1. Introduction
Cancers of the brain and central nervous system (CNS) are comprised of a varied group of pathology stemming from brain and spinal cord tissue, with the World Health Organization (WHO) classifying over 150 differing brain tumor subtypes [
1]. Intracranial and spinal cord malignancies have remained steadfast amongst ranking within the most fatal cancers, estimating that only esophageal, lung, hepatic and pancreatic tumors stand ahead of CNS tumor pathology in 5-year mortality rates [
2]. While CNS cancers are considered rare when contrasted to other classifications of malignancy (with statistics showing that CNS cancer comprised 1.7% of global cancer cases as compared to 12.2% for cancers of the lung), their place amongst the most fatal cancers has been stout for several years [
3,
4]. While the 5-year survival rates of brain and CNS cancer are enduringly morbid, mild improvements in the frequency of intracranial malignancy cases have been noted in recent decades, as exemplified by the 0.8% decrease in incidence noted between 2008 and 2017 within North America [
5].
The choice of treatment regimen against brain tumors most often anchors on information received from histopathologic analysis of cancerous tissue, an approach that can even be applied intra-operatively to guide for necessity of retrieving additional tissue for biopsy or improvement of margins with regards to tumor resection and overall morbidity and mortality outcomes [
6]. Examples of such growing technique modalities include Raman spectroscopy, frozen sectioning, and confocal laser endomicroscopy [
7].
Despite being regarded by some as one of the oldest medical specialties in human history (with some historians arguing for the evidence of neurosurgical techniques performed dating back to 10,000 BC), neurosurgery still stands as one of the most dynamic fields within medicine today [
8]. Given the wide variety in etiology, clinical presentation, and pathohistological features touted amongst the variety of potential intracranial and CNS tumors, neurosurgery continues to discover new avenues for greater treatment interventions and more efficient intraoperative analyses. Given that the current prevalence of said pathology has continued to remain primarily stagnant (with only marginal reductions in case prevalence since 2008), it is imperative that current clinicians remain vigilant in aiming to appreciate the medley of possible treatment approaches that are practically applicable in the world of neurosurgical techniques. This review specifically aims to discuss advancements in intraoperative histology in the realm of neurosurgery, highlighting the role of these innovations in improving patient outcomes [
3,
4].
2. Frozen Sectioning and Cytologic Preparations
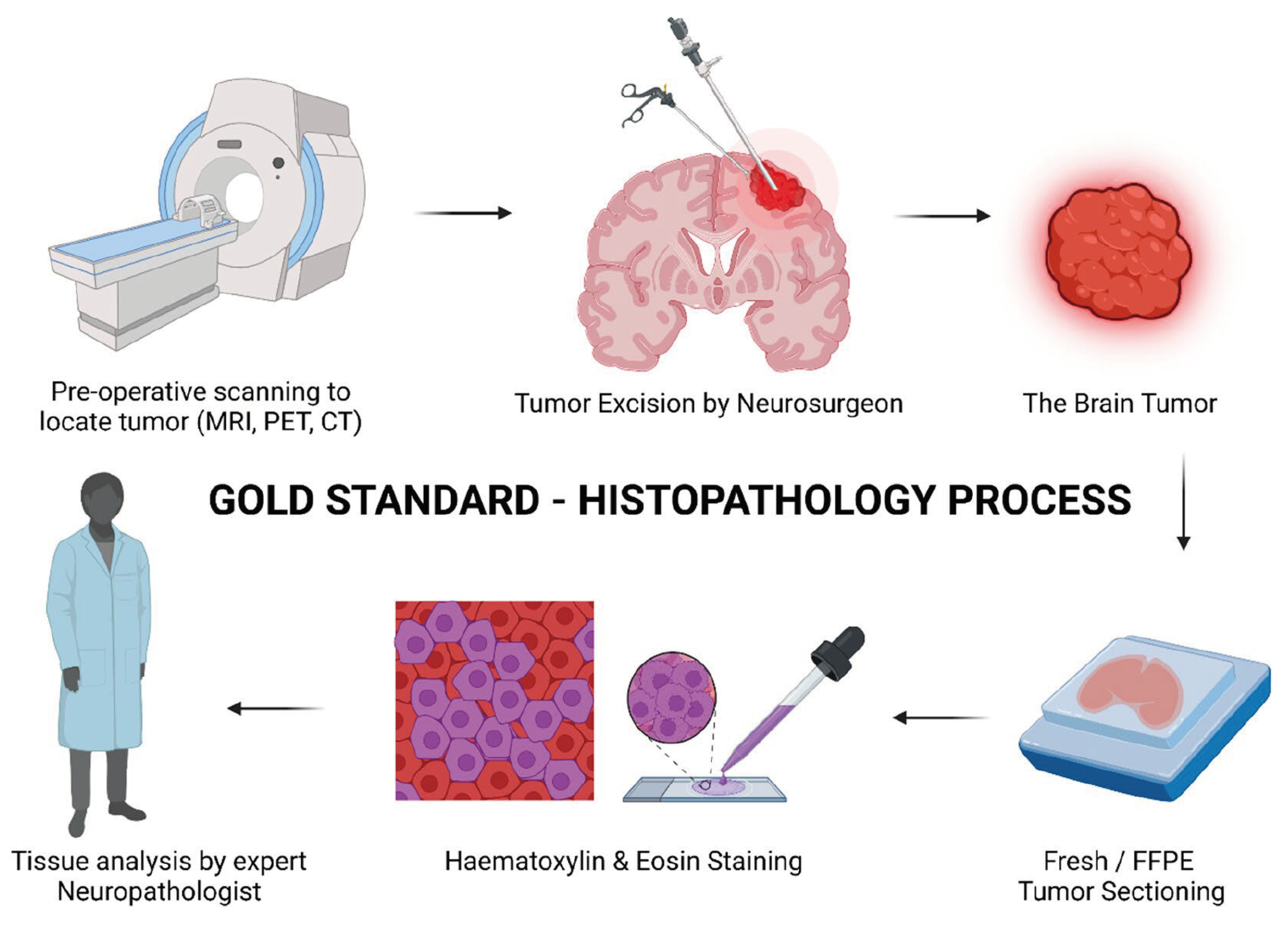
Intraoperative analysis of tissue samples is crucial to allow for a surgeon to determine established treatment protocols and margins in the neurosurgical setting. Proper analysis of tissue requires hardening of the matrix to allow for sectioning. This led to the introduction of frozen sectioning into the field of intraoperative pathology (
Figure 1). The first instance of this technique dates to 1905 when Dr. Louis B. Wilson simply used cold winter air to help freeze his samples [
9]. Dr. Wilson then progressed to using the CO
2 microtome and staining samples with methylene blue before many other advancements were made to the current state of frozen cytology [
10]. The most current form of frozen sectioning includes the utilization of the cryostat set between -20 to -30 degrees Celsius and frozen aerosol sprays to help set the sample [
11]. A study by Novis and Zarbo found that 90% of frozen sectioning block turnaround times were within 20 minutes from the time the pathologist received the sample [
12]. This fast turnaround time has allowed for the expansion of intraoperative diagnosis of lesions, rather than waiting for the traditional pathology report after the operation has been completed.
