1. Introduction
One of the main goals expressed in the United Nations Agenda 2030 [
1] is to im-prove the state of nutrition combined with sustainable agriculture in order to ensure a healthy and quality life for all individuals regardless of their age, gender or economic power. All these parameters are related to the fact that there are currently several risk factors associated with diseases that are related to eating habits. With the growing world population, currently 8 billion and estimated that by 2080 will be about 10.4 billion [
2], it becomes worrying to project the amount of food that will need to be produced to ensure a healthy diet, both in terms of quantity and quality.
According to Food-based Dietary Guidelines of the Food and Agriculture Organization of the United Nations (FAO) a sustainable diet goes beyond nutrition and the environment, is more complex, including economic and socio-cultural dimensions [
3]. In this sense, the search for sustainable food production systems that enable the implementation of resilient agricultural practices, in view to increase productivity and production, but maintaining a balanced ecosystem, fit for climate change, and which also improve land and soil quality, is becoming inevitable [
1].
Grape pomace, composed of skin, seed and stem, is a disposal from the winemaking industry and has in its constitution bioactive compounds with antimicrobial, anti-inflammatory [
4], antioxidant properties [
5], anti-cancer effects and also beneficial cardiovascular and hepatic effect [
4,
6,
7], besides other important nutritional components such as fibers [
8], unsaturated fatty acids [
9], proteins [
10], and may have low total sugars [
11] depending on the type of grape, wine, and its production process [
10]. The metal composition, including micronutrients, is of high importance in food quality assurance and safety [
12].
As previously described in several papers, grape pomace presents in its composition phenolic compounds, such as anthocyanins, catechins, flavonol glycosides, phenolic acids, and alcohols, also fibers, namely lignin, cellulose, hemicellulose, all these compounds being pointed out as being remarkable for health [
13].
Two distinct cultivars of Vitis vinifera L., specifically the white Arinto and the red Touriga Nacional varieties, were subjected to comparative analysis to elucidate their chemical and nutritional profiles. This investigation aimed to delineate both the com-monalities and divergences in their compositions, thereby providing insights into their unique organoleptic and chemical attributes. These findings hold significant promise for innovative applications within the realm of food technology, leveraging the distinct characteristics inherent to each varietal.
This study contributes significantly to the broader dissemination of findings that reinforce the data supporting the valorization and repurposing of grape pomace as a potential novel ingredient in human food. It underscores the capability for sustainable utilization of this by-product, highlighting its value in food technology through innovative reuse strategies.
2. Materials and Methods
2.1. Grape Pomace Samples
Pomace samples derived from two distinct Vitis vinifera L. varietals, namely Touriga Nacional and Arinto, from red and wine production, respectively, were systematically harvested from the Alentejo region in 2018. Post-collection, these samples underwent a meticulous drying process in a J.P. Selecta (Barcelona, Spain) oven, maintained at 60 °C for a duration of 24 hours. Subsequently, the dried samples were finely milled using a Moulinex domestic blade grinder to achieve a uniform granular consistency. To preserve their integrity and prevent degradation, the milled pomace, named Grape Pomace Flour (GPF) was securely stored in airtight propylene containers at a con-trolled temperature of 4 °C, thereby ensuring their stability until the commencement of analytical evaluations.
2.2. Chemical and Nutritional Composition of Grape Pomace Fluor
2.2.1. Nitrogen and Extractable Phosphorus Composition
The determination of nutrients concentrations in the samples (nitrates, NOx; ammonia, NH
4, extractable phosphorus, PO
4) was performed by UV/vis spectroscopy using specific colorimetric methods implemented in a Skalar SANplus Segmented Flow Auto-Analyzer (Breda, Netherlands). NOx and NH
4 were determined, respectively, according to Strickland and Parsons (1972) [
14] and to Koroleff (1976) [
15], upon KCl extraction, following Bryant and Wright’s method [
16]. Extractable phosphorus was determined according to Murphy and Riley (1962) [
17], upon Na
2CO
3 extraction, following Olsen´s method [
18]. All procedures were adapted to segmented flow analysis.
2.2.2. Nutritional Composition of Grape Pomace Fluor
The quantification of total ash content in the samples was accurately executed through a process of incineration. This procedure involved the use of a J.P. Selecta-Horn (Barcelona, Spain) muffle furnace, operating at a temperature of 550 °C, in strict accordance with the guidelines stipulated in the Portuguese Norm NP 518 [
19]. Concurrently, the moisture content of each sample was rigorously assessed. This entailed weighing the samples, followed by desiccation in a J.P. Selecta (Barcelona, Spain) oven with forced air circulation set at 105 °C. The process was continued until a constant weight was achieved, adhering to the established procedures delineated in NP 875 [
20]. To ensure accuracy and reproducibility, all assays were conducted in triplicate.
Utilizing a conversion factor of 6.25, the protein content in GPF samples was ascertained by quantifying total nitrogen, employing the Kjeldahl method. This analysis was conducted in accordance with the procedures outlined in ISO 16634, which pro-vides a standardized approach for nitrogen determination in food products. This method is critical for accurately determining the protein content in GPF samples, offering vital insights into their nutritional composition [
21].
Using nuclear magnetic resonance express in ISO 8292:2008, the lipid content was quantify using previously dried at 50 °C and stabilized [
22].
Insoluble dietary fibers (insoluble hemicellulose, cellulose, and lignin) were deter-mined according to Goering & Van Soest [
23]. This procedure involves a sequential extraction with neutral and acid detergents, followed by a treatment with 72% sulfuric acid and incineration at 550 °C of the final residue. The difference in weight between the neutral and acid detergent extracts represents the insoluble hemicellulose fraction. The acid detergent residue contains lignin, cellulose, and ash. Treatment with 72% sulfuric acid hydrolyzes the cellulose and leaves lignin and ash. The final incineration of the residue in a muffle Heraeus Electronic (Shanghai, Singapore) makes it possible to determine the total content in lignin [
23,
24]. The soluble dietary fiber percentage was determined by subtracting the sum of the percentage contents of ash, fat, protein, simple sugars [
25] and insoluble dietary fiber from total percentage.
