1. Introduction
Sleep quality plays an essential role in multiple physical, behavioral, and cognitive functions [
1,
2,
3]
. An individual’s sleep quality can be described as satisfaction with all aspects of their sleep experience and is usually defined by four factors: sleep efficiency, sleep latency, sleep duration, and wakefulness following sleep start [
3]
. Universal guidelines have been debated regarding the appropriate sleep duration for young adults [
4]
. Through a consensus developed by the American Academy of Sleep Medicine (AASM) and the Sleep Research Society (SRS), it has been suggested that young adults need more than nine hours of sleep per night [
5]
, whereas the National Sleep Foundation in the US has released an updated recommendation stating that seven to eight hours of sleep is sufficient for the proper execution of daily activities [
6,
7]
. A recent study in the United Arab Emirates (UAE) showed that young adults in medical colleges have shorter sleep durations than the recommended sleep guidelines, with an average of five hours and twenty-four minutes [
8]
. Additionally, the literature reports that 40% of young adults suffer from poor sleep quality worldwide [
9]
.
Investigations of sleep quality’s effect on cognitive and motor functions have recently drawn more attention regarding various healthy and diseased populations [
10,
11,
12]
. According to several findings, poorer sleep quality leads to greater impairments in younger adults in reaction time, performance lapses, and frequency of unintentional sleep episodes when compared with older adults [
13]
. Generally, poor sleep quality affects performance during simple tasks and results in a decline in the level of attention while increasing the attentional effort required [
10,
14]
. Moreover, sleep deprivation tends to reduce attentional capacity and vary arousal levels, leading to difficulty in sustaining effort and multitasking performance [
10]
.
Gait is a complex rhythmic movement achieved through the association of multiple body systems [
15]
. The cerebellum and basal ganglia play important roles in the cognitive and automatic processes required for gait control through connections with the cerebral cortex and brainstem, respectively [
16]
. Studies have shown that low-quality sleep is linked to lower metabolic activity in the thalamo-basal ganglia circuit, the limbic and prefrontal regions, and the cerebellum [
17]
. Thus, such disruptions might alter gait control, resulting in gait disturbances and, eventually, falls [
16]
.
The effect of sleep quality on gait has been investigated in diseased populations, including elderly people with Parkinson’s disease [
18]
. Step width variability increases in patients with Parkinson’s disease who suffer from poor sleep quality [
18]
. The association between gait and sleep has also been investigated among community-dwelling elderly people given the expected decline in both sleep quality and alterations in gait patterns [
19]
. The literature has little information regarding the effects of sleep quality on gait in younger populations. However, it is known that sleep disorders occur at a younger age because of rapid lifestyle changes and growing responsibilities, including university and work [
20,
21]
.
Furthermore, gait control requires an adequate balance between the automatic and executive mechanisms [
19]
. An effective and common way to distinguish between these two mechanisms is by adding a dual task [
22]
. A dual task is an individual’s ability to perform two tasks at the same time in which they are required to coordinate their attention between both tasks while performing them simultaneously [
23]
. Usually, a decline in the performance of at least one of the tasks is expected while dual-tasking because of the capacity sharing theory, which proposes that when limited cognitive resources are shared simultaneously, a decrease in the efficiency of either task will be observed [
24]
. This performance decrement is known as the dual-task cost (DTC) [
25]
.
Evidence suggests that sleep-quality-related disorders might influence task performance or motor response while implementing dual tasks [
26]
. The literature shows that an individual’s ability to divide attention while multitasking declines when exposed to sleep deprivation and adopting a poor sleep schedule [
10]
. An investigation was conducted on both preterm and full-term children, revealing that poorer sleep quality and a higher number of awakenings after sleep onset correlate with poorer dual-task performance [
27]
.
There is a lack of literature regarding the association between sleep quality and the dual-task cost on gait spatiotemporal parameters in younger populations. Therefore, we investigated the association between sleep quality and gait spatiotemporal parameters following the addition of a dual task to young adults in the United Arab Emirates (UAE).
2. Materials and Methods
2.1. Study Design, Participants, and Setting
This cross-sectional observational study was conducted at the Physiotherapy Research Laboratory at the University of Sharjah between May 2023 and October 2023. A sample of 65 healthy young adults, (50.8% females and 49.2% males) with a mean age of 21.1±2.5 volunteered to participate in the study and signed an informed consent form. All participants were assessed between 12 pm and 5 pm on weekdays between Monday and Thursday. The inclusion criterion was participants aged between 18 to 25 years old. Undergraduate and graduate students were recruited from a university setting through a series of in-classroom announcements. The exclusion criteria were as follows: (1) being athletic or adopting a lifestyle with high-interval training; (2) any recent major surgeries performed within the previous 2 months; (3) any existing musculoskeletal, neurological, or cardiorespiratory disorders; (4) current intake of medications with side effects interfering with concentration and balance; (5) any visual impairments or unmanaged visual myopia, farsightedness, or astigmatism; (6) any vestibular or middle-ear disorders; (7) any cognitive impairments or sleep disorders; (8) any sensory impairment.
