Submitted:
01 April 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
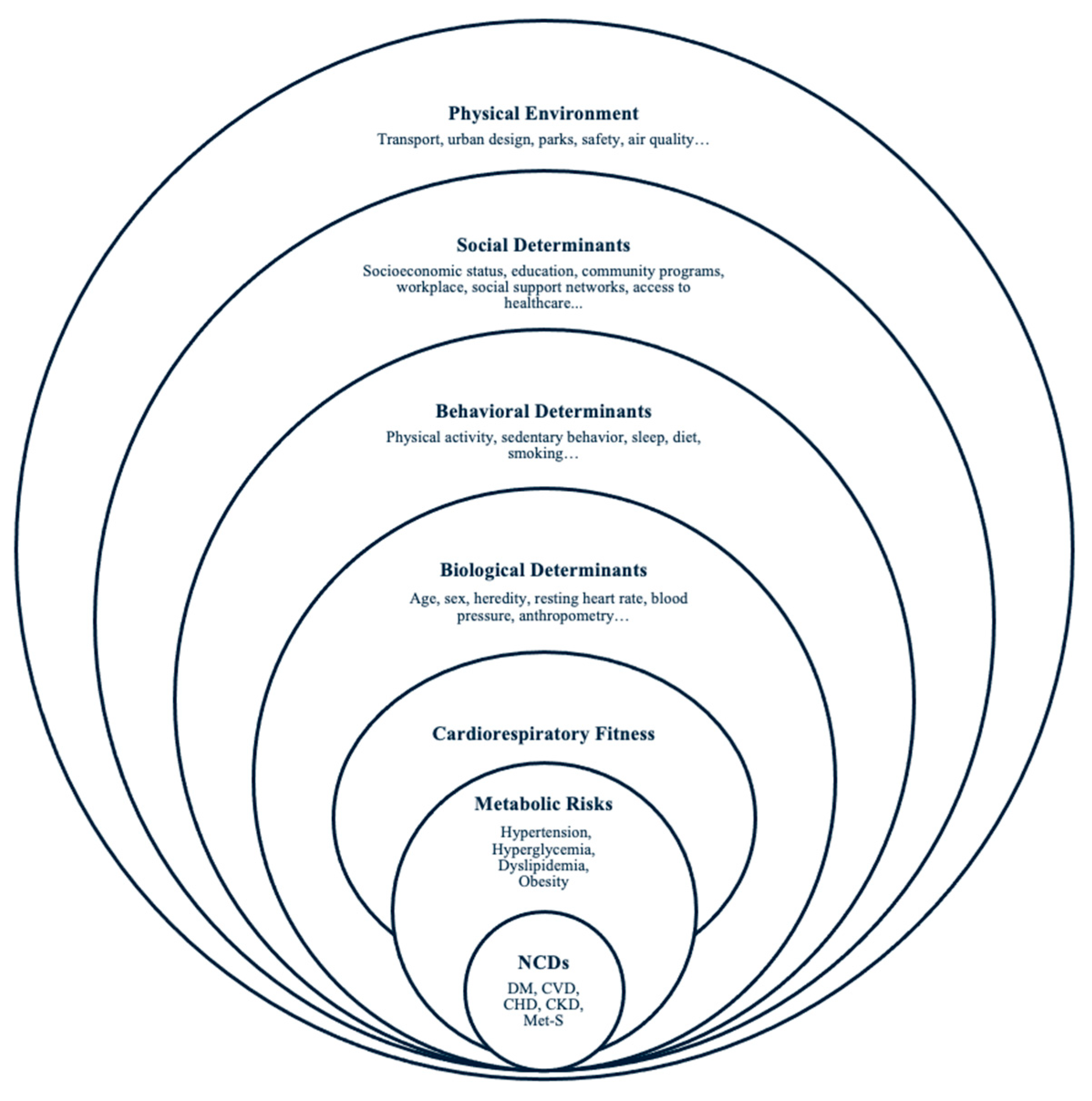
1. Introduction
Cardiorespiratory Fitness and Metabolic Health
Limitations of Measured CRF in Healthcare and Public Health
2. Literature Search
3. eCRF and the Incidence of Metabolic Risks
Hypertension
Hyperglycemia
Dyslipidemia
Obesity
4. Discussion
Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.B.D.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Li, Y.; Pan, A.; Wang, D.D.; Liu, X.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di Angelantonio, E.; Stampfer, M.; Willett, W.C.; et al. Impact of Healthy Lifestyle Factors on Life Expectancies in the US Population. Circulation 2018, 138, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 2015, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Myers, J. Cardiorespiratory Fitness and Its Place in Medicine. RCM 2023, 24. [Google Scholar] [CrossRef]
- Myers, J.; Ross, R. Implementing Cardiorespiratory Fitness as a Routine Measure in Health Care Settings. Journal of Clinical Exercise Physiology 2021, 10, 62–69. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P. Physical Activity, Sedentary Behaviours, and Cardiovascular Health: When Will Cardiorespiratory Fitness Become a Vital Sign? Can J Cardiol 2016, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Sui, X.; O'Connor, D.P.; Church, T.S.; Lee, D.C.; Artero, E.G.; Blair, S.N. Longitudinal cardiorespiratory fitness algorithms for clinical settings. American journal of preventive medicine 2012, 43, 512–519. [Google Scholar] [CrossRef]
- Prince, S.A.; Dempsey, P.C.; Reed, J.L.; Rubin, L.; Saunders, T.J.; Ta, J.; Tomkinson, G.R.; Merucci, K.; Lang, J.J. The Effect of Sedentary Behaviour on Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Medicine 2024. [Google Scholar] [CrossRef]
- Blair, S.N.; Kohl, H.W.; Paffenbarger, R.S.; Clark, D.G.; Cooper, K.H.; Gibbons, L.W. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA 1989, 262, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Watanabe, D.; Miyachi, M. Estimated standard values of aerobic capacity according to sex and age in a Japanese population: A scoping review. PLoS One 2023, 18, e0286936. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Myers, J.; Peterman, J.E.; Bonikowske, A.R.; Harber, M.P.; Medina Inojosa, J.R.; Lavie, C.J.; Squires, R.W. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND). Mayo Clin Proc 2022, 97, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Yang, B.; Du, Z.; Cai, M.; Sun, Z.; Zugel, M.; Michael Steinacker, J.; Schumann, U. Association Between Cardiorespiratory Fitness and Risk of Type 2 Diabetes: A Meta-Analysis. Obesity (Silver Spring) 2019, 27, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Isiozor, N.M.; Myers, J.; Seidu, S.; Khunti, K.; Laukkanen, J.A. Baseline and usual cardiorespiratory fitness and the risk of chronic kidney disease: A prospective study and meta-analysis of published observational cohort studies. Geroscience 2023, 45, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Mensink, G.B.M.; Keil, T.; Finger, J.D. Why are some people more fit than others? Correlates and determinants of cardiorespiratory fitness in adults: protocol for a systematic review. Syst Rev 2017, 6, 102. [Google Scholar] [CrossRef]
