1. Introduction
Pain, weakness, and sensory deficits, including numbness and paresthesia, are widely prevalent. Chronic pain affects 20.9% of all adults in the United States, [
1] reduced muscle strength is reported by 18% of those 60 years and above and 53% of those 80 and above, [
2] and peripheral neuropathy, a common cause of numbness and paresthesia, affects over 14.5 million Americans [
3]. Symptoms may be due to a wide variety of etiologies with the broadest classification schemes dividing causes into central or peripheral pathologies, with further differentiation between neurologic and musculoskeletal disease.
Because symptoms are relatively subjective and difficult to objectively characterize, a variety of diagnostic modalities are used to identify a diagnosis, including physical examination, imaging, and tissue sampling. While electromyography and nerve conduction studies have improved the ability to diagnose such conditions, muscle and nerve biopsies remain the gold standard for diagnosis in many neurologic and musculoskeletal conditions. However, as further studies into the molecular and genetic etiologies of such neurologic and musculoskeletal pathologies are performed, the need for invasive tissue sampling continues to diminish. We aim to discuss this controversy and highlight the role of neurosurgical intervention in nerve and muscle biopsies.
Muscle biopsies are typically performed later in the diagnostic workup, e.g., to confirm the presence of a muscular dystrophy or an inflammatory myositis. However, when the differential diagnosis is extensive, early muscle biopsy can quickly lead to a diagnosis and allow for earlier intervention. Muscle biopsies are done by a surgeon, commonly a neurosurgeon, in conjunction with a pathologist. Typically, an affected muscle is located by physical examination, although electrodiagnostic or magnetic resonance imaging (MRI) may also be used [
4]. Muscle biopsies are often performed under local anesthesia. The surgeon dissects down to the muscle and identifies a group of muscle fibers at the belly of the muscle, after which the surgeon excises in parallel to the length of the muscle fiber a sample approximately 1 centimeter (cm) in length and 0.5 cm in diameter. Once removed, the specimen is packaged for examination by a pathologist. Muscle biopsy typically produces no residual deficits [
4].
Nerve biopsies are comparable in that they are typically performed later in the diagnostic workup and are also done by a surgeon, commonly a neurosurgeon, in conjunction with a pathologist. Nerve biopsies are especially useful in diagnosing inflammatory neuropathies, autoimmune neuropathies (e.g., nerve vasculitides, chronic inflammatory demyelinating polyneuropathy), and hereditary neuropathies and can alter treatment in up to 60% of cases [
5]. Sensory nerves are the ideal targets rather than motor nerves, as nerve biopsies often result in temporary nerve damage and sensory deficits are tolerated better than motor weakness. As such, transient sensory loss, which is rarely permanent and infrequently associated with painful paresthesia, may occur after nerve biopsy. The sural nerve is the most common site of biopsy although many other nerves may also be sampled when indicated by physical examination, electrophysiological studies, or MRI [
5]. Like muscle biopsies, nerve biopsies are commonly done under local anesthesia, although they require a large incision to ensure safe and adequate dissection from all surrounding fat and connective tissue. After careful dissection, the nerve is isolated and extracted before undergoing extensive preparation for pathologist examination.
2. Nerve Biopsy Indications
Nerve biopsy plays an important diagnostic and evaluative role in cases of clear and unclear pathology [
6]. Nerve biopsy can also assist in attaining a definitive diagnosis in cases of vasculitis, neurosarcoidosis, neurolymphomatosis, amyloidosis and neuritic leprosy [
7,
8,
9,
10,
11]. In addition, nerve biopsy can be instrumental in cases of rapidly progressive peripheral neuropathy or peripheral neuropathy of an unexplained cause [
12,
13,
14,
15]. However, the diagnostic role for nerve biopsy may differ from one pathology to another (
Table 1) [
6]. Despite its variable importance, the many indications and clear diagnostic role make neurosurgical intervention for nerve biopsies an important aspect of the clinical decision making process.
2.1. Nerve Biopsy: High Importance
2.1.1. Vasculitis
Vasculitic neuropathy is a common indication for nerve biopsy with a reported sensitivity of 54.76% and reports of a 5.1% increase in diagnostic yield when underwent with a combined muscle biopsy [
17,
18,
19]. When underwent with a combined muscle and skin biopsy, cutaneous vasculitis was identified in 20% of suspected patients with vasculitic neuropathy [
20]. Besides evidence of potential diagnostic benefits of full thickness skin biopsy, different quantification stratagies have been explored to increase sensitivity and specificity for diagnosing and differentiating vascultic neuropathies [
20,
21,
22,
23]. Current diagnostic recommendations by the Peripheral Nerve Society dictate biopsy for definitive diagnosis of vasculitic neuropathy and non-systemic vasculitic neuropathy, with the exception of cases of proven systemic vasculitis or diabetic radiculoplexus neuropathy [
11,
24].
The clinical presenation of vasculitic neuropathy is described as multifocal sensorimotor or pure sensory, acute/subacute, painful, asymmetric neuropathy [
11]. Systemic vasculitic neuropathy involves small arteries and large arterioles whereas non-systemic vasculitic neuropathy more typically involves the small arterioles, venules, and capillaries [
16].
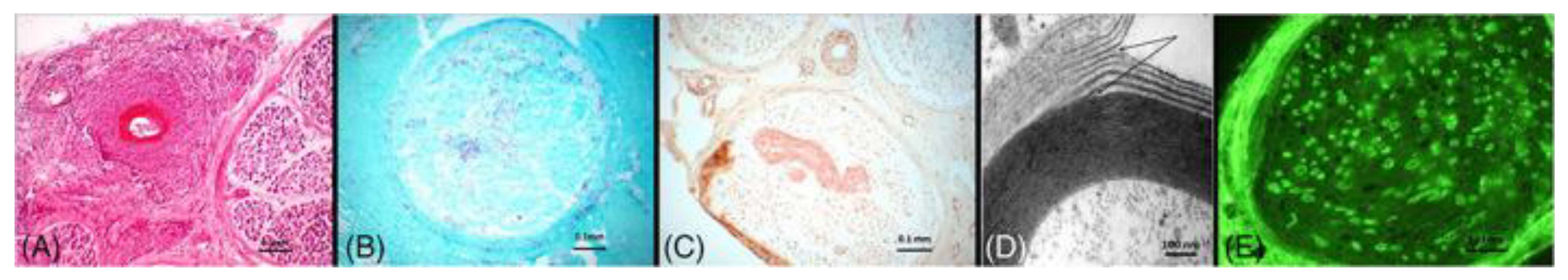
Diagnosis is made upon histologic confirmation of both inflammation within the vessel wall and vascular damage. Other histological findings including predominant axonal changes, perivascular inflammation, hemosiderin deposition, multifocal or asymmetric nerve fiber degeneration, neurovascularization, predominant axonal change or prominent axonal degeneration, and vascular deposition of IgM, fibrinogen, or complement can support a diagnosis of vasculitic neuropathy (
Figure 1) [
6,
11,
16,
25].
2.1.2. Neurolymphomatosis
Neurolymphomastosis is another neurological manifestation, but that of neoplastic nerve infiltration by a hematological malignancy [
7]. Nerve biopsy for diagnosis of neurolymphomastosis does not demonstrate 100% sensitivity, but a reported sensitivity of 88% because of inconsistent distribution of malignant cells [
26]. Biopsy is not need for diagnosis of neurolymphomastosis in cases of a known hematological malignancy presenting with symptoms of neurological manifestations. However, neuropathy experienced in cases of latent or hematological malignancies in remission may only be attributed to malignancy by nerve biopsy [
25]. Utilizing
polymerase chain reaction of biopsy attained lymphoid infiltrate is an additional diagnostic angle utilized to differentiate between inflammatory and malignant infiltrates through the assessment of clonality [
27].
Neurolymphomastosis presents with peripheral or cranial neuropathy, plexopathy, and radiculopathy associated with rapidly evolving, asymmetrically distributing, severe pain [
7].
Positive nerve biopsy will demonstrate malignant cells with non Hodgkin lymphoma representing the usual underlying hematological malignancy [
28].
2.1.3. Peripheral Nerve Tumors
Benign peripheral nerve tumors, commonly schwannomas, neurofibromas, perineurioma, and ganglioneuroma and malignant peripheral nerve tumors, arising denovo or from benign tumors, are another indication for nerve biopsy [
29,
30,
31,
32].
Presentation is that of peripheral neuropathy. Perineuriomas may also present clinically as a plexopathy, but typically present clinically as a mononeuropathy [
33].
Diagnosis typically requires identification of characteristic histological findings of the underlying disease with the use of the immunohistochemical stains S100, glial fibrillary acidic protein (GFAP), epithelial membrane antigen (EMA), and cluster differentiation 34 (CD34) [
29,
30,
31,
32].
2.1.4. Pseudoneoplastic Peripheral Nerve Tumors (Pseudotumors)
Pseudotumors are an important indication for nerve biopsy to attain a definitive diagnosis and differentiate the underlying pathology from a malignancy. Inflammatory pseudotunors and neuromuscular choristomas are specific types of pseudotunors within this rare symptomatic disease category [
34].
Typical presentation is progressive, painful mononeuropathy with associated sensory and strength loss. Nerve biopsy of inflammatory pseudotunors typically reveal chronic inflammatory infiltrates, increased vascularity and lipocytes, and interstitial fibrosis [
34,
35]. Nerve biopsy of neuromuscular choristomas usually demonstrate well differentiated muscle fibers, typically skeletal muscle fibers, interspersed among nerve fascicles.
2.1.5. Neuritic Leprosy
Pure neuritic leprosy is a subtype of leprosy that represents up to 8% of all leprosy cases [
9].
Nerve biopsy is the diagnostic gold standard, with a reported sensitivity of 33.3-75.9% of cases [
36,
37,
38]. Pure neuritic leprosy presents as an isolated peripheral neuropathy. Definitive diagnosis following confirmatory biopsy typically demonstrates acid fast bacilli for lepromatous leprosy and epithelioid caseating and noncaseating granulomas for tuberculoid leprosy. Acid fast bacilli are typically found within Schwan cells and foam cells
(Figure 1) [
9].
2.2. Nerve Biopsy: Medium Importance
2.2.1. Amyloidosis
Amyloid neuropathy is a neurological complication of amyloidosis mainly caused by the deposition of light chain or mutant transthyretin amyloid fibrils [
39]. Nerve biopsy for amyloidosis has a sensitivity range from 30-100%. Sural nerve biopsy specifically demonstrates a sensitivity of 80% in detecting mutant transthyretin amyloid fibrils [
40]. Definitive diagnosis criteria of light chain amyloidosis consists of histopathologic findings of amyloid with supporting evidence of amyloid protein composition [
41]. Definitive diagnosis of mutant transthyretin amyloidosis requires evidence of amyloid deposits from biopsy as well as a minimum of two symptoms associated with amyloidosis [
10]. Although many other test options exist for conclusive diagnosis of amyloidosis, mainly bone marrow testing and abdominal fat pad biopsy because of their sensitivity, nerve biopsy can provide a definitive diagnosis in cases of negative findings [
42,
43].