Total carbohydrates were determined gravimetrically according to the Munson and Walker procedure [
26]. This technique is based on the quantification of the cuprous oxide precipitate formed after copper(II) reduction, at high temperature and alkaline conditions, by the reducing sugars obtained after sugar acid inversion.
2.2.3. Metals and Semi-Metal Composition of Grape Pomace Fluor
The determination of metals aluminum (Al), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), lithium (Li), manganese (Mn), nickel (Ni), lead (Pb), and zinc (Zn) and semi-metal arsenic (As) concentrations in the samples was performed by atomic absorption spectrometry in a Solaar-Thermo elemental Thermo Fisher Scientific, Inc., Cambridge, United Kingdom, after digestion in a microwave oven ETHOS PLUS Milestone (Sorisole, Italy).The digestion was done with 10 mL of nitric acid in a three steps procedure: 5 min at 100 °C and 250 watts, 10 min at 180 °C and 800 watts and 20 min at 180 °C and 800 watts. The procedures were done acording EPA Method 3051 (2007) [
27]. Measurements were performed by validated procedures and their quality was controlled through the analysis of blank samples, control standards close to the LOQ, and in the middle of the calibration interval, duplicate samples, and spiked samples. Metrologically sound criteria were used to accept these controls [
12,
28].
Mercury (Hg)concentrations were directly measured in samples by atomic absorption spectrometry with thermal decomposition, using a Direct Mercury Analyzer (DMA Milestone, Sorisole, Italy). Blanks were prepared using the same procedure without sample. All reagents were of Merck Suprapure quality and pure water was MilliQ grade [
12,
29].
2.2.4. Phenolic Composition of Grape Pomace Flour
The extraction of phenolic compounds was performed in triplicate, according to Pérez-Ramírez et al (2018) [
30]. A hydroalcoholic solution and acetone/water mixture were used to ex-tract free phenolics. 0.5 g of freeze-dried grape pomace was mixed with 20 mL water:methanol (50:50, v/v, pH 2). The mixture was shaken at room temperature for 1 hour and the resulting ex-tract was centrifuged for 15 min at 4 °C and 10000 rpm (Dynamica Velocity 14R Refrigerated Centrifuge, Dynamic Scientific Ltd. (Livingston, United Kingdon). The residue was re-extracted with 20 mL of acetone/water (70:30, v/v), as described above. The supernatants of each extraction were combined, corresponding to the extractable phenolic compounds fraction.
Total phenolic content (TPC) on each sample was determined through the Folin-Ciocalteau colorimetric method [
31,
32]. The reaction mixture was prepared by mixing 15 µL of each extractable phenolic compounds fraction (or water for control) with 75 µL of Folin-Ciocalteau phenol rea-gent and 500 µL of distilled water in 2 mL Eppendorf tubes. The mixture was vortexed for 10 s, followed by the addition of 300 µL of 5% Na
2CO
3. The mixture was brought up to 1500 µL by adding 610 µL of distilled water and vortexed for another 10 s. After 30 min incubation at room temperature in the dark, the absorbance was measured at 750 nm. Results were expressed as mg gallic acid equivalents per g of dry weight (mg GAE/g DW).
Anthocyanins content in the extractable phenolic compounds fraction were analyzed HPLC-DAD [
33] on an Elite LaChrom Merck Hitachi HPLC, (Shanghai, China) composed of the following modules: quaternary pump (L-2130), auto-sampler (L-2200), thermostated column compartment (L2300) with a LiChrosphere® 100 LiChroCART® C-18 reverse-phase column (250 mm x 4 mm, i.d.; 5 µm), thermostated at 25 °C and a diode array detector (L-2455). The mobile phase consisted of HCOOH/H
2O (10/90, v/v) (solvent A) and HCOOH/ CH
3CN/H
2O (10/30/60, v/v/v) (solvent B). The flow rate was 1.0 mL.min
-1 with a linear gradient ranging from 80% of A to 15% in 70 min, then reaching 100% B in 5 min, a final isocratic gradient of 100% B during 10 min and a final re-equilibration isocratic gradient of 80% A for 5 min. Spectra were recorded between 220 nm to 600 nm and detection was carried out at 280 nm and 520 nm as the preferred wavelengths. Results were expressed as mg malvidin-3-O-glucoside equivalents/g dry weight (mg Mv3Glc/g DW).
To analyze the content on low molecular weight phenolic compounds, proanthocyanidins and flavonols, the extractable phenolic compounds fractions were evaporated to 1/3 of the origi-nal volume under vacuum at 37 °C on a rotary vacuum evaporator and the volume was adjusted with water to 20.0 mL. The fractions were extracted three times with 20.0 mL ethyl acetate and the resulting organic phases were combined and evaporated to dryness on a vacuum rotative evaporator at 37 °C. The residues were resuspended on 2.0 mL methanol and analyzed by HPLC-DAD [
34]. The mobile phase consisted of HCOOH/H
2O (10/90, v/v) (solvent A) and CH
3CN (solvent B). The flow rate was 0.5 mL.min-1 with a linear gradient ranging from 90% of A to 65% in 50 min, then reaching 100% B in 5 min, a final isocratic gradient of 100% B during 7 min and a final re-equilibration isocratic gradient of 90% A for 5 min. Spectra were recorded between 220 nm to 400 nm and detection was carried out at 280 nm as the preferred wavelength. Results were expressed as mg catechin equivalents/g dry weight (mg catechin/g DW).
