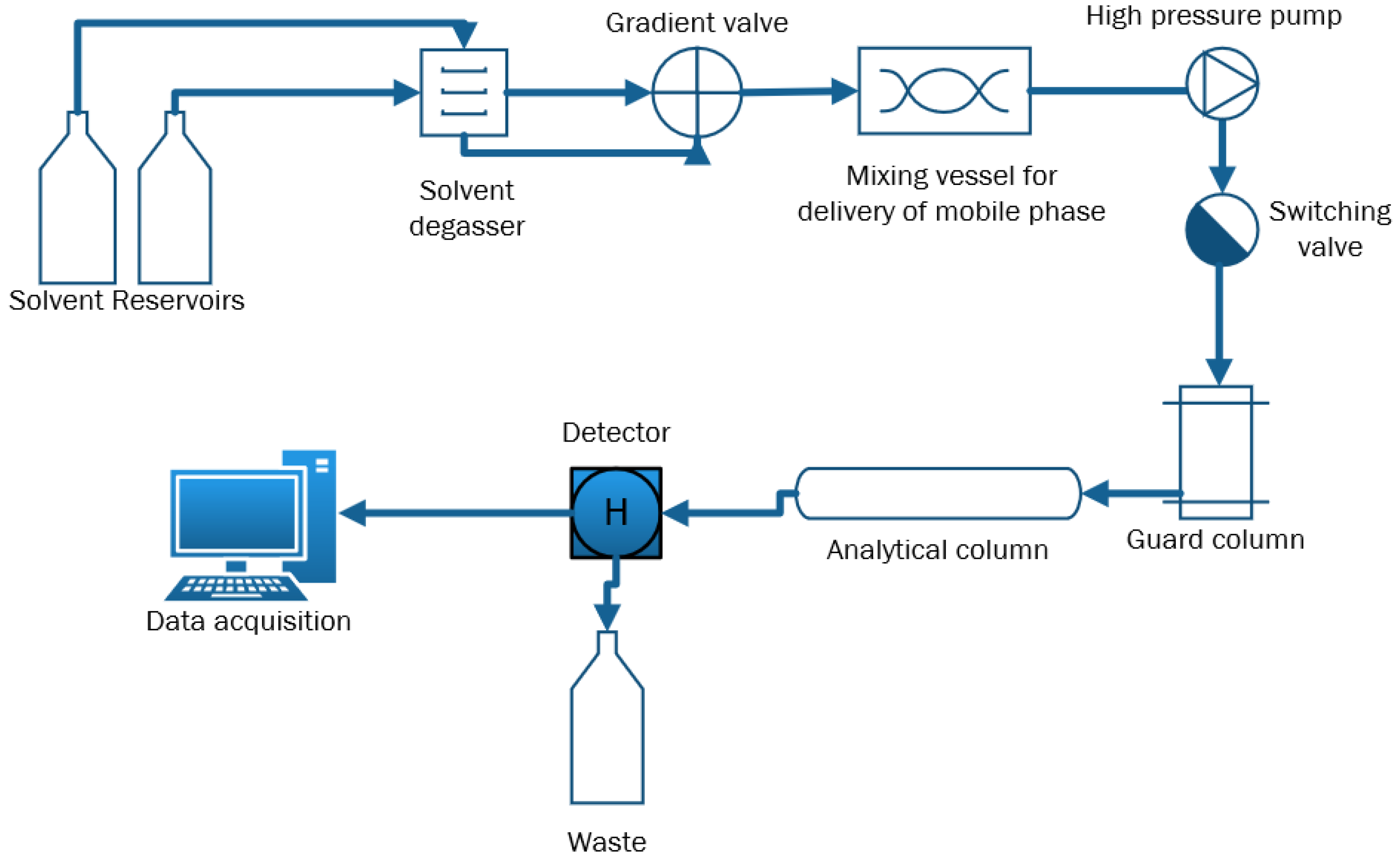
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Arts, I. C., & Hollman, P. C. Polyphenols and disease risk in epidemiologic studies. The American Journal of Clinical Nutrition 2005, 81(1), 317S-325S. [CrossRef]
- Balentine, D. A., Wiseman, S. A., & Bouwens, L. C. The chemistry of tea flavonoids. Critical Reviews in Food Science and Nutrition 1997, 37(8), 693-704. [CrossRef]
- Cabrera, C., Artacho, R., & Giménez, R. Beneficial effects of green tea—a review. Journal of the American College of Nutrition 2006, 25(2), 79-99. [CrossRef]
- Chowdhury, P., & Barooah, M. Tea Catechins: Beneficial Perspectives in Medicine, Food, and Cosmetics—A Review. Comprehensive Reviews in Food Science and Food Safety 2021, 20(2), 1334-1358.
