Submitted:
03 April 2024
Posted:
04 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Lonicera japonica Thunb. Extracts
2.2. Cell Line and Cell Culture
2.3. MMP Zymography
2.4. CCK-8 Assay
2.5. Quantitative Reverse Transcription–PCR (qRT-PCR)
2.6. Western blot Analysis
2.7. Cell migration Assay
2.8. Immunofluorescence Stain
2.9. Luciferase Assay
2.10. ELISA
2.11. Statistical Analysis
3. Results
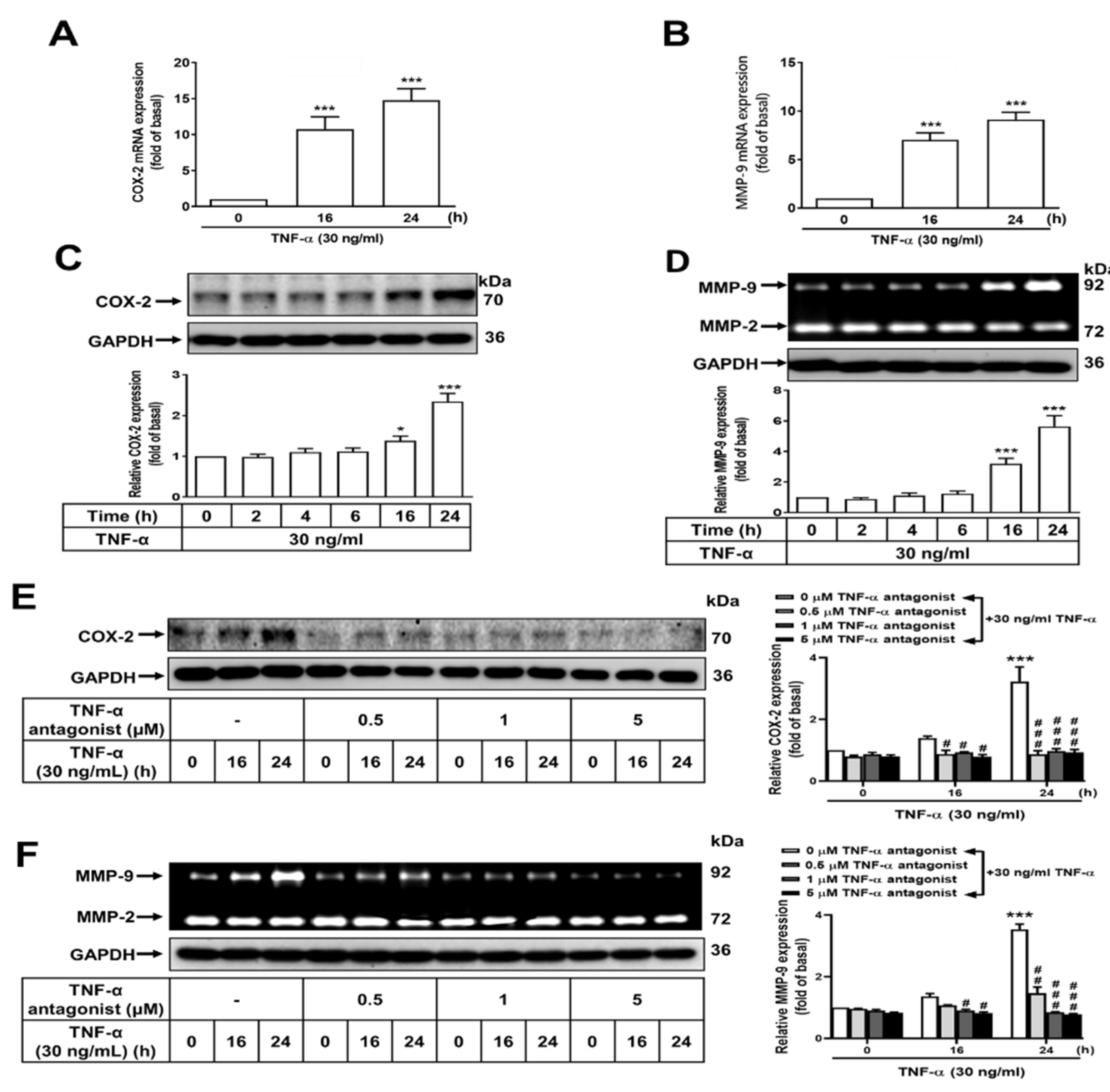
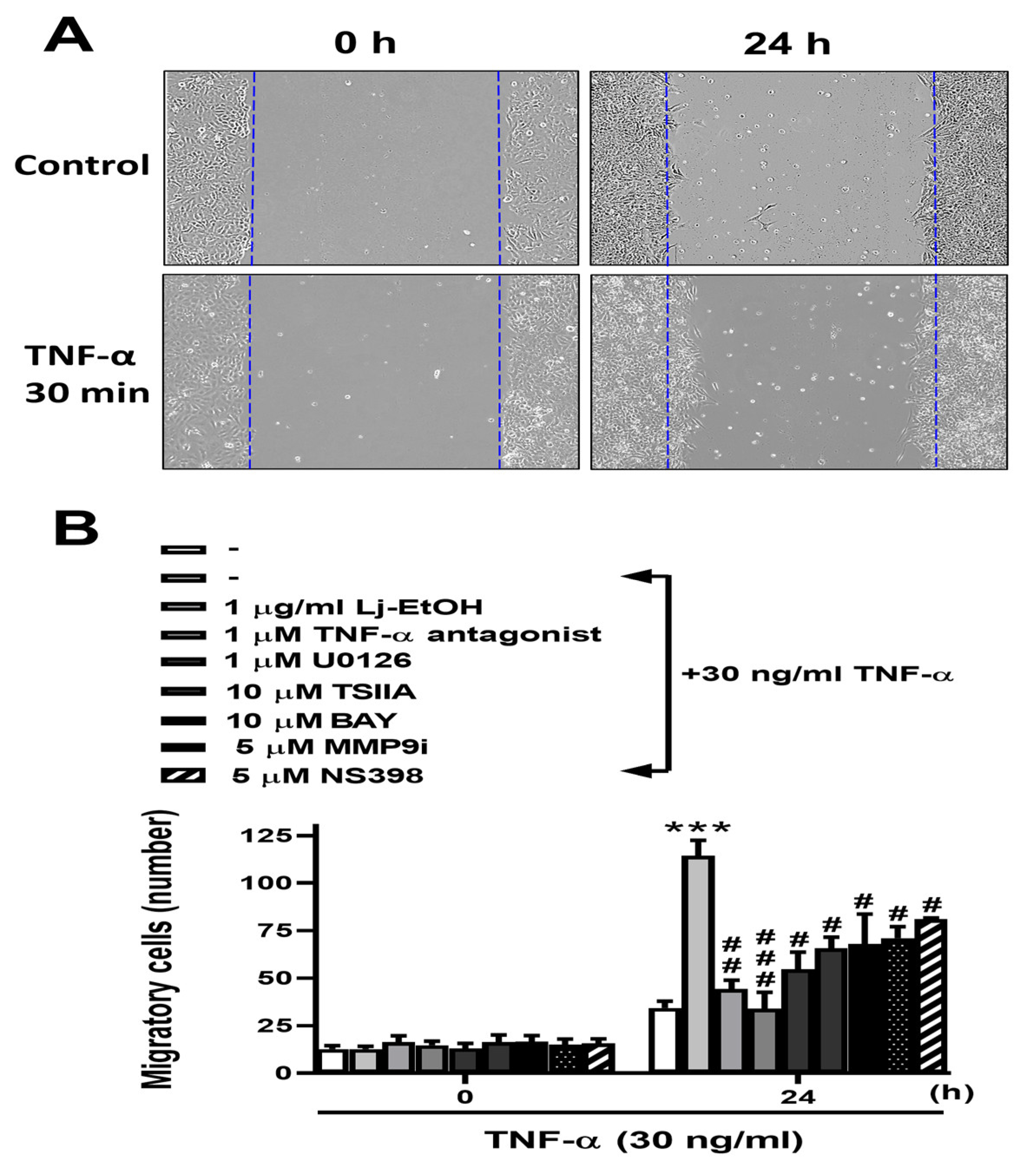
3.1. COX-2 and MMP-9 Expression upon TNF-α Treatment in GES-1 Cells
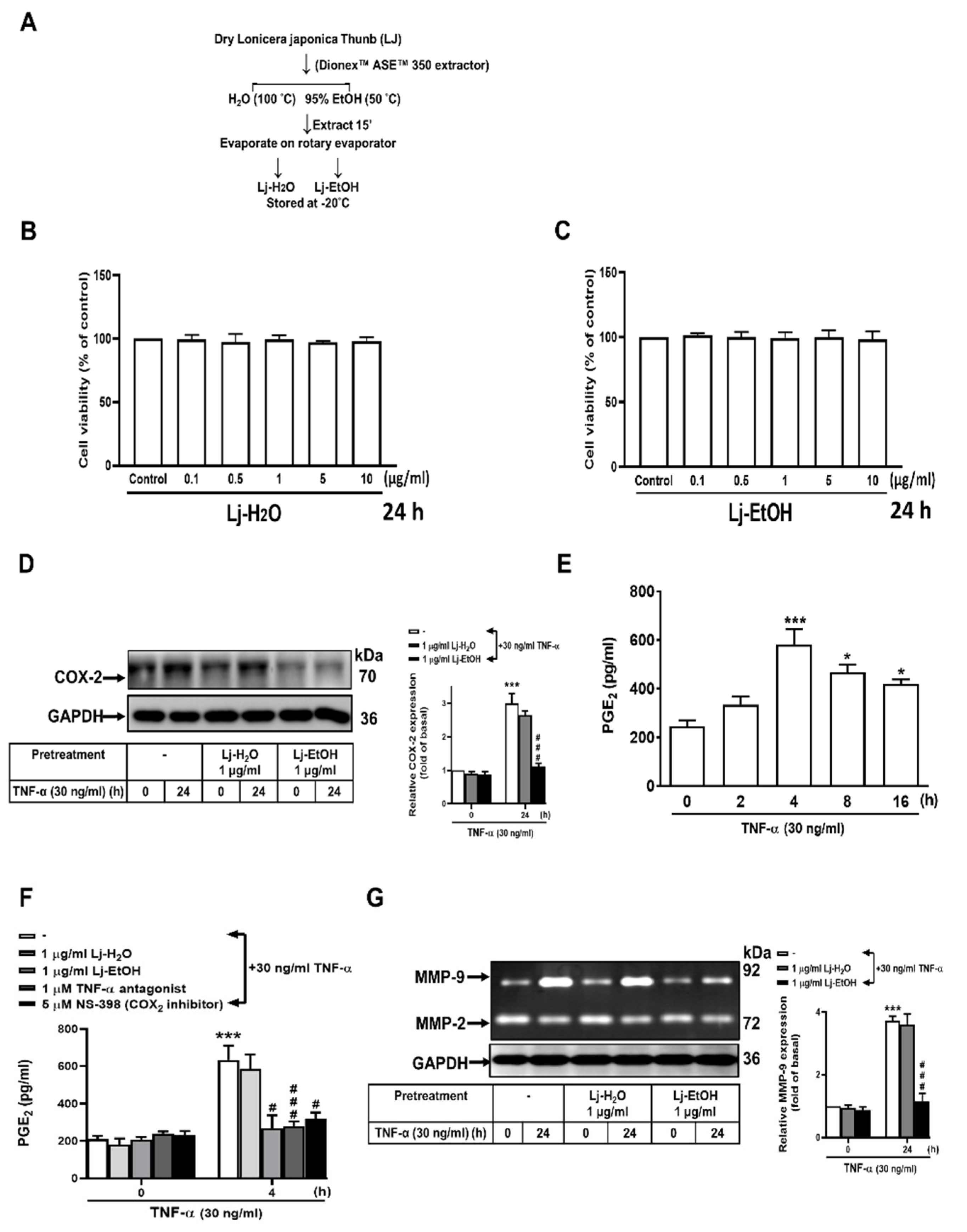
3.2. Effect of Lonicera japonica Thunb. Ethanol Extract on the Expressions of TNF-α-Induced COX-2, PGE2, and MMP-9 in GES-1 Cells
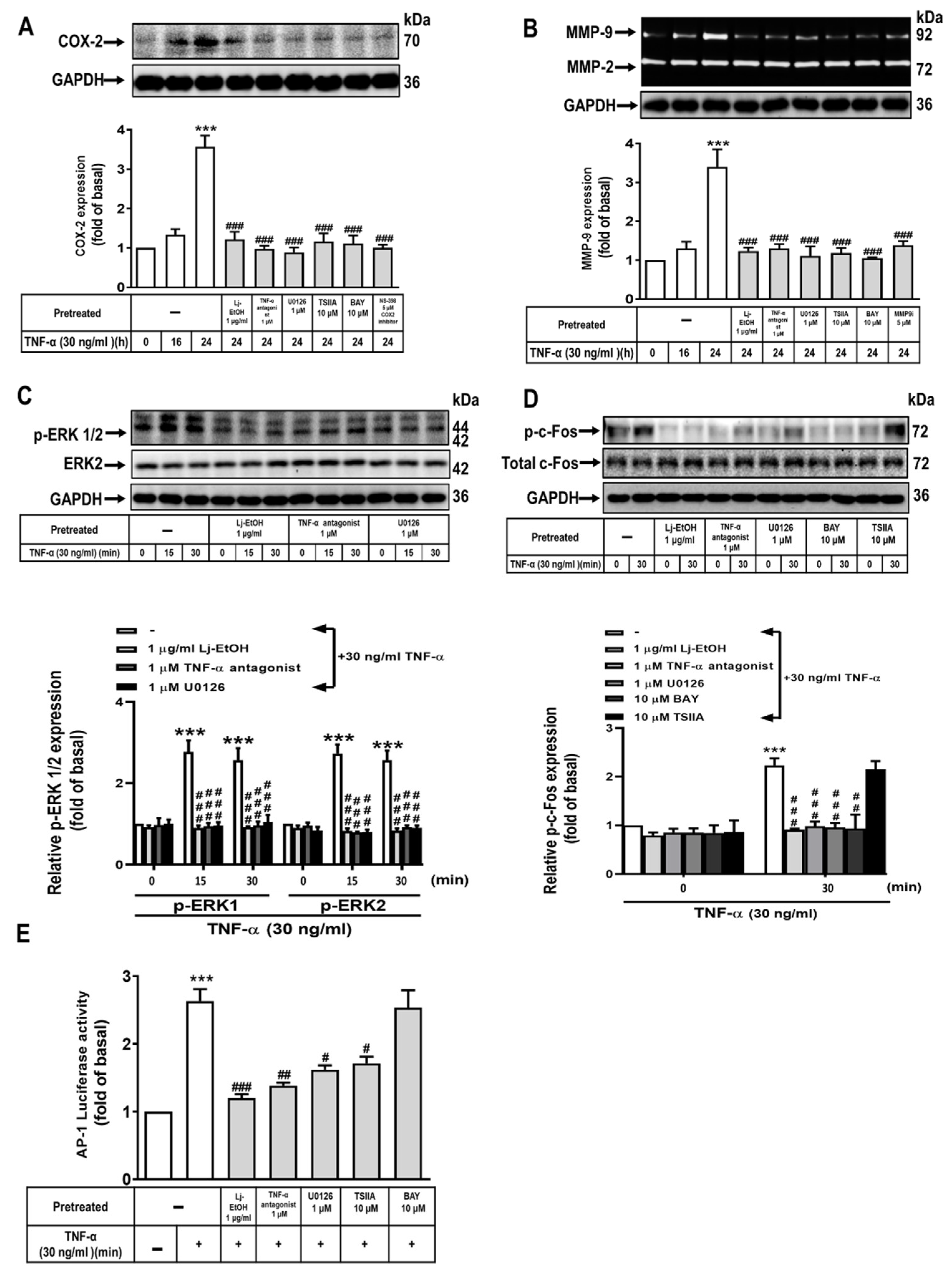
3.3. The Role of ERK1/2 and c-Fos in TNF-α-Stimulated COX-2 and MMP-9 Expression in Normal GES-1 Cells
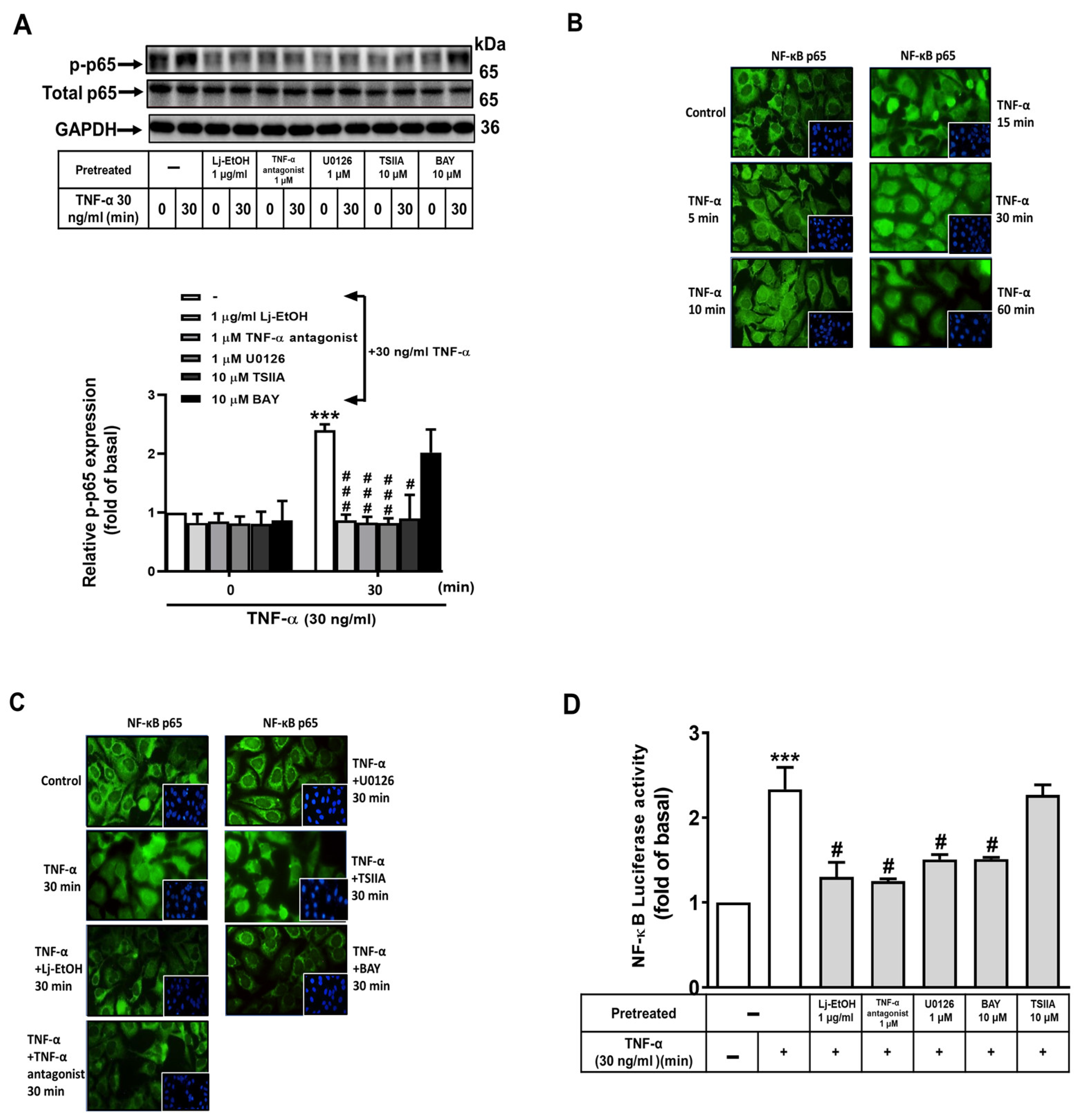
3.4. The Effect of NF-κB on TNF-α-Stimulated COX-2 and MMP-9 Expression in Normal GES-1 Cells
3.5. The Antimetastatic Activity of Ethanol Extract from Lonicera japonica Thunb. Was Evaluated In Vitro
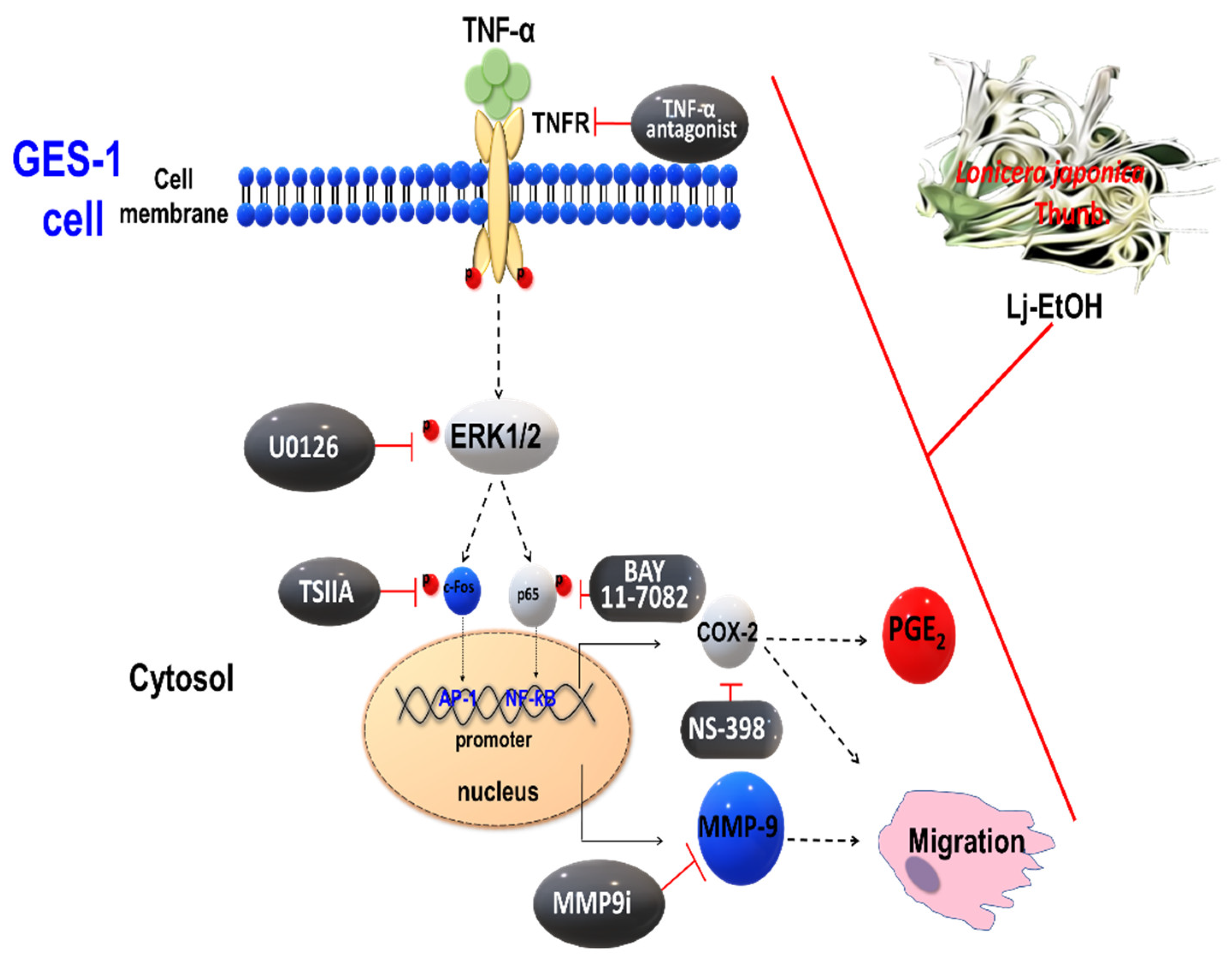
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol 2011, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Yang, Q.R.; Hao, J.B.; Li, W.D. [Research progress on pharmacological effects and their differences among the flowers, stems and leaves of Lonicera japonica]. Zhongguo Zhong Yao Za Zhi 2016, 41, 2422–2427. [Google Scholar] [CrossRef]