Frozen sectioning has been proven to be a fast intraoperative process, yet many individuals have questioned the accuracy of such procedures. A study performed by Khoddami et al. (2015) found an accuracy rate of 99.5% for the frozen sectioned samples compared to the gold standard of formalin-fixed and paraffin-embedded samples [
14]. The same article reported a sensitivity and specificity of 91.4% and 99.7%, respectively. The high level of accuracy associated with frozen sectioning was repeated in a study performed by Kang et al. (2019) which displayed an intraoperative accuracy of 98.8% compared to the permanent histological diagnosis [
15]. Another study demonstrated a high correlation of 95.6% between the intraoperative diagnosis and the final diagnosis [
16]. These results have demonstrated a high level of accuracy of intraoperative frozen sectioning, but several limitations must be considered while utilizing the technique.
It is an important distinction to make that the high level of accuracy of frozen sectioning does not make it a viable replacement for paraffin-embedded tissue techniques. One reason that it cannot be used as a replacement is the possibility of interpretative errors in frozen sections due to the freezing process distorting the architecture [
17]. This interpretative error occurs commonly in differentiating cells that are spindle shaped. This includes differentiating between cerebellopontine angle meningiomas and schwannomas [
18]. Also, frozen sectioning provides the possibility that oligodendroglioma is mischaracterized as a high-grade astrocytoma. The perinuclear halo traditionally seen with paraffin-embedded oligodendroglia-based cells is lost in the freezing process [
19]. The loss of a perinuclear halo along with freezing artifacts causes oligodendroglia to be angulated similar to astrocyte cells. Errors with frozen sectioning extend beyond these issues listed, but it is a valuable tool that can still provide accurate information. Proper anatomical knowledge, good surgical technique, and expert pathological interpretation of neural tissue can help reduce errors in differentiation between cell lines and structures [
20].
Overall, frozen sectioning is a valuable intraoperative tool to help provide guidance for the surgeon on margins, diagnosis, and possible complications. Limitations exist in the usage of frozen sectioning due to the possibility of artifact and tissue distortion created during the freezing process. However, close cooperation between the surgeon and the pathologist can help to limit errors. The pathologist should have the opportunity to look at radiologic images before the procedure to be able to compare histological findings to what is seen in imaging modalities. The combination of radiologic images and histology has been shown to improve accuracy and avoid interpretative errors with frozen sectioning [
11]. Continuing, proper selection of tumor for the procedure improves accuracy. Masses firmer in consistency, such as fibroblastic meningioma, showed better yields from frozen cytology than more friable masses [
21]. Lastly, usage of intraoperative frozen cytology along with other diagnostic techniques such as crush smear has been shown to improve diagnostic accuracy [
22]. In conclusion, proper usage of frozen cytology has been shown to be a valuable tool in the belt of neurosurgeons to provide greater guidance for intraoperative decision-making.
3. Raman histology
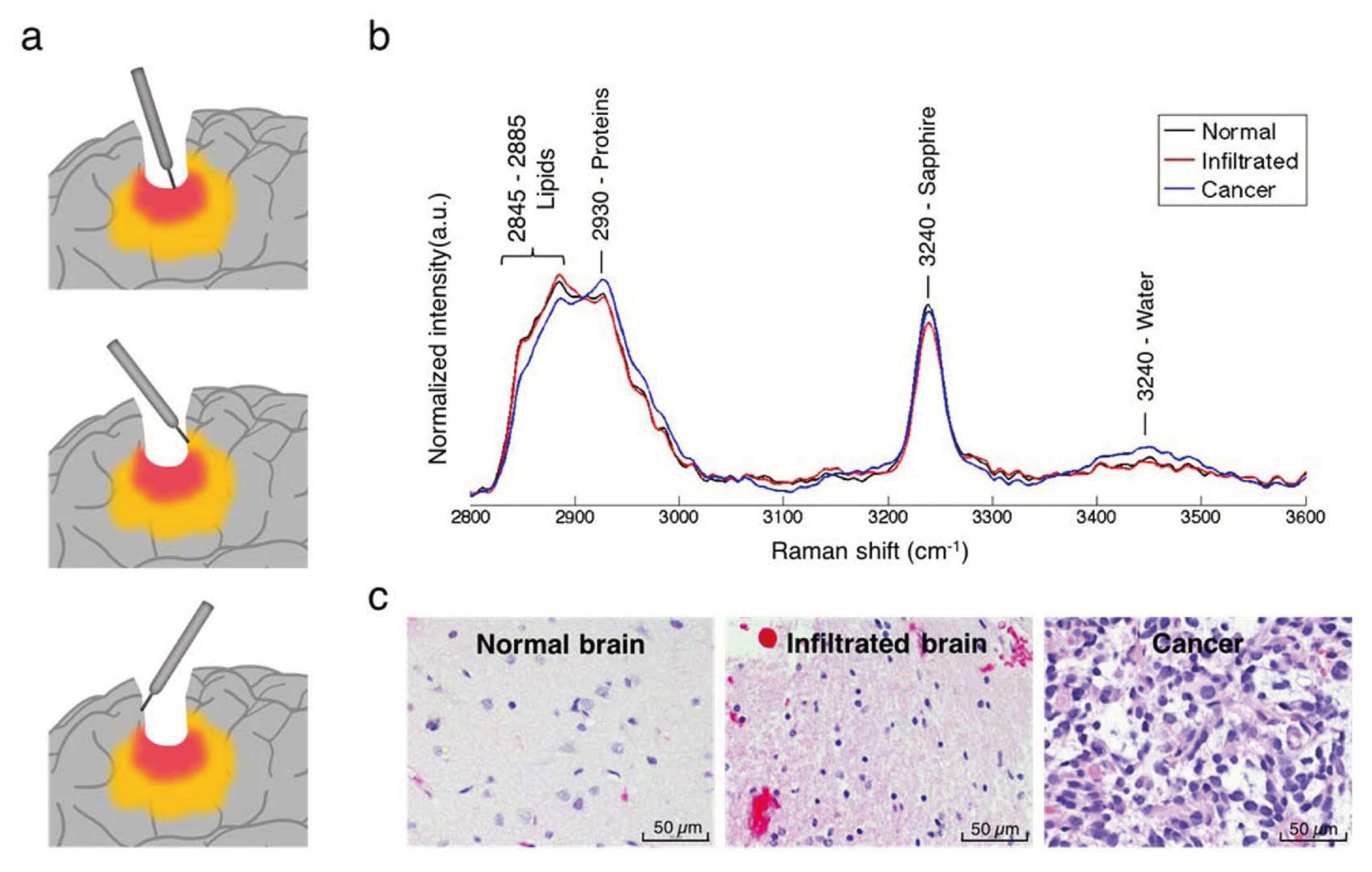
In recent years, the development of various intraoperative neurohistological analyses has emerged based on advancements in the application of Raman spectroscopy (
Figure 2). Raman spectroscopy was first developed in 2008 and is based on the Raman scattering phenomenon, which posits that spontaneous inelastic scattering of light will occur based on the molecular characteristics of the sample [
23,
24,
25]. Therefore, Raman spectroscopy allows for the rapid, non-destructive acquisition of label-free information directly from the sample’s chemical characteristics [
