Preprint
Review
BDNF Modulation by microRNAs: An Update on the Experimental Evidence
Altmetrics
Downloads
156
Views
42
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
02 April 2024
Posted:
02 April 2024
You are already at the latest version
Alerts
Abstract
Abstract: MicroRNAs can interfere with a protein function by suppressing their messenger RNA translation or the synthesis of its related factors. The function of brain-derived neurotrophic factor (BDNF) is essential to the proper formation and function of the nervous system and is seen to be regulated by many microRNAs. However, understanding how microRNAs influence BDNF actions within cells requires a wider comprehension of their integrative regulatory mechanisms. Aim: In this literature review, we have synthesized the evidence of microRNA regulation on BDNF in cells and tissues, and provided an analytical discussion about direct and indirect mechanisms that appeared involved in BDNF regulation by microRNAs. Methods: Searches were conducted on PubMed.gov using the terms “BDNF” AND “MicroRNA” and “brain-derived neurotrophic factor” AND “MicroRNA”, updated on September 1st, 2023. Papers without open access were requested from the authors. One hundred and seventy-one papers were included for review and discussion. Results and Discussion: The local regulation of BDNF by microRNAs involves a complex interaction between a series of microRNAs with target proteins that can either inhibit or enhance BDNF expression, at the core of cell metabolism. Therefore, understanding this homeostatic balance provides resources for the future development of vector-delivery-based therapies for the neuroprotective effects of BDNF.
Keywords:
Subject: Biology and Life Sciences - Neuroscience and Neurology
Introduction
MicroRNAs are a class of non-coding RNAs which do not code for proteins but carry out biological function by regulating cell proteome at the translational level. They can be expressed within the activation of a gene promoter or their own promoters (Lai, 2002; Smallridge, 2001), and play a regulatory role in protein synthesis by targeting and degrading RNA transcripts containing compatible nucleotide sequences (Gulyaeva & Kushlinskiy, 2016; Ha & Kim, 2014; Y. Lee et al., 2004; O’Brien et al., 2018; Shukla et al., 2011). MicroRNAs are seen to participate in the functional regulation at the distant synaptic sites in neuronal cells, and to modulate inflammatory mechanisms that lead to neurological diseases (Chen et al., 2024; Kaurani, 2024).
As a main expressed neurotrophin, the brain-derived neurotrophic factor (BDNF) plays essential roles in the development and maintenance of neural tissues (Labrador-Velandia et al., 2019; Trzaska et al., 2009). Signaling of the mature form of BDNF via tropomyosin receptor kinase (Trk) B, participates in neuronal survival, dendritogenesis, synaptogenesis, axon growth and synaptic function; meanwhile, release pro-BDNF isoform can bind with low affinity to p75 neurotrophin receptor (p75NTR) and lead to apoptosis. So that, a tight regulation of BDNF activity is necessary for the proper functioning of the central nervous system (CNS) (Bouron et al., 2006; de Assis & Hoffman, 2022; Ibarra et al., 2022).
Analyses in silico estimate hundreds of microRNAs as possible regulators of BDNF. However, with a 10–20% variability detected in predicted regulatory relationships between genes and microRNAs in human RefSeq data set, the effective regulation of BDNF mRNA transcripts by microRNAs in biological systems is much smaller (Rajewsky, 2006). In addition, the microRNA affinity for multiple targets and microRNA-microRNA interactions in a cell milieu influence on their regulation and cannot be predicted by computational logarithms. As the studies typically address only one or a few number of microRNAs in the experiments, it remains a challenge to design pre-clinical studies based on computational predictions (Lai, 2002; S. C. Li et al., 2010).
Regarding the above mentioned, and that understanding how microRNAs effectively regulate BDNF actions provide basis for the development of potential therapies against neurodegenerative conditions; we have collected all the available data on the post-transcriptional regulation of BDNF by microRNAs evidenced in experimental studies, and provided a synthesis of the regulatory mechanisms currently demonstrated.
Methods
In order to retire all the scientific publications possibly reporting data from the analysis of microRNAs and BDNF expression in a same biological system, a systematic search was conducted on PubMed.gov using the following combination of terms: [“BDNF” AND “MicroRNA”] OR [“brain-derived neurotrophic factor” AND “MicroRNA”]. All the available publications were retrieved and screened by abstract. Papers published without open-access were requested from the authors via email or ResearchGate. Studies containing data from BDNF and microRNA analyses in vivo or in vitro were considered for inclusion. The studies reporting data of microRNAs that did not influence on BDNF regulation, Reviews, articles not written in English, high throughput profiles and computational predictor studies, as well as those not made available by the authors were excluded from discussion during full text assessment. The last search update, performed in September 1th 2023, launched 314 papers published from 2006 to 2023 indexed in PubMed (see Figure 1). The studies selection was performed using the software Mendeley 1.19.8.
Two-hundred and ninety-seven articles were sought for retrieval according to the inclusion criteria of containing data from BDNF and microRNA analyses, after duplicates removal. Two hundred and sixty-one papers were assessed by full text. A total of 172 were found to include the analyses of BDNF and diverse microRNAs and were included in the qualitative synthesis, after exclusion criteria (Figure 1). A list of the studies and microRNAs involved in BDNF regulation was displayed in Table 1.
Results
Discussion
BDNF by microRNAs
A great number of microRNAs are able to target and influence BDNF activity (Figure 2). The post-transcriptional regulation of BDNF can influence on BDNF synthesis and activity in a non-specific manner throughout tissues. Moreover, in addition to the ability to target and degrade the transcripts of BDNF mRNA in a ‘direct regulation’, microRNAs can affect the activity of BDNF (either positively or negatively) via regulation of other factors, here referred as ‘indirect regulation’. Great part of research identifying BDNF-target microRNAs regard to investigations on the oncogenicity of BDNF/TrkB signal transduction in tumor cell growth and metastasis (Y. Li et al., 2023; Ni & Zhang, 2020). A list of microRNAs found to degrade BDNF mRNA transcripts in oncology research follows: miR-10a, miR-22, miR-204, miR-107, miR-382, miR-496, miR-497, miR-584, miR-744, miR-26a-1 and miR-26a-2 subtypes (Caputo et al., 2011; Cheng et al., 2018; B. Gao et al., 2017; L. Gao et al., 2018; Imam et al., 2012; J. D. Jiang et al., 2019; R. Li et al., 2018; Wu Li et al., 2015; Long et al., 2016; R. Ma et al., 2019; J. Y. Peng et al., 2016; D. Song et al., 2017; Y. Song et al., 2019; Z. Sun et al., 2019; P. Wang et al., 2017; Xia et al., 2016; Xiang et al., 2016; A. J. Xu et al., 2017; Yan et al., 2015; Xiao yu Zhang et al., 2018). The evidences that miR-206 is able to suppress BDNF synthesis in diverse tissues such as the cardiac muscles, the skeletal muscles and the endothelial tissue elucidate a role for microRNAs in a tissue-tissue communication, although their actions might be locally regulated (Guan et al., 2021; S. T. Lee et al., 2012; Mu et al., 2015; D. Peng et al., 2022; Solomon et al., 2019; W. Sun et al., 2017; Tapocik et al., 2014; Tian et al., 2014; W. Tu et al., 2022; M. Wang et al., 2018; Xie et al., 2017; Xing et al., 2018; X. Yang et al., 2014; H. Zhao et al., 2019).
Some microRNAs can be are exported from cells by membrane-derived vesicles, lipoproteins, and other ribonucleoprotein complexes and travel through the blood stream reaching recipient cells in distant tissues (Boon & Vickers, 2013), providing a communication between disparate cell types and diverse biological mechanisms and homeostatic pathways. Our data collection reports a number of microRNAs whose circulating levels are increased in inflammatory conditions and that demonstrably suppress BDNF synthesis in the CNS, namely: miR-1, miR-128, miR-182-5p, miR-195-5p and miR-451a (F. Fang et al., 2022; Jian Gao et al., 2022; Giordano et al., 2020; J. C. Ma et al., 2015; Misiorek et al., 2020). Among those circulating microRNAs which suppress BDNF synthesis, some were found involved in the physiopathology of neuropsychiatric disorders and disease such as anxiety/depression – miR-182, miR-206-3p, miR-1-3p, MiR-16, miR-124, miR-432 and miR-182 (Bai et al., 2012; Ding et al., 2022; Y. Fang et al., 2018; Y. J. Li et al., 2013; Miao et al., 2018; Y. X. Sun et al., 2013; Xiaonan Zhang et al., 2021; Z. Zhang et al., 2022), Schizophrenia - miR-16, miR-195 and miR-30a-5p (Asadi et al., 2022; Mellios et al., 2008, 2009; Pan et al., 2021), Parkinson’s disease - miR-494-3p (C. Deng et al., 2020) and Dementia - miR-10a, miR-34a-5p, miR-204 and miR-613 (Cui et al., 2017; Ge et al., 2018; Giannotti et al., 2014; Lambert et al., 2003; Wei Li et al., 2016; Ji Chao Ma et al., 2018; Ryan et al., 2013; B. W. Wu et al., 2018). Together, those findings suggest a mechanistic crosstalk driven by microRNAs between inflammation in peripheral systems and neurodegenerative processes.
MicroRNAs play crucial roles in immunoinflammatory reactions. In normal conditions, the CNS parenchyma is not exposed to peripheral immune cells or robust inflammatory responses and microglia and astrocytes remain quiescent. However, upon stress, astrocytes and microglia transiently activate and produce chemokines and cytokines, and other small molecule messengers (prostaglandins, nitric oxide, reactive oxygen species - ROS) which contribute to the inflammatory response and subsequent restoration of CNS homeostasis (Jingdan Zhang et al., 2023). The study by (Kynast et al., 2013) identified that miR-124 is constitutively expressed in neuron of the dorsal horn in spinal cord, where its elevation is associated with a decrease in BDNF levels. While a decrease in miR-124 levels lead elevation in MeCP2 and BDNF expression levels. From a different perspective, the studies by (Duclot & Kabbaj, 2017; Wenqian Yang et al., 2019) demonstrated that miR-124 is able to attenuate an acute increase in pro-inflammatory factors in the CNS, by suppressing the early growth response 1 (EGR1) and preventing a decline in BDNF expression. Conversely, (Yu et al., 2022) showed that BDNF administration increased the expression levels of miR-3168, and suppressed the secretion of interleukin (IL)-1β, TNF-α, and IL-6 in the activated macrophage.
Another mechanism by which microRNAs indirectly modulate BDNF synthesis in inflammatory conditions involve the Let-7 miRNA family (Roush & Slack, 2008), which include let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i, miR-98 and miR-202 (Y. Ma et al., 2021). Dysregulation of let-7 leads to a less differentiated cellular state and cell-based diseases such as cancer. Cho and colleagues (2015) investigation in neural tissue reported that let-7a levels increase in microglia following the accumulation of ROS and pro-inflammatory cytokines. The data indicated that let-7a participates in reducing nitrite production while increasing the levels of inducible NO synthase and IL-6. Anti-inflammatory events that accompanied an upregulation in BDNF expression levels. Alternatively, (Nguyen et al., 2018) detected that miR-let-7i suppresses the synthesis of progesterone receptor membrane component 1, reducing progesterone-inducible release of BDNF by astrocytes. Such reduction has a negative effect on neuronal tissue recovery. These findings show that Let-7 members might exert specific roles that positively or negatively affect BDNF function in CNS parenchyma.
Although the regulation of a protein function by microRNAs mostly always depend on their nucleotide sequence to target mRNA transcripts present in a same micro environment; in silico predictions of BDNF target microRNAs are not always confirmed in biological systems. Meanwhile, some experiments have pointed out that microRNA targeting of BDNF mRNA is selectively guided by their prime untranslated region (3′-UTR) (Caputo et al., 2011; Shrestha et al., 2019; Varendi et al., 2014; Jun Zhang et al., 2018). Having noted the presence of two variants of 3’ UTR regions in the mRNA transcripts of BDNF, which exert an influence on their cellular trafficking/localization (Lekk et al., 2023), the mechanistic regulation of BDNF by microRNA within a cell might as well occurs in a local specificity manner, a least in cells that express the two BDNF mRNA 3’ UTR isoforms.
Neuroplasticity and BDNF Regulation by microRNAs
The expression of BDNF is present in progenitor cells from the early embryonic phase and in neural tissue throughout the whole lifespan. It participation in essential processes such as dendritogenesis, axonal innervation and synaptogenesis, neuronal growth and survival guaranties the maintenance and proper functioning of the neuronal tissue (Barde et al., 1987; Citri & Malenka, 2008; Robinson, 1996).
A growing number of microRNAs have been identified as direct regulators of BDNF in the neural tissue. Here, we list some of the microRNAs that target and degrade BDNF mRNA transcripts and modulate BDNF actions in processes such as neuronal cell growth, differentiation and proliferation: miR-1, miR-1b, miR-1-3p, miR-10a, miR-10b, miR-10a-5p, miR-15a, miR-16, miR-26a, miR-34a, miR-103, miR-125b, miR-125b-5p, miR-30a-5p, miR-34a-5p, miR-140, miR-139-5p, miR-155, miR-191, miR-181, miR-186, miR-191, miR-191-5p, miR-191a-5p, miR-204-5p, miR-206, miR-210, miR-210-3p miR-211, miR-216a-5p, miR-219, miR-636-3p, miR-365, miR-375, miR-551b-5p, miR-937, miR-497 and the miR-497a subtypes (Aili et al., 2016; Angelucci et al., 2011; Burckhardt et al., 2019; Croce et al., 2013; Darcq et al., 2015; L. Deng et al., 2022; Duan et al., 2018; Ehinger et al., 2021; Fu et al., 2017; Lin Gao et al., 2020; J. J. Hu et al., 2020; X. M. Hu et al., 2016; Huan et al., 2021; Hung et al., 2019; Hutchison et al., 2013; Y. Jiang & Zhu, 2015; Ke et al., 2021; Hongxia Li et al., 2021; Xiaojie Li et al., 2021; Xiu-juan Li, 2018; S. P. Liang et al., 2019; B. Lin et al., 2021; H. Liu et al., 2020; X. Liu et al., 2020; Zhen Liu et al., 2015; Lu et al., 2018; Lv et al., 2018; Miao et al., 2019; Mohammadipoor-Ghasemabad et al., 2019; Nagpal et al., 2013; Neumann et al., 2015, 2016; Niu et al., 2021; Panta et al., 2019; Scheen et al., 2015; B. Su et al., 2022; Tang et al., 2021; Z. Tu et al., 2017; F. Wang et al., 2020; Li Wang et al., 2020; Linxiao Wang et al., 2022; P. Wang et al., 2017; G. Wu et al., 2020; Y. Wu et al., 2021; H. Xu et al., 2020; W. Xu et al., 2022; Y. Xu et al., 2021; S. Yi et al., 2016; Yongguang et al., 2022; Zeng et al., 2016; Zhai et al., 2022; Jing Zhang et al., 2014; Jun Zhang et al., 2018; K. Zhang et al., 2017; T. Zhang et al., 2020; Xiao yu Zhang et al., 2018; P. Zhao et al., 2021; X. Zhao et al., 2019).
