1. Introduction
Canine leishmaniasis caused by the protist
Leishmania infantum (CanL) is a metazoonosis transmitted by phlebotomine sandfly vectors, endemic in the regions surrounding the Mediterranean basin [
1,
2,
3]. In domestic dogs, CanL is a serious disorder that can be lethal if not adequately treated. Lesions are associated with a generalized inflammatory reaction and include peripheral lymphadenopathy and hepatosplenomegaly. In addition, dermatological signs include alopecia, dermatitis, onychogryphosis and epistaxis. Renal disease is severe and may progress from nephrotic syndrome to chronic renal failure [
4,
5,
6,
7,
8].
In addition to domestic dogs, numerous cases of wild mammals carrying
Leishmania antibodies have been reported, including carnivores of different genera, bats, rodents, and lagomorphs, among others [
9,
10,
11,
12,
13,
14,
15]. The importance of CanL should not only be addressed from the point of view of animal welfare, but as a real public health problem, since humans are also a definitive host of
Leishmania and can undergo the potentially fatal visceral form of the disease. The relevance of this fact is reflected in the outbreak detected in Madrid (Spain) during the period 2009 to 2011, in which hares and rabbits were reported as necessary reservoirs of leishmaniasis cases suffered by humans with an estimated incidence of 55.7 cases/100, 000 inhabitants [
16,
17]. Therefore, it is of the utmost importance to prevent and eventually to eradicate the disease in all possible scenarios where it may arise, which implies close collaboration between authorities and health professionals.
Due to their phylogenetic similarity with dogs, and their coexistence in environments where vectors reproduce, it is to be expected that wolves are potential reservoirs of
Leishmania. To this, must be added their social habits and their proximity to rural areas where dogs still play important guarding, herding or hunting roles. Since the first description of leishmaniasis caused by
L. infantum (MON-1 strain) in wolves in Croatia in 2008 [
18], studies on the existence of this disease have been sporadic, unsystematic and in most cases performed on remains of animals killed in hunts, traffic accidents or poisoned. In these animal remains, typical clinical signs of CanL found in dogs have been described, such as chronic dermatitis, hair loss, scabs, skin erosions, ulcerations, cachexia, orchitis, lymphadenopathy, hepatomegaly and splenomegaly. In previous studies carried out to determine the endemicity of CanL in wolves in northwestern Spain, high seropositivity values were obtained both in animal remains in the province of Asturias [
19,
20] and in animals under semi-captivity during the period 2018-2022 in the province of Zamora [
21]. In the latter case, the incidence of CanL was around 50%, using a PCR-based diagnostic system. This percentage reached up to 57% when adding the positives found in other wolves captured alive, sampled and subsequently released.
To date, apart from wild animals exhibited in zoos and circuses, no case of CanL in wolves that has been diagnosed and treated in semi-captivity conditions has been reported [
22]. Due to the exceptional facilities offered by the Iberian Wolf Environmental Education Centre "Félix Rodríguez de la Fuente", which houses three wolf packs under sanitary control by specialized personnel, vector-borne diseases can be detected in conditions close to nature, which allows for their treatment. The endemicity of the vectors transmitting leishmaniasis in the region [
23,
24] opens the possibility of the occurrence of cases whose seroprevalence has already been reported in wolves, but which so far have not been reported as clinical cases. In the present report we present the case of a wolf diagnosed with canine leishmaniasis, which was treated in time, normalized its seroprevalence and healed the wounds related to the infection.
2. Case Report
The case report is about a female Iberian wolf (Canis lupus signatus) born on June 8th, 2012, known as Dakota, housed in semi-captivity conditions at the Iberian Wolf Center "Félix Rodríguez de la Fuente" (IWC), in Robledo de Sanabria, Zamora (Spain). The IWC is an Environmental Education Centre with the aim of informing the public about the biology, conservation, relations with society, tourism, ecology, etc. of the Iberian wolf. IWC is geographically located in the "Sierra de La Culebra" at the northwest of Castilla y León (Spain) 41º59'35''N, 6º34'25''W, at 965 m altitude, a natural area of Community Interest (
https://centrodellobo.es/). The natural environment of the wolf specimens living in the Centre is a pine forest (Pinus sylvestris) of 20 Ha in surface divided into six enclosures intercommunicated with heathland and some chestnut, birch and ash trees. The difference in height between the lowest and the highest part of the enclosures is 50 m.
