Introduction
Closure of a patent foramen ovale (PFO) with a percutaneous occluder device has been around for over 30 years.1 A dedicated device for the PFO, the Amplatzer PFO Occluder, was first used on September 10, 1997.2 As practically the only device in interventional cardiology, it has not needed, and therefore not seen, any significant iteration since its first-in-man use. To date, the Amplatzer PFO Occluder has gathered an impressive record to have worked virtually flawlessly in hundreds of thousands if not millions of patients. It comes as no surprise that the device has begot a fair number of duplicates and derivatives.
PFO Closure Procedure
Closing a PFO with an Amplatzer occluder or a comparable device is extremely simple, innocuous, and effective. After a short and steep learning curve, it can be accomplished by any invasive cardiologist within less than 30 min. There is no necessity for sedation of patients. In fact, there is no reason for them not to leave the catheterization laboratory on foot holding the venous femoral puncture like one is holding the elbow after donating blood. There are no physical restrictions after such a PFO closure and even sports including underwater diving are possible already on the same day. Medical treatment after the procedure can be stripped down to 100 mg of acetylsalicylic acid daily for a month or two without preloading or loading dose. Later problems to be looked for are so rare that no further checkups are medically required. Nonetheless for ruling out a clinically significant residual shunt (to be expected in less than 10% of cases), it is recommendable to perform a transthoracic contrast echocardiogram (TTE) with a bubble test at the end of a sustained Valsalva maneuver after 3 to 6 months. To also reliably uncover small residual shunts (to be expected in about 20% of cases, but clinically irrelevant in most instances) or significant thrombi on the device (a risk of less than 1 in 500), a contrast transesophageal echocardiogram (TEE) is required. Most physicians and patients want to make sure that everything is perfect and follow-up contrast TTEs or TEEs are standard in the majority of institutions.
The intervention in its economical and frugal version is a same-day procedure, guided solely by fluoroscopy. The patient usually arrives at the catheterization laboratory with a venous line, having received some prophylactic intravenous or oral antibiotics. The intervention starts with a local anesthesia at the right groin, a small skin incision, and an intravenous bolus of 5000 units of heparin.
The femoral vein is punctured, and a regular 0.035” U-tip guidewire is inserted through the puncture needle. In nearly half the cases, the guidewire spontaneously passes the PFO into the left atrium when advanced up from the inferior vena cava through the right atrium. If not, it pays out to recross the right atrium a couple of times, because the wire may not pass at the first but at one of the following attempts. If the wire fails to pass, a 4 to 6 French (F) multipurpose catheter is inserted over the wire without a sheath. It may be necessary to actually screw the catheter through the skin and any tense subcutaneous tissue to be able to enter the vein over a regular guidewire. The multipurpose catheter is then parked at the level of the diaphragm pointing to the left shoulder, i.e., to 2 o’clock, in a frontal view. The wire is advanced several times deep into the right atrium until it arrives at the 2 o’clock position. This means usually that he left atrium is reached. In the frontal view, a similar wire trace may appear with an inadvertent route through the right ventricle into the left pulmonary artery. This path, however, almost invariably causes ventricular ectopy during wire advancement. Checking the wire position in a left anterior oblique or lateral view, the erroneous passage through the right ventricle into a pulmonary artery is identified by a bow of the wire on the left side of the screen close to the sternum. The correct passage through the PFO into the left atrium shows the wire straight on the right side of the screen in that view.
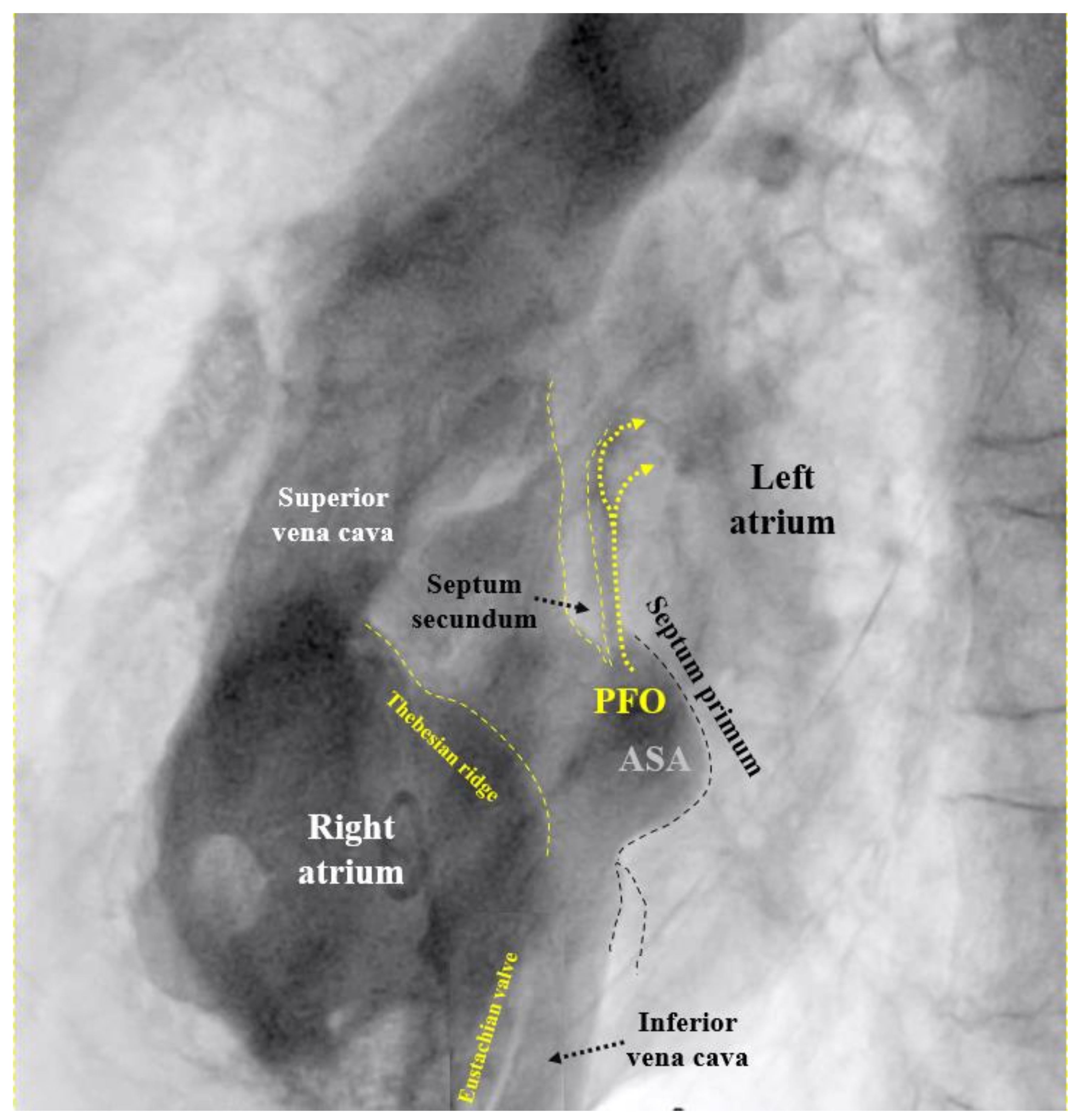
If the PFO cannot be passed despite changing direction and position of the multipurpose catheter, the U-tip wire may be removed and the tip straightened by scratching the back of the curve with the thumb nail. Should the now straight wire still not pass, the PFO is searched for with the tip of the multipurpose catheter. If even that fails, a right atriogram with contrast medium is performed to understand the anatomy (
Figure 1). The initial attempt to pass the atrial septum with a U-tip wire tries to take advantage of the fact that the U-tip wire can only pass through the slit-like PFO. A straight wire or a catheter may inadvertently pass through a tiny atrial septal defect (ASD) in the septum primum. Small cribriform ASDs often go unnoticed in prior echocardiograms.
