1. Introduction
Multiple sclerosis is a chronic neuroinflammatory autoimmune disease in which autoreactive T cells and B cells infiltrate the blood-brain barrier and wrongly attack the myelin sheath, resulting in demyelination and axonal damage in the central nervous system (CNS). The condition typically manifests as a Relapsing-Remitting MS (RRMS), although the majority of patients advance to Secondary Progressive MS (SPMS) which is characterized by disease worsening without signs of remission (Lorscheider et al., 2016). About 10-15% of MS patients are initially diagnosed with Primary Progressive MS (PPMS), where the disease steadily worsens without any intervals of remission (Faissner et al., 2019, Oh & Bar-Or, 2022).
Currently, the therapeutic landscape of RRMS revolves around disease-modifying treatments (DMTs) that aim to reduce pathology related to relapses to achieve a favorable clinical course (Oh & Bar-Or, 2022). However, despite a long list of approved DMTs, patients still face the progression of disease and worsening of disabilities. Unfortunately, the DMT’s benefit in RRMS patients are typically not seen in patients with SPMS and PRMS (Faissner et al., 2019; Oh & Bar-Or, 2022). There is increasing evidence to suggest that the accumulation of axonal loss and neurodegeneration, in association with the local inflammation, leads to irreversible neurological disabilities associated with the clinical advancement in MS. Currently, drugs that can promote neural repair are absent (Absinta, Lassmann & Trapp, 2020; Klotz, Antel & Kuhlmann, 2023). Thus, there is a need to develop novel therapies that could provide solutions to the existing unmet needs of MS patients.
2. CNS is not Immune Privileged
Although the frequency of adaptive immune cells in healthy brain tissue is low, there is a steady state of lymphocyte migration across the blood-brain border, which is often incorrectly described as blood-brain barrier (Engelhardt & Ransohoff, 2012). In addition to low level migration into brain tissue, there is also a population of CNS-resident TRM cells that, in experimental models, can cause local tissue damage even in the absence of further T cell infiltration from the blood (Vincenti et al., 2022). However, the infiltration of pathogenic T cells from the blood into the CNS plays an important role in the progression of MS. Hence, reducing the migration of T cells across the blood-brain border has been a successful strategy in the management of patients with RRMS. The antibody natalizumab blocks the a4 integrin subunit of VCAM-1 and MDdCAM-1, and thus disrupts T cell adhesion to endothelial cells, resulting in the inhibition of T cell infiltration into brain and spinal cord (Engelhardt & Ransohoff, 2012). Although Natalizumab, and other disease modifying biologics that prevent T cell infiltration, have been effective in RRMS, these interventions are ineffective in SPMS and PPMS. Mechanistically, prevention of T cell infiltration can reduce inflammation and cell damage caused by autoreactive immune cells during the RRMS phase of disease, but it cannot reverse extensive demyelination and tissue damage caused by resident TRM cells. Despite the success of treatment with biologics, the current approaches do not enhance tissue repair or stimulate remyelination in patients with MS.
3. Treg Biology and its Role in MS
Treg cells are a subset of CD4+ T cells that are vital to prevent autoimmunity. FoxP3 is the master transcription factor for Treg cells, and inherited defects in the FoxP3 gene lead to the failure of Treg development which results in fatal autoimmunity in mice (Clark et al., 1999) and in the Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) syndrome in humans (Bennett et al., 2001). While the majority of Treg cells develop naturally in the thymus, some can be reprogrammed from conventional CD4+ T cells in peripheral tissues. The T cell receptor (TCR) of thymus-derived Treg cells have been selected for high affinity to self-peptides (Jordan et al., 2001), and these cells play an important role in maintaining systemic tolerance. By contrast, peripherally-induced pTreg are formed in response low affinity self-peptides and in response to commensal microbiota and food antigens, and these cells play a role in tissue specific hemostasis and tolerance (Raffin, Vo & Bluestone, 2019). In vitro Treg induction from conventional CD4+ T cells has been repeatedly demonstrated by TGF-β and IL-2 to mirror the pTreg lineage commitment pathway (Chen et al., 2003), yet these iTreg cells are prone to lose FoxP3 expression and thus their Treg functionality. This so-called Treg plasticity is to a large extent under the epigenetic control of the Treg -Specific Demethylated Region (TSDR). While the TSDR is completely demethylated in tTreg cells (Toker et al., 2013), it is partially methylated in pTreg and iTreg (Dhamne et al., 2013), which may favor loss of FoxP3 expression in these cells after in vitro restimulation (Floess et al., 2007; Toker et al., 2013). Similarly, activated effector T cells that can temporarily upregulate FoxP3 have a TSDR that is largely methylated (Baron et al., 2007).