The IWC maintains a stable population of fourteen wolves housed in semi captivity conditions, distributed in three established wolf packs. The wolves are housed in three different enclosures, interacting with each other as well as with members of the management team. Routine management of the specimens housed at the IWC includes daily inspection during the administration of the food ration, where any signs of disease, injury or behavioral disturbances are reported to specialist veterinary staff. In addition, an annual examination of the specimens is carried out under sedation. In this case, a comprehensive protocol including blood sampling for routine analysis and systematic inspection of each animal is carried out. Finally, all specimens are subjected to a strict schedule of external parasite removal with one pipette of Frontline
® (containing 6.7 mg/kg body weight fipronil and 6 mg/kg body weight (S)-metopreno), internal deworming with one tablet per 10 kg body weight of DrontaPLUS SABOR
® (containing 15 mg febantel, 14.4 mg pyrantel embonate and 5 mg praziquantel, per kg body weight) and a vaccination protocol (
Table 1).
Since 2013, the annual protocol shown in
Table 1 includes the administration of an anti-rabies vaccine (Rabisyva Vp-13
®), and the vaccine Canigen DHPPiL
® to prevent parvovirosis, hepatitis, parainfluenza, distemper and leptospirosis). It is worth mentioning that there is a specific protocol for wolverines in which the tetravalent vaccine is applied at two months of age and a new revaccination one month later. This protocol may be subject to variations depending on the physiological situation of the animals, the existence of specific health alarms or regulatory constraints. Due to the increasing number of CanL cases recorded by veterinary practitioners in dogs in the region (unpublished results), the protocol was modified to include vaccination against leishmania in the IWC wolves with CaniLeish
® (Virbac), the only commercial vaccine available at that time. This vaccine was administered to all wolves housed at the IWC from May 15th, 2015. Then, vaccine boosters were administered annually according to the manufacturer's recommendation for dogs. Prior to vaccination, all wolves were sedated for blood sampling with medetomidine (40 µg/kg body weight; Dorbene Vet
®) and ketamine (5 mg/kg body weight; Anesketin
