Submitted:
02 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Procedures
2.2. Technical Characteristics of the Vaccinations Proposed
2.3. Questionnaires
2.4. Statistical Analysis
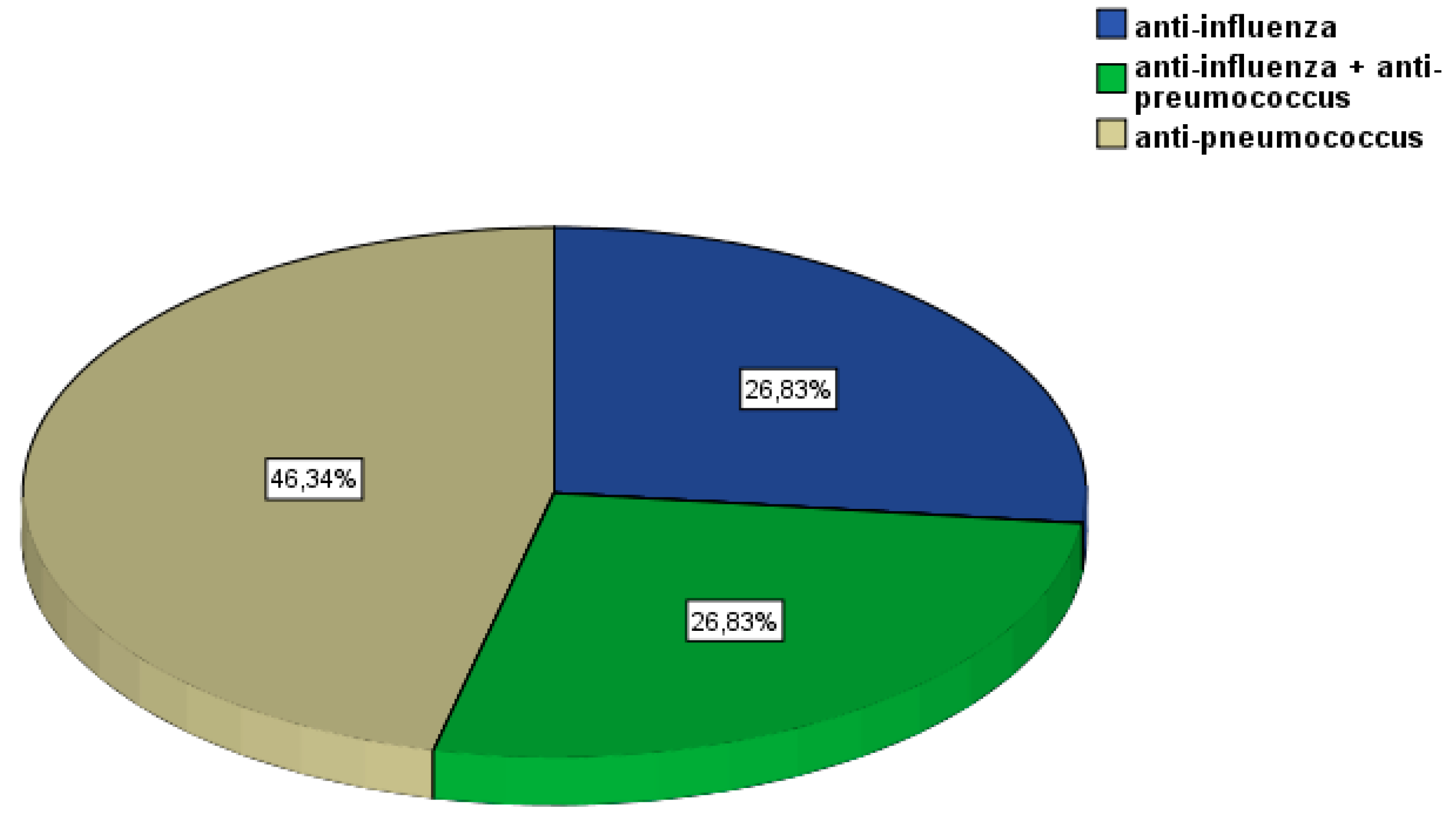
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Health, I.P.a.C.r.f.t.-s. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&codLeg=87997&parte=1%20&serie=null.
- (ECDC), E.C.f.D.C. Flunewseurope, WHO/Europe weekly Influenza update, available online: https://flunewseurope.org/ (accessed on 30th May).
- Epicenter, H.I.o.H.-. Epidemiology for public health and integrated surveillance report of influenza, available online: https://www.epicentro.iss.it/influenza/flunews (accessed on 30th May).
- Organization, W.H. Considerations for pneumococcal vaccination in older adults. Weekly Epidemiological Record 2021, 23, 217-228.