23,
24]. As most neuropathologies involve alterations at the molecular level, Raman technology has been increasingly efficacious in rapidly diagnosing and treating ischemic or traumatic brain injuries, neurodegenerative diseases, and brain tumors [
23]. Several techniques for enhancing Raman spectroscopy have been developed, as spontaneous Raman spectroscopy alone is not strong enough to provide high-resolution images. These methods include but are not limited to stimulated Raman histology (SRH), coherent anti-Stokes Raman scattering (CARS), surface-enhanced Raman scattering (SERS), and tip-enhanced Raman scattering (TERS) [
26]. For instance, compared to conventional methods, Stimulated Raman Histology (SRH) microscopy allows for rapid and accurate detection of the extent of brain tumor infiltration through high-resolution imaging [
27]. A review of existing literature indicates that as Raman histological techniques continue to develop and improve, they will become an essential element in the neurosurgeon’s armamentarium for accurate intraoperative diagnosis and decision-making [
28].
3.1. Indications
Raman technology has very promising indications in oncological neurosurgery, as it allows for the detection of microscopic cancer cell infiltration and therefore more accurate resection of brain tumors and diagnosis of tumor subtypes [
30]. Perhaps the primary advantage of Raman spectroscopy is its ability to rapidly produce histological images, allowing the neurosurgeon to achieve empirically clear tumor margins and enhancing the prognosis of neuro-oncologic patients [
30]. Raman-based imaging methods can also be used in the detection of neurodegenerative diseases such as Alzheimer Disease (AD). Raman spectroscopy has been shown to greatly improve the accuracy of diagnosing such disorders by detecting the presence of neurofibrillary tangles, amyloid-β plaques, and tau protein in tissues [
31]. A study by Ryzhikova et al. suggests that RS analysis of CSF may allow for early detection of Alzheimer’s, as they were able to diagnose AD with 84% specificity and sensitivity [
31,
32]. Another study by Lochoki et al. successfully used RA to detect amyloid-beta peptides in tissue samples from AD patients [
31,
33].
Raman-based imaging techniques may also be effective in analyzing ischemic metabolic changes in stroke and TBI patients. For example, the release of cytochrome C from damaged neurons can be measured by RS to elucidate the extent of ischemic cell death [
23].
3.2. Techniques
3.2.1. Scattered Raman Spectroscopy
Scattered Raman spectroscopy has promising clinical applications in the detection of healthy versus necrotic or diseased brain tissue as well as the detection of biomarkers that may indicate the stage of the pathology [
34]. Raman imaging uses monochromatic light that interacts with the vibrational modes of a sample, causing inelastic scattering of photons. The Raman phenomenon can be analyzed through Stokes or anti-Stokes scattering, where the scattered beams have a lower or higher frequency than that of the incident beam, respectively [
34,
35]. The resultant peaks represent the Raman spectrum and the energy shift is measured as the “Raman shift”, which indicates the various concentrations of specific molecules based on the strength of each spectroscopic peak. This allows each protein, lipid, and DNA molecule to be distinguished through its unique vibrational fingerprint [
34]. However, due to the relatively weak signal given by spontaneous Raman spectroscopy, various techniques have been developed to amplify the signal, including Stimulated Raman spectroscopy (SRS), coherent anti-Stokes Raman scattering (CARS) microscopy, surface-enhanced Raman scattering (SERS) microscopy, and tip-enhanced Raman scattering (TERS) microscopy [
26,
34]. Furthermore, the invention of the femtosecond laser has allowed for the development of other SRS techniques with minimal collateral tissue damage, as its pulse duration is a minuscule 10
-15 seconds [
36].
