1. Introduction
Cellulose nanofibres are a quite recent type of nanomaterials, which can be obtained by intensive mechanical treatment of cellulose fibres. The most common mechanical treatments are high-pressure homogenization (HPH), microfluidization, grinding, twin screw extrusion, cryo-crushing, and high-intensity ultrasonication [
1]. Usually, the cellulose fibres treated only by mechanical processes do not present a high level of conversion to nanofibres, even when a highly intensive treatment is applied; for example, cellulose fibres treated only by mechanical treatment using HPH with two passes only resulted in ca. 6% conversion to nanofibres [
2]. Even increasing the number of cycles, the yield in nanofibres is usually low; for example, Jeencham et al. reported a yield in nanofibers of 14.7% applying 15 cycles of HPH, when preparing CNF from cassava pulp [
3].
The chemical pre-treatment is a suitable way to improve nanofibrillation degree. Among the chemical pre-treatments commonly used, TEMPO-mediated oxidation is one of the most used. The nanofibres obtained from TEMPO-oxidized fibres consist of very thin (less than 10 nm) and long fibrils, which result in a material with high aspect ratio [
4]. The high aspect ratio and the crystallinity are key factors for the formation of stiff hydrogels at relatively low CNF concentrations [
5]. This favourable property enables the use of CNFs as rheology modifiers with potential application in paints, foods, cosmetics, suspending agents in mineral applications among others [
6].
Another pre-treatment suitable for the preparation of CNFs that emerged recently is cationization [
7]. The introduction of cationic groups, especially quaternary ammonium groups, into the cellulose structure, not only enhances the degree of fibrillation but also expands the type of applications due to the new CNF properties introduced by the cationic groups such as antimicrobial properties [
8]. Another recent application which presents high potential is the use of cationic CNFs as flocculants in wastewater treatment [
9]. The introduction of positively charged groups can be achieved by different processes, being the most common the direct cationization through the reaction of the cellulose fibres with 2,3-epoxypropyltrimethylammonium chloride (EPTAC) [
10], and a two-step cationization, using Girard's reagent T (GT) after oxidation by periodate [
7]. The first method, due to the nature of the modification (direct cationization in mild conditions) does not result in extended depolymerization of cellulose; contrary, the second method, in which strong oxidation is applied to form cellulose dialdehyde, being the cationic groups added in aldehyde moieties, results in large depolymerization of cellulose. On the other hand, the amount of cationic groups which can be introduced using the first method is quite limited, but for the second method high charge density CNFs can be obtained [
7]. As expected, the fibrillation degree obtained when applying the first or the second method is quite different, being the second method (for highly charged celluloses) able to produce nanofibres in larger extension.
The charge density, the fibrillation degree and the fibril dimensions have a deep impact on the rheology of CNF suspensions [
5] because the rheology of polymeric solutions or CNF suspensions is controlled by the physical entanglement of polymeric chains/cellulose nanofibres and their aggregation [
11,
12]. Nechyporchuk et al. point out that the CNF suspensions produced using mechanical fibrillation, with or without enzymatic pre-treatment (no surface chemical modification), possess highly flocculated structure and are not able to induce high increase in the rheology of suspensions, contrary to the ones containing surface modification such as, carboxylation, carboxymethylation, and quaternization [
13]. In the case of CNFs containing charged groups, in which the charge density could be affected by external stimulus, as it is the case of carboxylic groups in TEMPO CNFs, it is possible to tune the rheology of the suspensions by changing the pH conditions [
12,
14,
15]. In literature are reported works dealing with the study of different ways to control the rheology of TEMPO CNFs, proposing the addition of salts [
14,
15,
16], the ageing effect [
17], gelation induced by heat [
18], gelation induced by alcohol adding [
19], and the use of surfactants [
20].
On the other hand, Kopac et al. reported the effect of the quaternization reaction, its optimization and fibril characteristic effects on the rheology of cationic micro- and nanofibrillated cellulose [
21]. Moberg et al. studied the effects of particle/fibril dimensions and surface characteristics on the rheological properties of nanocellulose suspensions [
22]. They have concluded that the length (or the aspect ratio) of the particles played a very important role on the rheology of the suspensions.
However, no studies have been reported comparing the effect of different pre-treatments during the CNF preparation, and different contents of charged groups, on the rheology and gelation of CNF suspensions. In the present work, four different samples of negatively charged CNFs (TEMPO-oxidised), containing different levels of carboxylic groups and degrees of fibrillation, were compared for their rheological behaviour with five samples of cationic CNFs (one prepared by direct cationization and four by two-step cationization) containing different levels of cationic groups and degrees of fibrillation. Additionally, dynamic light scattering, and zeta potential determinations were performed to shed light on the aggregation extension and its impact on the gelation of CNF suspensions.
2. Results and Discussion
The type of pre-treatment during CNF production has a great impact on the properties of the obtained materials. For example, if no pre-treatment is used previous to mechanical fibrillation, the extension of nanofibres production is very limited. Moreover, the enzymatic pre-treatment can increase the degree of fibrillation, but not to very high levels. For example, the use of an enzymatic pre-treatment, followed by two HPH passes, was able to increase the degree of fibrillation from ca. 6% to ca. 17% for a bleached kraft
Eucalyptus pulp [
2,
23]. On the other hand, the use of chemical pre-treatments enables to obtain CNF suspensions with high degrees of fibrillation, up to 95% or more, using the same two passes in HPH.
