Submitted:
03 April 2024
Posted:
04 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Group. Inclusion and Exclusion Criteria
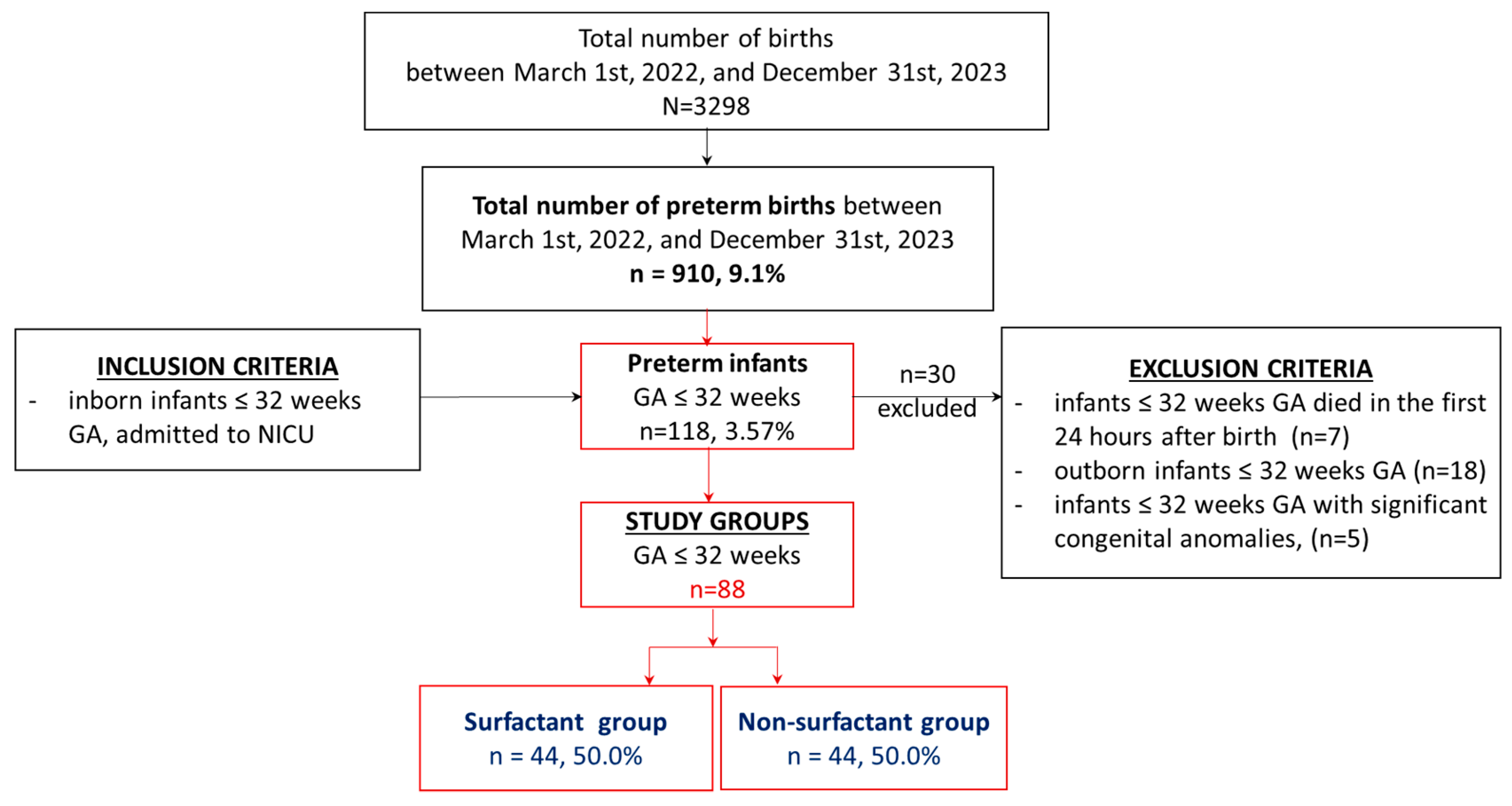
2.2. Study Design
2.3. Demographic and Clinical Data
2.4. Diagnosis and Surfactant Treatment of RDS
2.5. Evaluation of DA at 24 Hours after Birth
2.5.1. Clinical Evaluation of DA
2.5.2. Echocardiographic Assessment of DA
2.5.3. Head Ultrasound
2.5.4. Abdominal Ultrasounds
2.5.5. Cerebral and Mesenteric Oxygenation Monitoring
2.5.6. Blood Sample Collection (Laboratory Data)
2.6. Statistical Analysis
3. Results
Evaluation of the Clinical Parameters of the DUCTUS arteriosus
Predictive Analysis: The Influence of Surfactant Administration on Clinical Parameters of the Ductus Arteriosus
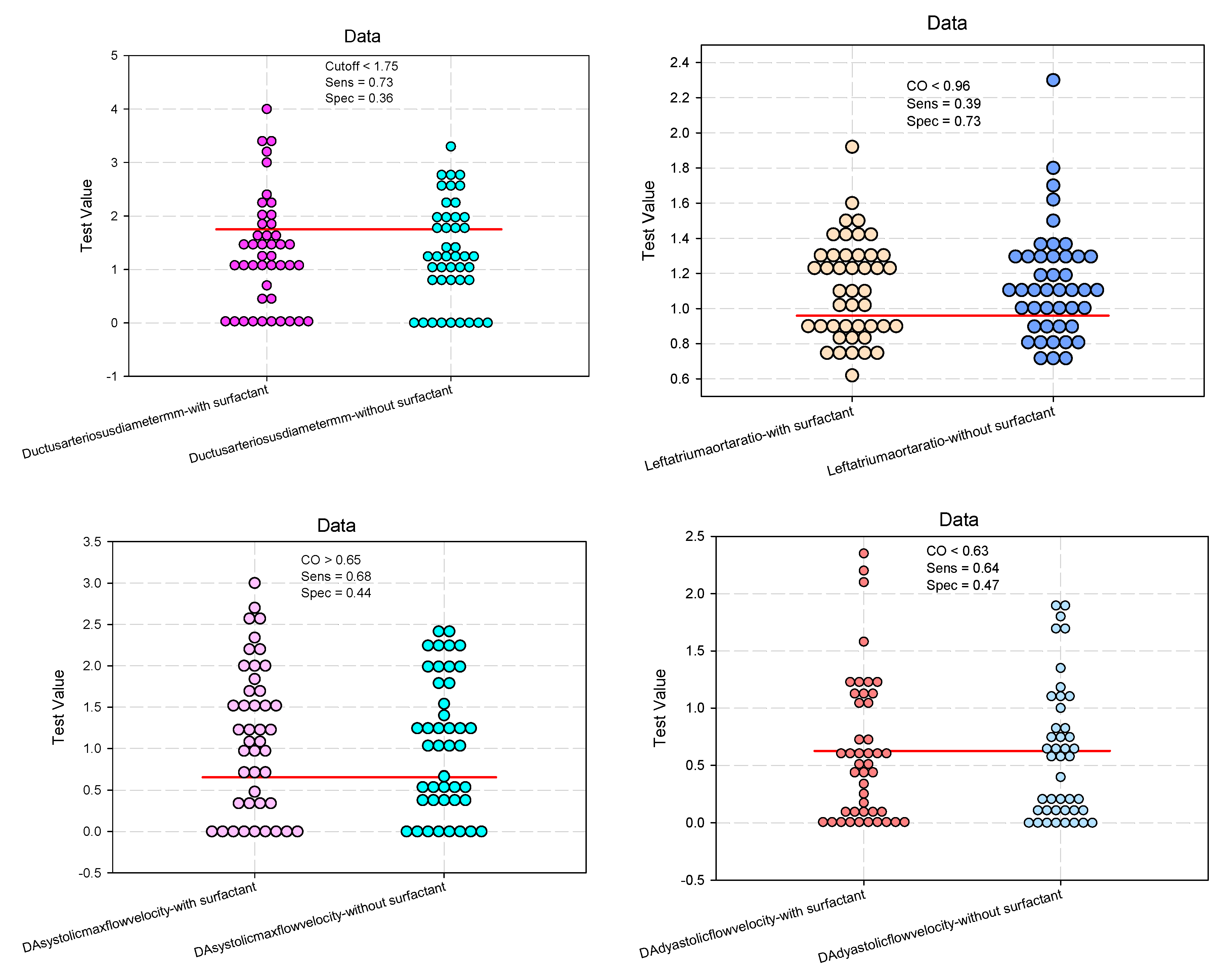
Evaluation of Echographic Parameters of the Arterial Duct
Predictive Analysis: The Effect of Surfactant on Echographic Parameters for Ductus Arteriosus Assessment.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Luca, D. Respiratory distress syndrome in preterm neonates in the era of precision medicine: A modern critical care-based approach. Pediatr Neonatol. 2021, 62 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Tana, M.; Tirone, C.; Aurilia, C.; Lio, A.; Paladini, A.; Fattore, S.; Esposito, A.; De Tomaso, D.; Vento, G. Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside. Children 2023, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Thekkeveedu, R.K.; El-Saie, A.; Prakash, V.; Katakam, L.; Shivanna, B. Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies. J. Clin. Med. 2022, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Polglase, G.R.; Miller, S.L.; Barton, S.K.; Kluckow, M.; Gill, A.W.; Hooper, S.B.; Tolcos, M. Respiratory support for premature neonates in the delivery room: effects on cardiovascular function and the development of brain injury. Pediatr. Res. 2014, 75, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.Y.; Miller, S.L.; Schmölzer, G.M.; Stojanovska, V.; Polglase, G.R. Respiratory Support of the Preterm Neonate: Lessons About Ventilation-Induced Brain Injury From Large Animal Models. Front. Neurol. 2020, 11, 862. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Course, C.; Chakraborty, M. Management of Respiratory Distress Syndrome in Preterm Infants In Wales: A Full Audit Cycle of a Quality Improvement Project. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Balázs, G.; Balajthy, A.; Seri, I.; Hegyi, T.; Ertl, T.; Szabó, T.; Röszer, T.; Papp, Á.; Balla, J.; Gáll, T.; et al. Prevention of Chronic Morbidities in Extremely Premature Newborns with LISA-nCPAP Respiratory Therapy and Adjuvant Perinatal Strategies. Antioxidants 2023, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; de Winter, J.P.; De Luca, D. Lung ultrasound-guided surfactant administration: time for a personalized, physiology-driven therapy. Eur. J. Pediatr. 2020, 179, 1909–1911. [Google Scholar] [CrossRef]