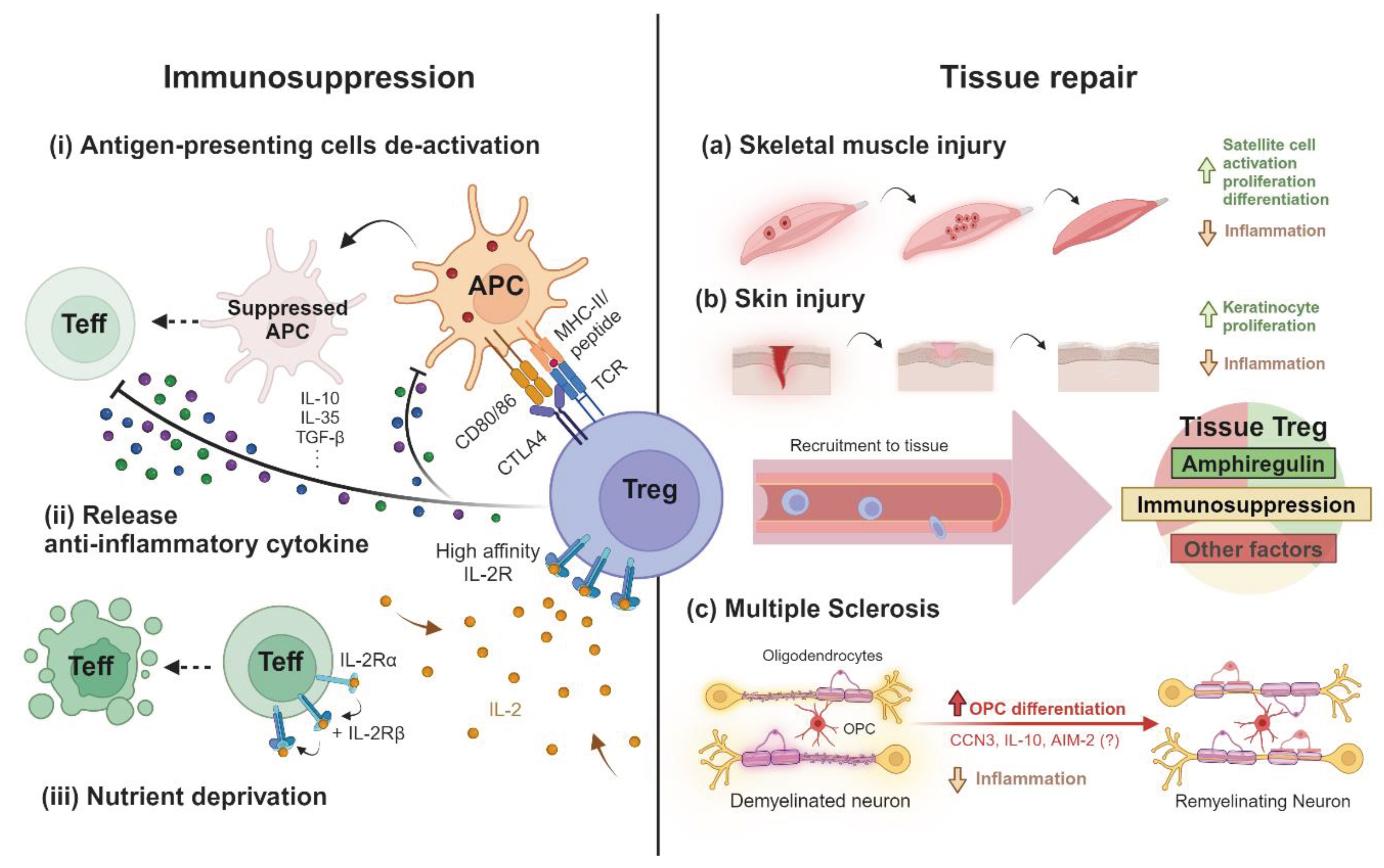
T
reg cells are equipped with multifaceted mechanisms to achieve their two major roles - immune suppression and tissue homeostasis (
Figure 1). Upon activation by cognate self-antigen and sensing a pro-inflammatory environment, T
reg cells are attracted to the site of inflammation, release anti-inflammatory cytokines and restrict further T cell activation by hindering antigen presentation. Additionally, local immunosuppression can be achieved by activated T
reg through depriving T-cell pro-survival nutrients and signals, such as IL-2, from the local environment (Duffy, Keating & Moalem-Taylor, 2019). The mechanisms by which T
reg can repair tissue damage are less well established. Tissue resident T
reg can have a direct regenerative ability, as demonstrated in skin and skeletal muscle (Li
et al., 2018). Amphiregulin, an EGFR ligand crucial to tissue repair, is dispensable for the suppressive function of T
reg cells (Arpaia
et al., 2015), while its production by T
reg was found to play a role during the chronic phase after stroke by accelerating neurological recovery (Ito
et al., 2019). Moreover, the observation that T
reg can promote oligodendrocyte proliferation and differentiation
in vitro, may explain how T
reg can enhance oligodendrocytes mediated repair of damaged CNS tissue
in vivo, as described by Crawford and colleagues (Crawford
et al., 2016). Mechanistic studies further revealed that CCN3, IL-10 and AIM2 expressed by T
reg play an important role in stimulating neuronal tissue repair (Dombrowski
et al., 2017; Naughton
et al., 2020; de la Vega Gallardo
et al., 2020; Chou, 2021).
