Introduction
The identification of genes and pathogens has paradigmatically changed our understanding of diseases. Traditionally, we attributed diseases to two primary categories: infectious diseases caused by pathogens and genetic diseases governed by Mendel's law of heredity, arising from genetic mutations. However, a significant group defies these classifications within the realm of diseases. This group encompasses disorders such as arteriosclerosis, type 2 diabetes, autoimmunity, chronic respiratory diseases, neurological diseases, and cancer. These diseases are classified as non-communicable diseases and constitute approximately 70% of global mortality, as estimated by the World Health Organization [
1]. The etiology of these diseases is presumed to be multifactorial, arising from a complex interplay of lifestyle, behavior, environmental influences, and genetic factors.
Moreover, research has focused on additional factors, such as the gut microbiome [
2] and epigenetics [
3]. Studies on pregnancy and transplantation medicine have also suggested the potential involvement of alloreactivity in the development of these diseases. This idea is mainly supported by the fact that allogeneic cells transferred during pregnancies persist in small numbers and remain viable for decades after childbirth. These cells are referred to as microchimerism.
Microchimerism
The existence of chimerism contradicts the inherent idea that cells exchanged between individuals cannot persist in a foreign immune system environment and should be rejected. Nevertheless, there is increasing evidence that most, if not all, individuals carry a small fraction of cells with an allogeneic origin [
4,
5,
6]. Microchimerism has been detected in the blood [
4] and multiple tissues, including the brain [
7], liver [
8], and pancreas [
9]. Furthermore, chimerism has been observed in cattle, pigs, and other animals, suggesting it is not a rare evolutionary occurrence [
10,
11]. The discovery of microchimerism fundamentally challenges our concept of alloreactivity, as it indicates that allogeneic interactions are not confined to pregnancy or childbirth but may persist long after these events. Considering the lifelong persistence of these cells, it has been hypothesized that alloreactivity may be involved in non-communicable diseases [
12,
13].
Autoimmune Diseases
Notably, a strong association exists between auto- and alloimmunity [
12,
14]. Chronic graft-versus-host disease (cGvHD) following allogeneic stem cell transplantation has similar clinical components as autoimmune diseases, such as dry mouth and eyes, joint stiffness, skin lesions, and fibrosis. Moreover, autoantibodies, which indicate autoimmune diseases, are frequently observed in patients with cGvHD. Antinuclear antibodies have been reported in approximately 65% of patients with cGvHD [
15]. Detecting anti-topoisomerase I antibodies in cGvHD [
16] is noteworthy as these antibodies are highly specific for systemic sclerosis [
17]. Moreover, anti-platelet-derived growth factor (anti-PDGF) receptor antibodies have been identified in nearly all patients with systemic sclerosis. These antibodies are suspected to be significant in the disease's pathogenesis due to their capacity to activate collagen gene expression [
18]. Similarly, anti-PDGF-receptor antibodies have been detected in approximately all patients with extensive cGvHD, whereas they are absent in controls without cGVHD [
19].
Additional direct evidence linking auto- and alloreactivity stems from discovering allogeneic DNA in the tissues of patients with autoimmune diseases. Fetal DNA has been found in the salivary glands of women with Sjögren's syndrome [
20], the skin lesions of women with systemic sclerosis [
21], and the thyroid tissue of women with autoimmune thyroiditis [
22,
23]. Conversely, myocardial cells of maternal origin have been found in newborns with lupus congenital heart block [
24]. However, the evidence of an association with autoimmune diseases is complicated by the fact that microchimeric cells can also be detected in healthy individuals [
20,
21,
22,
23].
Nevertheless, studies with inbred mouse models provide compelling evidence supporting alloreactivity's involvement in autoimmune diseases. The injection of immune cells from one inbred mouse strain into another frequently induces autoimmunity, characterized by typical clinical and histopathological patterns [
25].
Type 2 Diabetes
Approximately 60% of women with gestational diabetes develop type 2 diabetes later in life [
26]. Consequently, gestational diabetes represents one of the most significant risk factors for type 2 diabetes. Furthermore, gestational diabetes closely resembles type 2 diabetes in two clinical components: impaired glucose tolerance and insulin resistance. The development of gestational diabetes is attributed to the hormonal and metabolic changes that occur during pregnancy. However, pregnancy presents a unique immunological condition characterized by direct allogeneic cell contact at the fetomaternal interface and bidirectional exchange of antigens, antibodies, and cells. Fetal cells enter the maternal circulation during pregnancy and are not entirely removed after that [
27,
28]. It has been established that allogeneic encounters during pregnancy primarily result in immunological tolerance [
29,
30]; however, there is also evidence indicating that tolerance may be incomplete, with excessive alloreactivity being managed by peripheral tolerance [
31].