®), both administered by intramuscular injection. No anti-leishmanial antibody titres were found in any wolf by an indirect commercial ELISA method; LSH Ab Test Kit SensPERT
® (Vetall Laboratories).
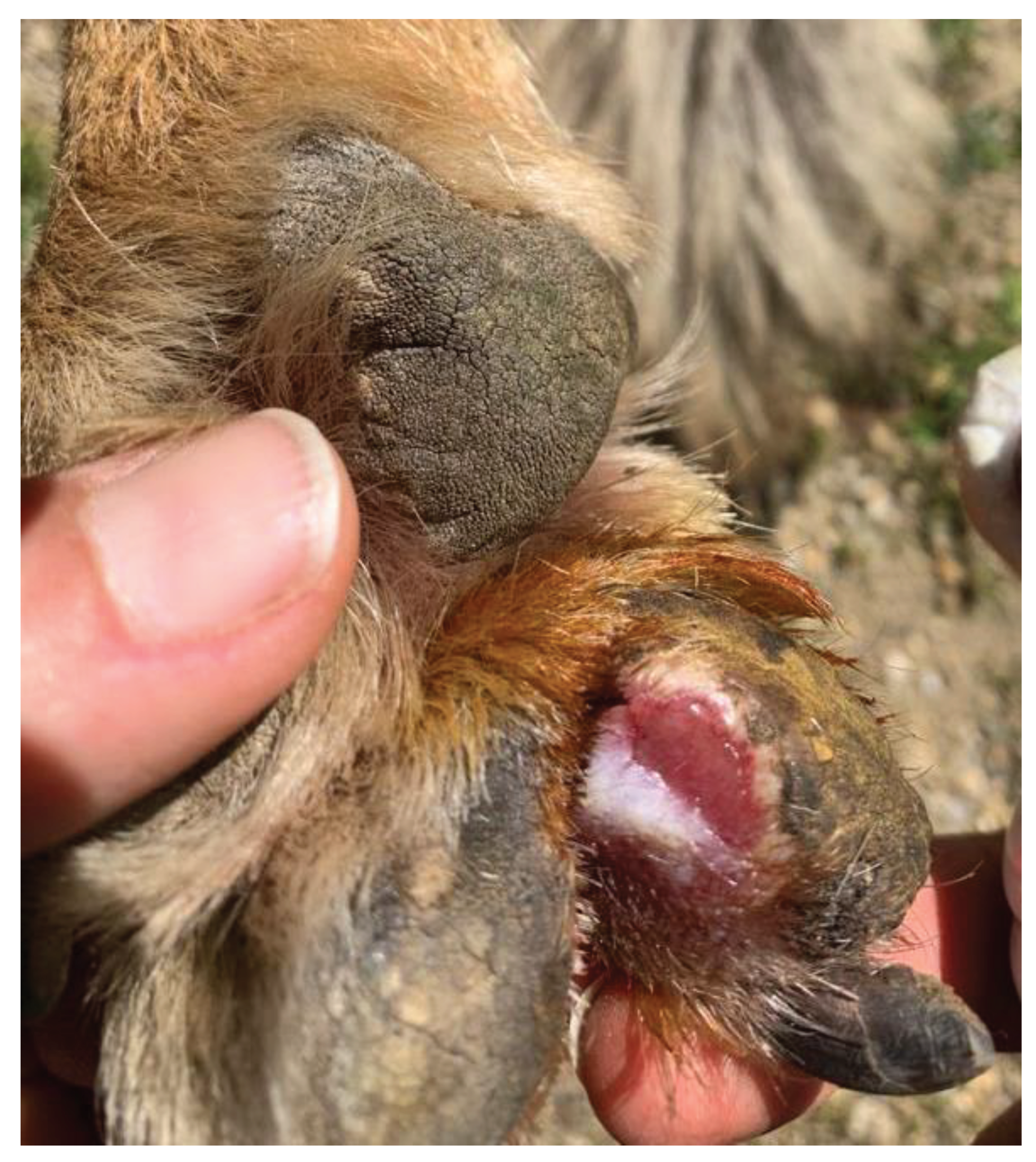
In February 2021, a case of lameness was observed in a 34.5 kg female wolf specimen that did not disappear with time. Since this animal was one of the most socialized wolves (means, easy to handle by keepers without sedation), a quick examination of the paws was performed, and an interdigital ulcerative wound was detected on the right forepaw (
Figure 1). Since the wound was detected during the pre-estrus period of the wolves, it was decided to isolate the animal and initiate a curative treatment based on daily washings, iodine cures and a single subcutaneous injection of 8 mg/kg body weight of cevofecin (Convenia
®) to prevent further infections.
3.1. Diagnosis and Initial Treatment of Leishmaniasis
On March 23, 2021, as no improvement was observed in the interdigital ulcerative wound after the treatment applied one month before, the specimen was subjected to a more exhaustive examination with the objective of obtaining an accurate diagnosis and establishing an appropriate treatment. For this purpose, the wolf was sedated following the protocol described above, and blood samples were collected for further analysis. No other notable clinical signs were observed at that time. Blood samples were collected by venoclysis from the cephalic vein, one tube with EDTA as anticoagulant for blood counts and another tube without anticoagulant for biochemical analysis and measurement of anti-Leishmania antibodies titres (
Table 2). As the ulcerative wounds on the right forepaw were compatible with early skin lesions of CanL, in addition to the analysis by indirect ELISA, a rapid test for Leishmania diagnosis (Speed Leish ELISA kit K
®) was included in the analysis (
Table 2). Speed Leish K
® detects circulating antibodies against L. infantum kinesins in blood, serum or plasma samples. Blood samples were sent to a veterinary laboratory for clinical analysis (Laboratorio de Análisis Clínicos Sagunto, Valencia, Spain). Also hair samples, an oral mucosal swab and two swabs from both ears were also collected for in-house PCR analysis to amplify a 131 base pair fragment of the kinetoplast minicircle of L. infantum. The results of this PCR were shown in a previous study describing the prevalence of L. infantum in wolves sampled in Northwestern Spain [
21].