Recent studies have uncovered some abnormalities of Treg in MS patients, yet it has been difficult to establish whether this is a cause or consequence of MS pathogenesis. Direct comparisons of the frequency of peripheral Treg and infiltrated Treg from cerebrospinal fluid between MS patients and healthy individuals had generated unconclusive results (Duffy et al., 2018). However, ex vivo phenotyping of peripheral Treg of MS patients revealed reduced FoxP3 expression and impaired suppressive activity (Viglietta et al., 2004; Haas et al., 2007). A study of untreated RRMS patients showed an increased number of Th1-like, IFN-γ-producing FoxP3+ T cells compared to healthy controls (Dominguez-Villar, Baecher-Allan, & Hafler, 2011). This functional plasticity could be due to the highly pro-inflammatory environment of CNS lesions, characterized by IFN-γ, TNF-α, IL-1β, IL-6 and IL-17 (Duffy et al., 2018). The use of animal models has provided more mechanistic understanding of the role of Treg cells in MS disease. Using the most widely used MS mouse model, Experimental Autoimmune Encephalomyelitis (EAE), it has been shown that Treg depletion or dysfunction can promote disease progression which can then be rescued by the adoptive transferr of functional Treg (Duffy et al., 2017; Stephens et al., 2009; Kim et al., 2018; Duffy, Keating & Moalem-Taylor, 2019). Considering the dual actions of Treg cells, it is likely that the beneficial effect of adoptive Treg therapy is the concerted result of immune suppressive activities and their ability to stimulate tissue repair.
4. Novel Treg Based Therapies for MS
To ultimately achieve an increased number of functional Treg in MS patient, a variety of non-cell-based and cell-based therapies are under development. Whilst the former aims at rescuing the patient’s own Treg repertoire, cell-based therapies replenish the patient with ex vivo expanded and potentially engineered Treg. The toolbox of non-cell based therapies comprises primarily three synergistic approaches, which are peptides for antigen-specific expansion, variants of IL-2 for preferential Treg survival, and small molecules for reinforcing functionality (Eggenhuizen, Ng & Ooi, 2020). Although these strategies have not yet entered the clinic, novel biologic designs aiming at combining all three keys of the toolbox are emerging. Particularly, a recent proof-of-concept study demonstrated the feasibility and efficacy of microparticles decorated with MHC-II molecules containing peptides of myelin oligodendrocyte glycoprotein (MOG) used as a Treg vaccine, which led to targeted expansion of MOG-specific Treg cells via the incorporation of a modified IL-2 molecule stimulating the IL-2 receptor of Treg, as well as simultaneous inhibition of effector T cell proliferation via the release of rapamycin (Rhodes et al., 2023). However, these types of strategies rely on the presence Treg with the vaccine-targeted specificity in MS patients, and they carry the risk of inadvertently expanding pathogenic effector T cells that have the same specificity. Thus, adoptive transfer of antigen-specific Treg represents an alternative therapeutic approach.
5. Antigen-Specific Treg Cell Therapy for MS and its Benefits
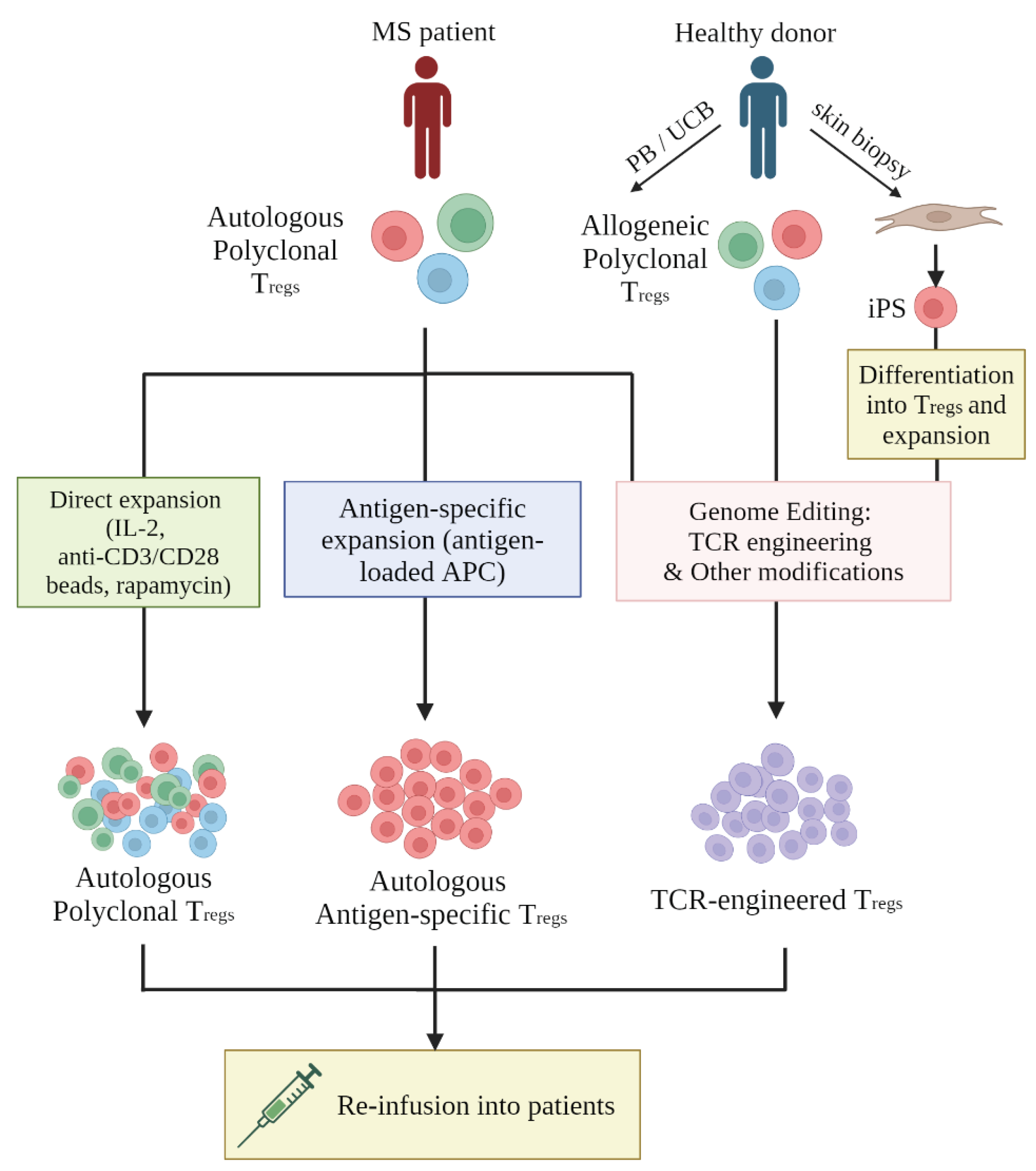
Therapeutic T
reg can be generated in three ways (
Figure 2), which include (i) polyclonal expansion or (ii) antigen-specific expansion of autologous T
reg obtained from patients, and (iii) genetic engineering of T
reg specificity (Raffin, Vo & Bluestone, 2019).