The identification of phenolic compounds was performed by HPLC-DAD/ESI-MS, using the same solvents, flow rate and gradient as for HPLC-DAD analysis (nonanthocyanic compounds). For anthocyanins analysis, solvents were HCOOH/H2O (1/99, v/v) (solvent A) and HCOOH/ CH3CN/H2O (1/30/69, v/v/v) (solvent B), with the same gradient as described for HPLC-DAD analysis. A Finnigan Surveyor series liquid chromatograph equipped with a Thermo Finnigan (Hypersyl Gold) C-18 reversed-phase column (150 mm x 4.6 mm, i.d.; 5 µm, Thermo Scientific), thermostated at 25 °C was used. Detection was carried out between 200 nm and 700 nm using a Finnigan Surveyor PDA Plus detector. Mass detection was made on a Finnigan LCQ DECA XP MAX Finnigan Cor. (San Jose, CA, USA) quadrupole ion trap equipped with an atmospheric pressure ionization (API) source using an electrospray ionization (ESI) source. The vaporizer and capillary voltages were 5 kV and 4 V, respectively. The capillary temperature was set at 325 °C. Nitrogen was used as both sheath and auxiliary gas flow rates of 80 and 30, respectively (in arbitrary units). Spectra were recorded in the negative- or positive-ion mode between m/z 120 and 2000. The mass spectrometer was programmed to do a series of three scans: a full mass, a zoom scan of the most intense ion in the first scan, and a MS-MS of the most intense ion using relative collision energies of 30 V and 60 V.
3. Results and Discussion
This study focused on analyzing the chemical and nutritional profiles of two varieties of Vitis vinifera L., Arinto and Touriga Nacional, to understand their unique properties. The study sheds light on the potential of grape pomace as a sustainable food ingredient, emphasizing its composition rich in beneficial compounds such as phenolics, unsaturated fatty acids, fiber, and low sugar content. This discussion will explore how these findings can contribute to food technology as well as sustainable agriculture, aligning with the goals of the United Nations 2030 Agenda to improve nutrition and promote sustainable practices. The repurposing of grape pomace represents a significant step towards innovative food solutions and the sustainable use of resources.
3.1. Nitrogen and Phosphorus Composition
The use of grape pomace can be a sustainable and a very versatile practice that can contribute to human nutrition when used as a food ingredient, and plant nutrition when used to pro-duce fertilizers. Grape pomace contains various organic and inorganic compounds, essential nutrients such as nitrogen, phosphorus, macro, and micronutrients that can enhance human health and nutrient cycling [
35], contributing to environmental sustainability.
Nitrogen and phosphorus are critical nutrients for plant growth, and their availability in soil directly influences crop yield and quality [
36]. Healthy, well-nourished plants are more resistant to diseases and pests. This can reduce the need for chemical pesticides and, consequently, minimize potential health risks associated with pesticide residues in food [
37]. The composition in nitrate, nitrite, ammonia nitrogen and phosphorus data for the GPF obtained from wine-making waste are shown
below.
As presented in
Table 1, the variance in nitrogen and phosphorus content between Arinto and Touriga Nacional grape pomace is noteworthy. The implications of these differences are substantial for the application of these by-products in agriculture, particularly as soil amendments aimed at enhancing plant growth.
Touriga Nacional GPF exhibits a higher concentration of both forms of nitrogen
- nitrate and nitrite, as well as ammonia
- compared to Arinto GPF. Given the crucial role of nitrogen in plant physiology, particularly in the synthesis of chlorophyll and amino acids, the elevated levels in Touriga Nacional GPF could more effectively support the photosynthetic process and the overall protein synthesis within plants [
38]. The implications of this higher nitrogen content may be reflected in more vigorous leaf development and an increase in plant biomass, a beneficial attribute for crops during the vegetative growth phase.
The difference in ammonia nitrogen content is particularly significant, as ammonia is directly incorporated into amino acids in the plant. This could suggest a faster response in plant growth when Touriga Nacional GPF is applied, due to the immediate availability of this form of nitrogen [
39] . This rapid assimilation can be advantageous in soils that are deficient in nitrogen or when quick growth is desired.
The phosphorus levels, although seemingly modest in absolute terms, are higher in the Touriga Nacional variety. Phosphorus availability is a key factor in plant energy transfer processes and in the formation of genetic material [
40]. The higher extractable phosphorus content in Touriga Nacional GPF may be particularly beneficial during the critical stages of plant development, such as root and flower formation, and could lead to an increase in fruit yield [
41].
It is evident that the nutrient profile of grape pomace has practical implications for sustainable agricultural practices. The use of such by-products not only contributes to a reduction in agricultural waste but also adds value through the recirculation of essential nutrients in the farming ecosystem. Further research could elucidate the optimal conditions and application rates for these pomace varieties to maximize their benefits in different crop systems.
3.2. Nutritional Composition of Grape Pomace Fluor
In the realm of oenological by-products, the
GPF exhibit contrasting nutritional profiles as detailed in
Table 2. This table provides an analytical breakdown of crucial nutritional constituents which are essential for evaluating the potential application of grape pomace flour in food products and health supplements.
The ash content, indicative of mineral presence, is notably higher in Touriga Nacional (5.49%) than in Arinto (4.02%). This remark suggests that Touriga Nacional
GPF could be a richer source of essential minerals, which is significant considering that mineral-rich feed has been associated with better bone health and overall growth in animals [
42].
Moisture content, which influences shelf life and microbial stability, is substantially higher in Arinto (8.88%) compared to Touriga Nacional (3.99%). This variation can impact the storage and preservation conditions required for these flours. Drier products like the Touriga Nacional GPF are generally more stable and less prone to microbial spoilage, which can be advantageous in feed formulations [
43].
The protein content is higher in Touriga Nacional (10.13%) compared to Arinto (8.38%). High-protein feeds are crucial for muscle development and overall growth in animals, and they also play a role in human diets for muscle maintenance and satiety [
44].
In terms of fats, Touriga Nacional is richer in both monounsaturated and polyunsaturated fatty acids, which have been linked to positive health outcomes in animals, including improved meat quality and antioxidant status [
45]. This suggests that incorporating Touriga Nacional grape pomace in animal diets could potentially enhance the nutritional value of the produced meat.