- Raschetti, R.; Yousef, N.; Vigo, G.; Marseglia, G.; Centorrino, R.; Ben-Ammar, R.; Shankar-Aguilera, S.; De Luca, D. Echography-Guided Surfactant Therapy to Improve Timeliness of Surfactant Replacement: A Quality Improvement Project. J. Pediatr. 2019, 212, 137–143.e1. [Google Scholar] [CrossRef]
- Raimondi, F.; Yousef, N.; Migliaro, F.; Capasso, L.; De Luca, D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr. Res. 2018, 90, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.-B.; Pang, L.; Yang, B.; Zhang, C.; Chen, X.-Y.; OuYang, J.-B.; Wu, C.-J. Lung Ultrasound for the Diagnosis and Management of Neonatal Respiratory Distress Syndrome: A Minireview. Front. Pediatr. 2022, 10, 864911. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Plavka, R.; Saugstad, O.D.; Simeoni, U.; Speer, C.P.; Vento, M.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology 2016, 111, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Behrman, R.E.; Clyman, R.I.; Jobe, A.; Heymann, M.; Ikegami, M.; Roman, C.; Payne, B.; Mauray, F. Increased shunt through the patent ductus arteriosus after surfactant replacement therapy. J. Pediatr. 1982, 100, 101–107. [Google Scholar] [CrossRef]
- Fujii, A.; Allen, R.; Doros, G.; O'Brien, S. Patent ductus arteriosus hemodynamics in very premature infants treated with poractant alfa or beractant for respiratory distress syndrome. J. Perinatol. 2010, 30, 671–676. [Google Scholar] [CrossRef]
- Vitali, F.; Galletti, S.; Aceti, A.; Aquilano, G.; Fabi, M.; Balducci, A.; Faldella, G. Pilot observational study on haemodynamic changes after surfactant administration in preterm newborns with respiratory distress syndrome. Ital. J. Pediatr. 2014, 40, 26–26. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Ruoss, J.L.; Stanford, A.H.; Lakshminrusimha, S.; McNamara, P.J. Hemodynamic consequences of respiratory interventions in preterm infants. J. Perinatol. 2022, 42, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.G.; McBrien, A. Ductus arteriosus and fetal echocardiography: Implications for practice. Semin. Fetal Neonatal Med. 2018, 23, 285–291. [Google Scholar] [CrossRef]
- Sung, S.I.; Chang, Y.S.; Kim, J.; Choi, J.H.; Ahn, S.Y.; Park, W.S. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23-28 weeks of gestation. PLOS ONE 2019, 14, e0212256. [Google Scholar] [CrossRef]