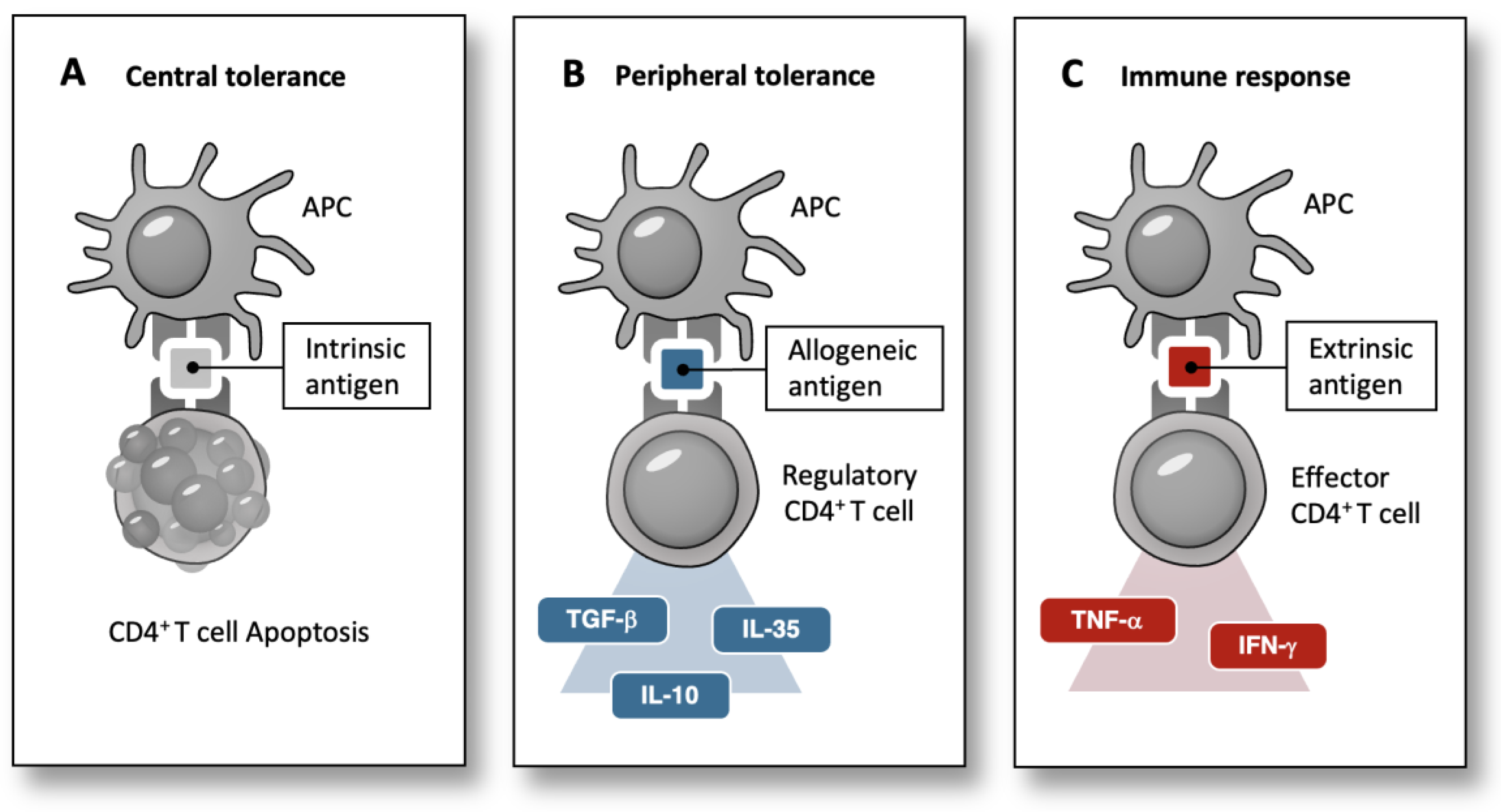
In contrast to central tolerance, which involves the thymic depletion of autoreactive immune cells (
Figure 1A), peripheral tolerance is closely associated with the secretion of cytokines, such as the transforming growth factor-β (TGF-β) in response to antigens (
Figure 1B). These cytokines can further affect the body, as TGF-β has been shown to regulate glucose tolerance and pancreatic islet β-cell function in mice [
32,
33]. Furthermore, increased TGF-β levels have been correlated with obesity in humans [
33]. Therefore, an alternative explanation for gestational diabetes could be that fetal allogeneic cells in the maternal body stimulate maternal regulatory T cells to produce cytokines, such as TGF-β, subsequently leading to gestational diabetes. Recently, a correlation between increased fetal cells in the maternal circulation and poor glucose control has been demonstrated in diabetic pregnancies [
34].
Moreover, eclampsia, a pregnancy-associated disorder characterized by hypertension, proteinuria, and thrombotic microangiopathy, leads to endovascular damage and organ dysfunction. In eclampsia, placental damage increases fetal cell translocation into the maternal blood and tissues [
35]. Pre-eclampsia is associated with gestational diabetes and a twofold increase in diabetes in later life [
36].
Arteriosclerosis
Human arteriosclerosis is a chronic inflammatory disorder with significant clinical manifestations, such as myocardial infarction and stroke [
37]. Similarly, graft arteriosclerosis, which often occurs following organ transplantation, is the primary clinical factor limiting the long-term survival of allogeneic organ grafts [
38]. The formation of arteriosclerotic plaques by allogeneic triggers is noteworthy. Perivascular inflammation and consecutive graft arteriosclerosis have been documented in heart, kidney, lung, and liver transplantations [
38,
39]. Histologically, there are similarities and differences between spontaneous and transplant arteriosclerosis [
40,
41,
42]. The accumulation of lymphocytes, macrophages in the artery wall, and lipids in atherosclerotic plaques are common features in spontaneous and transplant arteriosclerosis. Transplant arteriosclerosis exhibits more diffuse concentric intimal thickening compared with spontaneous arteriosclerosis. Furthermore, spontaneous arteriosclerosis typically develops over several decades, whereas transplant arteriosclerosis may develop within 1 or 2 weeks. However, calcification occurs late in transplant arteriosclerosis.