The dietary fiber composition is particularly interesting, with Touriga Nacional showing a significant amount of soluble fiber (14.3%) compared to Arinto (1.7%). Soluble fibers are beneficial for cardiovascular health and can aid in lowering blood cholesterol levels. They also play a role in gut health by influencing the gut microbiota, which is critical for both animals and humans [
46].
Finally, the total sugars are much higher in Arinto (31.3%) than in Touriga Nacional (4.7%). For animal feeds, lower sugar content is generally preferred to avoid rapid fermentation in the gut, which can lead to digestive disturbances [
47].
These findings suggest that Touriga Nacional GPF might be better suited for enhancing nutritional quality in animal feed, which could translate into health benefits for livestock and quality improvements in animal-derived food products. Future studies should aim to explore the practical applications of these and other flours in various dietary formulations, considering their nutrient profiles and the potential health benefits they may confer.
3.3. Metals and Semi-Metal Composition of Grape Pomace Fluor
These same samples were analyzed for their metal
and semi-metal content and the results
are shown in the
Table 3.
The metal content in GPF from the Arinto and Touriga Nacional varieties, as outlined in
Table 3, is significant for its inclusion of both non-essential and essential elements. These elements can influence the potential application of grape pomace in human diets and need to be assessed carefully considering their health implications [
12].
Non-essential elements such as aluminum (Al) and nickel (Ni) are present in both varieties of grape pomace, but lithium (Li) was not detected in any of the samples. Aluminum, despite being the most abundant metal in the earth's crust, is not required by the human body and, at elevated levels, is linked to health concerns such as neurotoxicity. The grape pomace of the Touriga Nacional variety shows higher aluminum content, which, while typical for plant materials, should be consumed within safe limits to avoid potential health risks. Nickel, also non-essential, is present in negligible amounts, posing minimal health risks, but their intake should still be monitored due to the potential for toxicity at higher levels.
The presence of heavy metals including arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) in both GPF calls for careful consideration. Mercury's undetectable levels are beneficial since it is known for its toxicity. For arsenic, cadmium, and lead, although detectable, their concentrations are relatively low. However, their potential for toxicity, even at low exposure levels, warrants regular monitoring to ensure safety for consumption and use in animal feeds.
In contrast, the essential elements, chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn), which are crucial trace metals, are present and beneficial for various biological functions. Touriga Nacional GPF, with its higher ash content of 5.49%, as compared to 4.02% in Arinto, suggests a richer mineral profile and may offer more significant dietary benefits. These elements are vital for a range of biological processes, including immune function and metabolic processes. However, it's essential to maintain a balanced intake of these metals to prevent the adverse effects of toxicity.
The ash content in grape pomace is an indicator of total mineral content, and the higher ash content in Touriga Nacional might enhance its value as a dietary supplement. Nevertheless, the levels of non-essential and heavy metals must be kept within acceptable safety margins [
12].
Incorporating grape pomace into the human diet aligns with sustainable dietary practices and can contribute to the circular economy by utilizing agro-industrial by-products. However, continual testing and adherence to safety standards are crucial for ensuring the health benefits of these products. The findings resonate with recent studies, highlighting the potential of grape pomace as a source of bioactive compounds and the need to manage waste products responsibly to minimize environmental impacts [
12,
48].
These findings, with its rich content of trace metals and potential health-promoting properties, could be a step towards more sustainable food-production practices if used within the safety standards set by the European Commission and other health regulatory bodies.
3.4. Phenolic Composition of Grape Pomace Flour
The following results
(Table 4) provides an insightful look into the phenolic content of Arinto and Touriga Nacional
GPF, offering a perspective on their potential functional uses and health implications. The rich profile of extractable phenolics, anthocyanins, proanthocyanidins, and flavonols in these
flours signifies a substantial bioactive potential.
In this way
, Arinto, with its higher extractable phenolics at 25.9 mg GAE/g DW, suggests a robust antioxidant capacity, which can be fundamental in countering oxidative stress and contributing to the prevention of chronic diseases [
4]. This high phenolic content may also be indicative of potential anti-inflammatory properties, given the links established between phenolic compounds and inflammation reduction [
49].
Touriga Nacional, while lower in overall extractable phenolics, contains anthocyanins at 1.62 mg Mv3Glcl/g DW. These compounds are not only crucial for their antioxidant activities but also for their reported benefits in cardiovascular health [
50]. The presence of anthocyanins could also play a role in modulating gut microbiota, which is increasingly recognized for its importance in overall health.
The proanthocyanidins in both pomaces, albeit at different levels, add to this anti-inflammatory narrative and have been noted for their contribution to cardiovascular health through various mechanisms, including the reduction of blood pressure and anti-atherosclerotic activity [
51].
Flavonols, with similar levels in both varieties, complement the beneficial effects of other phenolic compounds. Their presence has been associated with a reduced risk of certain cancers and cardiovascular diseases, due to their influence on cell signaling pathways and gene expression [
52].
The comprehensive analysis of these compounds and their concentrations in Arinto and Touriga Nacional grape pomace supports their potential incorporation into food products. This strategy could harness the health-promoting effects of these bioactive compounds, contributing to the growing field of functional foods and nutraceuticals.
