1. Introduction
Plastics are irreplaceable in modern life due to their versatility and wide range of applications. Over the past decades, global plastic production has exploded from 234 million tones (Mt) in 2000 to 460 Mt in 2019 [
1]. With the increase in plastic usage, plastic litter has become a significant threat to the environment. Furthermore, since the term ‘microplastics’ (MPs), which are less than 5 mm in size, surfaced [
2,
3], they have been recognized as a ubiquitous material and novel kind of pollutant to ecosystems [
4,
5]. Recent studies on the occurrence of MPs in environmental media have shown that MPs pollution is widespread, appearing in ocean [
6,
7], fresh water [
8,
9], air [
10], soil and sediments [
11,
12]. Due to the high resistant property of plastics to degradation, MPs accumulate through food chain and are consequently consumed by humans [
13]. Especially in seafoods, the bioaccumulation of MPs in the marine web may cause a problem for food safety from the perspective of the food chain [
14]. As exposure to MPs in human body becomes more concerning, analysis has focused on foods such as beverages [
15], milk [
16], seafoods [
17], fruits and vegetables [
18] , edible oils [
19,
20]. However, there is still a lack of occurrence data in the source of foods and harmonized identification methods, representing more data and research are necessary [
21].
Omega-3 fatty acids are polyunsaturated fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), containing carbon double bond in their omega location at their methyl terminal end. On account of their health benefits in human, they have become one of the most widely consumed dietary supplements. The global omega-3 market size accounted for USD 7.5 billion in 2022 and is projected to reach around USD 15.1 billion by the end of 2032 [
22]. Omega-3 supplement oils are mainly made from fish oils extracted from blue-backed fish such as herring and mackerel, but recently, consumer interest in plant-based ingredients made from algal oil for vegetarians and vegans has grown. Since the source of the omega-3 supplements is fish oil, there have been concerns that omega-3 supplements may contain MPs due to contamination and accumulation in marine organisms used for the raw material. However, to date, there have not been no reports evaluating the presence of MPs in omega-3 oils [
23]. In this context, it is necessary to understand the current level of MPs contamination in omega-3 supplements.
Internationally standardized methods for determining MPs are being developed for water matrices by ISO/TC 147/SC 2/JWG 1, but not yet for food or oil matrices. The standard methods for identifying and quantifying MPs utilize vibrational spectroscopic techniques equipped with microscopes such as microscope-Fourier-transform infrared (μFTIR), microscope-Raman (μRaman) [
24]. Using these methods, MPs can be identified comparing collected spectra with databases. Visual images of MPs, including their size and shape can also be obtained. Due to spectroscopic and time limitations, a practical cut-off size of 20 μm is applied for μFTIR and 5 μm for μRaman, to analyze entire filtrated area either by automated mapping or individual particle analysis. Considering the potentially low concentration of MPs in oil, the cut-off size of 5 μm was chosen using μRaman to achieve the highest possible detection limit in this study.
In this study, we aimed to investigate the presence of MPs in various omega-3 supplements available in the Korean market. We collected encapsulated omega-3 oil samples, which represent the form of product consumers typically take, as well as the raw oil itself stored the non-plastic bottles before encapsulation, provided by the manufacturer. Furthermore, these samples were categorized to animal-based (AB) and plant-based (PB) sources to examine potential differences in the presence of MPs based on the oil source. We conducted preliminary tests, including a simple recovery test, and rigorously controlled environmental factors to minimize possibility of MPs contamination in the laboratory.
2. Materials and Methods
2.1. Preparation of Omega-3 Oil Samples
Commercial encapsulated omega-3 oil samples from the Korean market, as well as raw material samples which are not encapsulated and provided directly from the manufacturer, were collected. Due to the confidentiality concerns, commercial brands are not disclosed. A total of 14 raw omega-3 oils and 21 encapsulated omega-3 oils were prepared. The raw oils and products were categorized into PB oils and AB oils based on their sources. The number of samples by sample type and based oil is listed in
Table 1.
Capsule oil samples were carefully cut using a metal scalpel, and the oil inside was poured into a pre-cleaned Petri dish. The sample amount was determined based on the manufacturer’s recommended serving size. It was calculated by subtracting the amount of the capsule from the total amount of the product (capsule + oil). Since the amount of oil in one serving capsule typically ranges from 2 g to 3 g, 2.5 g of raw oil was used for raw oil samples.
The cut capsules were washed using pasture pipet with pre-filtered acetone (Samchun chemicals., Korea) to take out remaining oil in capsule. The mixture of omega-3 oil sample and acetone was then poured into a filtration system and filtered directly through 5 μm silicon filter (10 mm x 10 mm square, SmartMembranes, Germany). Any remaining oil on the walls of the Petri dish and the filtration funnel was washed off with acetone. A total volume of 30 ml of acetone was used and to minimize any possible damage caused by acetone, the sampling was conducted quickly.
2.2. Determination of Microplastics by μRaman
Raman analysis was performed using an XploRA Plus confocal Raman microscope (Horiba, France) to detect microplastics. The Raman system and detailed parameters were same as our previous study [
25]. Briefly, a 532 nm laser and a 1024 x 256 pixel-cooled charge-coupled device (CCD) detector were used with 10 % laser power. The spectra range was set between 1020–3400 cm
-1 using a 1200 grooves/mm grating and the exposure time was set to 1 x 2
s. Mosaic images and collected spectra were processed using the Particlefinder
TM module in Labspec 6 software. All spectra were screened for plastics using the classical least square algorithm (CLS). Spectra of polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinylchloride (PVC), polyurethane (PU), polyamide (PA), poly (ethylene terephthalate) (PET) and poly (methyl methacrylate) (PMMA) were set as standard references for the CLS fitting. Each measured spectrum was calculated as the sum of all the references and the theoretical composition. The results of the CLS spectra were manually investigated to avoid missing data or false positives by the KnowitAll
TM spectrum matching software with a library of Raman. Only results with a minimum Hit Quality Index (HQI) of 70 % were classified as MPs.