Arteriosclerosis has also been observed during pregnancy, where acute atherosis of the spiral arteries at the fetomaternal interface closely resembles early-stage atherosclerosis [
43]. Acute atherosis is rare in healthy pregnancies but is frequently observed in abnormal pregnancies, such as those complicated by pre-eclampsia [
43,
44]. The role of alloreactivity in acute atherosclerosis has also been previously discussed [
44]; alloreactivity leads to transplant vasculopathy and is implicated in the etiology of spontaneous arteriosclerosis.
Cancer
Chronic infections caused by human papillomavirus, hepatitis B and C, and Helicobacter pylori contribute significantly to cancer development [
45]. In addition to viral oncogenes and cytokines, chronic inflammation is involved in cancer development and increases the risk of malignant transformation [
46]. Allogeneic cells have been found in various cancer tissues, including melanoma, breast, lung, thyroid, and brain tumors [
13,
47]. A malignant transformation caused by allogeneic cells is yet to be directly proven; however, their presence in tumor tissues has been extensively documented. Their active role in malignant transformation has been discussed in addition to the potential benefits of microchimerism, such as anti-tumor effects and tissue repair [
13,
47,
48].
Alloreactivity in Non-Communicable Diseases
In 1893, Georg Schmorl described fetal cells in the lungs of women who died of eclampsia [
49]. He was the first to postulate a connection between fetal cells in the maternal circulation and a disease. Over a century later, in 1996, J. Lee Nelson advanced the exploration of this idea by introducing the question, “Is some autoimmune disease auto-alloimmune or allo-autoimmune?” [
12]. Unfortunately, the low frequency of microchimeric cells prevents comprehensive tracking of their local and systemic effects. Even today, the theory that cells exchanged between individuals subsequently cause alloreactive effects in the host’s body remains speculative. None of the previously discussed correlations offer definitive evidence, leaving the hypothesis neither validated nor disproven.
Mathematical Aspects of Alloreactivity
Questioning whether microchimerism leads to disease goes beyond pinpointing a cause; it raises the intriguing question of whether diseases themselves might follow mathematical patterns, suggesting a deeper, rule-based structure to their occurrence and progression.
In contrast to microchimerism, alloreactivity and its role in health and disease have been more clearly defined. Focusing on alloreactivity, three fundamental mathematical concepts may help unravel its role in health and disease: relativity, set theory, and asymmetry. Importantly, these mathematical concepts of alloreactivity originate from concrete properties of the immune system.
The Immune System Works in a Relativistic Mode
Relativity, a fundamental concept in physics, elucidates the intricate relationships between space, time, mass, energy, and gravity. Notably, the principles of relativity extend beyond the realm of physics to biological systems [
50]. Based on the following findings, relativity is pivotal in the interaction between the immune system and genes: The immune system establishes a natural barrier against invasion, safeguarding the body against pathogens. Moreover, the immune system adapts to the body's genome, and during T-cell maturation, central immune tolerance eliminates T-cells in the thymus that react with self-antigens. This process, however, leaves an intriguing gap in immune surveillance (
Figure 2A). Consequently, the immune system contains an individual's immunological information, characterized by a complete set of specific immune cells devoid of a depleted set of self-reactive cells. Therefore, each person possesses a distinct set of self-antigens, representing their immunological frame of reference. Since there is an individual and no universal or privileged frame of reference, the immune system operates in a relativistic mode [
50].
A Relativistic Perspective on Alloreactivity
Across different species, a substantial genetic commonality exists, with most genes being shared. However, alloantigens are encoded outside the overlapping genetic pool. Alloreactivity is the general term for immune responses to alloantigens not found within an individual’s genome but instead encoded in the genomes of others within the same species. Alloantigens commonly arise due to genetic mutations. Notably, many blood group systems are founded on genetic variants, such as the Rhesus D blood group alloantigen resulting from a gene deletion and the H-Y alloantigen exclusively encoded on the Y chromosome. A central feature of alloantigens is their presence alongside genes shared by other individuals. Importantly, the information that triggers an alloreactive immune response is not contained within the host or donor genomes but depends on the exclusive antigen expression in one but not the other individual. Each immune system represents a distinct frame of reference encoded by an individual genome. Following transmission into a foreign body, the donor immune cells can detect host antigens not encoded by the donor’s genome. Therefore, the term “relativistic” implies that the ensuing effect defies explanation or prediction through the lens of a single genome. The personal genome provides only an individual frame of reference, and immunological incompatibility emerges in response to genomic differences between individuals.
In addition to minor alloantigens, variations in the major histocompatibility complex (MHC) determine alloreactivity; T-cells recognize a compound of the MHC and its presented antigen. T-cells may directly target MHC variants, but alterations in the MHC molecules lead to a shift in antigen selection. Therefore, transplantation of MHC-mismatched allogeneic organs or stem cells may lead to complex and unpredictable immunological interactions. This complexity can be markedly reduced if the immune system is considered a binary system that distinguishes self- and non-self-antigens, regardless of their MHC and minor antigen components. Since the term “non-self” pertains to a single individual other than oneself, the binary system, featuring two conditions, “self” and “non-self,” reflects the relativity of genes.