3.4.1. Anthocyanins Composition of Grape Pomace Flour
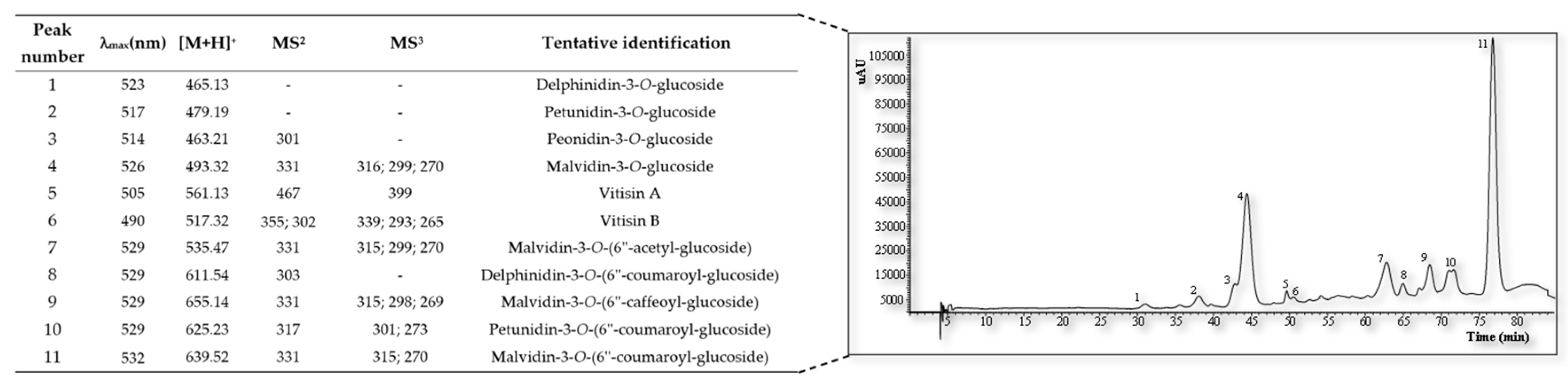
Figure 1 shows
the overlay of chromatogram and table of the anthocyanins of the GPF identify in fractions with no polyphenolys. This analysis provides a deeper insight into the bioactive components of these grape varieties, which are significant due to the numerous health benefits associated with anthocyanins. The results indicate the existence of delphinidin-3-
O-glucoside, delphinidin-3-
O-(6''-coumaroyl-glucoside), malvidin-3-
O-glucoside, mal-vidin-3-
O-(6''-acetyl-glucoside), , malvidin-3-
O-(6''-caffeoyl-glucoside), pe-onidin-3-
O-glucoside petunidin-3-
O-(6''-coumaroyl-glucoside), petunidin-3-
O-glucoside, mal-vidin-3-
O-(6''-coumaroyl-glucoside), vitisin A, and vitisin B in the analyzed samples.
Arinto GPF demonstrates a notable absence of anthocyanins, whereas Touriga Nacional is enriched with these potent bioactive compounds, such as delphinidin-3-
O-glucoside and petunidin-3-
O-glucoside. Their presence is correlated with significant health benefits, particularly in the context of cardiovascular health, due to their potent antioxidant capabilities. These compounds help in reducing oxidative stress, a key factor in the pathogenesis of cardiovascular diseases [
53].
Furthermore, the identification of malvidin-3-
O-glucoside and its derivatives suggests additional neuroprotective effects, which have been explored in recent studies for their role in preventing neurodegenerative disorders. These findings are indicative of the potential of grape pomace anthocyanins to impact cognitive functions positively and modulate gut microbiota, contributing to overall well-being [
53].
3.4.2. Proanthocyanidins and Flavonols Composition of Grape Pomace Flour
Proanthocyanidins are a class of compounds that have garnered attention for their potential to improve human health, including hypothesized systemic effects like hypoglycemic and lipid-lowering actions, and local anti-inflammatory actions on the intestinal epithelium. Their effects are primarily associated with their antioxidant properties, which are thought to contribute to a reduced risk of chronic diseases such as heart disease and diabetes [
54]. This class of polyphenolics is often studied for their implications in the nutraceutical field, hinting at the vast potential they hold for dietary supplementation and functional food development.
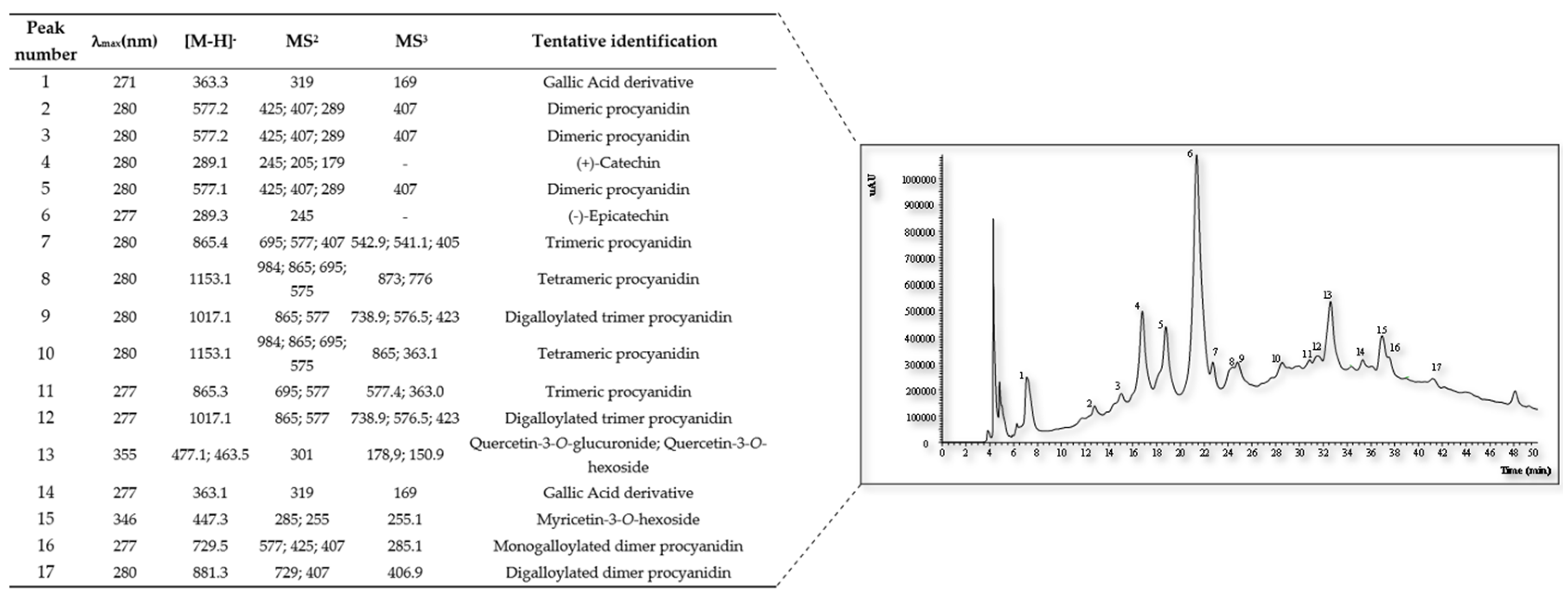
The results presented in
Figure 2 indicates the presence of several proanthocyanidins, flavonols, and phenolic acids, namely (+)-catechin, (-)-epicatechin, digalloylated dimer procyanidin, digalloylated trimer procyanidin, dimeric procyanidin, gallic acid derivative, monogalloylated dimer procyanidin, myricetin-3-o-hexoside, quercetin-3-o-glucuronide; quercetin-3-o-hexoside, tetrameric procyanidin, and trimeric procyanidin, each with a unique set of potential health effects.