The results of the indirect ELISA and the rapid test confirmed the presence of anti-leishmania anibodies (
Table 2). The antileishmanial circulating antibody response was initiated with a 1/640 titre by indirect ELISA, (negative control >1/80 dilution, according to the manufacturer). Also, the analysis of buccal and ear swab samples by PCR showed amplification of the expected kDNA band specific for L. infantum [
21].
After analysis of biochemical parameters and serum protein electrophoresis, the main findings were hypergammaglobulinemia, 18.3 g/L (range 3.3-10.6) and a significant increase in serum glutamic pyruvic transaminase (GPT), 652 U/L (range 10-65), which could indicate liver damage. The other biochemical parameters were within reference values, showing that there was no possible renal damage from infection. On the other hand, blood counts were within reference values for dogs (data not shown).
After cleaning and healing the paw wound as described previosuly, one external pipette of Frontline® was applied to prevent, as far as possible, reinfections of L. infantum transmitted by Phlebotomine flies. In addition, antibiotherapy with marbofloxacin (2mg/kg/day; Marbocyl®) was administered orally for 6 days to protect against opportunistic bacterial infections.
Immediately after confirming the diagnosis of CanL, a conventional antileishmanial treatment was applied by administering a combination of glucantime and allopurinol (gold standard of CanL treatments in dogs) [
3,
25]. The doses administered were 10 mg/kg body weight/day of allopurinol (Zyloric
®) (in two administrations every 12 hours) orally during 12 weeks, and 100 mg/kg body weight/day of meglumine antimoniate (Glucantime
®), by subcutaneous route. Although the “guidelines” indicate that the treatment with meglumine antimoniate should be 30 days [
25], in this case we have to stop after 6 days due to the non-tolerance of the administration and the aggressiveness that the animal began to show, presumably due to pain at the application site. Therefore, it was decided to change the parenteral treatment for a more friendly oral treatment based on miltefosine (2 mg/kg/day; Milteforan
®), for 30 days. The oral administration of any drug was carried out with the help of "treats" (cheese or sausages). At the end of this protocol, the interdigital wound had evolved favorably and healed completely
3.2. Evolution of the Clinical Case of Leishmaniasis
On September 24, 2021, a second sampling was performed to determine blood biochemical parameters (
Table 2). It was found that circulating antileishmania antibodies remained elevated, with a 1/340 titre. Mild hypergammaglobulinemia, 9.8 g/L (range 3.3-10.6 g/L), but 15, 6 % (range 6.0-13.0%) and significantly elevated serum GPT values, 102 U/L (range 10-65 U/L) were also detected. According to these values, it was decided to continue the treatment with allopurinol until the end of 2021, with the same regimen established in March 2021. Therefore, at that moment, the administration of allopurinol lasted 36 weeks.
This trend towards improvement was verified in the third and fourth examinations performed on May 6, 2022 and July 6, 2023, respectively. The antileishmania antibody titer decreased below positivity (PI > 1/80), although gamma globulinemia and GPT values remained slightly higher than the reference values for the species.
4. Discussion
The progress of Dakota's health status was optimal, responding to the established treatment. No clinical symptoms were observed after healing. Dakota's follow-up shows the typical alterations in the biochemical and haematological analyses detected in CanL-seropositive dogs. Hyperproteinemia [
26,
27], due to a hyperglobulinemia that corresponds to the synthesis of antibodies against Leishmania, stands out. Hyper-gammaglobulinaemia is a very characteristic alteration of CanL. An increase in the alpha-2-globulin fraction [
27], beta and gamma globulins [
26], alteration of the albumin/globulin ratio [
28] and a decrease in serum albumin (hypoalbuminaemia) have been described in dogs [
29]. In addition, monitoring serum electrophoretic values enables the evolution of the treated animal to be evaluated from the time of diagnosis and to assess how they progressively normalise because of the treatment administered. The albumin/globulin ratio was slightly lower than the reference values, which is consistent with a decrease in albumin concentration, possibly due to incipient damage to the renal glomerulus because of immunocomplex deposition. In severe cases, glomerulonephritis with albuminaemia may lead to nephrotic syndrome with severe hyperproteinemia, which was not observed in our case.