2.2. Sample Size
Power analysis for correlation was performed with G-Power (version 3.1.9.7) to determine a sufficient sample size. For α = 0.05, two-tailed; a power of 0.8; and an effect size of 0.05, a sample of at least 29 participants was required.
2.3. Ethical Considerations
This study was approved by the Research Institute of Medical & Health Sciences of the University of Sharjah (reference number: REC-23-02-23-04-PG). All participants were informed about the study’s aims and procedure, and a written informed consent form was obtained from each participant before the evaluation.
2.4. Study Tools and Outcome Measures
2.4.1. Sleep Assessment Questionnaires
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). The PSQI distinguishes between sleep quality types to form two sleep groups for comparison. This differentiation was achieved by measuring seven domains: (1) subjective sleep quality, (2) sleep latency, (3) sleep duration, (4) habitual sleep efficiency, (5) sleep disturbances, (6) the use of sleep medication, and (7) daytime dysfunction [
28]
. A total score equal to or less than 5 indicated generally good sleep quality, while scores greater than 5 indicated generally poor quality of sleep [
28]
.
2.4.2. BTS GAITLAB System
Gait spatiotemporal parameters were assessed using the BTS GAITLAB system. The BTS GAITLAB system provides optoelectronic data about gait using eight high-frequency infrared cameras (BTS SMART-DX EVO) that can detect reflective markers placed on the participant’s landmarks according to the desired protocol, two RGB video cameras for recording, and a kit reflective marker [
29,
30]
. The spatiotemporal parameters assessed included the gait velocity, cadence, step width, and stride length.
2.5. Study Procedure
Participants were asked to complete a demographics data sheet and the PSQI questionnaire. Subsequently, the participants were divided into two groups based on their PSQI scores: a good sleep quality group and a poor sleep quality group. Following this categorization, the participants underwent a baseline cognitive evaluation to assess their initial cognitive performance using the Short Orientation–Memory–Concentration Test (SOMCT CogTest). This test evaluates cognitive functions related to orientation, concentration, and memory [
31,
32]
. Before the gait analysis, all anthropometric data were acquired using the smart capture software in BTS GAITLAB [
33]
. Reflective markers were then placed on the participants’ landmarks according to anatomical points defined by the “Helen Hayes with Medial Markers” protocol [
34]
. Specifically, the protocol uses 22 reflective markers placed on the following landmarks: (1) the 7th cervical vertebra; (2) the right acromion; (3) the left acromion; (4) the 2nd sacral vertebra; (5) both anterior superior iliac spines (ASIS); (6) both greater trochanters; (7) both medial femoral epicondyles; (8) both lateral femoral epicondyles; (9) both shanks (approximately in the region of the fibular head); (10) both lateral malleoli; (11) both medial malleoli; (12) both calcaneus; and (13) both the 2nd and 3rd toe junctions [
34]
.
Following the preparatory procedures, the participants underwent a 3D gait analysis in which they were instructed to walk at their normal speed along a 10 m walkway as a single-task. The procedure was repeated twice; the first trial was used to familiarize the participants with the study method, and the second trial was included in the final data processing. The participants were then asked to walk according to the same instructions while simultaneously performing a cognitive dual task, which involved spelling a five-letter word backward. Data were processed using dedicated BTS software (Smart Analyzer, BTS Bioengineering, Milan, Italy) [
29]
.
2.6. Statistical Analysis
The normality of the data was tested using the Shapiro–Wilk test, and the descriptive variables are presented using mean ± standard deviation (SD). The DTC was calculated as the percentage of performance changes in gait parameters (gait velocity, cadence, step width, and bilateral stride length) under the two conditions (single-task and dual-task) using the following equation: {Dual Tasks − Single Tasks / Single Tasks × (100)} [
35]
. Positive results indicated a reduction in gait parameter performance after the addition of a dual task, while a negative result indicated improved performance while dual-tasking [
35]. The data were not normally distributed; thus, the Mann–Whitney U test was used to compare the differences in the DTC on gait variables between the two sleep quality groups (good sleep quality and poor sleep quality). Spearman rho correlation was used to correlate sleep quality and DTC on gait variables. Statistical analyses were performed using IBM-SPSS 22.0 (IBM Corp., Armonk, NY, USA), and the significance level was set at p < 0.05, and 95% confidence intervals were obtained.
