Introduction
Acute kidney injury (AKI) is a common and threatening condition among critically ill children, with some reports indicating mortality rates between 11% and 64% for pediatric patients requiring kidney replacement therapy [
1,
2]. Despite a variety of conditions that cause AKI, children, especially infants, submitted to cardiac surgery under cardiopulmonary bypass (CPB) are at increased risk of developing kidney failure. Accordingly, in a cohort including only infants, 56.1% of the patients submitted to CPB developed AKI within 5 days of surgery [
3].
Although not yet completely understood, AKI after CPB (AKI-CPB) presents a very unique pathophysiology. The current understanding about the pathogenesis of the condition states that AKI-CPB is anchored in the concomitant occurrence of, at least, four main processes: reduced kidney perfusion pressure, hemolysis, activation of proinflammatory pathways, and formation of microemboli [
4]. Firstly, CPB exposes the patients’ kidneys to a decrease of up to 30% in its perfusion pressure, a fact that, alongside CPB-induced hemolysis, favors ischemic damage to the kidney parenchyma [
5]. Simultaneously, CPB inherently promotes the development of microemboli, formations that are small enough to evade the bypass filters and damage kidney capillaries [
4]. Finally, these pathological phenomena, in association with the systemic inflammatory response experienced by the CPB patient, trigger ischemia-reperfusion injury of the kidneys, potentially leading to AKI-CPB [
4].
The definition of AKI has, since 2012, been unified by the Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group through the KDIGO Criteria as a predefined increase in serum creatinine (SCr) or decrease in urine output [
6]. As stated previously, oliguria is an important parameter for the identification of AKI. In addition, since oliguria is the clinical repercussion of an already symptomatic AKI, this sign is inherently late. Despite being a very cost-effective and available biomarker, SCr is also not able to early detect AKI. The findings point that SCr might only indicate AKI-CPB in pediatric patients 48 hours after the beginning of the injury [
7,
8].
For these reasons, the assessment of new biomarkers that early detect AKI-CPB emerges as a critical strategy for improving the management of this set of patients. More specifically, the assessment of the molecule in urinary samples has some practical advantages in comparison to serum ones, especially when considering children submitted to cardiac surgery under CPB. Most of these patients, due to the expectancy of long postoperative ICU admission, will have a urinary catheter, which facilitates urine collection, even for serial assessments, and makes it less invasive than the obtention of serum samples.
In this context, the Liver-type Fatty Acid-binding Protein (L-FABP), a 14 kDa protein from the superfamily of lipid-binding proteins, emerges as a promising biomarker for the prediction, diagnosis, and prognosis assessment of kidney function in patients with AKI post-CPB. The
L-FABP gene is responsive to hypoxic stress, thus urinary excretion of L-FABP reflects the ischemic stress of proximal tubular cells, associating with the severity of the ischemia [
9,
10]. In this regard, a prospective study with 1273 cardiac intensive care unit (ICU) patients, with a mean age of 68 years, identified urinary L-FABP (uL-FABP) as an independent predictor of AKI, and the use of this biomarker was able to improve early prediction of AKI [
11]. Therefore, the assessment of L-FABP levels emerges as a potential strategy for the prediction and early diagnosis of AKI post-CPB.
Hence, this systematic review and meta-analysis aimed to evaluate the potential value of uL-FABP in early predicting AKI among infants submitted to cardiac surgery with cardiopulmonary bypass.
Methods
Protocol Design and Registration
The research protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), identified as CRD42022318748. Throughout the entire development process of this review, adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [
12] (PRISMA) recommendations was rigorously maintained.
Eligibility Criteria
Observational studies employing a case-control, cohort, or cross-sectional design, as well as diagnostic test accuracy studies, were included in this systematic review. The focus was on assessing the predictive capability of uL-FABP for early detection of post-surgical AKI in pediatric patients (under 19 years of age) undergoing cardiac surgery with CPB. Articles published in English, Spanish, French, and Portuguese were considered for appropriate screening. Exclusion criteria were: studies including only adult population (older than 19 years), studies that did not analyze the development of AKI, studies with patients solely undergoing surgery without CPB, and studies that did not align with the research question.
Study Selection and Data Extraction
Following the systematic search and removal of duplicate records, six independent researchers proceeded to screen articles based on their titles and abstracts, obeying the predefined inclusion and exclusion criteria. Subsequently, the remaining articles underwent thorough reading in their entirety as the final step of the screening process. All papers that met the inclusion criteria were comprehensively reviewed.
Data extraction comprised the following variables: authorship, year, number of participants and grouping characteristics, study design, gender, age, characterization of data collection, inclusion and exclusion criteria, AKI definition criteria, and clinical outcomes (progression to dialysis, and death). Clinical and laboratory characteristics were also extracted and included: AKI stage stratification, prior CPB and CPB duration, pre-operatory risk adjustment in congenital heart surgery (RACHS) score, and uL-FABP values according to group and time after CPB. Finally, we also performed the extraction of synthesis metrics: area under the ROC curve (AUC) for the accuracy of AKI detection by uL-FABP, and correlation coefficients between uL-FABP levels and relevant clinical parameters.
Methodological Quality Evaluation of the Included Studies
Two researchers independently assessed the methodological quality of the included studies. We employed the JBI Critical Appraisal Checklist to evaluate methodological quality. Studies were assessed by the JBI Critical Appraisal Checklist for Diagnostic Test Accuracy Studies [
13] or by the JBI Critical Appraisal Checklist for Case-Control Studies [
14], according to study design. The detailed quality assessment process can be found in Supplementary
Table 1.