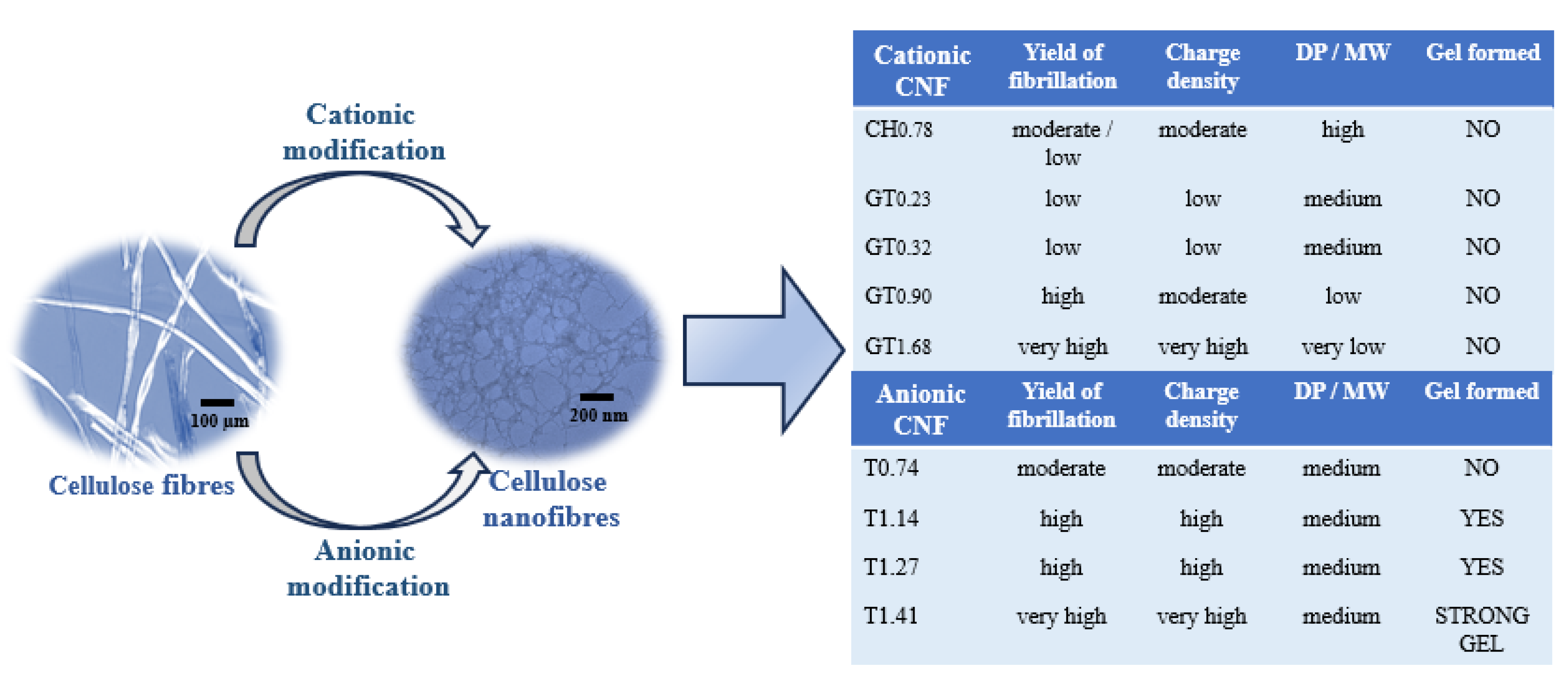
Table 1 summarizes the main properties of the prepared CNFs, by applying different chemical pre-treatments, followed by two passes in HPH, using as starting material bleached kraft
Eucalyptus pulp.
The level of nanofibrils generated by the intensive mechanical treatment (HPH) is clearly influenced by the concentration of oxidizing agents, sodium hypochlorite and periodate, used during the pre-treatments. It is possible to find a clear relation between the amount of oxidizing agent and the degree of fibrillation. Increasing oxidising agent led to higher charge densities (samples T1.41 and GT1.68), resulting in the swelling of the fibres, and consequently, higher contents of nanofibrils are formed. However, these exhaustive chemical pre-treatments resulted in a relevant decrease in the degree of polymerization of cellulose molecules. For example, the starting material (BEKP) presented a DP of ca. 2990, and the sample T1.41 showed a DP of ca. 365, being the decrease more pronounced when periodate oxidation followed by cationization with GT reagent was used (GT1.68 showed a DP of only ca. 26). As a consequence, the dimensions and shape of the formed nanofibrils are also highly affected, being the fibrils formed in the sample GT1.68 very short in length. One of the most valuable characteristics of CNFs is their high aspect ratio, which endows these materials with peculiar properties. One of these properties is the ability of materials with high aspect ratio to form gels at low concentration, due to the easy network formation caused by the entanglement of the thin and long fibrils.
Figure 1 presents digital photos of CNF samples with different charged groups at different contents.
From
Figure 1 it is possible to infer that the degree of fibrillation has a deep impact on the ability of suspensions of cellulose nanofibres to form gels; similarly, the aspect ratio of the fibrils has a key role in their formation of gels. The sample T0.74 presents a moderate level of fibrillation reducing the possibility of the formation of the entangled network at a concentration of 0.80 wt.%, and thus a gel was not obtained at the higher concentration tested. On the other hand, the samples with higher degrees of fibrillation (T1.14, T1.27 and T1.41) formed gel, at the same concentration, due to the higher content of fibrils with the capacity to contribute to the formation of the three-dimensional network and an increased capacity to resist to flow. The sample GT1.68 presents a fibrillation degree of ca. 100% and thus it should be expected to observe a strong gel for this suspension. However, due to the high destruction of the fibrils' integrity during the pre-treatment step, the formed nanofibrils present a short length and are not prone to form a network, being not observed the formation of a gel for all the tested concentrations. Long fibrils could touch each other and easily form a strong network; contrary, short fibrils couldn’t touch and the formation of highly viscous suspensions and/or gels could not be observed or was only observed for highly concentrated samples. The behaviour shown by the suspensions of CNFs follows the one observed for other polymers, where it was clearly demonstrated a high decrease in overlap concentration of the polymer as the MW increases [
24].
