Submitted:
03 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
- (1)
- Plant species, dominant species, height, number, and canopy density;
- (2)
- City, specific distribution point, longitude, latitude, sediment type, intertidal zone condition, and interference condition.
2.3. Representations of D. spathacea Population Dynamic Characteristics
2.3.1 Population Structure
2.3.2 Analysis of Quantitative Population Dynamics
2.3.3 Compilation of Static Life Table
2.3.4 Drawing of Survival Curve
2.3.5 Compilation of Population Survival Analysis Functions
2.3.6 Analysis of Time-Series
2.4. Description of Statistical Analysis
3. Results
3.1. Basic Information and Current Distribution Status of D. spathacea on Hainan Island, China
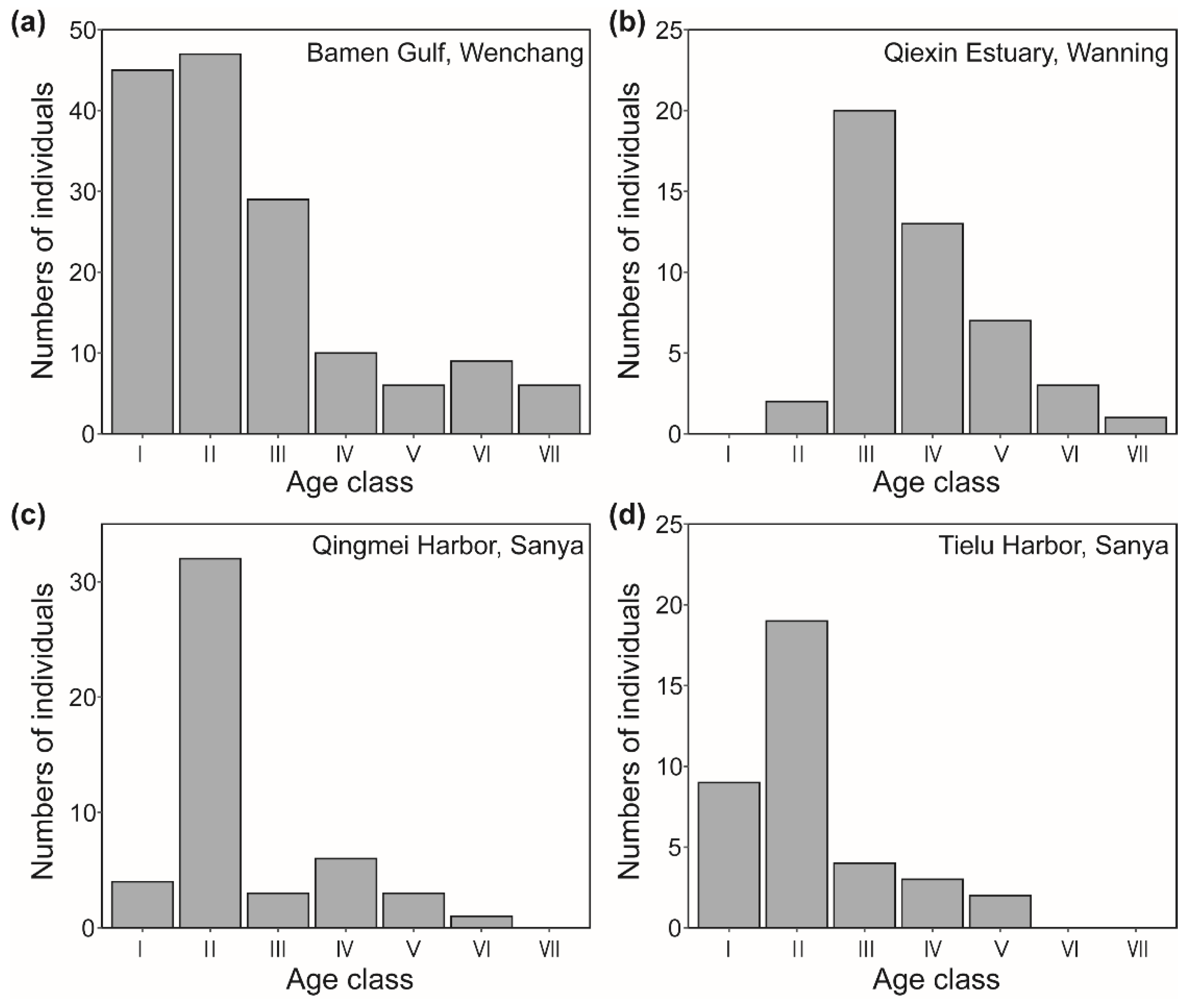
3.2. Age Class Structure Analysis of D. spathacea Population