3. Results
3.1. Demographics
This study included 65 homogenous participants with a mean body weight of 71.8
15.2 kg and a mean height of 169.15
8.9 cm. The total PSQI mean score was 6.46 ± 3.15, indicating generally poor sleep quality among the participants. Additional participant characteristics and baseline gait variables are shown in
Table 1.
3.2. The Dual-Task Cost on Gait Variables and the Difference Between Sleep Quality Groups
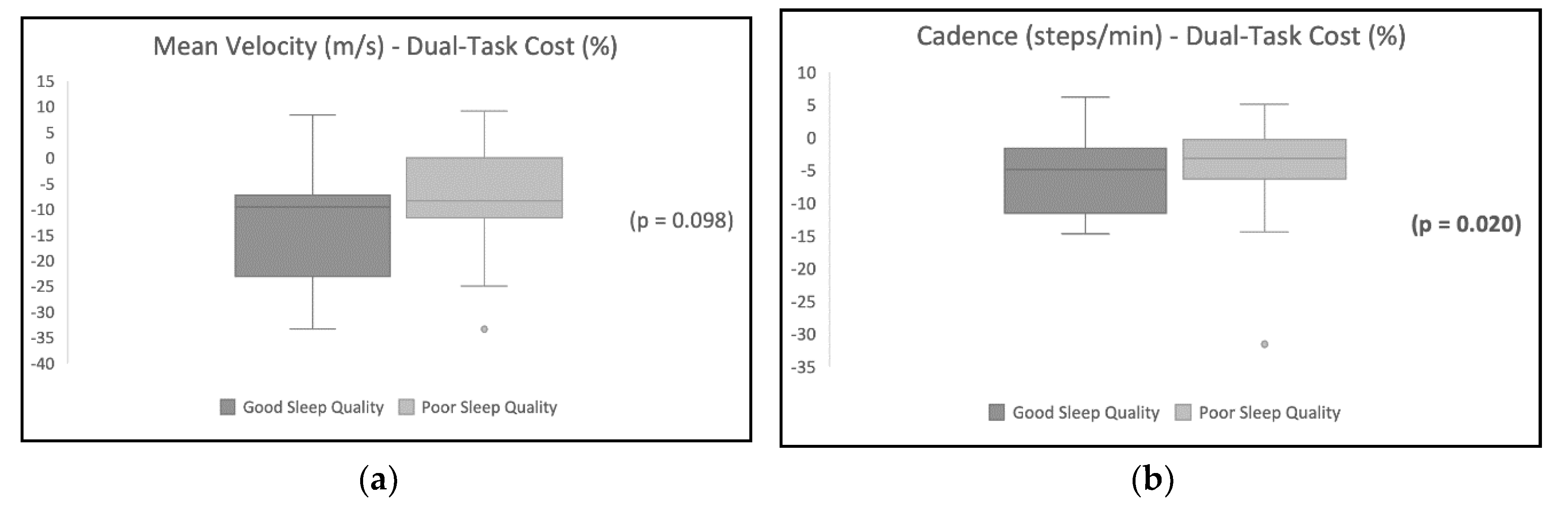
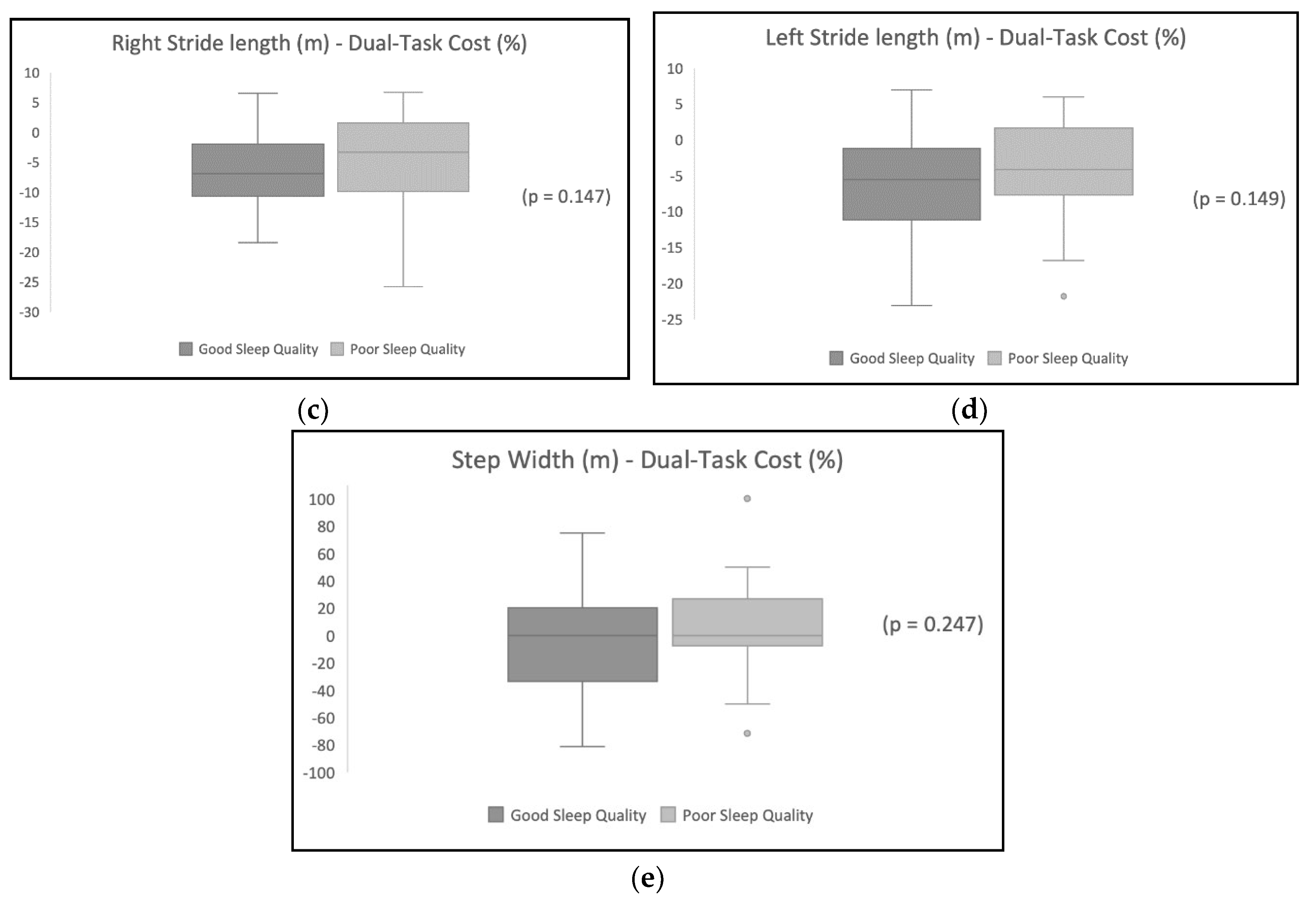
Table 2 shows the DTC on gait variables, and the results show a higher DTC for the participants with poor sleep quality.
Table 3 shows the difference in the DTC between the sleep quality groups. Cadence was significantly different in the good sleep quality group compared with the poor sleep quality group (p<0.05) after the addition of a dual task. Other variables were not statistically different between the two groups.
Figure 1 illustrates the distribution of the DTC’s effect on each gait variable for participants with poor sleep quality in comparison with participants with good sleep quality.
3.3. Correlation Between Sleep Quality and the Dual-Task Cost on Gait Variables.
Table 4 shows the variables’ correlation coefficients. The analysis shows a positive significant correlation between sleep quality and the DTC on gait mean velocity, cadence, and right and left lower limb stride lengths. However, there were no significant correlations between sleep quality and the DTC on step width (p > 0.05).
4. Discussion
This study investigated the association between sleep quality and gait spatiotemporal parameters following the addition of a dual task in young adults in the UAE. We hypothesized a decline in gait performance following the addition of a dual task, with the most prominent decrements observed in participants with poorer sleep quality. Our findings partially supported our hypothesis by revealing that the DTC had a greater effect on participants with poorer sleep quality; however, those with better sleep quality exhibited a lower DTC effect on gait, indicating that dual-tasking does not directly affect gait performance if the participant has relatively good sleep quality. Specifically, our analysis showed a significant difference in cadence between the two sleep quality groups. Moreover, we found a positive correlation between sleep quality and DTC on gait parameters, including mean velocity, cadence, and stride length. These significant correlations emphasize the growing body of evidence showing the effects of sleep quality on multitasking while walking.