- Zeiher, J.; Ombrellaro, K.J.; Perumal, N.; Keil, T.; Mensink, G.B.M.; Finger, J.D. Correlates and Determinants of Cardiorespiratory Fitness in Adults: a Systematic Review. Sports Med Open 2019, 5, 39. [Google Scholar] [CrossRef]
- Stokols, D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot 1996, 10, 282–298. [Google Scholar] [CrossRef]
- Zeiher, J.; Manz, K.; Kuntz, B.; Perumal, N.; Keil, T.; Mensink, G.B.M.; Finger, J.D. Individual and interpersonal correlates of cardiorespiratory fitness in adults - Findings from the German Health Interview and Examination Survey. Sci Rep 2020, 10, 445. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Lavie, C.J.; Zhang, J.; Sui, X. An Overview of Non-exercise Estimated Cardiorespiratory Fitness: Estimation Equations, Cross-Validation and Application. Journal of Science in Sport and Exercise 2019, 1, 38–53. [Google Scholar] [CrossRef]
- Tarp, J.; Stole, A.P.; Blond, K.; Grontved, A. Cardiorespiratory fitness, muscular strength and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetologia 2019, 62, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Artero, E.G.; Jackson, A.S.; Sui, X.; Lee, D.C.; O'Connor, D.P.; Lavie, C.J.; Church, T.S.; Blair, S.N. Longitudinal algorithms to estimate cardiorespiratory fitness: associations with nonfatal cardiovascular disease and disease-specific mortality. J Am Coll Cardiol 2014, 63, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, N.C.D.R.F. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Cabanas-Sanchez, V.; Artero, E.G.; Lavie, C.J.; Higueras-Fresnillo, S.; Garcia-Esquinas, E.; Sadarangani, K.P.; Ortola, R.; Rodriguez-Artalejo, F.; Martinez-Gomez, D. Prediction of cardiovascular health by non-exercise estimated cardiorespiratory fitness. Heart 2020, 106, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.H.; Gates, M.; Kokkinos, P.; Lavie, C.J.; Zhang, J.; Sui, X. Non-Exercise Estimated Cardiorespiratory Fitness and Incident Hypertension. Am J Med 2022, 135, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, R.J.; Musa Yola, I.; Shrout, T.A.; Mitchell, G.F.; Vasan, R.S.; Xanthakis, V. Association of Estimated Cardiorespiratory Fitness in Midlife With Cardiometabolic Outcomes and Mortality. JAMA Netw Open 2021, 4, e2131284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, J.; Yu, J.; Zhang, X. Cardiorespiratory fitness and metabolic risk in Chinese population: evidence from a prospective cohort study. BMC Public Health 2024, 24, 522. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, D.; Chen, S.; Duan, G. The association of cardiorespiratory fitness and the risk of hypertension: a systematic review and dose-response meta-analysis. J Hum Hypertens 2022, 36, 744–752. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Federation, I.D. IDF Diabetes Atlas; 2021.