Another aspect that can be found in blood tests of dogs with CanL is an elevated hepatic GPT [
29]. Increased GPT values can be interpreted as a consequence of severe liver damage, as the GPT value in the blood is directly proportional to the amount of damaged tissue. In the second analysis, 6 months after treatment, GPT levels were still above baseline values, although they had decreased significantly compared to the first measurement (102/65). In the third analysis, it was observed that the GPT continued to decrease to 93 U/L, until normalising below 65 U/L of baseline norms in the fourth sampling, indicating reversal of liver damage due to the treatment.
As Dakota's right forepaw wound and biochemical parameters were compatible with CanL, we performed an immunological test to detect antibodies against
Leishmania and determine that the wolf was indeed infected. In the first test, positivity was established at a serum dilution of 1/640, which confirmed the reaction of the animal's antibodies with the parasite antigens. Although the number of drugs to tackle leishmaniasis is not large [
30], the glucantime/alopurinol combination is considered the gold standard for CanL in dogs [
3,
25]. As described in the previous section drug combination was initiated - with the glucantime having to be replaced by miltefosine, as described below. By the second test, the antileishmania antibody titre had dropped to 1/320, by the third (1/160) and by the fourth (>1/80) the levels considered negative by the kit manufacturer were reached.
It should be noted that, although the wolves at the IWC were vaccinated with Canileish® - the only vaccine available against CanL in 2015 - several seropositive wolves had been observed in addition to the clinical case in Dakota. CaniLeish
® is an injectable formulation composed of excreted-secreted proteins from
L.
infantum (LiESP) supplemented with saponin-derived adjuvants such as QA-21. To our knowledge, CaniLeish
® had not been applied systematically in a wolf community under semi-captive conditions. In addition to promoting an increase in humoral IgG2 levels, CaniLeish
® induces a strong shift towards a Th1 immune response in dogs [
31,
32]. After primovaccination, blood levels of IG2 and IFN-γ persisted for approximately one full year, and this may be the reason why all recorded cases were mild [
31]. Since 2022, the IWC vaccination protocol has been using the Letifend
® vaccine, which has significant published results in terms of protection against CanL and is better tolerated by the animals [
33,
34].
When we initiated anti-leishmanial treatment in Dakota, immediately after serological diagnosis, we used the gold-standard dog combination glucantime and allopurinol, which we assumed would also work in wolves. However, the wolf developed aggressive behaviour towards the caretaker in charge of injecting glucantime subcutaneously (possibly because of pain at the injection site) and had to be replaced by miltefosine. Miltefosine, a second-choice drug, is the only drug that can be administered orally against leishmania, either as monotherapy or in combination with other leishmaniostatic drugs and is replacing antimony derivatives as the first-choice treatment for CanL in southern European countries [
1]. Several clinical trials show that miltefosine as monotherapy or in combination has a good therapeutic profile [
35], contributing to an improvement of most clinical symptoms in dogs up to two years after drug withdrawal [
36].
The allopurinol administration was maintained for the entire duration of Dakota's treatment. In dogs, allopurinol is administered orally in the treatment of CanL, either alone as monotherapy or in combination with other drugs [
3]. Allopurinol has leishmaniostatic effects, reducing the parasite load and thus preventing future relapses [
25], with little toxicity to the host. The duration of treatment is variable and is adjusted to the positive evolution of clinical signs. In general, good results are obtained after prolonged treatment (4-10 weeks) with the above regimen, but relapses usually occur 2-4 weeks after drug withdrawal [
37,
38]. In combination with other first-line drugs, glucantime or miltefosine, seropositivity is persistently reduced and signs of CanL can be effectively reversed without relapses.