- McNamara, P.J.; Sehgal, A. Towards rational management of the patent ductus arteriosus: the need for disease staging. Arch. Dis. Child. - Fetal Neonatal Ed. 2007, 92, F424–F427. [Google Scholar] [CrossRef]
- Rios, D.R.; Bhattacharya, S.; Levy, P.T.; McNamara, P.J. Circulatory Insufficiency and Hypotension Related to the Ductus Arteriosus in Neonates. Front. Pediatr. 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y. Echocardiographic Evaluation of Hemodynamics in Neonates and Children. Front. Pediatr. 2017, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Keusters, L.; Purna, J.; Deshpande, P.; Mertens, L.; Shah, P.; McNamara, P.J.; Weisz, D.E.; Jain, A. Clinical validity of systemic arterial steal among extremely preterm infants with persistent patent ductus arteriosus. J. Perinatol. 2020, 41, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kindler, A.; Seipolt, B.; Heilmann, A.; Range, U.; Rüdiger, M.; Hofmann, S.R. Development of a Diagnostic Clinical Score for Hemodynamically Significant Patent Ductus Arteriosus. Front. Pediatr. 2017, 5, 280. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Thewissen, L.; Austin, T.; da Costa, C.S.; de Boode, W.P.; Dempsey, E.; Kooi, E.; Pellicer, A.; Rhee, C.J.; Riera, J.; et al. Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now? Pediatr. Res. 2023. [CrossRef]
- Binder, C.; Urlesberger, B.; Avian, A.; Pocivalnik, M.; Müller, W.; Pichler, G. Cerebral and Peripheral Regional Oxygen Saturation during Postnatal Transition in Preterm Neonates. J. Pediatr. 2013, 163, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lim, J.; Shim, G.-H. Comparison of mortality and short-term outcomes between classic, intubation-surfactant-extubation, and less invasive surfactant administration methods of surfactant replacement therapy. Front. Pediatr. 2023, 11, 1197607. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, R.; Bohlin, K.; van Kaam, A.; Fuchs, H.; Danhaive, O. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr. Res. 2020, 88, 176–183. [Google Scholar] [CrossRef]
- Sehgal, A.; Mak, W.; Dunn, M.; Kelly, E.; Whyte, H.; McCrindle, B.; McNamara, P.J. Haemodynamic changes after delivery room surfactant administration to very low birth weight infants. Arch. Dis. Child. - Fetal Neonatal Ed. 2010, 95, F345–F351. [Google Scholar] [CrossRef]
- Kumar, A.; Lakkundi, A.; McNamara, P.J.; Sehgal, A. Surfactant and patent ductus arteriosus. Indian J. Pediatr. 2010, 77, 51–55. [Google Scholar] [CrossRef]