Statistical Analysis
The study data were extracted and organized using Microsoft Excel. In instances where statistical synthesis posed significant limitations, we opted for a narrative or graphical synthesis approach. Studies that reported the AUC for the accuracy of urinary L-FABP levels in detecting AKI were aggregated through a generic inverse variance multivariate meta-analysis. When original manuscripts lacked the 95% confidence intervals (95%CI) and standard error (SE) of AUC values, we computed the estimated 95%CI and SE utilizing the total number of included patients and the AUC values from each study individually. Both random and fixed/common models were evaluated and presented. Cochran’s Q-test and Higgins’ I² test were computed for each model. All data were presented as effect estimates with 95% confidence intervals. Analyses were conducted using the “meta” and “metafor” R statistical packages in R Studio, version 4.3.3, R Foundation for Statistical Computing, Vienna, Austria.
Results
Studies Design
Nine observational studies were included, published from 2008 to 2018 [
15,
16,
17,
18,
19,
20,
21,
22,
23]. In total, 1658 patients were analyzed. Seven studies were prospective [
15,
16,
18,
19,
20,
22,
23], and two were case-control studies [
17,
21]
. Sample sizes varied among the studies and ranged from 27 to 408 participants. 8 studies divided patients according to their gender [
15,
17,
18,
19,
20,
21,
22,
23]: out of 1250 patients, 638 (51.0%) were boys and 612 (49.0%) were girls. Characteristics of location, interventions, and sample sizes of the included studies are described in
Table 1.
Table 1.
Characteristics of location, interventions, and sample sizes of the included studies.
Table 1.
Characteristics of location, interventions, and sample sizes of the included studies.
| Author and Year |
Country |
Study Design |
Number of Participants |
Sample Sizes |
| AKI (%) |
Non-AKI (%) |
| Portilla et. al, 2008 |
United States |
Prospective Cohort |
40 |
21 (53%) |
19 (47%) |
| Krawczeski et. al, 2011 |
United States |
Prospective Cohort |
220 |
60 (27%) |
160 (73%) |
| Ivanisevic et. al, 2012 |
Serbia |
Case-control |
27 |
11 (41%) |
16 (59%) |
| Parikh et al., 2013 |
United States |
Prospective Cohort |
311 |
53 (17%) |
258 (83%) |
| Peco-Antic et. al, 2013 |
Serbia |
Prospective Cohort |
112 |
18 (16%) |
94 (84%) |
| Zappitelli et. al, 2015 |
Canada |
Prospective Cohort |
287 |
125 (43%) |
162 (57%) |
| Dong et. al, 2017 |
United States, China |
Prospective Cohort |
150 |
50 (33%) |
100 (67%) |
| Greenberg et. al, 2018 |
United States |
Prospective Cohort |
408 |
176 (43%) |
232 (57%) |
| Yoneyama et. al, 2020 |
Japan |
Prospective Cohort |
103 |
47 (46%) |
56 (54%) |
Inclusion criteria were similar between studies and comprised pediatric patients submitted to cardiac surgeries with the use of CPB. Exclusion criteria were disclosed for 8 articles and varied according to the paper, but severe and chronic kidney disease (CKD) was frequently reported: 7 of 8 studies excluded patients with CKD or kidney insufficiency [
15,
17,
18,
20,
21,
22,
23]; 2 studies excluded patients with congenital abnormalities of the kidney and urinary tract (CAKUT) [
15,
17]; 3 studies excluded patients who were taking nephrotoxic drugs [
15,
17,
18].
The definition of AKI also differed among studies. Seven articles utilized the KDIGO parameters of AKI, defined as a 50% increase or an absolute increase of 0.3 mg/dL in SCr levels [
16,
17,
18,
20,
21,
22,
23]. One article defined AKI as a 25% decrease of the glomerular filtration rate (GFR) estimated by Schwartz’s formula [
15]. One final paper defined AKI as the doubling of SCr or the need for acute dialysis [
19]. Finally, one paper divided AKI patients between two groups, according to disease progression [
16]. AKI with progression was defined as a progression in the AKI stage, or as a persisting stage 3 AKI, for at least 2 consecutive days. The baseline time-point was defined as the time immediately after surgery or at post-CPB ICU admission.
Population Data
Out of the 1658 patients, 561 (33.8%) developed AKI after CPB. The prevalence of AKI varied from 16.0% to 52.5% in the studies. In only one study the percentage of patients who developed AKI was over 50% [
18]. All studies divided AKI and non-AKI patients according to their sex. A total of 273 (44.6%) girls evolved with AKI versus 288 (45.1%) boys who developed the condition. In all articles, there was no significant difference in AKI occurrence between genders. The age of patients who developed AKI varied from 3.84 (0.36-28.8) months to 60.24 ±10.92 months. In non-AKI patients, the age varied from 7.2 (4.8-21.6) months to 57.6 ±56.4 months. Five studies [
19,
20,
21,
22,
23] revealed significant age difference between patients with and without AKI. Parikh’s et al.[
19], Zappitelli’s et al. [
22], and Yoneyama et al. [
23] studies showed a notably lower age for the AKI group when compared to the non-AKI group, Conversely, Krawczeski et al. [
20] and Dong et al. [
21] reported higher ages for the AKI group, when compared to the non-AKI group, being, respectively, 7.2 (4.8-21.6) months versus 17.4 (2.4-32.4) months. Details of baseline characteristics of the population are described in
Table 2.
Cardiopulmonary Bypass Duration
All nine studies compared the bypass duration between the AKI and non-AKI groups and showed a statistically significant differences. Patients who developed AKI had a mean time of bypass which ranged from 113 (84-172) to 240 (183-297) minutes. In comparison, children without AKI had a time of bypass from 82 ±9.7 to 126 ±65 minutes.