3.3. Quantitative Population Dynamic Analysis of D. spathacea Population
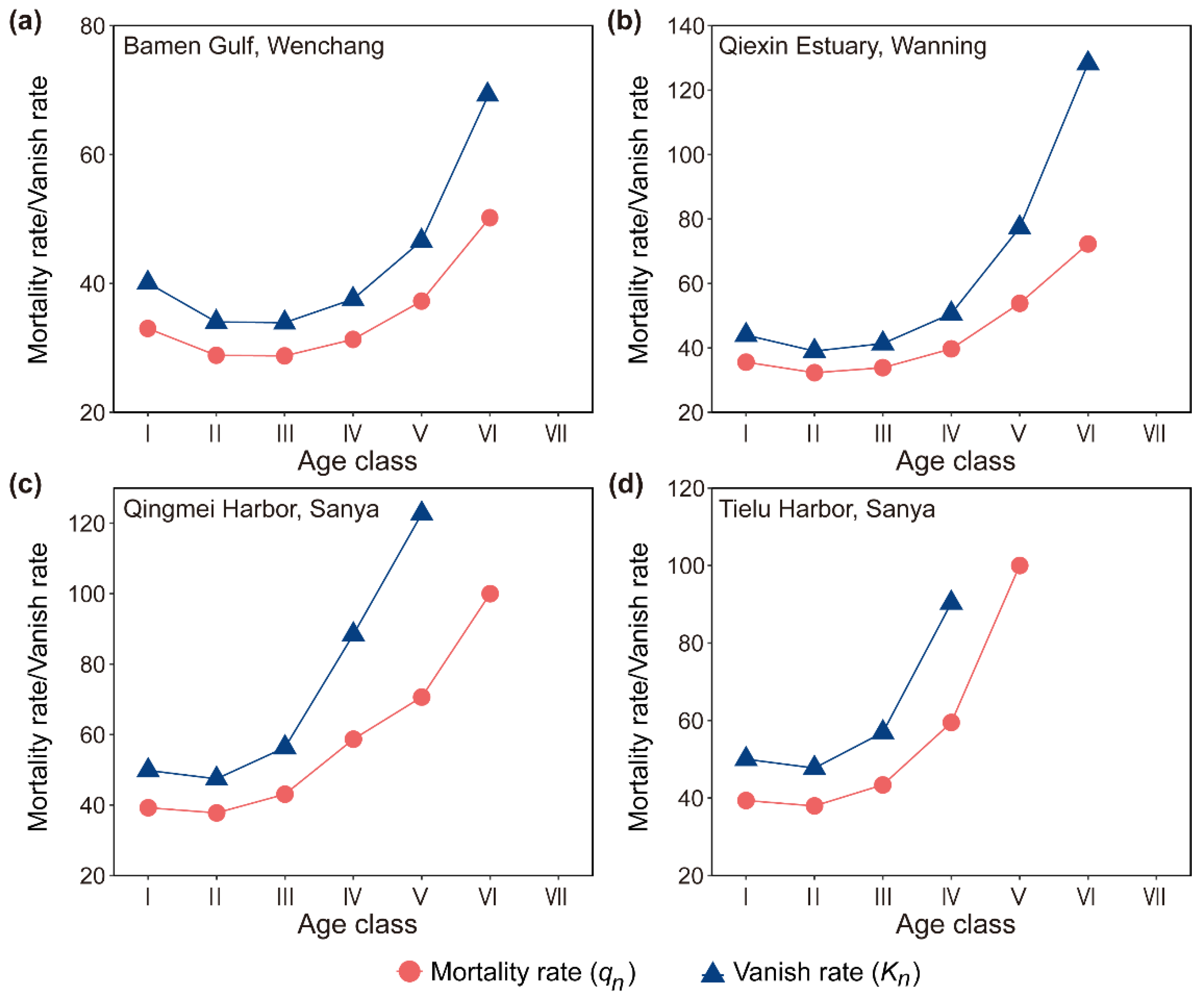
3.4. Analysis of Static Life Table of D. spathacea Population
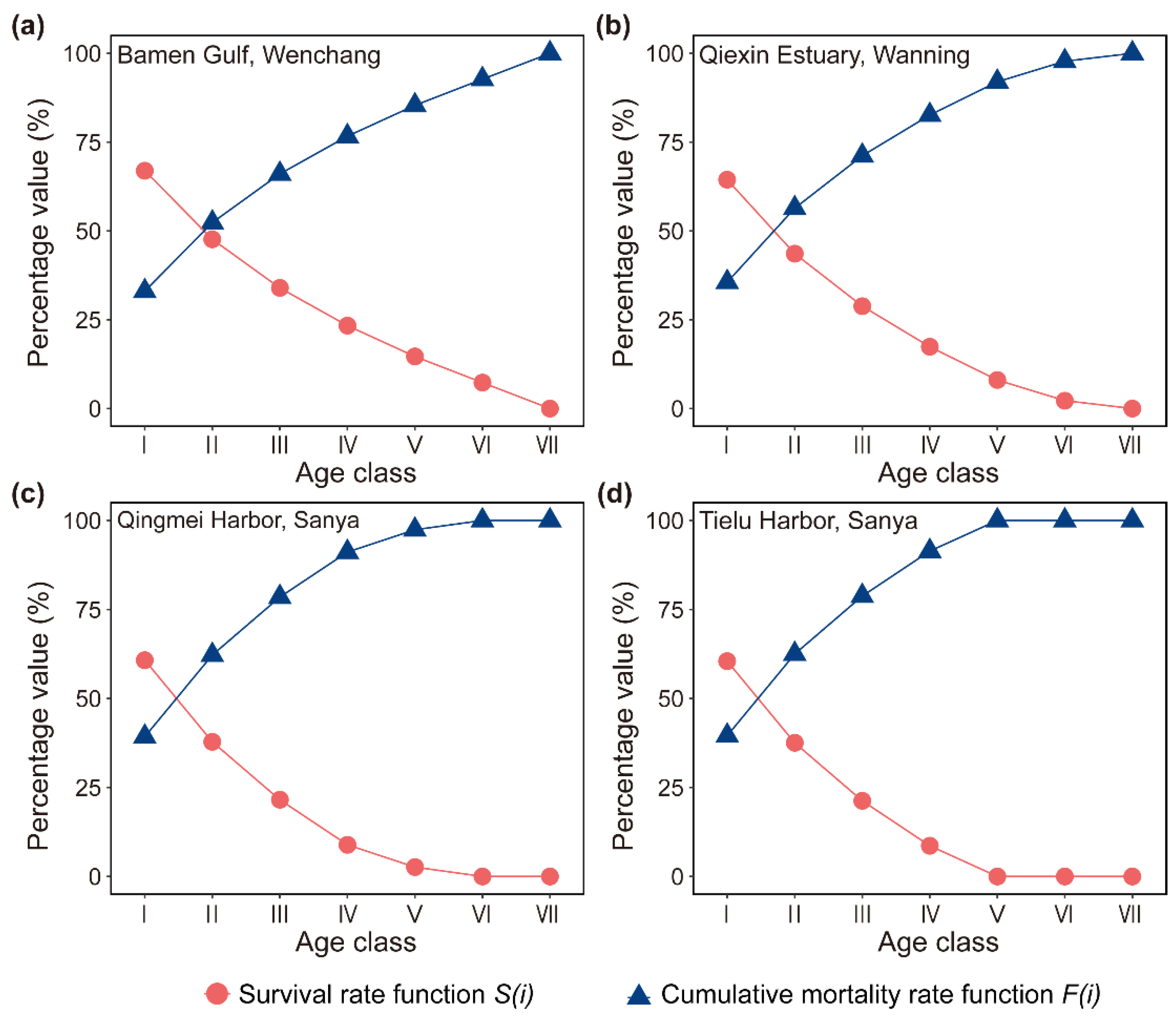
3.5. Survival Curve Analysis of D. spathacea Population
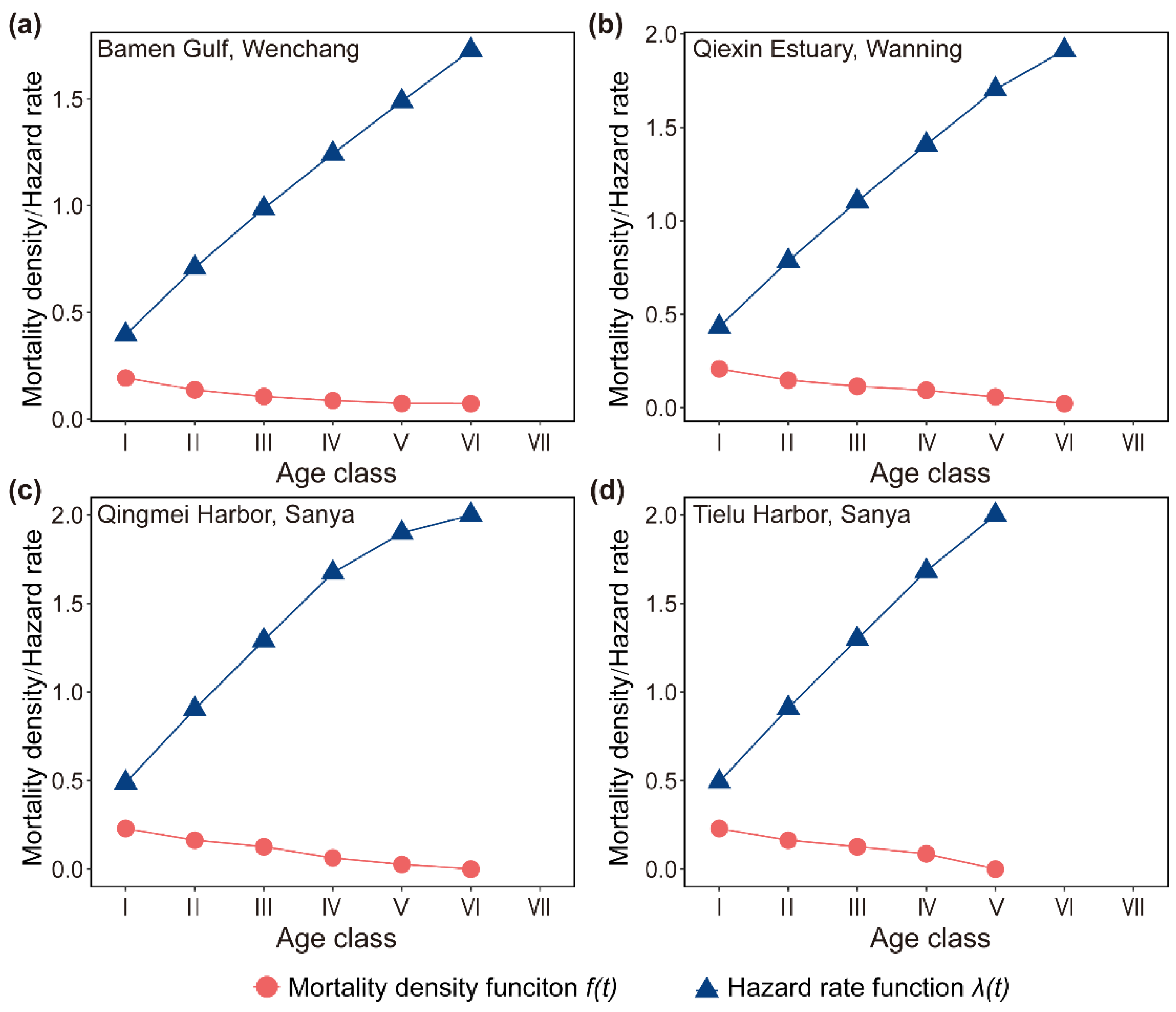
3.6. Population Survival Analysis of D. spathacea Population
3.7. Analysis of Age Class Time-Series of D. spathacea Population
4. Discussion
4.1. Analysis of D. spathacea Population Structure
4.2. Analysis of D. spathacea Population Dynamics
4.3. Recommendations of Conservation and Management for D. spathacea Population
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alongi, D.M. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Bayen, S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012, 48, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Feller, I.; Lovelock, C.; Berger, U.; McKee, K.; Joye, S.; Ball, M. Biocomplexity in Mangrove Ecosystems. Annu. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef]
- Lacerda, L.D. Mangrove ecosystems: function and management. Springer Verlag, Berlin. 2002.
- Wang, Y.S. Molecular ecology of mangroves. Beijing: Science Publishing Press. in Chinese. 2019.
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: a reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of climate change on mangrove ecosystems: a region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- Carugati, L.; Gatto, B.; Rastelli, E.; Martire, M.L.; Coral, C.; Greco, S.; Danovaro, R. Impact of mangrove forests degradation on biodiversity and ecosystem functioning. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef]
- Richards, D.R.; Friess, D.A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. USA 2016, 113, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Duke, N.C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; Koedam, N.; Lee, S.Y.; Marchand, C.; Nordhaus, I.; Dahdouh-Guebas, F. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Wang,W. ; Wang, M. The mangroves of China; Science Press: Beijing, China, 2007. [Google Scholar]
- Lin, P. A review on the mangrove research in China. J. Xiamen Univ. (Natural Sci.) 2001, 40, 592–603. [Google Scholar]
- Mahesh, G.; Patil, K.H. Dynamics of seedling distribution of Dolichandrone spathacea(L.f.)K.Schum. A proceeding of national conference on recent advances in physics and natural sciences. Maharashtra, India: Karmaveer Bhaurao Patil College, Urun, Islampur, 2018.