Amyloid neuropathy may present with motor symptoms but mainly presents as a sensory neuropathy with autonomic symptoms [
44,
45]. Variability in symptoms and presentation can be attributed to the focal nature of the disease. Because of this, serial sections are examined to establish the diagnosis. Expected findings are unmyelinated or small myelinated nerve fiber loss. Hematoxylin & Eosin or Congo red staining may reveal endo- and epineuria connective tissue deposition of amyloid. Endo- and epineural thickening of blood vessels may also be seen
(Figure 1) [
46].
2.2.2. Neurosarcoidosis
Neurosarcoidosis is a neurological manifestation that can attribute to the neuropathy and other neurological symptoms experienced by up to 16% of patients diagnosed with sarcoidosis [
47,
48].
Definitive diagnosis requires nerve biopsy if clinical manifestations and imaging findings are suggestive of neurosarcoidosis [
49].
If extraneural sarcoidosis is evident on biopsy in the absence of positive nerve biopsy findings, a diagnosis of probable neurosarcoidis may be made when the clinical presentation and imaging findings are supportive [
8,
48,
50].
The neurological manifestations of sarcoidosis include cranial, optic, and peripheral neuropathy, endocrine and hypothalamic dysfunction, seizures, meningitis, myelopathy, and myopathy [
16,
19,
24,
47,
48,
49,
50].
Confirmatory nerve biopsy demonstrates non-caseating granulomas, typically found in the epi- and perineurium, that are independent of other etiologies such as leprosy and tuberculosis [
49].
2.2.3. IgG4 Related Perineural Disease
IgG4 related perineural disease is a rare disease caused by IgG4 positive plasma cell infiltration of tissue within the peripheral nervous system. Definitive diagnosis requires nerve biopsy confirmation of IgG4 positive plasma cell infiltrate [
51]. Patients may present with multifocal neuropathy and reported biopsy findings have been variable [
52,
53,
54]. Axonal and myelin degeneration, epineural infiltration of plasma cells, eosinophils, and lymphocytes, and myelinated nerve fiber reduction all represent a spectrum of features reported for different patient cases [
52,
53,
55,
56].
2.2.4. Paraneoplastic Syndromes
Paraneoplastic syndromes (PNS) can manifest as complex neurological syndromes that can affect multiple parts of the nervous system, including the CNS, neuromuscular junction (NMJ), and peripheral nervous system [
57]. They are caused by malignancies which are often occult and thus found as a result of clinical symptoms attributed to the syndrome [
58]. Paraneoplastic syndromes affect around 8% of individuals with cancer, but are most common in small-cell lung cancer where around 5% of patients are affected by PNS, most commonly Lambert-Eaton myasthenic syndrome.
Clinical presentations vary with each neurologic syndrome but may include cognitive and personality changes, cranial nerve deficits, ataxia, paresthesia, weakness, or numbness [
58]. Common PNS include limbic encephalitis and paraneoplastic cellular degeneration within the CNS, Lambert-Eaton myasthenic syndrome and myasthenia gravis within the NMJ, and autonomic neuropathy and subacute sensory neuropathy within the peripheral nervous system [
59]. The pathogenesis of these syndromes is poorly understood, but current research supports evidence of the cross-reactivity of nervous system antigens that are also ectopically produced by the tumor, producing an immunological response. Thus, paraneoplastic antibodies, or onconeural antibodies, can develop against target antigens [
57]. Additionally, diagnosis is strengthened by the presence of onconeural antibodies in the CSF or serum, which can be highly specific for tumors that may be in earlier stages and not otherwise detected [
58]. However, around 30% of patients with PNS may not have detectable antibodies in the serum or CSF. Thus, diagnostic methods focus on imaging (CT/MRI/PET) to determine the source of malignancy, serologies, EEG, nerve conduction studies, electromyography, as well as CSF analysis for inflammation.
PNS becomes a more “definite” or “probable” diagnosis when patients demonstrate clinical presentations, the presence of cancer, and positive onconeural antibodies [
58]. Current diagnostic procedures do not often include a nerve biopsy to aid diagnosis, as most syndromes rely on nerve conduction or EMG when symptoms of weakness and neuropathy are involved and can be evaluated with less invasive techniques. However, in a case study in 2020, a sural nerve biopsy demonstrating lymphocytic microvasculitis (with both B and T cells) was crucial for a patient presenting with progressive upper and lower limb weakness. The biopsy, along with positive antibodies (anti-Hu and anti-CV2), led suspicion towards paraneoplastic syndrome and subsequent testing including a lymph node biopsy, helped diagnose small cell lung cancer confined to a solitary lymph node as the source of malignancy [
60]. Thus, nerve biopsies in the setting of unclear etiologies of peripheral neuropathies, may be beneficial in steering diagnosis of paraneoplastic syndromes with unique presentations and previous negative testing.
2.3. Nerve Biopsy: Low Importance
2.3.1. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
CIDP presents with monophasic, progressive, or relapsing symmetrical sensory and motor deficits both proximally and distally [
61,
62].
Although nerve biopsies can be utilized in cases of patients presenting with limited features of demyelination,
diagnosis of CIDP is less reliant on nerve biopsy because of the easy access and availability of supporting criteria from other tests. Investigations have demonstrated that amongst patients diagnosed with CIDP, nerve biopsy may be utilized as the supportive criteria in only 3% of patients [
63].
The main use of nerve biopsy in the context of CIDP is for atypical presentations, when patients are treatment resistant, or to rule out other etiologies of the presenting symptoms [
64].
2.3.2. Paraproteinemic Neuropathy
Paraproteinemic neuropathy is a relatively large class of neuropathy causing disorders that are less reliant on nerve biopsy. Nerve biopsy in paraproteinemic neuropathies is mainly used to support a causal relationship between the presence of specific paraproteins and the clinical presentation of neuropathy. Typical identification of pathologic paraproteins, and their distinguishment form coincidental non-disease-causing paraproteins, is done through the visualization of paraprotein binding to nerve components using indirect immunofluorescence. In addition, high titers of IgM antibodies against ganglioside Q1b and myelin associated glycoprotein alleviate the need for nerve biopsy to determine the cause of the presenting demyelinating neuropathy [
6,
65].
Nerve biopsy can assist in specific diagnosis confirmation of cryoglobulinemic neuropathy. Nerve biopsy in these cases will typically reveal large fiber axonal degeneration. Concaminant features of demyelination, vasculopathy, and vasculitis are also common [
66]. Amorphous material can also be visualized within vacuoles in myelin sheaths [
66,
67].
Nerve biopsy can also assist in confirming paraproteinemic neuropathy in cases where IgM antibodies are visualized against neural antigens including GM1, GM2, GD1a, and GD1b. Nerve biopsy can also be utilized to assist in confirmatory diagnosis of cases where IgG or IgA antibodies are present, visualization of complement binding to myelin or widely spaced myelin, or visualization of immunoglobulin binding to myelin
(Figure 1). Specific features on nerve biopsy can be specific to different types of paraproteinemic neuropathy [
68,
69,
70,
71,
72,
73,
74,
75,
76,
77]. However, because these disorders are less reliant on biopsy for diagnosis, specificity is rarely utilized clinically for diagnosis and confirmation [
6,
65].
2.3.3. Adult Polyglucosan Body Disease
Adult polyglucosan body disease is typically diagnosed by genetic testing followed by glycogen branching enzyme activity assays [
78]. Nerve biopsy is only indicated if enzyme activity assay results are ambiguous. Reported biopsy findings included polyglucosan bodies, however these findings were also found in patients without the disease [
6,
79,
80].
2.3.4. Lysosomal & Perioxisomal Storage Disorders
Nerve biopsy is only indicated in the presence of atypical manifestations/presentations or if usual testing fails to determine a highly suspected diagnosis of neuropathy secondary to lysosomal and peroxisomal storage disorders [
81]. Nerve biopsy may reveal Zebra or Tuff stone bodies, a result of intralysosomal material accumulation in Schwan cells and neurons, onion bulb structures in patients with Refsum’s disease, or prismatic inclusions in patients with leukodystrophies and other sphingolipidoses [
6,
81].
2.3.5. Pure Motor Neuropathy
Pure motor neuropathy can be difficult to differentiate from progressive muscular atrophy. In such cases, nerve biopsy can assist in diagnosis. Nerve biopsy of pure motor neuropathy has demonstrated 95% sensitivity when biopsy is conducted on the motor branch of the obturator nerve [
82].
Biopsy findings suggestive of pure motor neuropathy include high regenerative activity, findings suggestive of demyelination and remyelination, and deposits of axonal inclusions or amyloid. Low regenerative activity and axonal degeneration are more consistent with a motor neuron disease and not a pure motor neuropathy [
6,
82].
2.3.6. Diabetic Neuropathy
Nerve biopsy in cases of suspected diabetic neuropathy can be indicated in the presence of atypical manifestations/presentations or to determine the true etiology of the patient’s symptoms when other superimposed disorders, such as an ischemic inflammatory process, may be occurring [
83].
Typical cases of diabetic neuropathy do not require morphological confirmation [
44].
2.3.7. Cryptogenic Neuropathy
Similarly, nerve biopsy in cases of cryptogenic neuropathy is not typically indicated but may be useful in cases of high suspicion where extensive workup is inconclusive [
13,
15].
Nerve biopsy findings are expected to yield 0-37% of useful diagnostic information [
84,
85].
2.3.8. Hereditary Neuropathy
Owing to next generation sequencing, nerve biopsy does not play an important diagnostic role in patients with hereditary neuropathies. Specific select cases may benefit from nerve biopsy, but other diagnostic tools are sufficient in most cases [
86].
2.3.9. Other Neuropathies
Consistent with what has been discussed for all nerve biopsy indications of low importance, several other neuropathies that are not reliant on nerve biopsy for diagnosis may still benefit from nerve biopsy in select cases. This includes toxic neuropathies from specific medications, neuropathy secondary to environmental or industrial exposure, diffuse infiltrative lymphocytosis syndrome, and chronic idiopathic sensory axonal neuropathy [
87,
88,
89,
90,
91,
92,
93]. Nerve biopsy in such cases may assist in diagnostic clarification, in cases of rapid progression or atypical presentations, or exploration of disease progression and potential treatments [
94,
95,
96].