3.2.2. Stimulated Raman Scattering (SRS) Microscopy
Stimulated Raman scattering (SRS) microscopy utilizes two lasers, the pump beam, and the Stokes beam, to amplify the Raman signal given by specific bonds in the sample and generate image contrast [
37]. Inverted microscopes with two lenses are usually used for SRS; the first lens projects beams onto the sample and the second funnels the photons into the detector [
25]. The Stokes beam is typically a fixed wavelength while the Pump beam is a tunable wavelength and both pulse at a rate of 80 MHz. The Stokes and Pump beams must overlap for SRS to occur; this is achieved through the microscope’s dichroic mirror and optical delay line [
38]. The Stokes beam is then reduced to a fixed frequency of 1-20 MHz using an optic modulator and optic filters to elucidate the SRS signal [
25,
38]. A large area photodiode detector and optical filters are then used to retrieve the unmodulated beam from the overlapping Stokes and Pump beams, and the signal is finally converted to a histological image through specialized computer software [
38].
3.2.3. Coherent Anti-Strokes Raman Scattering (CARS)
Coherent anti-Stokes Raman scattering (CARS) microscopy is one method that was developed to overcome the imaging speed limitations of spontaneous Raman scattering [
39]. As in SRS, the Pump and Stokes beams concurrently excite the sample such that the frequency difference between the beams corresponds to the vibrational frequency of a specific chemical bond in the sample. However, in addition to stimulated Raman gain (ω
s) and loss (ω
p), CARS produces two much stronger signals: coherent anti-Stokes Raman scattering ((ω
p−ω
s) + ω
p) and coherent Stokes Raman scattering (ω
p−(ω
p−ωs)) frequencies [
39]. For this reason, CARS is significantly more sensitive than spontaneous Raman spectroscopy and allows for extremely rapid imaging [
40]. Furthermore, the nonlinear nature of CARS allows for 3-D sectioning of thick tissues, and tissue damage is minimized as CARS occurs in the ground electric state [
40].
3.2.4. Surface-Enhanced Raman Scattering (SERS) Microscopy
Another method of enhancing the sensitivity of spontaneous Raman scattering is surface-enhanced Raman scattering (SERS) microscopy [
38]. The SERS method was discovered serendipitously when it was observed that a roughened silver electrode significantly increased the Raman signal of an adsorbed pyridine sample [
41]. SERS utilizes a metal probe to absorb molecules and concentrate electromagnetic energy through surface plasmons, therefore significantly enhancing the scattering of photons [
42,
43]. The probe must be composed of a transition metal, a noble metal, or a semiconductor to allow for molecules to be rapidly analyzed on the surface of the metal [
43]. The use of such metal probes enhances the incident light and therefore increases Raman scattering, providing a faster and clearer image [
38].
3.2.5. Tip-Enhanced Raman Scattering (TERS) Microscopy
Tip-enhanced Raman spectroscopy (TERS) operates based on the chemical sensitivity of the SERS method, with the added benefit of high spatial resolution at the sub-nanometer level via scanning probe microscopy (SPM) [
44]. Like SRS, CARS, and SERS, TERS is rapid, accurate, and non-destructive. The TERS method involves a sharp metal SPM tip with an enhanced electromagnetic field at the apex due to the resonance of surface plasmons, thereby significantly augmenting the Raman signal of molecules in its vicinity [
44]. This increased signal strength allows TERS to overcome the diffraction limit of SRS and SERS and convey improved sub-diffraction limit spatial resolution [
45].
3.3. Outcomes
Several ex vivo studies have found Raman spectroscopy to be highly specific and highly sensitive; Zhang et al. reported the detection of brain tumors by Raman spectroscopy (RS) to be 97% sensitive and 98.5% specific [
46,
47]. Another ex vivo study by Aguiar et al. resulted in 97.4% sensitivity and 100% specificity in distinguishing meningiomas, medulloblastomas, and glioblastomas from health tissue [
46,
48]. These highly promising ex vivo results have laid the foundation for in vivo use of Raman spectroscopy. In a study of 13 glioma patients, RS was used to detect invasive cancer cells that would not be detected with T1 or T2 MRI. It was found that RS was able to intraoperatively detect residual grade 2-4 gliomas with 93% sensitivity and 91% specificity [
49].
In 2017, Orringer et al. described the first application of intraoperative stimulated Raman scattering (SRS) microscopy and developed the stimulated Raman histology (SRH) interpretation technique, which utilizes SRS images to produce simulated hematoxylin and eosin (H&E) stains [
24]. They found that in a sample of 30 patients the intraoperative use of SRH has over 92% accuracy when compared to conventional histological methods in diagnosing brain tumors, and 90% accuracy in elucidating tumor subtypes. In another study on the efficacy of SRH, they created a residual network to predict the presence of tumor cells, non-tumor cells, and low-quality images. Compared to the neuropathologist’s analysis of the samples, the SRH-based residual network predicted the presence of tumor cells with 90.2% accuracy in under four minutes [
24].
The use of Coherent anti-Stokes Raman scattering (CARS) has also illustrated promising results by significantly decreasing the time required for image collection. In a landmark study by Evans et al., the speed of generating CARS images was found to be 30 images per second, compared to a 30-minute CARS image collection time in 1999 [
50]. This reduction in image generation time greatly enhances intraoperative decision-making as well as patient prognoses.
Furthermore, Surface-enhanced Raman scattering (SERS) microscopy has been shown to significantly improve prognosis in post-resection glioma. Han et al. used a SERS scanner intraoperatively until all Raman signals were completely absent in the tissue, and longitudinal MRI analysis indicated that this SERS-guided resection greatly reduced glioma recurrence rate in rat models. SERS has also been used to distinguish acidic margins of gliomas, as extracellular acidosis is a marker for cancer cell infiltration [
51]. Jin et al. used a SERS navigation system to locate these acidic margins in animal models as well as in excised tissue from glioma patients and found that the level of acidity correlates with the level of cancer cell proliferation and density. The post-operative survival of animal models was greatly enhanced compared to conventional methods, indicating a promising prognosis for glioma patients [
51].