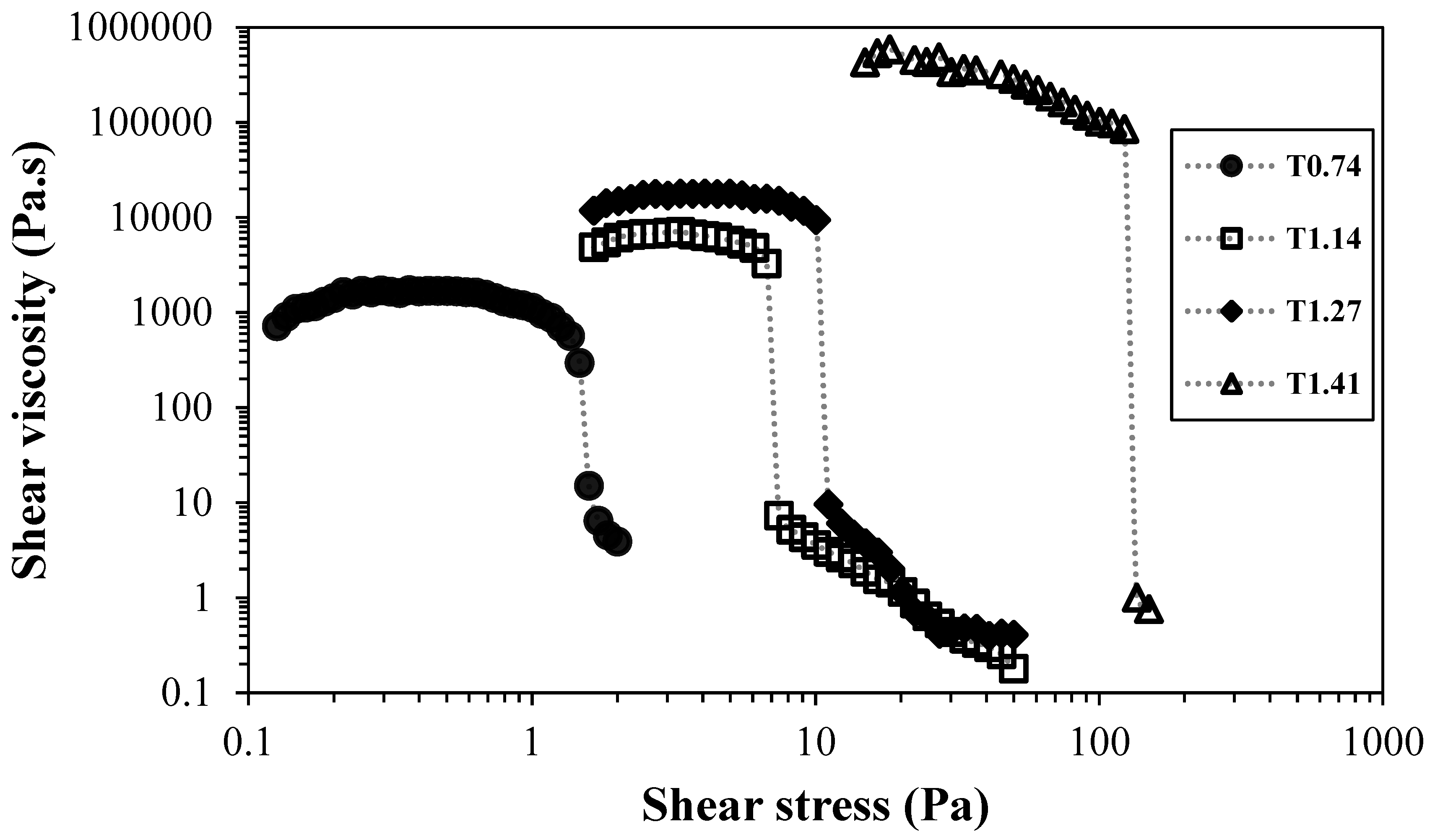
Figure 2 presents the flow curves of suspensions of TEMPO-oxidised CNFs, at ca. 0.80 wt.% and neutral pH.
The increase of the amount of sodium hypochlorite used during the pre-treatment of TEMPO-oxidised samples led to higher fibrillation of the cellulose fibres and this resulted in higher viscosity of the samples (at low shear rate values). The trend obtained shows an increase in shear viscosity of the samples at low stresses, as well as a shift to higher stress values for the yield stress of the samples, from ca. 1 Pa for the sample with lower charge density, to ca. 100 Pa for the sample with higher charge density. Undoubtedly, the increase in