Some studies showed that Sonic hedgehog (Shh) is able to relief the suppression exerted by miR-206 on BDNF synthesis, which led to an enhancement in BDNF-TrkB signaling during the processes of differentiation and innervation in muscle cells (Miura et al., 2012; Radzikinas et al., 2011). Shh is a key signaling molecule in the embryonic morphogenesis and organization of nervous system. Its signaling via receptor Patched mediated Smoothened receptor complex is putative to the development of neural tube; whereas, abnormal activation of Shh signaling implicates in various types of cancers (Odent et al., 1999). This indirect and positive effect of Shh on BDNF activity seem to be involved in a complex and phasic destabilization of cell homeostasis during differentiation in mesenchymal cells.
BDNF binding to TrkB receptors at neuronal cells surface lead to the dimerization and transphosphorylation of a critical regulator of actin dynamics in the axons and dendrites named LIM Domain Kinase 1 (LIMK1). This occurs independently of TrkB kinase activity. The LIMK1 mRNA transcript is a target for miR-134 in the axon and dendrite cell compartments, and is able to annul BDNF/TrkB-induced protein synthesis during the synaptic activity, whenever TrkB activation is not sufficient to surpass miR-134 suppression on LIMK1. This suggest that miR-134 actively participates in competitive synapses formation; and establishes a role for this microRNA in the fine tune regulation of neuroplasticity processes (Dong et al., 2012; Jun Gao et al., 2010; Han et al., 2011; Kim et al., 2019; Kumari et al., 2016; M. Li et al., 2015; Schratt et al., 2006).
Several microRNAs were found to target different components of BDNF-TrkB signaling intracellular cascades, consequently decreasing the activity of cAMP response element binding (CREB) protein, leading to a decrease in BDNF gene expression. The investigation by (Thomas et al., 2017) identified that miR-137 regulates the levels of various proteins within the PI3K-Akt-mTOR pathway in neurons, namely: p55g, PTEN, Akt2, GSK3b, mTOR, and rictor. And this negatively affects BDNF- induced dendritic outgrowth. In addition, the miR-221, miR-383 and miR-199a-5p were shown to suppress the synthesis of Wnt2, which is a glycoprotein with essential roles in the embryonic development and dendrite development (Wayman et al., 2006). The neuronal activity enhances CREB-dependent transcription of Wnt2, which in turn, stimulates dendritic arborization. Both Wnt2 and BDNF are CREB-responsive genes and so Wn2t suppression results in a decrease in BDNF expression possibly via a Wnt2/CREB/BDNF axis (Lian et al., 2018; S. Liu et al., 2021; Zheng Liu et al., 2021). Additionally, some microRNAs were reported as negatively correlated with the levels of BDNF in studies, i.e. miR-183/96 (Hongyang Li et al., 2015; C. R. Lin et al., 2014), miR-134 (Huang et al., 2017; Shen et al., 2018, 2019), and miR-182-5p (F. Fang et al., 2022; C. Li et al., 2022).
Cell Metabolism and BDNF Regulation by microRNAs
The post-transcriptional regulation of proteins elicits compensatory mechanisms to maintain transcriptional activity of essential proteins involved in cell energy homeostasis. The integrative regulation of a number of proteins in the core of cell metabolism homeostasis affects BDNF gene expression by various manners, including it self-regulation via autocrine and/or paracrine TrkB signaling. BDNF/TrkB activation leads to the activation of several small G proteins in addition to the pathways regulated by mitogen-activated protein kinase (MAPK), PI 3-kinase (PI-3K), and phospholipase-Cγ (PLCγ) (Numakawa et al., 2010). Meanwhile, as miR-101 suppresses MAPK phosphatases 1 (which dephosphorylates p38, JNK and ERK), it has a positive effect on ERK phosphorylation and the downstream activation of BDNF expression in cortical neurons (Y. Zhao et al., 2017).
The activity of AMP-activated protein kinase (AMPK) and CREB represent the axis of cell energy metabolism. A compensatory increase in CREB activity following a decrease in the concentrations of BDNF and methyl CpG binding protein 2 (MeCP2) was evidenced in the brain of 132/212 KO mice (Hernandez-Rapp et al., 2015; Klein et al., 2007). While MeCP2 is a nuclear protein that may function as both a transcriptional activator or repressor, it works as a stabilizer of BDNF expression patterns and cell homeostasis (Buist et al., 2021; Pejhan et al., 2020; Vuu et al., 2023). Another compensatory effect is seen for BDNF in dendritogenesis when inhibition of miR-15a, and the consequent relief of BDNF supression, can rescue dendritic maturation deficits in MeCP2-deficient neurons (Y. Gao et al., 2015). Further, upregulation in BDNF gene expression accompanies an increase in the expression of miR-132/212 cluster; both of which target and suppress MeCP2 mRNA translation. Suppression of miR-132 and miR-212 on MeCP2 relieves its repression on BDNF expression. By this manner, the expression of BDNF and miR-132 and miR-212 represent a self-regulatory homeostatic mechanism that involves the nuclear protein MeCP2 at the core of cell metabolism (Chen-Plotkin et al., 2012; Im et al., 2010; Jimenez-Gonzalez et al., 2016; Kawashima et al., 2010; Klein et al., 2007; Y. Liang et al., 2016; Marler et al., 2014; Mendoza-Viveros et al., 2017; M. Su et al., 2015; Wibrand et al., 2012; L. T. Yi et al., 2014).
The enzymatic activity of the histone deacetylase Sirtuin 1 (SIRT1) in the nicotinamide adenine dinucleotide (NAD)-dependent deacetylation of histones is crucial to protect cells from oxidative stressors. SIRT1 activates the expression of mitochondrial DNA genes related to mitochondrial biogenesis, ATP generation and cell proliferation. It was detected in experiments that SIRT1 is able to inhibit miR-134 expression by directly binding to its inhibitory elements. Whereas, SIRT1 deficiency and high levels of miR-134 result in a downregulation of CREB and BDNF expression, and a negative effect on neuronal survival/plasticity. Another indirect mechanism by which miR-134 negatively affects BDNF function in the core of cell metabolism (Jun Gao et al., 2010; Huang et al., 2015, 2017; Shao et al., 2015; Shen et al., 2019). Finally, the study by (Oikawa et al., 2015) showed that the guanine nucleotide binding protein alpha inhibitor 1 (GNAI1), an adenylate cyclase inhibitor which regulates the ATP conversion to cAMP, is a target of miR-124. In physiological conditions, suppression of GNAI1 by miR-124 increases in cAMP activity and leads to an upregulation of BDNF expression via cAMP/PKA/CREB pathway. Indeed, alterations in cell metabolism and microRNA environment reflect on the regulation of BDNF.
MiR-124 suppression on BDNF activity negatively influence on neuronal plasticity in various brain regions such as hippocampus and striatum (Bahi, 2016, 2017; Bahi et al., 2014; Bahi & Dreyer, 2013; Chandrasekar & Dreyer, 2009). More recently, (Wei Yang et al., 2020) identified that the miR-124 targets CREB mRNA, consequently downregulating BDNF expression, and alters BDNF function via targeting to various gene transcripts downstream TrkB signaling, e.g. PI3K, Akt3 and Ras (Kang et al., 2022). Likewise, miR-124 negatively influence on BDNF signal transduction via suppression of glucocorticoid receptors (S. S. Wang et al., 2017; L. T. Yi et al., 2018), known to enhance TrkB signaling pathways (de Assis & Gasanov, 2019). From another perspective, by testing different exercise intensities, (Mojtahedi et al., 2013) showed that miR-124 levels increase with the intensity, and this increase is amplified in strenuous intensity. BDNF and TrkB also increased but not in strenuous the intensity exercise. The findings indicate a threshold beyond which changes metabolic demands evoke an acute rise in miR-124 levels and suppression.
Amongst the indirect effects seen for microRNA on BDNF, (B. Jiang et al., 2016) study registered that miR-9 upregulates BDNF expression in retinal ganglion cells by suppressing the restrictive silencer factor/RE1-silencing transcription factor (REST), a transcription repressor whose suppression is required for neuronal cell differentiation. Similarly, miR-29c has a positive effect on BDNF expression levels by targeting DNA methyltransferase 3 (G. Yang et al., 2015). The miR-705 was also found in a positive correlation with BDNF levels in ischemic injured brain (Ji et al., 2017). Further, BDNF administration increases miR-214 expression during embryonic stem cells differentiation into endothelial cells (Descamps et al., 2018); and to promote vascular endothelial growth factor-C- dependent lymph angiogenesis by suppressing miR-624-3p in human chondrosarcoma cells (C. Y. Lin et al., 2017).
Final Considerations
Regulation of BDNF by microRNAs involves a dynamic regulation of basic proteins that integrate core mechanisms in neuronal cell homeostasis. This complexity represents a relevant limitation in research towards development of therapeutic strategies. Nevertheless, based on the collection of data, using multiple microRNAs that cooperatively influence on BDNF function might be a prospective strategy for future studies addressing vector-delivery based treatments.
Funding
No funding declared.
Conflicts of Interest
No conflict of interest declared.
References
- Aili, A., Chen, Y., & Zhang, H. (2016). MicroRNA-10b suppresses the migration and invasion of chondrosarcoma cells by targeting brain-derived neurotrophic factor. Molecular Medicine Reports, 13(1), 441–446. [CrossRef]
- Angelucci, F., Croce, N., Spalletta, G., Dinallo, V., Gravina, P., Bossù, P., Federici, G., Caltagirone, C., & Bernardini, S. (2011). Paroxetine rapidly modulates the expression of brain-derived neurotrophic factor mRNA and protein in a human glioblastoma-astrocytoma cell line. Pharmacology, 87(1–2), 5–10. [CrossRef]
- Asadi, M. R., Gharesouran, J., Sabaie, H., Moslehian, M. S., Dehghani, H., Arsang-Jang, S., Taheri, M., Mortazavi, D., Hussen, B. M., Sayad, A., & Rezazadeh, M. (2022). Assessing the expression of two post-transcriptional BDNF regulators, TTP and miR-16 in the peripheral blood of patients with Schizophrenia. BMC Psychiatry, 22(1), 771. [CrossRef]
- Bahi, A. (2016). Sustained lentiviral-mediated overexpression of microRNA124a in the dentate gyrus exacerbates anxiety- and autism-like behaviors associated with neonatal isolation in rats. Behavioural Brain Research, 311, 298–308. [CrossRef]
- Bahi, A. (2017). Hippocampal BDNF overexpression or microR124a silencing reduces anxiety- and autism-like behaviors in rats. Behavioural Brain Research, 326, 281–290. [CrossRef]
- Bahi, A., Chandrasekar, V., & Dreyer, J. L. (2014). Selective lentiviral-mediated suppression of microRNA124a in the hippocampus evokes antidepressants-like effects in rats. Psychoneuroendocrinology, 46, 78–87. [CrossRef]
- Bahi, A., & Dreyer, J. L. (2013). Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. European Journal of Neuroscience, 38(2), 2328–2337. [CrossRef]
- Bai, M., Zhu, X., Zhang, Y., Zhang, S., Zhang, L., Xue, L., Yi, J., Yao, S., & Zhang, X. (2012). Abnormal Hippocampal BDNF and miR-16 Expression Is Associated with Depression-Like Behaviors Induced by Stress during Early Life. PLoS ONE, 7(10), e46921. [CrossRef]
- Barde, Y. A., Davies, A. M., Johnson, J. E., Lindsay, R. M., & Thoenen, H. (1987). Brain derived neurotrophic factor. Progress in Brain Research, 71(C), 185–189. [CrossRef]
- Boon, R. A., & Vickers, K. C. (2013). Intercellular Transport of MicroRNAs. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(2), 186–192. [CrossRef]
- Bouron, A., Boisseau, S., De Waard, M., & Peris, L. (2006). Differential down-regulation of voltage-gated calcium channel currents by glutamate and BDNF in embryonic cortical neurons. European Journal of Neuroscience, 24(3), 699–708. [CrossRef]
- Buist, M., Fuss, D., & Rastegar, M. (2021). Transcriptional regulation of mecp2e1-e2 isoforms and bdnf by metformin and simvastatin through analyzing nascent rna synthesis in a human brain cell line. Biomolecules, 11(8). [CrossRef]
- Burckhardt, M. A., Abraham, M. B., Mountain, J., Coenen, D., Paniora, J., Clapin, H., Jones, T. W., & Davis, E. A. (2019). Improvement in psychosocial outcomes in children with type 1 diabetes and their parents following subsidy for continuous glucose monitoring. Diabetes Technology and Therapeutics, 21(10), 538–545. [CrossRef]
- Caputo, V., Sinibaldi, L., Fiorentino, A., Parisi, C., Catalanotto, C., Pasini, A., Cogoni, C., & Pizzuti, A. (2011). Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS ONE, 6(12). [CrossRef]
- Chandrasekar, V., & Dreyer, J. L. (2009). microRNAs miR-124, let-7d and miR-181a regulate Cocaine-induced Plasticity. Molecular and Cellular Neuroscience, 42(4), 350–362. [CrossRef]
- Chen-Plotkin, A. S., Unger, T. L., Gallagher, M. D., Bill, E., Kwong, L. K., Volpicelli-Daley, L., Busch, J. I., Akle, S., Grossman, M., Van Deerlin, V., Trojanowski, J. Q., & Lee, V. M. Y. (2012). TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. Journal of Neuroscience, 32(33), 11213–11227. [CrossRef]