- Lanza, F.L. A review of gastric ulcer and gastroduodenal injury in normal volunteers receiving aspirin and other non-steroidal anti-inflammatory drugs. Scand J Gastroenterol Suppl 1989, 163, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.D.; Zheng, Z.D.; Liu, D.W.; Liu, Y.Y.; Shan, X.J. [Effects of Nasea on prevention of gastrointestinal side effects caused by chemotherapeutic drugs]. Zhonghua Yi Xue Za Zhi 2003, 83, 1180–1182. [Google Scholar] [PubMed]
- Zhang, X.Y.; Zhang, P.Y.; Aboul-Soud, M.A. From inflammation to gastric cancer: Role of Helicobacter pylori. Oncol Lett 2017, 13, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ernst, P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther 1999, 13 (Suppl 1), 13–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.W.; Nam, K.H.; Han, S.H.; Kim, J.W.; Ahn, S.H.; Park, D.J.; Lee, K.W.; Lee, H.S.; Kim, H.H. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer 2017, 20, 602–611. [Google Scholar] [CrossRef]
- Naito, Y.; Ito, M.; Watanabe, T.; Suzuki, H. Biomarkers in patients with gastric inflammation: a systematic review. Digestion 2005, 72, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Cui, X.; Sun, P.; Wang, X. Regulatory Roles of Tumor Necrosis Factor-alpha-Induced Protein 8 Like-Protein 2 in Inflammation, Immunity and Cancers: A Review. Cancer Manag Res 2020, 12, 12735–12746. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, F.; Beutler, B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996, 334, 1717–1725. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, H.; Cao, A.; Cao, L.; Hu, X. Cytokine TNF-alpha promotes invasion and metastasis of gastric cancer by down-regulating Pentraxin3. J Cancer 2020, 11, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Ishikawa, T.; Yoshida, G.J.; Naoi, K.; Maeda, Y.; Naka, K.; Ju, X.; Yamada, Y.; Minamoto, T.; Mukaida, N. , et al. TNF-alpha/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 2014, 33, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
