1. Introduction
Living organisms acquire their strength, durability, and some degree of flexibility from bones, which consist of collagen and calcium phosphate apatite crystals [
1]. Bone, a mineralized connective tissue, boasts a repertoire of four distinct cell types: osteoblasts, bone lining cells, osteocytes, and osteoclasts [
2]. It holds pivotal roles within the body, including facilitating locomotion, providing structural support, and safeguarding delicate tissues, as well as serving as a reservoir for calcium and phosphate while also hosting bone marrow [
3]. Despite that, Conditions such as osteoporosis, osteoarthritis, and fractures become more common as people grow older, posing substantial challenges in clinical settings with increased demand for materials to repair bone. Efforts to address these bone-related issues have evolved from traditional autogenous and allogeneic bones to modern polymer materials and tissue-engineered bones. Ongoing scientific and clinical research in related fields is dedicated to advancing solutions for bone-related diseases [
4,
5]. In a clinical context, bone defects are typically treated with autologous bone grafts and allograft bone grafts. Autologous bone grafting is often regarded as the "gold standard" due to its biological benefits and cost-effectiveness [
6]. Nevertheless, these methods encounter issues such as limited donor bone volume, complications after autologous bone graft procedures, and a relatively high occurrence of immune rejection associated with allograft [
7,
8].
Subsequently, it is crucial to have a comprehensive understanding of the intricate biomechanics of natural bone and a basic knowledge of commercially available bone grafts, to facilitate successful treatment planning [
9]. As a consequence, bone stands as the second most commonly transplanted tissue on a global scale, with a minimum of four million surgeries annually employing bone grafts and substitute materials [
10,
11,
12]. Therefore, bone tissue engineering has become a promising option to traditional approaches in treating bone defects, offering solutions to overcome their drawbacks. Several techniques, including the utilization of bioceramics, mesenchymal stem cells, and the incorporation of diverse drugs within bioceramic substrates, employing advanced methodologies such as 3D printing and laser sintering, have been devised to fabricate composite scaffolds [
13,
14].
The term 'bioceramics' denotes biocompatible ceramics materials and find application in biomedical or clinical contexts [
15]. Their chemical composition influences the degradability, biocompatibility, and bioactivity of bioceramics when implanted in vivo [
16]. Various biomaterials have been employed as scaffolds, providing an option for the regeneration of bone. An ideal scaffold should exhibit an interlinked porous configuration, controlled biodegradability, mechanical characteristics akin to bone, biocompatibility, and pores sized to facilitate cell proliferation and nutrient diffusion. The porosity of the scaffold significantly impacts cellular adherence, proliferation, revascularization, optimal nourishment, and other factors influencing cellular behavior [
17,
18,
19]. The limitations encompass brittleness, inadequate fracture toughness, exceptionally low elasticity, and exceedingly high stiffness [
19,
20].
Some reports suggest that between 5% and 10% of all bone fractures, and even in some cases, up to 50%, lead to delayed or unsuccessful healing [
21]. To address these challenges, several research studies have concentrated on improving bone regeneration by applying (mesenchymal stromal cells) MSCs derived from different connective tissues [