- Scheen, A.; Louis, R.; Lancellotti, P.; Jouret, F.; Delwaide, J.; Moutschen, M. [Pneumococcal vaccination among at-risk groups with comorbidities : guidelines from the "Belgian Superior Health Council" and reimbursement criteria]. Rev Med Liege 2023, 78, 665-673.
- (WHO), W.H.O. In Global Influenza Programme,, available online: https://www.who.int/teams/global-influenza-programme/vaccines#:~:text=A%20flu%20vaccine%20offers%20the,spread%20of%20flu%20to%20others.&text=The%20constantly%20evolving%20nature%20of,frequent%20reformulation%20of%20influenza%20vaccines, : (accessed on 30th May).
- (CDC), C.o.D.C.a.P. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season,. In available online: https://www.cdc.gov/mmwr/volumes/71/rr/rr7101a1.htm?s_cid=rr7101a1 (accessed on 30th May).
- 2019), N.V.P.P.P.-. available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 30th May).
- Bianchi, F.P.; Stefanizzi, P.; Cuscianna, E.; Di Lorenzo, A.; Migliore, G.; Tafuri, S.; Germinario, C.A. Influenza vaccine coverage in 6months-64 years-old patients affected by chronic diseases: A retrospective cohort study in Italy. Human Vaccines & Immunotherapeutics 2023, 19, 2162301.
- Capodici, A.; Salussolia, A.; La Fauci, G.; Di Valerio, Z.; Montalti, M.; Odone, A.; Costantino, C.; Larson, H.J.; Leask, J.; Lenzi, J. Influenza Vaccine Uptake in Italy—The 2022–2023 Seasonal Influenza Vaccination Campaign in Italy: An Update from the OBVIOUS Project. Vaccines 2024, 12, 297.
- Ministry of Health, I.v.-A.v.c. available onlie: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=19 (accessed on 30th May).
- Nichol, K.L. Improving influenza vaccination rates for high-risk inpatients. Am J Med. 1991 Dec;91(6):584-8. [CrossRef]
- Hinman, A.R.; Orenstein, W.A.; Mortimer, E.A., Jr. When, where, and how do immunizations fail? Ann Epidemiol 1992, 2, 805-812, . [CrossRef]
- Ahorsu, D.K.; Lin, C.Y.; Yahaghai, R.; Alimoradi, Z.; Broström, A.; Griffiths, M.D.; Pakpour, A.H. The mediational role of trust in the healthcare system in the association between generalized trust and willingness to get COVID-19 vaccination in Iran. Hum Vaccin Immunother 2022, 18, 1-8, . [CrossRef]
- Thomas RE, L.D. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2018 May 30;5(5):CD005188. [CrossRef]
- Region., H.D.o.t.S. Influenza vaccination campaign 2022/2023-involvement of GPs and PLS, available online: http://www.gurs.regione.sicilia.it/Gazzette/g22-44/g22-44.pdf (accessed on 30th May).
- Schmid, P., Rauber, D., Betsch, C., Lidolt, G., Denker, M.L.,. Barriers of Influenza Vaccination Intention and Behavior - A Systematic Review of Influenza Vaccine Hesitancy, 2005 - 2016. PLoS One. 2017 Jan 26;12(1):e0170550. [CrossRef]
- Veronese, N.; Zambon, N.; Noale, M.; Maggi, S. Poverty and Influenza/Pneumococcus Vaccinations in Older People: Data from The Survey of Health, Ageing and Retirement in Europe (SHARE) Study. Vaccines 2023, 11, 1422.
- Sammon, C.J., McGrogan, A., Snowball, J., de Vries, C.S. Factors associated with uptake of seasonal and pandemic influenza vaccine among clinical risk groups in the UK: an analysis using the General Practice Research Database. Vaccine. 2012 Mar 23;30(14):2483-9. [CrossRef]
- Malosh, R., Ohmit, S.E., Petrie, J.G., Thompson, M.G., Aiello, A.E., Monto, A.S.,. Factors associated with influenza vaccine receipt in community dwelling adults and their children. Vaccine. 2014 Apr 1;32(16):1841-7. [CrossRef]
- Fallucca, A.; Ferro, P.; Mazzeo, L.; Zagra, L.; Cocciola, E.; Oliveri, R.; Tuttolomondo, A.; Benfante, A.; Battaglia, S.; Scichilone, N. Impact of Actively Offering Influenza Vaccination to Frail People during Hospitalisation: A Pilot Study in Italy. Vaccines 2023, 11, 1829.