- Canedo, P.; Duraes, C.; Pereira, F.; Regalo, G.; Lunet, N.; Barros, H.; Carneiro, F.; Seruca, R.; Rocha, J.; Machado, J.C. Tumor necrosis factor alpha extended haplotypes and risk of gastric carcinoma. Cancer Epidemiol Biomarkers Prev 2008, 17, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.Y.; Xia, B.; Deng, C.S.; Xia, X.Q.; Xie, M.; Crusius, J.B.; Pena, A.S. Association of tumor necrosis factor genetic polymorphism with chronic atrophic gastritis and gastric adenocarcinoma in Chinese Han population. World J Gastroenterol 2004, 10, 1256–1261. [Google Scholar] [CrossRef]
- Harris, P.R.; Mobley, H.L.; Perez-Perez, G.I.; Blaser, M.J.; Smith, P.D. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology 1996, 111, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.G.; Chua, A.; Fan, X.J.; Keeling, P.W. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol 1995, 48, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.D.; Ulrich, C.M. COX-2 and gastric cancer: More on inflammation and neoplasia. Gastroenterology 2006, 130, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Echizen, K.; Hirose, O.; Maeda, Y.; Oshima, M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci 2016, 107, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.M.; Lin, H.C.; Yu, M.C.; Lin, W.J.; Chu, M.Y.; Tsai, C.C.; Cheng, C.Y. Anticancer Effects of Helminthostachys zeylanica Ethyl acetate Extracts on Human Gastric Cancer Cells through Downregulation of the TNF-alpha-activated COX-2-cPLA2-PGE(2) Pathway. J Cancer 2021, 12, 7052–7068. [Google Scholar] [CrossRef]