Finally, tip-enhanced Raman scattering (TERS) microscopy achieves both the enhancement of SERS and the high spatial resolution of SPM, thereby overcoming the diffraction limit for more accurate images [
52]. As TERS is a relatively new technique, the literature shows limited in vivo analysis of SERS in neurosurgery.
4. Confocal Laser Endomicroscopy
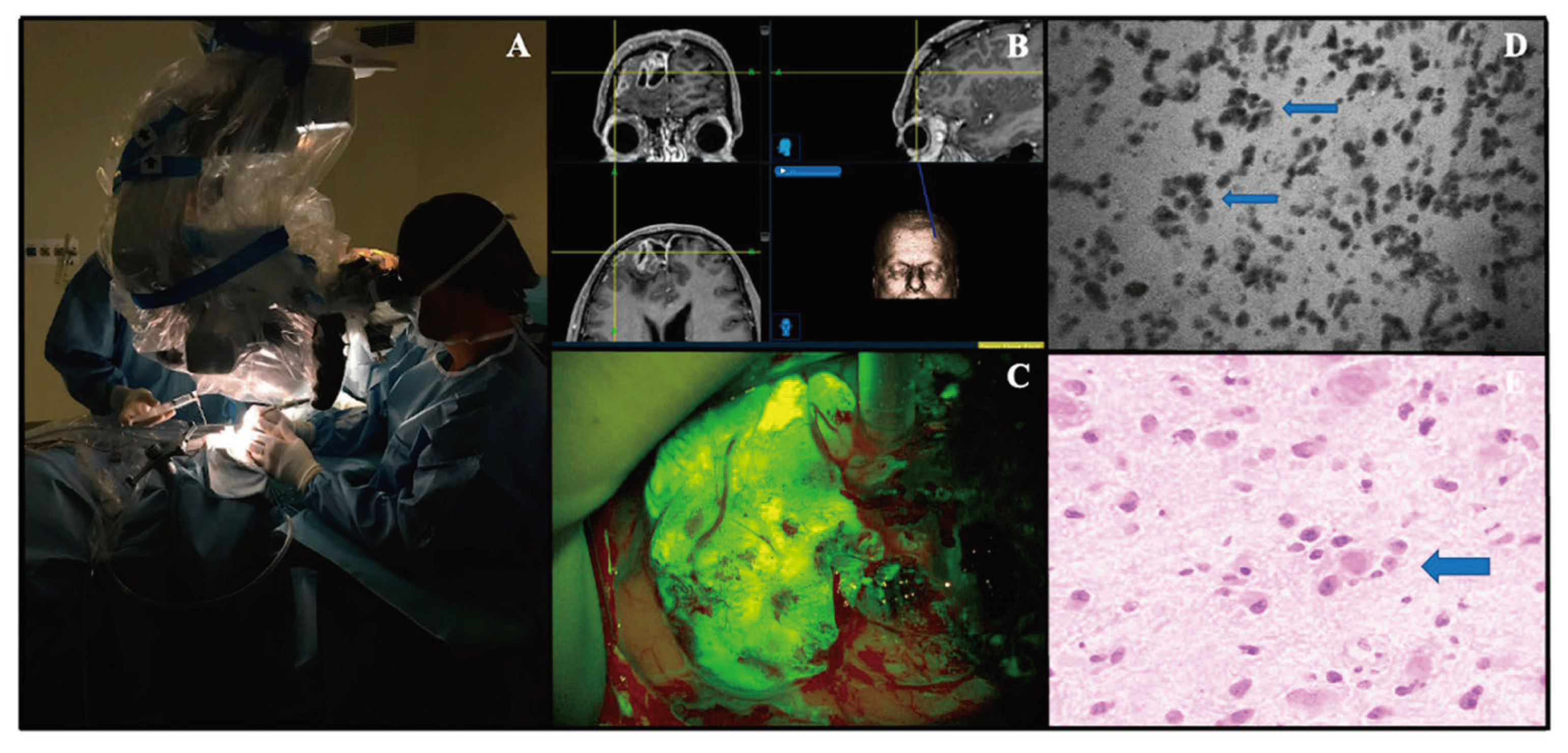
Confocal laser endoscopy has grown in popularity for intraoperative, real-time imaging (
Figure 3). The use of real-time imaging is especially useful in tumor resections as surgeons can effectively evaluate histoarchitecture and the need for resection of specific pieces of tissue. Using intraoperative imaging reduces the time required for tissue collection and standard biopsy procedures involving in-person pathologists. Instead, surgeons can employ the CLE in the OR and send the live camera feed to a pathologist remotely or in person for analysis. With a pathologist on alert, analysis of histoarchitecture can be done instantly. As such, the surgeon can more effectively execute the resection [
53]. CLE has been widely implemented in gastroenterology because of its remarkable accuracy in detecting neoplasia of the colon [
54]. However, the technology has yet to reach widespread practice in neurosurgery. The gold standard remains frozen sectioning.
4.1. Indications
Because CLE allows surgeons an insight into the histological and cytologic features of tissues, most indications are neoplastic in nature. It allows surgeons to determine if a tissue is dysplastic, neoplastic, or healthy. More specifically, CLE is used to investigate, monitor, and resect tissue samples from meningiomas, gliomas, and pituitary adenomas.
4.2. Techniques
CLE devices are classified as either probe-based or endoscope-based. Either way, the laser is directed until it is in direct contact with the tissue of interest. The laser works by sending light via a straight line into the tissue. Light rays then reflect off the tissue back towards the laser. Reflections are redirected through the same pinhole as the light source (thus the name confocal). The sensor is placed proximally to the pinhole, excluding all the light that is scattered or refocused out of the imaging plane. By doing so, the laser is able to increase the spatial resolution of the image [
56,
57]. An additional measure to increase resolution has been the advent of a photoactive contrast enhancer. Contrast is commonly fluorescein sodium [
58,
59].