- Canpolat, F.E.; Şimşek, G.K.; Webbe, J.; Büyüktiryaki, M.; Karaçağlar, N.B.; Elbayiyev, S.; Kutman, H.G.K. Late Administration of Surfactant May Increase the Risk of Patent Ductus Arteriosus. Front. Pediatr. 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, R.; Bohlin, K.; van Kaam, A.; Fuchs, H.; Danhaive, O. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr. Res. 2020, 88, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bugiera, M.; Szczapa, T.; Sowińska, A.; Roehr, C.C.R.; Szymankiewicz-Bręborowicz, M. Cerebral oxygenation and circulatory parameters during pressure-controlled vs volume-targeted mechanical ventilation in extremely preterm infants. Adv. Clin. Exp. Med. 2020, 29, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
| Study Group (n = 88) | Odd Ratio (OR) / Risk ratio (RR) | ||||
|---|---|---|---|---|---|
| Group 1 (Surfactant) (n = 44) |
Group 2 (Non- Surfactant) (n = 44) |
p-value | OR (95%CI) RR (95%CI) |
p-value | |
| Dependent variable: Surfactant. Independent variable: Maternal age, GA, BW, gender, SGA, PROM, ACT, vaginal delivery Apgar score, pH, BE, pO2, lactate, Need of intubation at birth, RDS | |||||
| Maternal age #, mean (SD) |
30.2 (7.7) | 27.3 (6.9) | 0.075 | 1.055 (0.994–1.119) 1.000 (0.658–1.519) |
0.078 |
| GA §, (weeks), median (IQR) |
28 (25–29) | 30 (29–31) | <0.001* | 0.539 (0.405–0.716) 0.991 (0.571–0.993) |
<0.001* |
| BW §, (g) median (IQR) |
875.0 (700-1195) | 1260 (1100–1400) | <0.001* | 0.997 (0.995–0.998) 0.631 (0.000–0.947) |
<0.001* |
| Male gender ‡, n (%) male (n=53) / female (n=35) |
29 / 15 (54.7 / 42.9) |
24 / 20 (45.3 / 57.1) |
0.275 |
1.611 (0.681–3.810) 1.262 (0.836–1.906) |
0.277 |
| SGA ‡, n (%) SGA (Yes n=22 / No n=66) |
13 / 31 (59.1 / 46.9) | 9 / 35 (40.9 / 53.1) | 0.332 | 1.631 (0.613–4.336) 1.296 (0.747–2.249) |
0.325 |
| PROM # (hours) mean (SD) |
42.2 (92.51) | 32.6 (100.63) | 0.639 | 1.001 (0.997–1.006) 0.999 (0.994–1.003) |
0.638 |
| ACT ‡, n (%) Yes n=59 / No n=29 |
23 / 12 (54.2 / 41.4) | 27 / 17 (45.8 / 58.6) | 0.256 | 1.679 (0.683–4.126) 1.281 (0.847–1.936) |
0.259 |
| Vaginal delivery ‡, n (%) Yes n=36 / C-section n=52 |
15 / 29 (41.7 / 55.8) | 21 / 23 (58.3 / 44.2) | 0.192 | 1.765 (0.747–4.169) 1.319 (0.874–1.990) |
0.192 |
| Apgar score 1 min § median (IQR) |
6.0 (3–7) | 7.0 (6–8) | <0.001* | 0.663 (0.521–0.844) 0.411 (0.121–0.700) |
<0.001* |
| Apgar score 5 min § median (IQR) |
7.0 (5–8) | 8.0 (8–9) | 0.003* | 0.705 (0.535–0.927) 0.510 (0.163–0.935) |
0.012* |
| Cord blood gases | |||||
| pH # mean (SD) |
7.06 (1.05) | 7.31 (0.10) | 0.001* | 0.472 (0.259–0.863) 0.368 (0.201–0.672) |
0.014* |
| BE # mean (SD) |
-6.36 (4.58) | -4.83 (3.44) | 0.079 | 0.907 (0.812–1.014) 0.854 (0.741–1.002) |
