Introduction
The diagnostic approach to gliomas has experienced a significant transformation due to recent revisions in the new World Health Organization (WHO) classification. The term "glioblastoma" (GBM) is now specifically assigned to IDH-wildtype tumors, and its diagnosis hinges on histological features. [
1]
The determination of prognosis in gliomas has been a critical aspect influencing treatment approaches. A decision-analytic method to define poor prognosis, as discussed by van Dijk et al. (2008), highlights the importance of adapting treatments based on prognostic markers. This methodology aids in identifying patients who may profit from more aggressive interventions, while sparing those with a better prognosis from burdensome treatments. [
2]
Newly diagnosed cases of glioblastoma present a challenging medical task, characterized by a five-year survival rate of only 7.2%. [
3] Some of the identified factors associated with poor survival outcomes in glioblastoma patients include increasing age, poor performance status (PS), and corticosteroid use. [
4]
Present studies highlight the importance of distinct blood markers, including Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Red Cell Distribution Width (RDW), as predictive factors for the progression of diverse tumors. NLR and PLR aid as indicators of the body's systemic inflammatory response, and ongoing research has investigated their associations with different cancer types, particularly focusing on colorectal and oropharyngeal cancers. [
5] Additionally, RDW which quantifies red blood cell size variability, is linked to cancer prognosis. Studies have found that elevated RDW levels are more common in cancer patients who did not survive, highlighting its significance as an indicator of prognosis. [
6]
Subsequently, different studies are highlighting the prognostic applicability of the NLR and PLR in the context of glioblastoma. The findings from these studies have consistently revealed that elevated levels of NLR and PLR before treatment are associated with less favorable outcomes in these patients. Notably, NLR seems to be a more decisive prognostic marker than PLR in certain studies. [
7,
8,
9]
At the present study, we gathered data on NLR, PLR, Red Cell Distribution Width-Coefficient of Variation (RDW-CV at various stages including pre-operatively, post-operatively, and prior to specific adjuvant treatments. Our objective was to investigate the potential influence of these hematological markers on the Overall Survival (OS) and Progression-Free Survival (PFS) in glioblastoma patients, thereby contributing to a deeper understanding of their prognostic significance in this context.
Methodology
This study aims to evaluate whether the values of NLR, PLR and RDW-CV at different treatment timepoints can predict outcomes in patients with glioblastomas.
It is a retrospective study, utilizing data collected from clinical records between January 1, 2016, and December 31, 2018, at Centro Hospitalar de São João in Porto.
The data gathered include patient demographics (age and sex), pre-operative Eastern Cooperative Oncology Group (ECOG) and Karnofsky Performance Scale (KPS) scores, date of glioblastoma diagnosis (marked by the first CT scan), presence of significant pre-operative deficits, and whether the lesion was in an eloquent area (responsible for critical functions, such as language, sensory processing, motor skills, vision, and cognition). We also, conducted a review of medical records to determine whether patients were undergoing corticosteroid therapy prior to their surgical procedures, leading to the identification of this patient cohort.
Additionally, NLR, PLR, and RDW-CV values were recorded at four specific timepoints: Pre-Surgery, Pre-First Progression after surgery, Pre-First Adjuvant Therapy, and Pre-Second Adjuvant Therapy. Most patients in our department were subjected to the Stupp protocol, which consists of a standard first-line treatment approach. This protocol typically involves radiotherapy and concurrent temozolomide chemotherapy. For second-line or adjuvant treatment options, a combination involving bevacizumab was mainly used. NLR was calculated by dividing the absolute count of neutrophils by the absolute count of lymphocytes, serving as a marker of inflammation and immune response. PLR, on the other hand, is determined by dividing the absolute count of platelets by the absolute count of lymphocytes. Previous articles focus on the prognostic role of RDW generally without specifying which measure (RDW-CV or Red Cell Distribution Width - Standard Deviation (RDW-SD)) was used. Our choice fell on the RDW-CV, a parameter that establishes a ratio of the Red Cell Distribution Width to the mean corpuscular volume, thereby providing a comparative measure of the variation in red blood cell size.
Information regarding the type of surgery (Biopsy, Total Surgical Removal, or Partial Removal), first and second-line adjuvant treatments, and the use of corticosteroids prior to surgery was also collected. Key outcomes measured were the dates of reoperation, tumor progression, death, OS, and PFS. Tumor progression was defined using criteria such as Response Assessment in Neuro-Oncology (RANO), which was applied to patients who underwent surgery and initially experienced tumor progress. Overall Survival was defined as the duration from either the diagnosis or initiation of treatment to the time of death from any cause. Progression-Free Survival is measured from the start of treatment until the observed disease progression or death from any cause, whichever occurs first.
The study included patients diagnosed with Glioblastoma IDH-Wildtype as per the WHO 2016 classification, aged 18 years or older, who underwent surgery and adjuvant treatment. It excluded patients with IDH mutant glioblastomas, missing data, surgeries predating 2016, or a history of other major diseases that could affect inflammatory markers, like previous cancer treatments, infections, or acute inflammation. This study's limitations include the retrospective nature, which might impact data completeness and quality, and the potential for selection and recall biases. The generalizability of findings might also be limited due to these factors.
The expected outcomes include identifying if high or low levels of NLR, PLR, and RDW are significant in predicting OS, their correlation with cancer stage, and evaluating their combinatorial prognostic value.
Data was analysed with SPSS, version 29.0 (IBM Corp., 2023). Descriptive statistics were presented as means and standard deviations for normally distributed variables and medians and quartiles otherwise. For categorical variables, frequencies and percentages were presented. Repeated measures ANOVA with Sidak multiple comparisons tests were used to analyze the variation of NLR, PLR and RDW-CV along the four treatment phases. In addition to the RM-ANOVA, univariate Cox regression analyses were performed to assess the impact of these hematological markers on OS and PFS. Survival analysis included Kaplan-Meier survival curves and log-rank tests and were used to further delineated the prognostic significance of these biomarkers, with proposed cut-offs for NLR and PLR validated against survival outcomes. Adjusted Cox regressions were then conducted, controlling for relevant covariates such as age, ECOG performance status, preoperative neurological deficit, type of surgery, and corticosteroid use. Log-minus-log plots were checked to verify Cox proportional hazard assumptions. Significance was deemed for p<0.05.
