1. Introduction
Immunotherapy has become an important clinical strategy in the treatment of cancer patients.
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that enhance antitumor immune activity by activating T-Cells [
1,
2].
The antitumor effects of ICIs have been demonstrated in several randomized clinical trials and ICIs are now available for the treatment of many malignant cancers such as lung cancer, melanoma, hepatocellular carcinoma, gastrointestinal cancer, etc.
Immune related adverse events (IRAEs) may be associated with ICIs and may occur at any time after initiation of ICIs treatment [
3].
Most IRAEs are mild and moderate and include skin rash, colitis, hepatitis, endocrine disorder, myositis, interstitial lung disorder, etc. [
3]. IRAEs involving nervous system are relatively uncommon and include Myastenia Gravis, Guillain-Barre syndrome, peripheral sensory motor neurophaty [
4].
ICIs associated autoimmune encephalitis is infrequent and this complication is more common during concurrent or sequential ICIs treatment and in patients with lung cancer [
5,
6]. Fifty patients with ICIs related autoimmune encephalitis were identified in a review of cases published from 2016 to 2022 [
4].
Herein, we report a case of autoimmune encephalitis in a patient with metastatic melanoma in complete remission after Pembrolizumab treatment.
2. Case Report
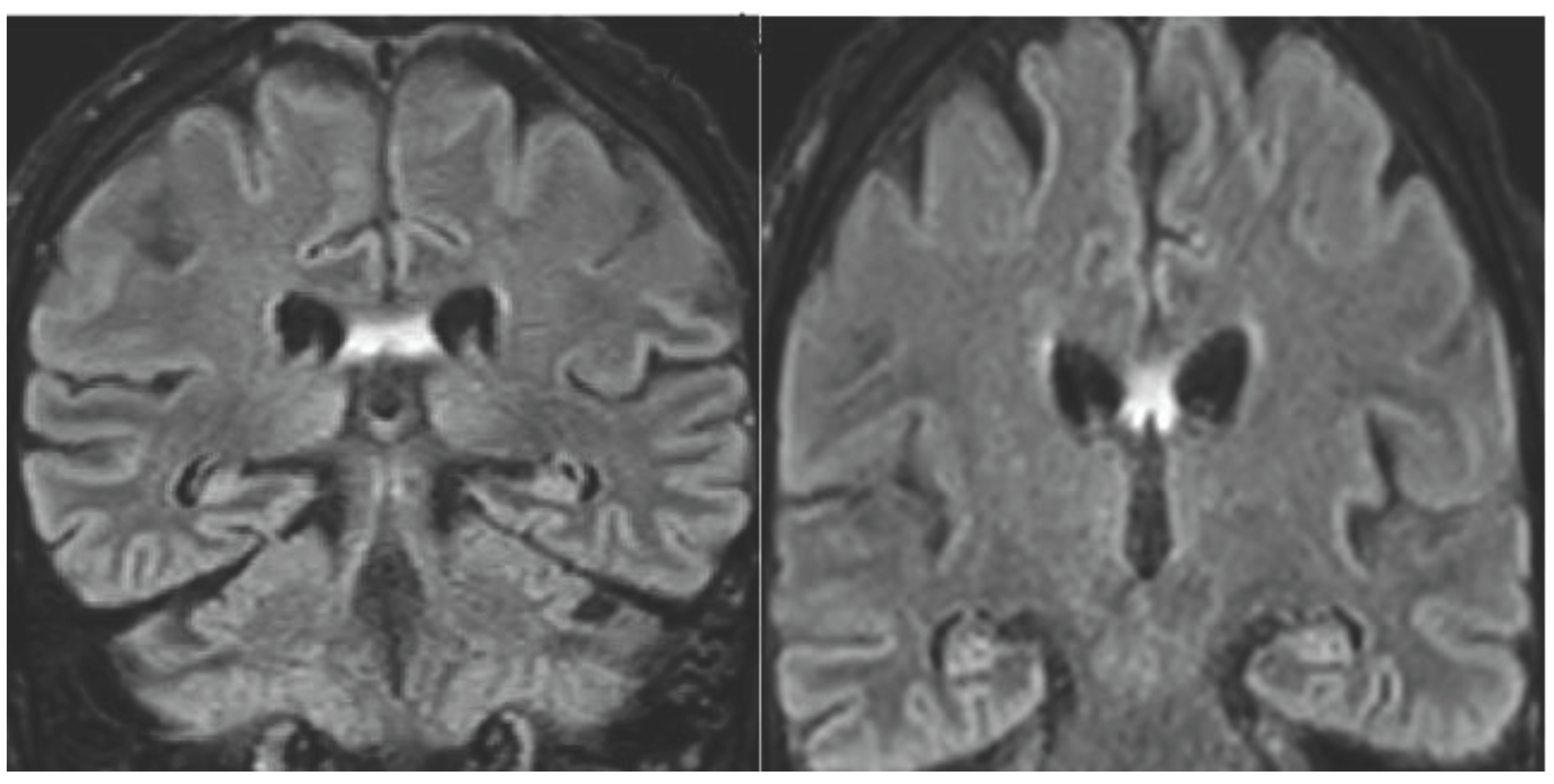
A 68-years-old man was referred to the neurologic department hospital of Piacenza (North Italy) in December 2023 with approximately a 3 months history of worsening gait, weakness, loss of appetite and confusional state. The patient was diagnosed with malignant melanoma on his left hand in April 2018. The melanoma lesion (negative for BRAF mutation) was excised and clinical staging was negative for metastasis. The patient, 3 years later, developed lung and liver metastasis, and a treatment with Pembrolizumab was then initiated in July 15, 2021. After six months of Pembrolizumab, restaging with Total Body Computerized Tomography (CT) and FDG-PET/CT showed complete remission. The treatment was continued for 14 months and then stopped for grade 3 diarrhea. The patient was in complete remission when, 10 months later the stop of Pembrolizumab therapy, he developed neurological symptoms confusion, altered mental state, progressive memory loss, gait disturbance. Neurological examination did not display focal deficits. Cognitive testing revealed MMS 18/30. Head Magnetic Resonance Imaging did not reveal brain metastasis, sing of carcinomatous meningitis or stroke and evidenced hyperintensity of the flair imaging in the fornix bilaterally (
Figure 1).