- Chen H, L.Q., Zhang M, Gu Z, Zhou X, Cao H, Wu F, Liang M, Zheng L, Xian J, Chen Q, Lin Q. Factors associated with influenza vaccination coverage and willingness in the elderly with chronic diseases in Shenzhen, China. Hum Vaccin Immunother. 2022 Nov 30;18(6):2133912. [CrossRef]
- Minardi V, G.R., Possenti V, Contoli B, Di Fonzo D, D'Andrea E, Masocco M. Influenza Vaccination Uptake and Prognostic Factors among Health Professionals in Italy: Results from the Nationwide Surveillance PASSI 2015-2018. Vaccines (Basel). 2023 Jul 10;11(7):1223. [CrossRef]
- Morganti, W.; Veronese, N.; Barbagelata, M.; Castagna, A.; Custodero, C.; Solimando, L.; Burgio, M.I.; Montana Lampo, S.E.; Seminerio, E.; Puleo, G. Validation of a Brief Form of the Self-Administered Multidimensional Prognostic Index: The SELFY-BRIEF-MPI Project. Journal of Clinical Medicine 2023, 12, 6026.
- Szilagyi, P.G.; Bordley, C.; Vann, J.C.; Chelminski, A.; Kraus, R.M.; Margolis, P.A.; Rodewald, L.E. Effect of patient reminder/recall interventions on immunization rates: a review. Jama 2000, 284, 1820-1827.
- McFadden, K., Seale, H.,. A review of hospital-based interventions to improve inpatient influenza vaccination uptake for high-risk adults, 2021 Jan 22;39(4):658-666. [CrossRef]
- Organization, W.H. Essential Immunization Program - Reducing Lost Opportunities to Immunize (MOV);. In available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/implementation/reducing-missed-opportunities-for-vaccination-(mov) (accessed on 30th May).
- Measles Immunization Status of Health Care Workers: A Cross-Sectional Study Exploring Factors Associated with Lack of Immunization According to the Health Belief Model. Vaccines (Basel) 2023;11(3):618. [CrossRef]
- Bakare, M.; Shrivastava, R.; Jeevanantham, V.; Navaneethan, S.D. Impact of two different models on influenza and pneumococcal vaccination in hospitalized patients. Southern medical journal 2007, 100, 140-145.
- Macias, L.M.; Sabato, S.; Obed, M.; Nannini, E.; Crinejo, A.; Losso, M. Effectiveness of influenza vaccination in an ambulatory cohort of patients with flu-like symptoms. International Journal of Infectious Diseases 2018, 73, 359.
- Pennant, K.N.; Costa, J.J.; Fuhlbrigge, A.L.; Sax, P.E.; Szent-Gyorgyi, L.E.; Coblyn, J.; Desai, S.P. Improving Influenza and Pneumococcal Vaccination Rates in Ambulatory Specialty Practices. Open Forum Infectious Diseases 2015, 2, . [CrossRef]
- Manabe, T.; Fujikura, Y.; Mizukami, K.; Akatsu, H.; Kudo, K. Pneumonia-associated death in patients with dementia: A systematic review and meta-analysis. PLoS One 2019, 14, e0213825, . [CrossRef]
- Ridda, I.; Dastouri, F.; King, C.; Yin, J.K.; Tashani, M.; Rashid, H. Vaccination of Older Adults with Dementia Against Respiratory Infections. Infect Disord Drug Targets 2014, 14, 133-139, . [CrossRef]
- Veronese, N.; Demurtas, J.; Smith, L.; Michel, J.P.; Barbagallo, M.; Bolzetta, F.; Noale, M.; Maggi, S. Influenza vaccination reduces dementia risk: A systematic review and meta-analysis. Ageing Res Rev 2022, 73, 101534, . [CrossRef]
- Gallini, A.; Coley, N.; Andrieu, S.; Lapeyre-Mestre, M.; Gardette, V. Effect of dementia on receipt of influenza vaccine: a cohort study in French older adults using administrative data: 2007-2012. Fundam Clin Pharmacol 2017, 31, 471-480, . [CrossRef]
- Antonelli Incalzi, R.; Consoli, A.; Lopalco, P.; Maggi, S.; Sesti, G.; Veronese, N.; Volpe, M. Influenza vaccination for elderly, vulnerable and high-risk subjects: a narrative review and expert opinion. Intern Emerg Med 2023, 10.1007/s11739-023-03456-9, . [CrossRef]
- Lee, J.K.; Lam, G.K.; Yin, J.K.; Loiacono, M.M.; Samson, S.I. High-dose influenza vaccine in older adults by age and seasonal characteristics: Systematic review and meta-analysis update. Vaccine: X 2023, 100327.