Results
In this study, we analyzed data from 117 patients. The average age was 61.51 years (SD = 11.47), with an age range from 19 to 85 years. The most common type of surgery conducted was Gross Total Removal, performed in 51 cases. Prevalence of preoperative neurologic deficit was 65.0% (n=76) and 78 (66.7%) patients were treated with corticosteroid in the preoperative period. Prevalence of tumor location in the eloquent area was 73.5% (n=86). The mean ECOG performance status pre-operation was 1.03 (SD = 0.88), and the mean KPS pre-operation was 83.85 (SD = 15.36). The mean OS duration for the cohort was approximately 16.69 months, and the average PFS duration was 8.25 months. [
Table 1] Re-operations were carried out in 7 patients (6%), with an average time to re-operation of approximately 7.03 months from the initial diagnosis.
In our cohort, patients undergoing Gross Total Removal without pre-operative neurological deficits or corticosteroid therapy demonstrated an average OS of 19.68 months and an average PFS of 10.70 months. These findings suggest a favorable prognosis in this specific subgroup, highlighting the potential impact of initial clinical status and surgical extend of removal on long-term outcomes.
In this study we observed significant variations in key hematological parameters. Specifically, the NLR and PLR showed noteworthy changes across different treatment phases, as revealed by our statistical analysis.
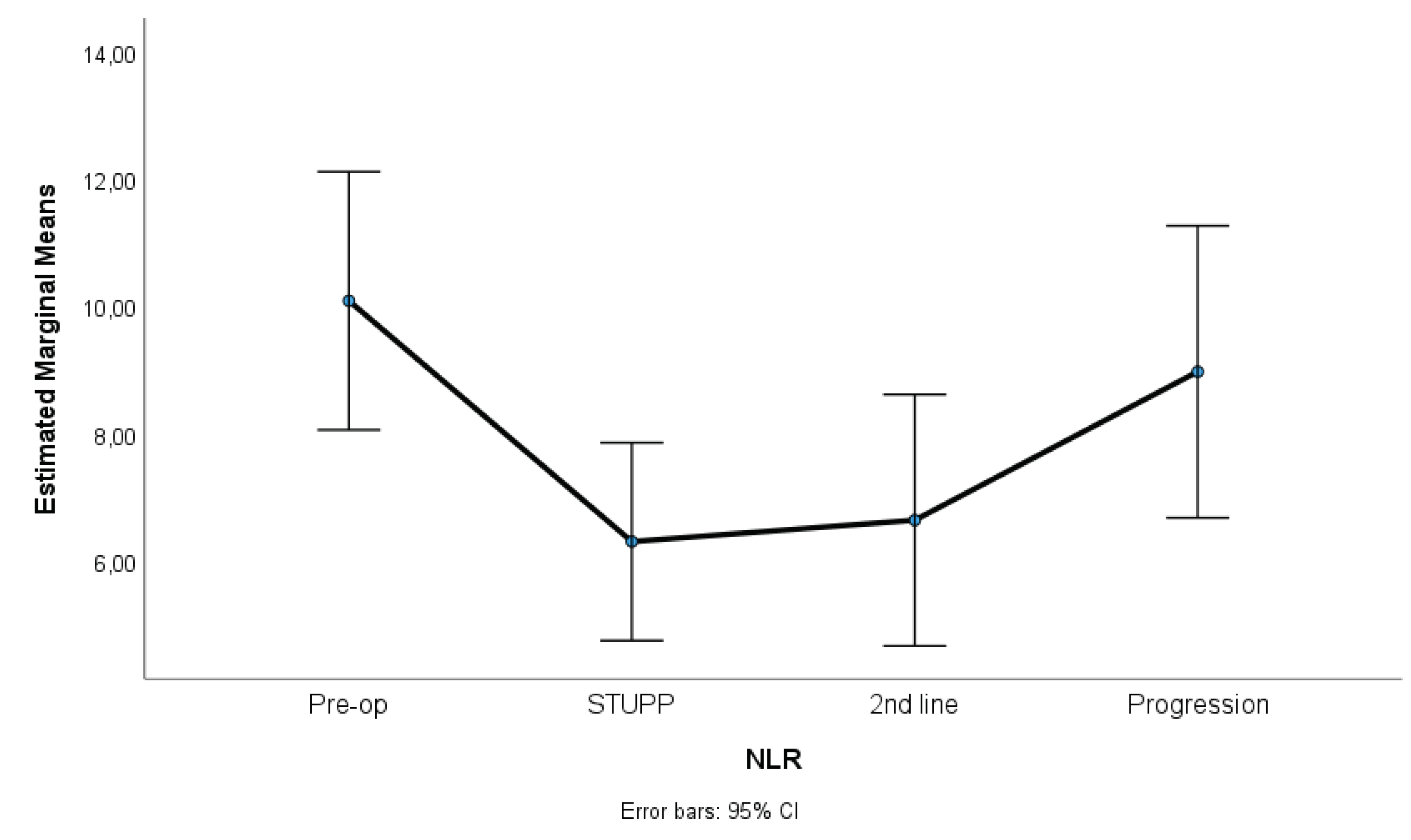
Figure 1,
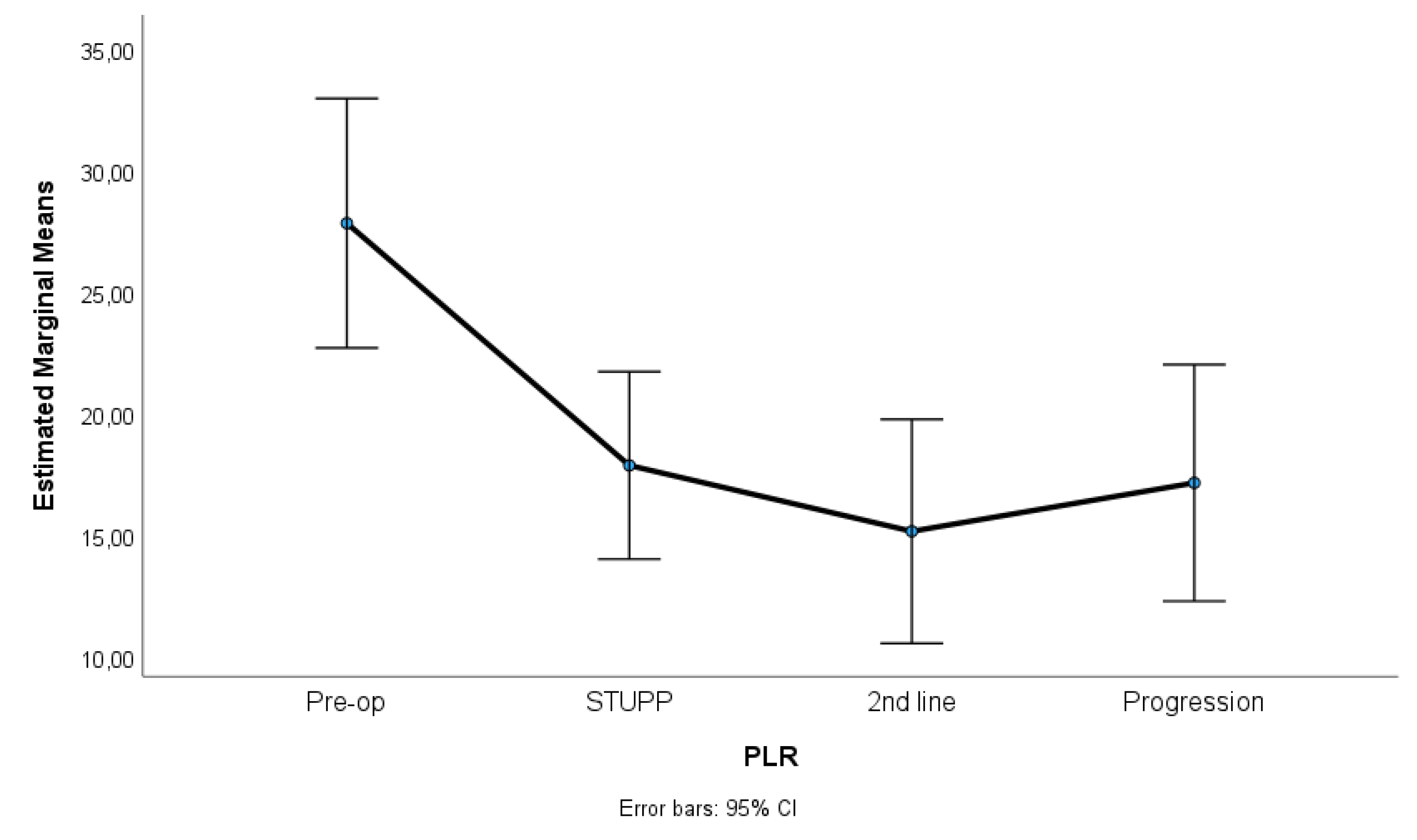
Figure 2 and
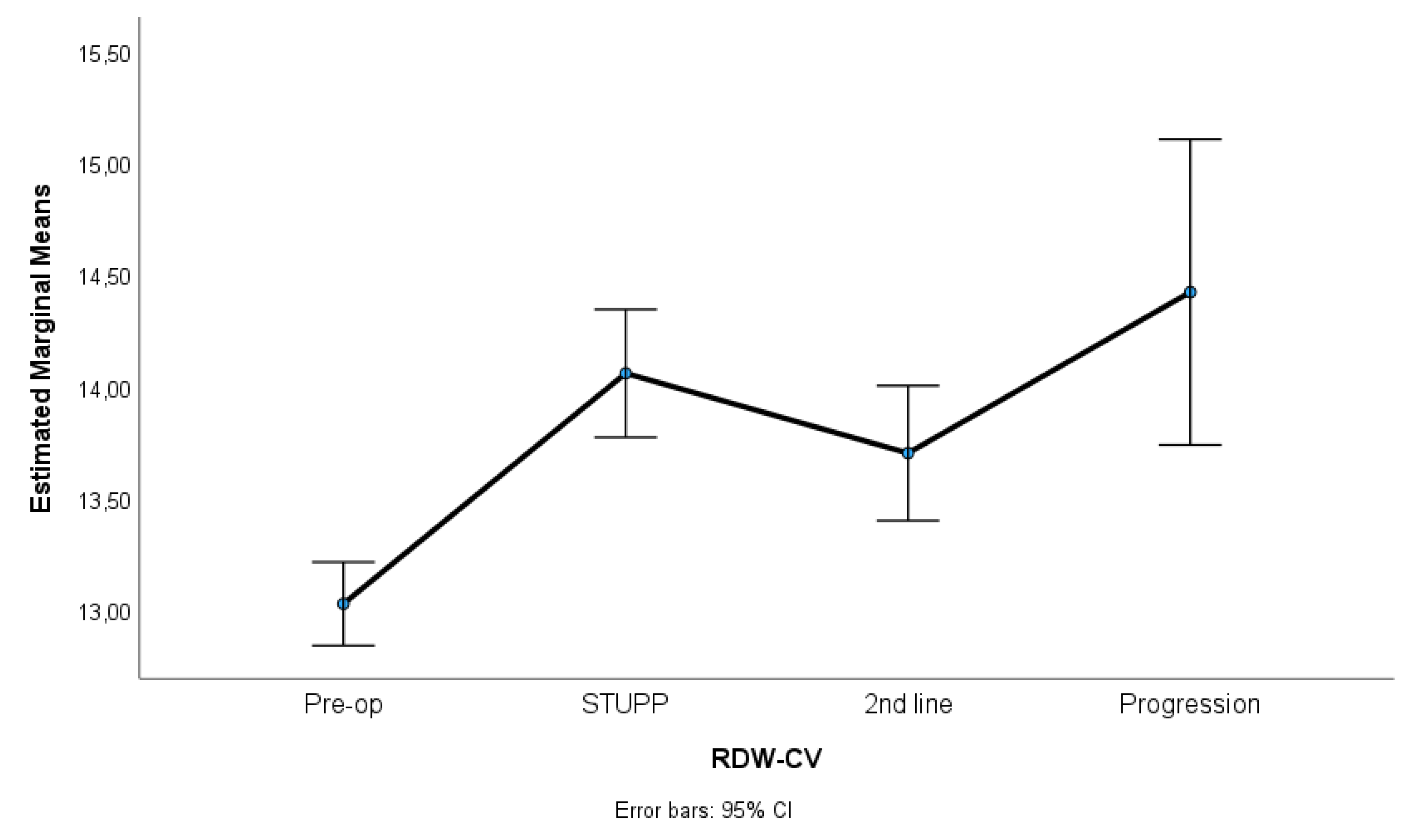
Figure 3 show NLR, PLR and RDW-C variation along treatment phases. NLR and PLR decreased after surgery, with some stabilization after STUPP phase. RDW-CV increased after surgery, showing a positive slope, despite a slight decrease during 2nd line treatment.