The observation that multiple autoantigens have been identified as the cause of MS, and more than one clone of autoreactive T cells can be found in patients (Bronge et al., 2022), raised the question whether adoptive transfer of polyclonal Treg is preferable over Treg cells with a single specificity. However, Treg cells with specificity for one specific antigen, for example myelin basic protein (MBP), can achieve local suppression of effector T cells present in the same microenvironment, even if these effector cells are specific for several other proteins of the neuronal myelin sheet. For example, experiments in the murine EAE model showed that Treg specific for MBP were able to suppress pathology caused by effector T cells specific for MOG (McGovern et al., 2022). The murine EAE model was also used to demonstrated that Treg with defined specificity are more potent than polyclonal Treg in controlling MS-like symptoms (Stephens et al., 2009; Kim et al., 2018), which is consistent with better disease control by antigen-specific Treg cells observed in other autoimmune animal models (Tang et al., 2004; Tarbell et al., 2004; Zhou et al., 2004; Fujio et al., 2006; Tsang et al., 2008). The improved efficacy of antigen-specific over polyclonal Treg is related to the enhanced antigen-driven migration of Treg into target tissues, and the continued TCR-stimulation at the site of pathology which is required for Treg to exert their optimal suppressive function (Eggenhuizen, Ng & Ooi, 2020).
6. TCR-Engineered Treg Versus CAR-Engineered Treg
A population of antigen-specific Treg can be produced by ex vivo stimulation with antigen, or by genetic Treg engineering. The frequency of naive Treg that are specific for a desired antigen is very low, and the expansion of these cells by in vitro stimulation is technically difficult and unreliable, compared to the robust process of genetic engineering of Treg specificity (Serra & Santamaria, 2019). Specificity engineering can be achieved by TCR and CAR (Chimeric Antigen Receptor) gene transfer. Both methods involve designing and transducing a transgenic receptor, yet each has its strengths and limitations. Whilst antigen recognition by transgenic TCR is restricted by the HLA genotype of patients, the antigen-binding domain of CAR is a synthetic single-chain variable antibody construct that allows antigen recognition irrespective of HLA. However, in contrast to TCRs, CARs can only recognize proteins present on the cell surface but not proteins in the cytosol or nucleolus, whereas HLA-presented peptides can be derived from any cellular protein for recognition by TCRs. Hence, the number of cellular proteins that can be targeted by TCRs is vastly greater than the proteins targetable by CARs. Currently, the preclinical development of CAR-Treg for MS is lagging behind the use of TCR-engineered Treg (Depil et al., 2020).
7. Source of Treg
As shown in
Figure 2, human T
reg cells can be isolated from Peripheral Blood (PB), Umbilical Cord Blood (UCB) or
in vitro differentiated from Induced Pluripotent Stem Cells (iPSC). Autologous T
reg from PB remain the most common source of T
reg used in patients (Eggenhuizen, Ng & Ooi, 2020). The results of a clinical trial in patients with type 1 diabetes produced safety data of autologous
ex vivo expanded polyclonal T
reg (Bluestone
et al., 2015), prompting the development of T
reg therapies for other autoimmune diseases, including MS. In addition, several studies with UCB-derived T
reg have demonstrated that they displayed superior phenotypic stability and greater TCR repertoire diversity compared to PB-derived T
reg (Seay
et al, 2017; Motwani
et al., 2020). As UCB biobanks expand, the use of HLA-matched allogeneic T
reg population for the treatment of GvHD, and possibility certain autoimmune conditions, becomes a realistic possibility (Motwani
et al., 2020).
iPSC is an attractive source for producing large numbers of ‘off the shelf’ Treg with defined specificity and phenotypic and functional features. However, Treg differentiation from iPSC is still at an early stage of development, and TCR+FoxP3 transduction combined with Notch-1 ligand stimulation was required to achieve Treg differentiation (Haque et al., 2012; Haque et al., 2016). The use of iPSC-derived Treg as ‘off the shelf’ medicines would require additional genetic engineering to avoid host-mediated recognition and rejection of the adoptively transferred cells (Raffin, Vo & Bluestone, 2019).