2.3. Cross-Check for Possible Misidentification of PE by μFTIR
μFTIR analysis was performed using an FTIR spectroscopy on a microscope (LUMOS II, Bruker Optics, USA), equipped with a 32 × 32 pixels focal plane array (FPA) detector. IR images were acquired in transmission mode at a spectral resolution of 12 cm-1 within a spectral range from 3800 to 900 cm-1, utilizing scan time one scan. μFTIR was specifically employed to distinguish particles that might be erroneously identified as PE using Raman spectroscopy.
2.4. Environmental Control to Prevent MPs Contamination
To prevent microplastic contamination, only glass and non-plastic materials were used during the sampling and filtering process. All sample preparation, pretreatment, and filtration steps in the laboratory were conducted inside a laminar flow bench equipped with a ULPA H14 filter (Sinan science industry, HSCV-1300) to mitigate contamination from indoor airborne microplastics. Solutions, including ultrapure water, chemical reagents, were pre-filtered using 0.7 µm GF/F filter (Whatman, UK) and then a 1 μm stainless steel filter (KIAST, Korea) prior to use. Glassware was cleaned with filtered ultrapure water before laboratory experiments. When moved outside the laminar flow bench, all samples were covered with aluminum foil to prevent any contamination. Nitrile gloves and cotton coats were worn during all processing steps to minimize the risk of contamination.
3. Results
3.1. Preliminary Tests with Simple Recovery Test
Omega-3 oil itself is a clear solution and can be directly filtrated through a silicon filter for analysis using μRaman or μFTIR. However, since omega-3 oil also has vibrational spectrum in μRaman and μFTIR, it is desirable to thoroughly remove it from the filter surface. Furthermore, after pouring the oil inside the capsule onto the petri dish, the remaining oil inside the capsule, as well as the petri dish and filtration funnel must be cleaned with a solvent. In first place, hot water containing nonionic surfactant was utilized as a washing solvent. However, the main issue with using a water-based solution was that the capsule was easily dissolved in water, making sampling difficult. Additionally, not only was the oil on the filter surface difficult to remove, but also the oil on the glassware such as petri dish and filtration funnel. For these reasons, we attempted acetone as cleansing solution instead.
Acetone may cause damage to some types of plastics such as PS and styrene copolymer, but is less likely to affect PP, PE and PET. A recent study reported a simplified method for extraction of MPs using acetone [
26]. Considering that the major determined species of MPs are PE and PP, acetone could be a good choice to remove oil due to its amphipathic characteristics. We aimed to minimize the sampling time shorter as much as possible to reduce the potential damage of polymers. The total sampling time per sample was less than 10 min including filtration and washing steps, meaning that MPs could be exposed to acetone for the same duration.
A simple recovery test was conducted using PE sphere MPs with a size range from 75 μm to 80 μm (Cospheric, USA). The PE spheres were added to the slide glass and manually counted using μRaman microscope. The PE spheres on the glass were transferred into the petri dish after washing the glass with acetone. Raw oil was pre-sampled on the petri dish before adding spheres to it. The PE sphere with oil were filtered and the number of spheres on the filter were counted. Initially, 118 PE spheres were added, and 117 spheres were counted on the filter (
Figure S1). The calculated recovery rate of microplastics in oil was over 99 %.
3.2. Procedural Blank Test and Limit of Detection (LOD)
The determination of the LOD was performed to establish the threshold for the number of MPs resulting from contamination, following the ‘best practice guidelines’ outlined by Schymanski et al. [
27] and also defined in ISO/DIS 16094-2 [
24] as the reporting limit (RL). This value is used to define the limit from which the laboratory can provide quantitative result. In this study, a particle size of 5 μm was applied as the lower limit due to the filter pore size and the instrumental limit for the μRaman in automatic mode. It is recommended to analyze 10 blank samples to determine LOD. The LOD was determined as follows:
Where
meanblanks is the average number of MPs identified in the procedural blanks and
sblanks is the standard deviation.
In this study, 12 procedural blank samples were analyzed. Procedural blank samples were prepared in the same manner as omega-3 oil sampling but not containing omega-3 oil. The obtained value was 0.3 ± 0.5 and the calculated LOD was 1.8 MPs, as tested in this study. This LOD demonstrates the cleanness of the studied environmental area, indicating that the contamination from airborne particles or apparatus is very low, allowing for the determination of MPs down to a minimum size of 5 μm in oil.