nanofibres with low thickness led to a strong reinforcement of the network, and the resistance to flow was increased about 100 times (at the same suspension concentration), due to the larger opportunity of the nanofibrils to physically interact with the neighbour ones and form highly entangled networks, which present high resistance to flow.
Independently of the CNF nanofiber content, the increase in the concentration of the CNF suspensions always led to an increase in the viscosity of the suspensions.
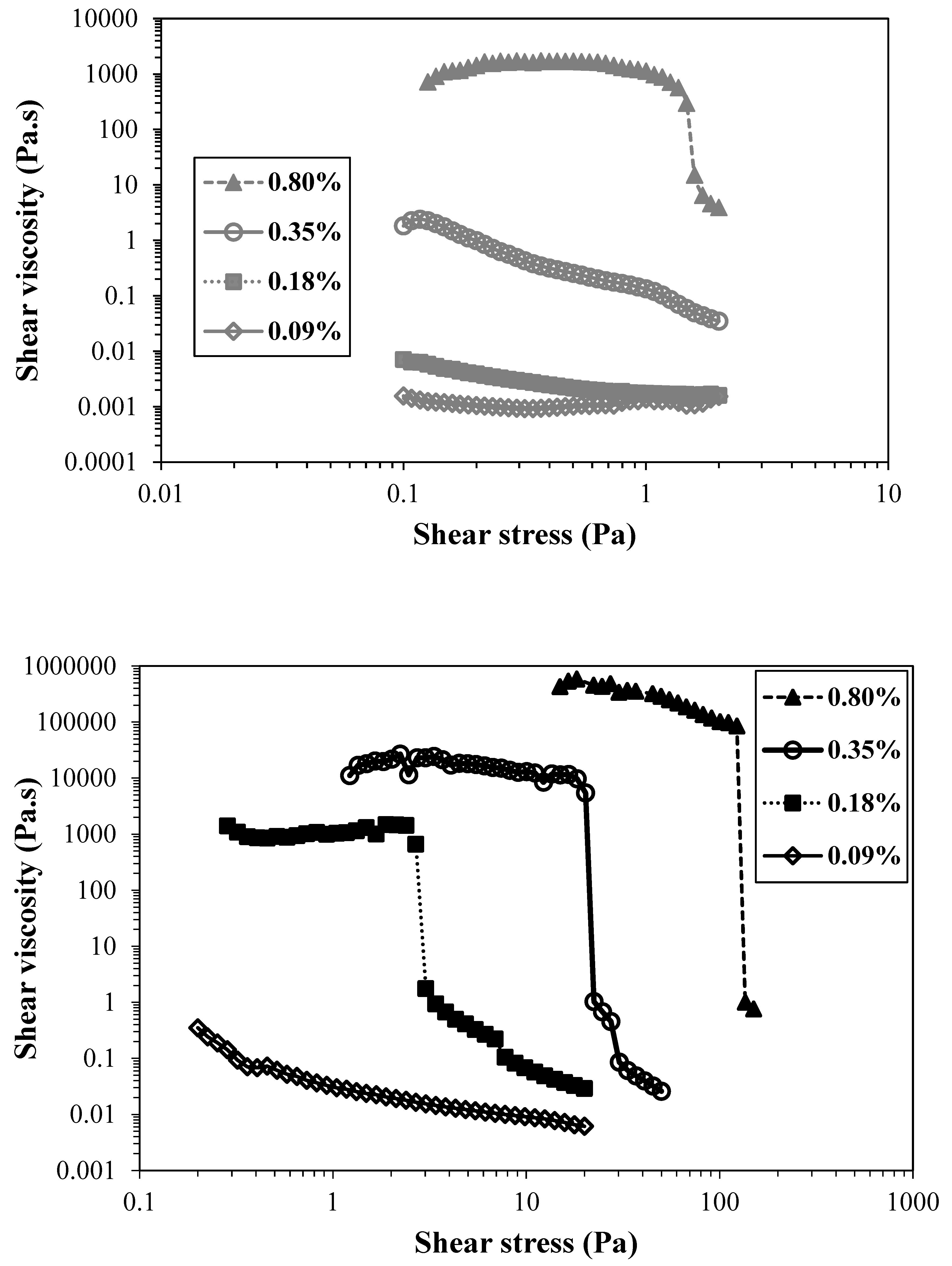
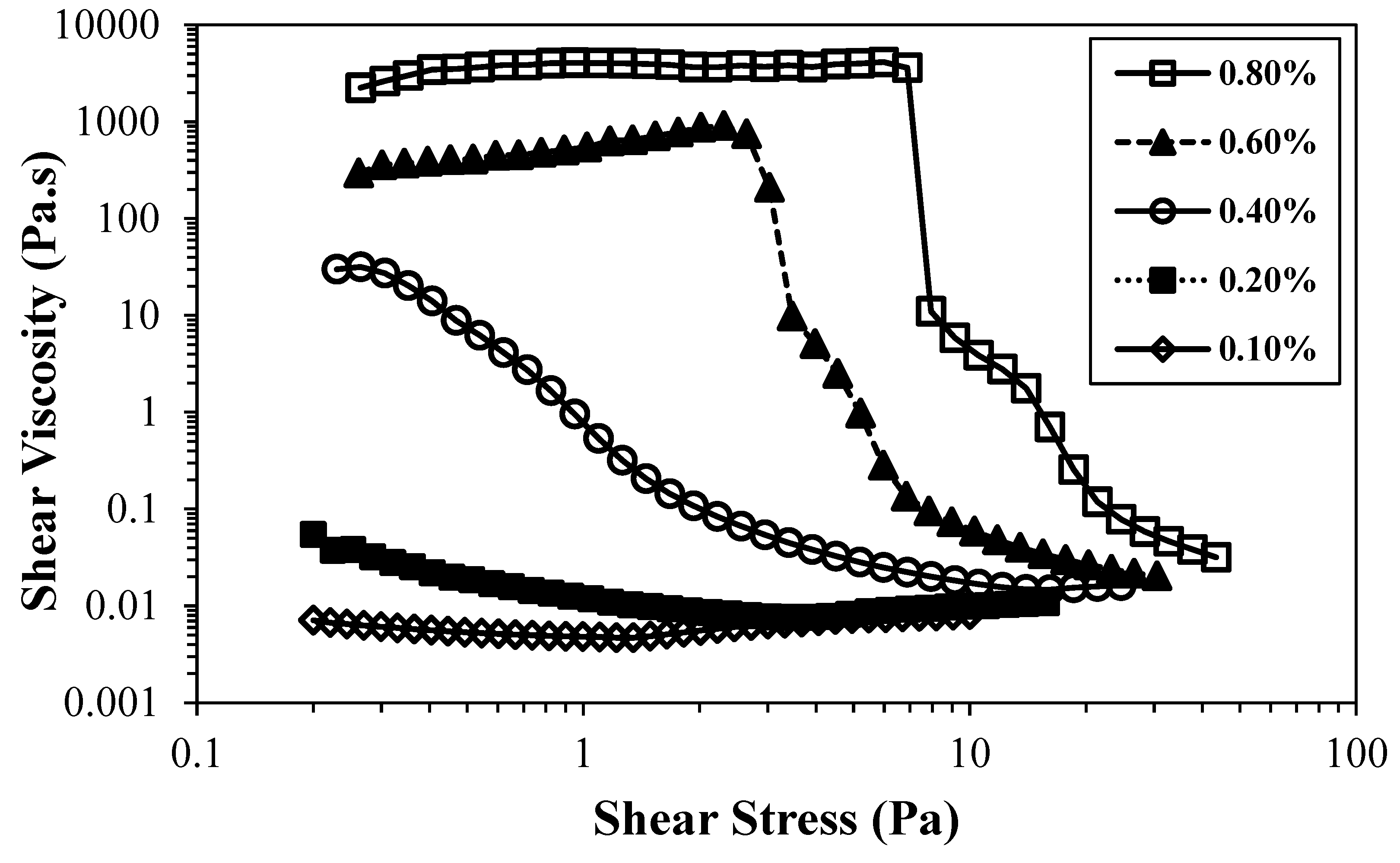
Figure 3 shows the flow curves of TEMPO-oxidised CNFs with lower (T0.74) and higher (T1.41) charge density.