- Chen, Y., Mateski, J., Gerace, L., Wheeler, J., Burl, J., Prakash, B., Svedin, C., Amrick, R., & Adams, B. D. (2024). Non-coding RNAs and neuroinflammation: implications for neurological disorders. Experimental Biology and Medicine (Maywood, N.J.), 249(February), 10120. [CrossRef]
- Cheng, F., Yang, Z., Huang, F., Yin, L., Yan, G., & Gong, G. (2018). microRNA-107 inhibits gastric cancer cell proliferation and metastasis by targeting PI3K/AKT pathway. Microbial Pathogenesis, 121, 110–114. [CrossRef]
- Cho, K. J., Song, J., Oh, Y., & Lee, J. E. (2015). MicroRNA-Let-7a regulates the function of microglia in inflammation. Molecular and Cellular Neuroscience, 68, 167–176. [CrossRef]
- Citri, A., & Malenka, R. C. (2008). Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology, 33(1), 18–41. [CrossRef]
- Croce, N., Gelfo, F., Ciotti, M. T., Federici, G., Caltagirone, C., Bernardini, S., & Angelucci, F. (2013). NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: A possible role in neuroprotection? Molecular and Cellular Biochemistry, 376(1–2), 189–195. [CrossRef]
- Cui, M., Xiao, H., Li, Y., Dong, J., Luo, D., Li, H., Feng, G., Wang, H., & Fan, S. (2017). Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1863(9), 2333–2341. [CrossRef]
- Darcq, E., Warnault, V., Phamluong, K., Besserer, G. M., Liu, F., & Ron, D. (2015). MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Molecular Psychiatry, 20(10), 1240–1250. [CrossRef]
- de Assis, G. G., & Gasanov, E. V. (2019). BDNF and Cortisol integrative system – Plasticity vs. degeneration: Implications of the Val66Met polymorphism. Frontiers in Neuroendocrinology, 55, 100784. [CrossRef]
- de Assis, G. G., & Hoffman, J. R. (2022). The BDNF Val66Met Polymorphism is a Relevant, But not Determinant, Risk Factor in the Etiology of Neuropsychiatric Disorders – Current Advances in Human Studies: A Systematic Review. Brain Plasticity, 8(2), 133–142. [CrossRef]
- Deng, C., Zhu, J., Yuan, J., Xiang, Y., & Dai, L. (2020). Pramipexole Inhibits MPP+-Induced Neurotoxicity by miR-494-3p/BDNF. Neurochemical Research, 45(2), 268–277. [CrossRef]
- Deng, L., Lai, S., Fan, L., Li, X., Huang, H., & Mu, Y. (2022). miR-210-3p suppresses osteogenic differentiation of MC3T3-E1 by targeting brain derived neurotrophic factor (BDNF). Journal of Orthopaedic Surgery and Research, 17(1), 1–9. [CrossRef]
- Descamps, B., Saif, J., Benest, A. V., Biglino, G., Bates, D. O., Chamorro-Jorganes, A., & Emanueli, C. (2018). BDNF (Brain-Derived Neurotrophic Factor) promotes embryonic stem cells differentiation to endothelial cells via a molecular pathway, including MicroRNA-214, EZH2 (Enhancer of Zeste Homolog 2), and eNOS (Endothelial Nitric Oxide Synthase). Arteriosclerosis, Thrombosis, and Vascular Biology, 38(9), 2117–2125. [CrossRef]
- Ding, J., Jiang, C., Yang, L., & Wang, X. (2022). Relationship and effect of miR-1-3p expression and BDNF level in patients with primary hypertension complicated with depression. Cellular and Molecular Biology, 68(1), 67–74. [CrossRef]
- Dong, Q., Ji, Y. S., Cai, C., & Chen, Z. Y. (2012). LIM Kinase 1 (LIMK1) interacts with Tropomyosin-related Kinase B (TrkB) and mediates Brain-derived Neurotrophic Factor (BDNF)-induced axonal elongation. Journal of Biological Chemistry, 287(50), 41720–41731. [CrossRef]
- Duan, W., Chen, Y., & Wang, X. R. (2018). MicroRNA-155 contributes to the occurrence of epilepsy through the PI3K/Akt/mTOR signaling pathway. International Journal of Molecular Medicine, 42(3), 1577–1584. [CrossRef]
- Duclot, F., & Kabbaj, M. (2017). The role of early growth response 1 (EGR1) in brain plasticity and neuropsychiatric disorders. Frontiers in Behavioral Neuroscience, 11(March), 1–20. [CrossRef]
- Ehinger, Y., Phamluong, K., Darevesky, D., Welman, M., Moffat, J. J., Sakhai, S. A., Whiteley, E. L., Berger, A. L., Laguesse, S., Farokhnia, M., Leggio, L., Lordkipanidzé, M., & Ron, D. (2021). Differential correlation of serum BDNF and microRNA content in rats with rapid or late onset of heavy alcohol use. Addiction Biology, 26(2), 1–12. [CrossRef]
- Fang, F., Zhang, X., Li, B., & Gan, S. (2022). miR-182-5p combined with brain-derived neurotrophic factor assists the diagnosis of chronic heart failure and predicts a poor prognosis. Journal of Cardiothoracic Surgery, 17(1), 88. [CrossRef]
- Fang, Y., Qiu, Q., Zhang, S., Sun, L., Li, G., Xiao, S., & Li, X. (2018). Changes in miRNA-132 and miR-124 levels in non-treated and citalopram-treated patients with depression. Journal of Affective Disorders, 227(November 2017), 745–751. [CrossRef]
- Fu, Y., Hou, B., Weng, C., Liu, W., Dai, J., Zhao, C., & Yin, Z. Q. (2017). Functional ectopic neuritogenesis by retinal rod bipolar cells is regulated by miR-125b-5p during retinal remodeling in RCS rats. Scientific Reports, 7(1), 1011. [CrossRef]
- Gao, B., Hao, S., Tian, W., Jiang, Y., Zhang, S., Guo, L., Zhao, J., zhang, G., Yan, J., & Luo, D. (2017). MicroRNA-107 is downregulated and having tumor suppressive effect in breast cancer by negatively regulating brain-derived neurotrophic factor. Journal of Gene Medicine, 19(12). [CrossRef]
- Gao, Jian, Liang, Z., Zhao, F., Liu, X., & Ma, N. (2022). Triptolide inhibits oxidative stress and inflammation via the microRNA-155-5p/brain-derived neurotrophic factor to reduce podocyte injury in mice with diabetic nephropathy. Bioengineered, 13(5), 12275–12288. [CrossRef]
- Gao, Jun, Wang, W. Y., Mao, Y. W., Gräff, J., Guan, J. S., Pan, L., Mak, G., Kim, D., Su, S. C., & Tsai, L. H. (2010). A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature, 466(7310), 1105–1109. [CrossRef]
- Gao, L., Yan, P., Guo, F. F., Liu, H. J., & Zhao, Z. F. (2018). MiR-1-3p inhibits cell proliferation and invasion by regulating BDNF-TrkB signaling pathway in bladder cancer. Neoplasma, 65(1), 89–96. [CrossRef]
- Gao, Lin, Feng, A., Yue, P., Liu, Y., Zhou, Q., Zang, Q., & Teng, J. (2020). Lncrna bc083743 promotes the proliferation of schwann cells and axon regeneration through mir-103-3p/bdnf after sciatic nerve crush. Journal of Neuropathology and Experimental Neurology, 79(10), 1100–1114. [CrossRef]
- Gao, Y., Su, J., Guo, W., Polich, E. D., Magyar, D. P., Xing, Y., Li, H., Smrt, R. D., Chang, Q., & Zhao, X. (2015). Inhibition of miR-15a promotes BDNF expression and rescues dendritic maturation deficits in MeCP2-deficient neurons. Stem Cells, 33(5), 1618–1629. [CrossRef]
- Ge, Q. Di, Tan, Y., Luo, Y., Wang, W. J., Zhang, H., & Xie, C. (2018). MiR-132, miR-204 and BDNF-TrkB signaling pathway may be involved in spatial learning and memory impairment of the offspring rats caused by fluorine and aluminum exposure during the embryonic stage and into adulthood. Environmental Toxicology and Pharmacology, 63(August), 60–68. [CrossRef]
- Giannotti, G., Caffino, L., Calabrese, F., Racagni, G., Riva, M. A., & Fumagalli, F. (2014). Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. International Journal of Neuropsychopharmacology, 17(4), 625–634. [CrossRef]
- Giordano, M., Trotta, M. C., Ciarambino, T., D’Amico, M., Galdiero, M., Schettini, F., Paternosto, D., Salzillo, M., Alfano, R., Andreone, V., Malatino, L. S., Biolo, G., Paolisso, G., & Adinolfi, L. E. (2020). Circulating MiRNA-195-5p and -451a in diabetic patients with transient and acute ischemic stroke in the emergency department. International Journal of Molecular Sciences, 21(20), 1–10. [CrossRef]
- Guan, W., Xu, D. W., Ji, C. H., Wang, C. N., Liu, Y., Tang, W. Q., Gu, J. H., Chen, Y. M., Huang, J., Liu, J. F., & Jiang, B. (2021). Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF. Pharmacological Research, 174, 105932. [CrossRef]
- Gulyaeva, L. F., & Kushlinskiy, N. E. (2016). Regulatory mechanisms of microRNA expression. Journal of Translational Medicine, 14(1), 1–10. [CrossRef]
- Ha, M., & Kim, V. N. (2014). Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology, 15(8), 509–524. [CrossRef]
- Han, L., Wen, Z., Lynn, R. C., Baudet, M. L., Holt, C. E., Sasaki, Y., Bassell, G. J., & Zheng, J. Q. (2011). Regulation of chemotropic guidance of nerve growth cones by microRNA. Molecular Brain, 4(1), 40. [CrossRef]
- Hernandez-Rapp, J., Smith, P. Y., Filali, M., Goupil, C., Planel, E., Magill, S. T., Goodman, R. H., & Hébert, S. S. (2015). Memory formation and retention are affected in adult miR-132/212 knockout mice. Behavioural Brain Research, 287, 15–26. [CrossRef]
- Hu, J. J., Qin, L. J., Liu, Z. Y., Liu, P., Wei, H. P., Wang, H. Y., Zhao, C. C., & Ge, Z. M. (2020). miR-15a regulates oxygen glucose deprivation/reperfusion (OGD/R)-induced neuronal injury by targeting BDNF. Kaohsiung Journal of Medical Sciences, 36(1), 27–34. [CrossRef]
- Hu, X. M., Cao, S. Bin, Zhang, H. L., Lyu, D. M., Chen, L. P., Xu, H., Pan, Z. Q., & Shen, W. (2016). Downregulation of miR-219 enhances brain-derived neurotrophic factor production in mouse dorsal root ganglia to mediate morphine analgesic tolerance by upregulating CaMKIIγ. Molecular Pain, 12, 1–12. [CrossRef]
- Huan, Z., Mei, Z., Na, H., Xinxin, M., Yaping, W., Ling, L., Lei, W., Kejin, Z., & Yanan, L. (2021). lncRNA MIR155HG Alleviates Depression-Like Behaviors in Mice by Regulating the miR-155/BDNF Axis. Neurochemical Research, 46(4), 935–944. [CrossRef]
- Huang, W., Cao, J., Liu, X., Meng, F., Li, M., Chen, B., & Zhang, J. (2015). AMPK Plays a Dual Role in Regulation of CREB/BDNF Pathway in Mouse Primary Hippocampal Cells. Journal of Molecular Neuroscience, 56(4), 782–788. [CrossRef]
- Huang, W., Meng, F., Cao, J., Liu, X., Zhang, J., & Li, M. (2017). Neuroprotective Role of Exogenous Brain-Derived Neurotrophic Factor in Hypoxia–Hypoglycemia-Induced Hippocampal Neuron Injury via Regulating Trkb/MiR134 Signaling. Journal of Molecular Neuroscience, 62(1), 35–42. [CrossRef]
- Hung, Y. Y., Huang, Y. L., Chang, C., & Kang, H. Y. (2019). Deficiency in androgen receptor aggravates the depressive-like behaviors in chronic mild stress model of depression. Cells, 8(9). [CrossRef]
- Hutchison, E. R., Kawamoto, E. M., Taub, D. D., Lal, A., Abdelmohsen, K., Zhang, Y., Wood, W. H., Lehrmann, E., Camandola, S., Becker, K. G., Gorospe, M., & Mattson, M. P. (2013). Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia, 61(7), 1018–1028. [CrossRef]
- Ibarra, I. L., Ratnu, V. S., Gordillo, L., Hwang, I., Mariani, L., Weinand, K., Hammarén, H. M., Heck, J., Bulyk, M. L., Savitski, M. M., Zaugg, J. B., & Noh, K. (2022). Comparative chromatin accessibility upon BDNF stimulation delineates neuronal regulatory elements. Molecular Systems Biology, 18(8), 1–25. [CrossRef]
- Im, H. I., Hollander, J. A., Bali, P., & Kenny, P. J. (2010). MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature Neuroscience, 13(9), 1120–1127. [CrossRef]
- Imam, J. S., Plyler, J. R., Bansal, H., Prajapati, S., Bansal, S., Rebeles, J., Chen, H. I. H., Chang, Y. F., Panneerdoss, S., Zoghi, B., Buddavarapu, K. C., Broaddus, R., Hornsby, P., Tomlinson, G., Dome, J., Vadlamudi, R. K., Pertsemlidis, A., Chen, Y., & Rao, M. K. (2012). Genomic Loss of Tumor Suppressor miRNA-204 Promotes Cancer Cell Migration and Invasion by Activating AKT/mTOR/Rac1 Signaling and Actin Reorganization. PLoS ONE, 7(12), e52397. [CrossRef]
- Ji, M., Wang, W., Li, S., & Hu, W. (2017). Implantation of bone mesenchymal stem cells overexpressing miRNA-705 mitigated ischemic brain injury. Molecular Medicine Reports, 16(6), 8323–8328. [CrossRef]
- Jiang, B., Gao, L., Lei, D., Liu, J., Shao, Z., Zhou, X., Li, R., Wu, D., Xue, F., Zhu, Y., & Yuan, H. (2016). Decreased expression of miR-9 due to E50K OPTN mutation causes disruption of the expression of BDNF leading to RGC-5 cell apoptosis. Molecular Medicine Reports, 14(5), 4901–4905. [CrossRef]
- Jiang, J. D., Zheng, X. C., Huang, F. Y., Gao, F., You, M. Z., & Zheng, T. (2019). MicroRNA-107 regulates anesthesia-induced neural injury in embryonic stem cell derived neurons. IUBMB Life, 71(1), 20–27. [CrossRef]
- Jiang, Y., & Zhu, J. (2015). Effects of sleep deprivation on behaviors and abnormal hippocampal BDNF/miR-10B expression in rats with chronic stress depression. International Journal of Clinical and Experimental Pathology, 8(1), 586–593. http://www.ncbi.nlm.nih.gov/pubmed/25755749.
- Jimenez-Gonzalez, A., García-Concejo, A., López-Benito, S., Gonzalez-Nunez, V., Arévalo, J. C., & Rodriguez, R. E. (2016). Role of morphine, miR-212/132 and mu opioid receptor in the regulation of Bdnf in zebrafish embryos. Biochimica et Biophysica Acta - General Subjects, 1860(6), 1308–1316. [CrossRef]
- Kang, E., Jia, Y., Wang, J., Wang, G., Chen, H., Chen, X., Ye, Y., Zhang, X., Su, X., Wang, J., & He, X. (2022). Downregulation of microRNA-124-3p promotes subventricular zone neural stem cell activation by enhancing the function of BDNF downstream pathways after traumatic brain injury in adult rats. CNS Neuroscience & Therapeutics, 28(7), 1081–1092. [CrossRef]
- Kaurani, L. (2024). Clinical Insights into MicroRNAs in Depression : Bridging Molecular Discoveries and Therapeutic Potential.