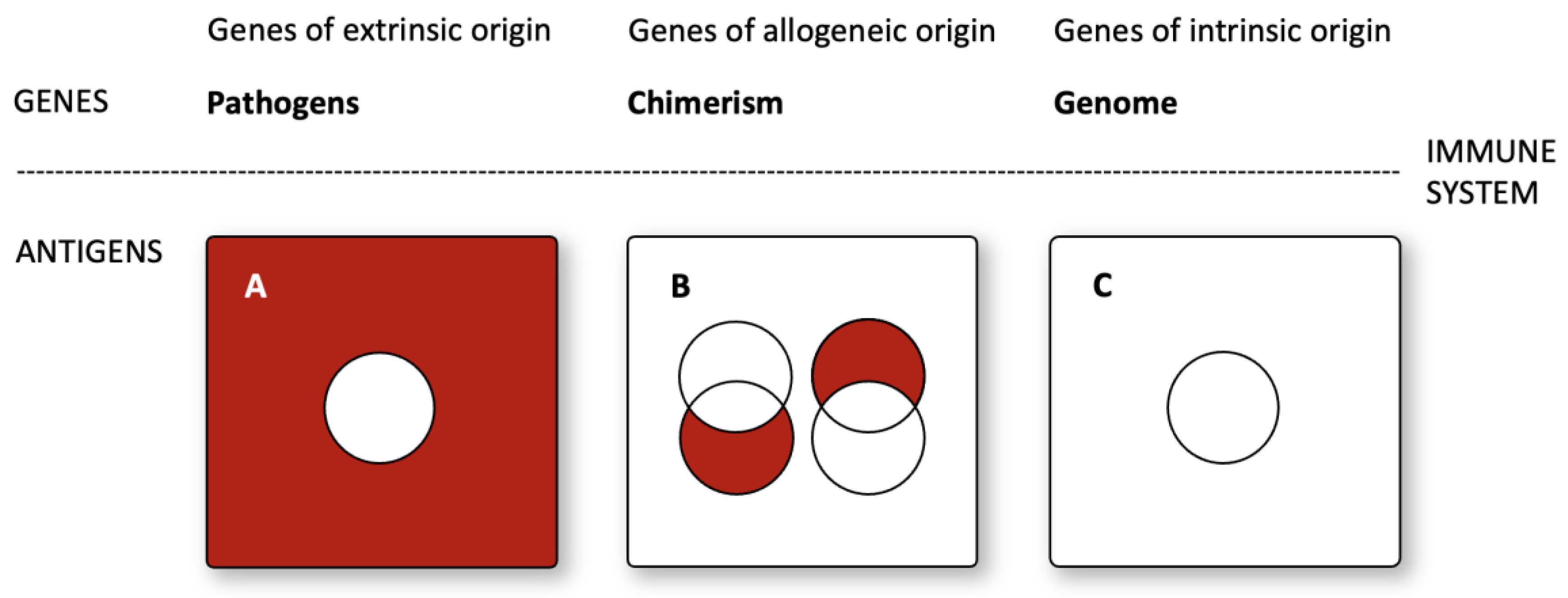
The genes are classified based on extrinsic, allogeneic, or intrinsic origins. The immune system then converts the genes into antigens.
(A) Within the universe of all existing antigens (red rectangle), central and peripheral tolerance leads to a gap in immune surveillance (white circle). Extrinsic antigens are defined as the absolute complement of self-antigens (white circles) within all antigens (red rectangles). Pathogens are extrinsic antigens.
(B) Following the transfer of cells into another individual, donor immune cells detect host antigens by recognizing host antigens (left red circle) in a graft-versus-host-like reaction. Therefore, allogeneic antigens can be defined as a set of host antigens (left red circle) that do not overlap the self-antigens of the donor (left white circle): allogeneic antigens of the host are the relative complement of host antigens (left red circle) without donor antigens (left white circle). Host immune cells can recognize donor antigens through the antigen recognition of donor antigens (right red circle). Therefore, allogeneic antigens can be defined as a set of donor antigens (right red circle) without the self-antigens of the host (right white circle): allogeneic antigens of the donor are the relative complement of the set of donor antigens (right red circle) without the host antigens (right white circle).
(C) Individual genomes encode intrinsic antigens (self-antigens). Central and peripheral tolerance deletes or silences self-reactive immune cells. Therefore, antigens of intrinsic origin do not induce an immune response (white circle).
A Mathematical Model of Disease
The interaction of T cells, antigen-presenting cells, and costimulatory signals commonly determines immune reactions. For this analysis, we will set aside the complex relationships of these cells and explore the situation from the antigen's perspective.
Alloantigens are defined by their absence in an individual’s genome but their presence in the genomes of others within the same species. This definition implies that alloantigens, and indeed antigens more broadly, can be precisely characterized using the rules of set theory and their ability to describe the inclusion and exclusion of elements within sets. In addition to a mathematical description, antigens can be categorized by set theory. Self-antigens encoded within an individual’s genome are of intrinsic origin (
Figure 2C). Pathogenic antigens, such as bacterial and viral antigens, are extrinsic (
Figure 2A). Antigens encoded by some, but not all, members of a species, such as the Rhesus-D-protein, are allogeneic (
Figure 2B). Therefore, antigens can be categorized based on their intrinsic, extrinsic, or allogeneic origins.
Moreover, this classification serves as a mathematical model of disease integrating genetic, infectious, and allogeneic diseases (
Figure 2). This model offers a mathematical approach by quantifying the relationships among these disease categories. It provides a mathematical framework for differentiating immune responses to alloantigens from those against extrinsic antigens. Furthermore, it accurately predicts that, unlike these antigens, the genome and genetic disorders are unlikely to trigger an immune reaction. Starting from intrinsic self-antigens that are genomically defined, extrinsic and allogeneic antigens can be mathematically calculated using two different axioms of set theory: the absolute and relative complements.