Table 2 shows means, standard errors and repeated measures ANOVA tests. A total of 62 patients were included in this analysis. Missing were listwise excluded.
Results indicated significant differences among treatment phases for NLR, p = 0.007, η2p = 0.06, PLR, p = 0.001, η2p = 0.23, and RDW-CV, p < 0.001, η2p = 0.67. Effect sizes (partial eta-squared) ranged from medium to high, suggesting a substantive impact of treatment phases on the variability of these parameters. Sidak multiple comparisons tests showed that pre-operative phase was significantly different of other treatment phases. These phases were not statistically different among themselves.
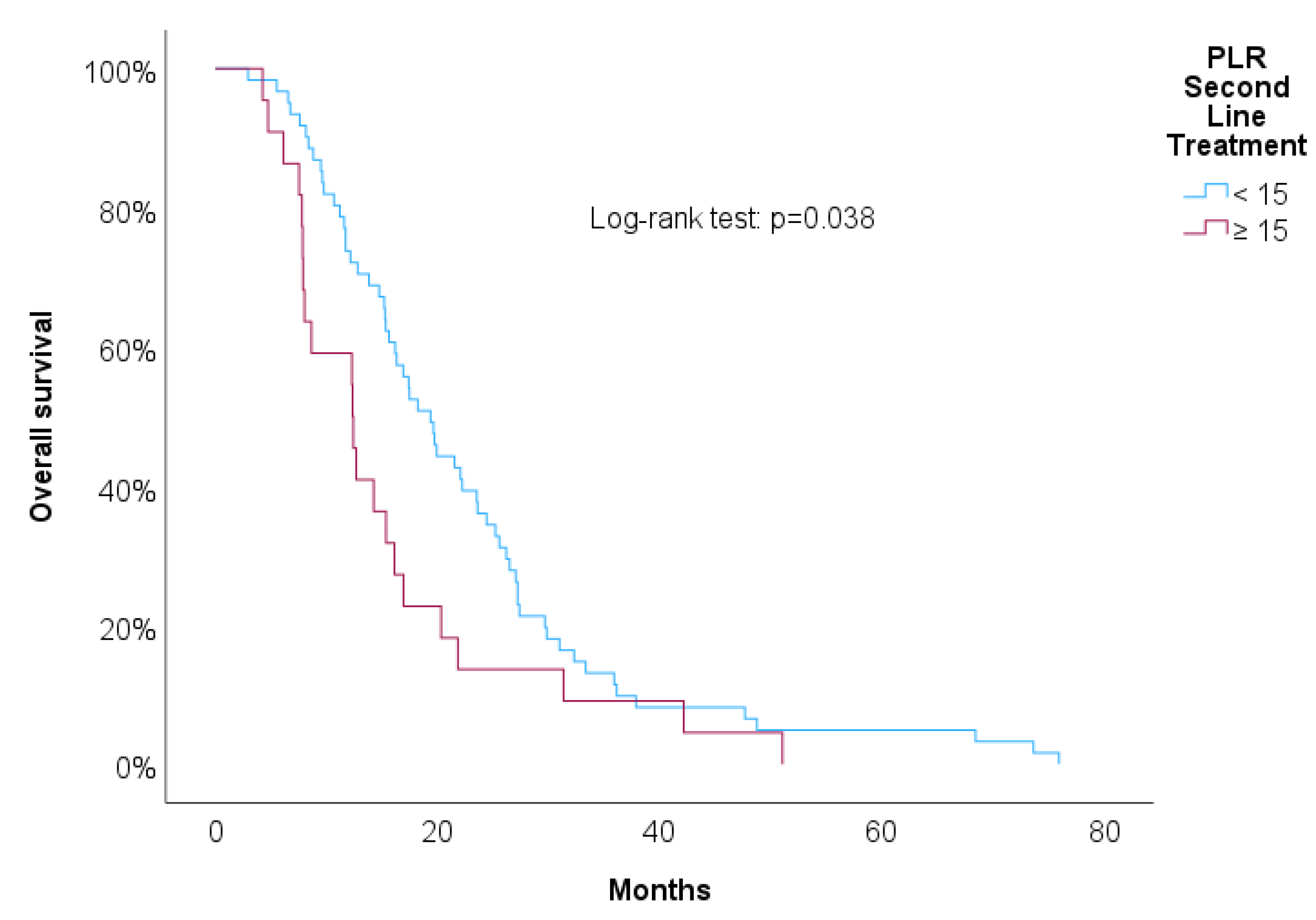
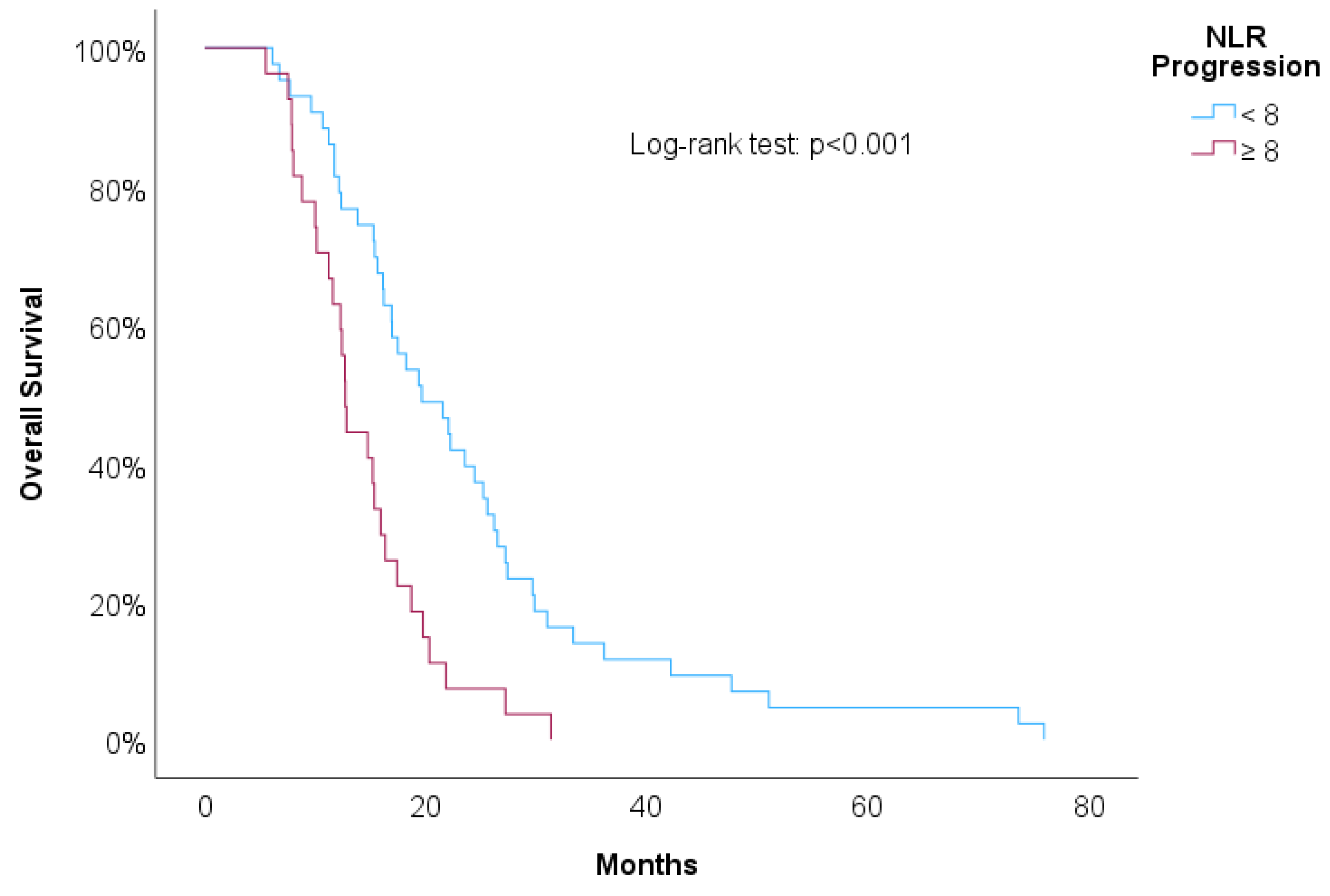
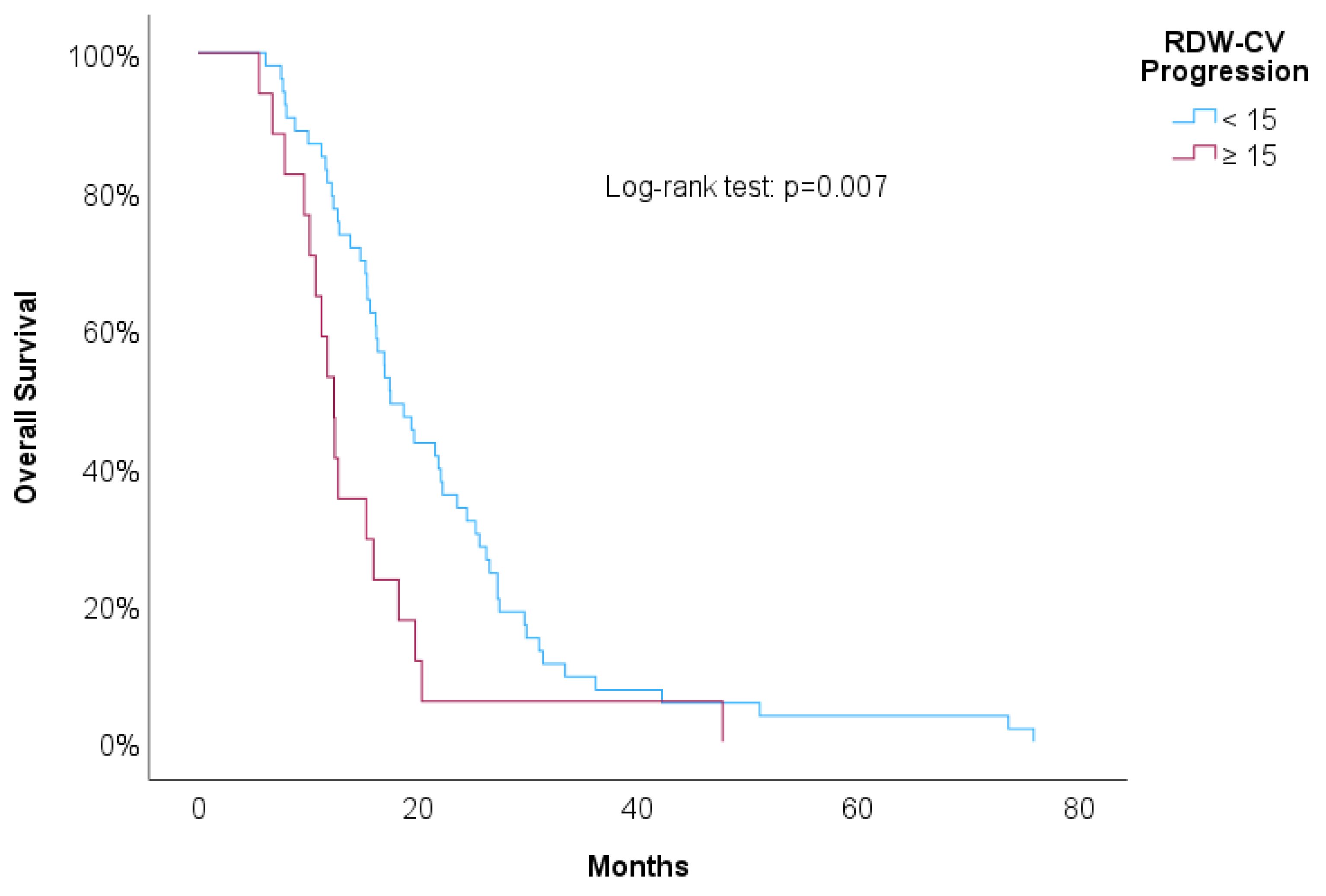
Table 3 shows univariate Cox regressions for OS and PFS. Cut-offs were proposed for significant associations. Increased risk of death was associated with higher NLR at 2nd line of treatment (HR=1.03, p=0.029), NLR at progression (HR=1.04, p=0.006) and RDW-CV at progression (HR=1.14, p=0.004). A result close to statistical significance was detected in PLR at 2nd line of treatment (HR=1.01, p=0.084). For these variables we propose cut-offs that were statistically significant when implemented in Kaplan-Meier survival curves.