The presence of catechin and epicatechin within the larger proanthocyanidin compounds enriches their profile and underscores the complexity of these bioactive structures.These compounds have been recognized for their role in the pigmentation of rice and for their beneficial health effects. The significance of these structures lies not only in their contribution to the aesthetic quality of food but also in their health-promoting properties [
55].
Furthermore, the detection of flavonols like quercetin-3-
O-glucuronide underscores the diverse nature of polyphenolic compounds present in grape pomace. Quercetin, a well-researched flavono
l, is known for its antioxidant capabilities, and recent findings continue to support its role in reducing inflammation and bolstering heart health [
4].
Taken together, these findings from
Figure 2 emphasize the richness of grape pomace in various phenolic compounds with promising health benefits. This composition may translate into practical applications in the food industry, where these compounds can be extracted and utilized to enhance the health value of food products. As the interest in functional foods continues to rise, grape pomace's rich polyphenolic content presents an excellent opportunity for the industry to turn a byproduct into a valuable resource for health-promoting food ingredients.
The present study points towards a promising avenue for the agricultural and food industries. Utilizing GPF, particularly from the Touriga Nacional variety, could offer a dual benefit: improving crop yields through soil amendment and delivering health-promoting phenolics through dietary means. The use of this byproduct may contribute to sustainable agricultural practices, capitalizing on the nutritional potential of grape pomace while aligning with environmental stewardship goals.
4. Conclusions
The incorporation of Grape Pomace Flour into our food systems presents a multifaceted approach to addressing key Sustainable Development Goals (SDGs) [
1] set by the United Nations Agenda 2030. As an innovative solution, GPF aligns remarkably with these global objectives, offering direct nutritional advantages and broader environmental benefits.
For combating hunger, particularly in regions affected by food scarcity, GPF emerges as a potent tool in addressing Zero Hunger (Goal 2). Its rich nutritional profile can significantly elevate nutritional standards, thus playing a crucial role in mitigating malnutrition challenges. This is particularly relevant in the context of ensuring food security and improving nutrition, as emphasized in the Agenda 2030.
The health benefits of GPF, endowed with antioxidants, fibers, and unsaturated fatty acids, align seamlessly with Good Health and Well-being (Goal 3). The promotion of such nutritious food aids in ensuring healthy lives and promoting well-being for all ages, underpinning the Agenda’s commitment to health and wellness.
In terms of Responsible Consumption and Production (Goal 12), GPF exemplifies sustainable practices by transforming winemaking by-products into valuable nutritional resources. This approach not only encourages sustainable consumption patterns but also underscores the importance of reducing waste and enhancing environmental responsibility. Furthermore, the application of GPF in composting contributes effectively to organic waste management and soil health, reinforcing the principles of sustainable consumption and production.
Moreover, GPF's role in Climate Action (Goal 13) is significant. By repurposing grape pomace, it reduces the carbon footprint associated with waste disposal, thereby contributing actively to climate change mitigation efforts.
Finally, regarding Life on Land (Goal 15), GPF supports sustainable agricultural practices that are crucial for the preservation of terrestrial ecosystems. Its production, which does not require arable land or irrigation water, differs substantially from traditional flour production methods, thus contributing to the conservation of natural resources and biodiversity.
In essence, GPF stands as a beacon of sustainable innovation, offering not only nutritional benefits but also contributing to a more sustainable, healthier, and better-nourished global population. Its broader integration and promotion are not just steps towards fulfilling specific goals of the United Nations Agenda 2030 but also represent a stride towards a more sustainable and equitable world.
Author Contributions
Conceptualization, M.N., P.P., and M.L.P.; methodology, M.N, P.P, C.P., C.B., E.M., A.L.F., M.P.D., M.L. and A.F.; validation, M.N., N.M., and P.P.; formal analysis, M.N., P.P., and M.L.P.; investigation, M.N., P.P., and M.L.P.; resources, M.N., P.P., and M.L.P.; data curation, M.N., and P.P.; writing original draft preparation, M.N., P.P., M.L.P, C.P., C,B., E.M., A.L.F., M.P.D., A.F., and N.M.; writing, M.N.; visualization, M.N., P.P., and M.L.P.; supervision, M.N.; project administration, M.N., P.P., and M.L.P.; funding acquisition, M.N., P.P., E.M., A.L.F., and M.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by the Foundation for Science and Technology – FCT, under the grants UIDB/04567/2020, UIDP/04567/2020 to CBIOS, and MEtRICs, through FCT/MCTES (
https://doi.org/592 10.54499/UIDB/04077/2020, https://doi.org/10.54499/UIDP/04077/2020).
Acknowledgments
The authors thank CARMIM for providing the fresh grape pomace samples for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Council of Europe Contribution to the United Nations 2030 agenda for sustainable development goals. Available online: https://www.coe.int/en/web/programmes/un-2030-agenda (acessed on 17 july 2023).