EEG showed slower asymmetric activity in the right cerebral hemisphere. Cerebrospinal fluid (CSF) examination showed signs of inflammation with a raised level of protein and lymphocyte count, without malignant cells. Viral PCR were negative. Anti-SOX1 antibodies were detected in serum and CSF. Total Body CT and PET/CT were negative for relapse of melanoma or other malignancy.
Autoimmune encephalitis was suspected as the patients has previously treated with Pembrolizumab and he did not fulfill the criteria for definite or possible paraneoplastic neurological syndrome since no evidence of malignant disease was found with Total Body CT and PET/CT, furthermore the clinical/laboratory findings were coherent with the recently published Canadian consensus guidelines for the diagnosis and treatment of autoimmune encephalitis in adults [
7]. The patient was then treated with 2 plasmapheresis sessions, then high doses of steroids (Methylprednisolone 1 g daily iv for 5 days followed by Prednisone 62.5 mg daily by mouth), intravenous immunoglobulins at 0.4 g/kg/day iv for 5 days. The neurological symptoms improved significantly and he underwent physical rehabilitation physiotherapy program, at the St. Antonino Clinic (Piacenza).
The patient was discharged from the St. Antonino Clinic in January 20, 2024, he is now well and his neurological status improved and he is followed for melanoma in complete remission and for autoimmune encephalitis with progressive improvement of neurologic symptoms with a performance status 1.
3. Discussion
Immune Checkpoint Inhibitors have demonstrated important results in many type of cancer. Inhibitors to cytotoxic T-Lymphocyte-associated antigen 4 (CTLA-4) and programmed death-1 (PD-1) and programmed death receptor ligand 1 (PD-L1) are currently used in patients with cancer [
1,
2,
8]. In these treatments, the primary mechanism of action is the generation of an immune response against cancer rather than performing actions directly at the tumor [
8]. This mechanism can allow a complete tumor regression associated with an improved quality of life compared with cytotoxic chemotherapy and or radiation.
It must be remembered that the 2018 Nobel Prize in Medicine and Physiology was awarded to James Allison and Tasuku Honjo for their studies and discoveries in cancer immunology based treatment [
9].
James Allison discovered the immunosuppressive molecule: cytotoxic T-lymphocyte antigen 4, and Tasuku Honjo discovered the programmed death molecule-1 on T cells.
The major escape mechanism of cancer cells is the suppression of T cell activation by CTLA-4 or by PD-1. The immune-checkpoint inhibitors promote the inhibition of these molecules and consequently the activation of the immune system against cancer cells.