- Kawashima, H., Numakawa, T., Kumamaru, E., Adachi, N., Mizuno, H., Ninomiya, M., Kunugi, H., & Hashido, K. (2010). Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience, 165(4), 1301–1311. [CrossRef]
- Ke, X., Huang, Y., Fu, Q., Lane, R. H., & Majnik, A. (2021). Adverse Maternal Environment Alters MicroRNA-10b-5p Expression and Its Epigenetic Profile Concurrently with Impaired Hippocampal Neurogenesis in Male Mouse Hippocampus. Developmental Neuroscience, 43(2), 95–105. [CrossRef]
- Kim, D. Il, Taylor, J. A., Tan, C. O., Park, H., Kim, J. Y., Park, S. Y., Chung, K. M., Lee, Y. H., Lee, B. S., & Jeon, J. Y. (2019). A pilot randomized controlled trial of 6-week combined exercise program on fasting insulin and fitness levels in individuals with spinal cord injury. European Spine Journal, 28(5), 1082–1091. [CrossRef]
- Klein, M. E., Lioy, D. T., Ma, L., Impey, S., Mandel, G., & Goodman, R. H. (2007). Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nature Neuroscience, 10(12), 1513–1514. [CrossRef]
- Kumari, A., Singh, P., Baghel, M. S., & Thakur, M. K. (2016). Social isolation mediated anxiety like behavior is associated with enhanced expression and regulation of BDNF in the female mouse brain. Physiology and Behavior, 158, 34–42. [CrossRef]
- Kynast, K. L., Russe, O. Q., Möser, C. V., Geisslinger, G., & Niederberger, E. (2013). Modulation of central nervous system-specific microRNA-124a alters the inflammatory response in the formalin test in mice. Pain, 154(3), 368–376. [CrossRef]
- Labrador-Velandia, S., Alonso-Alonso, M. L., Di Lauro, S., García-Gutierrez, M. T., Srivastava, G. K., Pastor, J. C., & Fernandez-Bueno, I. (2019). Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Experimental Eye Research, 185, 107671. [CrossRef]
- Lai, E. C. (2002). Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nature Genetics, 30(4), 363–364. [CrossRef]
- Lambert, C. P., Sullivan, D. H., & Evans, W. J. (2003). Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: A randomized controlled trial. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 58(2), 165–170. [CrossRef]
- Lee, S. T., Chu, K., Jung, K. H., Kim, J. H., Huh, J. Y., Yoon, H., Park, D. K., Lim, J. Y., Kim, J. M., Jeon, D., Ryu, H., Lee, S. K., Kim, M., & Roh, J. K. (2012). MiR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Annals of Neurology, 72(2), 269–277. [CrossRef]
- Lee, Y., Kim, M., Han, J., Yeom, K. H., Lee, S., Baek, S. H., & Kim, V. N. (2004). MicroRNA genes are transcribed by RNA polymerase II. EMBO Journal, 23(20), 4051–4060. [CrossRef]
- Lekk, I., Cabrera-Cabrera, F., Turconi, G., Tuvikene, J., Esvald, E. E., Rähni, A., Casserly, L., Garton, D. R., Andressoo, J. O., Timmusk, T., & Koppel, I. (2023). Untranslated regions of brain-derived neurotrophic factor mRNA control its translatability and subcellular localization. Journal of Biological Chemistry, 299(2), 102897. [CrossRef]
- Li, C., Lie, H., & Sun, W. (2022). Inhibitory effect of miR-182-5p on retinal neovascularization by targeting angiogenin and BDNF. Molecular Medicine Reports, 25(2), 61. [CrossRef]
- Li, Hongxia, Du, M., Xu, W., & Wang, Z. (2021). MiR-191 downregulation protects against isoflurane-induced neurotoxicity through targeting BDNF. Toxicology Mechanisms and Methods, 31(5), 367–373. [CrossRef]
- Li, Hongyang, Gong, Y., Qian, H., Chen, T., Liu, Z., Jiang, Z., & Wei, S. (2015). Brain-derived neurotrophic factor is a novel target gene of the has-miR-183/96/182 cluster in retinal pigment epithelial cells following visible light exposure. Molecular Medicine Reports, 12(2), 2793–2799. [CrossRef]
- Li, M., Armelloni, S., Zennaro, C., Wei, C., Corbelli, A., Ikehata, M., Berra, S., Giardino, L., Mattinzoli, D., Watanabe, S., Agostoni, C., Edefonti, A., Reiser, J., Messa, P., & Rastaldi, M. P. (2015). BDNF repairs podocyte damage by microRNA-mediated increase of actin polymerization. Journal of Pathology, 235(5), 731–744. [CrossRef]
- Li, R., Shang, J., Zhou, W., Jiang, L., Xie, D., & Tu, G. (2018). Overexpression of HIPK2 attenuates spinal cord injury in rats by modulating apoptosis, oxidative stress, and inflammation. Biomedicine and Pharmacotherapy, 103, 127–134. [CrossRef]
- Li, S. C., Chan, W. C., Hu, L. Y., Lai, C. H., Hsu, C. N., & Lin, W. chang. (2010). Identification of homologous microRNAs in 56 animal genomes. Genomics, 96(1), 1–9. [CrossRef]
- Li, Wei, Li, X., Xin, X., Kan, P.-C., & Yan, Y. (2016). MicroRNA-613 regulates the expression of brain-derived neurotrophic factor in Alzheimer’s disease. BioScience Trends, 10(5), 372–377. [CrossRef]
- Li, Wu, He, Q. Z., Wu, C. Q., Pan, X. Y., Wang, J., Tan, Y., Shan, X. Y., & Zeng, H. C. (2015). PFOS Disturbs BDNF-ERK-CREB Signalling in Association with Increased MicroRNA-22 in SH-SY5Y Cells. BioMed Research International, 2015. [CrossRef]
- Li, Xiaojie, Yuan, L., Wang, J., Zhang, Z., Fu, S., Wang, S., & Li, X. (2021). MiR-1b up-regulation inhibits rat neuron proliferation and regeneration yet promotes apoptosis via targeting KLF7. Folia Neuropathologica, 59(1), 67–80. [CrossRef]
- Li, Xiu-juan. (2018). Long non-coding RNA nuclear paraspeckle assembly transcript 1 inhibits the apoptosis of retina Müller cells after diabetic retinopathy through regulating miR-497/brain-derived neurotrophic factor axis. Diabetes and Vascular Disease Research, 15(3), 204–213. [CrossRef]
- Li, Y. J., Xu, M., Gao, Z. H., Wang, Y. Q., Yue, Z., Zhang, Y. X., Li, X. X., Zhang, C., Xie, S. Y., & Wang, P. Y. (2013). Alterations of Serum Levels of BDNF-Related miRNAs in Patients with Depression. PLoS ONE, 8(5), e63648. [CrossRef]
- Li, Y., Wei, C., Wang, W., Li, Q., & Wang, Z. C. (2023). Tropomyosin receptor kinase B (TrkB) signalling: targeted therapy in neurogenic tumours. Journal of Pathology: Clinical Research, 9(2), 89–99. [CrossRef]
- Lian, N., Niu, Q., Lei, Y., Li, X., Li, Y., & Song, X. (2018). MiR-221 is involved in depression by regulating Wnt2/CREB/BDNF axis in hippocampal neurons. Cell Cycle, 17(24), 2745–2755. [CrossRef]
- Liang, S. P., Chen, Q., Cheng, Y. B., Xue, Y. Y., & Wang, H. J. (2019). Comparative effects of monosialoganglioside versus citicoline on apoptotic factor, neurological function and oxidative stress in newborns with hypoxic-ischemic encephalopathy. Journal of the College of Physicians and Surgeons Pakistan, 29(4), 324–327. [CrossRef]
- Liang, Y., Liu, Y., Hou, B., Zhang, W., Liu, M., Sun, Y. E., Ma, Z., & Gu, X. (2016). CREB-regulated transcription coactivator 1 enhances CREB-dependent gene expression in spinal cord to maintain the bone cancer pain in mice. Molecular Pain, 12. [CrossRef]
- Lin, B., Zhao, H., Li, L., Zhang, Z., Jiang, N., Yang, X., Zhang, T., Lian, B., Liu, Y., Zhang, C., Wang, J., Wang, F., Feng, D., & Xu, J. (2021). Sirt1 Improves Heart Failure Through modulating the NF-κB p65/microRNA-155/BNDF Signaling Cascade. Aging, 13(10), 14482–14498. [CrossRef]
- Lin, C. R., Chen, K. H., Yang, C. H., Huang, H. W., & Sheen-Chen, S. M. (2014). Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. European Journal of Neuroscience, 39(10), 1682–1689. [CrossRef]
- Lin, C. Y., Wang, S. W., Chen, Y. L., Chou, W. Y., Lin, T. Y., Chen, W. C., Yang, C. Y., Liu, S. C., Hsieh, C. C., Fong, Y. C., Wang, P. C., & Tang, C. H. (2017). Brain-derived neurotrophic factor promotes VEGF-C-dependent lymphangiogenesis by suppressing miR-624-3p in human chondrosarcoma cells. Cell Death and Disease, 8(8). [CrossRef]
- Liu, H., Wang, J., Yan, R., Jin, S., Wan, Z., Cheng, J., Li, N., Chen, L., & Le, C. (2020). Microrna-204-5p mediates sevoflurane-induced cytotoxicity in ht22 cells by targeting brain-derived neurotrophic factor. Histology and Histopathology, 35(11), 1353–1361. [CrossRef]
- Liu, S., Liu, Q., Ju, Y., & Liu, L. (2021). Downregulation of miR-383 reduces depression-like behavior through targeting Wnt family member 2 (Wnt2) in rats. Scientific Reports, 11(1), 1–15. [CrossRef]
- Liu, X., Cui, X., Guan, G., Dong, Y., & Zhang, Z. (2020). microRNA-192-5p is involved in nerve repair in rats with peripheral nerve injury by regulating XIAP. Cell Cycle, 19(3), 326–338. [CrossRef]
- Liu, Zhen, Wang, C., Wang, X., & Xu, S. (2015). Therapeutic Effects of Transplantation of As-MiR-937-Expressing Mesenchymal Stem Cells in Murine Model of Alzheimer’s Disease. Cellular Physiology and Biochemistry, 37(1), 321–330. [CrossRef]
- Liu, Zheng, Yang, J., Fang, Q., Shao, H., Yang, D., Sun, J., & Gao, L. (2021). MiRNA-199a-5p targets WNT2 to regulate depression through the CREB/BDNF signaling in hippocampal neuron. Brain and Behavior, 11(8). [CrossRef]
- Long, J., Jiang, C., Liu, B., Fang, S., & Kuang, M. (2016). MicroRNA-15a-5p suppresses cancer proliferation and division in human hepatocellular carcinoma by targeting BDNF. Tumor Biology, 37(5), 5821–5828. [CrossRef]
- Lu, Y., Huang, Z., Hua, Y., & Xiao, G. (2018). Minocycline Promotes BDNF Expression of N2a Cells via Inhibition of miR-155-Mediated Repression After Oxygen-Glucose Deprivation and Reoxygenation. Cellular and Molecular Neurobiology, 38(6), 1305–1313. [CrossRef]
- Lv, M., Yang, S., Cai, L., Qin, L. qiang, Li, B. yan, & Wan, Z. (2018). Effects of Quercetin Intervention on Cognition Function in APP/PS1 Mice was Affected by Vitamin D Status. Molecular Nutrition and Food Research, 62(24), 1–38. [CrossRef]
- Ma, J. C., Duan, M. J., Sun, L. L., Yan, M. L., Liu, T., Wang, Q., Liu, C. D., Wang, X., Kang, X. H., Pei, S. C., Zong, D. K., Chen, X., Wang, N., & Ai, J. (2015). Cardiac over-expression of microRNA-1 induces impairment of cognition in mice. Neuroscience, 299, 66–78. [CrossRef]
- Ma, Ji Chao, Duan, M. J., Li, K. X., Biddyut, D., Zhang, S., Yan, M. L., Yang, L., Jin, Z., Zhao, H. M., Huang, S. Y., Sun, Q., Su, D., Xu, Y., Pan, Y. H., & Ai, J. (2018). Knockdown of MicroRNA-1 in the Hippocampus Ameliorates Myocardial Infarction Induced Impairment of Long-Term Potentiation. Cellular Physiology and Biochemistry, 50(4), 1601–1616. [CrossRef]
- Ma, R., Zhu, P., Liu, S., Gao, B., & Wang, W. (2019). miR-496 suppress tumorigenesis via targeting BDNF-mediated PI3K/Akt signaling pathway in non-small cell lung cancer. Biochemical and Biophysical Research Communications, 518(2), 273–277. [CrossRef]
- Ma, Y., Shen, N., Wicha, M. S., & Luo, M. (2021). The roles of the let-7 family of micrornas in the regulation of cancer stemness. Cells, 10(9), 1–20. [CrossRef]
- Marler, K. J., Suetterlin, P., Dopplapudi, A., Rubikaite, A., Adnan, J., Maiorano, N. A., Lowe, A. S., Thompson, I. D., Pathania, M., Bordey, A., Fulga, T., Van Vactor, D. L., Hindges, R., & Drescher, U. (2014). BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. Journal of Neuroscience, 34(3), 969–979. [CrossRef]
- Mellios, N., Huang, H. S., Baker, S. P., Galdzicka, M., Ginns, E., & Akbarian, S. (2009). Molecular Determinants of Dysregulated GABAergic Gene Expression in the Prefrontal Cortex of Subjects with Schizophrenia. Biological Psychiatry, 65(12), 1006–1014. [CrossRef]
- Mellios, N., Huang, H. S., Grigorenko, A., Rogaev, E., & Akbarian, S. (2008). A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Human Molecular Genetics, 17(19), 3030–3042. [CrossRef]
- Mendoza-Viveros, L., Chiang, C. K., Ong, J. L. K., Hegazi, S., Cheng, A. H., Bouchard-Cannon, P., Fana, M., Lowden, C., Zhang, P., Bothorel, B., Michniewicz, M. G., Magill, S. T., Holmes, M. M., Goodman, R. H., Simonneaux, V., Figeys, D., & Cheng, H. Y. M. (2017). miR-132/212 Modulates Seasonal Adaptation and Dendritic Morphology of the Central Circadian Clock. Cell Reports, 19(3), 505–520. [CrossRef]