8. The Risks and Challenges of Antigen-Specific Treg Cell Therapy
The challenges of Treg-based adoptive cell therapy include common limitations of currently available techniques, and challenges specific to the treatment of MS. The first challenge is the yet inconclusive markers for the isolation of Treg cells with high purity, phenotypic stability, and continued functionality. At present the markers CD4+ CD25+ CD127-/low are frequently used to purify Treg. Unfortunately, staining for FoxP3, an intracellular protein, requires cell permeabilization and is therefore not suitable for the purification of life cells for adoptive therapy, although the vast majority of CD4+ CD25+ CD127-/low cells are FoxP3-positive and thus largely overcomes the inability of using FoxP3 for cell purification. However, Treg isolation is further complicated by the observation that FoxP3-positive Treg consist of distinct subsets with distinct functionality. For example, it was shown that tTreg cells are more resistant to phenotypic plasticity than pTreg cells (Miyao et al., 2012), yet any differentiating phenotypic markers remain to be elucidated in humans (Raffin, Vo & Bluestone, 2019). At present, we do not know which Treg subset is most suitable for dampening the neuroinflammation in the CNS in the context of MS.
9. Off-Target Toxicities
Retro and lentiviral gene transfer, which so far has been used in most human applications of CAR or TCR-T cell therapy, can cause genome toxicity by insertion mutagenesis, which has resulted in fatal side effects with gene engineered stem cells (Hacein-Bey-Abina et al., 2003), while similar side effects have not been seen with gene engineered T cells. Owing to the heterodimer nature of TCR alpha and beta chains, mis-paring between transgenic TCR and endogenous TCR is a risk associated with TCR but not CAR engineering. Experiments in murine T cell transfer models have shown that TCR mis-pairing can result in severe side effects in lymphodepleted recipient mice (Bendle et al., 2010). In patients, toxicities caused by TCR mis-pairing have not yet been described, although a large number of patients have been treated over the past years. Nevertheless, strategies for modifying transgenic TCR to avoid pairing with endogenous TCR, such cysteine-modification (Cohen et al., 2007), domain-swapping (Bethune et al., 2016), constant region modification (Sommermeyer & Uckert, 2010) and variable region modification (Thomas et al., 2019), all reduce the risk of mis-pairing. The CRISPR/Cas9 precision gene-editing tool changed the landscape, as it allows specific disruption of TRAC and TRBC loci, which encode the TCRα and TCRβ chain, respectively. As recently demonstrated, the disruption of endogenous TCR expression and insertion of therapeutic CAR or TCR constructs into the TRAC locus has improved the in vivo functionality of engineered T cell products in preclinical models (Eyquem et al., 2017; Roth et al., 2018).
10. Systemic Immunosuppression
Inevitably, any drugs with immunosuppressive activity afford a risk of systemic immunosuppression, whereupon the likelihood of opportunistic infection or malignancy will be elevated. Being a cell therapy, adoptively transferred Treg cells are expected to persist long-term and therefore require careful assessment of possible side effects.
Safety features developed for TCR/CAR engineering for cancer cell therapy can be translated into controlling unwanted systemic immunosuppression of Treg-based therapy. One strategy is to use a suicide gene to control the in vivo survival and/or functionality of the adoptively transferred Treg cells. Numerous ‘suicide switched’ have been designed and assessed for their efficacy and safety in preclinical studies and in patients, exemplified by truncated EGFR (Paszkiewicz et al., 2016) and inducible Caspase 9 (Mestermann et al., 2019). Another approach to regulating Treg function in vivo is the introduction of synthetic receptors. The requirement of IL-2 for Treg proliferation, survival and function makes IL-2 a promising manipulative pathway (Chinen et al., 2016). Recently, an elegant study has demonstrated that a genetically modified IL-2 receptor binds selectively to a synthetic IL-2 molecule that contains a ‘matched’ modification, while wild type IL-2 does not bind to the modified receptor. Hence, engineered T cells expressing the modified IL-2 receptor responded in vivo only to administered of synthetic IL-2 containing the ‘matched’ modification but not to endogenous IL-2 (Sockolosky et al., 2018). This technology could be used to engineer Treg whose survival and function in vivo is controlled by the time and dose of synthetic IL-2 administration.
11. Plasticity of Functions
The functional plasticity of Treg cells has been demonstrated repeatedly both in vitro and in vivo following adoptive transfer. Upon cues from inflammatory cytokines and IL-2 deficiency, antigen-specific Treg cells can adopt an alternative transcriptional program and become effector T cells that may contribute to tissue pathology (Sawant et al., 2014). For example, in the murine EAE model researchers identified a population of exFoxP3-Treg that had lost expression of FoxP3 which was associated with the production of the effector cytokine IFNg (Bailey-Bucktrout et al., 2013). However, genetic engineering can be used to reinforce the Treg identity by forcing FoxP3 expression. This can be achieved by delivering a genetic construct that drives FoxP3 expression from a constitutively active promotor (McGovern et al., 2022), or by inserting a constitutively active enhancer/promotor into genome proximal to the FoxP3 gene (Honaker et al., 2022). Both approaches lead to sustained level of FoxP3 expression which ‘locks’ Treg cells into a stable phenotype. Both approaches can also be utilized to force FoxP3 expression in conventional CD4+ T cells and convert them into Treg-like cells that display suppressive activity in vitro and in vivo (Wright et al., 2009).