We hypothesized that adding a dual task would have detrimental effects on gait spatiotemporal parameters because of capacity sharing theory [
24]. However, our results showed that velocity, cadence, and stride lengths improved in the total sample, regardless of the participant’s sleep quality, following the addition of a dual task. These results are in line with a previous cohort that also noted improved performance when dual-tasking was combined with walking [
36]
. One possible explanation is that the dual task provided may not have sufficiently challenged the participants’ cognitive resources, as walking is an automatic motor task that typically requires minimal use of attention-demanding executive control centers [
37]
. Moreover, adding a cognitive task could have shifted attention from motor control related to walking, thereby enhancing the consistency and automaticity of the walking patterns in response [
38]
. An increase in speed while performing a concurrent dual task with walking was also observed in a previous study in which individuals showed an increase in gait velocity while carrying shopping bags in a simulated setting [
39]
. The authors hypothesized that paying more attention to a concurrent task would result in a more spontaneous pattern leading to walking at higher speeds [
39]
.
On the other hand, our results showed that the dual task addition had a negative effect on step width. Step width for most participants decreased following the addition of a dual task. Wider step width has also been shown to improve lateral stability during walking, leading to a steadier gait [
40]
; thus, a narrowed step width may decrease the base of support and postural stability. Previous research has shown that increased step width is associated with a decrease in the mediolateral local and orbital stability of the C7 vertebra, indicating a decline in the stability and consistency of mediolateral trunk motion [
40]
. Similar recent results showed a decreased step width during a typical conversational dual task [
41]
.
Furthermore, our analysis shows a significant difference in the DTC on cadence between the good and poor sleep quality groups. The literature shows that adding a dual task has a negative impact on gait spatiotemporal parameters, including cadence, among healthy and diseased populations [
42]
. The addition of a dual task to walking among older community-dwelling individuals has a negative effect on gait speed, cadence, and stride length [
42]
. Generally, previous research has focused only on studying the effects of adding cognitive and motor dual tasks on gait control [
15,
25,
26,
38,
39,
41,
42,
43]. However, the results of the present study provide a unique contribution to the literature, as there have been no investigations comparing the DTC on gait spatiotemporal parameters between good and poor sleep qualities among younger populations.
Supporting the increased DTC on the poor sleep quality group, our investigation shows a positive correlation between the DTC on gait mean velocity, cadence, and bilateral stride lengths and the total PSQI score. This can be interpreted as a decline in mean velocity, cadence, and bilateral stride lengths after adding a dual task in individuals with poor sleep quality. These results are in line with those of a previous study that investigated the effect of sleep deprivation on divided attention performance in 30 healthy males exposed to 40 hours of continuous sleep deprivation [
10]
. The results of this study showed that the more individuals were deprived of sleep, the more performance deteriorated performance during tasks [
10]
. Sleep quality’s effect on cognitive function has also been studied in preterm and full-term children, and it was found that the poorer the child’s sleep quality, the higher the number of awakenings after sleep onset and the more reduced dual-task performance was [
27]
.
Our study also shows that 56.9% of the participants had poor sleep quality. Similar results have been reported multiple times over the previous decades [
44,
45]
. The literature also reports that poor sleep quality is associated more with females than with males, with a higher incidence of nighttime wakefulness and reduced sleep duration and efficiency [
46,
47]
. These findings are aligned with our results, in which females comprised 62.6% of the poor sleep quality group.
Limitations of the Study
This study had a few limitations that justify further discussion and that should be addressed in future works. Because all participants had a higher education level, our results cannot be generalized to populations with lower educational levels. Additionally, as the poor sleep quality group was mostly females, gait performance deterioration following the addition of a dual task might have been due to the biomechanical disadvantage of height, as males are generally taller than females [
48]
; thus, further research thoroughly investigating each gender would be recommended. Furthermore, although we used a valid and reliable subjective sleep assessment tool, future studies with more objective sleep assessments are necessary to further investigate the effects of sleep quality, such as the duration of each sleep cycle phase. Further recommendations include adding a triple-task to challenge neural resources since the population consisted mostly of young, healthy, educated adults.
5. Conclusions
We identified statistically significant associations between poor sleep quality and reductions in gait performance during the addition of a dual task. Our results show that studies of sleep quality effects should be initiated at a younger age, as the percentage of sleep-related issues has recently increased in this population [
11,
21]
. Future studies with more objective sleep assessment tools are recommended to further understand the association between specific sleep disorders and changes in performance.
Author Contributions
Conceptualization, D.J. and K.M.; methodology, K.M. and D.J.; data curation, D.J.; investigation, D.J.; formal analysis, K.M., D.J., Z.S.A.; writing – original draft preparation, D.J. and K.M.; writing – review and editing, K.M., D.J., and Z.S.A.; supervision, K.M.; project administration, K.M.