- Zaccardi, F.; O'Donovan, G.; Webb, D.R.; Yates, T.; Kurl, S.; Khunti, K.; Davies, M.J.; Laukkanen, J.A. Cardiorespiratory fitness and risk of type 2 diabetes mellitus: A 23-year cohort study and a meta-analysis of prospective studies. Atherosclerosis 2015, 243, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qie, R.; Han, M.; Huang, S.; Wu, X.; Zhang, Y.; Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; et al. Independent and joint associations of non-exercise cardiorespiratory fitness and obesity with risk of type 2 diabetes mellitus in the Rural Chinese Cohort Study. Nutr Metab Cardiovasc Dis 2022, 32, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, C.; McCurdy, A.; Lamboglia, C.G.; Wohlers, B.; Pham, A.N.Q.; Sivak, A.; Spence, J.C. The extent to which family physicians record their patients' exercise in medical records: a scoping review. BMJ Open 2020, 10, e034542. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.; Visentini-Scarzanella, M.; Sawada, S.; Sui, X.; Myers, J. Estimating Cardiorespiratory Fitness Without Exercise Testing or Physical Activity Status in Healthy Adults: Regression Model Development and Validation. JMIR Public Health Surveill 2022, 8, e34717. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.A.; Kim, Y.; Kenyon, J.; Visentini-Scarzanella, M.; Sawada, S.S.; Sui, X.; Lee, I.M.; Myers, J.N.; Lavie, C.J. Association between Estimated Cardiorespiratory Fitness and Abnormal Glucose Risk: A Cohort Study. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Hooker, S.P.; Lee, I.M.; Church, T.S.; Colabianchi, N.; Lee, C.D. and Blair, S.N. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care 2008, 31, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, D.; Qi, Y. Global Trends in the Epidemiology and Management of Dyslipidemia. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Sui, X.; Liu, J.; Zhou, H.; Kokkinos, P.F.; Lavie, C.J.; Hardin, J.W.; Blair, S.N. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol 2015, 65, 2091–2100. [Google Scholar] [CrossRef]
- Breneman, C.B.; Polinski, K.; Sarzynski, M.A.; Lavie, C.J.; Kokkinos, P.F.; Ahmed, A.; Sui, X. The Impact of Cardiorespiratory Fitness Levels on the Risk of Developing Atherogenic Dyslipidemia. Am J Med 2016, 129, 1060–1066. [Google Scholar] [CrossRef]
- Collaboration, N.C.D.R.F. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Kodama, S.; Horikawa, C.; Fujihara, K.; Heianza, Y.; Hirasawa, R.; Yachi, Y.; Sugawara, A.; Tanaka, S.; Shimano, H.; Iida, K.T.; et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol 2012, 176, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, J.; Guo, Z.; Yu, J.; Zhang, X.; Ge, H.; Zhu, Y. Estimated cardiorespiratory fitness and incident risk of cardiovascular disease in China. BMC Public Health 2023, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Grandes, G.; Agullo-Ortuno, M.T.; Gomez-Cantarino, S. Changes in Cardiorespiratory Fitness and Probability of Developing Abdominal Obesity at One and Two Years. Int J Environ Res Public Health 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Grandes, G.; Sanchez, A.; Montoya, I.; Torcal, J.; group, P. Cardiorespiratory fitness and development of abdominal obesity. Prev Med 2019, 118, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kannel, W.B.; Kohl, H.W.; Goodyear, N.; Wilson, P.W. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol 1989, 129, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology (Sunnyvale) 2016, 6. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 38, 27–46. [Google Scholar] [CrossRef]
- Shi, J.; Norgeot, B. Learning Causal Effects From Observational Data in Healthcare: A Review and Summary. Front Med (Lausanne) 2022, 9, 864882. [Google Scholar] [CrossRef]
- Gander, J.C.; Sui, X.; Hebert, J.R.; Lavie, C.J.; Hazlett, L.J.; Cai, B.; Blair, S.N. Addition of estimated cardiorespiratory fitness to the clinical assessment of 10-year coronary heart disease risk in asymptomatic men. Prev Med Rep 2017, 7, 30–37. [Google Scholar] [CrossRef]