ICIs offered a new and effective treatment against cancer; effectively before the advent of ICIs, the therapeutic standard for treating cancer was based on surgery, chemotherapy, radiation therapy and recently also on target therapy [
9]. Ipilinumab was the first immune checkpoint inhibitor available against cancer, this anti-CTLA-4 antibody was able to produce durable response and survival in patients with metastatic melanoma [
10]. ICIs allow a new mechanism against cancer cells that is based on an indirect approach rather than a direct effect determined by other treatments like chemotherapy or radiation. ICIs are monoclonal antibodies that act blocking the inhibitory molecule involved in regulation of immune response pathways such as programmed cell death 1 (e.g.
, Cemiplimab, Nivolumab, Pembrolizumab), or programmed cell death protein ligand 1 (e.g.
, Atezolizumab, Avelumab, Durvalumab) or cytotoxic T-lymphocyte-associated protein 4 (e.g.
, Ipilinumab). It must be emphasized that immune checkpoint inhibitors by activating T cells can trigger a large spectrum of autoimmune responses that are currently referred to as immune-related adverse events [
11]. These adverse events deeply differ from the toxicities caused by cytotoxic chemotherapy or by molecular targeted drugs, it must be kept in mind that time to toxicity my be delayed and not predictable as seen with conventional anticancer chemotherapy or radiation therapy [
11]. The incidence and pattern of IRAEs are different according to the type of ICIs used: hypophysitis and colitis were more frequent with Ipilinumab, while diabetes and pneumonia were more frequent with anti-PD1/PD-L1, in a large review that analyzed 16 485 patients treated with ICIs in randomized clinical trials [
12].
However real-world data indicate that the incidence of ICIs-related IRAEs may be higher than previously highlighted in clinical trials [
13]. Rates of toxicity are higher with CTLA-4 inhibitor when compared with PD1/PD-L1 inhibitors and IRAEs can affect any organ system [
13]. Risk factors for IRAEs are little known, however there is evidence that IRAEs are more frequent and occur faster in patients with pre-existing autoimmune disease, such as psoriasis, rheumatoid arthritis, vasculitis, intestinal bowel disease, systemic lupus erythematosus and patients with underlying autoimmune disease should be managed by a multidisciplinary team [
13]. The combination therapy of anti-CTLA-4 and anti PD1/PD-L1 favors a greater incidence and high-grade IRAEs [
13].
The management of IRAEs is based predominantly on retrospective study and glucocorticoids represent first line therapy for most ICIs induced adverse events; though glucocorticoids may be associated with reduction of anti-tumor effect of ICIs, low doses of steroids seems do not affect the tumor response and the survival of cancer patients treated with ICIs.
For patients with IRAEs not responsive to glucocorticoids, treatment with monoclonal antibodies targeting TNF-α such as Infliximab, was effective in autoimmune colitis and pneumonia during ICIs therapy [
13].
Timing of ICIs toxicity should be considered with caution since IRAEs can occur late during the treatment with ICIs or after treatment discontinuation [
13], as reported in our case.
This case describes Pembrolizumab-associated autoimmune encephalitis in a 68-year old male with metastatic melanoma in complete remission. It is very interesting that autoimmune encephalitis was diagnosed 10 months later the Pembrolizumab therapy cessation.
In our patient the presence of anti-SOX1 antibody was detected in both serum and CSF as reported in a previous cases of Immune Checkpoint Inhibitors associated autoimmune encephalitis [
4].
In adult anti-SOX1 antibodies are classified as onconeural antibodies and were firstly reported in patients with Lambert-Eaton Myasthenic syndrome (LEMS) small cell lung cancer (SCLC) and were considered a marker of SCLC related paraneoplastic syndrome [
14]. The main clinical manifestations of LEMS are characterized by predominantly proximal muscle weakness, absent or very reduced tendon reflex, other symptoms are constipation, dry mouth, erectile dysfunction [
14].
Symptom of LEMS can precede by months or even years the diagnosis of small cell lung cancer. The detection of LEMS related antibodies favored, in recent years, the diagnosis of this rare autoimmune neuromuscolar junction disorder and a series of report have demonstrated that SOX-1 antibodies, called initially anti-glial nuclear antibody (AGNA) are associated with Lambert-Eaton myasthenic syndrome [
14,
15]. However, subsequently anti-SOX1 antibodies also were detected in patients with various neurological disorders, predominantly with immune-mediated diseases [
15] as in patients with ICIs associated autoimmune encephalitis [
4].