- Miao, Z., Mao, F., Liang, J., Szyf, M., Wang, Y., & Sun, Z. S. (2018). Anxiety-Related Behaviours Associated with microRNA-206-3p and BDNF Expression in Pregnant Female Mice Following Psychological Social Stress. Molecular Neurobiology, 55(2), 1097–1111. [CrossRef]
- Miao, Z., Zhang, J., Li, Y., Li, X., Song, W., Sun, Z. S., & Wang, Y. (2019). Presence of the pregnant partner regulates microRNA-30a and BDNF levels and protects male mice from social defeat-induced abnormal behaviors. Neuropharmacology, 159. [CrossRef]
- Misiorek, J. O., Schreiber, A. M., Urbanek-Trzeciak, M. O., Jazurek-Ciesiołka, M., Hauser, L. A., Lynch, D. R., Napierala, J. S., & Napierala, M. (2020). A Comprehensive Transcriptome Analysis Identifies FXN and BDNF as Novel Targets of miRNAs in Friedreich’s Ataxia Patients. Molecular Neurobiology, 57(6), 2639–2653. [CrossRef]
- Miura, P., Amirouche, A., Clow, C., Bélanger, G., & Jasmin, B. J. (2012). Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206. Journal of Neurochemistry, 120(2), 230–238. [CrossRef]
- Mohammadipoor-Ghasemabad, L., Sangtarash, M. H., Sheibani, V., Sasan, H. A., & Esmaeili-Mahani, S. (2019). Hippocampal microRNA-191a-5p Regulates BDNF Expression and Shows Correlation with Cognitive Impairment Induced by Paradoxical Sleep Deprivation. Neuroscience, 414, 49–59. [CrossRef]
- Mojtahedi, S., Kordi, M. R., Hosseini, S. E., Omran, S. F., & Soleimani, M. (2013). Effect of treadmill running on the expression of genes that are involved in neuronal differentiation in the hippocampus of adult male rats. Cell Biology International, 37(4), 276/a-283/a. [CrossRef]
- Mu, Y., Zhou, H., Wu, W. J., Hu, L. C., & Chen, H. B. (2015). Dynamic expression of miR-206-3p during mouse skin development is independent of keratinocyte differentiation. Molecular Medicine Reports, 12(6), 8113–8120. [CrossRef]
- Nagpal, N., Ahmad, H. M., Molparia, B., & Kulshreshtha, R. (2013). MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis, 34(8), 1889–1899. [CrossRef]
- Neumann, E., Brandenburger, T., Santana-Varela, S., Deenen, R., Köhrer, K., Bauer, I., Hermanns, H., Wood, J. N., Zhao, J., & Werdehausen, R. (2016). MicroRNA-1-associated effects of neuron-specific brain-derived neurotrophic factor gene deletion in dorsal root ganglia. Molecular and Cellular Neuroscience, 75, 36–43. [CrossRef]
- Neumann, E., Hermanns, H., Barthel, F., Werdehausen, R., & Brandenburger, T. (2015). Expression changes of microRNA-1 and its targets Connexin 43 and brain-derived neurotrophic factor in the peripheral nervous system of chronic neuropathic rats. Molecular Pain, 11(1). [CrossRef]
- Nguyen, T., Su, C., & Singh, M. (2018). Let-7i inhibition enhances progesterone-induced functional recovery in a mouse model of ischemia. Proceedings of the National Academy of Sciences of the United States of America, 115(41), E9668–E9677. [CrossRef]
- Ni, J., & Zhang, L. (2020). Cancer cachexia: Definition, staging, and emerging treatments. Cancer Management and Research, 12, 5597–5605. [CrossRef]
- Niu, Y., Wan, C., Zhang, J., Zhang, S., Zhao, Z., Zhu, L., Wang, X., Ren, X., Wang, J., & Lei, P. (2021). Aerobic exercise improves VCI through circRIMS2/miR-186/BDNF-mediated neuronal apoptosis. Molecular Medicine, 27(1), 4. [CrossRef]
- Numakawa, T., Suzuki, S., Kumamaru, E., Adachi, N., Richards, M., & Kunugi, H. (2010). BDNF function and intracellular signaling in neurons. Histology and Histopathology, 25(2), 237–258. [CrossRef]
- O’Brien, J., Hayder, H., Zayed, Y., & Peng, C. (2018). Overview of microRNA biogenesis, mechanisms of actions, and circulation. In Frontiers in Endocrinology (Vol. 9, Issue AUG, pp. 1–12). [CrossRef]
- Odent, S., Attié-Bitach, T., Blayau, M., Mathieu, M., Augé, J., Delezoïde, A. L., Le Gall, J. Y., Le Marec, B., Munnich, A., David, V., & Vekemans, M. (1999). Expression of the Sonic hedgehog (SHH ) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Human Molecular Genetics, 8(9), 1683–1689.
- Oikawa, H., Goh, W. W. B., Lim, V. K. J., Wong, L., & Sng, J. C. G. (2015). Valproic acid mediates miR-124 to down-regulate a novel protein target, GNAI1. Neurochemistry International, 91, 62–71. [CrossRef]
- Pan, S., Feng, W., Li, Y., Huang, J., Chen, S., Cui, Y., Tian, B., Tan, S., Wang, Z., Yao, S., Chiappelli, J., Kochunov, P., Chen, S., Yang, F., Li, C. S. R., Tian, L., Tan, Y., & Elliot Hong, L. (2021). The microRNA-195 - BDNF pathway and cognitive deficits in schizophrenia patients with minimal antipsychotic medication exposure. Translational Psychiatry, 11(1). [CrossRef]
- Panta, A., Pandey, S., Duncan, I. N., Duhamel, S., & Sohrabji, F. (2019). Mir363-3p attenuates post-stroke depressive-like behaviors in middle-aged female rats. Brain, Behavior, and Immunity, 78(August 2018), 31–40. [CrossRef]
- Pejhan, S., Del Bigio, M. R., & Rastegar, M. (2020). The MeCP2E1/E2-BDNF-miR132 Homeostasis Regulatory Network Is Region-Dependent in the Human Brain and Is Impaired in Rett Syndrome Patients. Frontiers in Cell and Developmental Biology, 8(August). [CrossRef]
- Peng, D., Wang, Y., Xiao, Y., Peng, M., Mai, W., Hu, B., Jia, Y., Chen, H., Yang, Y., Xiang, Q., Su, Z., Zhang, Q., & Huang, Y. (2022). Extracellular vesicles derived from astrocyte-treated with haFGF14-154 attenuate Alzheimer phenotype in AD mice. Theranostics, 12(9), 3862–3881. [CrossRef]
- Peng, J. Y., An, X. P., Fang, F., Gao, K. X., Xin, H. Y., Han, P., Bao, L. J., Ma, H. D., & Cao, B. Y. (2016). MicroRNA-10b suppresses goat granulosa cell proliferation by targeting brain-derived neurotropic factor. Domestic Animal Endocrinology, 54, 60–67. [CrossRef]
- Radzikinas, K., Aven, L., Jiang, Z., Tran, T., Paez-Cortez, J., Boppidi, K., Lu, J., Fine, A., & Ai, X. (2011). A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. Journal of Neuroscience, 31(43), 15407–15415. [CrossRef]
- Rajewsky, N. (2006). Microrna target predictions in animals. Nature Genetics, 38(6S), S8–S13. [CrossRef]
- Robinson, M. (1996). Timing and regulation of trkB and BDNF mRNA expression in placode-derived sensory neurons and their targets. European Journal of Neuroscience, 8(11), 2399–2406. [CrossRef]
- Roush, S., & Slack, F. J. (2008). The let-7 family of microRNAs. Trends in Cell Biology, 18(10), 505–516. [CrossRef]
- Ryan, K. M., O’Donovan, S. M., & McLoughlin, D. M. (2013). Electroconvulsive stimulation alters levels of BDNF-associated microRNAs. Neuroscience Letters, 549, 125–129. [CrossRef]
- Scheen, A. J., Schmitt, H., Jiang, H. H., & Ivanyi, T. (2015). Individualizing treatment of type 2 diabetes by targeting postprandial or fasting hyperglycaemia: Response to a basal vs a premixed insulin regimen by HbA1c quartiles and ethnicity. Diabetes and Metabolism, 41(3), 216–222. [CrossRef]
- Schratt, G. M., Tuebing, F., Nigh, E. A., Kane, C. G., Sabatini, M. E., Kiebler, M., & Greenberg, M. E. (2006). A brain-specific microRNA regulates dendritic spine development. Nature, 439(7074), 283–289. [CrossRef]
- Shao, Y., Yu, Y., Zhou, Q., Li, C., Yang, L., & Pei, C. gang. (2015). Inhibition of miR-134 Protects Against Hydrogen Peroxide-Induced Apoptosis in Retinal Ganglion Cells. Journal of Molecular Neuroscience, 56(2), 461–471. [CrossRef]
- Shen, J., Li, Y., Qu, C., Xu, L., Sun, H., & Zhang, J. (2019). The enriched environment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive impairment by activating the SIRT1/miR-134 signaling pathway in hippocampus. Journal of Affective Disorders, 248(August 2018), 81–90. [CrossRef]
- Shen, J., Xu, L., Qu, C., Sun, H., & Zhang, J. (2018). Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behavioural Brain Research, 349(2010), 1–7. [CrossRef]
- Shrestha, S., Phay, M., Kim, H. H., Pouladvand, P., Lee, S. J., & Yoo, S. (2019). Differential regulation of brain-derived neurotrophic factor (BDNF) expression in sensory neuron axons by miRNA-206. FEBS Open Bio, 9(2), 374–383. [CrossRef]
- Shukla, G. C., Singh, J., & Barik, S. (2011). MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Molecular and Cellular Pharmacology, 3(3), 83. [CrossRef]
- Smallridge, R. (2001). Gene expression: A small fortune. Nature Reviews Molecular Cell Biology, 2(12), 867. [CrossRef]
- Solomon, M. G., Griffin, W. C., Lopez, M. F., & Becker, H. C. (2019). Brain Regional and Temporal Changes in BDNF mRNA and microRNA-206 Expression in Mice Exposed to Repeated Cycles of Chronic Intermittent Ethanol and Forced Swim Stress. Neuroscience, 406, 617–625. [CrossRef]
- Song, D., Diao, J., Yang, Y., & Chen, Y. (2017). MicroRNA-382 inhibits cell proliferation and invasion of retinoblastoma by targeting BDNF-mediated PI3K/AKT signalling pathway. Molecular Medicine Reports, 16(5), 6428–6436. [CrossRef]
- Song, Y., Wang, G., Zhuang, J., Ni, J., Zhang, S., Gye, Y., & Xia, W. (2019). MicroRNA-584 prohibits hepatocellular carcinoma cell proliferation and invasion by directly targeting BDNF. Molecular Medicine Reports, 20(2), 1994–2001. [CrossRef]
- Su, B., Cheng, S., Wang, L., & Wang, B. (2022). MicroRNA-139-5p acts as a suppressor gene for depression by targeting nuclear receptor subfamily 3, group C, member 1. Bioengineered, 13(5), 11856–11866. [CrossRef]
- Su, M., Hong, J., Zhao, Y., Liu, S., & Xue, X. (2015). MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA-132 in rats with depression. Molecular Medicine Reports, 12(4), 5399–5406. [CrossRef]
- Sun, W., Zhang, L., & Li, R. (2017). Overexpression of miR-206 ameliorates chronic constriction injury-induced neuropathic pain in rats via the MEK/ERK pathway by targeting brain-derived neurotrophic factor. Neuroscience Letters, 646, 68–74. [CrossRef]
- Sun, Y. X., Yang, J., Wang, P. Y., Li, Y. J., Xie, S. Y., & Sun, R. P. (2013). Cisplatin regulates SH-SY5Y cell growth through downregulation of BDNF via miR-16. Oncology Reports, 30(5), 2343–2349. [CrossRef]
- Sun, Z., Guo, X., Zang, M., Wang, P., Xue, S., & Chen, G. (2019). Long non-coding RNA LINC00152 promotes cell growth and invasion of papillary thyroid carcinoma by regulating the miR-497/BDNF axis. Journal of Cellular Physiology, 234(2), 1336–1345. [CrossRef]
- Tang, X. W., Chu, D., Yan, S. N., Yin, Y. F., Bian, Q., Weng, B., Chen, B., & Ran, M. L. (2021). MiR-191 promotes the porcine immature Sertoli cell proliferation by targeting the BDNF gene through activating the PI3K/AKT signaling pathway. Yi Chuan = Hereditas, 43(7), 680–693. [CrossRef]
- Tapocik, J. D., Barbier, E., Flanigan, M., Solomon, M., Pincus, A., Pilling, A., Sun, H., Schank, J. R., King, C., & Heilig, M. (2014). MicroRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. Journal of Neuroscience, 34(13), 4581–4588. [CrossRef]
- Thomas, K. T., Anderson, B. R., Shah, N., Zimmer, S. E., Hawkins, D., Valdez, A. N., Gu, Q., & Bassell, G. J. (2017). Inhibition of the Schizophrenia-Associated MicroRNA miR-137 Disrupts Nrg1α Neurodevelopmental Signal Transduction. Cell Reports, 20(1), 1–12. [CrossRef]
- Tian, N., Cao, Z., & Zhang, Y. (2014). MiR-206 decreases brain-derived neurotrophic factor levels in a transgenic mouse model of Alzheimer’s disease. Neuroscience Bulletin, 30(2), 191–197. [CrossRef]
- Trzaska, K. A., King, C. C., Li, K. Y., Kuzhikandathil, E. V., Nowycky, M. C., Ye, J. H., & Rameshwar, P. (2009). Brain-derived neurotrophic factor facilitates maturation of mesenchymal stem cell-derived dopamine progenitors to functional neurons. Journal of Neurochemistry, 110(3), 1058–1069. [CrossRef]