- Chowdhury, P., & Barooah, M. Production and application of tea polyphenols: Present scenario and future perspectives. Food Research International 2021, 140, 110053.
- Chu, K. O., & Wang, C. C. Recent progress of natural products from traditional Chinese medicine in cancer drug discovery. European Journal of Medicinal Chemistry 2020, 204, 112595.
- Daglia, M. Polyphenols as antimicrobial agents. Current Opinion in Biotechnology 2012, 23(2), 174-181. [CrossRef]
- Lungu, I., Huzum, B., Humulescu, I. A., Cioanca, O., Morariu, D., Șerban, I. L., & Hancianu, M. Flavonoids as promising therapeutic and dietary agents. The Medical-Surgical Journal 2020, 124(1), 151-156.
- . Choung, M. G., Hwang, Y. S., Lee, M. S., Lee, J., Kang, S. T., & Jun, T. H. Comparison of extraction and isolation efficiency of catechins and caffeine from green tea leaves using different solvent systems. International journal of food science & technology 2014, 49(6), 1572-1578. [CrossRef]
- Dong, P., & Zhang, Y. Study on extraction of tea polyphenols from tea by ultrasonic method. Food Science and Technology 2018, 43(3), 62-65.
- Fraga, C. G., Galleano, M., Verstraeten, S. V., & Oteiza, P. I. Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular aspects of medicine 2010, 31(6), 435-445. [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. Journal of Agricultural and Food Chemistry 2014, 62(36), 9485-9502. [CrossRef]
- García-Ruiz, A., Baig, M. N., & Rodríguez-Pérez, C. Optimization of the ultrasound-assisted extraction of catechins from green tea using green solvents. Molecules 2018, 23(7), 1687.
- Hollman, P. C., & Katan, M. B. Health effects and bioavailability of dietary flavonols. Free Radical Research 1999, 31(s1), S75-S80. [CrossRef]
- Jabeen, A., & Hussain, S. Green tea (Camellia sinensis) polyphenols: Nutraceutical perspectives. Food Science and Technology International 2017, 23(7), 555-567.
- Lungu I. L., Batir-Marin D., Panainte A., Mircea C. , Tuchilus C., Stefanache A., Szasz F. A., Grigorie D., Robu S., Cioanca O., Hancianu M., Catechin-Zinc-Complex: Synthesis, Characterization and Biological Activity Assessment. Farmacia 2023, 71(4).
- Jeon, J. H., Lim, D. W., Lee, J. W., & Kim, Y. T. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Korean Journal of Physiology and Pharmacology 2018, 22(3), 227-236.
- Katiyar, S. K., & Agarwal, R. Green tea prevents non-melanoma skin cancer by enhancing DNA repair. Archives of Dermatology 2002, 138(10), 1409-1413.
- Khan, N., & Mukhtar, H. Tea polyphenols for health promotion. Life Sciences 2007, 81(7), 519-533. [CrossRef]
- Kumazawa, S., Taniguchi, M., Suzuki, Y., Shimura, M., & Kwon, M. S. Structure-activity relationships of catechins and their derivatives. Antioxidants 2009, 6(4), 1-8.
- Lambert, J. D., Sang, S., & Yang, C. S. Possible controversy over dietary polyphenols: Benefits vs risks. Chemical Research in Toxicology 2007, 20(4), 583-585. [CrossRef]
- Lee, S. C., Chan, W. K., Lee, T. W., Lam, C.I., & Wong, C. K. Preparation of catechins-rich extract from green tea. US Patent 2002 No. 6,440,422.
- Lin, J. K., & Lin-Shiau, S. Y. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Molecular Nutrition & Food Research 2006, 50(2), 211-217. [CrossRef]
- Loo, B. M. Simplified high-performance liquid chromatographic method for the quantitation of tea catechins. Journal of Chromatography A 1998, 793(1-2), 53-57.