4.3. Outcomes
Focusing on image quality, brightness, contrast, and resolution are parameters to examine. Brightness and contrast have been shown to be higher for in vivo imaging that uses CLE relative to ex vivo images that use traditional biopsy methods [
58,
60]. Another non-clinical measure critical to CLE is the reduction of time spent on tissue analysis. In 2016, Martirosyan et al. conducted one of the first preclinical tests for feasibility. The study showed a decrease in time as the system was only in use for an average of 15.7 minutes; a marked improvement from the typical 20+ minutes required for frozen histology [
61].
Feasibility of CLE was further confirmed by determining comparable, if not better, accuracy with decreased time spent in analysis [
62,
63,
64]. First, CLE was compared to frozen histology in ex vivo studies. In doing so, concordance was found to be 80% for determining correct diagnosis and 93.3% for categorizing patterns at the tumor core [
65].
Only a handful of studies have been performed to show the use of CLE in vivo. In the first, intraoperative CLE was shown to correctly diagnose neoplasia while reducing operation time. The device was reported as easy to use due to its similarity to other microsurgical instruments [
66].
A later study showed in a 30-patient sample that CLE could be used with nearly identical accuracy as frozen sectioning and permanent histology. The diagnostic accuracy, sensitivity, and specificity reported for CLE vs. frozen section were 94%, 94%, and 100%; for CLE vs. permanent histology 92%, 90%, and 94%, respectively [
67].
5. Conclusions
The field of neurosurgery faces ongoing challenges in the diagnosis and treatment of CNS malignancies. Intraoperative histology has emerged as a critical tool in guiding surgical decision-making, with techniques such as frozen sectioning, Raman histology, and Confocal Laser Endomicroscopy (CLE) offering invaluable insights into tumor pathology. Frozen sectioning provides rapid intraoperative analysis in tumor resection, and while highly accurate, tissue distortion and interpretive errors remain challenges. Stimulated Raman histology (SRH) offers high-resolution imaging and has demonstrated efficacy in the diagnosis of brain tumors and their subtypes. Surface-enhanced Raman scattering (SERS) microscopy has shown promise in detecting tumor margins and reducing glioma recurrence rates, and tip-enhanced Raman scattering (TERS) microscopy conveys enhanced spatial resolution. Coherent anti-Stokes Raman scattering (CARS) microscopy allows for rapid imaging and improved intraoperative decision-making, and Confocal Laser Endomicroscopy (CLE) enables real-time visualization of histoarchitecture and therefore precise tumor resection. incorporation of these intraoperative histological techniques into clinical practice holds great potential for improving patient outcomes in neurosurgical oncology. Continued investigation and refinement of these methods are essential to further advance their utility and address existing limitations. By harnessing the power of intraoperative histology, neurosurgeons can strive towards more precise and personalized treatments for patients with CNS malignancies.
Author Contributions
Conceptualization, A.M.; methodology, A.M. and B.L.; validation, A.M. and B.L.; investigation, A.M., E.S., C.W., Z.K., K.N.; writing—original draft preparation, A.M., E.S., C.W., Z.K., K.N., B.L.; writing—review and editing, A.M., E.S., C.W., Z.K., K.N., B.L.; visualization, A.M., B.L.; supervision, A.M., B.L.; project administration, A.M., B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiology Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: a global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef]
- Ilic, I.; Ilic, M. International patterns and trends in the brain cancer incidence and mortality: An observational study based on the global burden of disease. Heliyon 2023, 9, e18222. [Google Scholar] [CrossRef]
- Cioffi, G.; Waite, K.A.; Edelson, J.L.; Kruchko, C.; Ostrom, Q.T.; Barnholtz-Sloan, J.S. Changes in survival over time for primary brain and other CNS tumors in the United States, 2004–2017. J. Neuro-Oncology 2022, 160, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Mannen H: Historical review of development of neuro-historogical techniques and three-dimensional reconstruction of individual neurons. In: Clinical Neurology. 2006.
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nikova A, Birbilis T: The Basic Steps of Evolution of Brain Surgery. Maedica (Bucur). 2017, 12.
- Gal, A.A. The Centennial Anniversary of the Frozen Section Technique at the Mayo Clinic. Arch. Pathol. Lab. Med. 2005, 129, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Wheeler TM: ORIGIN AND DEVELOPMENT OF AMERICAN SURGICAL PATHOLOGY. Trans Am Clin Climatol Assoc. 2020, 131:326–34.
- Jaafar H: Intra-operative frozen section consultation: concepts, applications and limitations. Malays J Med Sci. 2006, 13:4–12.
- A Novis, D.; Zarbo, R.J. Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-Probes study of 32868 frozen sections in 700 hospitals. Arch Pathol Lab Med. 1997, 121, 559–67. [Google Scholar]
- Murugappan, S.; Tofail, S.A.M.; Thorat, N.D. Raman Spectroscopy: A Tool for Molecular Fingerprinting of Brain Cancer. ACS Omega 2023, 8, 27845–27861. [Google Scholar] [CrossRef]
- Khoddami M, Akbarzadeh A, Mordai A, Bidari-Zerehpoush F, Alipour H, Samadzadeh S, Alipour B: Diagnostic accuracy of frozen section of central nervous system lesions: a 10-year study. Iran J Child Neurol. 2015, 9:25–30.