Definition of Non-Self-Antigens as Absolute Complement by Set Theory
B- and T-cells commonly recognize non-self-antigens, such as bacterial or viral antigens. The set theory provides a useful framework for classifying non-self-antigens. Extrinsic antigens, such as those from pathogens, represent the absolute complement of self-antigens (
Figure 2A). Mathematically, a set of extrinsic antigens encompasses the entire spectrum without a set of self-antigens. These antigens are not encoded in an individual's genome; therefore, this set includes all viral and bacterial antigens and allogeneic antigens, such as the Rhesus protein. Allogeneic antigens are categorized as extrinsic; however, they can be further defined as follows:
Definition of Allogeneic Antigens as Relative Complement by Set Theory
Allogeneic antigens represent a specific subset of non-self-antigens. During the exchange of cells between individuals, both immune and non-immune cells encounter foreign immune systems and antigens. When fetal immune cells enter the maternal circulation, the immunogenic targets can be more precisely characterized using another principle of set theory: the set of allogeneic minor antigens is defined as a relative complement of the host’s (maternal) antigens without the self-antigens of the (fetal) donor (
Figure 2B, left) and vice versa (
Figure 2B, right).
Alloreactivity is a bidirectional process that manifests in response to maternal and fetal antigens. Maternal genes not present in the fetal genome encode proteins that can serve as allogeneic antigens. Consequently, when fetal immune cells enter the maternal circulation, they may recognize maternal antigens that are not contained in the set of self-antigens in a graft-versus-host-like reaction. Conversely, maternal immune cells or antibodies entering the fetal circulation may recognize fetal non-self-antigens in a host-versus-graft response, considering the fetus as a graft within the female’s body. These interactions have clinical implications. For instance, following immunization with the Rhesus-D-protein during a first pregnancy, a Rhesus-negative woman's Rhesus-D-specific antibodies can destroy the blood cells of Rhesus-positive babies in subsequent pregnancies. This potentially fatal condition can be prevented by administering Rhesus prophylaxis to mothers who are Rhesus-negative.
Asymmetry as a Marker of Alloreactivity
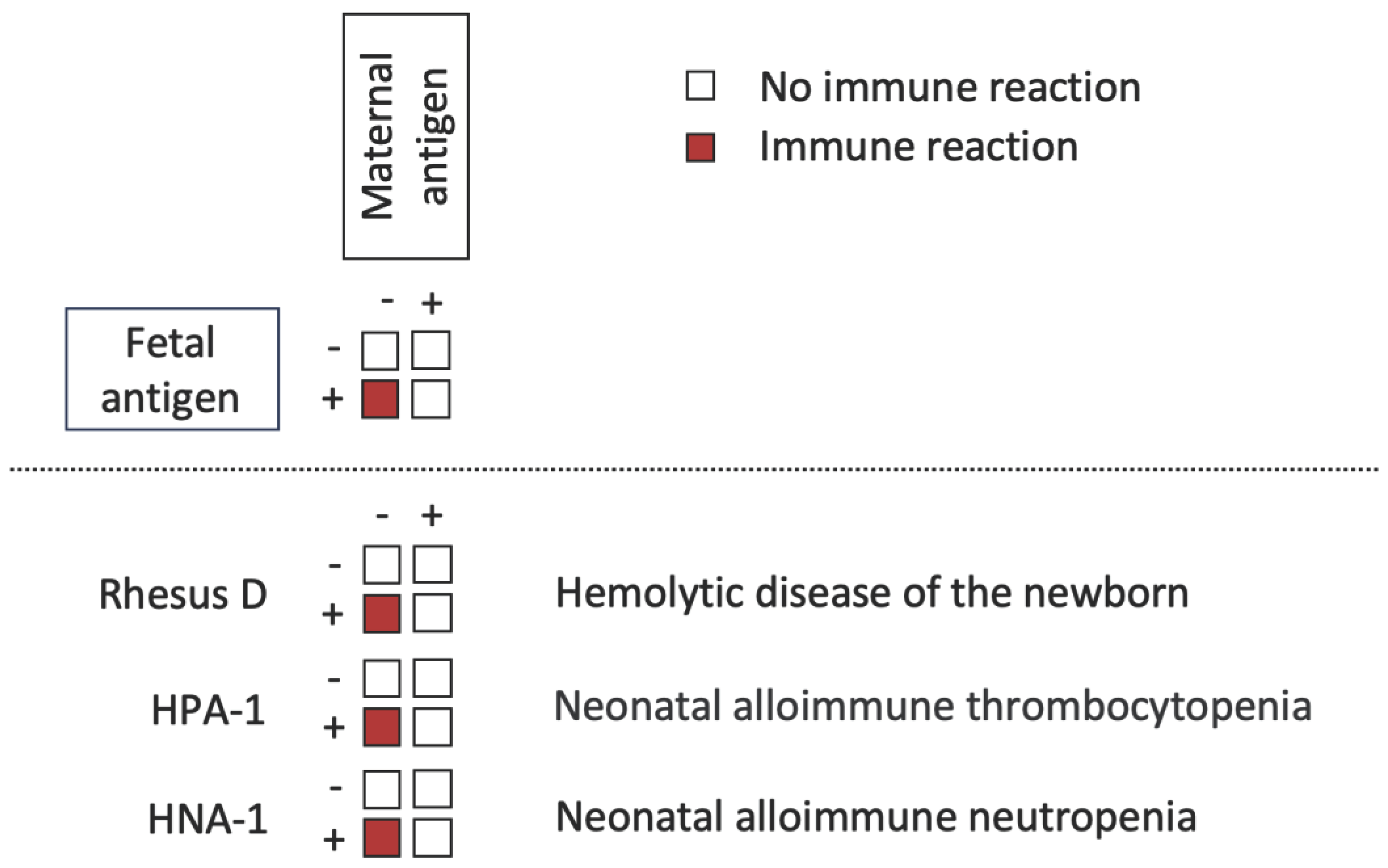
Our diverse genetic backgrounds often result in unpredictable immunological interactions when cells are transferred from one individual to another. Asymmetry can reveal alloreactivity in specific scenarios. It arises due to the direction of the immune system; antigen-negative individuals respond to antigens that are not encoded in their genomes. For instance, when Rhesus D-negative mothers are immunized during pregnancy with a Rhesus D-positive child, it can lead to hemolytic disease in the newborn in subsequent pregnancies. Notably, other examples of alloreactive diseases include neonatal alloimmune thrombocytopenia, primarily caused by the human platelet antigen-1 (HPA-1), and neonatal alloimmune neutropenia, which depends on the human neutrophil antigen-1 (HNA-1) expression in the fetus but not in the mother (
Figure 3).