12. Conclusions
With its multifaceted immunosuppressive functions and remyelinating capacity, Treg cells are undoubtedly an ideal cellular candidate for MS immunotherapy. Adoptive therapy with engineered Treg is now at the exciting stage where the first human clinical trials are ongoing, and will soon provide eagerly awaited feasibility, safety and efficacy data. Success of Treg therapy will depend on the selection of suitable target antigens that stimulate the suppressive activity and the tissue repair function of Treg at the local site of disease, without any systemic impairment of the immune system.
References
- Absinta, M.; Lassmann, H.; Trapp, B.D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Bucktrout, S.L.; Martinez-Llordella, M.; Zhou, X.; Anthony, B.; Rosenthal, W.; Luche, H.; Fehling, H.J.; Bluestone, J.A. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity 2013, 39, 949–962. [Google Scholar] [CrossRef]
- Baron, U.; Floess, S.; Wieczorek, G.; Baumann, K.; Grützkau, A.; Dong, J.; Thiel, A.; Boeld, T.J.; Hoffmann, P.; Edinger, M.; et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur. J. Immunol. 2007, 37, 2378–2389. [Google Scholar] [CrossRef]
- Bennett, C.L.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature Genetics 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Bendle, G.M.; Linnemann, C.; Hooijkaas, A.I.; Bies, L.; de Witte, M.A.; Jorritsma, A.; Kaiser, A.D.M.; Pouw, N.; Debets, R.; Kieback, E.; et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nature Medicine 2010, 16, 565–570. [Google Scholar] [CrossRef]
- Bethune, M.T.; Gee, M.H.; Bunse, M.; Lee, M.S.; Gschweng, E.H.; Pagadala, M.S.; Zhou, J.; Cheng, D.; Heath, J.R.; Kohn, D.B.; et al. Domain-swapped T cell receptors improve the safety of TCR gene therapy. eLife 2016, 5. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Science Translational Medicine 2015, 7, 315ra189. [Google Scholar] [CrossRef] [PubMed]
- Bronge, M.; Högelin, K.A.; Thomas, O.G.; Ruhrmann, S.; Carvalho-Queiroz, C.; Nilsson, O.B.; Kaiser, A.; Zeitelhofer, M.; Holmgren, E.; Linnerbauer, M.; et al. Identification of four novel T cell autoantigens and personal autoreactive profiles in multiple sclerosis. Sci. Adv. 2022, 8, eabn1823. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.-J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Chou, W.-C.; Guo, Z.; Guo, H.; Chen, L.; Zhang, G.; Liang, K.; Xie, L.; Tan, X.; Gibson, S.A.; Rampanelli, E.; et al. AIM2 in regulatory T cells restrains autoimmune diseases. Nature 2021, 591, 300–305. [Google Scholar] [CrossRef]
- Clark, L.B.; Appleby, M.W.; E Brunkow, M.; E Wilkinson, J.; Ziegler, S.F.; Ramsdell, F. Cellular and molecular characterization of the scurfy mouse mutant. . 1999, 162, 2546–54. [Google Scholar]
- Cohen, C.J.; Li, Y.F.; El-Gamil, M.; Robbins, P.F.; Rosenberg, S.A.; Morgan, R.A. Enhanced Antitumor Activity of T Cells Engineered to Express T-Cell Receptors with a Second Disulfide Bond. Cancer Res 2007, 67, 3898–3903. [Google Scholar] [CrossRef]
- Crawford, A.H.; Tripathi, R.B.; Foerster, S.; McKenzie, I.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D.; Franklin, R.J. Pre-Existing Mature Oligodendrocytes Do Not Contribute to Remyelination following Toxin-Induced Spinal Cord Demyelination. Am. J. Pathol. 2016, 186, 511–516. [Google Scholar] [CrossRef]
- Gallardo, N.d.l.V.; Penalva, R.; Dittmer, M.; Naughton, M.; Falconer, J.; Moffat, J.; de la Fuente, A.G.; Hombrebueno, J.R.; Lin, Z.; Perbal, B.; et al. Dynamic CCN3 expression in the murine CNS does not confer essential roles in myelination or remyelination. Proc. Natl. Acad. Sci. 2020, 117, 18018–18028. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nature Reviews Drug Discovery 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Dhamne, C.; Chung, Y.; Alousi, A.M.; Cooper, L.J.N.; Tran, D.Q. Peripheral and Thymic Foxp3+ Regulatory T Cells in Search of Origin, Distinction, and Function. Front. Immunol. 2013, 4, 253. [Google Scholar] [CrossRef]
- Dombrowski, Y.; O'Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Zhao, C.; Naughton, M.; et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017, 20, 674–680. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Baecher-Allan, C.M.; Hafler, D.A. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nature Medicine 2011, 17, 673–675. [Google Scholar] [CrossRef]
- Duffy, S.S.; Keating, B.A.; Perera, C.J.; Moalem-Taylor, G. The role of regulatory T cells in nervous system pathologies. Journal of Neuroscience Research 2017, 96, 951–968. [Google Scholar] [CrossRef]