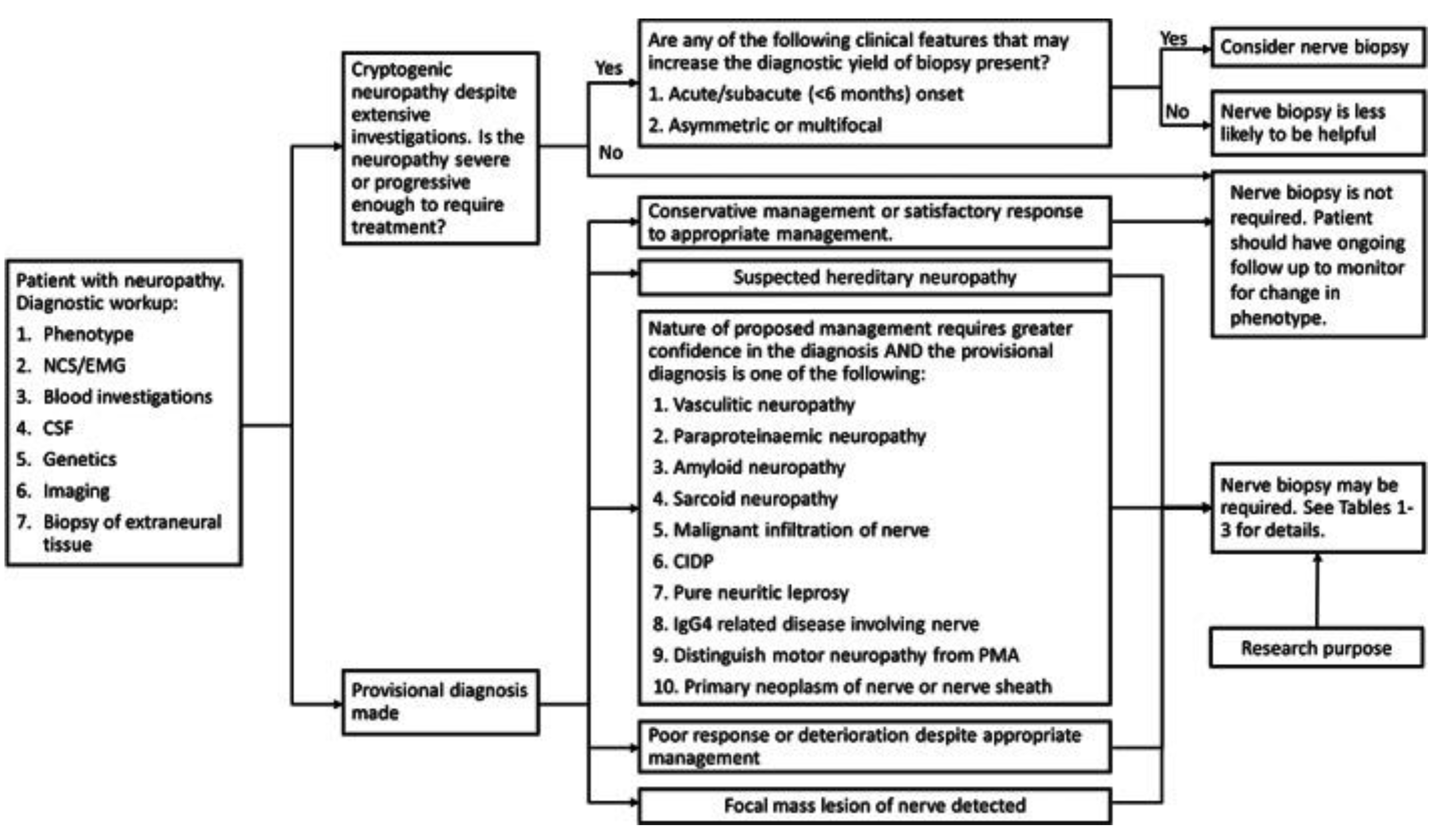
Figure 2 demonstrates a proposed decision tree for performing nerve biopsies and
Table 2 demonstrates when biopsy is indicated for a suspected diagnosis.
3. Muscle Biopsy Indications
Muscle biopsy is an important component in disease evaluation and diagnosis [
97]. Muscle biopsy is specifically required to definitively diagnose hereditary disorders such as muscular and myotonic dystrophies, congenital myopathies, channelopathies, primary metabolic disorders, disorders of carbohydrate and lipid metabolism, and acquired myopathies such as inflammatory, toxic/drug-induced, endocrine, and systemic illness-associated myopathies [
98]. Muscle biopsy also plays a significant role in the general evaluation of patients with neuromuscular disease, the progression and course of a disease, and the differentiation of neurogenic and myogenic disorders. Considering the many indications and its large evaluative and diagnostic role, neurosurgical intervention for muscle biopsies remains an important component of medical reasoning and decision making.
3.1. Muscle Biopsy: High Indication
3.1.1. Polyarteritis Nodosa (PAN)
Polyarteritis nodosa (PAN) is a medium-sized vessel, immune complex related vasculitis that causes necrosis [
99].
PAN can be caused by viral infections like Hepatitis B, Hepatitis C, HIV, CMV, and Parvovirus B19. However, majority of cases are idiopathic. This condition affects multiple systems in the body, with central nervous system involvement being associated with higher mortality. PAN diagnosis can be made following histological confirmation of muscle, skin, or nerve biopsy of the affected area [
100].
PAN can affect blood vessels systemically, with the potential of hemorrhage or ischemia in any organ because of the abundance of blood vessels throughout the body [
101].
Clinically, PAN can manifest with systemic, cutaneous, neurological, gastrointestinal, urologic, ophthalmologic, cardiac, and respiratory symptoms. The most common neurological manifestations are mononeuritis multiplex occurring in 38-72% of cases, peripheral neuropathy occurring in 74% of cases, central nervous system involvement occurring in 2-28% of cases, and cranial nerve palsy in <2% of cases [
100]
.
Combined muscle and nerve biopsies were effective in providing histologic confirmation of vasculitis in 83% of cases, whereas muscle biopsies alone were only effective at confirming vasculitis in 65% of cases. Histopathology typically demonstrates inflammatory infiltrates; lymphocytes, macrophages, neutrophils, and eosinophils. Granulomas and giant cells are not observed, but fibrinoid necrosis is typically seen in active lesions. Early in the disease course, fibrinoid necrosis will present with neutrophil involvement, followed by lymphocyte and macrophage involvement. In advanced lesions, neoangiogenesis and vascular remodeling will be seen with intimal hyperplasia and diffuse fibrotic changes in the vessel wall. Thrombosis can typically appear, as well as microaneurysms due to severe vessel wall injury [
100].
3.1.2. Dystrophinopathy
Dystrophinopathies are X-linked muscle diseases that result in muscle atrophy and fibrosis. The underlying pathophysiology of dystophinopathies involves a deficiency in the dystrophin protein [
102].
The most common dystrophinopathies are Duchenne and Becker muscular dystrophies. Duchenne’s muscular dystrophy (DMD) is recognized by an absence of dystrophin and dystrophin glycoprotein complex. This deficiency results in membrane fragility, excessive permeability, improper calcium homeostasis, and oxidative damage. The combined impact of these deficiencies result in muscle cell necrosis [
103].
Becker’s muscular dystrophy is a less severe disease, categorized by nonfunctional or decreased production of dystrophin. Because the underlying pathology is less severe, muscular atrophy and other clinical features progress modestly [
104].
Although genetic testing has grown in popularity for its ability to detect 95% of pathogenic variants of DMD, muscular biopsy remains to be a reliable diagnostic adjunct to genetic testing. A 2018 study of diagnosis of DMD, found that the number of muscular biopsies for DMD have remained at a stable level since 1997. They also found that muscle biopsy can provide enhanced diagnostic capacity for patients with later symptom onset, comorbidities, or a pervious normal DMD genetic test [
105].
Furthermore, muscle biopsies provide a better understanding of the disease phenotype through quantification of the dystrophin within the tissue.
Duchenne and Becker muscular dystrophies present relatively similarly, mainly distinguished by the timing of symptom presentation. Duchenne typically presents between the ages of 1.2-8 years, while Becker typically presents between the ages of 5-60 years [
105].
Short stature is common, but developmental milestones are achieved at a normal or slightly delayed pace prior to symptom onset. Infants commonly present with mild hypotonia and poor head control. The clinical presentation usually consists of muscle weakness and difficulty with ambulation. Patients will have difficulty moving up stairs, difficulty running, and are prone to falls. Weakness is typically more prominent in proximal muscles of the lower limb [
103].
Enlargement of calves due to muscular atrophy and adipose replacement in pseudohypertrophy is seem classically in this condition.
Confirmation of diagnosis is typically done through muscle biopsy of the quadriceps femoris and gastrocnemius. On muscle biopsy endomysial connective tissue proliferation, disorganized degeneration and regeneration of myofibers, and muscle fiber necrosis with a mononuclear infiltrate are expected findings. Muscle fiber necrosis will be present, involving characteristic replacement with adipose tissue as seen in pseudohypertrophy [
103].
3.1.3. Trichinosis
Trichinella are nematode parasites that infect humans most commonly from the ingestion of larvae in undercooked, infected meat. They can be found in pigs, boars, and horses who have been infected from eating rats or food containing T. spiralis, T. nativa, or T. britovi. There are an estimated 10,000 cases around the world, but the incidence in the United States has dramatically decreased after the introduction of laws requiring proper cooking of hog food. Once the larvae have been ingested, they develop into adults, begin to mate in the intestines, then releases more larvae. Larvae will travel systemically and invade muscle cells causing a multitude of effects [
106].
Muscle biopsy is the only way to prove absolute certainty of infection [
107]
.
Infection with Trichinella can cause neurotrichinellosis which presents with encephalopathy along with neuromuscular and ocular disturbances [
107].
Neurologic symptoms present in 10-20% of infected patients. Clinical presentation can include meningitis, encephalitis, paresis and paralysis [
108].
Imaging studies will show nodular multifocal hypodensities in patients who are serologically positive [
107]
.