- United Nations, Peace, dignity and equality on a healthy planet. Available online: https://www.un.org/EN/global-issues/population (acessed on 17 july 2023).
- Food Agriculture Organization of the United Nations. Available on line: https://www.fao.org/nutrition/education/food-dietary-guidelines/background/sustainable-dietary-guidelines/en/ (acessed on 17 july 2023).
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H-B. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [CrossRef]
- Ueda, J.M.; Griebler, K.R.; Finimundy, T.C.; Rodrigues, D.B.; Veríssimo, L.; Pires, T.C.S.P.; Gonçalves, J.; Fer-nandes, I.P.; Pereira, E.; Barros, L. Heleno, S.A.; Calhelha, R.C. Polyphenol Composition by HPLC-DAD-(ESI-)MS/MS and Bioactivities of Extracts from Grape Agri-Food Wastes. Molecules 2023, 28, 7368. [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [CrossRef]
- Pan, F., Liu, Y., Liu, J., Wang, E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC advances 2019, 9(19), 10842–10853. [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [CrossRef]
- Ju, Y.-L.; Liu, M.; Zhao, H.; Meng, J.-F.; Fang, Y.-L. Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries. Molecules 2016, 21, 1354. [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Grape pomace, an undervalued by-product: industrial reutilization with-in a circular economy vision. Rev Environ Sci Biotechnol 2023, 22, 739-773. [CrossRef]
- Costa, G. N., Tonon, R. V., Mellinger-Silva, C., Galdeano, M. C., Iacomini, M., Santiago, M. C., Almeida, E. L., Freitas, S. P. (2019). Grape seed pomace as a valuable source of antioxidant fibers. Journal of the Science of Food and Agriculture 2019, 99(10), 4593–4601. [CrossRef]
- Pereira, P.; Palma, C.; Ferreira-Pêgo, C.; Amaral, O.; Amaral, A.; Rijo, P.; Gregório, J.; Palma, L.; Nicolai, M. Grape Pomace: A Potential Ingredient for the Human Diet. Foods 2020, 9(12), 1772. [CrossRef]
- Silva, A., Silva, V., Igrejas, G., Aires, A., Falco, V., Valentão, P., & Poeta, P.). Phenolic compounds classifica-tion and their distribution in winemaking by-products. Eur Food Res Technol 2023, 249(2), 207-239. [CrossRef]
- Strickland, J.D.H. and Parsons, T.R. A Practical Handbook of Seawater Analysis. 2nd edition. Ottawa, Canada, Fisheries Research Board of Canada. Bulletin Fisheries Research Board of Canada, 1972, 167, 310. [CrossRef]
- Koroleff, F. Determination of ammonia and Determination of silicon. In Methods of Seawater Analysis; Grasshoff, K., Ed.; Verlag Chemie: New York, 1976, 126−158.
- International Organization for Standardization ISO/TS14256-1 Soil quality — Determination of nitrate, nitrite and ammonium in field-moist soils by extraction with potassium chloride solution. ISO: Geneva, Switzerland, 2003.
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31−36. [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dep. of Agric. Circ. 939, 1954.
- Instituto Português da Qualidade. Norma NP 518 Comissão Técnica; C410/CT41 - Cereais e leguminosas. Determinação do teor de cinza. Processo de incineração a 550ºC. Instituto Português da Qualidade: Lisboa, Portugal,1986.
- Instituto Português da Qualidade. Norma NP 875; Comissão Técnica: C 370/CT 37. Alimentos para animais. Determinação do teor de humidade (3ª edição). Instituto Português da Qualidade: Lisboa, Portugal, 1994.
- International Organization for Standardization. ISO 1871¸2009 Food and feed products — General guidelines for the determination of nitrogen by the Kjeldahl method Comissão Técnica ISO/TC34(ICS67.050. ISO: Geneva, Switzerland, 2009.
- International Organization for Standardization ISO 8292:2008 Animal and vegetable fats and oils - Determination of solid fat content by pulsed NMR - Part 1: Direct method . ISO: Geneva, Switzerland, 2008.
- Goering, H.R.; Van Soest, P.J. Forage fiber analyses. Agricultural Handbook No. 379. United States Department of Agricultural, Washington, 1970; pp 1-20.
- Mongeau, R.; Brooks, S.P.J. Dietary Fiber: Determination. In Caballero, B., Finglas, P. M., & Toldrá, F. Encyclopedia of Food and Health. Academic Press, 2016, pp 383-391. [CrossRef]
- Sanders, P., Ernste-Nota, V., Visser, K., van Soest, J., Brunt, K. The Determination of Sugars in Dairy Products: Development of a New Standard Method for the International Dairy Federation and the Internal Organization for Standardization. Journal of AOAC International 2017, 100(5), 1577–1581. [CrossRef]
- Instituto Português da Qualidade. Norma NP-1419. Comissão Técnica: C 310/CT 31. Frutos, Produtos Hortícolas e Seus Derivados. Determinação de Açúcares Totais, dos Açúcares Redutores e dos Açúcares não Redutores (Sacarose). Técnica de Munson and Walker. Instituto Português da Qualidade: Lisboa, Portugal, 1987.
- Element, C. A. S. Method 3051 A microwave assisted acid digestion of sediments, sludges, soils, and oils. Z. Für Anal. Chem 2007, 111, 362-366.
- Cordeiro R, Rosa C, Bettencourt da Silva R. Measurements recovery evaluation from the analysis of independent reference materials: Analysis of different samples with native quantity spiked at different levels. Accredit. Qual. Assur. 2018; 23, 57–71.