0.085 |
| pO2 # mean (SD) |
35.3 (17.73) | 39.9 (15.7) | 0.203 | 0.983 (0.958–1.009) 0.936 (0.990–1.002) |
0.195 |
| Lactate § median (IQR) |
3.60 (1.60–6.40) | 2.15 (1.65–3.35) | 0.0592 | 1.004 (0.951–1.060) 1.001 (0.987–1.015) |
0.893 |
| Need of intubation at birth‡, n (%) (Yes n=25 / No n=63) |
22/22 (88/34.9) | 3/41 (12/65.1) | <0.001* | 13.67 (3.67–50.79) 13.66 (4.16–62.38) |
0.001* |
| RDS (Silverman) § median (IQR) |
5.5 (5–6) | 3 (2–4) | <0.001* | 2.872 (1.858–4.440) 2.348 (2.216–3.560) |
<0.001* |
|
Group 1 (Surfactant) (n = 44) |
Group 2 (Non- Surfactant) (n = 44) |
p-value |
OR (95%CI) RR (95%CI) |
p-value | |
| Dependent variable: Need of MV, Duration of MV, Duration of CPAP, NICU days, Deaths. Independent variables: surfactant | |||||
| Need of MV ‡ (after 72h), n (%) Yes / No |
28/16 (63.6/36.4) | 5/39 (11.4 / 88.6) | <0.001* | 0.179 (0.076–0.420) 0.593 (0.500–0.703) |
<0.001* |
| Duration of MV §, (hours) median (IQR) |
240 (154–396) | 108 (100–160) | 0.0327* | 0.059 (0.008–0.423) 0.161 (0.030–0.852) |
0.002* |
| Duration of CPAP §, (hours) median (IQR) |
160 (72–243) | 72 (24–160) | 0.007* | 0.318 (0.139–0.729) 0.532 (0.332–0.854 |
0.007* |
| NICU days § median (IQR) |
24.5 (13–34) | 14.5 (8.25–19) | <0.001* | 0.226 (0.104–0.492) 0.401 (0.257–0.625) |
<0.001* |
| Deaths ‡, n (%) | 7 / 37 (15.9 / 84.1) | 2 / 42 (4.5 / 95.5) | 0.0785 | 0.252 (0.049–1.288) 0.286 (0.063–1.300) |
0.098 |
| Study group (n = 88) | Odd Ratio (OR) / Risk ratio (RR) |
||||
|---|---|---|---|---|---|
| Group 1 (Surfactant) (n = 44) |
Group 2 (Non-Surfactant) (n = 44) |
p-value | OR (95%CI) RR (95%CI) |
p-value | |
| Independent variable: surfactant. | |||||
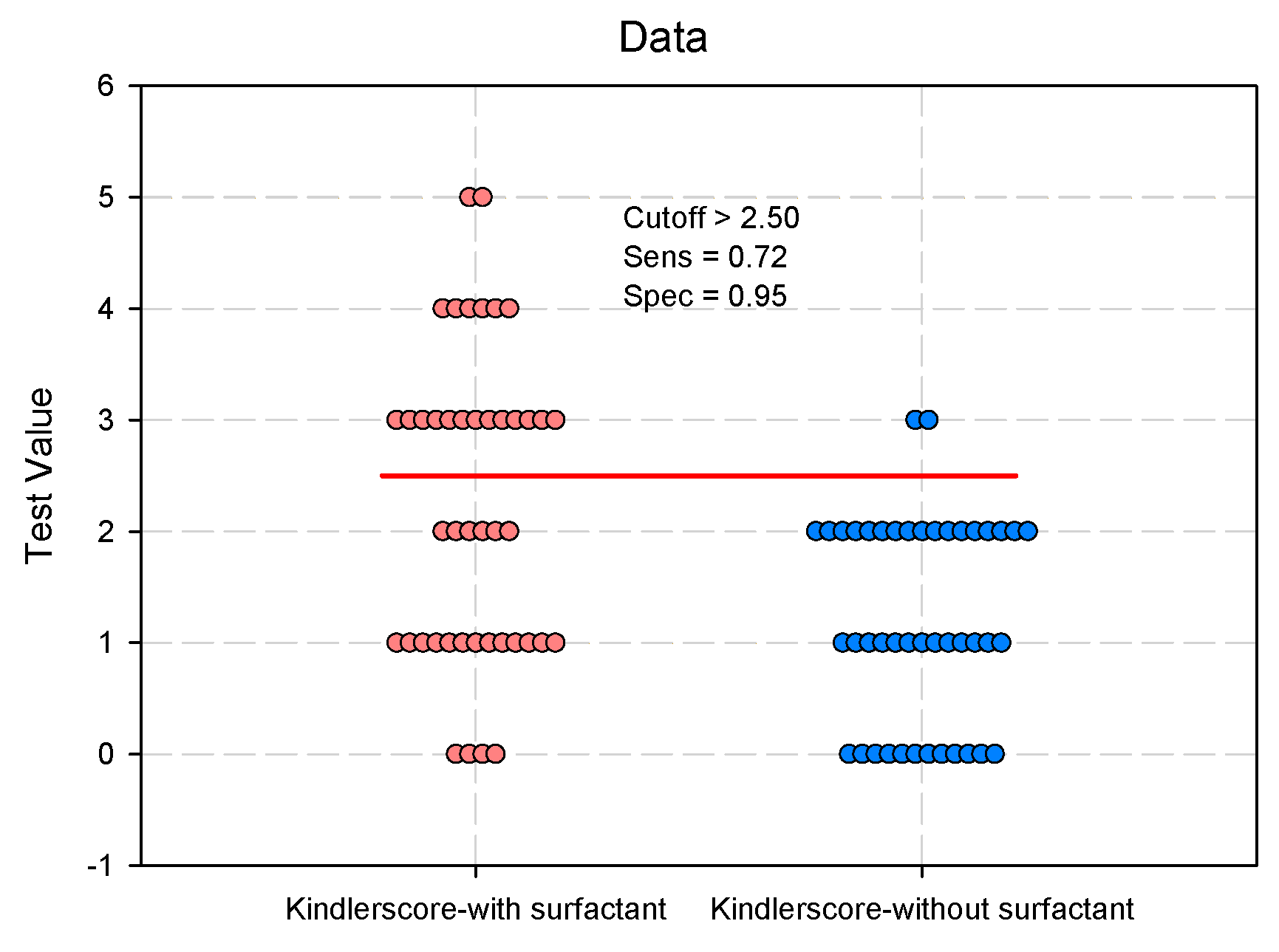
| Kindler Score § 0/1/2/3/4/5, n(%) median (IQR) |
4/13/6/13/6/2 (9.1/29.6/13.6/29.5/13.6/4.6) 2 (1–3) |
12/13/17/2/0/0 (27.3/29.6/38.6/4.6/0/0) 1 (0–2) |
<0.001* | 0.406 (0.256–0.643) 0.240 (0.108–0.532) |
<0.001* |
| Cardiac murmur ‡, n(%) Yes / No |
9 / 35 (20.5 / 79.5) | 6 / 38 (13.6 / 86.4) | 0.3937 | 0.667 (0.259–1.715) 0.614 (0.198–1.902) |
0.398 |
| Tachycardia>160/min ‡, n (%) Yes / No |
18 / 26 (40.9 / 59.1) | 7 / 37 (15.9 / 84.1) | 0.0084* | 0.389 (0.181–0.837) 0.273 (0.100–0.748) |
0.012* |
| Blood pressure #, mean (SD) | |||||
| Pre-ductal systolic pressure | 59.8 (11.9) | 64.2 (11.6) | 0.0783 | 0.593 (0.416–0.844) 0.605 (0.341–1.074) |
0.086 |
| Post-ductal systolic pressure | 58.1 (11.0) | 62.7 (12.0) | 0.0656 | 0.642 (0.455–1.907) 0.748 (0.427–1.310) |
0.231 |
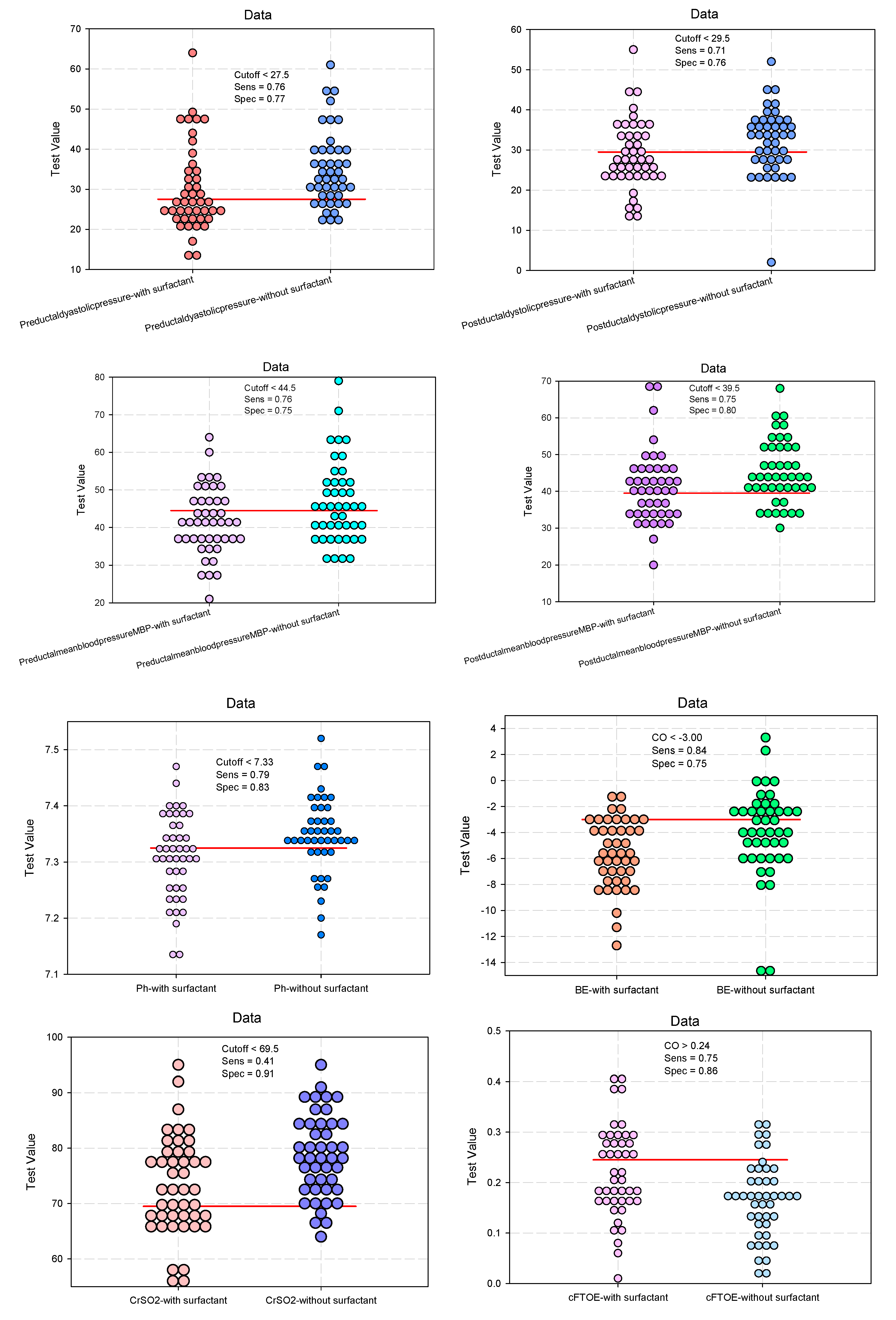
| Pre-ductal diastolic pressure | 29.9 (10.6) | 34.8 (9.3) | 0.0231* | 0.443 (0.307–0.640) 0.505 (0.283–0.900) |