RACHS Score
The RACHS score was assessed in 8 studies [
15,
16,
17,
19,
20,
21,
22,
23], of which 7 compared results between the AKI and non-AKI groups. In these 7 studies, 364 out of 1210 (30.0%) patients developed AKI. The classification of RACHS scores varied between the AKI and non-AKI groups and was available for 1208 patients. A RACHS score of ≤ 3, indicating a lower complexity of cardiac surgery, was more commonly observed in the non-AKI group (95.8%) as compared to the AKI group (89.2%) in these seven studies.
Clinical Outcomes
The need for dialysis was assessed by 3 studies [
15,
16,
17], which comprised 547 children. Of those, 10 (1.8%) patients required dialysis. Only 2 studies compared the need for this type of kidney replacement therapy between AKI and non-AKI groups [
15,
17]. In these papers, the difference between the AKI and non-AKI groups was statistically significant, and all 7 patients who needed dialysis belonged to the AKI group, corresponding to 24.1% of all AKI patients. In the third study [
16], the need for dialysis was only compared between two AKI groups: AKI with progression and AKI without progression. All three patients requiring dialysis were in the group with disease progression, reaching a statistically significant difference between the groups. Additionally, only one study combined dialysis and death as a single composed outcome [
19]. In this study, 9 out of 303 (3.0%) children progressed to the composed outcome, with no statistically significant difference between groups (p=0.19).
The progression to death, alone, was analyzed in 4 studies [
15,
16,
17,
22], with a total of 834 children; of those, 14 (1.7%) died. Only 2 studies compared the progression to death between the AKI and non-AKI groups [
15,
17]. In one study, all four deaths occurred among patients with AKI, representing 16.7% of the AKI group, reaching statistical significant difference (p<0.0001). However, in the second study, two deaths occurred: one among AKI patients and one among non-AKI patients, indicating no significant difference between the groups. In the third study [
16], the comparison focused solely on the need for dialysis between two AKI groups: AKI with progression and AKI without progression. However, there was no statistically significant difference in the occurrence of death between the two groups.
Urinary L-FABP
Six studies described levels of uL-FABP in patients with and without AKI at various time points after CPB surgery [
16,
18,
19,
20,
21,
23]. Five of these studies compared uL-FABP levels between patients who developed AKI and those who did not [
18,
19,
20,
21,
23]. The remaining study focused on comparing uL-FABP levels between AKI patients who showed disease progression and those who did not [
16].
At baseline, two studies [
15,
23] revealed significantly elevated levels of uL-FABP among patients with AKI compared to those without AKI (p<0.05; p<0.01). However, at this time point, there was no difference in uL-FABP levels between groups in four studies [
17,
18,
20,
21]. At 2 hours post-CPB, two independent studies reported higher uL-FABP levels in the AKI group. Nonetheless, this difference was not observed in two other studies [
20,
21]. At 4 hours post-CPB, two separate studies [
18,
23] indicated significantly higher levels of uL-FABP in the AKI group compared to the non-AKI group (p<0.05; p<0.01). Similar results were observed at 6 hours post-CPB when four separate studies [
15,
17,
20,
21] reported elevated levels of uL-FABP in the AKI group compared to the non-AKI group (p<0.05; p<0.0001; p<0.05; p<0.05). At the 12-hour time-point, four distinct studies [
18,
20,
21,
23] consistently reported substantially elevated levels of uL-FABP in the AKI group compared to the non-AKI group. Finally, at 24 hours post-CPB, four studies identified higher levels of uL-FABP in the AKI group [
15,
20,
21,
23]. However, one study reported no difference between the two groups at this time point [
17].
Two studies identified higher uL-FABP levels in the AKI group in the time interval of 0 to 6 hours post-CPB (P<0.05) [
19,
22] and one study also identified this difference in the time interval of 6 to 12 hours post-CPB [
19]. One study [
16] compared uL-FABP levels between AKI patients who showed disease progression and those who did not and pointed out that elevated levels of uL-FABP were significantly higher in AKI patients with disease progression (p<0.05). The peak uL-FABP values varied among the studies, occurring at different time-points, ranging from 4 to 12 hours after the procedure. The values at the peak also exhibited significant variation, with reported values ranging from 264.76 (175.90-398.50) ng/ml to 791 ±349 ng/ml.
Correlations of uL-FABP with Relevant Clinical Parameters
The reviewed studies also established several correlations between uL-FABP levels and various clinical parameters of the included patients. The length of hospital stay showed significant correlations with uL-FABP levls at 4 hours [
18] (r = 0.57825; p =0.0017), 6 hours [
15,
17,
20] (r = 0.722; p<0.0001; er = 0.248, p<0.05 and r = 0.37; p < 0.05 ), 12 hours [
18] (r = 0.53568; p =0.0004), and 24 hours [
15] (r = 0.424; p< 0.05) post-CPB.
The percentage change in postoperative serum creatinine correlated with uL-FABP levels at 2 hours [
15,
17] (r = 0.486; p < 0.01; r=0.680, p=0.001)
, 4 hours [
18] (r = 0.465; p= 0.0025), 6 hours [
15,
20] (r=0.567; p<0.01; r=0.33, p<0.05), 12 hours [
18] (r =0.479; p=0.0017) and 24 hours [
15] (r =0.466; p< 0.05) post-CPB. Furthermore, urinary L-FABP also demonstrated correlations with CPB time at 2 hours [
15,
17] (r= 0.509; p<0.05; r=0.408, p=0.045) and 6 hours [
17,
20] (r=0.498, p=0.036; r = 0.33, p<0.05), aortic cross-clamping time at 2 hours [
15,
17] (r=0.650; p<0.01; r=0.536, p=0.032), and the Risk Adjustment for Congenital Heart Surgery (RACHS) score at 2 hours [
15] (r = 0.785; p < 0.01) and 6 hours [
20] (r = 0.21, p<0.05) post-surgery.