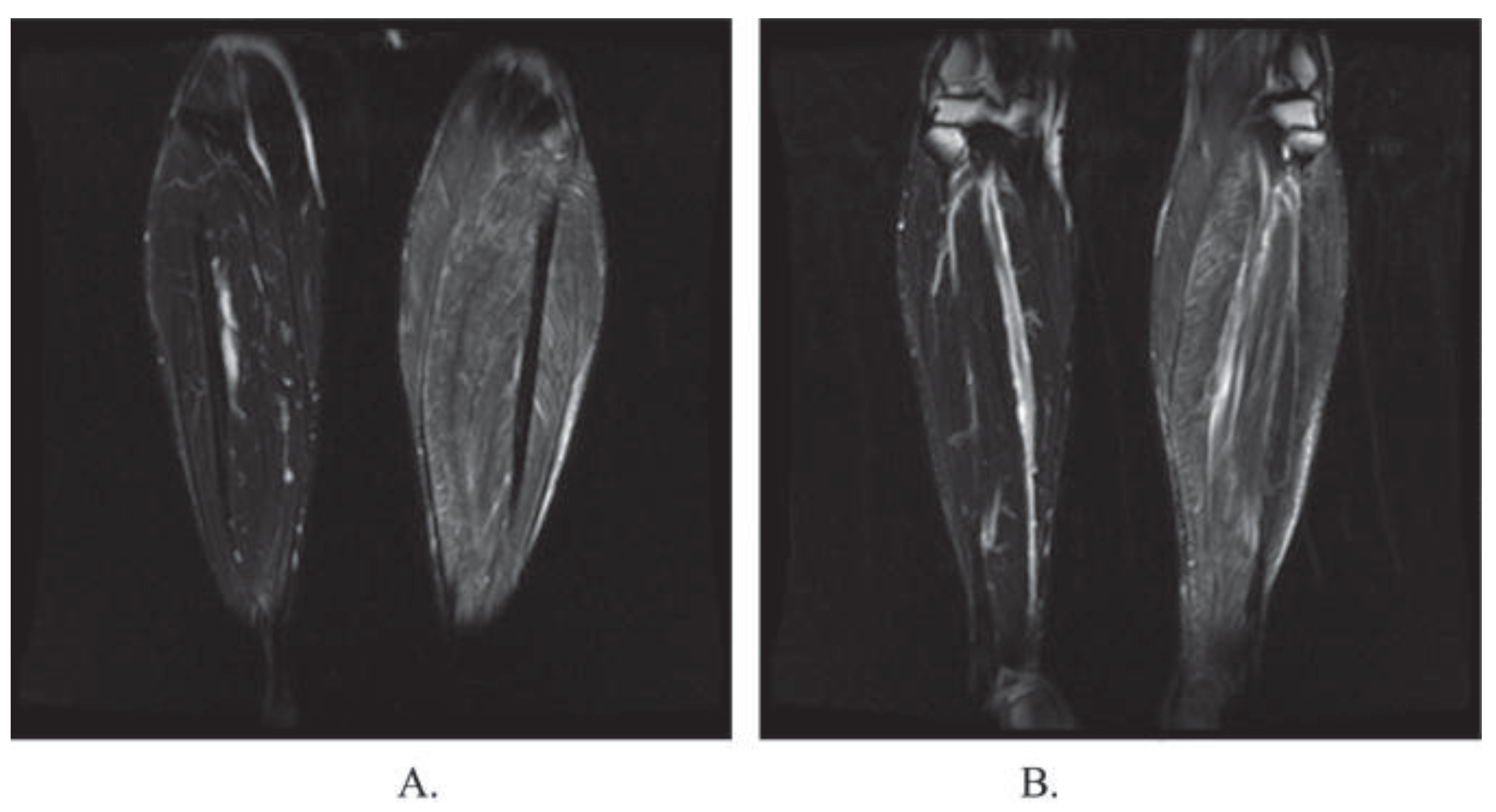
Muscle biopsy is typically done in skeletal muscle, most commonly the diaphragm, extraocular, laryngeal, deltoid, gastrocnemius, and intercostal muscles (
Figure 3). Histology will reveal larvae approximately 1mm long surrounded by intracellular membrane-bound vacuoles. These membrane-bound vacuoles are surrounded by new blood vessels and an eosinophilic infiltrate. Infiltrates are most prominent around dying parasites, which result in calcification and scaring [
106]
.
3.2. Muscle Biopsy: Low Indication
3.2.1. Chloroquine Toxicity
Chloroquine is approved for use in the treatment of certain strains of malaria including P. falciparum, P. ovale, P. vivax, and P. malariae. Chloroquine works by preventing the polymerization of heme into hemozoin. This disruption in the metabolism of heme stops the malarial parasite from using hemozoin as a food source for proliferation [
109].
Alternative indications for chloroquine are dermatomyositis, sarcoidosis, SLE, and some connective tissue diseases. Chloroquine can be used in these alternative diagnoses because of their modification of the immune system. Toxicity can affect cardiac and skeletal muscle, and muscle biopsy can be used as a diagnostic instrument to confirm the diagnosis [
110].
Chloroquine toxicity can cause neuromyopathy that presents as slowly progressing, painless proximal weakness. As toxicity progress, patients experience muscle atrophy that is worse in the legs than arms. Toxicity can reduce sensation and stretch reflexes in muscles, most prominently at the ankles. In terms of dosing, neuromyopathy usually occurs in patients taking 500mg/d for a year or greater but has been documented in patients taking doses as low as 200mg/d. A study that tracked prevalence of myopathy in patients taking antimalarials over the course of 3 years found that the incidence of myopathy was 9.2% [
111].
Laboratory features of chloroquine toxicity will reveal elevated creatine kinase (CK) levels. On nerve conduction studies, reduction of amplitude and reduced velocities can be observed [
112].
Histology of a muscle biopsy will show autophagic vacuoles in 50% of skeletal and cardiac muscle, but type 1 fibers seem to be affected to a greater extent. The vacuoles seen will stain positive for acid phosphatase. When using electron microscopy, vacuoles contain concentric lamellar myeloid debris and curvilinear structures [
110]
.
3.2.2. Amiodarone Toxicity
Amiodarone is the most prescribed anti-arrhythmic medication in the United States [
113].
It is indicated for the treatment of ventricular arrhythmias but is routinely used off-label to treat atrial fibrillation and to prevent ventricular tachyarrhythmias. Amiodarone is a class III antiarrhythmic that blocks potassium currents that repolarize the myocardial cell in phase 3 of the action potential. Amiodarone also blocks beta 1 adrenergic receptors, calcium channels, and sodium channels. Therefore, it has properties of all four classes of anti-arrhythmic medications [
113].
The diagnosis of toxicity can be confirmed by muscle biopsy.
Amiodarone toxicity presents as a neuromyopathy with severe proximal and distal weakness. It also causes sensory loss and reduced stretch reflexes. Typically, the legs are more affected than the arms. The toxic effects are more significant in patients with hypothyroidism from amiodarone use and with renal insufficiency. It is important to note that the risk of myopathy is increased for patients who are also on a statin. Laboratory test will show an elevated CK level and nerve conduction study will show reduced amplitude and slowed conduction. These nerve conduction study (NCS) findings are especially prominent in the lower extremities.
Muscle biopsy will reveal dispersed fibers with autophagic vacuoles. Neurogenic atrophy will be observed, especially in distal muscles. Electron microscopy will reveal myofibrillar disorganization and autophagic vacuoles that are filled with myeloid debris [
111]
.
3.2.3. Pompe Disease
Pompe disease is an inherited glycogen storage disease that causes deficiency of lysosomal acid alpha-glucosidase [
114,
115].
Deficiency of this enzyme causes glycogen to deposit inside of lysosomes within muscular tissue. The incidence of Pompe disease is approximately 1 in 40,000 in the US [
115].
Although muscle biopsy is not the only way to diagnose the condition, it can be used to recognize certain unusual variations in the disease. Muscle biopsy may play a particular role in the diagnosis of underreported variants that may be resistant to enzyme replacement therapy [
114].
Pompe disease can present early in life as the infantile phenotype or later in life as the late onset phenotype. The infantile phenotype presents with symptoms before the age of 1. These symptoms include hypotonia, muscle weakness, motor delay, cardiomegaly, hepatomegaly, and respiratory failure. The late onset phenotype exhibits symptoms in childhood or beyond. These include symptoms of proximal muscle weakness, and respiratory failure. Progression in the late onset phenotype is typically slower [
115].
Muscle biopsy stained with hematoxylin and eosin will show glycogen containing vacuoles that are nonspecific for glycogen storage diseases. The glycogen vacuoles will be in the lysosomes of muscle cells and will stain positively with periodic acid-Shiff [
114,
116]
. Lipofuscin inclusions can also be seen in patients with Pompe disease. These changes in muscle cells are absent in 20-30% of patients who have the late onset of Pompe Disease. Due to the low specificity of these findings, muscle biopsy is not the preferred method of diagnosis [
114,
115].
4. Emerging Biopsy Techniques
4.1. Image-Guided Biopsies
Using imaging modalities like computer tomography (CT), magnetic resonance neurography (MRN), or ultrasound (US) to guide the needle to the precise location has been shown to improve precision and accuracy, increase diagnostic yield, and reduce procedure time. Use of imaging also allows for smaller incisions, improved patient comfort, and decreased complication rates compared to traditional biopsy techniques.
The use of magnetic resonant imaging to visualize neurons, also called MRN, has seen a growing list of techniques, indications, and diagnostic applications in the past two decades [
117,
118]. MRNs depict the entire nerve in 3 dimensions, provide excellent soft tissue contrast, and functions without operator skill limits.
The soft tissue contrast allows for visualization of downstream muscle injury and high contrast resolution between surrounding fat and vascular structures, making it an excellent modality to guide nerve biopsy [
119].
US excels in its provision of real-time visualization and comparison of nerves, portability, and low cost, while still allowing for dynamic imaging with high spatial resolution [
120,
121]. It furthermore operates without ionizing radiation and does not require contrast to operate. Conversely, it does come with certain limitations, chiefly the depth of range, soft tissue contrasts, and a dependability on operator skillset. Despite these limitations, developments in high-resolution US provide valuable neuro-metrics which serve to guide diagnostics and biopsy [
122].
While CT scans have also historically been used to visualize nerves, [
123,
124] US and MRN have emerged as the most far-reaching modalities for peripheral nerve imaging and often provide complimentary information [
125,
126,
127]. The decision of which modality to use for biopsy ultimately depends on the target nerve’s depth and accessibility, imaging availability, and the clinician’s preference.