0.021* |
| Post-ductal diastolic pressure | 28.7 (8.6) | 32.2 (8.1) | 0.0178* | 0.293 (0.192–0.448) 0.389 (0.214–0.709) |
0.002* |
| Pre-ductal MAP | 41.6 (8.9) | 46.5 (10.7) | 0.0210* | 0.437 (0.300–0.637) 0.555 (0.312–0.987) |
0.001* |
| Post-ductal MAP | 41 (9.8) | 45.3 (8.6) | 0.0336* | 0.393 (0.266–0.580) 0.692 (0.393–0.841) |
0.001* |
| Blood gases | |||||
| pH #, mean (SD) | 7.31 (0.08) | 7.35 (0.07) | 0.0165* | 0.422 (0.289–0.616) 0.677 (0.480–0.955) |
0.005* |
| pH<7.25 ‡, n (%) | 12 / 32 (27.3 / 72.7) | 7 / 37 (15.9 / 84.1) | 0.1951 | 0.505 (0.177–1.435) 0.583 (0.254–1.342) |
0.195 |
| pO2 #, mean (SD) | 47.5 (13.2) | 47.8 (9.1) | 0.9031 | 0.594 (0.420–1.140) 0.766 (0.544–1.077) |
0.125 |
| pCO2 #, mean (SD) | 42.2 (11.4) | 40.1 (10) | 0.3544 | 1.368 (0.970–1.930) 1.211 (0.861–1.704) |
0.074 |
| BE #, mean (SD) | -5.61 (2.64) | -3.96 (3.41) | 0.0125* | 0.578 (0.406–0.823) 0.678 (0.480–0.958) |
0.028* |
| Lactate §, median (IQR) | 1.65 (1.30–2.55) | 1.70 (1.25–2.00) | 0.3247 | 0.713 (0.505–1.007) 0.823 (0.583–1.161) |
0.055 |
| SpO2 #, mean (SD) | |||||
| Pre-ductal | 94.2 (3.01) | 94.8 (2.45) | 0.3155 | 0.486 (0.336–1.102) 0.713 (0.504–1.008) |
0.056 |
| Post-ductal | 94.0 (3.00) | 94.9 (2.60) | 0.1329 | 0.622 (0.420–1.085) 0.802 (0.569–1.130) |
0.207 |
| FiO2 #, mean (SD) | 30.5 (13.29) | 26.3 (6.39) | 0.0618 | 0.667 (0.471–1.146) 0.944 (0.667–1.335) |
0.745 |
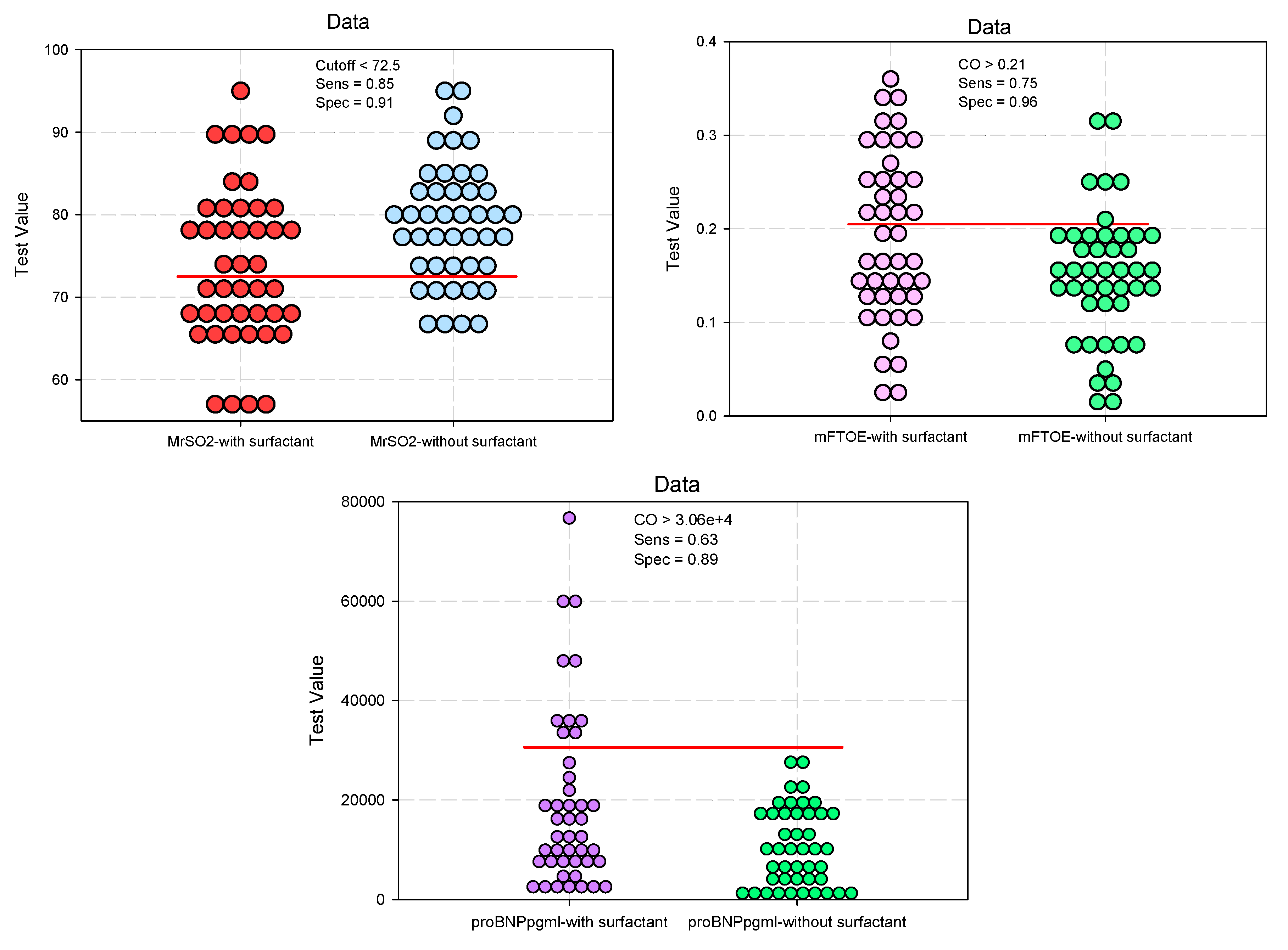
| CrSO2 #, mean (SD) | 73.14 (8.89) | 78.59 (7.42) | 0.0024* | 0.636 (0.447–0.905) 0.690 (0.485–0.980) |
0.012* |
| cFTOE #, mean (SD) | 0.22 (0.09) | 0.17 (0.08) | 0.0024* | 2.454 (1.670–3.605) 1.997 (1.387–2.874) |
<0.001* |
| MrSO2 #, mean (SD) | 73.8 (9.52) | 78.9 (7.35) | 0.0055* | 0.430 (0.294–0.629) 0.502 (0.348–0.725) |
<0.001* |
| mFTOE §, median (IQR) | 0.18 (0.13–0.26) | 0.15 (0.12–0.19) | 0.0345* | 2.305 (1.586–3.350) 1.749 (1.225–2.495) |
0.002* |
| Troponin #, mean (SD) | 34.37 (34.6) | 33.07 (29.4) | 0.8496 | 1.170 (0.508–3.124) 1.020 (0.457–3.007) |
0.069 |
| NT-proBNP §, median (IQR) | 12962.6 (7333.7–25934.8) | 9621.6 (3463.2–17381.8) |
0.0247* | 2.995 (1.973–4.545) 3.250 (2.114–4.996) |
<0.001* |
| Clinical parameters: evaluation of the ductus arteriosus Independent variable: surfactant |
Area under the Curve (AUC) |
Std. Error |
p-Value | 95%Confidence Interval for AUC |
||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Kindler Score | 0.780 | 0.052 | 0.0001* | 0.678 | 0.882 | |
| Tachycardia | 0.654 | 0.064 | 0.0161* | 0.528 | 0.779 | |
| Blood pressure | Pre-ductal diastolic pressure | 0.679 | 0.058 | 0.0037* | 0.565 | 0.793 |