- Lu, H., Meng, X., & Yang, C. S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metabolism and Disposition 2003, 31(5), 572-579. [CrossRef]
- Maalik, A., Khan, F. A., & Mumtaz, A. Health implications of tea: Herbal and green tea. Journal of Food Processing & Beverages 2019, 7(1), 6-11.
- Nakachi, K., Suemasu, K., Suga, K., Takeo, T., Imai, K., Higashi, Y., & Fukuda, S. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Journal of Epidemiology & Community Health 1998, 52(6), 377-381. [CrossRef]
- Nehlig, A. Effects of coffee/caffeine on brain health and disease: What should I tell my patients? Practical Neurology 2018, 18(2), 133-139.
- Newman, D. J., & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products 2012, 75(3), 311-335. [CrossRef]
- Niu, Y., Gong, Y., Xie, T., & Wu, C. Optimization of enzyme assisted extraction of catechins from green tea using response surface methodology. Journal of Food Engineering 2008, 83(1), 9-16.
- Pan, M. H., & Ho, C. T. Chemopreventive effects of natural dietary compounds on cancer development. Chemical Society Reviews 2008, 37(11), 2558-2574. [CrossRef]
- Pandey, K. B., & Rizvi, S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity 2009, 2(5), 270-278. [CrossRef]
- Patel, R. K., & Patel, N. J. Review on green tea constituents and its hidden benefits. International Journal of Pharmacy and Pharmaceutical Sciences 2015, 7(2), 30-35.
- Peng, X., Zhang, G., Liao, Y., Gong, D., & Wang, B. Extraction of catechins from green tea and the stability study of extracts. US Patent 2002 No. 6,428,741.
- Pietta, P. G., Simonetti, P., & Gardana, C. Catechin metabolites after intake of green tea infusions. BioFactors 1998, 8(1-2), 111-118. [CrossRef]
- Qiu, Y., Li, S., Wang, J., & Zhang, Q. Progress in the determination and extraction of catechins in tea. Journal of Food Science and Technology 2020, 37(4), 123-130.
- Sökmen, M., Demir, E., & Alomar, S. Y. Optimization of sequential supercritical fluid extraction (SFE) of caffeine and catechins from green tea. The Journal of Supercritical Fluids 2018, 133, 171-176. [CrossRef]
- Ray, P. D., Huang, B. W., & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling 2012, 24(5), 981-990. [CrossRef]
- Sanada, T., & Okano, M. Systematic extraction of catechins from green tea using ascorbic acid and basic methanol treatment. Journal of Chromatography A 2003, 1011(1-2), 189-199.
- Sang, S., & Lambert, J. D. Ho C-T, Yang C-S, 7th International Symposium on Tea Science and Human Health, 2011: The chemistry and biotransformation of tea constituents 2011. [CrossRef]
- Scalbert, A., Manach, C., Morand, C., Rémésy, C., & Jiménez, L. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition 2005, 45(4), 287-306. [CrossRef]
- Shimizu, M., Deguchi, T., Lim, J. T., & Moriwaki, H. Kopelovich L, Weinstein IB, Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 2008, 29(1), 105-111. [CrossRef]
- Si, W., & Gong, J. Green tea and its extracts in cancer prevention and therapy. Frontiers in Bioscience 2019, 24(1), 1535-1549. [CrossRef]
- Steinmann, J., & Buer, J. Pietschmann T, Steinmann E, Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. British Journal of Pharmacology 2009, 168(5), 1059-1073.
- Su, Y. L., Leung, L. K., & Huang, Y. Chen Z-Y, Stability of tea theaflavins and catechins. Food Chemistry 2003, 83(2), 189-195. [CrossRef]
- Wang, J., & Zhang, Q. Determination of catechins in tea by high performance liquid chromatography. China Tea 2018, 40(1), 7-10.
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS), et al. Scientific opinion on the safety of green tea catechins. EFSA Journal 2018, 16(4), e05239.
- Teng, H., Chen, L., & Huang, Q. Chemical characteristics of tea catechins. Food Science 2018, 39(9), 313-319.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