3.3. Determination of Microplastics in Omega-3 Oil Samples
The determination of MPs was performed using pre-tested method for omega-3 oil samples. An example of mosaic image of filter and detected particles, along with their spectra, is depicted in
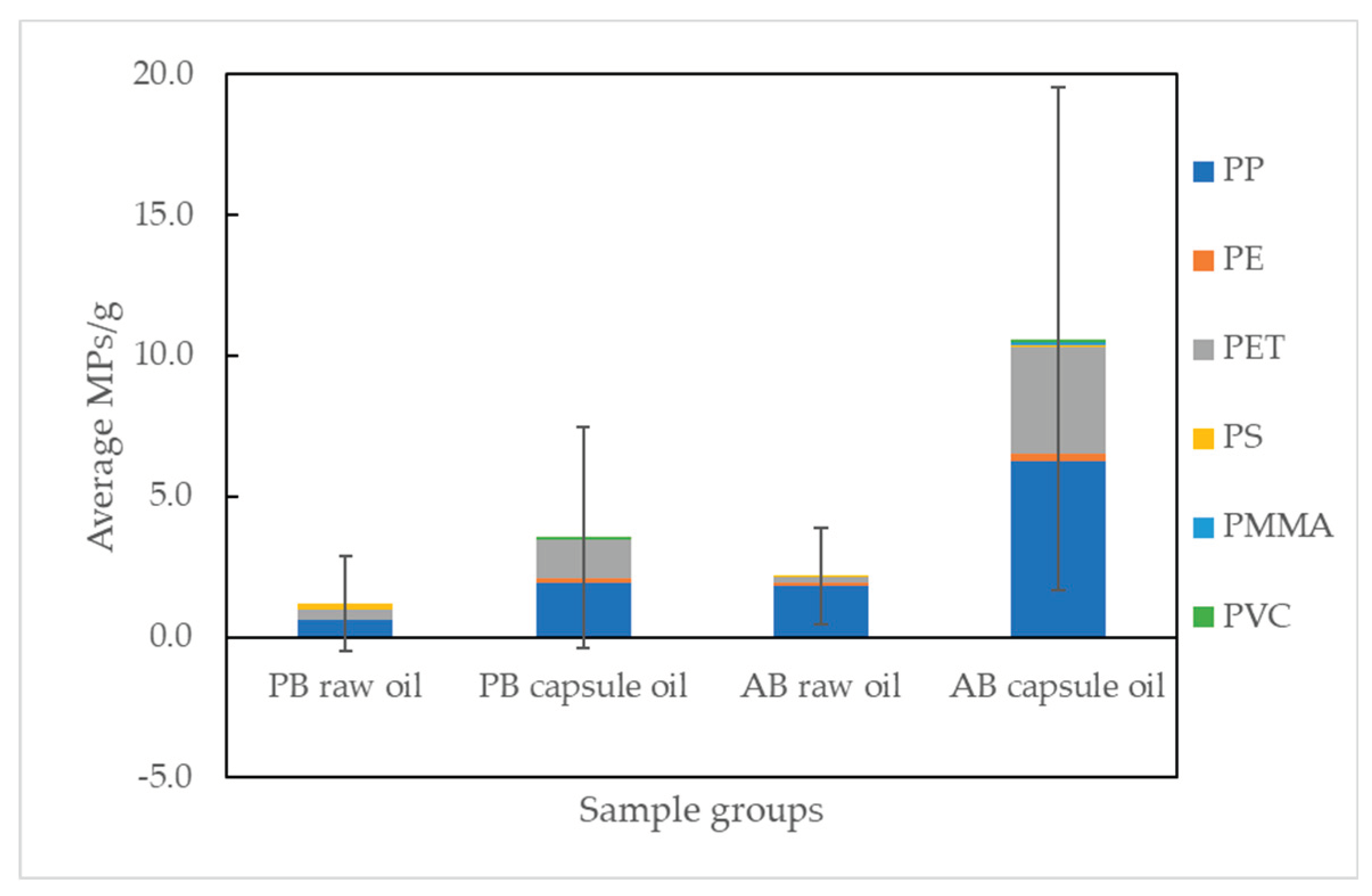
Figure 1. The average number of MPs and the standard deviation for each sample group (see
Table 1) are shown in
Figure 2. For comparison between groups, the results were presented as the average number of MPs per gram of sample, with individual data normalized accordingly. The average number of MPs per gram was 1.2 ± 1.7 MPs/g for PB raw oil and 2.2 ± 1.7 MPs/g for AB raw oil. When considering the raw oil alone, there was no significant difference in the abundance of MPs between the two sources (p > 0.05). During the oil processing steps, filtration and centrifugation were applied to refine and clean the oil. Typically, the oil undergoes multi-step cleaning, including filtration at 100 μm and 0.5 to 1 μm. Therefore, it is noteworthy that the source of the raw oil has no effect on MPs pollution in terms of MPs ingestion.
Whereas, the average number of MPs per gram for PB capsule oil and AB capsule oil were 3.5 ± 3.9 MPs/g and 10.6 ± 8.9 MPs/g, respectively. The number of MPs in capsule oil was indicated to be 3 to 5 times greater than that in the raw oil. This results clearly show that the main reason for MPs occurrence in the final product is not due to the oil itself but rather the encapsulation process. Additionally, it can be observed that the standard deviation of each sample group is high compared to the average value, indicating that the contamination of MPs depends on the cleanness of the manufacturing process, including encapsulation.