- Palma, C.; Morgado, V.; Bettencourt da Silva, R. J. N. B. Top-down evaluation of matrix effects uncertainty. Talanta 2019, 192, 278−287. [CrossRef]
- Pérez-Ramírez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Comprehensive Charac-terization of Extractable and Nonextractable Phenolic Compounds by High-Performance Liquid Chromatog-raphy–Electrospray Ionization–Quadrupole Time-of-Flight of a Grape/Pomegranate Pomace Dietary Sup-plement. J. Agric. Food Chem. 2018, 66(3), 661-673. [CrossRef]
- Muchagato Maurício, E., Rosado, C., Duarte, M. P., Fernando, A. L., Díaz-Lanza, A. M. Evaluation of Industrial Sour Cherry Liquor Wastes as an Ecofriendly Source of Added Value Chemical Compounds and Energy. Waste Biomass Valor 2020, 11, 201–210. [CrossRef]
- Şen, U.; Viegas, C.; Duarte, M.P.; Maurício, E.M.; Nobre, C.; Correia, R.; Pereira, H.; Gonçalves, M. Maceration of Waste Cork in Binary Hydrophilic Solvents for the Production of Functional Extracts. Environments 2023, 10, 142. [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Brás, N. F.; Ga-meiro, P.; Coelho, P.; Bessa, L. J.; Oliveira, J.; Fernandes, I. Anthocyanin-Related Pigments: Natural Allies for Skin Health Maintenance and Protection. Antioxidants 2021, 10(7), 1038. [CrossRef]
- Teixeira, N.; Mateus, N. de Freitas, V.; Oliveira, J., Wine industry by-product: Full polyphenolic characterization of grape stalks. Food Chem. 2018, 268, 110-117. [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; Nziguheba, G.; Ojima, D.; Palm, C.A.; Robertson, G.P.; Sanchez, P.A.; Townsend, A.R.; Zhang, F. S. Agriculture. Nutrient imbalances in agricultural development. Science 2009. 324(5934), 1519–1520. [CrossRef]
- Asmala, E.; Saikku, L.; Vienonen, S. Import-export balance of nitrogen and phosphorus in food, fodder and fertilizers in the Baltic Sea drainage area. Science Total Environ, 2011 409(23), 4917–4922. [CrossRef]
- Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., & Hens, L. (2016). Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health, 2016 4, 148. [CrossRef]
- Havlin, J. L., Tisdale, S. L., Nelson, W. L., & Beaton, J. D. (2020). *Soil Fertility and Fertilizers: An Introduction to Nutrient Management* (9th ed.). Pearson.
- Jones Jr, J. B. (2012). *Plant Nutrition and Soil Fertility Manual* (2nd ed.). CRC Press.
- Schnepf, A., & Leitner, D. Soil Phosphorus: Life, Death, and Resurrection. Plants 2020, 9(2), 267. [CrossRef]
- Morton, L. W., and St. John, R. A. (2020). Sustainable Horticulture in Semiarid Dry Lands. Springer.
- Alfaia, C.M.; Costa, M.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A.M. Use of Grape By-Products to Enhance Meat Quality and Nutritional Value in Monogastrics. Foods 2022, 11, 2754. [CrossRef]
- Rockenbach, I. I., Gonzaga, L. V., Rizelio, V. M., Gonçalves, A. E. D. S. S., Genovese, M. I., Fett, R. Phenolic compounds content and antioxidant activity in seed and skin of grape pomace from four grape varieties cultivated in Southern Brazil. Food Chem. 2011, 127 174–179. [CrossRef]
- Zhao, L., Huang, Y., Du, M. Farm animals for studying muscle development and metabolism: dual purposes for animal production and human health. Animal Frontiers 2019, 9(3), 21–27. [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Anim. 2018, 12, 246–255. [CrossRef]
- Taladrid, D., Rebollo-Hernanz, M., Martin-Cabrejas, M. A., Moreno-Arribas, M. V., Bartolomé, B. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants (Basel, Switzerland) 2023, 12(4), 979. [CrossRef]
- Jha, R., Mishra, P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: a review. J Animal Sci Biotechnol 2021, 12, 51. [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [CrossRef]
- Almanza-Oliveros, A., Bautista-Hernández, I., Castro-López, C., Aguilar-Zárate, P., Meza-Carranco, Z., Rojas, R., Michel, M. R., Martínez-Ávila, G. C. G. Grape Pomace-Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods (Basel, Switzerland) 2024, 13(4), 580. [CrossRef]
- Wallace, T. C. Anthocyanins in Cardiovascular Disease. Advances in Nutrition 2011, 2(1), 1-7. [CrossRef]
- Tresserra-Rimbau, A., Lamuela-Raventos, R. M., Moreno, J. J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochemical pharmacology 2018, 156, 186–195. [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [CrossRef]
- Scalbert, A., Williamson, G. Dietary intake and bioavailability of polyphenols. Journal of Nutrition 2020, 130, 2073S-2085S. [CrossRef]
- 56. Liang Zhao, Yan Huang, Min Du, Farm animals for studying muscle development and metabolism: dual purposes for animal production and human health, Animal Frontiers, Volume 9, Issue 3, July 2019, Pages 21–27, . [CrossRef]
- Moya, P. J., Fernández-Panchón, M. S., & de la Hoz, L. (2021). Vitis vinifera Leaves Extracts as Natural Preservatives in Cosmetic Formulations. Molecules 2020, 26(14), 4261. [CrossRef]
- Kyraleou, M., Kallithraka, S., Theodorou, N., Teissedre, P. L., & Kotseridis, Y. (2020). Changes in tannin composition of Syrah grape skins and seeds during fruit ripening under contrasting water conditions. Molecules, 25(18), 4325. [CrossRef]
- Alfaia, C. M., et al. (2022). Foods, 11(18), 2754. [CrossRef]
- Serra, X., et al. (2019). Use of grape by-products as a source of dietary fibre and phenolic compounds in growing lambs. Animal Feed Science and Technology, 247, 24-33. [CrossRef]
- Fernández-López, J., et al. (2020). A Circular Economy Model of Green Technologies for Agri-food By-products Based on Bio-refineries. Waste and Biomass Valorization, 11, 4643–4657.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).