- Tu, W., Yue, J., Li, X., Wu, Q., Yang, G., Li, S., Sun, Q., & Jiang, S. (2022). Electroacupuncture Alleviates Neuropathic Pain through Regulating miR-206-3p Targeting BDNF after CCI. Neural Plasticity, 2022, 1–15. [CrossRef]
- Tu, Z., Li, Y., Dai, Y., Li, L., Lv, G., Chen, I., & Wang, B. (2017). MiR-140/BDNF axis regulates normal human astrocyte proliferation and LPS-induced IL-6 and TNF-α secretion. Biomedicine and Pharmacotherapy, 91, 899–905. [CrossRef]
- Varendi, K., Kumar, A., Härma, M. A., & Andressoo, J. O. (2014). MIR-1, miR-10b, miR-155, and miR-191 are novel regulators of BDNF. Cellular and Molecular Life Sciences, 71(22), 4443–4456. [CrossRef]
- Vuu, Y. M., Roberts, C. T., & Rastegar, M. (2023). MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism. International Journal of Molecular Sciences, 24(4). [CrossRef]
- Wang, F., Zhu, J., Zheng, J., Duan, W., & Zhou, Z. (2020). MiR-210 enhances mesenchymal stem cell-modulated neural precursor cell migration. Molecular Medicine Reports, 21(6), 2405–2414. [CrossRef]
- Wang, Li, Liu, W., Zhang, Y., Hu, Z., Guo, H., Lv, J., & Du, H. (2020). Dexmedetomidine had neuroprotective effects on hippocampal neuronal cells via targeting lncRNA SHNG16 mediated microRNA-10b-5p/BDNF axis. Molecular and Cellular Biochemistry, 469(1–2), 41–51. [CrossRef]
- Wang, Linxiao, Zhou, Y., Chen, X., Liu, J., & Qin, X. (2022). Long-term iTBS promotes neural structural and functional recovery by enhancing neurogenesis and migration via miR-551b-5p/BDNF/TrkB pathway in a rat model of cerebral ischemia-reperfusion injury. Brain Research Bulletin, 184, 46–55. [CrossRef]
- Wang, M., Tang, X., Li, L., Liu, D., Liu, H., Zheng, H., Deng, W., Zhao, X., & Yang, G. (2018). C1q/TNF-related protein-6 is associated with insulin resistance and the development of diabetes in Chinese population. Acta Diabetologica, 55(12), 1221–1229. [CrossRef]
- Wang, P., Meng, X., Huang, Y., Lv, Z., Liu, J., Wang, G., Meng, W., Xue, S., Zhang, Q., Zhang, P., & Chen, G. (2017). MicroRNA-497 inhibits thyroid cancer tumor growth and invasion by suppressing BDNF. Oncotarget, 8(2), 2825–2834. [CrossRef]
- Wang, S. S., Mu, R. H., Li, C. F., Dong, S. Q., Geng, D., Liu, Q., & Yi, L. T. (2017). microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 79(May), 417–425. [CrossRef]
- Wayman, G. A., Impey, S., Marks, D., Saneyoshi, T., Grant, W. F., Derkach, V., & Soderling, T. R. (2006). Activity-Dependent Dendritic Arborization Mediated by CaM-Kinase I Activation and Enhanced CREB-Dependent Transcription of Wnt-2. Neuron, 50(6), 897–909. [CrossRef]
- Wibrand, K., Pai, B., Siripornmongcolchai, T., Bittins, M., Berentsen, B., Ofte, M. L., Weigel, A., Skaftnesmo, K. O., & Bramham, C. R. (2012). MicroRNA regulation of the synaptic plasticity-related gene Arc. PLoS ONE, 7(7), e41688. [CrossRef]
- Wu, B. W., Wu, M. S., & Guo, J. D. (2018). Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer’s disease. Journal of Cellular Physiology, 233(7), 5281–5292. [CrossRef]
- Wu, G., Li, X., Li, M., & Zhang, Z. (2020). Long non-coding RNA MALAT1 promotes the proliferation and migration of Schwann cells by elevating BDNF through sponging miR-129-5p. Experimental Cell Research, 390(1). [CrossRef]
- Wu, Y., Yang, S., Zheng, Z., Pan, H., Jiang, Y., Bai, X., Liu, T., Deng, S., & Li, Y. (2021). MiR-191-5p Disturbed the Angiogenesis in a Mice Model of Cerebral Infarction by Targeting Inhibition of BDNF. Neurology India, 69(6), 1601–1607. [CrossRef]
- Xia, H., Li, Y., & Lv, X. (2016). MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. International Journal of Oncology, 49(4), 1325–1333. [CrossRef]
- Xiang, L., Ren, Y., Li, X., Zhao, W., & Song, Y. (2016). MicroRNA-204 suppresses epileptiform discharges through regulating TrkB-ERK1/2-CREB signaling in cultured hippocampal neurons. Brain Research, 1639, 99–107. [CrossRef]
- Xie, B., Liu, Z., Jiang, L., Liu, W., Song, M., Zhang, Q., Zhang, R., Cui, D., Wang, X., & Xu, S. (2017). Increased Serum miR-206 Level Predicts Conversion from Amnestic Mild Cognitive Impairment to Alzheimer’s Disease: A 5-Year Follow-up Study. Journal of Alzheimer’s Disease, 55(2), 509–520. [CrossRef]
- Xing, Q., Shan, Z., Gao, Y., Mao, J., Liu, X., Yu, J., Sun, H., Fan, C., Wang, H., Zhang, H., & Teng, W. (2018). Differential Expression of MicroRNAs and miR-206-Mediated Downregulation of BDNF Expression in the Rat Fetal Brain Following Maternal Hypothyroidism. Hormone and Metabolic Research, 50(9), 696–703. [CrossRef]
- Xu, A. J., Fu, L. N., Wu, H. X., Yao, X. L., & Meng, R. (2017). MicroRNA-744 inhibits tumor cell proliferation and invasion of gastric cancer via targeting brain-derived neurotrophic factor. Molecular Medicine Reports, 16(4), 5055–5061. [CrossRef]
- Xu, H., Jia, Z., Ma, K., Zhang, J., Dai, C., Yao, Z., Deng, W., Su, J., Wang, R., & Chen, X. (2020). Protective effect of mesenchymal stromal cell-derived exosomes on traumatic brain injury via miR-216a-5p. Medical Science Monitor, 26. [CrossRef]
- Xu, W., Li, X., Chen, L., Luo, X., Shen, S., & Wang, J. (2022). Dexmedetomidine pretreatment alleviates ropivacaine-induced neurotoxicity via the miR-10b-5p/BDNF axis. BMC Anesthesiology, 22(1). [CrossRef]
- Xu, Y., Fu, Z., Gao, X., Wang, R., & Li, Q. (2021). Long non-coding RNA XIST promotes retinoblastoma cell proliferation, migration, and invasion by modulating microRNA-191-5p/brain derived neurotrophic factor. Bioengineered, 12(1), 1587–1598. [CrossRef]
- Yan, H., Wu, W., Ge, H., Li, P., & Wang, Z. (2015). Up-regulation of miR-204 enhances anoikis sensitivity in epithelial ovarian cancer cell line via brain-derived neurotrophic factor pathway in vitro. International Journal of Gynecological Cancer, 25(6), 944–952. [CrossRef]
- Yang, G., Song, Y., Zhou, X., Deng, Y., Liu, T., Weng, G., Yu, D., & Pan, S. (2015). DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor. Molecular Medicine Reports, 12(1), 1435–1442. [CrossRef]
- Yang, Wei, Liu, M., Zhang, Q., Zhang, J., Chen, J., Chen, Q., & Suo, L. (2020). Knockdown of miR-124 Reduces Depression-like Behavior by Targeting CREB1 and BDNF. Current Neurovascular Research, 17(2), 196–203. [CrossRef]
- Yang, Wenqian, Guo, Q., Li, J., Wang, X., Pan, B., Wang, Y., Wu, L., Yan, J., & Cheng, Z. (2019). microRNA-124 attenuates isoflurane-induced neurological deficits in neonatal rats via binding to EGR1. Journal of Cellular Physiology, 234(12), 23017–23032. [CrossRef]
- Yang, X., Yang, Q., Wang, X., Luo, C., Wan, Y., Li, J., Liu, K., Zhou, M., & Zhang, C. (2014). MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. NeuroMolecular Medicine, 16(3), 594–605. [CrossRef]
- Yi, L. T., Li, J., Liu, B. Bin, Luo, L., Liu, Q., & Geng, D. (2014). BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. Journal of Psychiatry and Neuroscience, 39(5), 348–359. [CrossRef]
- Yi, L. T., Mu, R. H., Dong, S. Q., Wang, S. S., Li, C. F., Geng, D., & Liu, Q. (2018). miR-124 antagonizes the antidepressant-like effects of standardized gypenosides in mice. Journal of Psychopharmacology, 32(4), 458–468. [CrossRef]
- Yi, S., Yuan, Y., Chen, Q., Wang, X., Gong, L., Liu, J., Gu, X., & Li, S. (2016). Regulation of Schwann cell proliferation and migration by MIR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Scientific Reports, 6. [CrossRef]
- Yongguang, L., Xiaowei, W., Huichao, Y., & Yanxiang, Z. (2022). Gastrodin promotes the regeneration of peripheral nerves by regulating miR-497/BDNF axis. BMC Complementary Medicine and Therapies, 22(1), 45. [CrossRef]
- Yu, H. C., Huang, H. Bin, Tseng, H. Y. H., & Lu, M. C. (2022). Brain-Derived Neurotrophic Factor Suppressed Proinflammatory Cytokines Secretion and Enhanced MicroRNA(miR)-3168 Expression in Macrophages. International Journal of Molecular Sciences, 23(1), 570. [CrossRef]
- Zeng, L.-L., He, X.-S., Liu, J.-R., Zheng, C.-B., Wang, Y.-T., & Yang, G.-Y. (2016). Lentivirus-Mediated Overexpression of MicroRNA-210 Improves Long-Term Outcomes after Focal Cerebral Ischemia in Mice. CNS Neuroscience & Therapeutics, 22(12), 961–969. [CrossRef]
- Zhai, Y., Liu, B., Mo, X., Zou, M., Mei, X., Chen, W., Huang, G., & Wu, L. (2022). Gingerol ameliorates neuronal damage induced by <scp>hypoxia-reoxygenation</scp> via the <scp>miR</scp> -210/ <scp>brain-derived</scp> neurotrophic factor axis. The Kaohsiung Journal of Medical Sciences, 38(4), 367–377. [CrossRef]
- Zhang, Jing, Guo, X., Shi, Y. W., Ma, J., & Wang, G. F. (2014). Intermittent hypoxia with or without hypercapnia is associated with tumorigenesis by decreasing the expression of brain derived neurotrophic factor and miR-34a in rats. Chinese Medical Journal, 127(1), 43–47. [CrossRef]
- Zhang, Jingdan, Li, A., Gu, R., Tong, Y., & Cheng, J. (2023). Role and regulatory mechanism of microRNA mediated neuroinflammation in neuronal system diseases. Frontiers in Immunology, 14(August), 1–12. [CrossRef]
- Zhang, Jun, Liu, Z., Pei, Y., Yang, W., Xie, C., & Long, S. (2018). MicroRNA-322 Cluster Promotes Tau Phosphorylation via Targeting Brain-Derived Neurotrophic Factor. Neurochemical Research, 43(3), 736–744. [CrossRef]
- Zhang, K., Wu, S., Li, Z., & Zhou, J. (2017). MicroRNA-211/BDNF axis regulates LPS-induced proliferation of normal human astrocyte through PI3K/AKT pathway. Bioscience Reports, 37(4). [CrossRef]
- Zhang, T., Liu, C., & Chi, L. (2020). Suppression of miR-10a-5p in bone marrow mesenchymal stem cells enhances the therapeutic effect on spinal cord injury via BDNF. Neuroscience Letters, 714, 134562. [CrossRef]
- Zhang, Xiao yu, Tang, X. yi, Li, N., Zhao, L. min, Guo, Y. li, Li, X. su, Tian, C. jie, Cheng, D. jun, Chen, Z. chang, & Zhang, L. xian. (2018). GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sciences, 212, 93–101. [CrossRef]
- Zhang, Xiaonan, Xue, Y., Li, J., Xu, H., Yan, W., Zhao, Z., Yu, W., Zhai, X., Sun, Y., Wu, Y., Li, Y., Gui, L., Yu, D., Xiao, Z., & Yin, S. (2021). The involvement of ADAR1 in antidepressant action by regulating BDNF via miR-432. Behavioural Brain Research, 402. [CrossRef]
- Zhang, Z., Xia, D. jian, & Xu, A. ding. (2022). Therapeutic effect of fastigial nucleus stimulation is mediated by the microRNA-182 & microRNA-382/BDNF signaling pathways in the treatment of post-stroke depression. Biochemical and Biophysical Research Communications, 627, 137–145. [CrossRef]
- Zhao, H., Li, Y., Chen, L., Shen, C., Xiao, Z., Xu, R., Wang, J., & Luo, Y. (2019). HucMSCs-Derived miR-206-Knockdown Exosomes Contribute to Neuroprotection in Subarachnoid Hemorrhage Induced Early Brain Injury by Targeting BDNF. Neuroscience, 417, 11–23. [CrossRef]
- Zhao, P., Li, X., Li, Y., Zhu, J., Sun, Y., & Hong, J. (2021). Mechanism of miR-365 in regulating BDNF-TrkB signal axis of HFD/STZ induced diabetic nephropathy fibrosis and renal function. International Urology and Nephrology, 53(10), 2177–2187. [CrossRef]
- Zhao, X., Shu, F., Wang, X., Wang, F., Wu, L., Li, L., & Lv, H. (2019). Inhibition of microRNA-375 ameliorated ketamine-induced neurotoxicity in human embryonic stem cell derived neurons. European Journal of Pharmacology, 844, 56–64. [CrossRef]
- Zhao, Y., Wang, S., Chu, Z., Dang, Y., Zhu, J., & Su, X. (2017). MicroRNA-101 in the ventrolateral orbital cortex (VLO) modulates depressive-like behaviors in rats and targets dual-specificity phosphatase 1 (DUSP1). Brain Research, 1669, 55–62. [CrossRef]
Figure 1.
Search flow chart.