Once the PFO is crossed, the guidewire is kept wherever it spontaneously wound up, be that the left atrium itself, the left atrial appendage (LAA), or a pulmonary vein. In the cases it was used, the multipurpose catheter is now removed. In tall patients, a regular length guidewire may be too short to manually keep the guidewire tip in the left atrium while exiting the multipurpose catheter. A small caliber syringe or a power injector can be connected to the multipurpose catheter once the wire has disappeared into the proximal end of the catheter to forcefully inject saline or contrast medium while the multipurpose catheter is fully withdrawn. This keeps the wire put in the left atrium. Particularly if a second person injects or a power injector is used, the tip of the multipurpose catheter has to be manually stopped by the operator as soon as it exits the skin to avoid uncontrolled ejection of the catheter accompanied by a shower to the environment with the injected fluid.
As soon as the multipurpose catheter is removed, the puncture site is inspected for exclusively venous bleeding. If there is arterial bleeding, a new puncture will be required. It has to be assumed that the femoral vein was inadvertently entered by traversing an artery. For obvious reasons, it must be avoided to insert the 8 to 10 F sheath required for device delivery over a wire passing through an artery into the femoral vein. In the mentioned frequent cases, the wire had passed directly through the PFO without using a multipurpose catheter, it is equally important to check the access path into the femoral vein before fully inserting the delivery sheath. This is done by advancing only the tapered tip of the sheath and then withdrawing it briefly to ascertain venous-only bleeding.
The sheath required for device implantation can be inserted over the regular guidewire in place. For passing through the skin into the femoral vein, a screwing rotation is recommended to avoid wire kinking. After that, the path is straight and no more resistance will occur that would require additional stiffness of the guidewire.
Once in the left atrium, the obturator (thick-wall inner part) of the sheath is removed and backflow of arterial blood from the left atrium is ascertained with the outer end of the sheath held at or below atrial level at the side of the patient. This is where the sheath should be kept and handled throughout the entire case.
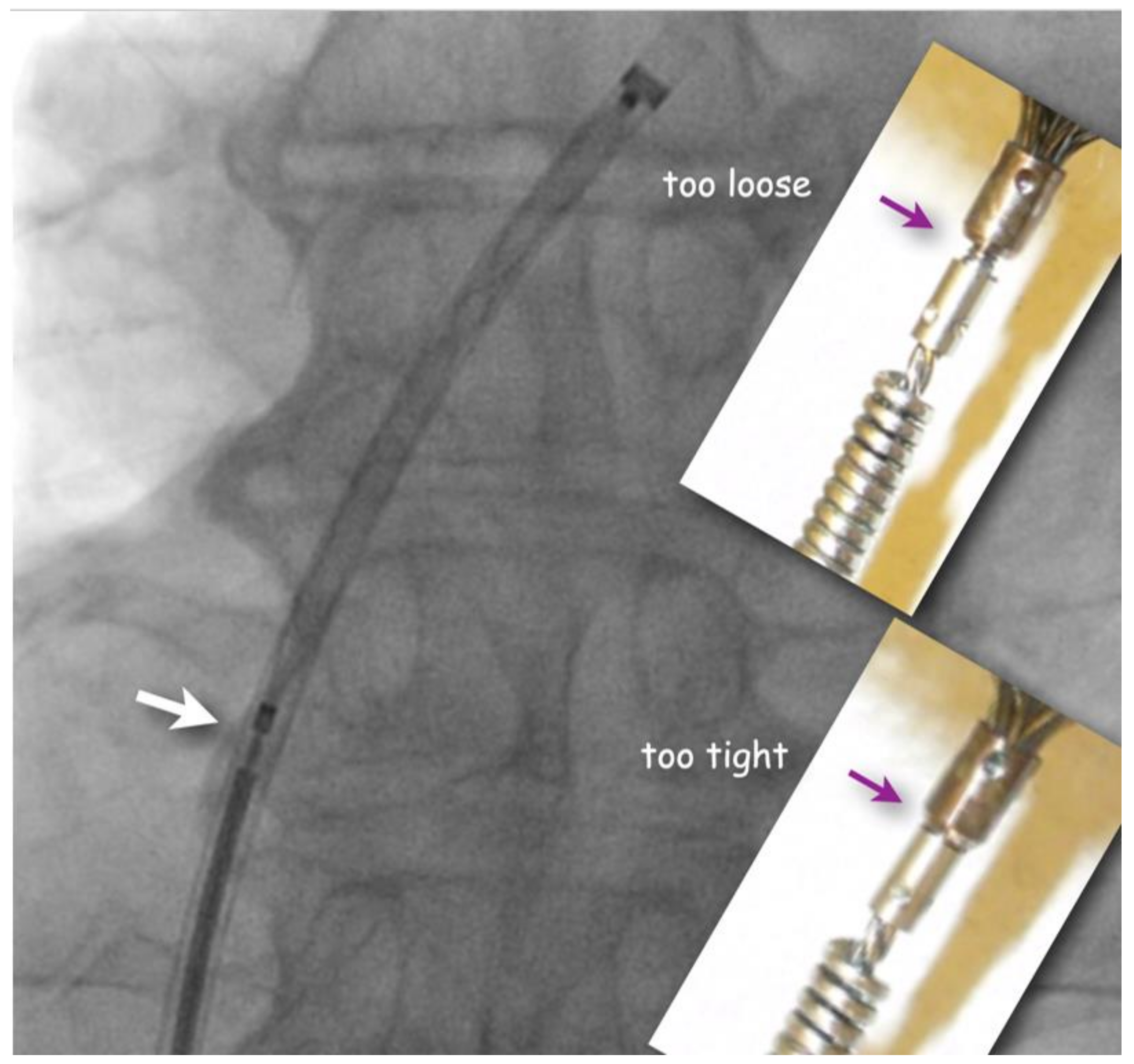
The flushed short loader sheath containing the selected preloaded occluder with its tip just peeking out is connected to the backbleeding sheath and the device is advanced to the distal tip of the sheath without any simultaneous flushing. Before exiting the sheath, it is made sure that the screw used for fixing the original Amplatzer device and most of its derivatives during delivery be still properly tightened, but not too much. A small gap ought to be visible (
Figure 2). If there is no gap, one or two full left (counterclockwise) turns are recommended during which usually an unblocking click is felt. This assures that the screw is not tightened overly fast so that the occluder would swivel with the loader cable rather than being released when the unscrewing is attempted at the end of occluder positioning. Too generous a gap documents inadvertent partial unscrewing of the occluder on the way up through the sheath. That needs to be corrected with turning right (clockwise) on the loading cable. In the extremely rare case that the screw is seen to be completely disconnected, the device is not implanted but pulled out of the sheath with a snare and reattached properly before reinsertion and implantation.
A small gap (white arrow) between pusher screw-collar and screw-nut on the device guarantees that the occluder is still safely attached but will be easily detachable once positioned. The inserts show screw positions that should be corrected either with turning the pusher cable clockwise (if too loose) or counterclockwise (if too tight) before advancing the occluder out of the sheath.
The remaining steps are done in a left anterior oblique fluoroscopic projection. The left disc is pushed out of the sheath. It may not be clearly visible at first, but the waist of the device is now shown at the tip of the sheath. The system is then pulled back as a unit. As soon as the left disc aligns to the atrial septum, it will infallibly become visible in its profile in the left anterior oblique projection. Now, the right disc is released by keeping the device snugly aligned to the septum and by withdrawing the sheath until proximal to the screw. At this moment, everything is relaxed (no more pulling on sheath or device) and the right disc will automatically adjust to the right side of the septum or it can be pushed there.