- Kang, M.; Chung, D.H.; Kim, N.R.; Cho, H.Y.; Ha, S.Y.; Lee, S.; An, J.; Seok, J.Y.; Yie, G.-T.; Yoo, C.J.; et al. Intraoperative Frozen Cytology of Central Nervous System Neoplasms: An Ancillary Tool for Frozen Diagnosis. J. Pathol. Transl. Med. 2019, 53, 104–111. [Google Scholar] [CrossRef]
- Uematsu, Y.; Owai, Y.; Okita, R.; Tanaka, Y.; Itakura, T. The usefulness and problem of intraoperative rapid diagnosis in surgical neuropathology. Brain Tumor Pathol. 2007, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chand, P.; Amit, S.; Gupta, R.; Agarwal, A. Errors, limitations, and pitfalls in the diagnosis of central and peripheral nervous system lesions in intraoperative cytology and frozen sections. J. Cytol. 2016, 33, 93–97. [Google Scholar] [CrossRef]
- Plesec, T.P.; Prayson, R.A. Frozen Section Discrepancy in the Evaluation of Central Nervous System Tumors. Arch. Pathol. Lab. Med. 2007, 131, 1532–1540. [Google Scholar] [CrossRef]
- Mitra, S.; Kumar, M.; Sharma, V.; Mukhopadhyay, D. Squash preparation: A reliable diagnostic tool in the intraoperative diagnosis of central nervous system tumors. J. Cytol. 2010, 27, 81–5. [Google Scholar] [CrossRef] [PubMed]
- Gandour-Edwards, R.F.; Donald, P.J.; Boggan, J.E. Intraoperative Frozen Section Diagnosis in Skull Base Surgery. J. Neurol. Surg. Part B: Skull Base 1993, 3, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Savargaonkar P, Farmer PM: Utility of intra-operative consultations for the diagnosis of central nervous system lesions. Ann Clin Lab Sci. 2001, 31:133–9.
- Duvuru, P.; Rao, S.; Rajkumar, A.; Ehtesham, M.D. Challenges in neurosurgical intraoperative consultation. Neurol. India 2009, 57, 464–8. [Google Scholar] [CrossRef]
- Terrones, O.; Olazar-Intxausti, J.; Anso, I.; Lorizate, M.; Nieto-Garai, J.A.; Contreras, F.-X. Raman Spectroscopy as a Tool to Study the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2023, 24, 2384. [Google Scholar] [CrossRef]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Wadiura, L.I.; Kiesel, B.; Roetzer-Pejrimovsky, T.; Mischkulnig, M.; Vogel, C.C.; Hainfellner, J.A.; Matula, C.; Freudiger, C.W.; Orringer, D.A.; Wöhrer, A.; et al. Toward digital histopathological assessment in surgery for central nervous system tumors using stimulated Raman histology. Neurosurg. Focus 2022, 53, E12. [Google Scholar] [CrossRef] [PubMed]
- Hollon, T.; Lewis, S.; Freudiger, C.W.; Sunney Xie, X.; Orringer, D.A. Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg. Focus 2016, 40, E9. [Google Scholar] [CrossRef] [PubMed]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.-A.; Obaid, S.; Marple, E.; Urmey, K.; Trudel, D.; Soulez, G.; et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hollon, T.; Orringer, D.A. Label-free brain tumor imaging using Raman-based methods. J. Neuro-Oncology 2021, 151, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, J.C.; Wang, Z.; Huang, S. Raman Spectroscopy on Brain Disorders: Transition from Fundamental Research to Clinical Applications. Biosensors 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikova, E.; Ralbovsky, N.M.; Sikirzhytski, V.; Kazakov, O.; Halamkova, L.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Raman spectroscopy and machine learning for biomedical applications: Alzheimer’s disease diagnosis based on the analysis of cerebrospinal fluid. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2020, 248, 119188. [Google Scholar] [CrossRef]
- Lochocki, B.; Morrema, T.H.J.; Ariese, F.; Hoozemans, J.J.M.; de Boer, J.F. The search for a unique Raman signature of amyloid-beta plaques in human brain tissue from Alzheimer's disease patients. Anal. 2020, 145, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, J.C.; Wang, Z.; Huang, S. Raman Spectroscopy on Brain Disorders: Transition from Fundamental Research to Clinical Applications. Biosensors 2022, 13, 27. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Martin-Hirsch, P.L.; Martin, F.L. Progress and Challenges in the Diagnosis of Dementia: A Critical Review. ACS Chem. Neurosci. 2018, 9, 446–461. [Google Scholar] [CrossRef]
- Stern, D.; Schoenlein, R.W.; Puliafito, C.A.; Dobi, E.T.; Birngruber, R.; Fujimoto, J.G. Corneal Ablation by Nanosecond, Picosecond, and Femtosecond Lasers at 532 and 625 nm. Arch. Ophthalmol. 1989, 107, 587–592. [Google Scholar] [CrossRef]
- Orillac C, Hollon T, Orringer DA: Clinical Translation of Stimulated Raman Histology. 225–36.10. 1007.