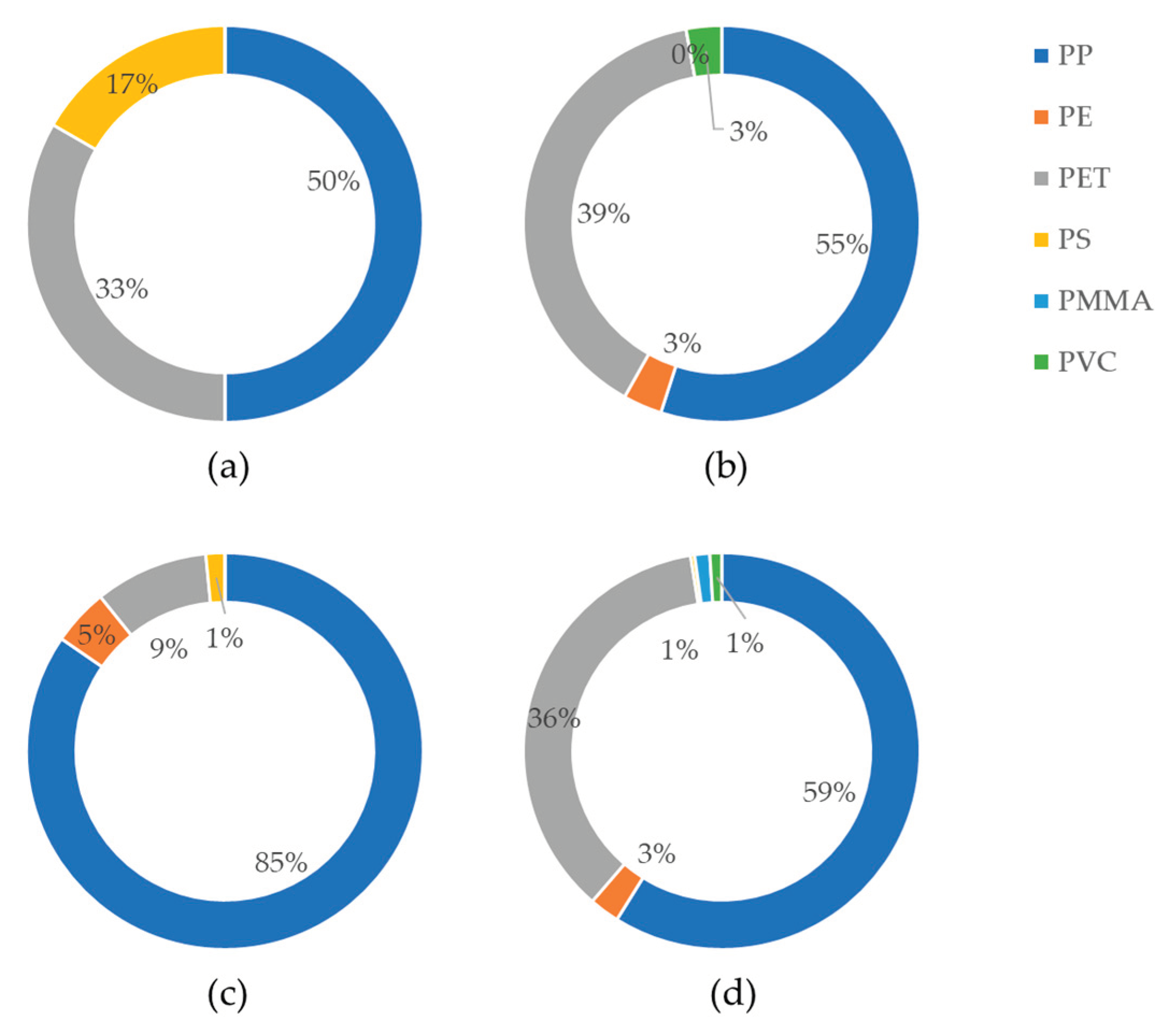
As depicted in
Figure 2 and
Figure 3, PP was the most abundant species, accounting for more than 50 % of the total MPs across all groups. In addition to that, PET was found in both capsule oil sample groups, representing a large portion of the detected MPs, accounting for more than 36 %. PP and PET were predominant species in omega-3 oils (83-95 %). This indicates that PET can be easily contaminated during the encapsulation process. Moreover, considering the increased number of PP in the capsule oil, PP is also the major source of MPs contamination during the encapsulation process. Furthermore, it can be inferred that the relatively lower number of MPs found on average in PB capsule oil may be attributed to the fact that PB oil, being a more recent development, is likely produced in facilities with inherently cleaner environments for MPs. There is no direct evidence of contamination from the encapsulation process because capsule oils are not directly matched with their raw oil and different sample numbers of each group. However, as a result, it can be concluded that final products consumed by consumers may contain MPs, regardless of how clean the raw material is. There may be concerns in experimental aspect that the different sampling method of raw oil and capsule oil can lead to differences. But, at least, contamination from the laboratory was checked by investigating procedural blank tests. The occurrence of MPs from the packaging or the packaging environment is common and easily predictable problem. Thus, it could be more important to control the packaging-derivative contamination to avoid MPs exposure through food intake.
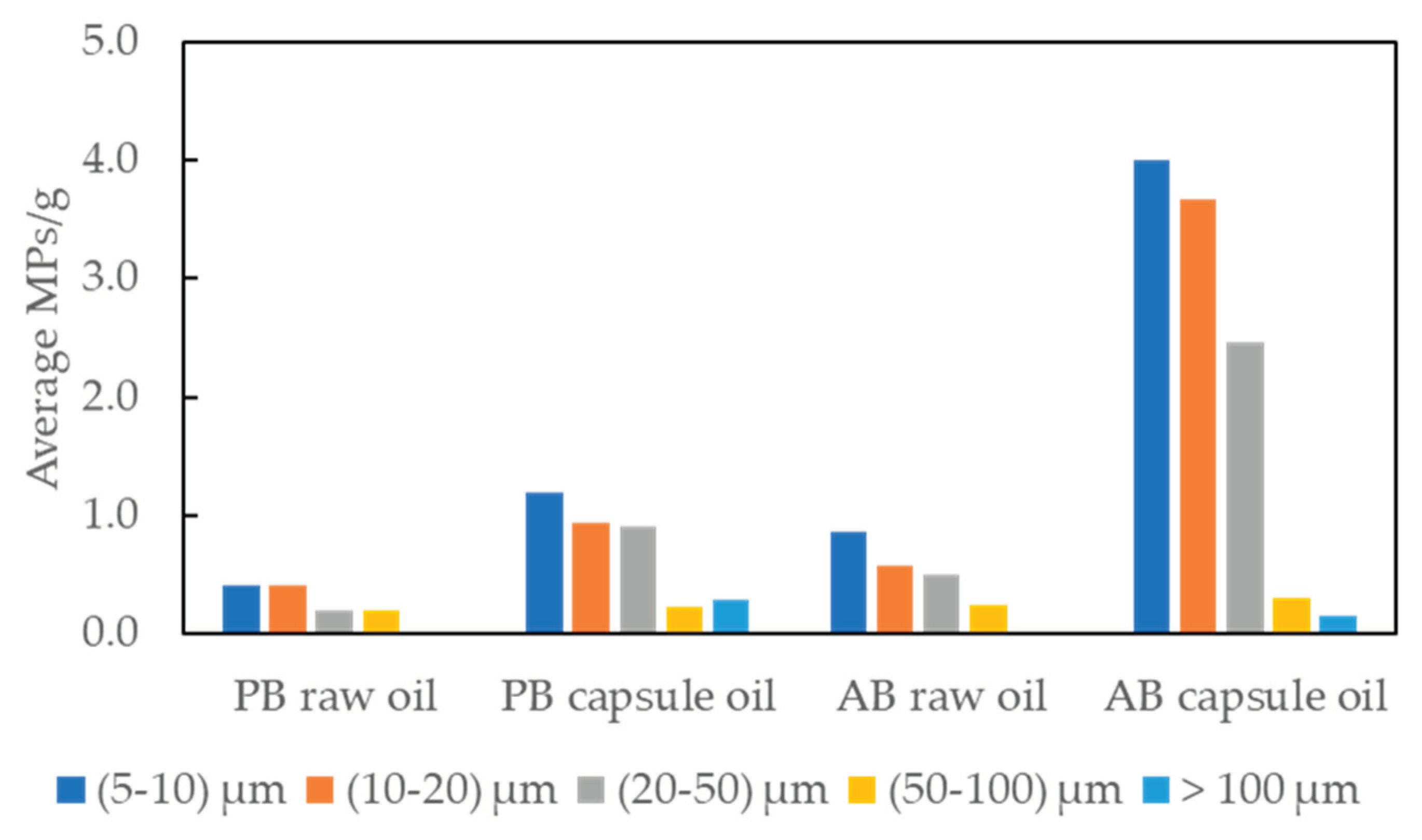
As can be seen in
Figure 4 and