6. Conclusions
In the current clinical report of leishmaniasis in a semi-captive housed female wolf, the only adverse observed effect found was the presence of an interdigital ulceration on the right forepaw. Blood tests showed hypergammaglobulinaemia and increased blood GPT, but no signs of renal damage were observed. The diagnosis was obtained by an ELISA blood test for antileishmanial antibodies confirmed by a previously reported in-house PCR analysis of mouth and ears swabs. The animal responded rapidly to treatment with a glucantime/alopurinol regimen. Intramuscular glucantime had to be replaced after few administrations by the oral administration of miltefosine due to the animal's aggressiveness caused by the pain associated at the site of the injection. In our case, a good clinical response to treatment was detected after starting the anti-Leishmania treatment, healing the wound and restoring all biochemical parameters to normal values.
Author Contributions
“Conceptualization, M.M.V. and R.B.F.; methodology, M.M.V., J.M.G.; validation, M.M.V. and R.B.F.; formal analysis, M.M.V.; investigation, J.M.G.; resources, J.P.A. and T.Y.M.; data curation, M.M.V. and R.B.F.; writing—original draft preparation, R.B.F.; writing—review and editing, R.B.F. and M.M.V.; supervision, M.M.V. and R.B.F. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
Ethic Committee Name: Comité de Ética de Experimentación Animal (Centro Temático del Lobo Ibérico). Approval Code: nº Registro Núcleo Zoológico Nacional 008184; approval date: 10-06-2013.
Acknowledgments
The authors acknowledge the Iberian Wolf Center "Félix Rodríguez de la Fuente" (IWC), in Robledo de Sanabria, Zamora (Spain), Center Management, Veterinary care and management team of the Iberian Wolf Center, and the Junta of Castilla y León.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gramiccia, M.; Gradoni, L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol 2005, 35, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Mattin, M.J.; Solano-Gallego, L.; Dhollander, S.; Afonso, A.; Brodbelt, D.C. The frequency and distribution of canine leishmaniasis diagnosed by veterinary practitioners in Europe. Vet J 2014, 200, 410–419. [Google Scholar] [CrossRef]
- Reguera, R.M.; Morán, M.; Pérez-Pertejo, Y.; García-Estrada, C.; Balaña-Fouce, R. Current status on prevention and treatment of canine leishmaniasis. Vet Parasitol 2016, 227, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K. Update on the diagnosis and management of Leishmania spp infections in dogs in the United States. Top Companion Anim Med 2010, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Aroch, I. Canine leishmaniasis: a diagnostic and clinical challenge. Vet J 2008, 175, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Saridomichelakis, M.N. An update on the diagnosis and treatment of canine leishmaniasis caused by Leishmania infantum (syn. L. chagasi). Vet J 2014, 202, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Cardoso, L.; Pennisi, M.G.; Oliva, G.; Baneth, G. Canine leishmaniosis – new concepts and insights on an expanding zoonosis: part two. Trends Parasitol 2008, 24, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Scalone, A.; Foglia-Manzillo, V.; Gramiccia, M.; Pagano, A.; Di Muccio, T.; Gradoni, L. Incidence and time course of Leishmania infantum infections examined by parasitological, serologic, and nested-PCR techniques in a cohort of naive dogs exposed to three consecutive transmission seasons. J Clin Microbiol 2006, 44, 1318–1322. [Google Scholar] [CrossRef]
- Azami-Conesa, I.; Gómez-Muñoz, M.T.; Martínez-Díaz, R.A. A systematic review (1990–2021) of wild animals infected with zoonotic Leishmania. Microorganisms 2021, 9, 1101. [Google Scholar] [CrossRef]