A sex-dependent immunological imbalance is another example of asymmetry resulting from alloreactivity. The male genome carries genes on the Y chromosome that are absent in the female genome. This genomic distinction results in a sex-dependent immunological imbalance and has clinical implications for donor selection in human stem cell transplantation. It is widely acknowledged that using a female donor for a male recipient should be avoided, as this combination significantly increases the risk of graft-versus-host disease [
51]. The HY antigen encoded in the Y chromosome has been identified as a relevant alloantigen in transplantations. In inbred mouse strains, such as C57BL/6, all individuals are genetically identical except males, who possess the Y chromosome. The HY antigen prompts female mice to reject skin grafts from male donors, whereas they tolerate female skin grafts, and male mice tolerate both female and male skin grafts [
52]. This asymmetric pattern may help uncover the contribution of alloreactivity to human diseases.
The fetus can be considered as a graft for the mother. Allogeneic immune responses occur when the lymphocytes of an antigen-negative mother recognize the antigen of an antigen-positive child (red squares). No immune reactions occur if the mother and child are antigen negative, both are antigen positive, or if the mother is antigen positive and the child is antigen negative (white squares). Examples include hemolytic disease of the newborn, in which lymphocytes of a Rhesus D-negative mother recognize the Rhesus D-antigen on the child’s red blood cells; neonatal alloimmune neutropenia, in which lymphocytes of an HPA-1-negative mother recognize the HPA-1-antigen on the child’s platelets; and neonatal alloimmune thrombocytopenia, in which lymphocytes of an HNA-1-negative mother recognize the HNA-1-antigen on the child’s neutrophils.
HNA-1, Human neutrophil antigen 1; HPA-1, Human platelet antigen 1; Rhesus D, Rhesus blood group antigen D.
Discussion
The genome encodes linear information stored within the solid molecular structure of the DNA. However, spontaneous mutations introduce dynamism into this otherwise static condition, maintaining an ongoing process where the DNA is subject to continuous modifications throughout evolution. Consequently, the genetic diversity has increased over time. During pregnancy, genetic disparities between mothers and children gain relevance and are primarily shaped by alloreactivity. Nevertheless, the continuous maternal effector immune cell response to fetal alloantigens would lead to severe fetal damage during pregnancy. This underscores the predominance of alloantigen tolerance within the immune system before birth [
29]. However, it is crucial to consider three important aspects that suggest a limit of allogeneic tolerance: First, pregnancy directly proves that genomic variability, such as blood group incompatibility, can cause immunological incompatibility and subsequent pregnancy loss. Second, maternal microchimerism fosters peripheral tolerance to non-inherited maternal antigens and positively affects offspring survival in the next generation [
6]. Conversely, this strongly implies a detrimental effect on the offspring of mothers lacking this acquired tolerance. Third, peripheral tolerance is, to some extent, incomplete, as it involves the secretion of cytokines, such as TGF-β (
Figure 1B).
Immunosuppression can be discontinued three months after allogeneic stem cell transplantation. This is widely accepted as strong evidence that stem cell transplantation results in tolerance. However, cGvHD is a common adverse effect and acute, chronic, and transfusion-associated GvHD have demonstrated that transferred immune cells can cause lethal damage to the host [
53]. Remarkably, the extent of cGvHD following allogeneic stem cell transplantation increases with the sex, relationship, and ethnic backgrounds of the donor and host. Therefore, cGvHD is a gradual process, reflecting the accumulation of immunological incompatibility with increasing genetic distance [
54,
55]. Notably, relativity operates with modest effects at lower volumes and markedly amplifies effects at higher volumes. When tolerance is incomplete, accumulating immunological incompatibility may limit genomic variability within a species [
56]. Therefore, alloreactivity, like an evolutionary clock, may be an immunological counterbalance to genomic variability.
Allogeneic immune responses during pregnancy, transfusion, and transplantation have been extensively studied. Naturally occurring alloreactive diseases include various conditions, such as hemolytic diseases in newborns, neonatal alloimmune neutropenia, and neonatal alloimmune thrombocytopenia. Notably, most red blood cells, neutrophils, and thrombocyte alloantigens are based on single nucleotide polymorphisms, underscoring the exceptional sensitivity of the immune system in detecting the smallest differences between the two genomes. The substantial and increasing number of alloantigens (> 300) in red blood cells, neutrophils, and platelets suggests the existence of additional undiscovered alloantigens in blood and tissues.