- Duffy, S.S.; Keating, B.A.; Moalem-Taylor, G. Adoptive Transfer of Regulatory T Cells as a Promising Immunotherapy for the Treatment of Multiple Sclerosis. Front. Neurosci. 2019, 13, 1107. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg Enhancing Therapies to Treat Autoimmune Diseases. International Journal of Molecular Sciences 2020, 21, 7015. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; van der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.-D.; Bopp, T.; Schmitt, E.; et al. Epigenetic Control of the foxp3 Locus in Regulatory T Cells. PLOS Biol. 2007, 5, e38–e38. [Google Scholar] [CrossRef] [PubMed]
- Fujio, K.; Okamoto, A.; Araki, Y.; Shoda, H.; Tahara, H.; Tsuno, N.H.; Takahashi, K.; Kitamura, T.; Yamamoto, K. Gene Therapy of Arthritis with TCR Isolated from the Inflamed Paw. J. Immunol. 2006, 177, 8140–8147. [Google Scholar] [CrossRef]
- Haas, J.; Fritzsching, B.; Trübswetter, P.; Korporal, M.; Milkova, L.; Fritz, B.; Vobis, D.; Krammer, P.H.; Suri-Payer, E.; Wildemann, B. Prevalence of Newly Generated Naive Regulatory T Cells (Treg) Is Critical for Treg Suppressive Function and Determines Treg Dysfunction in Multiple Sclerosis. J. Immunol. 2007, 179, 1322–1330. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.-L.; Fraser, C.C.; Cavazzana-Calvo, M.; et al. A Serious Adverse Event after Successful Gene Therapy for X-Linked Severe Combined Immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef]
- Haque, M.; Song, J.; Fino, K.; Sandhu, P.; Song, X.; Lei, F.; Zheng, S.; Ni, B.; Fang, D.; Song, J. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Sci. Rep. 2016, 6, 20588. [Google Scholar] [CrossRef]
- Haque, R.; Lei, F.; Xiong, X.; Bian, Y.; Zhao, B.; Wu, Y.; Song, J. Programming of Regulatory T Cells from Pluripotent Stem Cells and Prevention of Autoimmunity. J. Immunol. 2012, 189, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Honaker, Y.; Hubbard, N.; Xiang, Y.; Fisher, L.; Hagin, D.; Sommer, K.; Song, Y.; Yang, S.J.; Lopez, C.; Tappen, T.; et al. Gene editing to induce FOXP3 expression in human CD4 + T cells leads to a stable regulatory phenotype and function. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.S.; Boesteanu, A.; Reed, A.J.; Petrone, A.L.; Holenbeck, A.E.; Lerman, M.A.; Naji, A.; Caton, A.J. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001, 2, 301–306. [Google Scholar] [CrossRef]
- Kim, Y.C.; Zhang, A.-H.; Yoon, J.; Culp, W.E.; Lees, J.R.; Wucherpfennig, K.W.; Scott, D.W. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. Journal of Autoimmunity 2018, 92, 77–86. [Google Scholar] [CrossRef]
- Klotz, L.; Antel, J.; Kuhlmann, T. Inflammation in multiple sclerosis: consequences for remyelination and disease progression. Nature Reviews Neurology 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, J.; Martino, M.M.; Lui, K.O. Regulatory T-Cells: Potential Regulator of Tissue Repair and Regeneration. Frontiers in Immunology 2018, 9. [Google Scholar] [CrossRef]
- Lorscheider, J.; Buzzard, K.; Jokubaitis, V.; Spelman, T.; Havrdova, E.; Horakova, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P.; et al. Defining secondary progressive multiple sclerosis. Brain 2016, 139, 2395–2405. [Google Scholar] [CrossRef]
- McGovern, J.; Holler, A.; Thomas, S.; Stauss, H.J. Forced Fox-P3 expression can improve the safety and antigen-specific function of engineered regulatory T cells. J. Autoimmun. 2022, 132, 102888. [Google Scholar] [CrossRef]
- Mestermann, K.; Giavridis, T.; Weber, J.; Rydzek, J.; Frenz, S.; Nerreter, T.; Mades, A.; Sadelain, M.; Einsele, H.; Hudecek, M. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Science Translational Medicine 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Miyao, T.; Floess, S.; Setoguchi, R.; Luche, H.; Fehling, H.; Waldmann, H.; Huehn, J.; Hori, S. Plasticity of Foxp3+ T Cells Reflects Promiscuous Foxp3 Expression in Conventional T Cells but Not Reprogramming of Regulatory T Cells. Immunity 2012, 36, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Motwani, K.; Peters, L.D.; Vliegen, W.H.; El-sayed, A.G.; Seay, H.R.; Lopez, M.C.; Baker, H.V.; Posgai, A.L.; Brusko, M.A.; Perry, D.J.; et al. Human Regulatory T Cells From Umbilical Cord Blood Display Increased Repertoire Diversity and Lineage Stability Relative to Adult Peripheral Blood. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Naughton, M.; Moffat, J.; Georgios Eleftheriadis Nira Young, A.; Falconer, J.; Hawkins, K.; Pearson, B.; Perbal, B.; Hogan, A.E.; et al. CCN3 is dynamically regulated by treatment and disease state in multiple sclerosis. Journal of Neuroinflammation 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Bar-Or, A. Emerging therapies to target CNS pathophysiology in multiple sclerosis. Nature Reviews Neurology 2022, 18, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, P.J.; Fräßle, S.P.; Srivastava, S.; Sommermeyer, D.; Hudecek, M.; Drexler, I.; Sadelain, M.; Liu, L.; Jensen, M.C.; Riddell, S.R.; et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Investig. 2016, 126, 4262–4272. [Google Scholar] [CrossRef] [PubMed]