- St-Pierre, Y.; Couillard, J.; Van Themsche, C. Regulation of MMP-9 gene expression for the development of novel molecular targets against cancer and inflammatory diseases. Expert Opin Ther Targets 2004, 8, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Bernegger, S.; Jarzab, M.; Wessler, S.; Posselt, G. Proteolytic Landscapes in Gastric Pathology and Cancerogenesis. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yu, M.C.; Cheng, L.C.; Chu, M.Y.; Huang, T.H.; Yeh, T.S.; Tsai, M.M. Quercetin exerts anti-inflammatory effects via inhibiting tumor necrosis factor-alpha-induced matrix metalloproteinase-9 expression in normal human gastric epithelial cells. World J Gastroenterol 2022, 28, 1139–1158. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Liu, P.S.; Tsai, M.M.; Chen, J.L.; Wang, S.J.; Hsieh, H.L. The COX-2-derived PGE(2) autocrine contributes to bradykinin-induced matrix metalloproteinase-9 expression and astrocytic migration via STAT3 signaling. Cell Commun Signal 2020, 18, 185. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chen, J.L.; Liu, P.S.; Tsai, M.M.; Wang, S.J.; Hsieh, H.L. Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-delta-dependent ROS signal. J Neuroinflammation 2020, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.M.; Chen, J.L.; Lee, T.H.; Liu, H.; Shanmugam, V.; Hsieh, H.L. Brain Protective Effect of Resveratrol via Ameliorating Interleukin-1beta-Induced MMP-9-Mediated Disruption of ZO-1 Arranged Integrity. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Tsai, M.M.; Tsai, C.Y.; Huang, C.G.; Ou, Y.H.; Hsieh, C.C.; Hsieh, H.L.; Wang, C.S.; Lin, K.H. DEK Is a Potential Biomarker Associated with Malignant Phenotype in Gastric Cancer Tissues and Plasma. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Wu, M.S.; Chiang, E.P.; Chen, Y.J.; Chen, C.J.; Chi, N.H.; Shih, Y.T.; Chen, G.H.; Lin, J.T. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res 2007, 13, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Khoi, P.N.; Yin, H.; Sah, D.K.; Kim, N.H.; Lian, S.; Jung, Y.D. Sulforaphane Suppresses the Nicotine-Induced Expression of the Matrix Metalloproteinase-9 via Inhibiting ROS-Mediated AP-1 and NF-kappaB Signaling in Human Gastric Cancer Cells. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, Z.; Li, Y.; Zheng, J.; Dong, G.; Wang, W.; Ji, G. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer 2011, 129, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Complement Altern Med 2012, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.W.; Park, D.; Kwon, K.S.; Lee, D.H.; Jang, M.J.; Park, S.K.; Kim, J.Y. BST-104, a Water Extract of Lonicera japonica, Has a Gastroprotective Effect via Antioxidant and Anti-Inflammatory Activities. J Med Food 2019, 22, 140–151. [Google Scholar] [CrossRef]
- Yu, J.Q.; Wang, Z.P.; Zhu, H.; Li, G.; Wang, X. [Chemical constituents of Lonicera japonica roots and their anti-inflammatory effects]. Yao Xue Xue Bao 2016, 51, 1110–1116. [Google Scholar] [PubMed]
- Kim, S.; Choi, M.G.; Lee, H.S.; Lee, S.K.; Kim, S.H.; Kim, W.W.; Hur, S.M.; Kim, J.H.; Choe, J.H.; Nam, S.J. , et al. Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules 2009, 14, 4300–4311. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Sie, S.S.; Kuan, T.C.; Lin, C.S. Identifying the regulative role of NF-kappaB binding sites within promoter region of human matrix metalloproteinase 9 (mmp-9) by TNF-alpha induction. Appl Biochem Biotechnol 2013, 169, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Wu, M.S.; Lin, J.T.; Chen, C.C. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol 2005, 175, 8242–8252. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, F.; Ferroni, P.; Palmirotta, R.; Portarena, I.; Formica, V.; Roselli, M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo 2007, 21, 147–161. [Google Scholar] [PubMed]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front Microbiol 2021, 12, 719877. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mu, C.; Kazybay, B.; Sun, Q.; Kutzhanova, A.; Nazarbek, G.; Xu, N.; Nurtay, L.; Wang, Q.; Amin, A. , et al. Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv 2021, 28, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Amin, A. Ketoconazole-induced testicular damage in rats reduced by Gentiana extract. Exp Toxicol Pathol 2008, 59, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Murali, C.; Mudgil, P.; Gan, C.Y.; Tarazi, H.; El-Awady, R.; Abdalla, Y.; Amin, A.; Maqsood, S. Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma. Sci Rep 2021, 11, 7062. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, Y.; Abdalla, A.; Hamza, A.A.; Amin, A. Safranal Prevents Liver Cancer Through Inhibiting Oxidative Stress and Alleviating Inflammation. Front Pharmacol 2021, 12, 777500. [Google Scholar] [CrossRef]