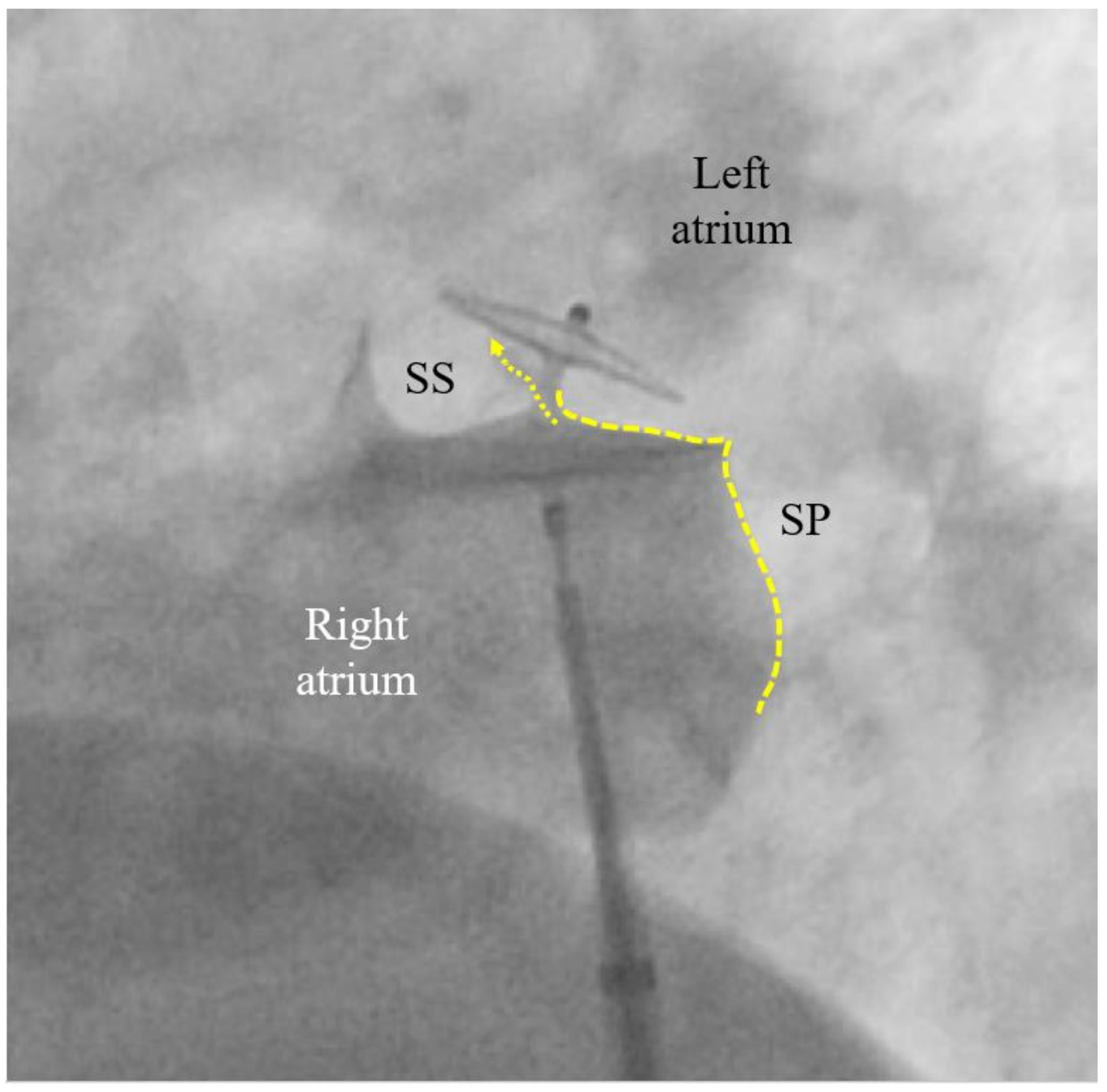
A contrast medium injection is done after flushing the sheath and after adjusting the projection so that both discs are seen in perfect profile without any overlap. It will show all necessary details (
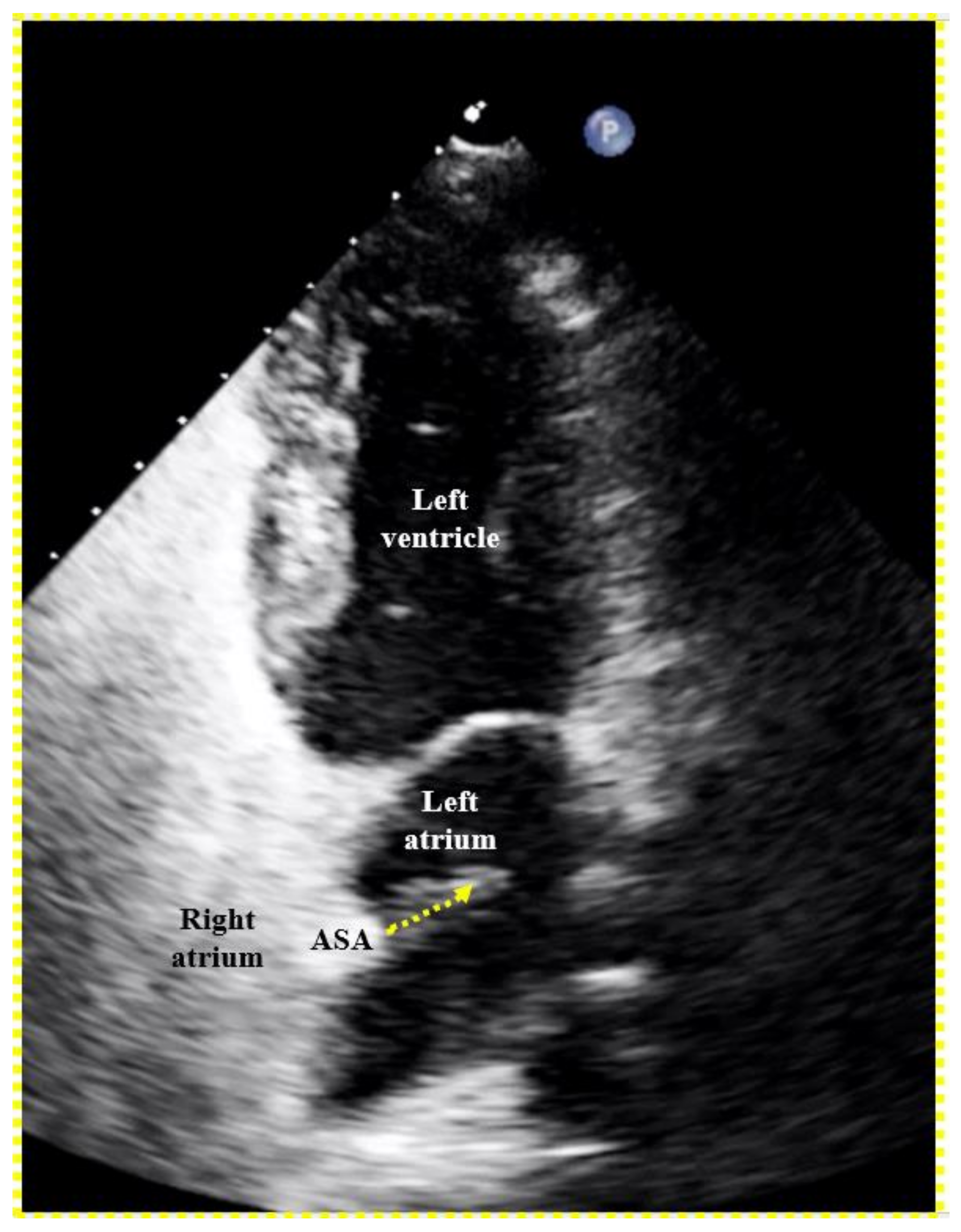
Figure 3). The device is then released, i.e., unscrewed from the pusher cable or liberated by whatever attachment and release mechanism is used with alternative occluders. A further contrast medium injection after readjusting the projection is required only if the device moves significantly upon release. This typically happens in the presence of an atrial septal aneurysm (ASA) or due to significant position distortion by sheath or pusher gear before release (
Figure 3).
The left (cranial) part of the 25/18 mm Amplatzer PFO Occluder is correctly biting into the septum secundum (SS). That is called a positive Pacman sign and is required for safe release. The original channel of the PFO is now blocked (dotted arrow). The septum primum (SP) is indicated with a dotted line. Its part caudal to the occluder is ideal for possibly later requiredfluoroscopy-guided transseptal punctures. A pre-release situation like this will usually have the device swivel up to a quarter turn clockwise upon release. The final occluder position is expected more vertical and parallel to the atrial septum with no more dent in the SP.
The patient can comfortably and safely hold the groin after having been helped to place the fingers in the right position before or after withdrawing the sheath. In a supine position, very little pressure is required to prevent bleeding by withholding the venous pressure of less than 10 mmHg. A hematoma cannot form with such a low pressure. Some operators close the skin nick with a figure of 8 suture. Once stitches or a finger block the only possible blood exit, the one through the skin, the femoral vein can no longer shed blood. When standing up, the venous pressure abruptly increases to the weight of the blood column from the tip of the head to the groin. That corresponds to about 60 mmHg, requires a more robust manual compression (a just applied figure of 8 suture is no longer sufficient), and also carries the risk of a hematoma or copious bleeding to the outside through an unstitched skin nick.