- Suman, D.O. Mangrove management: Challenges and guidelines. In coastal wetlands: An integrated ecosystem approach, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tian, G.H.; Li, M.; Yang, X.B.; Liao, B.W.; Liu, W.H.; Lei, Z.S. Study on Introduction of Dolichandrone spathacea on Qi-ao Island. J. Anhui Agr. Sci. 2011, 39, 9618–9619. [Google Scholar]
- Nguyen, V.T.; Do, L.Q.; Nguyen, T.A.; Nguyen, T.T.; Ho, N.A.; Tran, V.C.; Tran, T.P.T. New cycloartanes and new iridoids from Dolichandrone spathacea collected in the mangrove forest of Soc Trang province, Vietnam. J. Asian Nat. Prod. Res. 2017, 20, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Chandra Prasad, P.R.; Reddy, C.; Redy, S.H.; Dutt, C.B.S. Folkore medicinal plants of North Andaman Island, India. Fitoterapia 2008, 79, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Wiart, C. Medicinal plants of the Asia-Pacific: Drugs for the future? WSP Singapore, Singapore, 2006.
- Ho, P.H. Medicinal trees in Vietnam; Young Publishers: Ho Chi Minh, Vietnam, 2006. [Google Scholar]
- Cheng, C.; Ke, X.; Lang, T.; Zhong, C.; Lv, X.; Zhang, M.; Chen, Y.; Fang, Z.; Zhou, H.; Chen, Y. Current Status and Potential Invasiveness Evaluation of an Exotic Mangrove Species, Laguncularia racemosa (L.) C.F. Gaertn, on Hainan Island, China. Forests 2023, 14, 2036. [Google Scholar] [CrossRef]
- Hsieh, C.M.; Zhong, G.F. Hainan–The island of South Sea. A new province in China. Geosci. J. 1990, 20, 385–391. [Google Scholar]
- Li, M.; Lee, S. Mangroves of China: a brief review. For. Ecol. Manag. 1997, 96, 241–259. [Google Scholar] [CrossRef]
- Wang, B.; Yu, S. Handbook of Plant Community Experiments; Guangdong Education Press: Guangzhou, China, 1996. [Google Scholar]
- Hong, W.; Liu, J.; Wu, C.Z. A study on structure and spatial distribution pattern of Castanopsis hystrix population Sci. Silvae Sin. 2001, 31, 6–10, (In Chinese with English abstract). [Google Scholar]
- Frost, I.; Rydin, H. Spatial patem and size distribution of the anima-dispersed tree Quercus robur in two spruce-dominated forests. Ecoscience, 2000, 7, 38–44. [Google Scholar] [CrossRef]
- Shen, S.; Ma, H.; Wang, Y.; Wang, B.; Shen, G. Structure and dynamics of natural populations of the endangered plant Euryodendron excelsum HT Chang. Front. Forest. China, 2009, 4, 14–20. [Google Scholar] [CrossRef]
- Farahat, E.A. Age structure and static life tables of the endangered Juniperus phoenicea L. in North Sinai Mountains, Egypt: implication for conservation. J. Mt. Sci. 2020, 17, 2170–2178. [Google Scholar] [CrossRef]
- Hett, J.M.; Loucks, O.L. Age Structure Models of Balsam Fir and Eastern Hemlock. J. Ecol. 1976, 64, 1029. [Google Scholar] [CrossRef]
- Deevey, E.S. Life Tables for Natural Populations of Animals. Q. Rev. Biol. 1947, 22, 283–314. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z. (. A unified survival-analysis approach to insect population development and survival times. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harcombe, P.A. 1987; 37.