Accuracy of uL-FABP to Predict Post-CPB AKI
All nine studies [
15,
16,
17,
18,
19,
20,
21,
22,
23] examined the predictive potential of uL-FABP levels for AKI development at various post-CPB time points.
Ivanisevic [17] and colleagues pointed out that, when combining the uL-FABP levels with a clinical model comprised by age, sex, weight, CPB time, and aorta clamp time, the predictive ability of the model was improved to an AUC of 0.94 at 2 hours, 0.99 at 6 hours, and 0.97 at 24 hours post-CPB [
17]. The predictive ability of uL-FABP combined with the clinical model was higher than that of the clinical model alone. Similarly,
Krawczeski [20] and colleagues pointed out that, when combined with a clinical model including age and CPB time, uL-FABP levels predicted AKI development with an AUC of 0.73 at 2 hours, 0.77 at 6 hours, 0.81 at 12 hours, and 0.79 at 24 hours. However, combining these parameters did not improve predictive ability beyond that of the clinical model alone.
Moreover, Portilla and colleagues [
18] identified that, at 4 hours post-CPB, uL-FABP levels could predict, alone, the development of AKI with an AUC of 0.81 (p=0.0265). Two studies [
19,
22] showed that uL-FABP levels were able to predict the development of AKI with AUCs of 0.70 ±0.04 and 0.65 (0.58–0.71) in the time interval of 0 to 6 hours post-CPB and with an AUC of 0.71 ±0.04 in the time interval of 6 to 12 hours post-CPB [
19]. Furthermore, uL-FABP levels were also found to be predictive of AKI progression, demonstrating an optimism-corrected AUC of 0.71[