| Post-ductal diastolic pressure | 0.645 | 0.059 | 0.0183* | 0.529 | 0.761 | |
| Pre-ductal MAP | 0.617 | 0.059 | 0.0376* | 0.501 | 0.734 | |
| Post-ductal MAP | 0.646 | 0.058 | 0.0179* | 0.531 | 0.761 | |
| Blood gases | pH | 0.654 | 0.059 | 0.0122* | 0.539 | 0.770 |
| BE | 0.682 | 0.056 | 0.0032* | 0.571 | 0.793 | |
| CrSO2 | 0.682 | 0.056 | 0.0031* | 0.571 | 0.793 | |
| cFTOE | 0.672 | 0.057 | 0.0052* | 0.560 | 0.785 | |
| MrSO2 | 0.668 | 0.058 | 0.0064* | 0.555 | 0.782 | |
| mFTOE | 0.630 | 0.059 | 0.0347* | 0.513 | 0.748 | |
| NT-proBNP | 0.638 | 0.058 | 0.0247* | 0.523 | 0.754 | |
| Linear Regression: adjustment for gestational age (GA) |
Unstandardized Coefficients | Standardized Coefficients | t | p-Value | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| Kindler Score | 1.023 | 0.249 | 0.406 | 4.115 | <.001* |
| Tachycardia | 0.236 | 0.093 | 0.265 | 2.546 | 0.013* |
| Blood pressure | |||||
| Pre-ductal diastolic pressure | -4.800 | 2.134 | -0.236 | -2.250 | 0.027* |
| Post-ductal diastolic pressure | -3.568 | 1.782 | -0.211 | -2.002 | 0.041* |
| Pre-ductal MAP | -4.784 | 2.109 | -0.238 | -2.268 | 0.026* |
| Post-ductal MAP | -4.227 | 1.958 | -0.227 | -2.159 | 0.034* |
| Blood gases | |||||
| pH | -0.037 | 0.015 | -0.251 | -2.400 | 0.019* |
| BE | -1.676 | 0.657 | -0.265 | -2.548 | 0.013* |
| CrSO2 | -5.320 | 1.731 | -0.315 | -3.073 | 0.003* |
| cFTOE | 0.054 | 0.018 | 0.309 | 3.016 | 0.003* |
| MrSO2 | -5.043 | 1.802 | -0.289 | -2.799 | 0.006* |
| mFTOE | 0.040 | 0.017 | 0.243 | 2.323 | 0.023* |
| NT-proBNP | 8515.645 | 2893.041 | 0.303 | 2.943 | 0.004* |
| Study Group (n = 88) | Odd Ratio (OR) / Risk ratio (RR) |
||||
|---|---|---|---|---|---|
| Group 1 (Surfactant) (n = 44) |
Group 2 (Non-Surfactant) (n = 44) |
p-value | OR (95%CI) RR (95%CI) |
p-value | |
| Independent variable: surfactant; dependent variable: diameter, direction of shunt, LA/Ao ratio, DA flow velocity, DA treated after 72 h | |||||
| Diameter (mm) #, mean (SD) | 1.32 (1.04) | 1.32 (0.92) | 0.983 | 1.092 (0.779–1.531) 1.002 (0.715–1.406) |
0.990 |
| Small <1.5 mm ‡, n (%) | 18 (40.9) | 18 (40.9) | 0.828 | - | - |
| Moderate 1.5-3 mm ‡, n (%) | 14 (31.8) | 16 (36.4) | 0.652 | - | - |
| Large > 3 mm ‡, n (%) | 5 (11.4) | 1 (2.3) | 0.204 | - | - |
| Closed ‡, n (%) | 7 (15.9) | 9 (20.5) | 0.580 | 0.736 (0.247–2.189) | 0.814 |
| Direction of shunt‡, n (%) | |||||
| L-R: Left to right shunt | 34 (77.3) | 34 (77.3) | 0.799 | 1.0 (0.797–1.254) 1.0 (0.369–2.210) |
0.921 |
| Bidirectional | 2 (4.5) | 2 (4.5) | 0.691 | 1.0 (0.135–7.434) 1.0 (0.147–6.786) |
0.934 |