4.2. Optical Biopsy
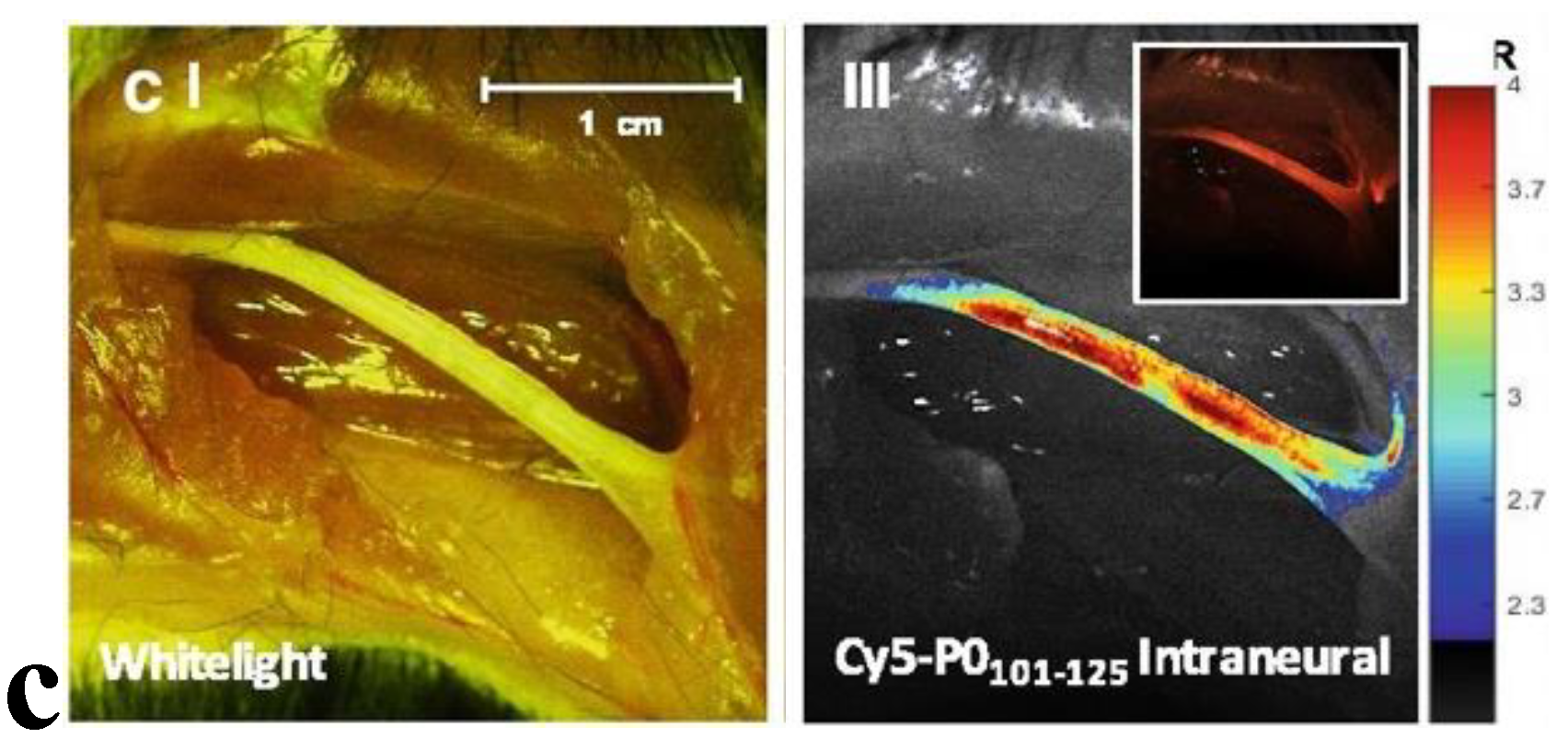
Optical biopsy involves the use of specialized instruments that provide real-time microscopic images of tissues without the need to remove and process samples in a traditional manner. Techniques such as optical coherence tomography (OCT), confocal laser endomicroscopy, and fluorescence-guided biopsy have been explored in various settings for this purpose.
OCT uses low-coherence light to capture micrometer-resolution two- and three-dimensional images from within optical scattering media. Of note, this technique has been used in animal models to obtain high-resolution images of sciatic nerves, [
128,
129] to monitor microvasculature flow around peripheral nerves as they are electrically stimulated, [
130,
131] and to capture functional images of nerves [
132]. The non-invasive nature of this technique makes biopsies of nerves possible without causing damage or impairing function, making studies such as those to visualize retinal nerve fibers in diabetic patients possible [
133,
134].
Another technique within the vein of optical biopsies is confocal microscopy. While outperformed by OCT in depth of visualization and general clinical utility, this technique provides higher quality images of superficial nerves. It’s notably been used to assess peripheral neuropathy in-vivo by visualizing epidermal nerves and corneal nerves [
135,
136,
137]. While its application has seen a particular focus on small corneal nerve fibers to detect diabetic peripheral neuropathy, [
135,
139,
140] it may be potentially useful in other neuropathologies, such as Parkinson’s Disease,[
141] Friedreich’s ataxia,[
142] or amyotrophic lateral sclerosis [
143].
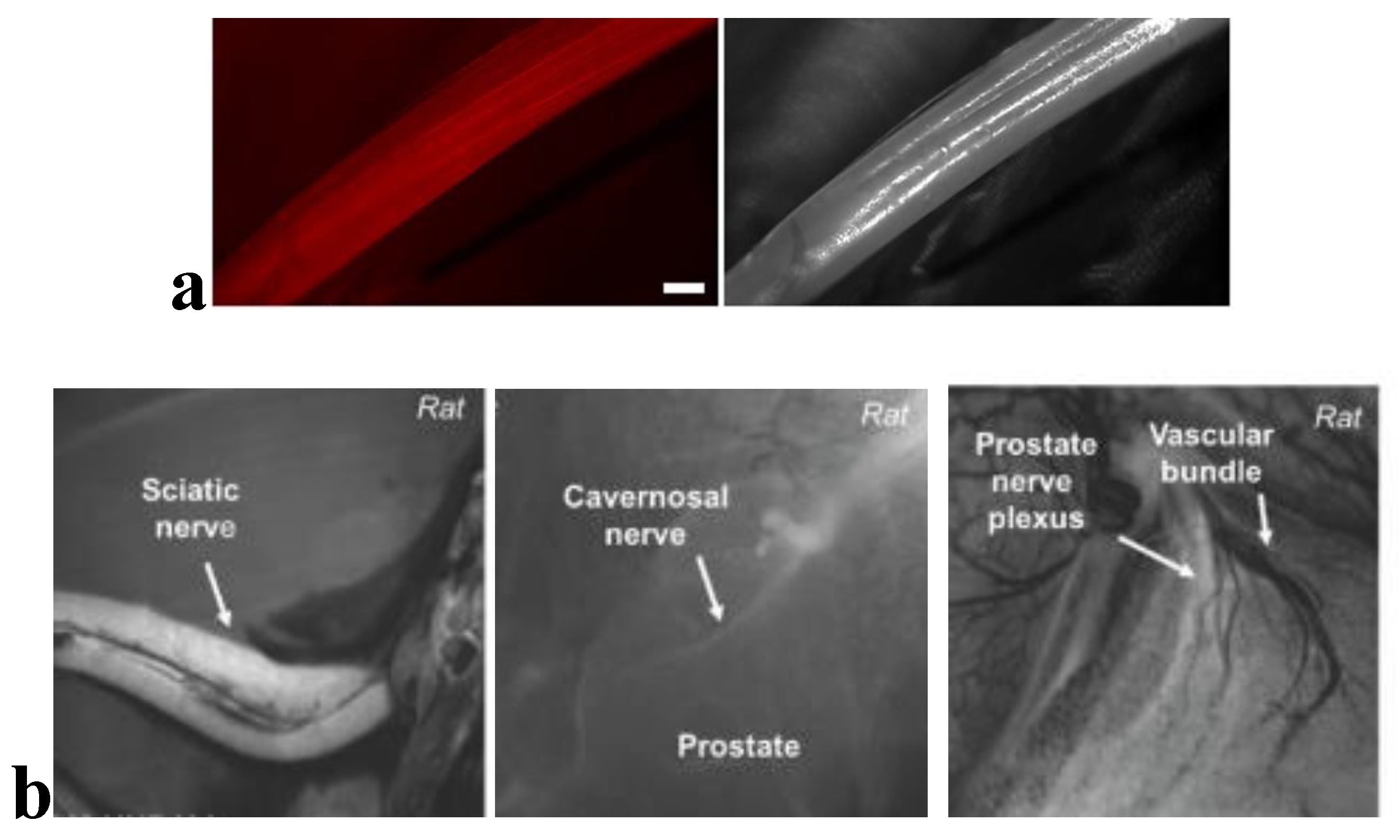
Fluorescence-guided biopsy, which involves the use of nerve-specific fluorescent agents, can enhance the accuracy of tissue sampling and resection (
Figure 4). While many studies focus on the use of fluorescent-guided imaging in the operative setting,[
144] the potential application to nerve biopsies is self-evident. One such technique targets Nav1.7 sodium channels, found in high density in peripheral nerves, with a selective fluorescent peptide. Using a surgical fluorescent microscope ex-vivo, researchers were able to simulate a clinical scenario which identified the peripheral nerves via fluorescent highlights [
145]. The growing success of fluorescence imaging has led many to seek out novel markers or strategies to visualize neurons in surgery or for biopsy [
146,
147].
4.3. Minimally Invasive & Target Fasicular Biopsy
Utilizing smaller instruments and techniques designed to reduce trauma, these biopsies aim to obtain tissue samples with minimal surgical intervention. Several studies have investigated how more superficial peroneal nerve and peroneus brevis muscle combined biopsies allow for the evaluation of both nerve and muscle tissues with high yield, sensitivity, and specificity for vasculitis neuropathies and other conditions [
152,
153]. The procedure's minimal invasiveness allows for reduced morbidity compared to traditional sural nerve biopsy, highlighting a progression towards less invasive biopsy methods for nerve tissues without compromising diagnostic value.
Another approach, coined “targeted fascicular biopsy”, targets one or a few fascicles within a nerve, rather than taking a larger portion of the nerve. The goal of targeting smaller sensory and motor branches is to minimize the risk of worsening neurologic function secondary to biopsy, while still providing enough histopathological tissue to be diagnostic. This is accomplished by combining neuroimaging, clinical examination, and electrophysiological studies to identify the ideal nerve target, access the nerve as minimally as possible, and biopsy for pathological examination [
154]. Selective and precise targeting allows for biopsy of a redundant or expendable motor branch rather than resecting a fascicle from the parent nerve. This strategy has been applied to biopsy the brachial plexus [
155] and the sciatic nerve and its major branches [
156] among many others [
154,
157].
4.4. Shear Wave Elastography (SWE)
SWE is a diagnostic imaging modality that quantitatively assesses tissue stiffness by measuring the velocity of shear waves induced by an acoustic radiation force impulse. The propagation speed of these shear waves, which is directly proportional to tissue rigidity, provides clinicians with precise metrics of studied nerves [
158]. SWE is gaining traction as a non-invasive technique to evaluate peripheral nerve integrity and pathology, potentially circumventing the need for invasive nerve biopsies.
Several studies are geared towards cataloguing the elastic properties of healthy nerves for future reference and comparative assessment. This growing catalogue currently includes the ulnar nerve,[
159] radial nerve,[
160] and median nerve [
161] among many others. Other studies, such as this one by Durand et al, 2021, have started comparing the elastic properties of healthy nerves against neuropathic nerves, in this case comparing entrapped ulnar nerves post decompression against the contralateral non-operative side [
162].
Overall, SWE offers a non-invasive means to obtain quantitative data on tissue stiffness, potentially aiding in both diagnosis and monitoring of various neuropathies while bypassing the risks of traditional surgical biopsy.