- Liu, D.; Guo, Z.; Cui, X.; Fan, C. Estimation of the Population Dynamics of Taxus cuspidata by Using a Static Life Table for Its Conservation. Forests 2023, 14, 2194. [Google Scholar] [CrossRef]
- Lang, T.; Pan, L.; Liu, B.; Guo, T.; Hou, X. Vegetation Characteristics and Response to the Soil Properties of Three Medicinal Plant Communities in Altay Prefecture, China. Sustainability 2020, 12, 10306. [Google Scholar] [CrossRef]
- Pala, N.A.; Negi, A.K.; Gokhale, Y.; Bhat, J.A.; Todaria, N.P. Diversity and regeneration status of Sarkot Van Panchyat in Garhwal Himalaya, India. J. For. Res. 2012, 23, 399–404. [Google Scholar] [CrossRef]
- Kramer, A.T.; Ison, J.L.; Ashley, M.V.; Howe, H.F. The Paradox of Forest Fragmentation Genetics. Conserv. Biol. 2008, 22, 878–885. [Google Scholar] [CrossRef]
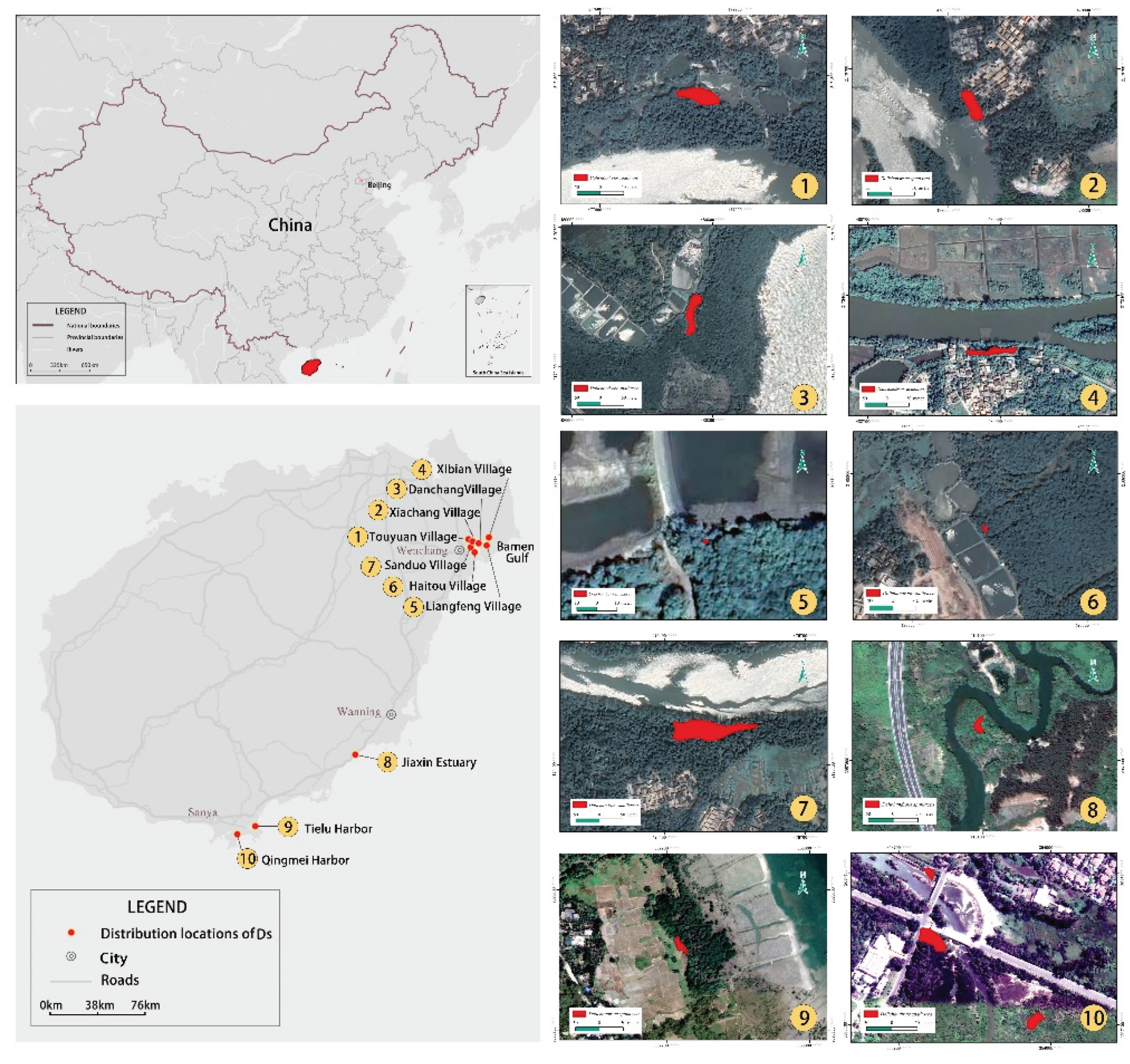
| DBH range | Level |
| DBH ≤ 2.5 cm | I |
| 2.5 cm < DBH ≤ 7.5 cm | II |
| 7.5 cm < DBH ≤ 12.5 cm | III |
| 12.5 cm < DBH ≤ 17.5 cm | IV |
| 17.5 cm < DBH ≤ 22.5 cm | V |
| 22.5 cm < DBH ≤ 27.5 cm | VI |
| DBH > 27.5 cm | VII |
| City | Distribution Point | Longitude | Latitude | Altitude (m) |
Quantity | Canopy Density | Dominant Tree Species | Tide Situation | Sediment Type | Disturbance Type | |
| Wenchang | Bamen Gulf | Touyuan Village | 110°47′19″E | 19°37′42″N | 2.5 | 13 | 0.9 | S. caseolaris, B. sexangula | Spring Tides | Silty | Dam |
| Xiachang Village | 110°47′43″E | 19°37′22″N | 6.5 | 24 | 0.7 |
S. caseolaris, B. sexangula, S. paracaseolaris |
Spring Tides | Sandy and Silty | Roadblock | ||
| Danchang Village | 110°48′42″E | 19°37′18″N | 3.2 | 36 | 0.8 |
R. stylosa, B. sexangula |
Spring Tides | Silty | Dam | ||
| Xibian Village |
110°53′40″E | 19°38′50″N | 10.2 | 17 | 0.7 |
C. nucifera, S. caseolaris, H. tiliaceus |
No Tides | Silty | Aquaculture | ||
| Liangfeng Village | 110°51′34″E | 19°37′43″N | 3.5 | 1 | 0.5 | R. apculata, C. tagal | No Tides | Sandy and Silty | Dam | ||
| Haitou Village | 110°47′46″E | 19°36′03″N | 2.6 | 2 | 0.6 | R. stylosa, X. granatum | No Tides | Sandy and Silty | Dam | ||
| Sanduo Village | 110°46′32″E | 19°37′17″N | 3.4 | 59 | 0.9 | H. littoralis, D. spathacea, C. nucifera | Spring Tides | Silty | Aquaculture | ||