| No shunt | 7 (15.9) | 10 (22.7) | 0.416 | 0.643 (0.220–1.880) 0.919 (0.748–1.128) |
0.722 |
| LA/Ao ratio #, mean (SD) | 1.12 (0.28) | 1.14 (0.31) | 0.664 | 0.790 (0.446–1.398) 0.827 (0.467–1.463) |
0.418 |
| <1.5 ‡, n (%) | 40 (90.9) | 39 (88.6) | 0.724 | - | - |
| 1/5-2.1 ‡, n (%) | 4 (9.1) | 4 (9.1) | 0.710 | - | - |
| >2 | 0 (0) | 1 (2.3) | 0.237 | - | - |
| DA flow velocity | |||||
| Systolic #, mean (SD) | 1.14 (0.88) | 1.05 (0.81) | 0.597 | 1.318 (0.750–2.314) 1.257 (0.716–2.205) |
0.426 |
| restrictive DA ‡, n (%) | 10 (22.7) | 9 (20.5) | 0.795 | 1.144 (0.414–3.162) 1.029 (0.827–1.282) |
0.818 |
| unrestrictive DA ‡, n (%) | 25 (56.8) | 27 (61.4) | 0.828 | 0.828 (0.354–1.940) 0.895 (0.541–1.480) |
0.739 |
| Diastolic #, mean (SD) | 0.62 (0.63) | 0.61 (0.58) | 0.911 | 1.167 (0.669–2.036) 1.064 (0.611–1.854) |
0.587 |
| DA treated after 72 h ‡, n (%) | 12 (27.3) | 9 (20.9) | 0.489 | 1.417 (0.526–3.812) 1.087 (0.857–1.379) |
0.805 |
| Clinical parameters: evaluation of the ductus arteriosus Independent variable: surfactant |
Area under the Curve AUC (95%CI) |
Std. Error |
p-Value | 95%Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Diameter (mm) | 0.501291 | 0.062204 | 0.983355 | 0.3794 | 0.6232 |
| LA/Ao ratio | 0.51188 | 0.062329 | 0.847787 | 0.3897 | 0.6340 |
| DA flow velocity | |||||
| Systolic | 0.523784 | 0.062364 | 0.702437 | 0.4016 | 0.6460 |
| Diastolic | 0.512949 | 0.062504 | 0.835232 | 0.3904 To | 0.6355 |
| Linear Regression: adjustment for gestational age (GA) |
Unstandardized Coefficients |
Standardized Coefficients |
t | p-Value | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| Diameter (mm) | 0.028 | 0.210 | 0.014 | 0.134 | 0.894 |
| LA/Ao ratio | -0.026 | 0.064 | -0.043 | -0.401 | 0.689 |
| DA flow velocity | |||||
| Systolic | 0.101 | 0.181 | 0.060 | 0.558 | 0.578 |
| Diastolic | 0.019 | 0.131 | 0.016 | 0.149 | 0.882 |
| Study Group (n = 88) | |||
|---|---|---|---|
| Group 1 (Surfactant) (n = 44) |
Group 2 (Non-Surfactant) (n = 44) |
p-value | |
| HEAD ULTRASOUNDS | |||
|
ACA PSV §, median (IQR) EDV §, median (IQR) RI #, mean (SD) |
24.7 (19.2-31.15) 7.60 (4.6-8.3) 0.73 (0.12) |
23.75 (20.65-26.65) 7.10 (5.55-8.20) 0.71 (0.09) |
0.603 0.717 0.329 |
| ABDOMINAL ULTRASOUNDS | |||
|
CT PSV #, mean (SD) EDV #, mean (SD) RI #, mean (SD) |
44.8 (21.1) 12.1 (8.38) 0.73 (0.1) |
46.1 (16.1) 13.8 (7.31) 0.70 (0.08) |
0.743 0.312 0.246 |
|
SMA PSV #, mean (SD) EDV §, median (IQR) RI #, mean (SD) |
35.7 (16.92) 9.8 (6.45–11.7)) 0.71 (0.64-0.75) |
33.9 (10.05) 9.15 (7.85–10.7) 0.70 (0.67-0.77) |
0.547 0.485 0.628 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