As expected, the viscosity of the suspensions increased when the suspension concentration was raised, both for the less charged CNFs and for the more charged ones. However, the increment in viscosity values was not with the same magnitude. For the CNF with the lowest charge, the increment in the viscosity was about six orders of magnitude, and for the highly charged CNF sample, the increment was ca. eight orders of magnitude. Similarly, the overlap concentration (transition from a diluted to a semi-diluted regime) occurs in lower concentration values for the sample with higher charge density, due to the larger amount of thin and long nanofibres able to form an entangled network at lower concentration, ca. 0.09 wt.%. On the other hand, the sample with lower charge density only attains similar values of viscosity for the higher concentration and the overlap concentration is estimated to be between 0.18 and 0.35 wt.%, where a significative increase in viscosity is observed, due to the starting of CNF entanglements and consequent viscosity raise.
Being the TEMPO-oxidised CNFs negatively charged, by the presence of carboxylic groups introduced in the carbon C6 of cellulose molecules through the sodium hypochlorite oxidation, mediated by TEMPO radical, the behaviour of the CNFs in suspension will be deeply affected by the protonation/deprotonation of the carboxylic groups present.
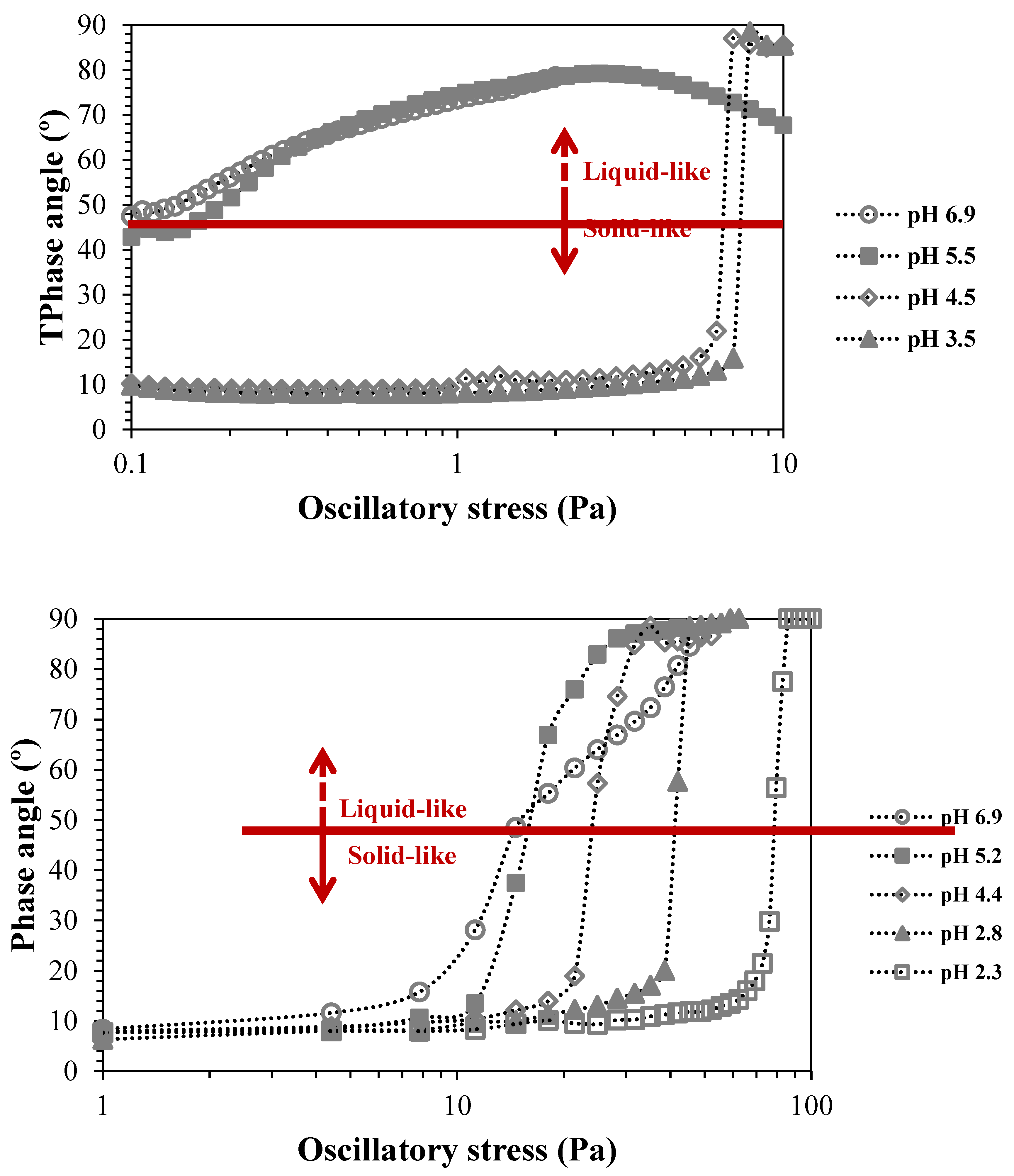
Figure 4 depicts the effect of the change of the suspension pH, and consequently the change in carboxylic groups protonation when pH is decreased, in the yield stress of the CNF suspensions.
The decrease in the pH of the suspensions and the consequent decrease in the negative charge of CNFs increased the CNF aggregation and led to a shift in the yield stress of the suspensions. For example, for the suspension of T0.74, the yield stress value goes from 0.1 Pa to ca. 8 Pa with the decrease of the pH from 6.9 to 3.5. A further decrease in the pH of the suspensions of T0.74 resulted in the precipitation of the respective CNF due to the very low negative charge and extensive aggregation. The large decrease of negative charges, by protonation of carboxylic groups led to a large loss of counterion entropy, resulting in extensive aggregation and low dispersibility of the nanofibrils, and consequent precipitation. Contrary, for the highly charged CNF, the decrease in pH of the suspension, until pH 2.3, did not result in CNF precipitation. It was observed that the effect of pH decrease is more progressive, and the larger shift in yield stress values only occurs for the transition from pH 2.8 to pH 2.3, contrary to the suspension of T0.74 where the main change happens for the pH transition from 5.5 to 4.5. This could be explained by the higher number of negative sites in the T1.41 sample, which even facing a decrease in negative charges by lowering the pH still presents a high enough number to avoid extensive aggregation of the nanofibrils. The larger effect observed for the sample with a lower charge density occurs close to the pKa of carboxylic groups (ca. 4.8 [
25]), with approximately 50% of the carboxylic groups being protonated at this pH value. Due to the low substituent group content, this decrease in 50% of charged groups is enough to give rise to extensive aggregation, and a further decrease in pH led to phase separation (precipitation).