Recently SOX-1 antibodies were detected in a 3-year-old girl, that presented with ataxia and dysmetria, she had a recent varicella infection. Tests performed: magnetic resonance imaging of the brain and spinal cord, EEG, blood and urine tests, and lumbar puncture did not show signs of encephalitis, only anti-SOX1 antibodies were identified in both serum and CSF. The patient showed favorable clinical course, with rapid improvement of symptoms. This case report represents that SOX-1 antibodies may be found also in childhood after viral infections like varicella [
16]. Within this cases of ICIs relevated encephalitis, Gao Y. et al. [
4] reviewing English literature in the Pub Med database published to 2022 found 50 cases of ICIs-associated autoimmune encephalitis, the majority of these cases had lung cancer 20/50 (40%). Interestingly, the time from the beginning of immune checkpoint inhibitor therapy to autoimmune symptoms onset ranged from 4 days to 18 months (median 3 months). In this review of the literature the treatments performed for autoimmune encephalitis were steroids, intravenous Immunoglobulins, plasmapheresis and Rituximab. Thirty-one patients / 50 (62%) improved, 13 patients / 50 (26%) did not improve and 6 patients (12%) died. Our patient was treated with plasma exchange, high doses of steroids and intravenous Immunoglobulins with a slow, but progressive improvement. More recently Fonesca E. et al. [
6] reported in a retrospective cohort study, in Spain, neurological adverse events related to immune-checkpoint inhibitors and included 64 patients in their study. Forty-five / 64 (70,3 %) patients showed encephalopathy. In these patients neurological symptoms developed very soon (median 8 weeks) after treatment with an immune-checkpoint inhibitors when compared with our case in which neurological symptoms developed 10 months after cessation the treatment with Pembrolizumab.
In patients reported by Fonesca et al. [
6] twelve / 45 patients with encephalopathy (27%) had definite paraneoplastic or autoimmune encephalitis, 24 / 45 (53%) had encephalitis without antibodies and 9 / 45 (20%) showed encephalopathy without central nervous system (CNS) inflammation changes. Around 40% of the entire group of patients died during the study period and 12 of the 27 patients who died, the cause of death was the neurological immune-related adverse event, and interestingly most of death occurred during the first month after the onset of symptoms related to adverse events. The mortality risk was associated with lung cancer when compared with other cancers and in patients with encephalopathy without evidence of CNS inflammation or combined myocarditis, myasthenia and myositis [
6].
Farina et al. [
17] performed a large multicenter retrospective cohort study of patients in France in order to asses neurological-ICIs-adverse events and in this series of 147 cases, 51 patients (34.7%) with CNS involvement were described, while in 87/147 patients (59.2%) the peripheral nervous system was involved. In all 51 patients with CNS involvement by IRAEs, cerebrospinal fluid examination excluded the presence of malignant cells; MRI of the brain excluded the presence of CNS metastasis. Anti neuronal/glial antibodies were detected in 64.4% tested patients, SOX1 antibodies were detected in 3 patients. ICIs were discontinued due to neurologic adverse events in 99.5% of the patients, the median delay from first ICI dose to the last ICI dose was 40 days (range 0-760). In 51.1% of the patients that withdrawal ICIs a cancer progression was documented and 11.5% of patients were rechallenged with ICIs and 2/17 (11.7%) showed a neurologic relapse. The neurologic adverse events were treated with steroids in 93% of the patients, intravenous immunoglobulin were done in 36.8%, plasma exchange in 15.3%. Mortality rates increased gradually at 6, 12 and 18 months of follow-up and 32.7% of patients died during follow-up. The main causes of death were cancer progression in 35.4% of patients, neurological toxicity (31.2%), other causes (20.8%). Interestingly, the most frequent cause of death was neurological toxicity in the first 3 months after neurological toxicity in the first 3 months after neurological adverse event onset (64.3%) and cancer progression after 3 months (47.1%).
In this study, neurological recovery after IRAEs was possible even in case with severe presentation, in patients with melanoma and in cases with myositis / neuromuscular junction disorders. In these series older age and paraneoplastic-like syndromes showed poor neurological outcome.
In a retrospective study at the Mayo Clinic, 16 patients with encephalitis related to ICIs were reported. The majority of these patients received immunosuppressive treatment and ICIs was discontinued in 97%. The unfavorable outcome was described in 39% of the entire reported series, and it was associated with higher severity degree at onset, shorter period from ICIs to the onset of neurological symptoms [
18].