| Wanning | Jiaxin Estuary | 110°10′36″E | 18°36′02″N | 3.5 | 46 | 0.9 |
D. spathacea, A. catechu, N. fruticans |
No Tides | sandy and Silty | Desertification | |
| Sanya | Tielu Harbor | 109°42′07″E | 18°15′47″N | 4.2 | 37 | 0.7 |
R. apculata, S. alba, L. racemosa |
Spring Tides | Sandy | – | |
| Qingmei Harbor | 109°37′03″E | 18°13′57″N | 5.2 | 49 | 0.6 |
L. racemosa, C. tagal, R. apculata |
Spring Tides | Sandy | Roadblock | ||
| Distribution Point | Variation Index of D. spathacea Population Dynamics (%) | |||||||||
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | Vpi | V'pi | Pmax | |
| Bamen Gulf (Wenchang) | -4.26 | 38.30 | 65.52 | 40.00 | -33.33 | 33.33 | 100.00 | 27.46 | 0.65 | 2.38 |
| Qiexin Estuary ( Wanning ) | -100.00 | -90.00 | 35.00 | 46.15 | 57.14 | 66.67 | 100.00 | 38.22 | 5.46 | 14.29 |
| Qingmei Harbor (Sanya) | -52.63 | 78.95 | 25.00 | 33.33 | 100.00 | 100.00 | 100.00 | 38.55 | 2.75 | 7.14 |
| Tielu Harbor (Sanya) | -52.63 | 78.95 | 25.00 | 33.33 | 100.00 | 100.00 | 100.00 | 38.55 | 2.75 | 7.14 |
| Distribution Point | Age class | ax | lx | lgax | dx | qx (%) | Lx | Tx | ex | Kx (%) | Px (%) |
| Bamen Gulf (Wenchang) | I | 52 | 1000 | 3.946 | 330 | 33.0 | 835 | 2402 | 2.402 | 40.1 | 67.0 |
| II | 35 | 670 | 3.545 | 193 | 28.8 | 573 | 1567 | 2.339 | 34.0 | 71.2 | |
| III | 25 | 477 | 3.205 | 137 | 28.8 | 408 | 994 | 2.085 | 33.9 | 71.2 | |
| IV | 18 | 339 | 2.866 | 106 | 31.3 | 286 | 586 | 1.725 | 37.6 | 68.7 | |
| V | 12 | 233 | 2.490 | 87 | 37.3 | 190 | 300 | 1.287 | 46.6 | 62.7 | |
| VI | 8 | 146 | 2.024 | 73 | 50.2 | 110 | 110 | 0.749 | 69.7 | 49.8 | |
| VII | 4 | 73 | 1.326 | – | – | – | – | – | – | – | |
| Qiexin Estuary ( Wanning ) | I | 45 | 1000 | 3.806 | 356 | 35.6 | 718 | 1943 | 1.943 | 44.0 | 64.4 |
| II | 29 | 644 | 3.366 | 208 | 32.3 | 540 | 1225 | 1.902 | 39.0 | 67.7 | |
| III | 20 | 436 | 2.976 | 148 | 33.9 | 305 | 685 | 1.572 | 41.4 | 66.1 | |
| IV | 13 | 288 | 2.562 | 115 | 39.7 | 231 | 380 | 1.319 | 50.6 | 60.3 | |
| V | 8 | 174 | 2.056 | 94 | 53.9 | 98 | 149 | 0.859 | 77.3 | 46.1 | |
| VI | 4 | 80 | 1.283 | 58 | 72.3 | 51 | 51 | 0.639 | 128.3 | 27.7 | |
| VII | 1 | 22 | 1.000 | – | – | – | – | – | – | – | |