- Hoshi, S.-l.; Shono, A.; Seposo, X.; Okubo, R.; Kondo, M. Cost-effectiveness analyses of 15-and 20-valent pneumococcal conjugate vaccines for Japanese elderly. Vaccine 2022, 40, 7057-7064.
- Zhang, Y.-Y.; Tang, X.-F.; Du, C.-H.; Wang, B.-B.; Bi, Z.-W.; Dong, B.-R. Comparison of dual influenza and pneumococcal polysaccharide vaccination with influenza vaccination alone for preventing pneumonia and reducing mortality among the elderly: A meta-analysis. Human Vaccines & Immunotherapeutics 2016, 12, 3056-3064.
- Capasso, M.; Bianchi, M.; Caso, D. Psychosocial Factors Influencing Parents’ Acceptance of COVID-19 Vaccination for Their Children: An Italian Cross-Sectional Study. Vaccines 2024, 12, 317.
- Rao, S., Fischman, V., Moss, A., Ziniel, S.I., Torok, M.R., McNeely, H., Hyman, D., Wilson, K.M., Dempsey, A.F.,. Exploring provider and parental perceptions to influenza vaccination in the inpatient setting. Influenza Other Respir Viruses. 2018 May;12(3):416-420. [CrossRef]
- Wennekes, M.; Eilers, R.; Caputo, A.; Gagneux-Brunon, A.; Gavioli, R.; Nicoli, F.; Quatrehomme, M.; Vokó, Z.; Timen, A. Vaccines for older adults; the low-hanging fruit of disease prevention. European Journal of Public Health 2022, 32, ckac131. 407.
- Vermeir, P.; Vandijck, D.; Degroote, S.; Peleman, R.; Verhaeghe, R.; Mortier, E.; Hallaert, G.; Van Daele, S.; Buylaert, W.; Vogelaers, D. Communication in healthcare: a narrative review of the literature and practical recommendations. International journal of clinical practice 2015, 69, 1257-1267.
- Verger, P.; Fressard, L.; Collange, F.; Gautier, A.; Jestin, C.; Launay, O.; Raude, J.; Pulcini, C.; Peretti-Watel, P. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine 2015, 2, 891-897.
- Killian, M.; Detoc, M.; Berthelot, P.; Charles, R.; Gagneux-Brunon, A.; Lucht, F.; Pulcini, C.; Barbois, S.; Botelho-Nevers, E. Vaccine hesitancy among general practitioners: evaluation and comparison of their immunisation practice for themselves, their patients and their children. European Journal of Clinical Microbiology & Infectious Diseases 2016, 35, 1837-1843.
| Variable | Values | Mean values or frequency |
|---|---|---|
| Age | ||
| Mean age (SD) | 75.4 (7.9) (range: 60-96) | |
| 60-70 years | 24.4 | |
| 70-80 years | 46.3 | |
| 80-90 years | 24.4 | |
| >90 years | 4.9 | |
| Gender | ||
| F | 68.3 | |
| M | 31.7 | |
| Frailty status | ||
| Robust | 29.3 | |
| Pre-frailty | 50.0 | |
| Frailty | 19.5 | |
| Missing | 1.2 | |
| Ambulatory | ||
| Cognitive disorders | 65.9 | |
| Endocrinology | 26.8 | |
| Caregivers | 7.3 | |
| Civil status | ||
| Married | 57.3 | |
| Widow | 35.4 | |
| Single | 7.3 | |
| Educational level | ||
| Elementary | 45.1 | |
| Medium school | 26.8 | |
| High school | 12.2 | |
| Degree | 6.1 | |
| No scholarity | 8.5 | |
| Smoking status | ||
| Actual | 12.2 | |
| Former | 35.4 | |
| Never | 52.2 |
| Variable | Values | Influenza | Pneumococcus |
|---|---|---|---|
| Influenza last 5 years | |||
| Yes | 56.1 | - | |
| Number of seasons with influenza symptoms | - | ||
| 1 | 48.9 | ||
| 2 | 26.7 | ||
| 3 | 13.3 | ||
| 4 | 11.1 | ||
| Suggested by GP | |||
| Yes | 82.9 | 13.4 | |
| No | 9.8 | 48.8 | |
| Don’t know/remember | 7.3 | 37.8 | |
| Vaccination in the past | |||
| Yes | 78.0 | 7.3 | |
| No | 19.5 | 90.2 | |
| Don’t know/remember | 2.4 | 2.4 | |
| If no, what’s the reason | |||
| Not suggested by GP | 18.9 | 29.5 | |
| Felt not efficacious | 6.3 | 5.9 | |
| Fear of adverse events | 74.8 | 47.0 | |
| Other reasons | - | 17.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