- Sun, X.Y.; Ma, R.L.; He, J.; Ding, Y.S.; Rui, D.S.; Li, Y.; Yan, Y.Z.; Mao, Y.D.; Liao, S.Y.; He, X.; et al. Updating Framingham CVD risk score using waist circumference and estimated cardiopulmonary function: a cohort study based on a southern Xinjiang population. BMC Public Health 2022, 22, 1715. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, J.; Kang, H. Adding Estimated Cardiorespiratory Fitness to the Framingham Risk Score and Mortality Risk in a Korean Population-Based Cohort Study. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Franklin, B.A.; Wedig, I.J.; Sallis, R.E.; Lavie, C.J.; Elmer, S.J. Physical Activity and Cardiorespiratory Fitness as Modulators of Health Outcomes: A Compelling Research-Based Case Presented to the Medical Community. Mayo Clin Proc 2023, 98, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Lamming, L.; Pears, S.; Mason, D.; Morton, K.; Bijker, M.; Sutton, S.; Hardeman, W.; Team, V.B.I.P. What do we know about brief interventions for physical activity that could be delivered in primary care consultations? A systematic review of reviews. Prev Med 2017, 99, 152–163. [Google Scholar] [CrossRef] [PubMed]
- van der Wardt, V.; di Lorito, C.; Viniol, A. Promoting physical activity in primary care: a systematic review and meta-analysis. Br J Gen Pract 2021, 71, e399–e405. [Google Scholar] [CrossRef] [PubMed]
- Milton, K.; Cavill, N.; Chalkley, A.; Foster, C.; Gomersall, S.; Hagstromer, M.; Kelly, P.; Kolbe-Alexander, T.; Mair, J.; McLaughlin, M.; et al. Eight Investments That Work for Physical Activity. J Phys Act Health 2021, 18, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, L.; Rodrigues, D.; Pereira, A.M.; Ribeiro, R.T.; Boavida, J.M. Use of Electronic Health Records and Geographic Information Systems in Public Health Surveillance of Type 2 Diabetes: A Feasibility Study. JMIR Public Health Surveill 2016, 2, e12. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Kelley, R.R. Machine Learning in Epidemiology and Health Outcomes Research. Annu Rev Public Health 2020, 41, 21–36. [Google Scholar] [CrossRef]
| First Author, Year of Publication |
Mean Follow-Up Years from Baseline (±SD) | Cohort | Location and Sample Size | Sex | Mean Age (±SD) |
eCRF model | Metabolic Risk Outcomes |
|---|---|---|---|---|---|---|---|
| Lee et al., 2021 | 15 | Framingham Offspring Study (FOS) | America 2962 |
M&F | 66.2 (8.6) |
Jackson | Incidence of SBP ≥140/ DBP ≥90 mm Hg, Incidence of DM fasting glucose level of 126 mg/dL or higher, nonfasting glucose level of 200 mg/dL or higher, or the use of hypoglycemic medications. |
| Patel et al., 2022 | 5 | Aerobics Center Longitudinal Study (ACLS) | America 5513 |
M&F | 42.8 (9.0) |
Jackson | Incidence of resting SBP ≥130/DBP ≥80 mm Hg or self-reported, physician-diagnosed hypertension. |
| Cabanas-Sánchez et al., 2022 |
5.7 (4.4) |
Taiwan MJ Cohort (TMJC) | Taiwan 200039 |
M&F | 38.5 (12.1) |
Jackson | Incidence of SBP ≥140/ DBP ≥90 mm Hg, serum total cholesterol ≥240 mg/dL, and fasting blood glucose ≥126mg/dL. Atherogenic dyslipidemia was defined as triglycerides≥150 mg/dL and HDL-C <40 mg/dL in men and <50 mg/dL in women. |
| Zhao et al., 2022 | 6.01(Median) | Rural Chinese Cohort Study (RCCS) | China 11825 |
M&F | 51.0 (8.5) | Jackson | Incidence of DM was defined as fasting plasma glucose 7.0 mmol/L or current treatment with anti-diabetes medication or a self-reported history of DM, gestational diabetes mellitus, or diabetes due to other causes. |
| Sloan et al., 2023 | 4.87 (4.58) |
Aerobics Center Longitudinal Study (ACLS) | America 8602 |
M&F | 43.0 (8.9) |
Sloan | Incidence of prediabetes (impaired fasting glucose) or DM as fasting plasma glucose concentrations of 100 to 125 and ≥126 mg/dL, respectively. Those who self-reported DM or hypoglycemic medication during a follow-up were also classified as having abnormal glucose. |
| Liu et al., 2024 | 4 (Median) | China Health and Retirement Longitudinal Study (CHARLS) | China 4862 |
M&F | 58.6 (9.4) |
Jackson | Change in resting SBP, DBP, fasting triglycerides, high-density lipoprotein, total cholesterol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