| Qingmei Harbor (Sanya) | I | 38 | 1000 | 3.647 | 392 | 39.2 | 804 | 1803 | 1.803 | 49.8 | 60.8 |
| II | 23 | 608 | 3.149 | 230 | 37.8 | 493 | 999 | 1.644 | 47.7 | 62.2 | |
| III | 15 | 378 | 2.674 | 163 | 43.1 | 297 | 506 | 1.339 | 56.3 | 56.9 | |
| IV | 8 | 215 | 2.111 | 126 | 58.7 | 152 | 210 | 0.974 | 88.4 | 41.3 | |
| V | 3 | 89 | 1.227 | 63 | 70.7 | 57 | 57 | 0.647 | 122.7 | 29.3 | |
| VI | 1 | 26 | 0.000 | 26 | 100.0 | 13 | 13 | 0.500 | – | 0.0 | |
| VII | 0 | 0 | – | – | – | – | – | – | – | – | |
| Tielu Harbor (Sanya) | I | 24 | 1000 | 3.184 | 394 | 39.4 | 803 | 1782 | 1.782 | 50.0 | 60.6 |
| II | 15 | 606 | 2.683 | 230 | 38.0 | 491 | 979 | 1.614 | 47.7 | 62.0 | |
| III | 9 | 376 | 2.206 | 163 | 43.4 | 295 | 487 | 1.295 | 56.9 | 56.6 | |
| IV | 5 | 213 | 1.637 | 127 | 59.5 | 150 | 193 | 0.905 | 90.3 | 40.5 | |
| V | 2 | 86 | 0.734 | 86 | 100.0 | 43 | 43 | 0.500 | – | 29.3 | |
| VI | 0 | 0 | – | 0 | – | 0 | 0 | – | – | – | |
| VII | 0 | 0 | – | – | – | – | – | – | – | – |
| Distribution Point | Nx = N0 e-bx | Nx = N0 x-b |
| Bamen Gulf (Wenchang) | Nx = 5.0355e-0.16x, R² = 0.9235 | Nx = 4.6354x-0.457, R² = 0.7586 |
| Qiexin Estuary ( Wanning ) | Nx =4.986e-0.19x, R² = 0.9314 | Nx = 4.3919x-0.491, R² = 0.7819 |
| Qingmei Harbor (Sanya) | Nx = 5.435e-0.281x, R² = 0.887 | Nx = 4.295x-0.633, R² = 0.7297 |
| Tielu Harbor (Sanya) | Nx = 5.3633e-0.357x, R² = 0.8766 | Nx = 3.9627x-0.301, R² = 0.7149 |
| Age class | Bamen Gulf (Wenchang) | Qiexin Estuary ( Wanning ) | Qingmei Harbor ( Sanya ) | Tielu Harbor (Sanya) | ||||||||||||
| Initial Number | M2 | M4 | M6 | Initial Number |
M2 | M4 | M6 | Initial Number | M2 | M4 | M6 | Initial Number | M2 | M4 | M6 | |
| I | 45 | – | – | – | 0 | – | – | – | 4 | – | – | – | 9 | – | – | – |
| II | 47 | 46 | – | – | 2 | 1 | – | – | 32 | 18 | – | – | 19 | 14 | – | – |
| III | 29 | 38 | – | – | 20 | 11 | – | – | 3 | 18 | – | – | 4 | 12 | – | – |
| IV | 10 | 20 | 33 | – | 13 | 17 | 9 | – | 6 | 5 | 11 | – | 3 | 4 | 9 | – |
| V | 6 | 8 | 23 | – | 7 | 10 | 11 | – | 3 | 5 | 11 | – | 2 | 3 | 7 | – |
| VI | 9 | 8 | 14 | 24 | 3 | 5 | 11 | 8 | 1 | 2 | 3 | 8 | 0 | 1 | 2 | 6 |
| VII | 6 | 8 | 8 | 18 | 1 | 2 | 6 | 8 | 0 | 1 | 3 | 8 | 0 | 0 | 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