5. Conclusions
Neurosurgical intervention for nerve and muscle biopsies remains relevance in the current diagnostic landscape for many neurological and musculoskeletal disorders. Although biopsy may not be required for definitive diagnosis in certain conditions, the role of biopsy may still be essential when dealing with atypical cases and as a supportive point of evidence for diagnosis. In addition, several emerging techniques have been explored in the literature to guide diagnostics and biopsy, conduct less invasive biopsies, and reduce risks of worsening neurologic function and other symptoms secondary to biopsy.
Author Contributions
Conceptualization, A.M. and B.L.; Methodology, A.M. and B.L.; Writing – Original Draft, A.M., T.C., E.M., and R.A.; Writing – Review & Editing, A.M., T.C., E.M., R.A., and B.L.; Visualization, A.M. and B.L.; Supervision, A.M. and B.L.; Project administration, A.M. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Any research article describing a study involving humans should contain this statement. Please add “Informed consent was obtained from all subjects involved in the study.” OR “Patient consent was waived due to REASON (please provide a detailed justification).” OR “Not applicable.” for studies not involving humans. You might also choose to exclude this statement if the study did not involve humans. Written informed consent for publication must be obtained from participating patients who can be identified (including by the patients themselves). Please state “Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rikard SM, Strahan AE, Schmit KM, Guy GP. Chronic Pain Among Adults — United States, 2019–2021. MMWR Morb Mortal Wkly Rep. 2023;72(15). [CrossRef]
- Looker AC, Wang CY. Prevalence of reduced muscle strength in older U.S. adults: United States, 2011-2012. NCHS Data Brief. 2015;(179).
- AHRQ. Effectiveness of Treatments for Diabetic Peripheral Neuropathy. SubStance. Published online 2016.
- Joyce NC, Oskarsson B, Jin LW. Muscle biopsy evaluation in neuromuscular disorders. Phys Med Rehabil Clin N Am. 2012;23(3). [CrossRef]
- Mellgren SI, Lindal S. Nerve biopsy - some comments on procedures and indications. Acta Neurol Scand. 2011;124:64-70. [CrossRef]
- Nathani D, Spies J, Barnett MH, et al. Nerve biopsy: Current indications and decision tools. Muscle Nerve. 2021;64(2). [CrossRef]
- Grisariu S, Avni B, Batchelor TT, et al. Neurolymphomatosis: An International Primary CNS Lymphoma Collaborative Group report. Blood. 2010;115(24). [CrossRef]
- Stern BJ, Royal W, Gelfand JM, et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018;75(12). [CrossRef]
- Hui M, Uppin MS, Challa S, Meena AK, Kaul S. Pure neuritic leprosy: Resolving diagnostic issues in acid fast bacilli (AFB)-negative nerve biopsies: A single centre experience from South India. Ann Indian Acad Neurol. 2015;18(3). [CrossRef]
- Adams D, Suhr OB, Hund E, et al. First european consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29. [CrossRef]
- Collins MP, Dyck PJB, Gronseth GS, et al. Peripheral nerve society guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: Executive summary. In: Journal of the Peripheral Nervous System. Vol 15. ; 2010. [CrossRef]
- Chia L, Fernandez A, Lacroix C, Adams D, Planté V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain. 1996;119(4). [CrossRef]
- Lubec D, Müllbacher W, Finsterer J, Mamoli B. Diagnostic work-up in peripheral neuropathy: An analysis of 171 cases. Postgrad Med J. 1999;75(890). [CrossRef]
- Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31(1). [CrossRef]
- Pasnoor M, Dimachkie MM, Barohn RJ. Cryptogenic sensory polyneuropathy. Neurol Clin. 2013;31(2). [CrossRef]
- Gwathmey KG, Tracy JA, Dyck PJB. Peripheral Nerve Vasculitis: Classification and Disease Associations. Neurol Clin. 2019;37(2). [CrossRef]
- Vrancken AFJE, Gathier CS, Cats EA, Notermans NC, Collins MP. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol. 2011;18(1). [CrossRef]
- Rohatgi S, Dave D, Khan F. Nonsystemic vasculitic neuropathy. Medical Journal of Dr DY Patil Vidyapeeth. Published online 2020. [CrossRef]
- Hui M, Meena A, Rajasekhar L, et al. Vasculitic neuropathy: A retrospective analysis of nerve biopsies and clinical features from a single tertiary care center. Ann Indian Acad Neurol. 2019;22(2). [CrossRef]
- Masuda H, Misawa S, Arai K, et al. Combined nerve/muscle/skin biopsy could increase diagnostic sensitivity for vasculitic neuropathy. Clin Exp Neuroimmunol. 2015;6(3). [CrossRef]
- Hadden RDM, Collins MP, Živković SA, et al. Vasculitic peripheral neuropathy: Case definition and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine. 2017;35(11). [CrossRef]
- Üçeyler N, Braunsdorf S, Kunze E, et al. Cellular infiltrates in skin and sural nerve of patients with polyneuropathies. Muscle Nerve. 2017;55(6). [CrossRef]
- Üçeyler N, Devigili G, Toyka K V., Sommer C. Skin biopsy as an additional diagnostic tool in non-systemic vasculitic neuropathy. Acta Neuropathol. 2010;120(1). [CrossRef]
- Weis J, Brandner S, Lammens M, Sommer C, Vallat JM. Processing of nerve biopsies: A practical guide for neuropathologists. Clin Neuropathol. 2012;31(1). [CrossRef]
- Duchesne M, Mathis S, Corcia P, et al. Value of nerve biopsy in patients with latent malignant hemopathy and peripheral neuropathy: A case series. Medicine (United States). 2015;94(3). [CrossRef]
- Ramirez-Zamora A, Morales-Vidal S, Chawla J, Biller J. Autopsy proven peripheral nervous system neurolymphomatosis despite negative bilateral sural nerve biopsy. Front Neurol. 2013;4 DEC. [CrossRef]
- Duchesne M, Roussellet O, Maisonobe T, et al. Pathology of nerve biopsy and diagnostic yield of PCR based clonality testing in neurolymphomatosis. J Neuropathol Exp Neurol. 2018;77(9). [CrossRef]
- Shree R, Goyal MK, Modi M, et al. The diagnostic dilemma of neurolymphomatosis. Journal of Clinical Neurology (Korea). 2016;12(3). [CrossRef]
- Bishop AJ, Zagars GK, Torres KE, Bird JE, Feig BW, Guadagnolo BA. Malignant Peripheral Nerve Sheath Tumors: A Single Institution’s Experience Using Combined Surgery and Radiation Therapy. Am J Clin Oncol. 2018;41(5):465-470. [CrossRef]
- Kourea HP, Bilsky MH, Leung DHY, Lewis JJ, Woodruff JM. Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumors: A clinicopathologic study of 25 patients and 26 tumors. Cancer. 1998;82(11). [CrossRef]
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10). [CrossRef]
- Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6). [CrossRef]
- Mauermann ML, Amrami KK, Kuntz NL, et al. Longitudinal study of intraneural perineuriomaa benign, focal hypertrophic neuropathy of youth. Brain. 2009;132(8). [CrossRef]
- Niederhauser BD, Spinner RJ, Jentoft ME, Everist BM, Matsumoto JM, Amrami KK. Neuromuscular choristoma: Characteristic magnetic resonance imaging findings and association with post-biopsy fibromatosis. Skeletal Radiol. 2013;42(4). [CrossRef]
- Mauermann ML, Scheithauer BW, Spinner RJ, et al. Inflammatory pseudotumor of nerve: Clinicopathological characteristics and a potential therapy. In: Journal of the Peripheral Nervous System. Vol 15. ; 2010. [CrossRef]
- Garbino JA, Ura S, Belone A de FF, Marciano LHSC, Fleury RN. Clinical and diagnostic aspects of the primarily neural leprosy. Hansenol Int. 2004;29(2).
- Suneetha S, Arunthathi S, Kurian N, Chacko CJG. Histological changes in the nerve, skin and nasal mucosa of patients with primary neuritic leprosy. Acta Leprol. 2001;12(1).
- de Freitas MR, Nascimento OJ, Drago MJ, de Freitas AR, Hahn MD. [Ulnar nerve palsy in leprosy without skin changes: biopsy of the superficial branch of the ulnar nerve in the hand]. Arq Neuropsiquiatr. 1998;56(3B):585-594. [CrossRef]
- Loavenbruck AJ, Singer W, Mauermann ML, et al. Transthyretin amyloid neuropathy has earlier neural involvement but better prognosis than primary amyloid counterpart: an answer to the paradox? Ann Neurol. 2016;80(3). [CrossRef]
- Koike H, Hashimoto R, Tomita M, et al. Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: a practical analysis. Amyloid. 2011;18(2):53-62. [CrossRef]
- Vaxman I, Gertz M. Recent Advances in the Diagnosis, Risk Stratification, and Management of Systemic Light-Chain Amyloidosis. Acta Haematol. 2019;141(2). [CrossRef]
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol. 2017;176(5):728-742. [CrossRef]
- De Larrea CF, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14). [CrossRef]
- Said Ǵerard. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59(10). [CrossRef]
- Mazzeo A, Russo M, Di Bella G, et al. Transthyretin-Related Familial Amyloid Polyneuropathy (TTR-FAP): A Single-Center Experience in Sicily, an Italian Endemic Area. J Neuromuscul Dis. 2015;2(s2):S39-S48. [CrossRef]
- Vital C, Vital A, Bouillot-Eimer S, Brechenmacher C, Ferrer X, Lagueny A. Amyloid neuropathy: a retrospective study of 35 peripheral nerve biopsies. J Peripher Nerv Syst. 2004;9(4):232-241. [CrossRef]
- Burns TM, Dyck PJB, Aksamit AJ, Dyck PJ. The natural history and long-term outcome of 57 limb sarcoidosis neuropathy cases. J Neurol Sci. 2006;244(1-2). [CrossRef]
- Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol. 2017;264(5). [CrossRef]
- Said G, Lacroix C, Planté-Bordeneuve V, et al. Nerve granulomas and vasculitis in sarcoid peripheral neuropathy. A clinicopathological study of 11 patients. Brain. 2002;125(2). [CrossRef]
- Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429-436. [CrossRef]
- Inoue D, Zen Y, Sato Y, et al. IgG4-related perineural disease. Int J Rheumatol. 2012;2012. [CrossRef]
- Suzuki Y, Shiraishi M, Yamada K, Doi M, Kato M, Hasegawa Y. [A case of refractory IgG4-related peripheral neuropathy with severe axonal damage]. Rinsho Shinkeigaku. 2016;56(5):323-327. [CrossRef]
- Waheed W, Nickerson J, Ambaye AB, Babi MA, Tandan R. IgG4-related neuromyopathy associated with recurrent pleural effusion. J Clin Neuromuscul Dis. 2015;16(4). [CrossRef]
- Yokoi S, Kawagashira Y, Ohyama K, et al. Mononeuritis multiplex with tumefactive cellular infiltration in a patient with reactive lymphoid hyperplasia with increased immunoglobulin G4-positive cells. Hum Pathol. 2014;45(2). [CrossRef]
- Zhan L, Fan M, Cai N, Cai B. Combination of autoimmune pancreatitis and peripheral neuropathy on an IgG4-related disease patient with 4 years following-up. J Neuroimmunol. 2020;348. [CrossRef]
- Baptista B, Casian A, Gunawardena H, D’Cruz D, Rice CM. Neurological Manifestations of IgG4-Related Disease. Curr Treat Options Neurol. 2017;19(4). [CrossRef]
- Thapa B, Mahendraker N, Ramphul K. Paraneoplastic Syndromes.; 2024.