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 2019, 20, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.R.; Tzeng, S.Y.; Iglesias, M.; Lee, D.; Storm, K.; Neshat, S.Y.; VanDyke, D.; Lowmaster, S.M.; Spangler, J.B.; Raimondi, G.; et al. Bioengineered particles expand myelin-specific regulatory T cells and reverse autoreactivity in a mouse model of multiple sclerosis. Sci. Adv. 2023, 9, eadd8693. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Puig-Saus, C.; Yu, R.; Shifrut, E.; Carnevale, J.; Li, P.J.; Hiatt, J.; Saco, J.; Krystofinski, P.; Li, H.; et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 2018, 559, 405–409. [Google Scholar] [CrossRef]
- Sawant, D.V.; Vignali, D.A.A. Once a Treg, always a Treg? Immunol. Rev. 2014, 259, 173–191. [Google Scholar] [CrossRef]
- Serada, S.; Fujimoto, M.; Mihara, M.; Koike, N.; Ohsugi, Y.; Nomura, S.; Yoshida, H.; Nishikawa, T.; Terabe, F.; Ohkawara, T.; et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. 2008, 105, 9041–9046. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Santamaria, P. Antigen-specific therapeutic approaches for autoimmunity. Nat. Biotechnol. 2019, 37, 238–251. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Trotta, E.; Parisi, G.; Picton, L.; Su, L.L.; Le, A.C.; Chhabra, A.; Silveria, S.L.; George, B.M.; King, I.C.; et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 2018, 359, 1037–1042. [Google Scholar] [CrossRef]
- Sommermeyer, D.; Uckert, W. Minimal Amino Acid Exchange in Human TCR Constant Regions Fosters Improved Function of TCR Gene-Modified T Cells. J. Immunol. 2010, 184, 6223–6231. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.A.; Malpass, K.H.; Anderton, S.M. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In Vitro–expanded Antigen-specific Regulatory T Cells Suppress Autoimmune Diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef]
- Tarbell, K.V.; Yamazaki, S.; Olson, K.; Toy, P.; Steinman, R.M. CD25+ CD4+ T Cells, Expanded with Dendritic Cells Presenting a Single Autoantigenic Peptide, Suppress Autoimmune Diabetes. J. Exp. Med. 2004, 199, 1467–1477. [Google Scholar] [CrossRef]
- Thomas, S.; Mohammed, F.; Reijmers, R.M.; Woolston, A.; Stauss, T.; Kennedy, A.; Stirling, D.; Holler, A.; Green, L.; Jones, D.; et al. Framework engineering to produce dominant T cell receptors with enhanced antigen-specific function. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Toker, A.; Engelbert, D.; Garg, G.; Polansky, J.K.; Floess, S.; Miyao, T.; Baron, U.; Düber, S.; Geffers, R.; Giehr, P.; et al. Active Demethylation of the Foxp3 Locus Leads to the Generation of Stable Regulatory T Cells within the Thymus. The Journal of Immunology 2013, 190, 3180–3188. [Google Scholar] [CrossRef]
- Tsang, J.Y.-S.; Tanriver, Y.; Jiang, S.; Xue, S.-A.; Ratnasothy, K.; Chen, D.; Stauss, H.J.; Bucy, R.P.; Lombardi, G.; Lechler, R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Investig. 2008, 118, 3619–3628. [Google Scholar] [CrossRef]
- Viglietta, V.; Baecher-Allan, C.; Weiner, H.L.; Hafler, D.A. Loss of Functional Suppression by CD4+CD25+Regulatory T Cells in Patients with Multiple Sclerosis. The Journal of Experimental Medicine 2004, 199, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, I.; Page, N.; Steinbach, K.; Yermanos, A.; Lemeille, S.; Nunez, N.; Kreutzfeldt, M.; Klimek, B.; Di Liberto, G.; Egervari, K.; et al. Tissue-resident memory CD8 + T cells cooperate with CD4 + T cells to drive compartmentalized immunopathology in the CNS. Science Translational Medicine 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cheng, X.-X.; Xue, J.Z.; Xue, S.-A. Emerging Strategies in TCR-Engineered T Cells. Front. Immunol. 2022, 13, 850358. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Notley, C.A.; Xue, S.-A.; Bendle, G.M.; Holler, A.; Schumacher, T.N.; Ehrenstein, M.R.; Stauss, H.J. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proceedings of the National Academy of Sciences of the United States of America 2009, 106, 19078–19083. [Google Scholar] [CrossRef]
- Zhou, P.; Borojevic, R.; Streutker, C.; Snider, D.; Liang, H.; Croitoru, K. Expression of Dual TCR on DO11.10 T Cells Allows for Ovalbumin-Induced Oral Tolerance to Prevent T Cell-Mediated Colitis Directed against Unrelated Enteric Bacterial Antigens. J. Immunol. 2004, 172, 1515–1523. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).