Funding
This research received no external funding.
Institutional Review Board Statement
This research was approved by the Research Institute of Medical & Health Sciences of the University of Sharjah (reference number: REC-23-02-23-04-PG).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study after they were provided with the detailed study procedure.
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Miletínová, E.; Bušková, J. Functions of Sleep. Physiol Res, 2021, 177–182. [CrossRef]
- Sletten, T. L.; Weaver, M. D.; Foster, R. G.; Gozal, D.; Klerman, E. B.; Rajaratnam, S. M. W.; Roenneberg, T.; Takahashi, J. S.; Turek, F. W.; Vitiello, M. V.; et al. The Importance of Sleep Regularity: A Consensus Statement of the National Sleep Foundation Sleep Timing and Variability Panel. Sleep Health, 2023, 9 (6), 801–820. [CrossRef]
- Nelson, K. L.; Davis, J. E.; Corbett, C. F. Sleep Quality: An Evolutionary Concept Analysis. Nurs Forum (Auckl), 2022, 57 (1), 144–151. [CrossRef]
- Furtado, F.; Gonçalves, B. da S. B.; Abranches, I. L. L.; Abrantes, A. F.; Forner-Cordero, A. Chronic Low Quality Sleep Impairs Postural Control in Healthy Adults. PLoS One, 2016, 11 (10), e0163310. [CrossRef]
- Watson, N. F.; Badr, M. S.; Belenky, G.; Bliwise, D. L.; Buxton, O. M.; Buysse, D.; Dinges, D. F.; Gangwisch, J.; Grandner, M. A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep, 2015. [CrossRef]
- Banks, S.; Dinges, D. F. Behavioral and Physiological Consequences of Sleep Restriction. J Clin Sleep Med, 2007, 3 (5), 519–528.
- Chaput, J.-P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping Hours: What Is the Ideal Number and How Does Age Impact This? Nat Sci Sleep, 2018, Volume 10, 421–430. [CrossRef]
- Meer, H.; Jeyaseelan, L.; Sultan, M. A. Sleep Quality and Emotional State of Medical Students in Dubai. Sleep Disord, 2022, 2022, 1–6. [CrossRef]
- Kasović, M.; Štefan, A.; Štefan, L. The Associations Between Objectively Measured Gait Speed and Subjective Sleep Quality in First-Year University Students, According to Gender. Nat Sci Sleep, 2021, Volume 13, 1663–1668. [CrossRef]
- Chua, E. C.-P.; Fang, E.; Gooley, J. J. Effects of Total Sleep Deprivation on Divided Attention Performance. PLoS One, 2017, 12 (11), e0187098. [CrossRef]
- Telzer, E. H.; Fuligni, A. J.; Lieberman, M. D.; Galván, A. The Effects of Poor Quality Sleep on Brain Function and Risk Taking in Adolescence. Neuroimage, 2013, 71, 275–283. [CrossRef]
- Brandolim Becker, N.; Jesus, S. N. de; Viseu, J. N.; Stobäus, C. D.; Guerreiro, M.; Domingues, R. B. Depression and Quality of Life in Older Adults: Mediation Effect of Sleep Quality. International Journal of Clinical and Health Psychology, 2018, 18 (1), 8–17. [CrossRef]
- Duffy, J. F.; Willson, H. J.; Wang, W.; Czeisler, C. A. Healthy Older Adults Better Tolerate Sleep Deprivation Than Young Adults. J Am Geriatr Soc, 2009, 57 (7), 1245–1251. [CrossRef]
- Massar, S. A. A.; Lim, J.; Sasmita, K.; Chee, M. W. L. Sleep Deprivation Increases the Costs of Attentional Effort: Performance, Preference and Pupil Size. Neuropsychologia, 2019, 123, 169–177. [CrossRef]
- Umemura, G. S.; Pinho, J. P.; Duysens, J.; Krebs, H. I.; Forner-Cordero, A. Sleep Deprivation Affects Gait Control. Sci Rep, 2021, 11 (1), 21104. [CrossRef]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J Mov Disord, 2017, 10 (1), 1–17. [CrossRef]
- Klumpers, U. M. H.; Veltman, D. J.; van Tol, M.-J.; Kloet, R. W.; Boellaard, R.; Lammertsma, A. A.; Hoogendijk, W. J. G. Neurophysiological Effects of Sleep Deprivation in Healthy Adults, a Pilot Study. PLoS One, 2015, 10 (1), e0116906. [CrossRef]