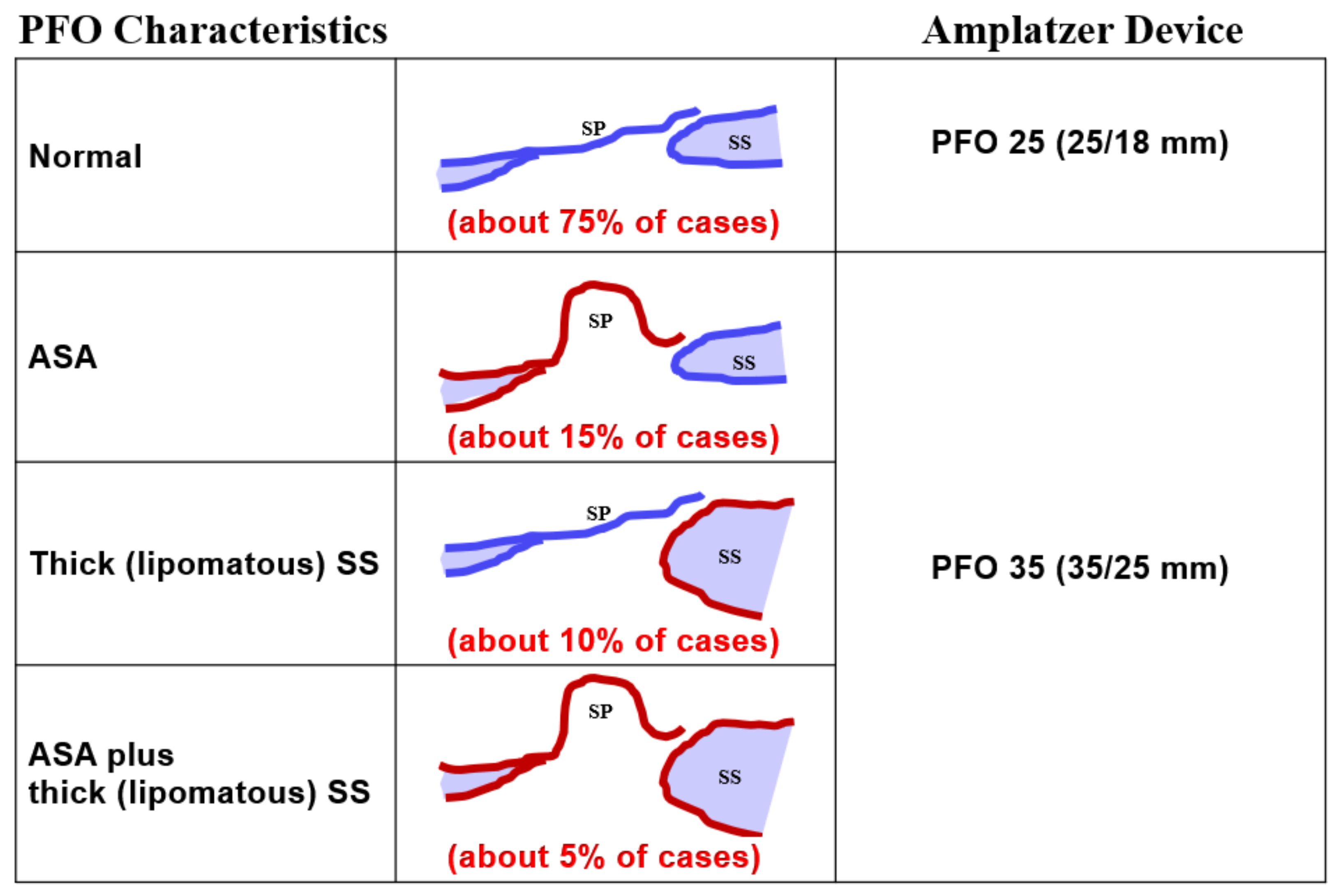
Regarding occluder size, practically all PFOs can be fit with either a 25 mm PFO occluder (right disc 25 mm, left disc 18 mm) or a 35 mm PFO occluder (35 mm, 25 mm), when using Amplatzer occluders (
Figure 4). The 35 mm occluder will be required in about 25% of cases, those with either a marked frailty and mobility of the septum primum (generally referred to as ASA), a lipomatous (thick triangular) septum secundum, or both. The production and use of occluders with a smaller left disc than the right disc makes sense. The flap-valve-like PFO will be pulled shut even by a small left disc. A smaller disc on the left side reduces overall contact with the pulsating aorta, the most common cause of the rare but ominous erosion of a free atrial wall by a PFO occluder. The right disc must be large enough to avoid disengagement from the septum secundum. That problem is also called unhinging from the Pacman position and is the consequence of too small a right disc in the presence of an ASA, a lipomatous septum secundum, or both.
3
When and How to Screen for a PFO
Venous thromboembolism, the prerequisite for a PFO to do harm, is hardly an issue until late adolescence but its incidence exponentially increases with older age4,5 and with morbidity. Oral contraception represents an early potential harbinger of paradoxical embolism in the presence of a PFO. Migraine in young people constitutes a compelling indication for PFO screening. PFO closure for migraine has a good chance for symptomatic relief6 and offers, as a collateral bonus, lifelong primary prevention – mechanical vaccination7 – against later paradoxical embolism.
To screen for dangerous PFOs, i.e., large ones or those associated with an ASA,
8 a Eustachian valve,
9–12 or a Chiari network,
13 a bubble test during a TTE suffices (
Figure 5). The contrast liquid has to be injected at the end of a sustained Valsalva maneuver. A Valsalva maneuver or pressing into the belly by a medical person during TEE if a sedated patient cannot perform a Valsalva maneuver, blocks the backflow of the venous blood into the thorax. Both atria get volume depleted within 10 to 20 s. Upon Valsalva release, the venous blood gushes into the right atrium first, thereby pushing the PFO open towards the still underfilled left atrium.
The venous inflow into the right atrium from the inferior vena cava is directed onto the PFO, even more so in the presence of a Eustachian valve9–12 or a Chiari network.13 The inflow of the superior vena cava is not. Hence, injecting the contrast medium in a leg vein would be ideal.14,15 Yet, this is impractical and under most circumstances an arm vein has to do. A state-of-the-art bubble test during a TEE or a transcranial Doppler examination16 in adddition detects small PFOs that harbor a smaller (albeit still relevant) risk for paradoxical embolism.
Ear oximetry represents an interesting PFO screening technique first published over 20 years ago.17 Regrettably, it has not been further pursued since 2013.18 It is the only screening method that could be applied in school class rooms or shopping malls. The proband performs a sustained Valsalva maneuver with a graphical oximeter attached to an ear lobe. Upon release of the Valsalva maneuver when any large PFO will briefly open, a small tsunami of venous blood will rush through the systemic circulation and reach the ear lobe. The oxygen saturation curve dips momentarily. The magnitude of the dip corresponds to the importance of the PFO.18
Obviously even with such a simple test, medical logistics and funding are lacking to screen the world population. Having said that, every situation bringing the PFO to mind should be considered a reason and opportunity for a screening test. In the realm of neurology that pertains to migraine, particularly but not exclusively if associated with aura, and to any cerebral event (not only to those called cryptogenic in young and otherwise healthy patients). The PFO causes more paradoxical embolism in old and sick people because of their propensity for venous thromboembolism, the prerequisite for paradoxical embolism. The absolute respective risk for PFO carriers increases geometrically with age and disease. The relative risk decreases due to aggregated hazard of accumulating competitive causes for systemic arterial occlusions. In the realm of cardiology, one should look for a PFO and close it with every myocardial infarction (MI) that looks even remotely embolic. Concomitant atherosclerosis does by no means acquit the PFO. It rather heightens its risk to cause an MI. The prevalence of both, atherosclerosis and venous thrombosis, grows in unison with age and comorbidities. Cardiologists should suspect – based on the blood volume distribution in the systemic circulation – one PFO associated MI per every three PFO associated cerebral events identified by neurologists. Add to this that PFO associated cerebral events are grossly underdiagnosed as they are still only looked for in the young and otherwise fit patients, notably the patient group with the lowest absolute respective risk.