16] (0.6, 0.8). The detailed AUCs of uL-FABP to predict AKI at different post-CPB time-points are described in
Table 3.
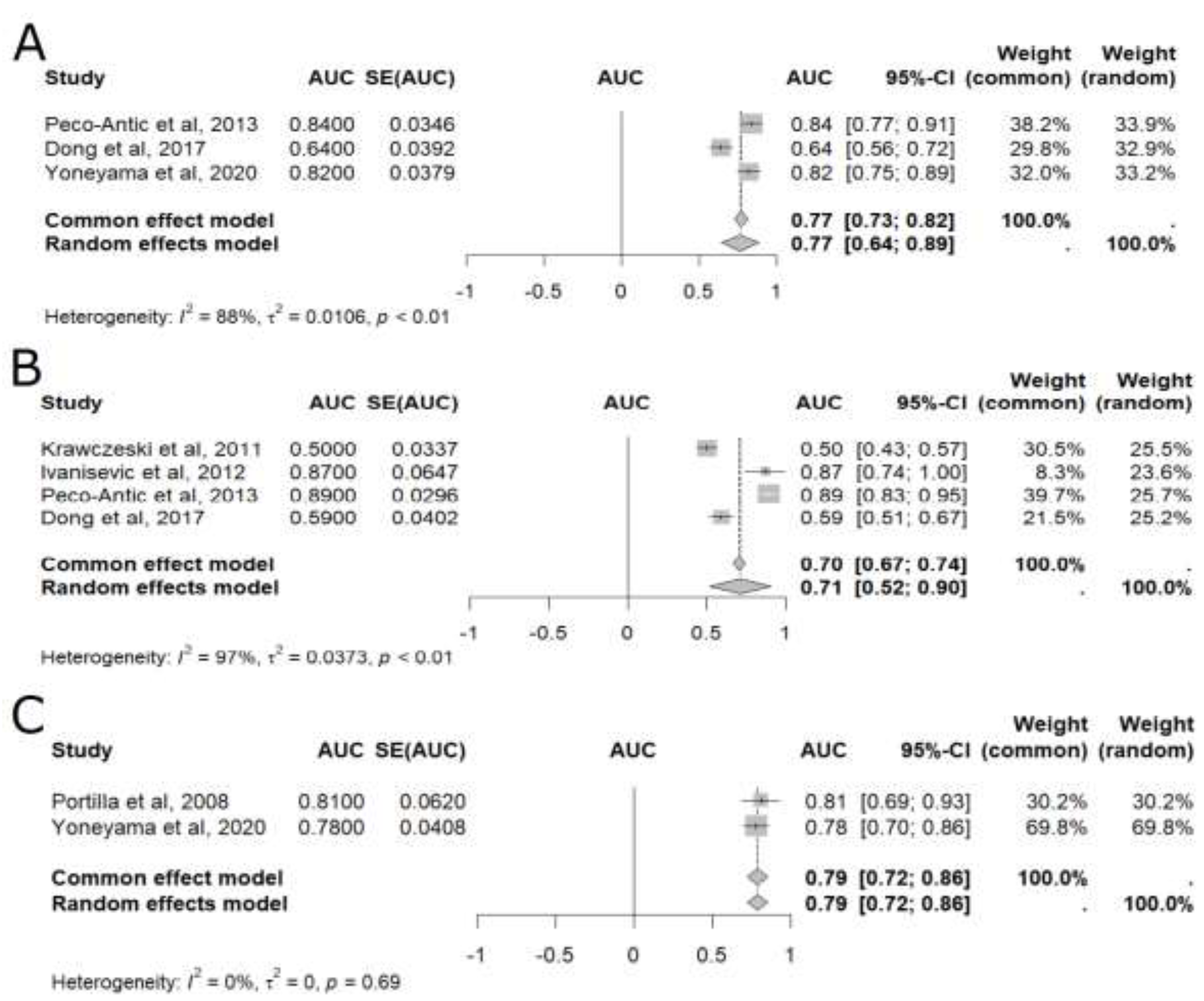
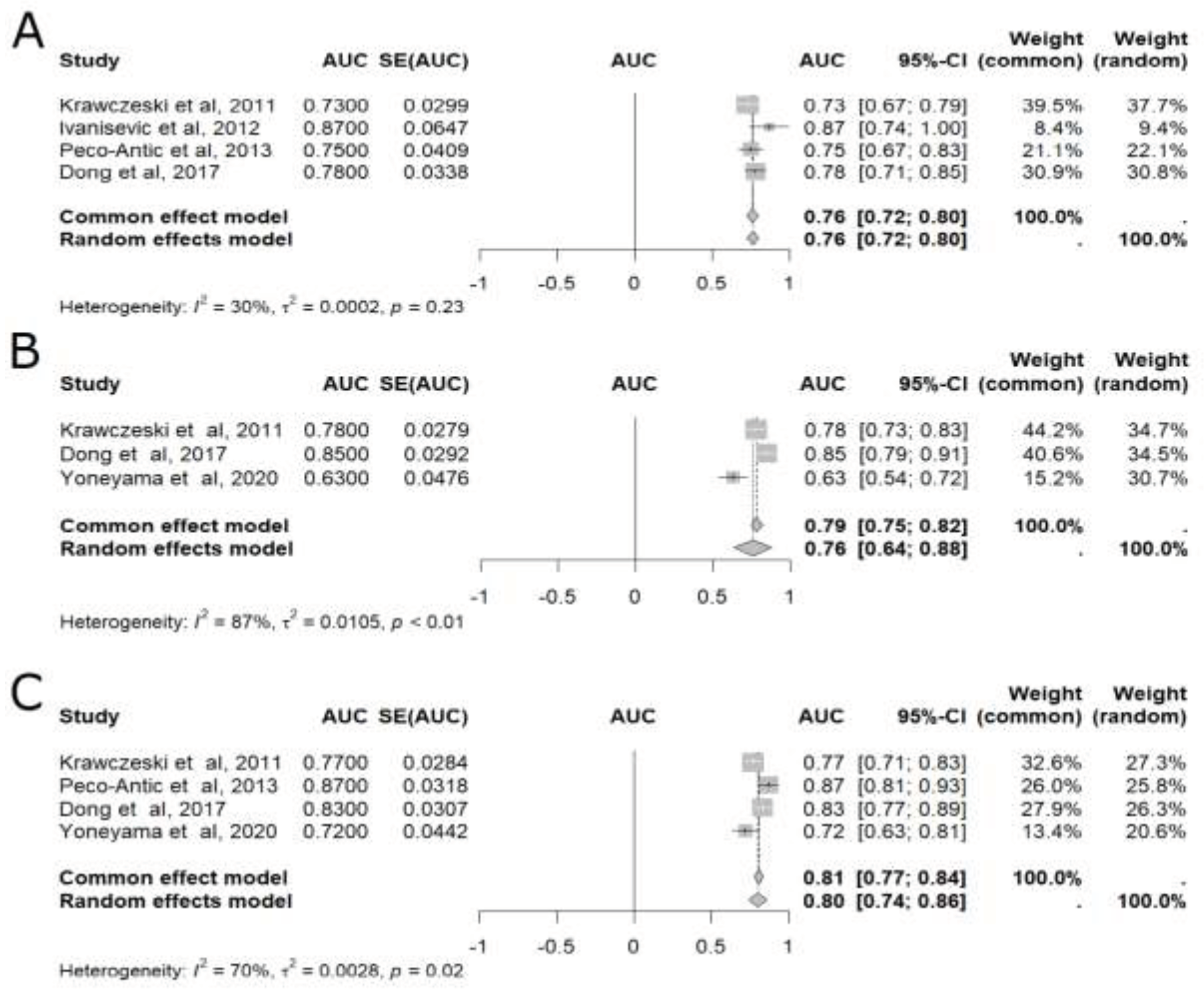
In the meta-analysis of pooled AUC results [
15,
17,
18,
20,
21,
23], uL-FABP was able to predict AKI at six different time points: baseline, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours after CPB (
Figure 2 and
Figure 3), with 24 hours presenting the highest accuracy (AUC=0.80, 95%CI: 0.74–0.86), as shown in
Figure 3 [
15,
20,
21,
23]. Although less accurate, uL-FABP still presented satisfactory results at baseline [
15,
21,
23] (AUC: 0.77, 95% CI 0.64-0.89; n= 365), 2 hours [
15,
17,
20,
21] (AUC=0.71, 95%CI: 0.52–0.90; n= 509), and 4 hours [
18,
23] (AUC = 0.79, 95%CI: 0.72-0.86; n= 143), as shown in
Figure 2. Significant results were also found at 6 hours [
15,
17,
20,
21] (AUC=0.76, 95%CI: 0.72–0.80; n= 509) and 12 hours [
20,
21,
23] (AUC= 0.76, 95% 0.64-0.88; n= 473), as also displayed in
Figure 3.
Figure 1.
Flow diagram depicting the screening process performed.
Figure 1.
Flow diagram depicting the screening process performed.
Figure 2.
Meta-analysis of the area under the ROC curve (AUC) values of studies that evaluated the accuracy of plasmatic liver-type fatty acid-binding protein (L-FABP) in detecting acute kidney injury (AKI) in children after cardiopulmonary bypass (CPB). (A) Baseline meta-analysis; (B) Two-hour meta-analysis; (C) Four-hour meta-analysis. 95% CI: 95% confidence interval; AUC: area under the ROC curve; SE: standard error of the mean.
Figure 2.