- Ma, F.; Chen, Y.; Li, J.; Qing, H.P.; Wang, J.D.; Zhang, Y.L.; Long, B.G.; Bai, Y. Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol 2010, 16, 5629–5634. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, C.; Wang, R.; Li, X.; Guo, S.; Zhang, W.; Liu, B. The metabolic profile elucidation of Lonicera japonica flos water extract and the metabolic characteristics evaluation of bioactive compounds in human gastrointestinal tract in vitro. J Pharm Biomed Anal 2022, 219, 114906. [Google Scholar] [CrossRef] [PubMed]
- Lambrou, G.I.; Hatziagapiou, K.; Vlahopoulos, S. Inflammation and tissue homeostasis: the NF-kappaB system in physiology and malignant progression. Mol Biol Rep 2020, 47, 4047–4063. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, C.; Huang, X.H.; Shen, C.L.; Li, L.; Zhang, W.; Yao, C.Z. Aspirin suppresses TNF-alpha-induced MMP-9 expression via NF-kappaB and MAPK signaling pathways in RAW264.7 cells. Exp Ther Med 2017, 14, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.C.; Chen, S.E.; Ho, T.H.; Peh, H.C.; Liu, W.B.; Tiantong, A.; Nagahata, H.; Chang, C.J. Involvement of TNF-alpha and MAPK pathway in the intramammary MMP-9 release via degranulation of cow neutrophils during acute mammary gland involution. Vet Immunol Immunopathol 2012, 147, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.J.; Kim, J.R.; Jin, U.H.; Choi, H.S.; Chang, Y.C.; Lee, Y.C.; Kim, S.H.; Lee, I.S.; Moon, T.C.; Chang, H.W. , et al. Deoxypodophyllotoxin, flavolignan, from Anthriscus sylvestris Hoffm. inhibits migration and MMP-9 via MAPK pathways in TNF-alpha-induced HASMC. Vascul Pharmacol 2009, 51, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.R.; Park, B.Y. Involvement of MAPK/NF-kappaB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed Pharmacother 2017, 95, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Sharma, S.; Mittal, A.; Mittal, A. Recent Progress in Selective COX-2 Inhibitor Formulations and Therapeutic Applications-. A Patent Review (2012-2022). Mini Rev Med Chem 2023, 10. [CrossRef]
- Ma, J.; Zhang, Y.; Sugai, T.; Kubota, T.; Keino, H.; El-Salhy, M.; Ozaki, M.; Umezawa, K. Inhibition of Cellular and Animal Inflammatory Disease Models by NF-kappaB Inhibitor DHMEQ. Cells 2021, 10. [Google Scholar] [CrossRef]
- Qi, X.; Yu, Y.; Wang, X.; Xu, J.; Wang, X.; Feng, Z.; Zhou, Y.; Xiao, H.; Sun, L. Structural characterization and anti-oxidation activity evaluation of pectin from Lonicera japonica Thunb. Front Nutr 2022, 9, 998462. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Williams, C.M. A pilot dose-response study of the acute effects of haskap berry extract (Lonicera caerulea L.) on cognition, mood, and blood pressure in older adults. Eur J Nutr 2019, 58, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, N.; Noh, G.T.; Lee, J.Y.; Lee, D.H. The Efficacy and Safety of GCWB104 (Flos Lonicera Extract) in Functional Dyspepsia: A Single-Center, Randomized, Double-Blind, Placebo-Controlled Study. Gut Liver 2020, 14, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.M. Honeysuckle contact dermatitis. Cutis 1993, 51, 424. [Google Scholar] [PubMed]
- Tae, J.; Han, S.W.; Yoo, J.Y.; Kim, J.A.; Kang, O.H.; Baek, O.S.; Lim, J.P.; Kim, D.K.; Kim, Y.H.; Bae, K.H. , et al. Anti-inflammatory effect of Lonicera japonica in proteinase-activated receptor 2-mediated paw edema. Clin Chim Acta 2003, 330, 165–171. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