Figure S2, there was a trend of increasing numbers of MPs detected as the size range decreased. The distribution of size classification was consistent across all groups, with the most dominant size ranged from 5 to 10 μm for every sample group, comprising 33 % to 40 % of the total. Notably, the size range of 5 to 20 μm accounted for 60 % to even 73 %. Considering the practical size limit of μFTIR is 20 μm, these results reveal the effectuality of μRaman for analyzing MPs in omega-3 oil. MPs smaller than 5 μm were not considered in this study due to technical limitations and the time constraints. However, it is implied that MPs of smaller size can be more prevalent in the samples, necessitating further investigation and enhancement of technical and analytical capability in the future.
Figure 1.
An example of mosaic image (right), detail image of an identified MP (center), and comparison between acquired and reference spectra (left).
Figure 1.
An example of mosaic image (right), detail image of an identified MP (center), and comparison between acquired and reference spectra (left).
Figure 2.
Average numbers of MPs (MPs/g) with standard deviation and polymer composition of each omega-3 sample group (Plant-based (PB) and Animal-based (AB)).
Figure 2.
Average numbers of MPs (MPs/g) with standard deviation and polymer composition of each omega-3 sample group (Plant-based (PB) and Animal-based (AB)).
Figure 3.
Distribution by polymer composition of: (a) Plant-based (PB) raw oil; (b) Plant-based (PB) capsule oil; (c) Animal-based (AB) raw oil; (d) Animal-based (AB) capsule oil.
Figure 3.
Distribution by polymer composition of: (a) Plant-based (PB) raw oil; (b) Plant-based (PB) capsule oil; (c) Animal-based (AB) raw oil; (d) Animal-based (AB) capsule oil.
Figure 4.
Distribution by particle size of the identified MPs in omega-3 oil samples ((Plant-based (PB) and Animal-based (AB)).
Figure 4.
Distribution by particle size of the identified MPs in omega-3 oil samples ((Plant-based (PB) and Animal-based (AB)).