- Franco, A.O.; Davies, C.R.; Mylne, A.; Dedet, J.P.; Gállego, M.; Ballart, C.; Gramiccia, M.; Gradoni, L.; Molina, R.; Gálvez, R.; Morillas-Márquez, F.; Barón-López, S.; Pires, C.A.; Afonso, M.O.; Ready, P.D.; Cox, J. Predicting the distribution of canine leishmaniasis in western Europe based on environmental variables. Parasitology 2011, 138, 1878–1891. [Google Scholar] [CrossRef]
- Muñoz-Madrid, R.; Belinchón-Lorenzo, S.; Iniesta, V.; Fernández-Cotrina, J.; Parejo, J.C.; Serrano, F.; Monroy, I.; Baz, V.; Gómez-Luque, A.; Gómez-Nieto, L.C. First detection of Leishmania infantum kinetoplast DNA in hair of wild mammals: application of qPCR method to determine potential parasite reservoirs. Acta Trop 2013, 128, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, M.; Arzamani, K.; Zarei, Z.; Akhoundi, B.; Hajjaran, H.; Raeghi, S.; Heidari, Z.; Motavalli-Haghi, S.M.; Elikaee, S.; Mousazadeh-Mojarrad, A.; Kakoei, Z. Canine visceral leishmaniasis in wild canines (fox, jackal, and wolf) in northeastern Iran using parasitological, serological, and molecular methods. J Arthropod Borne Dis 2016, 10, 538–545. [Google Scholar]
- Helhazar, M.; Leitão, J.; Duarte, A.; Tavares, L.; da Fonseca, I.P. Natural infection of synathropic rodent species Mus musculus and Rattus norvegicus by Leishmania infantum in Sesimbra and Sintra--Portugal. Parasit Vectors 2013, 6, 88. [Google Scholar] [CrossRef]
- Peters, W.; Bryceson, A.; Evans, D.A.; Neal, R.A.; Kaye, P.; Blackwell, J.; Killick-Kendrick, R.; Liew, F.Y. Leishmania infecting man and wild animals in Saudi Arabia. 8. The influence of prior infection with Leishmania arabica on challenge with L. major in man. Trans R Soc Trop Med Hyg 1990, 84, 681–689. [Google Scholar] [CrossRef]
- Gradoni, L.; Pozio, E.; Gramiccia, M.; Maroli, M.; Bettini, S. Leishmaniasis in Tuscany (Italy): VII. Studies on the role of the black rat, Rattus rattus, in the epidemiology of visceral leishmaniasis. Trans R Soc Trop Med Hyg 1983, 77, 427–431. [Google Scholar] [CrossRef]
- Molina, R.; Jiménez, M.I.; Cruz, I.; Iriso, A.; Martín-Martín, I.; Sevillano, O.; Melero, S.; Bernal, J. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet Parasitol 2012, 190, 268–271. [Google Scholar] [CrossRef]
- Jiménez, M.; González, E.; Martín-Martín, I.; Hernández, S.; Molina, R. Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania infantum in the focus of Madrid, Spain? Vet Parasitol 2014, 202, 296–300. [Google Scholar] [CrossRef]
- Beck, A.; Beck, R.; Kusak, J.; Gudan, A.; Martinkovic, F.; Artukovic, B.; Hohsteter, M.; Huber, D.; Marinculic, A.; Grabarevic, Z. A case of visceral leishmaniosis in a gray wolf (Canis lupus) from Croatia. J Wildl Dis 2008, 44, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Zanet, S.; Espí, A.; Pegoraro de Macedo, M.R.; Gortázar, C.; Ferroglio, E. Leishmania in wolves in northern Spain: A spreading zoonosis evidenced by wildlife sanitary surveillance. Vet Parasitol 2018, 255, 26–31. [Google Scholar] [CrossRef]
- Sobrino, R.; Ferroglio, E.; Oleaga, A.; Romano, A.; Millan, J.; Revilla, M.; Arnal, M.C.; Trisciuoglio, A.; Gortázar, C. Characterization of widespread canine leishmaniasis among wild carnivores from Spain. Vet Parasitol 2008, 155, 198–203. [Google Scholar] [CrossRef]