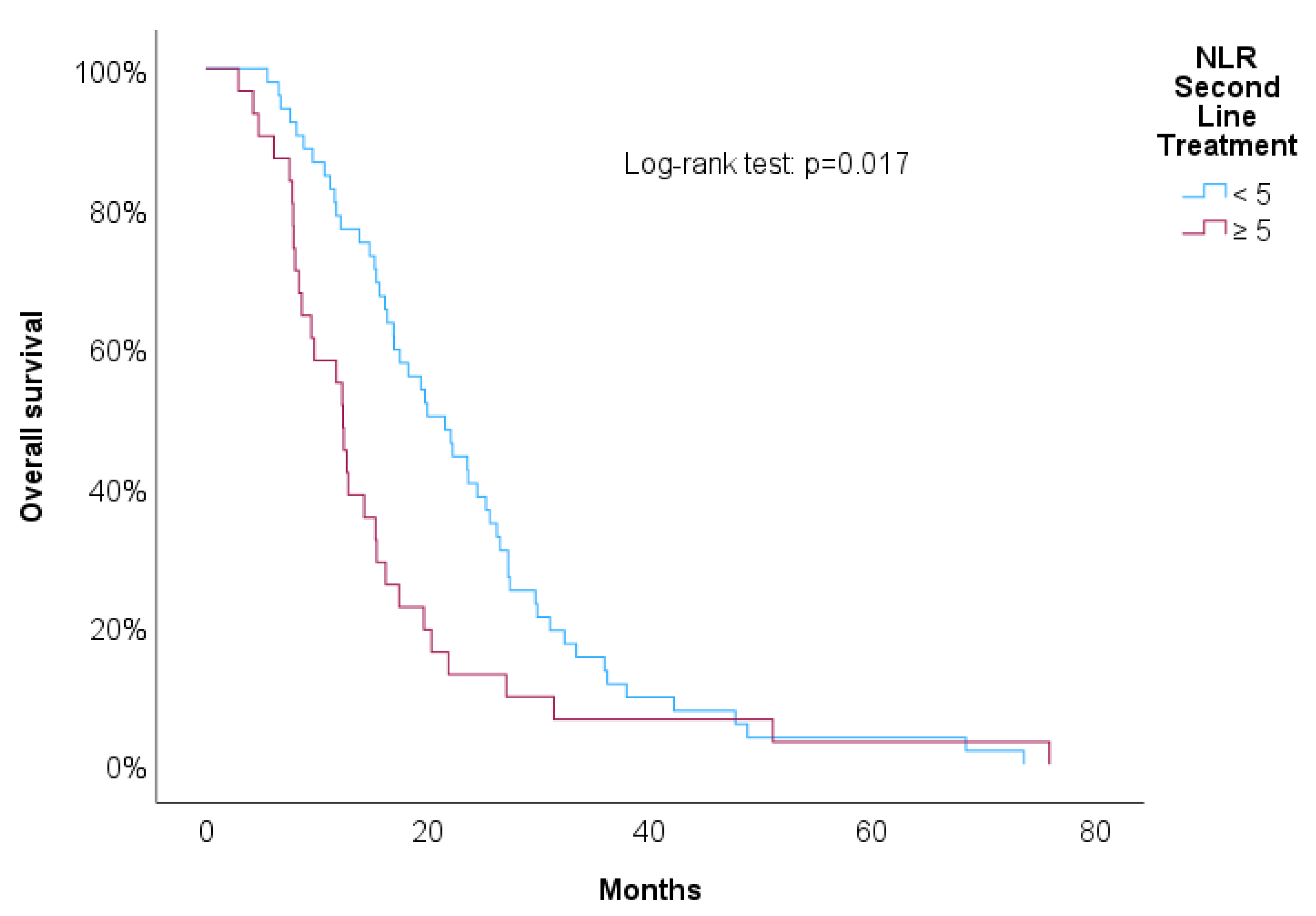
Figure 4,
Figure 5,
Figure 6 and
Figure 7 showed increased overall survival for NLR at 2nd line of treatment < 5 (p<0.017), for PLR at 2nd line of treatment <15 (p=0.038), for NLR at progression (p<0.01) and for RDW-CV at progression (p=0.007).
Table 4 presents results for adjusted Cox regressions for proposed cut-offs adjusted for the following covariates: age, ECOG, preoperative neurologic deficit, surgery, and preoperative corticosteroid use. After adjusting for covariates all predictors maintained the association with overall survival. NLR ≥ 5 at the 2nd line of treatment was associated with increased risk of mortality at t+1 at the rate of 88% more risk (aHR=1.88, p=0.008), PLR ≥ 5 at the 2nd line of treatment was associated with almost twice the risk of mortality at t+1 (aHR=1.93, p=0.012), NLR ≥ 15 at progression was associated with 2.22 times more risk of mortality at t+1 (aHR=2.22, p=0.004) and RDW-CV ≥ 8 at progression was associated with 2.30 times more risk of mortality at t+1 (aHR=2.30, p=0.013). Patients with higher KPS were associated with lower risk of mortality (HR=0.98, p=0.001, 95% CI= [0.96-0.99]).