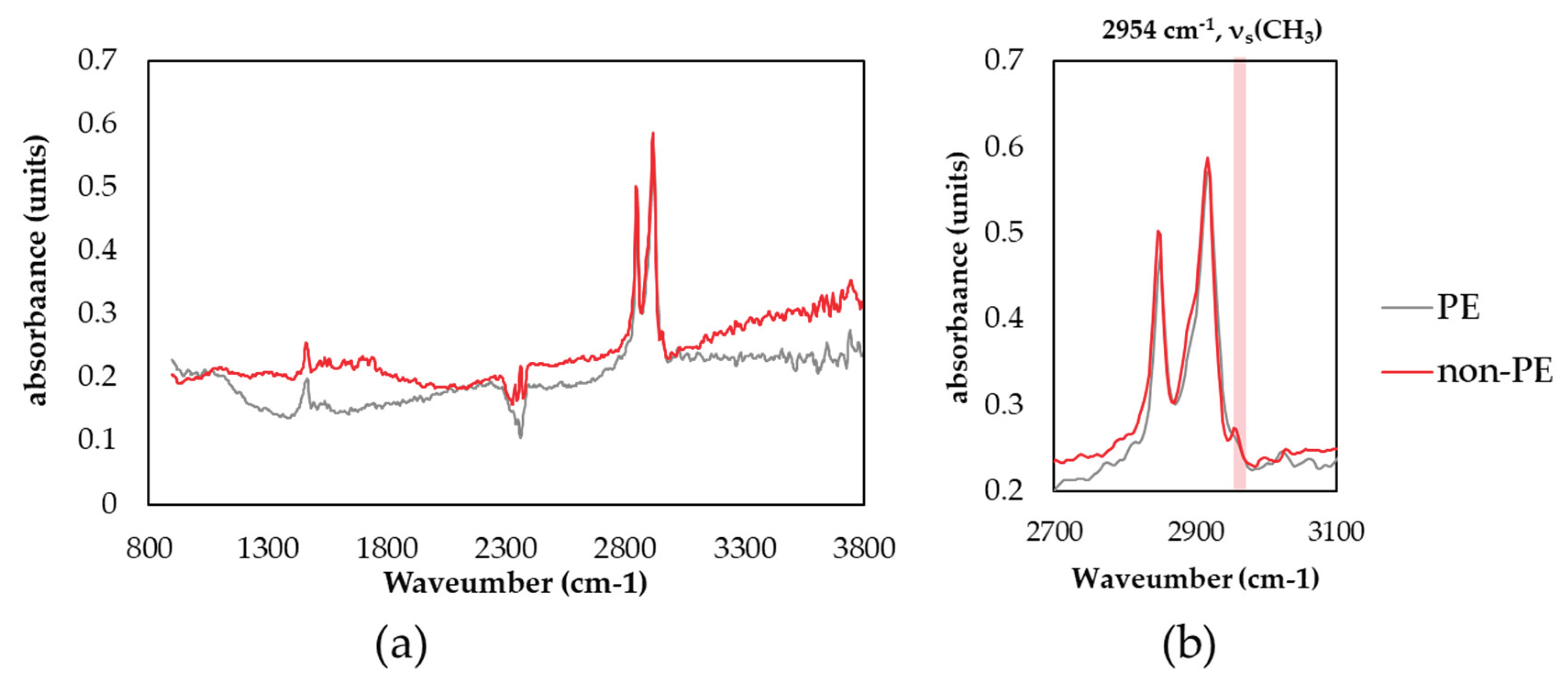
3.4. Identification of PE Spectrum and Check with μFTIR
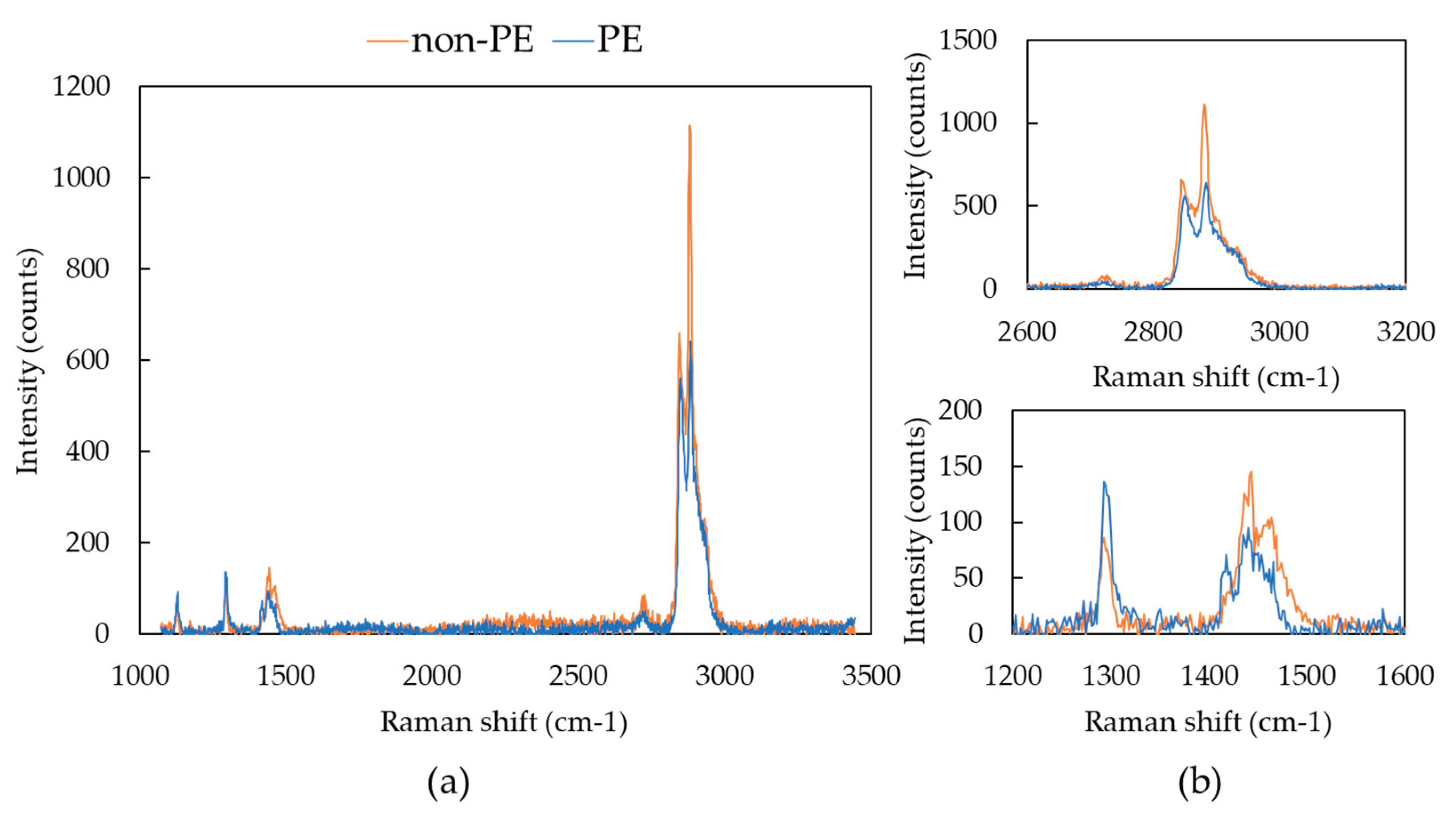
As mentioned in the under-developed test method standard ISO/DIS 16094-2, a Raman spectrum of PE is very similar to that of hydrocarbon compounds such as oil, wax, stearates, requiring attention to false-positive problem [
24]. Analyzing the entire filter area takes a long time, so we allocated 2 s for individual particle analysis. The total analysis time was at least 5 to 6 h for all particles greater than 5 μm on the filter. Thus, there is a limitation to get clearer spectra of individual particle with a high signal-to noise ratio. In some samples, a number of spectra that could easily be mistaken for PE were observed, which appeared not to be PE (expressed ‘not PE’). Raman Spectra of reference PE and non- PE particle are shown in
Figure 5. Characteristic wavenumbers for PE in Raman spectroscopy were observed at 1127 cm
-1 for C-C stretch, 1293 cm
-1 for CH
2 twisting, three bands at 1417 cm
-1, 1439 cm
-1 and 1465 cm
-1 for CH
2 bending and 2849 cm
-1 and 2883 cm
-1 for C-H stretching [
28,
29]. The difference of Raman spectra between not PE and PE can be seen at several points. First, the intensity of CH
2 bending at 1417 cm
-1 is low, resembling almost two bands rather than three bands. Second, intensity at 1293 cm
-1 is lower than the three bands between 1417~1465 cm
-1. This difference is can also be observed in low molecular hydrocarbon such as oil or wax. Moreover, intensity of 2960 cm
-1 shoulder is higher and it also can be seen compared to the ratio with the intensity of 2883 cm
-1.