- Merino Goyenechea, J.; Castilla Gómez de Agüero, V.; Palacios Alberti, J.; Balaña Fouce, R.; Martínez Valladares, M. Occurrence of leishmaniasis in Iberian wolves in northwestern Spain. Microorganisms 2023, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- Sastre, N.; Francino, O.; Ramírez, O.; Enseñat, C.; Sánchez, A.; Altet, L. Detection of Leishmania infantum in captive wolves from Southwestern Europe. Vet Parasitol 2008, 158, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol 1990, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Barriga, D.; Ruiz-Arrondo, I.; Estrada Peña, R.; Lucientes, J.; Delacour-Estrella, S. Phlebotomine sand flies (Diptera, Psychodidae) from Spain: an updated checklist and extended distributions. ZooKeys 2022, 1106, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Corso, R.; Galiero, G.; Cerrone, A.; Muzj, P.; Gravino, A.E. Long-term follow-up of dogs with leishmaniosis treated with meglumine antimoniate plus allopurinol versus miltefosine plus allopurinol. Parasit Vectors 2015, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniasis due to Leishmania infantum/chagasi. Vet Pathol 2014, 51, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Ravicini, S.; Rossi, G.; Roura, X. Serum concentrations of the derivatives of reactive oxygen metabolites (d-ROMs) in dogs with leishmaniosis. Vet J 2010, 186, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, P.; Oliva, G.; Luna, R.D.; Gradoni, L.; Ambrosio, R.; Cortese, L.; Scalone, A.; Persechino, A. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec 1997, 141, 539–543. [Google Scholar] [CrossRef]
- Petanides, T.A.; Koutinas, A.F.; Mylonakis, M.E.; Day, M.J.; Saridomichelakis, M.N.; Leontides, L.S.; Mischke, R.; Diniz, P.; Breitschwerdt, E.B.; Kritsepi, M.; Garipidou, V.A.; Koutinas, C.K.; Lekkas, S. Factors associated with the occurrence of epistaxis in natural canine leishmaniasis (Leishmania infantum). J Vet Intern Med 2008, 22, 866–872. [Google Scholar] [CrossRef]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and promising novel drug candidates against visceral leishmaniasis. Pure Appl Chem 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Moreno, J.; Vouldoukis, I.; Schreiber, P.; Martin, V.; McGahie, D.; Gueguen, S.; Cuisinier, A.M. Primary vaccination with the LiESP/QA-21 vaccine (CaniLeish) produces a cell-mediated immune response which is still present 1 year later. Vet Immunol Immunopathol 2014, 158, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Velez, R.; Domenech, E.; Rodríguez-Cortés, A.; Barrios, D.; Tebar, S.; Fernández-Arévalo, A.; Aguilar, R.; Dobaño, C.; Alberola, J.; Cairó, J.; Gállego, M. Evaluation of canine leishmaniosis vaccine CaniLeish® under field conditions in native dog populations from an endemic Mediterranean area-A randomized controlled trial. Acta Trop 2020, 205, 105387. [Google Scholar] [CrossRef] [PubMed]
- Cacheiro-Llaguno, C.; Parody, N.; Renshaw-Calderón, A.; Osuna, C.; Alonso, C.; Carnés, J. Vaccination with LetiFend® reduces circulating immune complexes in dogs experimentally infected with L. infantum. Vaccine 2020, 38, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Pistocchini, E.; Ritondo, B.L.; Roncada, P.; Palma, E. di Cave, D.; Mattei, M.; Britti, D. Immunoprophylaxis pharmacotherapy against canine leishmaniosis: A systematic review and meta-analysis on the efficacy of vaccines approved in European Union. Vaccine 2020, 38, 6695–6703. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanga, M.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug–membrane interactions. Antimicrob Agents Chemother 2005, 49, 2677–2686. [Google Scholar] [CrossRef]
- Woerly, V.; Maynard, L.; Sanquer, A.; Eun, H.M. Clinical efficacy and tolerance of miltefosine in the treatment of canine leishmaniasis. Parasitol Res 2009, 105, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy--challenges and opportunities. Clin Microbiol Infect 2011, 17, 1478–1483. [Google Scholar] [CrossRef]
- Ouellette, M.; Drummelsmith, J.; Papadopoulou, B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat 2004, 7, 257–266. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).