Meta-analysis of the area under the ROC curve (AUC) values of studies that evaluated the accuracy of plasmatic liver-type fatty acid-binding protein (L-FABP) in detecting acute kidney injury (AKI) in children after cardiopulmonary bypass (CPB). (A) Baseline meta-analysis; (B) Two-hour meta-analysis; (C) Four-hour meta-analysis. 95% CI: 95% confidence interval; AUC: area under the ROC curve; SE: standard error of the mean.
Figure 3.
Meta-analysis of the area under the ROC curve (AUC) values of studies that evaluated the accuracy of plasmatic liver-type fatty acid-binding protein (L-FABP) in detecting acute kidney injury (AKI) in children after cardiopulmonary bypass (CPB). (A) Six-hour meta-analysis; (B) Twelve-hour meta-analysis; (C) Twenty four-hour meta-analysis. 95% CI: 95% confidence interval; AUC: area under the ROC curve; SE: standard error of the mean.
Figure 3.
Meta-analysis of the area under the ROC curve (AUC) values of studies that evaluated the accuracy of plasmatic liver-type fatty acid-binding protein (L-FABP) in detecting acute kidney injury (AKI) in children after cardiopulmonary bypass (CPB). (A) Six-hour meta-analysis; (B) Twelve-hour meta-analysis; (C) Twenty four-hour meta-analysis. 95% CI: 95% confidence interval; AUC: area under the ROC curve; SE: standard error of the mean.
Quality Assessment
As for the quality assessment, diagnostic test studies (n=5) were evaluated by the JBI Critical appraisal checklist for diagnostic test accuracy studies [
13]. Five studies [
15,
18,
19,
22,
23] were considered of good quality and two [
16,
20] were considered of fair quality. The main defects were inappropriate exclusion (identified in 2 papers [
16,
20]), unclear information regarding whether the analysis of the reference standard test was blinded to the index test results (n=6) [
16,
18,
19,
20,
22,
23], and unclear information of whether the index test was interpreted blinded to the reference standard results (n=1) [
20]. Case-control studies (n=2) [
17,
21] were evaluated using the JBI Critical appraisal checklist for case-control studies [
14]. The studies were assessed as of good quality, but confounding factors and strategies to deal with those factors were not stated in either of them. The complete quality assessment can be found in Supplementary
Table 1 and
Table 2.
Discussion
AKI is a prevalent issue among pediatric patients undergoing cardiac surgery involving cardiopulmonary bypass (CPB), with an estimated incidence rate of 40% to 50% of all pediatric CPB procedures [
7]. In this sense, the early identification of AKI is crucial to improve patient outcomes within this specific group. However, the existing diagnostic criterion for AKI, which relies on SCr measurement and urinary output, is rather late. Consequently, the evaluation of novel biomarkers for AKI post-CPB has emerged as a promising strategy to enable early identification and subsequent treatment for these patients. Several biomarkers have been investigated for the early detection of AKI after surgeries with CPB. Our review focuses on uL-FABP obtained until 24 hours postoperatively.
A recent meta-analysis of diagnostic test accuracy has addressed the potential value of a group of biomarkers, including L-FABP, in serum and urinary samples of children submitted to cardiac surgery [
24]. However, our approach differs in some aspects from this previous study. First, our population is comprised exclusively of infants submitted to cardiac surgery with CPB; second, our approach focuses on levels of L-FABP in urinary samples only, not including serum measurements; and third, we focus on the early analysis of the biomarker, including data no later than 24 hours post-CPB.
Our data reinforce that, in the early hours after the kidney insult, uL-FABP levels are significantly increased in patients who will develop AKI. In our review, in patients that developed post-CPB AKI when compared to the non-AKI group, uL-FABP levels were already significantly increased at baseline [
23] and were consistently higher in the AKI group, at 4-, 6-, 12-, and 24-hours post-surgery [
15,
17,
18,
20,
21,
23]. Additionally, uL-FABP was found to reach peak concentration in the urine shortly after CPB, between 2- and 12-hours post-surgery [
15,
17,
18,
19,
20,
21], a finding that is in line with the capacity of uL-FABP to early indicate an increased risk of developing post-CPB AKI. Interestingly, uL-FABP was also significantly increased in patients who developed AKI stages 2 or 3 when compared to patients who exhibited AKI stage 1 or no AKI [
21]. Despite only one study addressing this type of direct comparison, this finding indicates a potential for urinary L-FABP to act both as a diagnostic and severity early biomarker for post-CPB AKI.
Levels of uL-FABP significantly correlated with various clinical parameters of severity, including length of hospital stay, percentage of change in postoperative serum creatinine, and pre-operative RACHS score. Correlations were also observed between uL-FABP levels and procedural parameters, such as CPB time, and aortic cross-clamping time. Despite most correlations were moderate, our data support the intrinsic relationship between uL-FABP and the magnitude of the acute insult to the kidneys, which ultimately results in AKI.
Our systematic review and meta-analysis suggest that early uL-FABP exhibits satisfactory accuracy to predict post-CPB AKI in children. In this matter, albeit assessed in few articles, uL-FABP obtained immediately after surgery (baseline uL-FABP) showed to be satisfactorily accurate to predict post-CPB AKI [
15,
21]. Urinary L-FABP was also valuable for prediction of AKI progression post-CPB [
16]. As a general tendency, the predictive capacity of uL-FABP increased in later post-CPB time points and reached its peak at 24 hours post-surgery, which is significantly earlier than the traditional biomarkers. The meta-analysis data reinforce this trend but, notably, uL-FABP alone presented significant and favorable accuracy in post-CPB time-points as early as immediately after surgery (baseline). In addition, although accuracy increased in later time-points, the meta-analysis shows that earlier post-CPB uL-FABP measurements had high accuracy and were not significantly different than the predictive capacity observed at the 24-hour threshold. Importantly, although only assessed by one study, uL-FABP associated with a robust clinical model presented excellent accuracy to predict AKI-CPB at very early post-surgery time-points. Despite the models comprising both uL-FABP and clinical parameters not resulting in a similarly high AUC in other papers, the association between this urinary biomarker and a robust clinical model may be a cost-effective way to reach consistent early detection of post-CPB AKI.