Contrary to the negatively charged CNFs produced in the present work, which contain charged groups without permanent charge, the cationic ones prepared contain quaternary ammonium groups (permanent charges) and thus the amount of charged groups in the latter CNFs does not suffer the effects of pH changes.
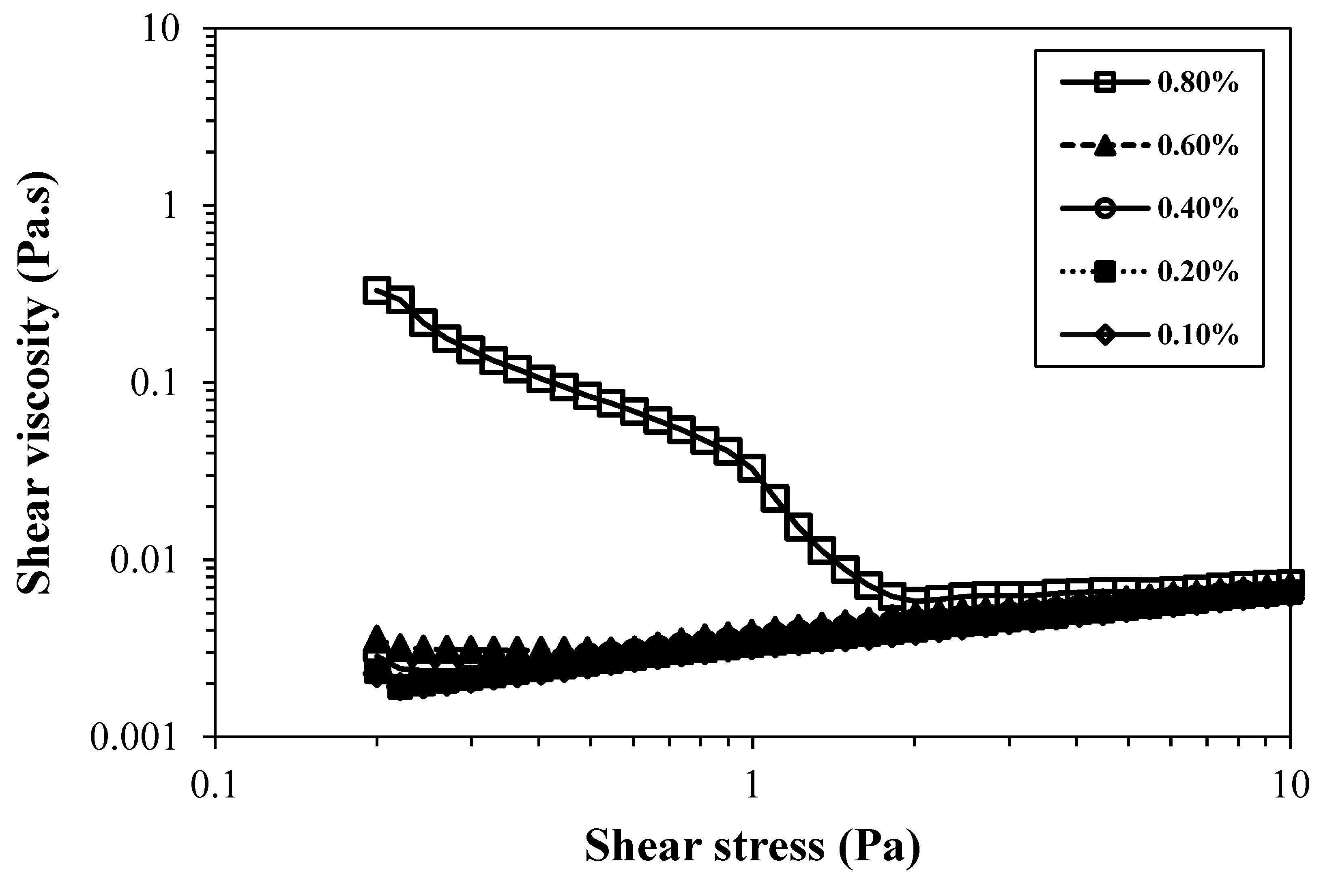
Figure 5 illustrates the flow curves of different cationic CNFs, at a concentration of 0.80 wt.%.
The sample CH0.78, prepared by direct cationization of cellulose with CHPTAC, even presenting a moderate degree of substitution and fibrillation was the one which showed the highest value for yield stress point, ca. 10 Pa, of all the cationic CNF suspensions tested. Comparing this sample with a negatively charged CNF sample with a similar charge density (T0.74) it is possible to observe that the cationic CNF suspension presents higher viscosity and yield stress. It is also important to note that sample T0.74 presents a higher degree of fibrillation (ca. 50%) almost twice the value obtained for CH0.78 (29%), A possible explanation for the higher viscosity obtained for a sample with lower fibrillation could be the low degradation of the cellulose molecules when CHPTAC is used for cellulose cationization and CNF preparation, being the DP obtained very close to that of native BEKP. Thus, the formed fibrils, even if not at a very high content, are long and could easily entangle with others and form a strong network. Besides, for the samples prepared by two-step cationization a different trend was observed compared to TEMPO-oxidised CNFs. Samples with a low degree of substitution (GT0.23 and GT0.32) presented a very low degree of fibrillation (6 and 12%, respectively) and did not form a gel, presenting therefore low to moderate viscosity values. The sample with an intermediate level of cationization (GT0.90) was the one of the GT series that showed higher values of viscosity, which can be attributed to the high level of fibrillation obtained, 98%. Making a comparison with a TEMPO-oxidised CNF sample with a similar degree of fibrillation (T1.41), the viscosity of the cationic CNF suspension is much lower, due to the greater destruction of cellulose structure induced by the intensive oxidation during the aldehyde formation; the DP of the sample GT0.90 is only 47 (64 times lower than the value obtained for the starting cellulose – BEKP present a DP of 2990). Conversely, the DP of the sample T1.41 is only 8 times lower than the DP observed for starting cellulose.