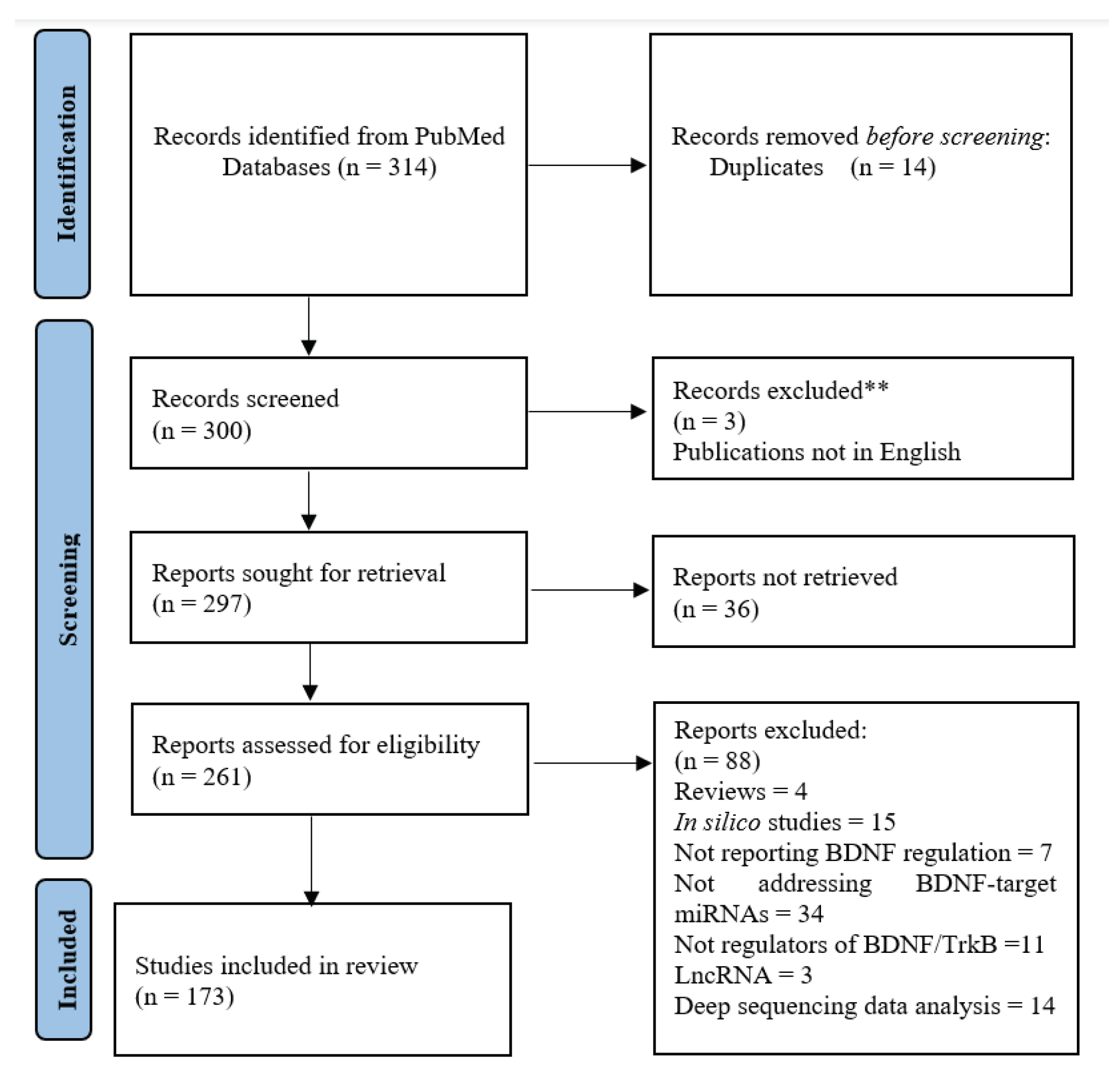
Figure 2.
BDNF regulation by microRNAs. Black lines; positive (arrows) and negative (cut lines) regulation predictions confirmed in tissue samples, Red lines: positive (arrows) and negative (cut lines) associations between microRNA and BDNF levels.
Figure 2.
BDNF regulation by microRNAs. Black lines; positive (arrows) and negative (cut lines) regulation predictions confirmed in tissue samples, Red lines: positive (arrows) and negative (cut lines) associations between microRNA and BDNF levels.
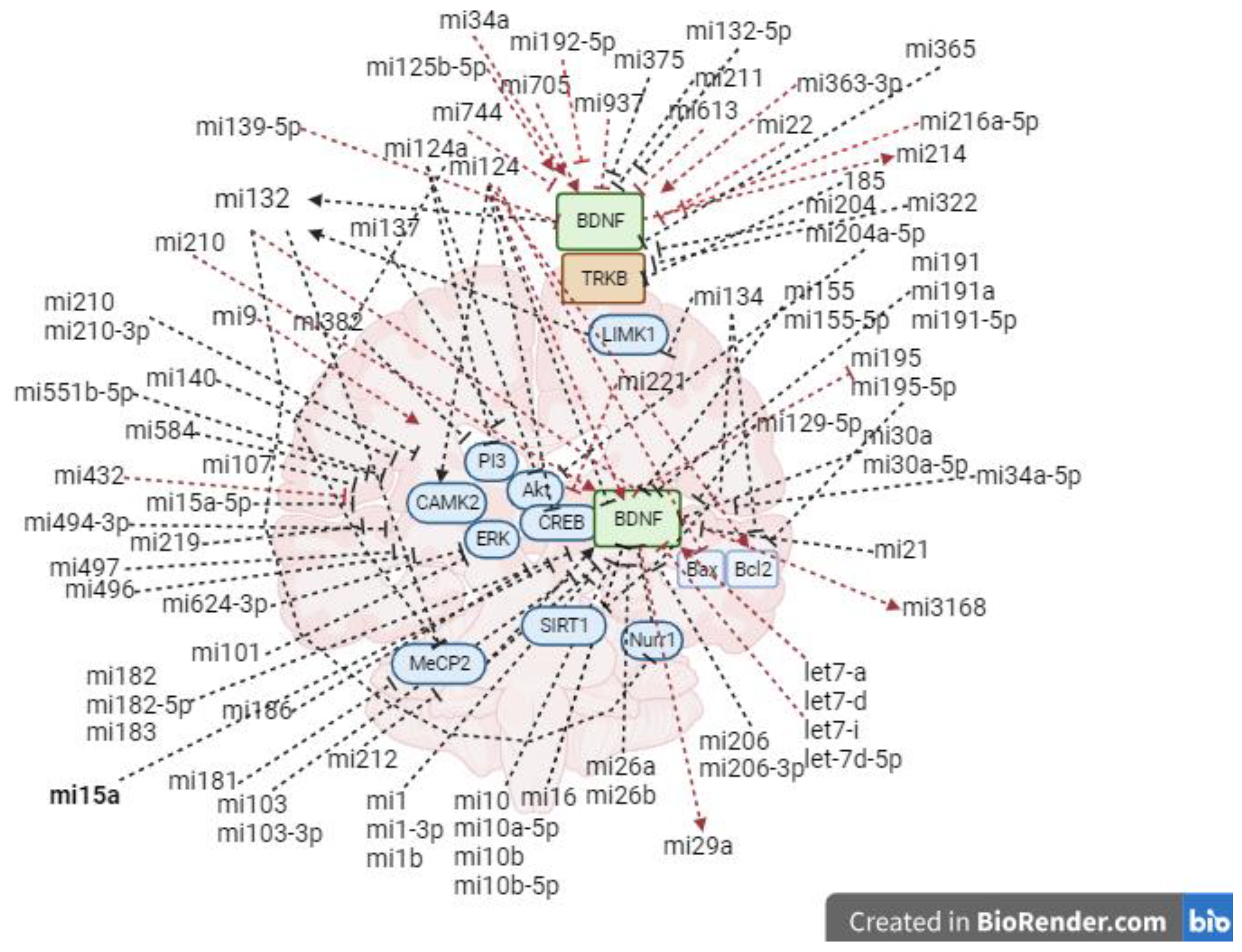
Table 1.
MicroRNAs that participate in BDNF regulation.
| 1 | Schratt, G. M., et al. (2006). https://doi.org/10.1038/nature04367 | miR-134 | |
| 2 | Klein, M. E., et al. (2007). https://doi.org/10.1038/nn2010 |
miR132 | |
| 3 | Mellios, N., et al. (2008). https://doi.org/10.1093/hmg/ddn201 |
miR-30a-5p miR-195 | |
| 4 | Mellios, N., et al. (2009). https://doi.org/10.1016/j.biopsych.2008.11.019 | miR-195 | |
| 5 | Chandrasekar, V., & Dreyer, J. L. (2009). https://doi.org/10.1016/j.mcn.2009.08.009 | miR-124 | |
| 6 | Im, H.-I., et al. (2010). https://doi.org/10.1038/nn.2615 |
MeCP2 | |
| 7 | Gao J. amd Wang, et al. (2010). https://doi.org/10.1038/NATURE09271 | miR-134 | |
| 8 | Kawashima, H., et al. (2010). https://doi.org/10.1016/j.neuroscience.2009.11.057 | miR-132 | |
| 9 | Han, L., et al. (2011). https://doi.org/10.1186/1756-6606-4-40 |
miR-134 | |
| 10 | Radzikinas, K., et al. (2011). https://doi.org/10.1523/JNEUROSCI.2745-11.2011 | miR-206 | |
| 11 | Angelucci, F., et al. (2011). https://doi.org/10.1159/000322528 |
miR30a-5p | |
| 12 | Caputo, V., et al. (2011). https://doi.org/10.1371/journal.pone.0028656 | miR-26a1/2 miR-26b | |
| 13 | Bai, M., et al. (2012). https://doi.org/10.1371/journal.pone.0046921 | miR-16 | |
| 14 | Lee, S. T., et al. (2012). https://doi.org/10.1002/ana.23588 |
miR-260 | |
| 15 | Imam, J. S., et al. (2012). https://doi.org/10.1371/journal.pone.0052397 | miR-204 | |
| 16 | Chen-Plotkin, A. S., et al. (2012). https://doi.org/10.1523/JNEUROSCI.0521-12.2012 | miR-132 | |
| 17 | Wibrand, K., et al. (2012). https://doi.org/10.1371/journal.pone.0041688 | miR-132 | |
| 18 | Miura, P., et al. (2012). https://doi.org/10.1111/j.1471-4159.2011.07583.x |
miR-206 | |
| 19 | Bahi, A., & Dreyer, J.-L. (2013). https://doi.org/10.1111/ejn.12228 | miR124a | |
| 20 | Hutchison, E. R., et al. (2013). https://doi.org/10.1002/glia.22483 | McCP2 | |
| 21 | SUN, Y.-X., et al. (2013). https://doi.org/10.3892/or.2013.2731 |
miR-16 | |
| 22 | Li, Y.-J., et al. (2013). https://doi.org/10.1371/journal.pone.0063648 | miR-182 | |
| 23 | Kynast, K. L., et al. (2013). https://doi.org/10.1016/j.pain.2012.11.010 | miRNA-124a | |
| 24 | Croce, N., et al. (2013). https://doi.org/10.1007/s11010-013-1567-0 |
NPY | |
| 25 | Ryan, K. M., et al. (2013). https://doi.org/10.1016/j.neulet.2013.05.035 | miR-212 | |
| 26 | Nagpal, N., et al. (2013). https://doi.org/10.1093/carcin/bgt107 |
miR-191 | |
| 27 | Mojtahedi, S., et al. (2013). https://doi.org/10.1002/cbin.10022 |
miR-124 | |
| 28 | Tapocik, J. D., et al. (2014). https://doi.org/10.1523/JNEUROSCI.0445-14.2014 | miR-206 | |
| 29 | Lin, C. R., et al. (2014). https://doi.org/10.1111/ejn.12522 |
miR-183 | |
| 30 | Tian, N., et al. (2014). https://doi.org/10.1007/s12264-013-1419-7 |
miR-206 | |
| 31 | Bahi, A., et al. (2014). https://doi.org/10.1016/j.psyneuen.2014.04.009 | miR124a | |
| 32 | Marler, K. J., et al. (2014). https://doi.org/10.1523/JNEUROSCI.1910-13.2014 | miRNA-132 | |
| 33 | Yang, X., et al. (2014). https://doi.org/10.1007/s12017-014-8312-z |
miR-206 | |
| 34 | Yi, L. T., et al. (2014). https://doi.org/10.1503/jpn.130169 |
miR-132 | |
| 35 | Giannotti, G., et al. (2014). https://doi.org/10.1017/S1461145713001454 | miR124 miR132 | |
| 36 | Varendi, K., et al. (2014). https://doi.org/10.1007/s00018-014-1628-x |
miR-1/206 miR-10 | |
| 37 | Zhang, J., et al. (2014). https://doi.org/10.3760/cma.j.issn.0366-6999.20131683 | miR- 34a | |
| 38 | Cho, K. J., et al. (2015). https://doi.org/10.1016/j.mcn.2015.07.004 | miR-Let-7a | |
| 39 | Li, H., et al. (2015). https://doi.org/10.3892/mmr.2015.3736 |
miR-183/96/182 | |
| 40 | Ma, J. C., et al. (2015). https://doi.org/10.1016/j.neuroscience.2015.04.061 | miR-1 | |
| 41 | Yang, G., et al. (2015). https://doi.org/10.3892/mmr.2015.3531 |
miR-29c | |
| 42 | Neumann, E., et al. (2015). https://doi.org/10.1186/s12990-015-0045-y |
miR-1 | |
| 43 | Li, W., et al. (2015). https://doi.org/10.1155/2015/302653 |
miR-22 | |
| 44 | Xiang, L., et al. (2015). https://doi.org/10.1016/j.brainres.2015.06.046 | miR-132 miR-132 | |
| 45 | Yan, H., et al. (2015). https://doi.org/10.1097/IGC.0000000000000456 | miR-204 | |
| 46 | Mu, Y., et al. (2015). https://doi.org/10.3892/mmr.2015.4456 |
miR-206-3p | |
| 47 | Li, M., et al. (2015). https://doi.org/10.1002/path.4484 |
miRNA-134 miR-132 | |
| 48 | Liu, Z., et al. (2015). https://doi.org/10.1159/000430356 |
miR-937 | |
| 49 | Su, M., et al. (2015). https://doi.org/10.3892/mmr.2015.4104 |
MeCP2 | |
| 50 | Hernandez-Rapp, J., et al. (2015). https://doi.org/10.1016/j.bbr.2015.03.032 | miR-132/212 | |
| 51 | Huang, W., et al. (2015). https://doi.org/10.1007/s12031-015-0500-2 |
miR-134 | |
| 52 | Oikawa, H., et al. (2015). http://doi.org/10.1016/j.neuint.2015.10.010 | miR-124 | |
| 53 | Jiang, Y., & Zhu, J. (2015). http://www.ncbi.nlm.nih.gov/pubmed/25755749 | miR-10B | |
| 54 | Shao, Y., et al. (2015). https://doi.org/10.1007/s12031-015-0522-9 |
miR-134 | |
| 55 | Gao, Y., et al. (2015). https://doi.org/10.1002/stem.1950 |
miR-15a+ | |
| 56 | Darcq, E., et al. (2015). https://doi.org/10.1038/mp.2014.120 |
miR-30a-5p | |
| 57 | Long, J., et al. (2016). https://doi.org/10.1007/s13277-015-4427-6 |
MiR-15a-5p | |
| 58 | Hu, X. M., et al. (2016). https://doi.org/10.1177/1744806916666283 | miR-219 | |
| 59 | Li, Y., et al. (2016). https://doi.org/10.1016/j.pnpbp.2015.09.004 | miR-182 | |
| 60 | Peng, J. Y., et al. (2016). https://doi.org/10.1016/j.domaniend.2015.09.005 | miR-10b | |
| 61 | Yi, S., et al. (2016). https://doi.org/10.1038/srep29121 |
miR-1 | |
| 62 | Bahi, A. (2016). https://doi.org/10.1016/j.bbr.2016.05.033 |
miR124a | |
| 63 | Xia, H., Li, Y., & Lv, X. (2016). https://doi.org/10.3892/ijo.2016.3628 | miR-107 | |
| 64 | Neumann, E., et al. (2016). https://doi.org/10.1016/j.mcn.2016.06.003 | miR-1 | |
| 65 | Kumari, A., et al. (2016). https://doi.org/10.1016/j.physbeh.2016.02.032 | miRNA-132 and miRNA-134 | |
| 66 | Liang, Y., et al. (2016). https://doi.org/10.1177/1744806916641679 | miRNA-212/132 | |
| 67 | Jiang, B., et al. (2016). https://doi.org/10.3892/mmr.2016.5810 | miR-9 | |
| 68 | Hang, P., et al. (2016) https://doi.org/10.7150/ijbs.15071 |
miRNA-195 | |
| 69 | Li, W., et al. (2016). https://doi.org/10.5582/bst.2016.01127 |
miR-613 | |
| 70 | Jimenez-Gonzalez, A., et al. (2016). https://doi.org/10.1016/j.bbagen.2016.03.001 | miR-212 and miR-132 | |
| 71 | Aili, A., Chen, Y., & Zhang, H. (2016). https://doi.org/10.3892/mmr.2015.4506 | miR-10b | |
| 72 | Zeng, L. L., et al. (2016). https://doi.org/10.1111/cns.12589 |
MiR-210 | |
| 73 | Xiang, L., et al. (2016). https://doi.org/10.1016/j.brainres.2016.02.045 | MiR-204 | |
| 74 | Cui, M., et al. (2017). https://doi.org/10.1016/j.bbadis.2017.06.021 | miR-34a-5p | |
| 75 | Thomas, K. T., et al. (2017). https://doi.org/10.1016/j.celrep.2017.06.038 | miR-137 | |
| 76 | Mendoza-Viveros, L., et al. (2017). https://doi.org/10.1016/j.celrep.2017.03.057 | miR-132/212 | |
| 77 | Xie, B., et al. (2017). https://doi.org/10.3233/JAD-160468 |
miR-206 | |
| 78 | Wang, P., et al. (2017). https://doi.org/10.18632/oncotarget.13747 | miR-497 | |
| 79 | Gao, B., et al. (2017). https://doi.org/10.1002/jgm.2932 |
miR-107 | |
| 80 | Tu, Z., et al. (2017). https://doi.org/10.1016/j.biopha.2017.05.016 | miR-140 | |
| 81 | Song, D., ett al. (2017). https://doi.org/10.3892/mmr.2017.7396 |
miR-382 | |
| 82 | Lin, C. Y., et al. (2017). https://doi.org/10.1038/cddis.2017.354 |
miR-624-3p | |
| 83 | Zhao, Y., et al. (2017). https://doi.org/10.1016/j.brainres.2017.05.020 | miR-101 | |