- Pelosof LC, Gerber DE. Paraneoplastic syndromes: An approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9). [CrossRef]
- Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: Pathogenesis and physiopathology. In: Brain Pathology. Vol 9. ; 1999. [CrossRef]
- Khan U, Warriach SA. Rare case: Paraneoplastic syndrome affecting peripheral nerves, associated with anti-collapsin-response mediator protein-5 (anti-CRMP5) antibodies, as early manifestation of small cell lung cancer confined to a solitary lymph node without evidence of lung mass on routine CT thorax. BMJ Case Rep. 2020;13(2). [CrossRef]
- Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010;9(4):402-412. [CrossRef]
- Kuwabara S, Misawa S. Chronic Inflammatory Demyelinating Polyneuropathy. Adv Exp Med Biol. 2019;1190:333-343. [CrossRef]
- Viala K, Maisonobe T, Stojkovic T, et al. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. Journal of the Peripheral Nervous System. 2010;15(1). [CrossRef]
- Rajabally YA, Stettner M, Kieseier BC, Hartung HP, Malik RA. CIDP and other inflammatory neuropathies in diabetes - Diagnosis and management. Nat Rev Neurol. 2017;13(10). [CrossRef]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Peripher Nerv Syst. 2010;15(3):185-195. [CrossRef]
- Nemni R, Corbo M, Fazio R, Quattrini A, Comi G, Canal N. Cryoglobulinaemic neuropathy. A clinical, morphological and immunocytochemical study of 8 cases. Brain. 1988;111 ( Pt 3):541-552. [CrossRef]
- Ciompi ML, Marini D, Siciliano G, et al. Cryoglobulinemic peripheral neuropathy: Neurophysiologic evaluation in twenty-two patients. In: Biomedicine and Pharmacotherapy. Vol 50. ; 1996. [CrossRef]
- Cerri F, Falzone YM, Riva N, Quattrini A. An update on the diagnosis and management of the polyneuropathy of POEMS syndrome. J Neurol. 2019;266(1):258-267. [CrossRef]
- Vallat JM, Magy L, Richard L, Sturtz F, Couratier P. Contribution of electron microscopy to the study of neuropathies associated with an IgG monoclonal paraproteinemia. Micron. 2008;39(2):61-70. [CrossRef]
- Vital C, Vital A, Ferrer X, et al. Crow-Fukase (POEMS) syndrome: A study of peripheral nerve biopsy in five new cases. Journal of the Peripheral Nervous System. 2003;8(3). [CrossRef]
- Vallat JM, Magy L, Sindou P, Magdelaine C, Cros D. IgG neuropathy: An immunoelectron microscopic study. J Neuropathol Exp Neurol. 2005;64(5). [CrossRef]
- Vallat JM, Tabaraud F, Sindou P, Preux PM, Vandenberghe A, Steck A. Myelin widenings and MGUS-IgA: an immunoelectron microscopic study. Ann Neurol. 2000;47(6):808-811.
- Ellie E, Vital A, Steck A, Boiron JM, Vital C, Julien J. Neuropathy associated with “benign” anti-myelin-associated glycoprotein IgM gammopathy: Clinical, immunological, neurophysiological pathological findings and response to treatment in 33 cases. J Neurol. 1996;243(1). [CrossRef]
- van de Mortel JPM, D’Sa S, Vrancken AFJE, Notermans NC, Vos JMI, Minnema MC. Polyneuropathy Associated with IgM Monoclonal Gammopathy; Advances in Genetics and Treatment, Focusing on Anti-MAG Antibodies. Hemato. 2022;3(4). [CrossRef]
- Magy L, Kaboré R, Mathis S, et al. Heterogeneity of Polyneuropathy Associated with Anti-MAG Antibodies. J Immunol Res. 2015;2015. [CrossRef]
- Bleasel AF, Hawke SHB, Pollard JD, McLeod JG. IgG monoclonal paraproteinaemia and peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1993;56(1). [CrossRef]
- Kawagashira Y, Koike H, Tomita M, et al. Morphological progression of myelin abnormalities in IgM-monoclonal gammopathy of undetermined significance anti-myelin-associated glycoprotein neuropathy. J Neuropathol Exp Neurol. 2010;69(11). [CrossRef]
- Mochel F, Schiffmann R, Steenweg ME, et al. Adult polyglucosan body disease: Natural history and key magnetic resonance imaging findings. Ann Neurol. 2012;72(3). [CrossRef]
- Klein CJ, Boes CJ, Chapin JE, et al. Adult polyglucosan body disease: Case description of an expanding genetic and clinical syndrome. Muscle Nerve. 2004;29(2). [CrossRef]
- Xu M, Pinto M, Sun C, et al. Expanded teased nerve fibre pathological conditions in disease association. J Neurol Neurosurg Psychiatry. 2019;90(2). [CrossRef]
- Mrak RE. The Big Eye in the 21st century: the role of electron microscopy in modern diagnostic neuropathology. J Neuropathol Exp Neurol. 2002;61(12):1027-1039. [CrossRef]
- Riva N, Iannaccone S, Corbo M, et al. Motor nerve biopsy: Clinical usefulness and histopathological criteria. Ann Neurol. 2011;69(1). [CrossRef]
- Lozeron P, Nahum L, Lacroix C, Ropert A, Guglielmi JM, Said G. Symptomatic diabetic and non-diabetic neuropathies in a series of 100 diabetic patients. J Neurol. 2002;249(5). [CrossRef]
- Rappaport WD, Valente J, Hunter GC, et al. Clinical utilization and complications of sural nerve biopsy. The American Journal of Surgery. 1993;166(3). [CrossRef]
- Deprez M, de Groote CC, Gollogly L, Reznik M, Martin JJ. Clinical and neuropathological parameters affecting the diagnostic yield of nerve biopsy. Neuromuscul Disord. 2000;10(2):92-98. [CrossRef]
- Duchesne M, Mathis S, Richard L, et al. Nerve biopsy is still useful in some inherited neuropathies. J Neuropathol Exp Neurol. 2018;77(2). [CrossRef]
- England JD, Gronseth GS, Franklin G, et al. Practice Parameter: The Evaluation of Distal Symmetric Polyneuropathy: The Role of Laboratory and Genetic Testing (An Evidence-Based Review). PM&R. 2009;1(1). [CrossRef]
- Chahin N, Temesgen Z, Kurtin PJ, Spinner RJ, Dyck PJB. HIV lumbosacral radiculoplexus neuropathy mimicking lymphoma: Diffuse infiltrative lymphocytosis syndrome (DILS) restricted to nerve? Muscle Nerve. 2010;41(2). [CrossRef]
- Estes ML, Ewing-Wilson D, Chou SM, et al. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med. 1987;82(3):447-455. [CrossRef]
- Pellissier JF, Pouget J, Cros D, De Victor B, Serratrice G, Toga M. Peripheral neuropathy induced by amiodarone chlorhydrate. A clinicopathological study. J Neurol Sci. 1984;63(2):251-266. [CrossRef]
- Little AA, Albers JW. Clinical description of toxic neuropathies. In: Handbook of Clinical Neurology. Vol 131. ; 2015. [CrossRef]
- Weis S, Büttner A. Neurotoxicology and drug-related disorders. In: Handbook of Clinical Neurology. Vol 145. ; 2018. [CrossRef]
- Katona I, Weis J. Diseases of the peripheral nerves. In: Handbook of Clinical Neurology. Vol 145. ; 2018. [CrossRef]
- Ubogu EE. Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol. 2015;130(4). [CrossRef]
- Querol L, Devaux J, Rojas-Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: Diagnostic and therapeutic implications. Nat Rev Neurol. 2017;13(9). [CrossRef]
- Rutchik J. Occupational medicine physician’s guide to neuropathy in the workplace, part 2: electromyography and cryptogenic and toxic neuropathy. J Occup Environ Med. 2009;51(5):622-625. [CrossRef]
- Walters J, Baborie A. Muscle biopsy: What and why and when? Pract Neurol. 2020;20(5). [CrossRef]
- Meola G, Bugiardini E, Cardani R. Muscle biopsy. J Neurol. 2012;259(4):601-610. [CrossRef]
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11. [CrossRef]
- Hernández-Rodríguez J, Alba MA, Prieto-González S, Cid MC. Diagnosis and classification of polyarteritis nodosa. J Autoimmun. 2014;48-49. [CrossRef]
- Hernández-Rodrígueza J, Hoffmanb GS. Updating single-organ vasculitis. Curr Opin Rheumatol. 2012;24(1). [CrossRef]
- Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: Roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev. 2015;96(1). [CrossRef]
- Venugopal V, Pavlakis S. Duchenne Muscular Dystrophy.; 2023.
- Thada PK, Bhandari J, Umapathi KK. Becker Muscular Dystrophy.; 2023.
- Carlson CR, Moore SA, Mathews KD. Dystrophinopathy muscle biopsies in the genetic testing ERA: One center’s data. Muscle Nerve. 2018;58(1). [CrossRef]
- Kumar V, AAK, & AJC. Robbins Basic Pathology. 10th ed. Elsevier - Health Sciences Division; 2017.
- Bruschi F, Brunetti E, Pozio E. Neurotrichinellosis. Handb Clin Neurol. 2013;114:243-249. [CrossRef]
- Nikolić S, Vujosević M, Sasić M, et al. Neurologic manifestations in trichinosis. Srp Arh Celok Lek. 1998;126(5-6).
- Goel P, Gerriets V. Chloroquine.; 2023.
- Pasnoor M, Barohn RJ, Dimachkie MM. Toxic myopathies. Neurol Clin. 2014;32(3). [CrossRef]
- Casado E, Graticós J, Tolosa C, et al. Antimalarial myopathy: An underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65(3). [CrossRef]
- Mastaglia FL, Papadimitriou JM, Dawkins RL, Beveridge B. Vacuolar myopathy associated with chloroquine, lupus erythematosus and thymoma. Report of a case with unusual mitochondrial changes and lipid accumulation in muscle. J Neurol Sci. 1977;34(3). [CrossRef]
- Florek JB, Lucas A, Girzadas D. Amiodarone.; 2023.