Although our systematic review and meta-analysis bring important considerations for the use of uL-FABP as a biomarker of post-CPB AKI in pediatric patients, our analysis harbors some limitations. First, as mentioned above, the limited number of studies using the same definitions for AKI increases the heterogeneity between the assessed clinical outcomes and hampers a more in-depth meta-analysis. Furthermore, the absence of some critical information in the manuscripts for the performance of the meta-analysis unfortunately reduced the overall number of included studies. This is especially true for the accuracy of uL-FABP at 4h post-CPB, which was only evaluated in two studies. Moreover, despite the overall good quality of the included studies, possible sources of bias were identified. In that sense, larger and well-designed studies are still needed to better evaluate the use of uL-FABP as an early biomarker in predicting post-CPB AKI.
Conclusion
In conclusion, uL-FABP emerges as a promising early biomarker for AKI following CPB in pediatric patients. Despite study limitations, its correlation with AKI development and severity suggests potential for enhancing timely intervention and improving patient outcomes. Moreover, results of the meta-analysis support that uL-FABP levels are capable of predicting AKI after CPB with good accuracy, especially after 24 hours of surgery. Nevertheless, further research is needed to standardize criteria and validate the clinical utility of uL-FABP.
Funding
This work was partially supported by Brazilian National Council of Research Development (CNPq – Grant # ), Coordination of High Education Level Personnel (CAPES), and Foundation of Research of Minas Gerais (FAPEMIG).
Authors’ contributions and acknowledgments
All authors contributed significantly to the conception, design, analysis, and interpretation of data. B.W., B.C.B., P.A.S.V.C., and A.C.S.S. conceptualized and designed the analysis. B.C.B., B.C.B., and A.D.S. were involved in data screening and extraction. B.W. and P.A.S.V.C. checked data extraction. B.W., P.A.S.V.C., and A.C.S.S. performed the statistical analysis. B.W., B.C.B., B.C.B., A.D.S., P.A.S.V.C., and A.C.S.S. were responsible for analysis and data interpretation. B.W., and B.S.B. evaluated the quality of the selected articles. B.W., B.C.B., B.C.B., and A.D.S. wrote the first draft. P.A.S.V.C. and A.C.S.S. made general supervision and revised the manuscript. All authors read and approved the final version of the manuscript. The order of authorship reflects a collective decision regarding the substantial intellectual contribution of each author to the study.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
- Hayes, L. W.; Oster, R. A.; Tofil, N. M.; Tolwani, A. J. Outcomes of Critically Ill Children Requiring Continuous Renal Replacement Therapy. J Crit Care 2009, 24 (3), 394–400. [CrossRef]
- Symons, J. M.; Chua, A. N.; Somers, M. J. G.; Baum, M. A.; Bunchman, T. E.; Benfield, M. R.; Brophy, P. D.; Blowey, D.; Fortenberry, J. D.; Chand, D.; Flores, F. X.; Hackbarth, R.; Alexander, S. R.; Mahan, J.; McBryde, K. D.; Goldstein, S. L. Demographic Characteristics of Pediatric Continuous Renal Replacement Therapy. Clinical Journal of the American Society of Nephrology 2007, 2 (4), 732–738. [CrossRef]
- Blinder, J. J.; Goldstein, S. L.; Lee, V.-V.; Baycroft, A.; Fraser, C. D.; Nelson, D.; Jefferies, J. L. Congenital Heart Surgery in Infants: Effects of Acute Kidney Injury on Outcomes. J Thorac Cardiovasc Surg 2012, 143 (2), 368–374. [CrossRef]
- Kumar, A. B.; Suneja, M.; Riou, B. Cardiopulmonary Bypass–Associated Acute Kidney Injury. Anesthesiology 2011, 114 (4), 964–970. [CrossRef]
- Ge, Y.; Behera, T. R.; Yu, M.; Xie, S.; Chen, Y.; Mao, H.; Xu, Q.; Zhao, Y.; Zhang, S.; Shen, Q. Higher Mean Arterial Pressure during Cardiopulmonary Bypass May Not Prevent Acute Kidney Injury in Elderly Patients Undergoing Cardiac Surgery. Int J Clin Pract 2022, 2022, 1–8. [CrossRef]
- Kellum, J.; Lameire, N.; Aspelin, P.; Barsoum, R.; Burdmann, E.; Goldstein, S. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl (2011) 2012, 2 (1), 1–138.