However, detecting the effects caused by alloreactivity remains difficult. Notably, most studies on microchimerism are based on quantifying allogeneic cells in blood or tissues; however, this approach does not provide insights into their function. In addition, the frequency of microchimeric T-cells is very low, suggesting that research on antibodies could be a more viable alternative. Antibodies are compelling evidence of a B-cell effector activity. As such, identifying antibodies from microchimeric B-cells or those targeting microchimeric antigens could offer a new perspective. The source of these antibodies can be identified through their distinct (non-self) allotype. Therefore, detecting antibodies with a foreign allotype indicates the immunological activity of microchimeric cells. For instance, patients with rheumatoid arthritis were found to produce antibodies targeting foreign immunoglobulin allotypes [
57,
58]. Moreover, the corresponding genes of these extrinsic immunoglobulins were detected in synovial tissues by polymerase chain reaction, suggesting a microchimerism involvement [
57]. Unfortunately, there are also limitations on antibody studies: antibodies targeting foreign antigens are continuously cleared from the bloodstream through their binding to their respective tissues. Notably, multiple other strategies can be used to explore the impact of alloreactivity on diseases, such as animal models for type 2 diabetes and arteriosclerosis, in which antigens can be actively defined.
Conclusion
The mathematical disease model presented here effectively integrates infectious, genetic, and alloreactive diseases. However, while this model does not prove that alloreactivity is involved in non-communicable diseases, it provides a valuable mathematical framework. This framework emphasizes that alloreactivity adheres to both predictable and quantifiable principles. Therefore, it can serve as a tool for evaluating the role of alloreactivity in health and disease. In this framework, diseases' primary origins are no longer attributed to misbehavior, dysfunction, or dysregulation. Instead, we understand that the immune system adapts and responds to the inherent relativity of genes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
I would like to thank Editage (
www.editage.com) for English language editing. However, Editage has not proofread the final version of this manuscript. Consequently, Editage holds no responsibility for the content, or any mistakes contained within.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hunter DJ, Reddy KS. Noncommunicable Diseases. N Engl J Med 2013, 369, 1336–1343. [Google Scholar] [CrossRef]
- Finlay, BB. Are noncommunicable diseases communicable? Science 2020, 367, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A 1996, 93, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Barinaga, M. Cells exchanged during pregnancy live on. Science 2002, 296, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Kinder JM, Jiang TT, Ertelt JM, Xin L, Strong BS, Shaaban AF, Way SS. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell 2015, 162, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Chan WF, Gurnot C, Montine TJ, Sonnen JA, Guthrie KA, Nelson JL. Male microchimerism in the human female brain. PLoS One 2012, 7, e45592. [Google Scholar] [CrossRef]
- Guettier C, Sebagh M, Buard J, Feneux D, Ortin-Serrano M, Gigou M, et al. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatology 2005, 42, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nelson JL, Gillespie KM, Lambert NC, Stevens AM, Loubiere LS, Rutledge JC, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet β cell microchimerism. Proc Natl Acad Sci U S A 2007, 104, 1637–1642. [Google Scholar] [CrossRef]
- Freeman, G. Explaining the freemartin: Tandler and Keller vs. Lillie and the question of priority. J Exp Zool B Mol Dev Evol 2007, 308, 105–112. [Google Scholar] [CrossRef]
- Bruere AN, Fielden ED, Hutchings H. XX/XY mosaicism in lymphocyte cultures from a pig with freemartin characteristics. N Z Vet J 1968, 6, 31–38. [Google Scholar] [CrossRef]
- Nelson, JL. Maternal-fetal immunology and autoimmune disease. Is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum 1996, 39, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola L, Cirello V, Beck-Peccoz P. Fetal microchimerism as an explanation of disease. Nat Rev Endocrinol 2011, 7, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lawley TJ, Peck GL, Moutsopoulos HM, Gratwohl AA, Deisseroth AB. Scleroderma, Sjögren-like syndrome, and chronic graft-versus-host disease. Ann Intern Med 1977, 87, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol 2006, 34, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Bell SA, Faust H, Mittermüller J, Kolb H, Meurer M. Specificity of antinuclear antibodies in scleroderma-like chronic graft-versus-host disease: clinical correlation and histocompatibility locus antigen association. BMJ 1996, 134, 848–854. [Google Scholar] [CrossRef]
- Hanke K, Dähnrich C, Brückner CS, Huscher D, Becker M, Jansen A, et al. Diagnostic value of anti-topoisomerase I antibodies in a large monocentric cohort. Arthritis Res Ther 2009, 11, R28. [Google Scholar] [CrossRef] [PubMed]
- Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 2006, 354, 2667–2676. [Google Scholar] [CrossRef]
- Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood 2007, 110, 237–241. [Google Scholar] [CrossRef]
- Endo Y, Negishi I, Ishikawa O. Possible contribution of microchimerism to the pathogenesis of Sjogren’s syndrome. Rheumatology 2002, 41, 490–495. [Google Scholar] [CrossRef]
- Artlett CM, Smith JB, Jimenez SA. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med 1998, 338, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa B, Srivatsa S, Johnson KL, Samura O, Lee SL, Bianchi DW. Microchimerism of presumed fetal origin in thyroid specimens from women: a case-control study. Lancet 2001, 358, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Lepez T, Vandewoestyne M, Deforce D. Fetal microchimeric cells in autoimmune thyroid diseases: harmful, beneficial or innocent for the thyroid gland? Chimerism 2013, 4, 111–118. [Google Scholar] [CrossRef]