- O’Dowd, S.; Galna, B.; Morris, R.; Lawson, R. A.; McDonald, C.; Yarnall, A. J.; Burn, D. J.; Rochester, L.; Anderson, K. N. Poor Sleep Quality and Progression of Gait Impairment in an Incident Parkinson’s Disease Cohort. J Parkinsons Dis, 2017, 7 (3), 465–470. [CrossRef]
- Agmon, M.; Shochat, T.; Kizony, R. Sleep Quality Is Associated with Walking under Dual-Task, but Not Single-Task Performance. Gait Posture, 2016, 49, 127–131. [CrossRef]
- Matthews, T.; Danese, A.; Gregory, A. M.; Caspi, A.; Moffitt, T. E.; Arseneault, L. Sleeping with One Eye Open: Loneliness and Sleep Quality in Young Adults. Psychol Med, 2017, 47 (12), 2177–2186. [CrossRef]
- Owens, J.; Au, R.; Carskadon, M.; Millman, R.; Wolfson, A.; Braverman, P. K.; Adelman, W. P.; Breuner, C. C.; Levine, D. A.; Marcell, A. V.; et al. Insufficient Sleep in Adolescents and Young Adults: An Update on Causes and Consequences. Pediatrics, 2014, 134 (3), e921–e932. [CrossRef]
- Saraiva, M.; Marouvo, J.; Fernandes, O.; Castro, M. A.; Vilas-Boas, J. P. Postural Control and Sleep Quality in Cognitive Dual Tasking in Healthy Young Adults. J (Basel), 2021, 4 (3), 257–265. [CrossRef]
- Deprez, S.; Vandenbulcke, M.; Peeters, R.; Emsell, L.; Amant, F.; Sunaert, S. The Functional Neuroanatomy of Multitasking: Combining Dual Tasking with a Short Term Memory Task. Neuropsychologia, 2013, 51 (11), 2251–2260. [CrossRef]
- Tombu, M.; Jolicœur, P. A Central Capacity Sharing Model of Dual-Task Performance. J Exp Psychol Hum Percept Perform, 2003, 29 (1), 3–18. [CrossRef]
- Mac-Auliffe, D.; Chatard, B.; Petton, M.; Croizé, A.-C.; Sipp, F.; Bontemps, B.; Gannerie, A.; Bertrand, O.; Rheims, S.; Kahane, P.; et al. The Dual-Task Cost Is Due to Neural Interferences Disrupting the Optimal Spatio-Temporal Dynamics of the Competing Tasks. Front Behav Neurosci, 2021, 15. [CrossRef]
- Silsupadol, P.; Shumway-Cook, A.; Lugade, V.; van Donkelaar, P.; Chou, L.-S.; Mayr, U.; Woollacott, M. H. Effects of Single-Task Versus Dual-Task Training on Balance Performance in Older Adults: A Double-Blind, Randomized Controlled Trial. Arch Phys Med Rehabil, 2009, 90 (3), 381–387. [CrossRef]
- Möhring, W.; Urfer-Maurer, N.; Brand, S.; Holsboer-Trachsler, E.; Weber, P.; Grob, A.; Lemola, S. The Association between Sleep and Dual-Task Performance in Preterm and Full-Term Children: An Exploratory Study. Sleep Med, 2019, 55, 100–108. [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C. M.; Colantonio, A. The Pittsburgh Sleep Quality Index as a Screening Tool for Sleep Dysfunction in Clinical and Non-Clinical Samples: A Systematic Review and Meta-Analysis. Sleep Med Rev, 2016, 25, 52–73. [CrossRef]
- Arippa, F.; Leban, B.; Monticone, M.; Cossu, G.; Casula, C.; Pau, M. A Study on Lower Limb Asymmetries in Parkinson’s Disease during Gait Assessed through Kinematic-Derived Parameters. Bioengineering, 2022, 9 (3), 120. [CrossRef]
- Giuntoli, M.; Scaglione, M.; Bonicoli, E.; Piolanti, N.; Puccioni, G.; Zepeda, K.; Giannini, E.; Marchetti, S.; Indelli, P. F. Intraoperative Load Sensing in Total Knee Arthroplasty Leads to a Functional but Not Clinical Difference: A Comparative, Gait Analysis Evaluation. J Funct Morphol Kinesiol, 2022, 7 (1), 23. [CrossRef]
- Wade, D. T.; Vergis, E. The Short Orientation–Memory–Concentration Test: A Study of Its Reliability and Validity. Clin Rehabil, 1999, 13 (2), 164–170. [CrossRef]