- Li, S.; Krawczeski, C. D.; Zappitelli, M.; Devarajan, P.; Thiessen-Philbrook, H.; Coca, S. G.; Kim, R. W.; Parikh, C. R. Incidence, Risk Factors, and Outcomes of Acute Kidney Injury after Pediatric Cardiac Surgery: A Prospective Multicenter Study*. Crit Care Med 2011, 39 (6), 1493–1499. [CrossRef]
- Ramesh, G.; Krawczeski, C. D.; Woo, J. G.; Wang, Y.; Devarajan, P. Urinary Netrin-1 Is an Early Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery. Clinical Journal of the American Society of Nephrology 2010, 5 (3), 395–401. [CrossRef]
- Susantitaphong, P.; Siribamrungwong, M.; Doi, K.; Noiri, E.; Terrin, N.; Jaber, B. L. Performance of Urinary Liver-Type Fatty Acid–Binding Protein in Acute Kidney Injury: A Meta-Analysis. American Journal of Kidney Diseases 2013, 61 (3), 430–439. [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A Novel Biomarker of Kidney Disease. Clinica Chimica Acta 2015, 445, 85–90. [CrossRef]
- Naruse, H.; Ishii, J.; Takahashi, H.; Kitagawa, F.; Nishimura, H.; Kawai, H.; Muramatsu, T.; Harada, M.; Yamada, A.; Motoyama, S.; Matsui, S.; Hayashi, M.; Sarai, M.; Watanabe, E.; Izawa, H.; Ozaki, Y. Predicting Acute Kidney Injury Using Urinary Liver-Type Fatty-Acid Binding Protein and Serum N-Terminal pro-B-Type Natriuretic Peptide Levels in Patients Treated at Medical Cardiac Intensive Care Units. Crit Care 2018, 22 (1), 197. [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009, 6 (7), e1000097. [CrossRef]
- Campbell JM; Klugar M; Ding S; Carmody DP; Hakonsen SJ; Jadotte YT; White S; Munn Z. Campbell JM, Klugar M, Ding S, Carmody DP, HakoChapter 9: Diagnostic Test Accuracy Systematic Reviews. In JBI Manual for Evidence Synthesis; Aromataris E, Munn Z, Eds.; 2020; Vol. 1.
- Moola S; Munn Z; Tufanaru C; Aromataris E; Sears K; Sfetcu R; Currie M; Qureshi R; Mattis P; Lisy K; Mu P-F. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris E, Munn Z, Eds.; 2020; Vol. 1.
- Peco-Antić, A.; Ivanišević, I.; Vulićević, I.; Kotur-Stevuljević, J.; Ilić, S.; Ivanišević, J.; Miljković, M.; Kocev, N. Biomarkers of Acute Kidney Injury in Pediatric Cardiac Surgery. Clin Biochem 2013, 46 (13–14), 1244–1251. [CrossRef]
- Greenberg, J. H.; Zappitelli, M.; Jia, Y.; Thiessen-Philbrook, H. R.; De Fontnouvelle, C. A.; Wilson, F. P.; Coca, S.; Devarajan, P.; Parikh, C. R. Biomarkers of AKI Progression after Pediatric Cardiac Surgery. Journal of the American Society of Nephrology 2018, 29 (5), 1549–1556. [CrossRef]
- Ivanišević, I.; Peco-Antić, A.; Vuličević, I.; Hercog, D.; Milovanović, V.; Kotur-Stevuljević, J.; Stefanović, A.; Kocev, N. L-FABP Can Be an Early Marker of Acute Kidney Injury in Children. Pediatric Nephrology 2013, 28 (6), 963–969. [CrossRef]
- Portilla, D.; Dent, C.; Sugaya, T.; Nagothu, K. K.; Kundi, I.; Moore, P.; Noiri, E.; Devarajan, P. Liver Fatty Acid-Binding Protein as a Biomarker of Acute Kidney Injury after Cardiac Surgery. Kidney Int 2008, 73 (4), 465–472. [CrossRef]
- Parikh, C. R.; Thiessen-Philbrook, H.; Garg, A. X.; Kadiyala, D.; Shlipak, M. G.; Koyner, J. L.; Edelstein, C. L.; Devarajan, P.; Patel, U. D.; Zappitelli, M.; Krawczeski, C. D.; Passik, C. S.; Coca, S. G. Performance of Kidney Injury Molecule-1 and Liver Fatty Acid-Binding Protein and Combined Biomarkers of Aki after Cardiac Surgery. Clinical Journal of the American Society of Nephrology 2013, 8 (7), 1079–1088. [CrossRef]
- Krawczeski, C. D.; Goldstein, S. L.; Woo, J. G.; Wang, Y.; Piyaphanee, N.; Ma, Q.; Bennett, M.; Devarajan, P. Temporal Relationship and Predictive Value of Urinary Acute Kidney Injury Biomarkers after Pediatric Cardiopulmonary Bypass. J Am Coll Cardiol 2011, 58 (22), 2301–2309. [CrossRef]
- Dong, L.; Ma, Q.; Bennett, M.; Devarajan, P. Urinary Biomarkers of Cell Cycle Arrest Are Delayed Predictors of Acute Kidney Injury after Pediatric Cardiopulmonary Bypass. Pediatric Nephrology 2017, 32 (12), 2351–2360. [CrossRef]
- Zappitelli, M.; Greenberg, J. H.; Coca, S. G.; Krawczeski, C. D.; Li, S.; Thiessen-Philbrook, H. R.; Bennett, M. R.; Devarajan, P.; Parikh, C. R. Association of Definition of Acute Kidney Injury by Cystatin C Rise with Biomarkers and Clinical Outcomes in Children Undergoing Cardiac Surgery. JAMA Pediatr 2015, 169 (6), 583–591. [CrossRef]
- Yoneyama, F.; Okamura, T.; Takigiku, K.; Yasukouchi, S. Novel Urinary Biomarkers for Acute Kidney Injury and Prediction of Clinical Outcomes After Pediatric Cardiac Surgery. Pediatr Cardiol 2020, 41 (4), 695–702. [CrossRef]
- Van den Eynde, J.; Schuermans, A.; Verbakel, J. Y.; Gewillig, M.; Kutty, S.; Allegaert, K.; Mekahli, D. Biomarkers of Acute Kidney Injury after Pediatric Cardiac Surgery: A Meta-Analysis of Diagnostic Test Accuracy. Eur J Pediatr 2022, 181 (5), 1909–1921. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).