Neglected screening for a PFO is only one of the reasons for underused closure of PFOs. Another reason is the false assumption that first PFO related events be benign and leave time for reaction. PFO case number one in the medical literature was fatal.19 PFO carriers as percentage of the population decrease with age,20,21 pointing to selective mortality of people with a PFO. Moreover in a propensity matched comparison among patients with a PFO and an index event, mortality was significantly lower over ten years of follow-up in the about 150 subjects with PFO closure than in the same number with purely medical treatment.22
The PFO direly needs to get the medical attention it deserves. PFO closure effectively addresses potentially life-threatening problems with an intervention comparable, in intricacy, to mending teeth. The completely normal life resumes already a couple of hours after the act. The technical success rate is virtually 100%. The long-term clinical efficacy is over 90%. Significant late problems are exotically rare. Actual costs of PFO closure amount to less than € 10,000.
Irrespective of the presence of established causes of cerebral events, such as atherosclerosis or atrial fibrillation (AF), the PFO must be looked for. It is a potential culprit for any such accident; the easiest to diagnose and to eliminate, for that matter. Interestingly, the term cryptogenic strokes initially encompassed patients with AF. Only later, AF was promoted to an established cause of stroke. That made sense but it makes equally sense and is overdue to classify a PFO as a non-cryptogenic stroke cause and also remove it from the basket of embolic stroke of undetermined source called ESUS.
23 Table 1 proposes an adapted classification of embolic events. The potential to cause an embolic event of paroxysmal AF with rare and short episodes is much smaller than that of a high-risk PFO. In both cases, the stroke cause is usually assumed and not proved, with rare exceptions.
24,25 The argument that AF requires oral anticoagulation (OAC) for life which at the same time protects against PFO mediated events can be rebutted in two ways. First, OAC provides only limited protection due to its notoriously poor compliance and required pauses. Second, easily combinable PFO and LAA closure (add only a few minutes and the cost of a second occluder)
26 obviates the need for OAC for AF with its incessantly accruing bleeding risk, exponentially climbing with age. The term mechanical vaccination applies to PFO
7 and LAA
27 closure and both are oblivious to any compliance issue.
Compelling and Established Indications for PFO Closure (Table 2)
At present, randomized data regarding to PFO closure for ischemic events are limited to patients under the age of 60 or 65 years after a cerebral ischemic event with no findable putative cause other than the PFO. As a consequence, PFO closure is exclusively recommended for such patients in all guidelines. To extrapolate indications to other embolic events in the systemic circulation, to primary prevention, or to patients who are older or have competing potential risks for systemic embolism breaches evidence-based medicine (EBM). Guidelines based on EBM so far inexcusably disregard that old and sick patients have a higher absolute risk of PFO associated events.28 At any age and in any setting, it is cynical and macabre to tell people at risk that they have to wait for a first event before this simple and efficacious preventive procedure will be performed.
Table 2 lists reasonable indications for PFO closure. They are all supported by data,
29 albethey statistically significant in randomized trials only for ischemic events
30 and migraine.
6 Yet, are we and the patients not better off with extrapolating randomized data without actually collecting them for cardiology’s simplest procedure with the best overall yield considering positive minus negative aspects? Think of the countless debilitating or even lethal events in control groups of future randomized trials that could and should have been prevented rather than just watched and counted in study spreadsheets.
28 Equally important, the positive effects for a given indication of PFO closure always include as collateral benefits the positive effects of all other indications. Close a PFO for migraine and the patient is vaccinated for life against paradoxical embolism into cerebral, coronary, ocular, visceral, or other systemic arteries. The other way around, close a PFO after any embolic event and the patient may be cured from migraine.
Table 2.
Proposal for PFO Closure Indications.
Table 2.
Proposal for PFO Closure Indications.
Therapeutic
- Migraine (particularly but no exclusively with aura)
- Vasospastic angina pectoris
- Exercise desaturation
- Platypnea orthodeoxia
- Sleep apnea
Primary prevention
- High-risk PFO
- Large gap
- Atrial septal aneurysm
- Eustachian valve
- Chiari network
- Family history for PFO problems
- Thromboembolism
- Pacemaker or defibrillator electrodes / other right heart catheters
- Surgery
- Major abdominal or thoracic
- Orthopedic
- Cerebral in sitting position
- Hypercoagulable blood disorders
- Carcinoid tumor
- Planned pregnancy
Secondary prevention
- Stroke or transient ischemic attack
- Myocardial infarction
- Ocular ischemia
- Visceral ischemia
- Peripheral ischemia
- Takotsubo event
- Decompression accident
- High-altitude pulmonary edema or mountain sickness
Profession, hobby, or life circumstances
- Deep sea diver
- Extreme mountain climber / highlander
- Brass musician
- Glass blower
- Tile setter
- Astronaut / pilot of air force jet or acrobat plane |
Therapy
Migraine. Migraine is the key indication for therapeutic PFO closure. Vasoactive triggers, foremost serotonin, are produced in the intestinal organs and get largely neutralized by the lung filter. If a gush of venous blood passes through a PFO, the systemic circulation experiences a serotonin tsunami that may activate hypersensitive receptors in the brain vessels. A cortical spreading depression can ensue from it.31 This corresponds to a transient vasoconstriction possibly provoking an aura and being followed by reactive vasodilation causing a headache. In case of a single hypersensitive receptor, the migraine symptoms are always identical. With several hypersensitive receptors, symptoms may vary. They can always pertain to the one same side in case all overreacting trigger receptors are located ipsilaterally.
PFO closure in migraine is yet another deplorable victim of EBM applied overzealously to the disadvantage of patients.28 There are a proved increased prevalence of PFOs among migraine patients32 and an uncontested increased prevalence of migraine among people with a PFO.33 An encrusted opinion prevails, particularly but not only among neurologists, that this be explainable by common genes. This theory is refuted by the fact that PFO closure improves some migraines. Almost all comparative or randomized trials investigating PFO device closure or medical treatment alone in patients with migraine showed a numerically conspicuous improvement of migraine with PFO closure. This was more pronounced in migraineurs with aura.34 In most non-randomized comparisons, the significance was statistically significant. The primary endpoints of all three major randomized trials were favorable for PFO closure but failed the predefined statistical significance. Most secondary endpoints showed statistically significant superiority for device closure of the PFO. High complexity of the migraine patients included, overly ambitious and ill-fated primary endpoints, and, in the studies with sham procedures, the equalizing placebo sham effect contributed to the missed statistical significances. Misquoting EBM, superiority trials missing their primary endpoints are repeatedly and erroneously used to bury alive a technique or a compound, despite proved noninferiority and numerical superiority.28 Neurological societies mistakably exclude PFO closure as an alternative or supplement of drug treatment for migraine, although the data leave no doubt that adopting PFO closure as a therapy option for migraine will not disadvantage patients. In fact, it offers them a good chance of improvement, even cure, not to mention that a mechanical vaccination as a simple and safe one-time measure is preferable to year-long medical treatment. The latter accrues side effects and cost for decades or for life.
The maintained neurological embargo on PFO closure in patients with migraine once again neglects that PFO closure in patients with migraine carries along for free the collateral benefit of lifelong protection against paradoxical embolism, the likes of stroke, MI, or even death. It also scotomizes meta-analyses of the randomized trials,35 inexplicably also the ones performed on individual patient data.6 Virtually all their examined endpoints depict statistically significant advantages of PFO closure in migraine.
It pays out to screen patients with typical migraine for a PFO and close the PFO if found. At least half the patients enjoy immediate and sustained improvement of migraine, a good percentage even get cured for good, and every single patient will be protected against paradoxical embolism through the now closed PFO for life.
An utterly disturbing case vignette of a 39-year-old nurse exemplifies the unfortunate prevailing attitude to the migraine and PFO issue. The patient was referred to me for refractory migraine for decades. Unfortunately, this happened more than 2 years after the she had been left permanently aphasic by a stroke. A PFO was found and percutaneously occluded. The migraine vanished instantly. Had she come to my attention years earlier, she would have overcome her disabling migraine already then. Most importantly, she would not have suffered the stroke and avoided the horrid struggle to educate 2 teenage sons and to function socially and professionally while being deprived of speech.
Vasospastic angina pectoris. Like migraine, vasospastic angina can be mediated by a PFO via serotonin tsunamis and treated by PFO closure in such cases.31 Even the patients without improvement regarding vasospastic angina are mechanically vaccinated against paradoxical embolism. They lack the direct improvement but are recompensed by the collateral benefit.