- Queally, V. R.; Evans, J. J.; McMillan, T. M. Accuracy in Scoring Vignettes Using the Mini Mental State Examination and the Short Orientation Memory Concentration Test. J Geriatr Psychiatry Neurol, 2010, 23 (3), 160–164. [CrossRef]
- Das, C. M.; Nagarajan, S.; Poonguzhali, S.; Mohanavelu, K. Biomechanical Characterization of Human GAIT Using EMG Parameters. J Phys Conf Ser, 2022, 2318 (1), 012012. [CrossRef]
- Tassani, S.; Tio, L.; Castro-Domínguez, F.; Monfort, J.; Monllau, J. C.; González Ballester, M. A.; Noailly, J. Relationship Between the Choice of Clinical Treatment, Gait Functionality and Kinetics in Patients With Comparable Knee Osteoarthritis. Front Bioeng Biotechnol, 2022, 10. [CrossRef]
- Schaefer, S.; Amico, G. Table Tennis Expertise Influences Dual-Task Costs in Timed and Self-Initiated Tasks. Acta Psychol (Amst), 2022, 223, 103501. [CrossRef]
- Patelaki, E.; Foxe, J. J.; Mazurek, K. A.; Freedman, E. G. Young Adults Who Improve Performance during Dual-Task Walking Show More Flexible Reallocation of Cognitive Resources: A Mobile Brain-Body Imaging (MoBI) Study. Cerebral Cortex, 2023, 33 (6), 2573–2592. [CrossRef]
- Clark, D. J. Automaticity of Walking: Functional Significance, Mechanisms, Measurement and Rehabilitation Strategies. Front Hum Neurosci, 2015, 9. [CrossRef]
- Wrightson, J. G.; Ross, E. Z.; Smeeton, N. J. The Effect of Cognitive-Task Type and Walking Speed on Dual-Task Gait in Healthy Adults. Motor Control, 2016, 20 (1), 109–121. [CrossRef]
- Kizony, R.; Levin, M. F.; Hughey, L.; Perez, C.; Fung, J. Cognitive Load and Dual-Task Performance During Locomotion Poststroke: A Feasibility Study Using a Functional Virtual Environment. Phys Ther, 2010, 90 (2), 252–260. [CrossRef]
- McAndrew Young, P. M.; Dingwell, J. B. Voluntary Changes in Step Width and Step Length during Human Walking Affect Dynamic Margins of Stability. Gait Posture, 2012, 36 (2), 219–224. [CrossRef]
- Latir, A.; Justine, M.; van Deursen, R. The Dual-Task Effects of Conversating While Walking on Gait Spatiotemporal Parameters in Healthy Young Adults. Malaysian Journal of Medicine and Health Sciences, 2022, 18 (s15), 227–232. [CrossRef]
- Guedes, R. C.; Dias, R. C.; Pereira, L. S. M.; Silva, S. L. A.; Lustosa, L. P.; Dias, J. M. D. Influence of Dual Task and Frailty on Gait Parameters of Older Community-Dwelling Individuals. Braz J Phys Ther, 2014, 18 (5), 445–452. [CrossRef]
- Camp, N.; Vagnetti, R.; Bisele, M.; Felton, P.; Hunter, K.; Magistro, D. The Effect of Cognitive Task Complexity on Healthy Gait in the Walking Corsi Test. Brain Sci, 2023, 13 (7), 1019. [CrossRef]
- Bruce, E. S.; Lunt, L.; McDonagh, J. E. Sleep in Adolescents and Young Adults. Clinical Medicine, 2017, 17 (5), 424–428. [CrossRef]
- Griggs, S.; Harper, A.; Hickman, R. L. A Systematic Review of Sleep Deprivation and Neurobehavioral Function in Young Adults. Applied Nursing Research, 2022, 63, 151552. [CrossRef]
- Fatima, Y.; Doi, S. A. R.; Najman, J. M.; Mamun, A. Al. Exploring Gender Difference in Sleep Quality of Young Adults: Findings from a Large Population Study. Clin Med Res, 2016, 14 (3–4), 138–144. [CrossRef]
- Li, S. H.; Graham, B. M.; Werner-Seidler, A. Gender Differences in Adolescent Sleep Disturbance and Treatment Response to Smartphone App–Delivered Cognitive Behavioral Therapy for Insomnia: Exploratory Study. JMIR Form Res, 2021, 5 (3), e22498. [CrossRef]
- Al-Makhalas, A.; Abualait, T.; Ahsan, M.; Abdulaziz, S.; Al Muslem, W. A Gender Based Comparison and Correlation of Spatiotemporal Gait Parameters and Postural Stability. Acta Biomed, 2023, 94 (2), e2023057. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).