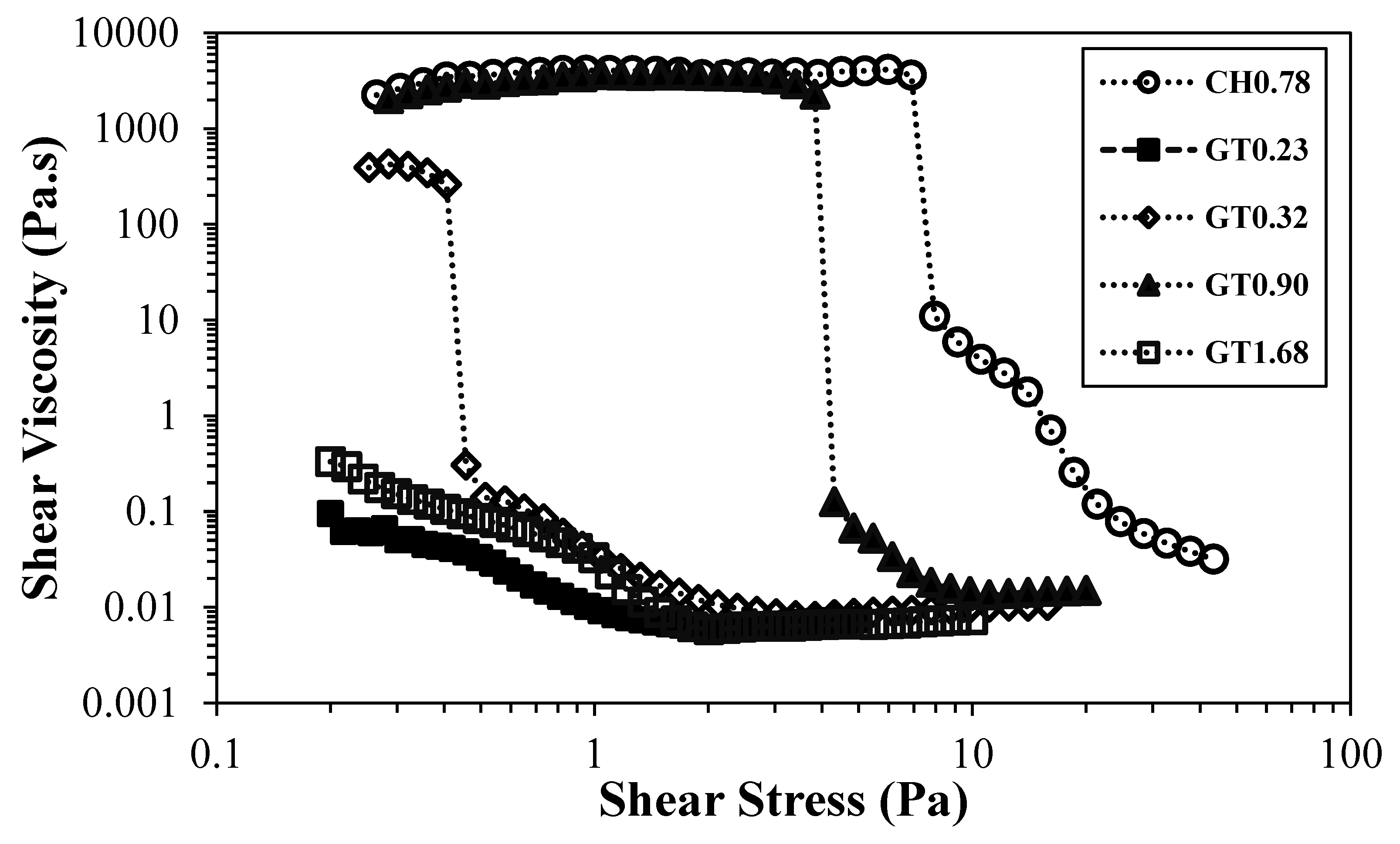
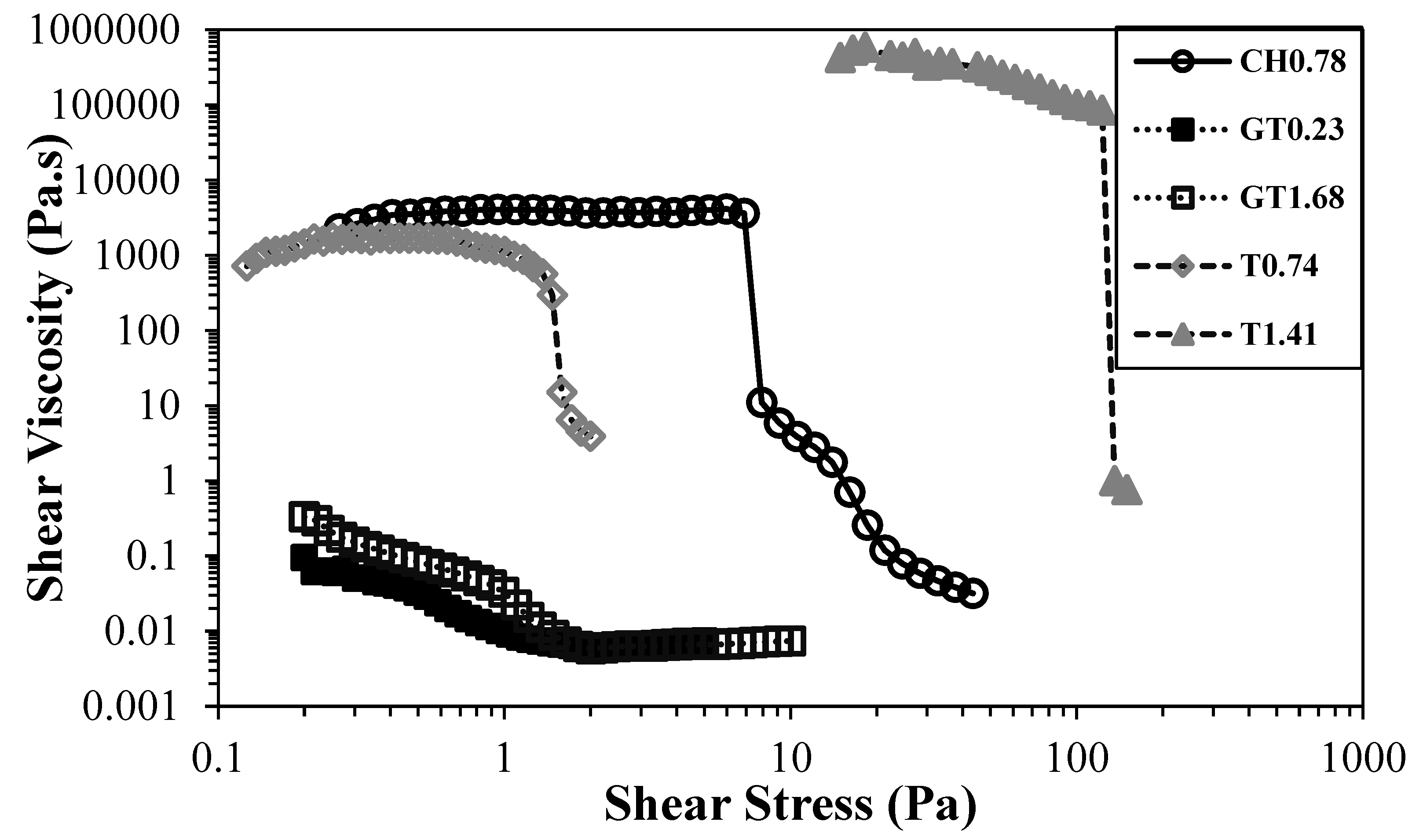
Figure 6 depicts the flow curves of CH0.78 and cationic CNF with the highest degree of substitution, for different CNF concentrations.
The difference between the samples is substantial, being the sample GT1.68 much less viscous than CH0.78, even though the sample prepared by direct cationization (CH0.78) presents a lower level of cationic groups and fibrillation degree. For samples obtained by direct cationization of cellulose hydroxyl groups, it was not possible to obtain higher degrees of cationization, even increasing the ratio cationizing agent/cellulose, due to some steric hindrance related to the position of the cellulose ring where the reaction occurs, carbon C6 [
7,
26]. Conversely, for the two-step cationization, it was possible to obtain high levels of cationization (up to 1.68 mmol/g) but an extensive degradation of the cellulose structure was observed. This destruction led to a shortening of the nanofibrils' length and resulted in a large increment of the overlap concentration (c*). The estimated value of c* for the different cationic samples prepared was quite different, varying from 0.13 wt.% (CH0.78) to 0.60 wt.% (GT1.68), having a clear influence also on the viscosity of the suspensions, as observed in
Figure 6.
Figure 7 represents the flow curves of CNF suspensions prepared by applying different pre-treatments for different levels of treatment.
From
Figure 7 it is clear that the pre-treatment applied, as well as the level of treatment, have a deep impact on the rheology of the CNF suspensions. Different trends were observed depending on the pre-treatment. For example, the oxidation of cellulose fibres, mediated by TEMPO radical (samples T0.74 and T1.41), led to an increase in the viscosity of the CNF suspensions and yield stress point, due to the higher level of nanofibrils produced, without a drastic destruction of the cellulose structure. Contrarily, the two-step cationization (samples GT0.90 and GT1.68) resulted in extensive degradation of the cellulose structure and the suspensions with higher levels of cationic groups were not able to form a gel or even a suspension with high viscosity. The length of nanofibrils is demonstrated to be a critical parameter for the formation of a 3D network and induces a high increase in viscosity. Long fibrils can easily entangle and form a robust network, which results in strong resistance to flow (sample CH0.78). Thus, the level of nanofibrils formed after HPH play a central role om the gelation of aqueous suspensions of charged CNF, but it was shown that this only happens if the level of shortening of CNF is not very high.