Exercise desaturation. Exercise desaturation occurs in some people when the PFO persistently gaps during strenuous activities, causing cyanosis and limiting exercise capacity.36 The phenomenon was documented in a third of 50 all-comers with a PFO.37 PFO closure improved it in most patients and protected all of them against later paradoxical embolism.
Platypnea orthodeoxia. The sitting position may distort the diaphragm cranially, especially in the obese elderly. This impacts on the heart in a way the PFO opens and stays patent till the body position is changed. Significant arterial desaturation ensues and is called platypnea orthodeoxia.36,38 PFO closure solves that and does away with the accompanying high risk of paradoxical embolism of thrombi of such patients.
Sleep apnea. Sleep apnea can be obstructive or central.39 The former type is characterized by increased venous backflow into the thorax during prolonged inspiration phases. A PFO is often opened by that. This increases the risk of paradoxical embolism and, at the same time, worsens central sleep apnea because the open PFO aggravates oxygen desaturation.40–42 PFO closure improves all that.
Primary Prevention
PFO closure for primary prevention deserves to be put on the agenda urgently. First it should be offered for people who, for whatever reason, are known to have a high-risk PFO.
High-risk PFO. As a side note, the label high-risk can well be employed for all PFOs presumed to have already caused an ischemic event and considered for secondary prevention. For primary prevention, high-risk PFOs are those with high-risk anatomical and physiological features. They are large PFOs, PFOs with an unprovoked right-to-left shunt, and PFOs associated with a flimsy septum primum, also called ASA,8 with a Eustachian valve9–12, or with a Chiari network.13 An ASA opens the PFO practically with every heart beat, thereby accumulating considerable overall right-to-left shunt time. A Eustachian valve or a Chiari network escort the clots from the inferior vena cava (where virtually all thrombi come from) directly through the PFO. All these features are associated with an increased PFO prevalence because they are congenital and hinder PFO closure in the first year of life.
Family history of PFO problems. Other candidates for PFO closure for primary prevention are people with one or more close blood relatives, e.g., twin, having suffered a PFO mediated stroke, MI, or other ischemic accident, or having experienced improvement of migraine after PFO closure.
Thromboembolism or hypercoagulable blood disorders. Patients with a history of thromboembolism or with hypercoagulability,43 should be offered primary PFO closure,44 even if they are under chronic OAC. OAC is notorious for compliance problems and there are medically imposed pauses. PFO closure knows neither compliance issues nor pauses.
Pacemaker or defibrillator electrodes, other right heart catheters, or vena cava filter. All foreign bodies in the venous circulation are potential sources of thromboembolism and place PFO carriers at increased jeopardy for paradoxical embolism. That also holds true for vena cava filters. They block large clots but not the small clots typical for PFO mediated systemic embolism. In fact, they, themselves, constitute a potential nidus for small emboli.
Major surgery. Time permitting, patients undergoing major surgical operations ought to be screened for a PFO. PFO closure should then precede surgery.45 It reduces the risk of a stroke from 9% to 1% within the first year after surgery. Neurosurgeons operating on sitting patients in order to reduce venous bleeds depending on gravity should make sure that there is no (unclosed) PFO.46 Brain veins opened during surgery have a propensity to catch air that risks to bypass the lung filter through a PFO to wind up in the systemic circulation. Being lighter than blood, air will tend to climb to the brain, topmost organ of the sitting patient.
Carcinoid tumor. Left heart valve problems with carcinoid tumors are more common if the patients have a PFO.47 These tumors typically produce serotonin in high enough doses to damage heart valves. In case the serotonin can shortcut the physiological neutralization during the lung passage, valve destruction not only pertains to the right but also to the left heart. There, it is clinically more devastating. PFO closure prevents that.
Planned pregnancy. Pregnancy is associated with an increased risk of thromboembolism and a rise in right atrial pressure. That enhances the deleterious potential of a PFO. Although there are no robust data to prove that the pregnancy risk be increased by the presence of a PFO, closing a PFO before a planned gravidity cannot be wrong, considering the collateral benefits.
Secondary Prevention
Stroke and transient ischemic attack (TIA). An embolic stroke differs from an embolic TIA only in the irreversibility of symptoms and that is subject to chance. No differences should be made in the indications for PFO screening and closure between the two. TIA symptoms may be mimicked by migraine. Especially in such cases, PFO closure being a valuable therapy for migraine and protecting against future real embolic strokes is the intervention of choice. The PFO cannot be acquitted in patients with a cerebral event presumed embolic on the basis that there are other potential stroke causes or because the patient is old or has comorbidities. The absolute yield, in contrast to the relative yield, to be expected with PFO closure increases with age and disease of the candidates. Compared to the old and sick, the young and so-called cryptogenic stroke patients have a lower risk of a PFO mediated paradoxical embolism. AF is not a contra-indication to PFO closure, either. Even under OAC, PFO closure is beneficial. Paradoxical embolism is more common with AF than without, as there are more clots in the venous circulation, and it is completely preventable by PFO closure but only partially by OAC. Extended screening for occult AF in young and otherwise healthy patients with a cerebral accident makes little sense. The yield is negligible. Even if AF is detected, it is unlikely that it was responsible for the event. A high-risk PFO is a much more likely culprit. In addition, a lengthy search for AF defers or even makes forget the initially planned PFO closure. Additional preventable yet un-prevented PFO mediated events may be the price for that. Finally, an obvious and attractive approach is to simultaneously close the PFO and the LAA. This puts an end to the indication for OAC and improves longevity twofold. Both, PFO closure22 and LAA closure – compared to warfarin48 or to novel oral anticoagulants49 – have shown a survival benefit.
MI. MI by paradoxical embolism through a PFO must be one of the most frequently misdiagnosed problems in cardiology.
50 This is particularly vexing because it is easily preventable by PFO closure. The cardiologists are the ones to blame for this oversight. In the wake of the bad example of neurologists who for nebulous reasons want to have excluded all other potential causes for stroke before scouting for a PFO, cardiologists think of all imaginable causes for a MI before considering paradoxical embolism. In the absence of obvious atherosclerosis (the prime suspect), spontaneous coronary dissection, protracted spasm, or drug abuse are pondered, searched for, or incriminated right off the bat, but not a PFO. The mentioned fact goes ignored that atherosclerosis, age, and systemic disorders promote rather than exclude paradoxical embolism of a venous clot. Only a minority of interventional cardiologists list a PFO among the acknowledged causes for MI. The amount of blood supply per minute to the coronary arteries is about 220 ml/minute at rest. This corresponds to almost a third of that to the brain (roughly 750 ml/minute). Both organs are equally jeopardized by the small clots typically passing through a PFO. Limbs and visceral organs require larger clots for serious ischemic harm. In brain and heart, there are silent embolisms thanks to unimportant areas, pre-existing tissue scars, or collateralized territories. Accounting for all that, one should attribute to a PFO roughly one MI per three cerebral events. This has yet to take into account that neurology significantly underdiagnoses PFO mediated cerebral events. Typically, they are ruled out by default in patients with the highest respective risk. The number of correctly diagnosed PFO mediated MIs should probably be at least hundredfold higher than it currently is. Any acute MI looking even remotely embolic, should prompt a search for a PFO, once the coronary situation is managed. This can be accomplished with a right heart catheterization at the end of the primary coronary percutaneous intervention. Rather than directly probing the PFO with a wire or a catheter as described above, a right atrial injection is preferable (
Figure 1). There is a small but concerning risk of a thrombus saddling the PFO,
24 part of which just broke off and caused the acute MI. Excluding or proving and closing a PFO in that way adds a few minutes to the coronary case and is doable day or night. It does not really increase the overall risk of the procedure but can do a lot of good. It makes sense in embolic looking MIs irrespective of whether or not they were indeed caused by the PFO. The next MI could well be and a mechanical vaccination against paradoxical embolism is never unwarranted. By the way, screening for a PFO with a right heart catheter is less uncomfortable for the patient than with a TEE. The characteristics of the PFO needed to select the appropriate device size (
Figure 4) can be equally well depicted with such a contrast medium study (
Figure 1). A combined coronary intervention and PFO closure is in the vetted interest of patients.