- Feeney EJ, Austin S, Chien YH, et al. The value of muscle biopsies in Pompe disease: Identifying lipofuscin inclusions in juvenile- and adult-onset patients. Acta Neuropathol Commun. 2014;2(1). [CrossRef]
- Morales A, Anilkumar AC. Glycogen Storage Disease Type II.; 2023.
- Leslie N, Bailey L. Pompe Disease.; 1993.
- Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review. Eur Radiol. 2018;28(2):698-707. [CrossRef]
- Ku V, Cox C, Mikeska A, MacKay B. Magnetic Resonance Neurography for Evaluation of Peripheral Nerves. J Brachial Plex Peripher Nerve Inj. 2021;16(1):E17-E23. [CrossRef]
- Nathani D, Spies J, Barnett MH, et al. Nerve biopsy: Current indications and decision tools. Muscle Nerve. 2021;64(2):125. [CrossRef]
- Loizides A, Gruber L, Peer S, Plaikner M, Gruber H. [Ultrasound-guided interventions on the peripheral nervous system]. Radiologe. 2017;57(3):166-175. [CrossRef]
- Strakowski JA. Ultrasound-Guided Peripheral Nerve Procedures. Phys Med Rehabil Clin N Am. 2016;27(3):687-715. [CrossRef]
- Singh KP, Singh Goindi A, Gupta K. Reference values for the cross-sectional area of normal radial nerve at two levels using high-resolution ultrasonography. J Ultrason. 2021;21(85):e112. [CrossRef]
- Pianta M, Chock E, Schlicht S, McCombe D. Accuracy and complications of CT-guided core needle biopsy of peripheral nerve sheath tumours. Skeletal Radiol. 2015;44(9):1341-1349. [CrossRef]
- Wang M, Zhou ZG, Du KP, et al. [Analysis of the safety and diagnostic efficiency of CT-guided percutaneous biopsy of pancreatic space-occupying lesions using large needle:comparison of trans-organ biopsy approach and non-trans-organ biopsy approach]. Zhonghua Yi Xue Za Zhi. 2023;103(5):364-369. [CrossRef]
- Holzgrefe RE, Wagner ER, Singer AD, Daly CA. Imaging of the Peripheral Nerve: Concepts and Future Direction of Magnetic Resonance Neurography and Ultrasound. J Hand Surg Am. 2019;44(12):1066-1079. [CrossRef]
- Pianta M, Chock E, Schlicht S, McCombe D. Accuracy and complications of CT-guided core needle biopsy of peripheral nerve sheath tumours. Skeletal Radiol. 2015;44(9):1341-1349. [CrossRef]
- Wang LG, Gibbs SL. Improving precision surgery: A review of current intraoperative nerve tissue fluorescence imaging. Curr Opin Chem Biol. 2023;76:102361. [CrossRef]
- Chlebicki CA, Lee AD, Jung W, et al. Preliminary Investigation on Use of High-Resolution Optical Coherence Tomography to Monitor Injury and Repair in the Rat Sciatic Nerve. Lasers Surg Med. 2010;42(4):306. [CrossRef]
- Choi D, Lee J, Jeon M, Kim J. In Vivo Fascicle Bifurcation Imaging of Rat Sciatic Nerve Using Swept-Source Optical Coherence Tomography. IEEE Access. 2018;6:7713-7718M, Kim J. In Vivo Fascicle Bifurcation Imaging of Rat Sciatic Nerve Using Swept-Source Optical Coherence Tomography. [CrossRef]
- Vasudevan S, Vo J, Shafer B, Nam AS, Vakoc BJ, Hammer DX. Toward optical coherence tomography angiography-based biomarkers to assess the safety of peripheral nerve electrostimulation. J Neural Eng. 2019;16(3):036024. [CrossRef]
- Monroy GL, Erfanzadeh M, Tao M, et al. Development of polarization-sensitive optical coherence tomography imaging platform and metrics to quantify electrostimulation-induced peripheral nerve injury in vivo in a small animal model. Neurophotonics. 2023;10(2). [CrossRef]
- Hope J, Goodwin M, Vanholsbeeck F. Optical coherence tomography imaging of evoked neural activity in sciatic nerve of rat. J Phys D Appl Phys. 2021;54(33):334002. [CrossRef]
- Sugimoto M, Sasoh M, Ido M, Narushima C, Uji Y. Retinal Nerve Fiber Layer Decrease during Glycemic Control in Type 2 Diabetes. J Ophthalmol. 2010;2010:1-6. [CrossRef]
- Akkaya S, Can E, Öztürk F. Comparison of the corneal biomechanical properties, optic nerve head topographic parameters, and retinal nerve fiber layer thickness measurements in diabetic and non-diabetic primary open-angle glaucoma. Int Ophthalmol. 2016;36(5):727-736. [CrossRef]
- Badian RA, Ekman L, Pripp AH, et al. Comparison of Novel Wide-Field In Vivo Corneal Confocal Microscopy With Skin Biopsy for Assessing Peripheral Neuropathy in Type 2 Diabetes. Diabetes. 2023;72(7):908-917. [CrossRef]
- Cosmo E, Midena G, Frizziero L, Bruno M, Cecere M, Midena E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J Clin Med. 2022;11(17). [CrossRef]
- Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017;15(1):15. [CrossRef]
- Petropoulos IN, Bitirgen G, Ferdousi M, et al. Corneal Confocal Microscopy to Image Small Nerve Fiber Degeneration: Ophthalmology Meets Neurology. Frontiers in Pain Research. 2021;2. [CrossRef]
- Jia X, Wang X, Wang X, et al. In Vivo Corneal Confocal Microscopy Detects Improvement of Corneal Nerve Parameters following Glycemic Control in Patients with Type 2 Diabetes. J Diabetes Res. 2018;2018. [CrossRef]
- Burgess J, Frank B, Marshall A, et al. Early Detection of Diabetic Peripheral Neuropathy: A Focus on Small Nerve Fibres. Diagnostics. 2021;11(2). [CrossRef]
- Che NN, Yang HQ. Potential use of corneal confocal microscopy in the diagnosis of Parkinson’s disease associated neuropathy. Transl Neurodegener. 2020;9(1). [CrossRef]
- Creigh PD, Mountain J, Sowden JE, et al. Measuring peripheral nerve involvement in Friedreich’s ataxia. Ann Clin Transl Neurol. 2019;6(9):1718-1727. [CrossRef]
- Wang HL, Fan DS, Zhang S, Liu ZY. [Corneal confocal microscopy detects small-fiber neuropathy in patients with amyotrophic lateral sclerosis]. Zhonghua Nei Ke Za Zhi. 2022;61(1):77-81. [CrossRef]
- Wang LG, Gibbs SL. Improving precision surgery: A review of current intraoperative nerve tissue fluorescence imaging. Curr Opin Chem Biol. 2023;76:102361. [CrossRef]
- Gonzales J, Demetrio De Souza Franca P, Jiang Y, et al. Fluorescence Imaging of Peripheral Nerves by a Nav1.7-Targeted Inhibitor Cystine Knot Peptide. Bioconjug Chem. 2019;30(11):2879-2888. [CrossRef]
- Hernández-Gil J, Chow CY, Chatras H, et al. Development and Validation of Nerve-Targeted Bacteriochlorin Sensors. J Am Chem Soc. Published online July 5, 2023. [CrossRef]
- Wei B, Su H, Chen P, et al. Recent advancements in peripheral nerve-specific fluorescent compounds. Biomater Sci. 2021;9(23):7799-781. [CrossRef]
- Massaad CA, Zhang G, Pillai L, Azhdarinia A, Liu W, Sheikh KA. Fluorescently-tagged anti-ganglioside antibody selectively identifies peripheral nerve in living animals. Sci Rep. 2015;5. [CrossRef]
- Hingorani D V., Whitney MA, Friedman B, et al. Nerve-targeted probes for fluorescence-guided intraoperative imaging. Theranostics. 2018;8(15). [CrossRef]
- Buckle T, Hensbergen AW, van Willigen DM, et al. Intraoperative visualization of nerves using a myelin protein-zero specific fluorescent tracer. EJNMMI Res. 2021;11(1). [CrossRef]
- Wang LG, Gibbs SL. Improving precision surgery: A review of current intraoperative nerve tissue fluorescence imaging. Curr Opin Chem Biol. 2023;76. [CrossRef]
- Collins MP, Mendell JR, Periquet MI, et al. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology. 2000;55(5):636-643. [CrossRef]
- Agadi JB, Raghav G, Mahadevan A, Shankar SK. Usefulness of superficial peroneal nerve/peroneus brevis muscle biopsy in the diagnosis of vasculitic neuropathy. J Clin Neurosci. 2012;19(10):1392-1396.y. [CrossRef]
- Marek T, howe BM, Amrami KK, Spinner RJ. From targeted fascicular biopsy of major nerve to targeted cutaneous nerve biopsy: Implementing clinical anatomy can catalyze a paradigm shift. Clinical Anatomy. 2018;31(5):616-621. [CrossRef]
- Capek S, Amrami KK, Dyck PJB, Spinner RJ. Targeted fascicular biopsy of the sciatic nerve and its major branches: rationale and operative technique. Neurosurg Focus. 2015;39(3):E12. [CrossRef]
- Capek S, Amrami KK, Dyck PJB, Spinner RJ. Targeted fascicular biopsy of the sciatic nerve and its major branches: rationale and operative technique. Neurosurg Focus. 2015;39(3). [CrossRef]
- Marek T, howe BM, Amrami KK, Spinner RJ. From targeted fascicular biopsy of major nerve to targeted cutaneous nerve biopsy: Implementing clinical anatomy can catalyze a paradigm shift. Clinical Anatomy. 2018;31(5):616-621. [CrossRef]
- Taljanovic MS, Gimber LH, Becker GW, et al. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics. 2017;37(3):855-870. [CrossRef]
- Bedewi MA, Kotb MA, Aldossary NM, Abodonya AM, Alhariqi BA, Swify SM. Shear wave elastography of the ulnar nerve at the forearm. Medicine. 2021;100(2):E24071. [CrossRef]
- Bedewi MA, Kotb MA, Aldossary NM, Abodonya AM, Saleh AK, Swify SM. Shear wave elastography of the radial nerve in healthy subjects. J Int Med Res. 2021;49(1). [CrossRef]
- Schrier VJMM, Lin J, Gregory A, et al. Shear wave elastography of the median nerve: A mechanical study. Muscle Nerve. 2020;61(6):826-833. [CrossRef]
- Durand S, Raffoul W, Christen T, Pedrazzi N. Post-Operative Assessment of Ulnar Nerve Tension Using Shear-Wave Elastography. Neurol Int. 2021;13(3):469-476. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).