- Brzozowski, K.; Matuszyk, E.; Pieczara, A.; Firlej, J.; Nowakowska, A.; Baranska, M. Stimulated Raman scattering microscopy in chemistry and life science – Development, innovation, perspectives. Biotechnol. Adv. 2022, 60, 108003. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Yi, R.; Liu, L.; Qu, J. Coherent Anti-Stokes Raman Scattering Microscopy and Its Applications. Front. Phys. 2020, 8. [Google Scholar] [CrossRef]
- Evans, C.L.; Xie, X.S. Coherent Anti-Stokes Raman Scattering Microscopy: Chemical Imaging for Biology and Medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 141–176. [Google Scholar]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Han, X.X.; Zhao, B.; Ozaki, Y. Surface-enhanced Raman scattering for protein detection. Anal. Bioanal. Chem. 2009, 394, 1719–1727. [Google Scholar] [CrossRef]
- Kumar, N.; Mignuzzi, S.; Su, W.; Roy, D. Tip-enhanced Raman spectroscopy: principles and applications. EPJ Tech. Instrum. 2015, 2, 9. [Google Scholar] [CrossRef]
- Li, Z.; Persits, N.; Gray, D.J.; Ram, R.J. Computational polarized Raman microscopy on sub-surface nanostructures with sub-diffraction-limit resolution. Opt. Express 2021, 29, 38027–38043. [Google Scholar] [CrossRef]
- Doran, C.E.; Frank, C.B.; McGrath, S.; Packer, R.A. Use of Handheld Raman Spectroscopy for Intraoperative Differentiation of Normal Brain Tissue From Intracranial Neoplasms in Dogs. Front. Veter- Sci. 2022, 8, 819200. [Google Scholar] [CrossRef]
- Zhang, R.R.; Kuo, J.S. Detection of Human Brain Tumor Infiltration With Quantitative Stimulated Raman Scattering Microscopy. Neurosurgery 2016, 78, N9–N11. [Google Scholar] [CrossRef]
- Aguiar, R.P.; Silveira, L.; Falcão, E.T.; Pacheco, M.T.T.; Zângaro, R.A.; Pasqualucci, C.A. Discriminating Neoplastic and Normal Brain Tissuesin VitroThrough Raman Spectroscopy: A Principal Components Analysis Classification Model. Photomed. Laser Surg. 2013, 31, 595–604. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Mercier, J.; St-Arnaud, K.; Guiot, M.-C.; Leblond, F.; Petrecca, K. Raman spectroscopy detects distant invasive brain cancer cells centimeters beyond MRI capability in humans. Biomed. Opt. Express 2016, 7, 5129–5137. [Google Scholar] [CrossRef]
- Evans, C.L.; Potma, E.O.; Puoris'Haag, M.; Côté, D.; Lin, C.P.; Xie, X.S. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl. Acad. Sci. 2005, 102, 16807–16812. [Google Scholar] [CrossRef]
- Jin, Z.; Yue, Q.; Duan, W.; Sui, A.; Zhao, B.; Deng, Y.; Zhai, Y.; Zhang, Y.; Sun, T.; Zhang, G.; et al. Intelligent SERS Navigation System Guiding Brain Tumor Surgery by Intraoperatively Delineating the Metabolic Acidosis. Adv. Sci. 2022, 9, 2104935. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, S.; Wang, R.; Sun, M. Tip-Enhanced Raman Spectroscopy. Anal. Chem. 2016, 88, 9328–9346. [Google Scholar] [CrossRef]
- Abramov, I.; Park, M.T.; Gooldy, T.C.; Xu, Y.; Lawton, M.T.; Little, A.S.; Porter, R.W.; Smith, K.A.; Eschbacher, J.M.; Preul, M.C. Real-time intraoperative surgical telepathology using confocal laser endomicroscopy. Neurosurg. Focus 2022, 52, E9. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.W.; Buchner, A.M.; Coron, E.; Woodward, T.A.; Raimondo, M.; Dekker, E.; Fockens, P.; Wallace, M.B. Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: a prospective study. Gastrointest. Endosc. 2011, 75, 525–533. [Google Scholar] [CrossRef]
- Restelli, F.; Pollo, B.; Vetrano, I.G.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef]
- Aisenberg, J. Gastrointestinal endoscopy nears “the molecular era”. Gastrointest. Endosc. 2008, 68, 528–530. [Google Scholar] [CrossRef]
- Wang, T.D.; Van Dam, J. Optical biopsy: A new frontier in endoscopic detection and diagnosis. Clin. Gastroenterol. Hepatol. 2004, 2, 744–753. [Google Scholar] [CrossRef]
- Xu, Y.; Abramov, I.; Belykh, E.; Mignucci-Jiménez, G.; Park, M.T.; Eschbacher, J.M.; Preul, M.C. Characterization of ex vivo and in vivo intraoperative neurosurgical confocal laser endomicroscopy imaging. Front. Oncol. 2022, 12, 979748. [Google Scholar] [CrossRef]
- Restelli, F.; Mathis, A.M.; Höhne, J.; Mazzapicchi, E.; Acerbi, F.; Pollo, B.; Quint, K. Confocal laser imaging in neurosurgery: A comprehensive review of sodium fluorescein-based CONVIVO preclinical and clinical applications. Front. Oncol. 2022, 12, 998384. [Google Scholar] [CrossRef]
- Kiesslich, R.; Neurath, M.F. Chromoendoscopy and Other Novel Imaging Techniques. Gastroenterol. Clin. North Am. 2006, 35, 605–619. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef]
- Breuskin, D.; Szczygielski, J.; Urbschat, S.; Kim, Y.-J.; Oertel, J. Confocal Laser Endomicroscopy in Neurosurgery—An Alternative to Instantaneous Sections? World Neurosurg. 2017, 100, 180–185. [Google Scholar] [CrossRef]
- Charalampaki, P.; Javed, M.; Daali, S.; Heiroth, H.-J.; Igressa, A.; Weber, F. Confocal Laser Endomicroscopy for Real-time Histomorphological Diagnosis. Neurosurgery 2015, 62, 171–176. [Google Scholar] [CrossRef]
- Pavlov, V.; Meyronet, D.; Meyer-Bisch, V.; Armoiry, X.; Pikul, B.; Dumot, C.; Beuriat, P.-A.; Signorelli, F.; Guyotat, J. Intraoperative Probe-Based Confocal Laser Endomicroscopy in Surgery and Stereotactic Biopsy of Low-Grade and High-Grade Gliomas. Neurosurgery 2016, 79, 604–612. [Google Scholar] [CrossRef]
- Acerbi, F.; Pollo, B.; De Laurentis, C.; Restelli, F.; Falco, J.; Vetrano, I.G.; Broggi, M.; Schiariti, M.; Tramacere, I.; Ferroli, P.; et al. Ex Vivo Fluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO® System) in Patients With Glioblastoma: Results From a Prospective Study. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: confocal laser endomicroscopy in neurosurgery. Clinical and user experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef]
- Abramov, I.; Park, M.T.; Belykh, E.; Dru, A.B.; Xu, Y.; Gooldy, T.C.; Scherschinski, L.; Farber, S.H.; Little, A.S.; Porter, R.W.; et al. Intraoperative confocal laser endomicroscopy: prospective in vivo feasibility study of a clinical-grade system for brain tumors. J. Neurosurg. 2023, 138, 587–597. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).