Ocular, visceral, or peripheral ischemia, takotsubo event, decompression accident, and high-altitude pulmonary edema or mountain sickness. The causative relationship of the PFO with ocular, visceral, or peripheral embolism is the same as that of cerebral and coronary ischemic events. Yet, with the exception of ocular embolism, larger clots are at stake. They are less likely to pass a regular PFO. A takotsubo event may be triggered by a PFO via a mechanism similar to the one described above for migraine or vasospastic angina pectoris. Decompression accident and high-altitude pulmonary edema or mountain sickness in the realm of PFOs are dealt with in the paragraph below.
Profession, Hobby, and Life Circumstances
Deep sea diver. Diving carries a risk for decompression accidents mainly by nitrogen bubbles. The more prevalent ones in the venous circulation are eliminated by the lung filter. A PFO increases the risk significantly that such bubbles bypass the filter to reach sensitive organs like the brain.51 Divers more commonly suffer cerebral events as evidenced by lacunar brain lesions on magnetic resonance imaging if they have a PFO.52 PFO closure is effective in preventing recurrent decompression events.51
Extreme mountain climber or highlander. High-altitude cerebral and pulmonary edema, also known as acute mountain sickness, is more common in people with a PFO.53,54 PFO closure deserves a chance to prevent or remedy that. It will improve the overall prognosis, for sure. Highlanders are known to develop chronic elevated right atrial pressures. Constriction of pulmonary arterioles occurs, secondary to low oxygen concentrations of ambient air, to slow down blood passage through the lungs for facilitating oxygen transfer to the erythrocytes. Elevated right atrial pressure perpetuates a situation similar to that at the release of a Valsalva maneuver and may keep a PFO open. Highlanders with a PFO are at a substantially increased risk for paradoxical embolism. They may experience a permanent right-to-left shunt through the PFO that worsens the situation by further decreasing oxygen saturation in the blood and leading to more pulmonary arterial vasoconstriction. The right atrial pressure increases further and the vicious cycle goes on.
Brass musician, glass blower, or tile setter. Brass musicians and glass blowers use Valsalva maneuvers for work. Activities in crouching positions with a not yet treated PFO render prone to oxygen desaturation and paradoxical embolism.
Astronaut or pilot of air force jet or acrobat plane. High accelerations represent a risk for paradoxical embolism in PFO carriers, but only until the PFO is occluded.
Synopsis
The PFO may well top the list of underestimated, poorly worked up, and therapeutically neglected cardiovascular health hazards. Ironically, among all risk factors and medical problems in cardiology, it is the easiest to get rid of. PFO device closure, the most simple and innocuous procedure of interventional cardiology with likely the best overall yield, is referred to as a mechanical vaccination as it is predominantly a preventive intervention. Notwithstanding, PFO closure is therapeutically effective regarding PFO related symptoms in a significant proportion of PFO carriers. A collateral benefit can be expected when closing a PFO to prevent a PFO related problem, such as ischemic cerebral, coronary, ocular, visceral, or peripheral paradoxical embolism, in terms of improvement of PFO induced migraine or exercise intolerance, etc., and vice versa. Close a PFO for one reason and you have eliminated for free and for sure all other PFO-related problems.
A PFO exists in perhaps 25% of the population, its particularly dangerous forms in presumably 5%. Screening for and closure of the PFO as primary prevention appears advantageous and cost-effective in at least the latter group.
It is high time to list in guidelines and textbooks the PFO as a serious candidate culprit for any ischemic cerebral, coronary, or other systemic event that could be embolic, particularly in people of age or with comorbidities. The common myth that a PFO is only to be looked for in otherwise healthy and young patients with suspicion of paradoxical embolism must be laid to rest once and for all: the sicker and older the patient, the higher the prevalence of venous thrombosis and, thus, the higher the absolute probability that the PFO causes an embolic event. Along these lines, PFO related embolic MIs are much more common than currently suspected, let alone diagnosed, especially in old and sick people. Per three emboli finding the brain, one will find a coronary artery based on the physiological systemic blood distribution.
Hardly will one ever regret to have closed a PFO; much more likely will one regret not to have closed a PFO.
It is not safe to wait for the first event before PFO closure. The first PFO strike may be fatal or leave behind irreversible and clinically devastating damage.
AF is not an important side effect of PFO closure. AF after PFO closure only occurs – in a form that is clinically relevant and needs treatment –in patients in whom AF was already imminent before. Chronic OAC that might be or become necessary because of pre-existing or new AF or a coagulation disorder provides incomplete protection against thrombo-embolism because of compliance problems and imposed pauses. PFO closure is supplementing OAC. It knows neither compliances issues nor pauses; and it causes no bleeding problems.
PFO closure will not inhibit later left atrial access for catheter-based interventions like ablation for AF, LAA closure, or mitral valve interventions. To the contrary, a well visible PFO occluder delineates, just caudal to it, a safe area for fluoroscopically guided transseptal puncture of the septum primum (
Figure 3).
55
Conflicts of Interest
No conflicts of interest.
References
- Bridges ND, Hellenbrand W, Latson L, Filiano J, Newburger JW, Lock JE. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992, 86, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Madhkour R, Meier B. Patent foramen ovale closure, a contemporary review. Structural Heart 2018, 2, 114–120. [Google Scholar] [CrossRef]
- Madhkour R, Wahl A, Praz F, Meier B. Amplatzer patent foramen ovale occluder: Safety and efficacy. Expert Rev Med Devices 2019, 16, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Anderson FA, Jr., Wheeler HB, Goldberg RJ; et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991, 151, 933–938. [Google Scholar] [CrossRef]
- Martinez C, Cohen AT, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: A population-based cohort study in patients without active cancer. Thromb Haemost. 2014, 112, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mojadidi MK KP, Mahmoud AN, Elgendy IY, Shapiro H, West B, Charles AC, Mattle HP, Sorensen S, Meier B, Silberstein SD, Tobis JM. Pooled analysis of PFO occluder device trials in patients with PFO and migraine. J Am Coll Cardiol 2021, 77, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Meier, B. Closure of the patent foramen ovale with dedicated Amplatzer occluders: Closing in on a mechanical vaccination. Catheter Cardiovasc Interv. 2008, 72, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Mas JL, Saver JL, Kasner SE; et al. Association of Atrial Septal Aneurysm and Shunt Size with Stroke Recurrence and Benefit from Patent Foramen Ovale Closure. JAMA Neurol. 2022, 79, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Schuchlenz HW, Saurer G, Weihs W, Rehak P. Persisting Eustachian valve in adults: Relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004, 17, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Goel SS, Tuzcu EM, Shishehbor MH; et al. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol. 2009, 103, 124–129. [Google Scholar] [CrossRef]
- Nakayama R, Takaya Y, Akagi T; et al. Identification of High-Risk Patent Foramen Ovale Associated with Cryptogenic Stroke: Development of a Scoring System. J Am Soc Echocardiogr. 2019, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Cai Q, Ahmad M. Eustachian valve, interatrial shunt, and paradoxical embolism. Echocardiography 2020, 37, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Schneider B, Hofmann T, Justen MH, Meinertz T. Chiari's network: Normal anatomic variant or risk factor for arterial embolic events? J Am Coll Cardiol. 1995, 26, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan R, Perlowski A, Shenoda M, Mojadidi MK, Agrawal H, Tobis JM. Sensitivity of brachial versus femoral vein injection of agitated saline to detect right-to-left shunts with Transcranial Doppler. Catheter Cardiovasc Interv. 2014, 84, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Tang PT, Cahill T, Rothwell PM, Ormerod OJ, Daniels MJ. Serial Shunt Evaluation Reveals Limitations of Contemporary Screening Studies for Patent Foramen Ovale. JACC Cardiovasc Interv. 2020, 13, 1269–1270. [Google Scholar] [CrossRef] [PubMed]
- Mojadidi MK, Roberts SC, Winoker JS; et al. Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: A bivariate meta-analysis of prospective studies. JACC Cardiovascular Imaging 2014, 7, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Karttunen V, Ventila M, Ikaheimo M, Niemela M, Hillbom M. Ear oximetry: A noninvasive method for detection of patent foramen ovale: A study comparing dye dilution method and oximetry with contrast transesophageal echocardiography. Stroke 2001, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Billinger M, Schwerzmann M, Rutishauser W; et al. Patent foramen ovale screening by ear oximetry in divers. Am J Cardiol. 2013, 111, 286–290. [Google Scholar] [CrossRef]
- Cohnheim, J. A handbook for practitioners and students. London: The New Sydenham Society; 1889.