Figure 8 summarizes the main properties of the CNF suspensions prepared and their influence on the rheology, referring to the formation or not of gels for suspensions containing 0.80 wt.% CNF in an aqueous medium.
3. Conclusions
The present work explored the effects of different pre-treatments used in the preparation of CNFs on the rheology of aqueous suspensions of CNFs containing different charged groups and different levels of modification. It was observed that TEMPO-oxidized CNFs can easily form strong and stiff gels, due to high levels of nanofibrillation and reasonable preservation of the length of the produced nanofibres. On the other hand, cationization of cellulose through a two-step procedure induces extensive degradation of CNF structure, leading to short nanofibres and cellulose chains with low DP. As a consequence, the suspensions of cationic CNF with high cationic charge density, GT1.68, did not form a gel at the highest concentration studied (0.80 wt.%), and this CNF was not even able to induce a significant increase in the viscosity of the suspensions. The direct cationization method, through CHPTAC grafting on the C6 of cellulose molecules, generated interesting results, enabling a moderate level of nanofibrillation without extensive structure degradation. The suspensions presented high viscosity but without the formation of a gel at 0.80 wt.%. From the obtained results it was possible to conclude that a high level of nanofibrillation combined with a not too low length of the obtained nanofibres are the key factors to form strong gels. The TEMPO CNFs could fulfil these requisites thus forming gels, whereas the cationic CNFs did not. The charge repulsion between charged groups of nanofibrils, and the associated entropy of the counterions, revealed to play a role in the rheology of the suspensions, but not so pronounced as the structure of the nanofibres. The content in charged groups was revealed to be very important in keeping the nanofibres in suspension and avoiding extensive aggregation and precipitation of nanofibers, when factors, such as the pH value in TEMPO-oxidized CNF suspensions, are modified and decrease the extent of those groups present in the CNF particles. In sum, the present work demonstrates the potential of the use of charged CNFs as rheology modifiers, being especially attractive the TEMPO-oxidised CNFs with high levels of carboxylic groups, which can form gels at low concentrations. On the other hand, cationic CNFs produced by direct cationization, and by two-step cationization with moderate levels of derivatization, also present good rheological properties, and additionally present other interesting properties such as antimicrobial activity.