These sample were analyzed using μFTIR to confirm whether the particles are PE or not. FT-IR spectra are shown in
Figure 6. Both spectra are quite similar, and even worse, fingerprint region cannot be seen clearly due to the low signal-to-noise ratio. In the FTIR spectra, characteristic band of CH
2 stretching can be observed at 2843 cm
-1 and 2913 cm
-1. The difference can be seen at the 2954 cm
-1 shoulder, which is clearly divided. This shoulder can be assigned as CH
3 stretching, which can appear at the methyl group of the end of the carbon chain. Ideal PE has a very low portion of methyl ends, but the portion increases in low molecular hydrocarbon, including oil, stearates and lipids [
30]. Combining the results of μFTIR and μRaman, this spectrum was excluded from the MPs results. The spectra of other such as PP, PET and PS showed clearer spectra than PE for identification, so the IR cross-check was not applied.
Figure 5.
Raman spectra of reference PE and non-PE particle found in omega-3 oil sample: (a)full measured spectrum range; (b)enlarged spectrum range.
Figure 5.
Raman spectra of reference PE and non-PE particle found in omega-3 oil sample: (a)full measured spectrum range; (b)enlarged spectrum range.
Figure 6.
FTIR spectra of reference PE and non-PE particle found in omega-3 oil sample: (a)full measured spectrum range; (b)enlarged spectrum range.
Figure 6.
FTIR spectra of reference PE and non-PE particle found in omega-3 oil sample: (a)full measured spectrum range; (b)enlarged spectrum range.
4. Conclusions
To our knowledge, this is the first study to demonstrate the presence of MPs in commercial omega-3 supplementary oil. Raw and capsule oils were sorted into categories based on their PB or AB sources. A simple filtration method employing acetone washing was applied, achieving around 99 % recovery rate with PE microspheres. μRaman was employed to the determination of MPs greater than 5 μm, and μFTIR was used for cross-checking the spectrum of PE. Rigorous environmental controls were maintained within the laboratory, and the calculated LOD from twelve procedural blanks was 1.8 MPs. The results revealed MPs concentration of 1.2 ± 1.7 MPs/g for PB raw oil, 2.2 ± 1.7 MPs/g for AB raw oil, 3.5 ± 3.9 MPs/g for PB capsule oil, and 10.6 ± 8.9 MPs/g for AB capsule oil. The results reveal that there is no significant difference in MP levels between PB and AB raw oils. Comparison between raw and capsule oils indicated that, MPs contamination primarily result from the encapsulation process rather than the source of raw material. PP and PET were predominant species detected (83 to 95 %) in factory. It was observed that there was a higher frequency of detection for MPs as their size decreased. This research gives a knowledge data for the occurrence of MPs in omega-3 oil by their sources with reliable methods. Based on the results, continuous development of test methods and further study of other functional foods will be required to cope human exposure and possible health effects.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: PE spheres used for recovery test: (a)before addition; (b) recovered PE spheres on filter.; Figure S2: Distribution by polymer size of: (a) PB raw oil; (b) PB capsule oil; (c) AB raw oil; (d) AB capsule oil.; Table S1: Numbers of MPs in omega-3 samples. ;
Author Contributions
Collected documents, drafted manuscripts and investigation, M.K.; analysis and data curation, S.P. and D.K.; writing—original draft preparation, J.K.; and conceptualization, supervision, writing—review and editing, J.J. and D.C.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- OECD Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD, 2022; ISBN 978-92-64-65494-5.
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838–838. [CrossRef]
- Microplastics - ECHA Available online: https://echa.europa.eu/hot-topics/microplastics (accessed on 27 February 2024).
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Marine Pollution Bulletin 2019, 138, 145–147. [CrossRef]
- The Plastics Transition • Plastics Europe. Plastics Europe.