Discussion
Our research on the Sequential Evaluation of Hematology Markers as a Prognostic Factor in Glioblastoma Patients has focused exclusively on a specific cohort of patients. We intentionally excluded glioblastomas with IDH mutations, directing our attention to the cohort of patients with IDH wild-type glioblastomas, which are known to have a poorer prognosis and representing similar tumor grade. We also acknowledge the diverse glioblastoma populations that have undergone various types of surgical interventions, specifically biopsy or partial resection. These procedures are recognized as prognostic influencers, impacting patient outcomes differently. Lastly, our study has taken into consideration the administration of corticosteroids before the calculation of NLR, PLR, and RDW values, recognizing these as potential confounding variables. Most notably, our study differentiates itself by examining the levels of these markers not just before surgery but also during the complete treatment course of glioblastomas. This includes periods during which, patients were undergoing first line or second line adjuvant treatments. By tracking these markers throughout the disease, we aim to provide a more comprehensive understanding of their prognostic value and their potential fluctuations in response to different therapeutic interventions.
The study in question involved the analysis of clinical data from 117 glioblastoma patients. There was a significant variation in the NLR, PLR, and RDW-CV throughout different treatment phases. After surgery, a decrease in NLR and PLR was observed, while RDW-CV levels increased. The analysis further revealed that higher levels of NLR and RDW-CV at the point of disease progression and at 2nd line adjuvant treatment phase were associated with an increased risk of mortality. Specifically, during the second line of treatment phase, patients with a NLR of 5 or higher and a PLR of 15 or higher were associated with a worse outcome. On the other hand, as glioblastoma progressed after surgery, an NLR of 8 or higher and a RDW-CV of 15 or higher were linked to a poorer prognosis. After adjusting for covariates all predictors maintained the association with overall survival. This suggests that higher levels of these biomarkers at different stages of the disease may predict more adverse outcomes for patients.
Recent investigations have concentrated on the role of inflammatory indicators such as counts of neutrophils, lymphocytes, and platelets, as well as ratios like the NLR and PLR, which are readily accessible through routine bloodwork. Nonetheless, the significance of these markers in predicting glioblastoma outcomes remains a subject of controversy. The contribution of persistent inflammation to the onset and expansion of cancer is recognized, particularly as it facilitates tumor development and affects vascularization. Typically, individuals with glioblastomas develop an increase in neutrophils and a reduction in lymphocytes, a condition often generated from the excessive production of G-CSF by the tumors, which in turn tips blood cell production in favor of granulocytes. [
10] An increased level of neutrophils and an elevated NLR have been associated with unfavorable prognoses in several types of cancer, GBM included. Likewise, the cell count of lymphocytes has not been a consistent prognostic tool for GBM, possibly because of the variation in lymphocyte types. [
9] High platelet levels can promote tumor growth and angiogenesis, which is the formation of new blood vessels. Platelets can release growth factors like Vascular Endothelial Growth Factor (VEGF), which is crucial for angiogenesis, thereby potentially aiding tumor growth and spread. [
9] The PLR may be suggestive of a prothrombotic state often associated with tumor growth and metastasis. Platelets can shield tumor cells from immune detection and destruction, promoting dissemination. [
11,
12]
Patients with glioma and higher RDW levels tend to have a less favorable prognosis. The underlying reasons for this correlation could be multifaceted, involving complex interactions between RDW and various inflammatory markers, cytokines like IL-6 and tumor necrosis factor-alpha, which are known to influence the behavior of tumor cells. [
13,
14] Additionally, increased RDW levels can lead to enhanced production of reactive oxygen species and contribute to tissue hypoxia, which may further complicate postoperative recovery and increase the likelihood of complications. [
15] The rise in RDW can be a consequence of elevated inflammatory cytokine levels, which in turn may lead to increased hepcidin production, affecting red blood cell synthesis and potentially leading to anemia. In patients with glioma, a higher RDW could reflect several underlying issues, such as inflammation, oxidative stress, and poor nutritional status, all of which can adversely affect the patient's health and treatment outcomes. [
16,
17]
Our study might be important in tailoring treatment decisions, or follow-up strategies. Firstly, the dynamic changes in these markers throughout different treatment phases can inform clinicians about the patient’s response to therapy. For instance, a post-surgical decrease in NLR and PLR may be indicative of a positive response to surgery. In contrast, increases in RDW-CV might necessitate closer monitoring. Secondly, the association of higher levels of NLR and RDW-CV with increased mortality risk can guide the intensity and type of adjuvant therapy. Patients with elevated levels of these markers might benefit from more aggressive or alternative treatment modalities. Thirdly, during the second line of treatment, the presence of high NLR and PLR levels could be used to identify patients at a higher risk of a worse outcome, potentially prompting earlier intervention or palliative care. Lastly, these markers could facilitate more personalized follow-up strategies, where patients with higher NLR and RDW-CV post-surgery might require more frequent imaging and clinical evaluation to detect early signs of progression.
Retrospective analyses are inherently subject to selection bias. Patients included may not represent the entire spectrum of glioblastoma cases, as those with missing data, surgeries predating 2016, patients with IDH mutation and other major diseases affecting inflammatory markers were also excluded. This selection could skew the results, as these factors could significantly alter the levels of the markers being studied. The single-center nature of the study may limit the applicability of the results to other settings. Variations in patient demographics, treatment protocols, and healthcare delivery systems could influence the biomarkers differently across institutions or populations such as the type of first line or second line adjuvant type treatment commonly applied in our department.
Lastly this research concentrated on two essential partial unanswered in the past years. Firstly, it explored the intricate relationship between hematological markers NLR, PLR an RDW-CV and the patient’s response to treatment. This aspect of the analysis aimed to recognize patterns that could potentially guide the personalization of therapeutic approaches, allowing for treatments to be more finely adjusted to the individual’s biological answer as shown by these markers. The second area of focus was the longitudinal variability of these indicators throughout the disease course, including throughout treatment. By assessing how NLR, PLR, and RDW fluctuated over time, we sought to gain perceptions into the disease's progression and the effectiveness of treatment modalities. [
18] This longitudinal analysis is pivotal, as it may uncover temporal patterns that might serve as early indicators of treatment success or failure and could potentially predict disease recurrence or progression.
Conclusion
Our study presented a detailed analysis of the prognostic significance of hematological markers such NLR, PLR and RDW-CV in patients with IDH-wildtype glioblastoma. We observed that variations in these markers at different treatment phases - specifically elevated levels of NLR and RDW-CV at the onset of second-line treatment and progression - were significantly associated with a decreased OS and PFS. We did not find statistically significant differences in preoperative marker levels. These findings highlight the potential of NLR, PLR, and RDW as accessible, non-invasive prognostic tools in the clinical management of glioblastoma. Future studies should focus on validating these markers in larger, multi-center cohorts and exploring the essential biological mechanisms driving their involvement with patient outcomes. Finally, understanding these dynamics could lead the way for more personalized and timely interventions, improving survival and quality of life for glioblastoma patients.