- Stevens AM, Hermes HM, Rutledge JC, Buyon JP, Nelson JL. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet 2003, 362, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Li W, Titov AA, Morel L. An update on lupus animal models. Curr Opin Rheumatol 2017, 29, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes 2015, 6, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Rijnink EC, Penning ME, Wolterbeek R, Wilhelmus S, Zandbergen M, van Duinen SG, et al. Tissue microchimerism is increased during pregnancy: a human autopsy study. MHR: Mol Hum Reprod 2015, 21, 857–864. [Google Scholar] [CrossRef]
- Cismaru CA, Pop L, Berindan-Neagoe I. Incognito: are microchimeric fetal stem cells that cross placental barrier real emissaries of peace? Stem Cell Rev Rep 2018, 14, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kinder JM, Stelzer IA, Arck PC, Way SS. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol 2017, 17, 483–494. [Google Scholar] [CrossRef]
- Shao TY, Kinder JM, Harper G, Pham G, Peng Y, Liu J, et al. Reproductive outcomes after pregnancy-induced displacement of preexisting microchimeric cells. Science 2023, 381, 1324–1330. [Google Scholar] [CrossRef]
- Alpdogan O, Van Den Brink MRM. Immune tolerance and transplantation. Semin Oncol 2012, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Lin HM, Lee JH, Yadav H, Kamaraju AK, Liu E, Zhigang D, et al. Transforming growth factor-β/Smad3 signaling regulates insulin gene transcription and pancreatic islet β-cell function. Journal of J Biol Chem 2009, 284, 12246–12257. [Google Scholar] [CrossRef] [PubMed]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 2011, 14, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Fjeldstad HE, Jacobsen DP, Johnsen GM, Sugulle M, Chae A, Kanaan SB, et al. Poor glucose control and markers of placental dysfunction correlate with increased circulating fetal microchimerism in diabetic pregnancies. J Reprod Immunol 2023, 159, 104114. [Google Scholar] [CrossRef] [PubMed]
- Hahn S, Hasler P, Vokalova L, van Breda SV, Than NG, Hoesli IM, et al. Feto-Maternal Microchimerism: The Pre-eclampsia Conundrum. Front Immunol 2019, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Wu P, Kwok CS, Haththotuwa R, Kotronias RA, Babu A, Fryer AA, et al. Pre-eclampsia is associated with a twofold increase in diabetes: a systematic review and meta-analysis. Diabetologia 2016, 59, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis - an inflammatory disease. N Engl J Med 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Merola J, Jane-Wit DD, Pober JS. Recent advances in allograft vasculopathy. Curr Opin Organ Transplant 2017, 22, 1–7. [Google Scholar] [CrossRef]
- McCaughan GW, Bishop GA. Atherosclerosis of the liver allograft. J Hepatol 1997, 27, 592–598. [Google Scholar] [CrossRef]
- Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res 2006, 99, 801–815. [Google Scholar] [CrossRef]
- Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef] [PubMed]
- von Rossum A, Laher I, Choy JC. Immune-mediated vascular injury and dysfunction in transplant arteriosclerosis. Front Immunol 2015, 5, 684. [Google Scholar] [CrossRef]
- Kim JY, Kim YM. Acute atherosis of the uterine spiral arteries: Clinicopathologic implications. J Pathol Transl Med 2015, 49, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Pitz Jacobsen D, Fjeldstad HE, Johnsen GM, Fosheim IK, Moe K, Alnæs-Katjavivi P, et al. Acute atherosis lesions at the fetal-maternal border: current knowledge and implications for maternal cardiovascular health. Front Immunol 2021, 12, 791606. [Google Scholar] [CrossRef]
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola L, Cirello V, Beck-Peccoz P. Fetal cell microchimerism in human cancers. Cancer Lett 2010, 287, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cirello V, Fugazzola L. Novel insights into the link between fetal cell microchimerism and maternal cancers. J Cancer Res Clin Oncol 2016, 142, 1697–1704. [Google Scholar] [CrossRef]
- Lapaire O, Holzgreve W, Oosterwijk JC, Brinkhaus R, Bianchi DW. Georg Schmorl on Trophoblasts in the Maternal Circulation. Placenta 2007, 28, 1–5. [Google Scholar] [CrossRef]
- Noble, D. A theory of biological relativity: no privileged level of causation. Interface Focus 2011, 2, 55–64. [Google Scholar] [CrossRef]
- Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood 2005, 1, 2973–2978. [Google Scholar] [CrossRef]
- Wachtel SS, Goldberg EH, Zuckerman E, Boyse EA. Continued expression of H-Y antigen on male lymphoid cells resident in female (Chimaeric) mice. Nature 1973, 13, 102–103. [Google Scholar] [CrossRef]
- Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, et al. A systematic review of transfusion-associated graft-versus-host disease. Blood 2016, 16, 406–414. [Google Scholar] [CrossRef]
- Martin PJ, Levine DM, Storer BE, Warren EH, Zheng X, Nelson SC, et al. Genome-wide minor histocompatibility matching as related to the risk of graft-versus-host disease. Blood 2017, 129, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med 2017, 377, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler M, Fisch, P. A role for microchimerism in obesity and evolution? Med Hypotheses 2012, 78, 528–532. [Google Scholar] [CrossRef]
- Grubb, R. Immunogenetic markers as probes for polymorphism, gene regulation and gene transfer in man - the Gm system in perspective. APMIS 1991, 99, 199–209. [Google Scholar] [CrossRef]
- Bjarnadottir M, Nathansson C, Balbin M, Eberhardt K, Aman P, Grubb R. Nucleotide sequences specific for nonnominal immunoglobulin allotypes in rheumatoid arthritis patients and in normal individuals and their expression in synovial tissue of rheumatoid arthritis patients. Exp Clin Immunogenet 1999, 16, 8–16. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).