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic Debris in the Open Ocean. Proc Natl Acad Sci U S A 2014, 111, 10239–10244. [CrossRef]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, Size and Polymer Composition of Marine Microplastics ≥ 10 Μm in the Atlantic Ocean and Their Modelled Vertical Distribution. Marine Pollution Bulletin 2015, 100, 70–81. [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Research 2019, 155, 410–422. [CrossRef]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. Pigments and Plastic in Limnetic Ecosystems: A Qualitative and Quantitative Study on Microparticles of Different Size Classes. Water Research 2016, 98, 64–74. [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environmental Pollution 2018, 234, 115–126. [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Soil: A Review on Methods, Occurrence, Sources, and Potential Risk. Science of The Total Environment 2021, 780, 146546. [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in Sediments: A Review of Techniques, Occurrence and Effects. Marine Environmental Research 2015, 111, 5–17. [CrossRef]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in Human Food Chains: Food Becoming a Threat to Health Safety. Science of The Total Environment 2023, 858, 159834. [CrossRef]
- Mercogliano, R.; Avio, C.G.; Regoli, F.; Anastasio, A.; Colavita, G.; Santonicola, S. Occurrence of Microplastics in Commercial Seafood under the Perspective of the Human Food Chain. A Review. J. Agric. Food Chem. 2020, 68, 5296–5301. [CrossRef]
- Sewwandi, M.; Wijesekara, H.; Rajapaksha, A.U.; Soysa, S.; Vithanage, M. Microplastics and Plastics-Associated Contaminants in Food and Beverages; Global Trends, Concentrations, and Human Exposure. Environmental Pollution 2023, 317, 120747. [CrossRef]
- Basaran, B.; Özçifçi, Z.; Akcay, H.T.; Aytan, Ü. Microplastics in Branded Milk: Dietary Exposure and Risk Assessment. Journal of Food Composition and Analysis 2023, 123, 105611. [CrossRef]
- Karami, A.; Golieskardi, A.; Ho, Y.B.; Larat, V.; Salamatinia, B. Microplastics in Eviscerated Flesh and Excised Organs of Dried Fish. Sci Rep 2017, 7, 5473. [CrossRef]
- Lazăr, N.-N.; Călmuc, M.; Milea, Ștefania-A.; Georgescu, P.-L.; Iticescu, C. Micro and Nano Plastics in Fruits and Vegetables: A Review. Heliyon 2024, 10, e28291. [CrossRef]
- Battaglini, E.; Miralles, P.; Lotti, N.; Soccio, M.; Fiorini, M.; Coscollà, C. Analysis of Microplastics in Commercial Vegetable Edible Oils from Italy and Spain. Food Chemistry 2024, 138567. [CrossRef]
- Guo, X.; Haochen Dai; Gukowsky, J.; Tan, X.; He, L. Detection and Quantification of Microplastics in Commercially Bottled Edible Oil. Food Packaging and Shelf Life 2023, 38, 101122. [CrossRef]
- van Raamsdonk, L.W.D.; van der Zande, M.; Koelmans, A.A.; Hoogenboom, R.L.A.P.; Peters, R.J.B.; Groot, M.J.; Peijnenburg, A.A.C.M.; Weesepoel, Y.J.A. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods 2020, 9, 72. [CrossRef]
- Omega 3 Market Size To Hit Around USD 15.1 Billion By 2032 Available online: https://www.precedenceresearch.com/omega-3-market (accessed on 5 March 2024).
- GOED GOED INDUSTRY ADVISORY: MICRO- AND NANOPLASTICS PARTICLE CONTAMINATION IN EPA/DHA OMEGA-3 OILS. GOED.
- ISO/DIS 16094-2 Available online: https://www.iso.org/standard/84460.html (accessed on 29 February 2024).
- Kim, S.H.; Kim, J.; Park, S.; Jung, J.; Kim, K.S.; Min, H.J. Identification and Characterization of Microplastics in Nasal Irrigation Fluids: A Preliminary Study. Int Forum Allergy Rhinol 2023, alr.23239. [CrossRef]
- Shalumon, C.S.; Ratanatamskul, C. A Novel Simplified Method for Extraction of Microplastic Particles from Face Scrub and Laundry Wastewater. Sci Rep 2023, 13, 14168. [CrossRef]
- Schymanski, D.; Oßmann, B.E.; Benismail, N.; Boukerma, K.; Dallmann, G.; von der Esch, E.; Fischer, D.; Fischer, F.; Gilliland, D.; Glas, K.; et al. Analysis of Microplastics in Drinking Water and Other Clean Water Samples with Micro-Raman and Micro-Infrared Spectroscopy: Minimum Requirements and Best Practice Guidelines. Anal Bioanal Chem 2021, 413, 5969–5994. [CrossRef]
- Gall, M.J.; Hendra, P.J.; Peacock, C.J.; Cudby, M.E.A.; Willis, H.A. Laser-Raman Spectrum of Polyethylene: Part 1. Structure and Analysis of the Polymer. Polymer 1972, 13, 104–108. [CrossRef]
- Kotula, A.P.; Meyer, M.W.; De Vito, F.; Plog, J.; Hight Walker, A.R.; Migler, K.B. The Rheo-Raman Microscope: Simultaneous Chemical, Conformational, Mechanical, and Microstructural Measures of Soft Materials. Review of Scientific Instruments 2016, 87, 105105. [CrossRef]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared Spectroscopy of Membrane Lipids. In Encyclopedia of Biophysics; Springer, Berlin, Heidelberg, 2013; pp. 1074–1081 ISBN 978-3-642-16712-6.
Table 1.
List of omega-3 oil samples.
Table 1.
List of omega-3 oil samples.
| Sample type |
Based oil |
The number of samples |
| Raw oil |
Plant-based (PB) |
2 |
| Animal-based AB) |
12 |
| Capsule oil |
Plant-based (PB) |
7 |
| Animal-based (AB) |
14 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).