| 84 | Zhang, K., et al. (2017). https://doi.org/10.1042/BSR20170755 |
miR-211 | |
| 85 | Bahi, A. (2017). https://doi.org/10.1016/j.bbr.2017.03.010 |
miR124a | |
| 86 | Xu, A. J., et al. (2017). https://doi.org/10.3892/mmr.2017.7167 | miR-744 | |
| 87 | Sun, W., et al. (2017). https://doi.org/10.1016/j.neulet.2016.12.047 | miR-206 | |
| 88 | Wang, S. S., et al. (2017). https://doi.org/10.1016/j.pnpbp.2017.07.024 | miR-124 | |
| 89 | (Wang et al., 2017) https://doi.org/10.3892/mmr.2017.8282 |
miR-103 | |
| 90 | Huang, W., et al. (2017). https://doi.org/10.1007/s12031-017-0907-z |
MiR-134 | |
| 91 | Ji, M., et al. (2017). https://doi.org/10.3892/mmr.2017.7626 |
miR-705 | |
| 92 | Fu et al., (2017) http://dx.doi.org/10.1038/s41598-017-01261-x |
miR-125b-5p | |
| 93 | Lian, N., et al. (2018). https://doi.org/10.1080/15384101.2018.1556060 | miR-221 | |
| 94 | Duan, W., et al. (2018). https://doi.org/10.3892/ijmm.2018.3711 | miR-155 | |
| 95 | Yi, L. T., et al. (2018). https://doi.org/10.1177/0269881118758304 | miR-124 | |
| 96 | Nguyen, T., et al. (2018). https://doi.org/10.1073/pnas.1803384115 | let-7i | |
| 97 | Cheng, F., et al. (2018). https://doi.org/10.1016/j.micpath.2018.04.060 | miR-107 | |
| 98 | Fang, Y., et al. (2018). https://doi.org/10.1016/j.jad.2017.11.090 | miR-124 | |
| 99 | Zhang, S., et al. (2018). https://doi.org/10.1016/j.neulet.2017.10.014 | miR-210-3p | |
| 100 | Xing, Q., et al. (2018). https://doi.org/10.1055/a-0658-2095 |
miR-206 | |
| 101 | Li, X. J. (2018). https://doi.org/10.1177/1479164117749382 |
miR-497 | |
| 102 | Lu, Y., et al. (2018). https://doi.org/10.1007/s10571-018-0599-0 |
miR-155 | |
| 103 | Miao, Z., et al. (2018). https://doi.org/10.1007/s12035-016-0378-1 |
miR-206-3p | |
| 104 | Descamps, B., et al. (2018). https://doi.org/10.1161/ATVBAHA.118.311400 | miR-214 | |
| 105 | Lv, M., Yet al. (2018). https://doi.org/10.1002/mnfr.201800621 |
miR-26a miR-125b miR-132 | |
| 106 | Ge, Q. Di, et al. (2018). https://doi.org/10.1016/j.etap.2018.08.011 | miR-132 miR-204 | |
| 107 | Shen, J., et al. (2018). https://doi.org/10.1016/j.bbr.2018.04.050 | miR-134 | |
| 108 | Wu, B. W., et al. (2018). https://doi.org/10.1002/jcp.26328 |
miR-10a | |
| 109 | Ma, J. C., et al. (2018). https://doi.org/10.1159/000494657 |
miR-1 | |
| 110 | Zhang, X. yu, et al. (2018). https://doi.org/10.1016/j.lfs.2018.09.002 | miR-10a | |
| 111 | Gao, L., et al. (2018). https://doi.org/10.4149/neo_2018_161128N594 | MiR-1-3p | |
| 112 | Zhang, J., et al. (2018). https://doi.org/10.1007/s11064-018-2475-1 |
miR-322 | |
| 113 | Jiang, J. D., et al. (2019). https://doi.org/10.1002/iub.1911 |
miR-107 | |
| 114 | Yang, W., et al. (2019). https://doi.org/10.1002/jcp.28862 |
miR-124 | |
| 115 | Shrestha, S., et al. (2019). https://doi.org/10.1002/2211-5463.12581 |
miRNA-206 | |
| 116 | Miao, Z., et al. (2019). https://doi.org/10.1016/j.neuropharm.2019.03.032 | miR-30a | |
| 117 | Song, Y., et al. (2019). https://doi.org/10.3892/mmr.2019.10424 | miR-584 | |
| 118 | Sun, Z., et al. (2019). https://doi.org/10.1002/jcp.26928 |
miR-497 | |
| 119 | Mohammadipoor-Ghasemabad, L., et al. (2019). https://doi.org/10.1016/j.neuroscience.2019.06.037 | miR-191a | |
| 120 | Hung, Y. Y., et al. (2019). https://doi.org/10.3390/cells8091021 | miR-204-5p | |
| 121 | Li, B. B., et al. (2019). https://doi.org/10.1016/j.chemosphere.2019.05.064 | miR-7 | |
| 122 | Ma, R.,et al. (2019). https://doi.org/10.1016/j.bbrc.2019.08.046 |
miR-496 | |
| 123 | Shen, J., et al. (2019). https://doi.org/10.1016/j.jad.2019.01.031 |
miR-134 | |
| 124 | Solomon, M. G., et al. (2019). https://doi.org/10.1016/j.neuroscience.2019.02.012 | miR-206 | |
| 125 | Zhao, X., et al. (2019). https://doi.org/10.1016/j.ejphar.2018.11.035 | miR-375 | |
| 126 | Panta, A., et al. (2019). https://doi.org/10.1016/j.bbi.2019.01.003 | miR-363-3p | |
| 127 | Zhao, H., et al. (2019). https://doi.org/10.1016/j.neuroscience.2019.07.051 | miR-206 | |
| 128 | Xie, W., et al. (2020). https://doi.org/10.1007/s11064-020-03013-2 |
miR-185 | |
| 129 | Deng, C., et al. (2020). https://doi.org/10.1007/s11064-019-02910-5 |
miR-494-3p | |
| 130 | Hu, J. J., et al. (2020). https://doi.org/10.1002/kjm2.12136 |
miR-15a | |
| 131 | Liu, X., et al. (2020). https://doi.org/10.1080/15384101.2019.1710916 | MiR-192-5p | |
| 132 | Zhang, T., et al. (2020). https://doi.org/10.1016/j.neulet.2019.134562 | miR-10a-5p | |
| 133 | Yang, W., et al. (2020). https://doi.org/10.2174/1567202617666200319141755 | miR-124 | |
| 134 | Wu, G., et al. (2020). https://doi.org/10.1016/j.yexcr.2020.111937 | miR-129-5p | |
| 135 | Liu, H., et al. (2020). https://doi.org/10.14670/HH-18-266 |
miR-204-5p | |
| 136 | Misiorek, J. O., et al. (2020). https://doi.org/10.1007/s12035-020-01899-1 |
miR-10a-5p | |
| 137 | Wang, F., et al. (2020). https://doi.org/10.3892/mmr.2020.11065 | mir-210 | |
| 138 | Wang, L., et al. (2020). https://doi.org/10.1007/s11010-020-03726-6 |
miR-10b-5p | |
| 139 | Gao, L., et al. (2020). https://doi.org/10.1093/JNEN/NLAA069 |
miR-103-3p | |
| 140 | Giordano, M., et al. (2020). https://doi.org/10.3390/ijms21207615 | miR-195-5p | |
| 141 | Xu, H., et al. (2020). https://doi.org/10.12659/MSM.920855 |
miR-216a-5p | |
| 142 | Pejhan et al., (2020) https://doi.org/10.3389/fcell.2020.00763 |
miR-132 | |
| 143 | Lin, B., et al. (2021). https://doi.org/10.18632/aging.103640 |
miR-155 | |
| 144 | Niu, Y., et al. (2021). https://doi.org/10.1186/s10020-020-00258-z |
miR-186 | |
| 145 | Zhang, X., et al. (2021). https://doi.org/10.1016/j.bbr.2020.113087 | miR-432 | |
| 146 | Huan, Z., et al. (2021). https://doi.org/10.1007/s11064-021-03234-z |
miR-155 | |
| 147 | Zhao, P., et al. (2021). https://doi.org/10.1007/s11255-021-02853-3 |
miR-365 | |
| 148 | Ke, X., et al. (2021). https://doi.org/10.1159/000515750 |
miR-10b-5p | |
| 149 | Xu, Y., et al. (2021). https://doi.org/10.1080/21655979.2021.1918991 | miR-191-5p | |
| 150 | Pan, S., et al. (2021). https://doi.org/10.1038/s41398-021-01240-x |
MiR-195 | |
| 151 | Li, H., et al. (2021). https://doi.org/10.1080/15376516.2021.1886211 | miR-191 | |
| 152 | Tang, Y., et al. (2021). https://doi.org/10.1371/journal.pone.0257280 | miR-155 | |
| 153 | Li, X., et al. (2021). https://doi.org/10.5114/fn.2021.105132 |
miR-1b | |
| 154 | Peng, D., et al. (2022). https://doi.org/10.7150/thno.70951 |
miR-206-3p | |
| 155 | Wu, Y., et al. (2021). https://doi.org/10.4103/0028-3886.333459 |
miR-191-5p | |
| 156 | Guan, W., et al. (2021). https://doi.org/10.1016/j.phrs.2021.105932 | miR-206-3p | |
| 157 | Zhang, Q., et al. (2021). https://doi.org/10.3389/fendo.2021.637384 | miR-103a-3p miR-10a-5p | |
| 158 | Ehinger, Y., et al. (2021). https://doi.org/10.1111/adb.12890 |
miR30a-5p miR-195-5p miR191-5p miR206-3p | |
| 159 | Fang, F., et al. (2022). https://doi.org/10.1186/s13019-022-01802-0 |
miR-182-5p | |
| 160 | Su, B., et al. (2022). https://doi.org/10.1080/21655979.2022.2059937 | miR-139-5p | |
| 161 | Yongguang, L., et al. (2022). https://doi.org/10.1186/s12906-021-03483-z |
miR-497 | |
| 162 | Tu, W., et al. (2022). https://doi.org/10.1155/2022/1489841 |
miR-206-3p | |
| 163 | Gao, J., et al. (2022). https://doi.org/10.1080/21655979.2022.2067293 | miR-155-5p | |
| 164 | Zhai, Y., et al. (2022). https://doi.org/10.1002/kjm2.12486 |
miR-210 | |
| 165 | Yu, H. C., et al. (2022). https://doi.org/10.3390/ijms23010570 |
miR-3168 | |
| 166 | Kang, E. M., et al. (2022). https://doi.org/10.1111/cns.13845 |
miR- 124-3p | |
| 167 | Li, C., et al. (2022). https://doi.org/10.3892/mmr.2021.12577 |
mir-182-5p | |
| 168 | Deng, L., et al. (2022). https://doi.org/10.1186/s13018-022-03315-x |
miR-210-3p | |
| 169 | Ding, J., Jiang, C., Yang, L., & Wang, X. (2022). https://doi.org/10.14715/CMB/2022.68.1.10 | miR-1-3p | |
| 170 | Ma, L., et al. (2022). https://doi.org/10.1038/s41398-022-02192-6 |
miR-132-5p | |
| 171 | Asadi, M. R., et al. (2022). https://doi.org/10.1186/s12888-022-04442-9 |
miR-16 | |
| 172 | Wang, L., et al. (2022). https://doi.org/10.1016/j.brainresbull.2022.03.002 | miR-551b-5p | |
| 173 | Zhang, Z., et al. (2022). https://doi.org/10.1016/j.bbrc.2022.05.038 | miR-382 miR-182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