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984, 59, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Tanzi A, Onorato O, Casilli F, Anzola G. Is the search for right-to-left shunt still worthwhile? Acta Neurol Scand. 2016, 133, 281–288. [Google Scholar] [CrossRef]
- Wahl A, Jüni P, Mono ML; et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation 2012, 125, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Elgendy AY SJ, Amin Z, Boudoulas KD, Carroll JD, Elgendy IY, Grunwald IQ, Gertz ZM, Hijazi ZM, Horlick EM, Kasner SE, Kent DM, Kumar P, Kavinsky CJ, Liebeskind DS, Lutsep H, Mojadidi MK, Messé SR, Mas JL, Mattle HP, Meier B, Mahmoud A, Mahmoud AN, Nietlispach F, Patel NK, Rhodes JF, Reisman M, Sommer RJ, Sievert H, Søndergaard L, Zaman MO, Thaler D, Tobis JM. Proposal for Updated Nomenclature and Classification of Potential Causative Mechanism in Patent Foramen Ovale-Associated Stroke. JAMA Neurol. 2020, 77, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Koullias GJ, Elefteriades JA, Wu I, Jovin I, Jadbabaie F, McNamara R. Images in cardiovascular medicine. Massive paradoxical embolism: Caught in the act. Circulation 2004, 109, 3056–3057. [Google Scholar] [CrossRef]
- Parekh A, Jaladi R, Sharma S, Van Decker WA, Ezekowitz MD. Images in cardiovascular medicine. The case of a disappearing left atrial appendage thrombus: Direct visualization of left atrial thrombus migration, captured by echocardiography, in a patient with atrial fibrillation, resulting in a stroke. Circulation 2006, 114, e513–e514. [Google Scholar]
- Koermendy D, Nietlispach F, Shakir S; et al. Amplatzer left atrial appendage occlusion through a patent foramen ovale. Catheter Cardiovasc Interv. 2014, 84, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Nietlispach F, Moarof I, Taramasso M, Maisano F, Meier B. Left atrial appendage occlusion. EuroIntervention. 2017, 13, AA78–AA84. [Google Scholar] [CrossRef] [PubMed]
- Meier B, Nietlispach F. Fallacies of Evidence-Based Medicine in Cardiovascular Medicine. Am J Cardiol. 2019, 123, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Meier B, Nietlispach F. The evil of the patent foramen ovale: We are seeing but the tip of the iceberg. Eur Heart J. 2018, 39, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Kent DM SJ, Kasner SE, Nelson J, Carroll JD, Chatellier G, Derumeaux G, Furlan AJ, Herrmann HC, Jüni P, Kim JS, Koethe B, Lee PH, Lefebvre B, Mattle HP, Meier B, Reisman M, Smalling RW, Soendergaard L, Song JK, Mas JL, Thaler DE. Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA 2021, 326, 2277–2286. [Google Scholar] [CrossRef]
- Ravi D, Tobis J, Parikh R, Aboulhosn J. A New Syndrome of Patent Foramen Ovale Inducing Vasospastic Angina and Migraine. J Am Coll Cardiol Case Reports 2024. [Google Scholar]
- Schwerzmann M, Nedeltchev K, Lagger F; et al. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology 2005, 65, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: A quantitative systematic review. Cephalalgia 2008, 28, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Ben-Assa E, Rengifo-Moreno P, Al-Bawardy R; et al. Effect of Residual Interatrial Shunt on Migraine Burden After Transcatheter Closure of Patent Foramen Ovale. JACC Cardiovasc Interv. 2020, 13, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Kheiri B, Abdalla A, Osman M; et al. Percutaneous Closure of Patent Foramen Ovale in Migraine: A Meta-Analysis of Randomized Clinical Trials. JACC Cardiovasc Interv. 2018, 11, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Mojadidi MK, Ruiz JC, Chertoff J; et al. Patent Foramen Ovale and Hypoxemia. Cardiol Rev. 2019, 27, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Devendra GP, Rane AA, Krasuski RA. Provoked exercise desaturation in patent foramen ovale & impact of percutaneous closure. J Am Coll Cardiol Intv. 2012, 5, 416–419. [Google Scholar]
- Hayek A, Rioufol G, Bochaton T; et al. Prognosis After Percutaneous Foramen Ovale Closure Among Patients with Platypnea-Orthodeoxia Syndrome. J Am Coll Cardiol. 2021, 78, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, O. Cheyne-Stokes Respiration in Chronic Heart Failure, Treatment with Adaptive Servoventilation Therapy. Circ J. 2012, 76, 2305–2317. [Google Scholar] [CrossRef]
- Agnoletti G, Iserin L, Lafont A, Sidi D, Desnos M. Obstructive sleep apnea and patent foramen ovale: Successful treatment of symptoms by percutaneous foramen ovale closure. J Interv Cardiol. 2005, 18, 393–395. [Google Scholar] [CrossRef] [PubMed]
- White JM, Veale AG, Ruygrok PN. Patent foramen ovale closure in the treatment of obstructive sleep apnea. J Invasive Cardiol. 2013, 25, E169–E171. [Google Scholar]
- Rimoldi SF, Ott S, Rexhaj E; et al. Patent Foramen Ovale Closure in Obstructive Sleep Apnea Improves Blood Pressure and Cardiovascular Function. Hypertension 2015, 66, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Buber J, Guetta V, Orion D; et al. Patent Foramen Ovale Closure among Patients with Hypercoagulable States Maintained on Antithrombotic Therapy. Cardiology 2021, 146, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Liu K, Song B, Palacios IF; et al. Patent Foramen Ovale Attributable Cryptogenic Embolism with Thrombophilia Has Higher Risk for Recurrence and Responds to Closure. JACC Cardiovasc Interv. 2020, 13, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Friedrich S, Ng PY, Platzbecker K; et al. Patent foramen ovale and long-term risk of ischaemic stroke after surgery. Eur Heart J. 2019, 40, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Fathi AR, Eshtehardi P, Meier B. Patent foramen ovale and neurosurgery in sitting position: A systematic review. Br J Anaesth. 2009, 102, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Douglas S, Oelofse T, Shah T, Rooney S, Arif S, Steeds RP. Patent foramen ovale in carcinoid heart disease: The potential role for and risks of percutaneous closure prior to cardiothoracic surgery. J Neuroendocrinol. 2023, 35, e13323. [Google Scholar] [CrossRef] [PubMed]
- Reddy VY, Sievert H, Halperin J; et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef]
- Gloekler S, Fürholz M, de Marchi S; et al. Left atrial appendage closure versus medical therapy in patients with atrial fibrillation: The APPLY study. EuroIntervention 2020, 16, e767–e774. [Google Scholar] [CrossRef]
- Raphael CE, Heit JA, Reeder GS; et al. Coronary Embolus: An Underappreciated Cause of Acute Coronary Syndromes. JACC Cardiovasc Interv. 2018, 11, 172–180. [Google Scholar] [CrossRef]
- Honek J, Sramek M, Honek T; et al. Patent Foramen Ovale Closure Is Effective in Divers: Long-Term Results From the DIVE-PFO Registry. J Am Coll Cardiol. 2020, 76, 1149–1150. [Google Scholar] [CrossRef] [PubMed]
- Billinger M, Zbinden R, Mordasini R; et al. Patent foramen ovale closure in recreational divers: Effect on decompression illness and ischaemic brain lesions during long-term follow-up. Heart 2011, 97, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- West BH, Fleming RG, Al Hemyari B; et al. Relation of Patent Foramen Ovale to Acute Mountain Sickness. Am J Cardiol. 2019, 123, 2022–2025. [Google Scholar] [CrossRef]
- Allemann Y, Hutter D, Lipp E; et al. Patent foramen ovale and high-altitude pulmonary edema. JAMA 2006, 296, 2954–2958. [Google Scholar] [CrossRef] [PubMed]
- Zaker-Shahrak R, Fuhrer J, Meier B. Transseptal puncture for catheter ablation of atrial fibrillation after device closure of patent foramen ovale. Catheter Cardiovasc Interv. 2008, 71, 551–552. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).