Funding
This Original article was not funded.
Financial Disclosure
We report no disclosures
Declaration of competing Interest
The authors declare that they have no identified competing financial interests or personal relationships that could have showed to influence the work reported in this paper.
Ethical approval
This work has been appreciated by the ethics committee. Given the retrospective nature of our study, which involved the analysis of existing data without directly engaging with patients or accessing identifiable personal health information, the need for patient consent was circumvented in accordance with ethical guidelines for research. The integrity of our research design aligns with established protocols that prioritize patient confidentiality and data protection, reflecting a commitment to uphold ethical standards while contributing valuable insights to the medical community.
References
- Torp, S. H., Solheim, O., & Skjulsvik, A. J. (2022). The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta neurochirurgica, 164(9), 2453–2464. [CrossRef]
- van Dijk, M. R., Steyerberg, E. W., & Habbema, J. D. (2008). A decision-analytic approach to define poor prognosis patients: a case study for non-seminomatous germ cell cancer patients. BMC medical informatics and decision making, 8, 1. [CrossRef]
- Wu, W., Klockow, J. L., Zhang, M., Lafortune, F., Chang, E., Jin, L., Wu, Y., & Daldrup-Link, H. E. (2021). Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacological research, 171, 105780. [CrossRef]
- Abedi, A. A., Grunnet, K., Christensen, I. J., Michaelsen, S. R., Muhic, A., Møller, S., Hasselbalch, B., Poulsen, H. S., & Urup, T. (2021). A Prognostic Model for Glioblastoma Patients Treated With Standard Therapy Based on a Prospective Cohort of Consecutive Non-Selected Patients From a Single Institution. Frontiers in oncology, 11, 597587. [CrossRef]
- Ng, W. W., Lam, S. M., Yan, W. W., & Shum, H. P. (2022). NLR, MLR, PLR and RDW to predict outcome and differentiate between viral and bacterial pneumonia in the intensive care unit. Scientific reports, 12(1), 15974. [CrossRef]
- Chen, W., Xin, S., & Xu, B. (2022). Value Research of NLR, PLR, and RDW in Prognostic Assessment of Patients with Colorectal Cancer. Journal of healthcare engineering, 2022, 7971415. [CrossRef]
- Wang, D. P., Kang, K., Lin, Q., & Hai, J. (2020). Prognostic Significance of Preoperative Systemic Cellular Inflammatory Markers in Gliomas: A Systematic Review and Meta-Analysis. Clinical and translational science, 13(1), 179–188. [CrossRef]
- Picarelli, H., Yamaki, V. N., Solla, D. J. F., Neville, I. S., Santos, A. G. D., Freitas, B. S. A. G., Diep, C., Paiva, W. S., Teixeira, M. J., & Figueiredo, E. G. (2022). The preoperative neutrophil-to-lymphocyte ratio predictive value for survival in patients with brain metastasis. O valor do índice neutrófilo-linfócito como preditor de sobrevida em pacientes com metástases cerebrais. Arquivos de neuro-psiquiatria, 80(9), 922–928. [CrossRef]
- Yang, C., Wen, H. B., Zhao, Y. H., Huang, W. H., Wang, Z. F., & Li, Z. Q. (2020). Systemic Inflammatory Indicators as Prognosticators in Glioblastoma Patients: A Comprehensive Meta-Analysis. Frontiers in neurology, 11, 580101. [CrossRef]
- Gupta, M. K., Yadav, G., Singh, Y., & Bhalekar, A. (2020). Correlation of the changing trends of red cell distribution width and serum lactate as a prognostic factor in sepsis and septic shock. Journal of anaesthesiology, clinical pharmacology, 36(4), 531–534. [CrossRef]
- Liu, X., & Wan, L. (2022). Molecular insights into the biochemical functions and signalling mechanisms of plant NLRs. Molecular plant pathology, 23(6), 772–780. [CrossRef]
- Bao, Y., Yang, M., Jin, C., Hou, S., Shi, B., Shi, J., & Lin, N. (2018). Preoperative Hematologic Inflammatory Markers as Prognostic Factors in Patients with Glioma. World neurosurgery, 119, e710–e716. [CrossRef]
- Bambury, R. M., Teo, M. Y., Power, D. G., Yusuf, A., Murray, S., Battley, J. E., Drake, C., O'Dea, P., Bermingham, N., Keohane, C., Grossman, S. A., Moylan, E. J., & O'Reilly, S. (2013). The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. Journal of neuro-oncology, 114(1), 149–154. [CrossRef]
- Han, S., Liu, Y., Li, Q., Li, Z., Hou, H., & Wu, A. (2015). Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC cancer, 15, 617. [CrossRef]
- Auezova, R., Ryskeldiev, N., Doskaliyev, A., Kuanyshev, Y., Zhetpisbaev, B., Aldiyarova, N., Ivanova, N., Akshulakov, S., & Auezova, L. (2016). Association of preoperative levels of selected blood inflammatory markers with prognosis in gliomas. OncoTargets and therapy, 9, 6111–6117. [CrossRef]
- Wang, D. P., Kang, K., Lin, Q., & Hai, J. (2020). Prognostic Significance of Preoperative Systemic Cellular Inflammatory Markers in Gliomas: A Systematic Review and Meta-Analysis. Clinical and translational science, 13(1), 179–188. [CrossRef]
- Nagula, P., Karumuri, S., Otikunta, A. N., & Yerrabandi, S. R. V. (2017). "Correlation of red blood cell distribution width with the severity of coronary artery disease-A single center study". Indian heart journal, 69(6), 757–761. [CrossRef]
- Zhao, C., Li, L. Q., Yang, F. D., Wei, R. L., Wang, M. K., Song, D. X., Guo, X. Y., Du, W., & Wei, X. T. (2020). A Hematological-Related Prognostic Scoring System for Patients With Newly Diagnosed Glioblastoma. Frontiers in oncology, 10, 591352. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).