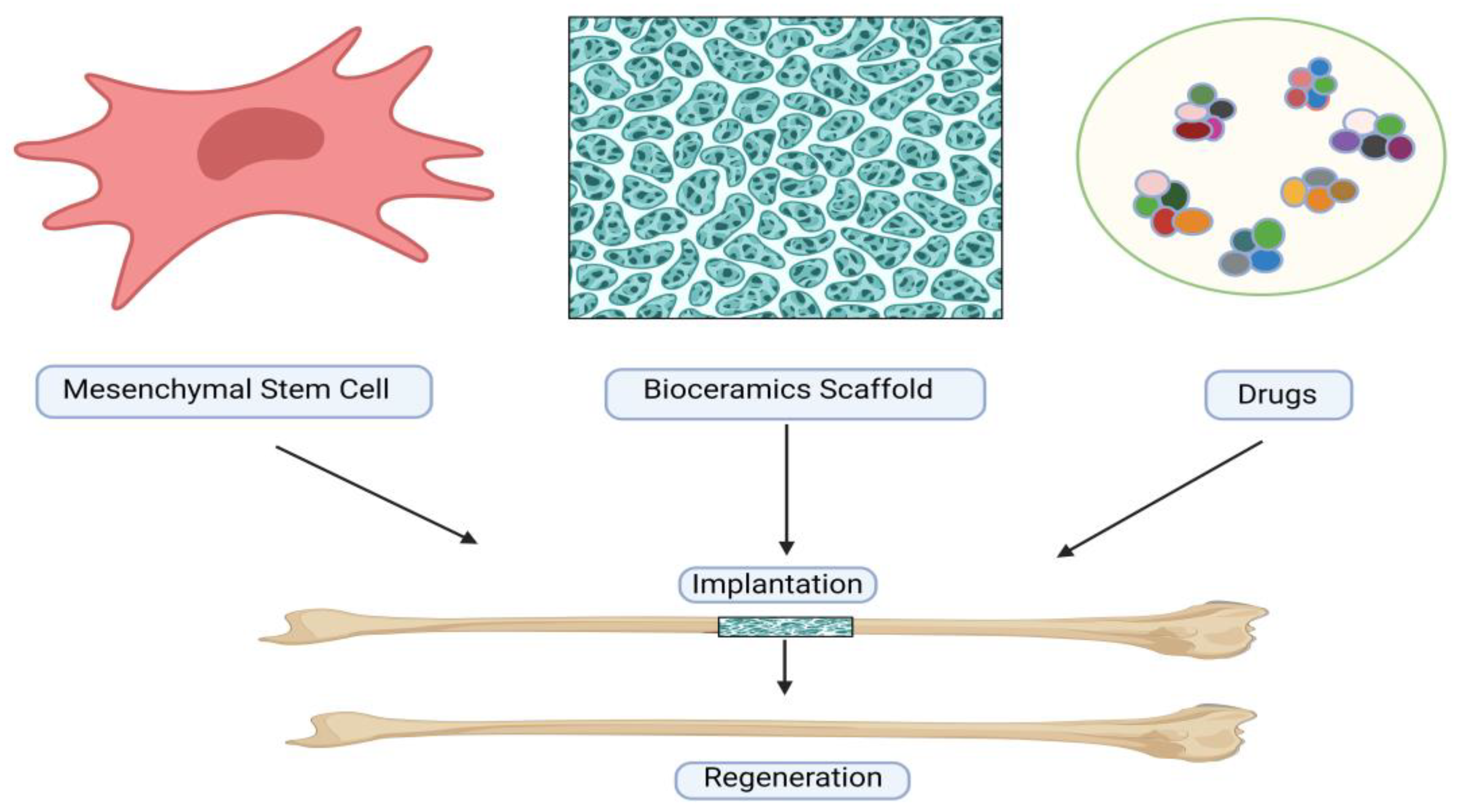
22]. Methods for in-vivo processing and culture have been developed to acquire an ample quantity of MSCs for therapeutic purposes. [
23] Moreover, researchers have integrated MSCs with diverse scaffolds and signaling factors to engineer viable "bone substitutes" that mimic essential characteristics of autologous bone grafts, thereby promoting enhanced bone regeneration (
Figure 1) [
24].
In the treatment of bone fractures and bone diseases, numerous beneficial therapeutic agents exist. However, since bones are distributed throughout the entire body, maintaining the blood concentration of therapeutic agents within a specific range is crucial to exert their pharmacological effects at the intended peripheral site. This often leads to undesirable systemic side effects, creating a narrow toxic-therapeutic window for the treatment of bone diseases. Consequently, there is a petition for the expansion of innovative drug delivery systems to mitigate these issues [
25]. In the context of treating bone fractures, sustained drug delivery systems are gaining attention. These systems consist of bio-compatible and biodegradable composites, incorporating bioactive materials and ceramic implants. They are currently undergoing successful evaluation in animal models as a promising approach for sustained drug release in the treatment of bone fractures [
26]. In the jurisdiction of biomaterial application, meticulous attention must be directed toward the pivotal factors governing the genesis of replacement bone tissue. These factors are bifurcated into biological determinants and mechanical stability requisites. Four fundamental components stand out in bone repair and rejuvenation: osteogenic cells, MSCs, growth factors facilitating osteoinduction, and scaffolding essential for osteoconduction.
In this review, we will explore the function of bioceramics for bone regeneration. Initially, we delve into the historical background and categorization of bioceramics. Subsequently, we examine the reactions observed in laboratory settings and the bone-forming capabilities observed in living organisms regarding the porous scaffolds' ability to promote bone growth. Following this, we reconnoiter how different biomolecules delivered within the scaffolds impact the integration of bone implants and the formation of new blood vessels. Lastly, we address the impact of environmental factors on bioceramics and the incorporation of drug delivery mechanisms and speculate on forthcoming advancements in scaffold technology for advancing bone tissue engineering practices.
2. History of Bioceramics
In 1920, the first documented successful use of a calcium phosphate reagent to treat a bone defect in a human patient was reported. [
27] In the 1950s, the focus was on using inert materials to avoid tissue interaction, solely based on non-toxicity. [
28] However, ceramics like zirconia and alumina triggered foreign body reactions, leading to encapsulation. [
29] The 1960s saw significant progress in bioceramics, with notable contributions from various researchers worldwide. [
30] In the 1980s, there was a shift towards using ceramics that interacted with the body, promoting bone formation. [
31,
32,
33] Today, the aim is to create porous ceramics serving as scaffolds for tissue self-regeneration. [
34,
35,
36] Calcium orthophosphates, especially hydroxyapatite, have been key materials since the 1980s. [
37] Currently, a range of biodegradable bioceramics has been effectively applied, although with certain limitations. Consequently, further research is warranted to address these constraints.
3. Classification of Bioceramics
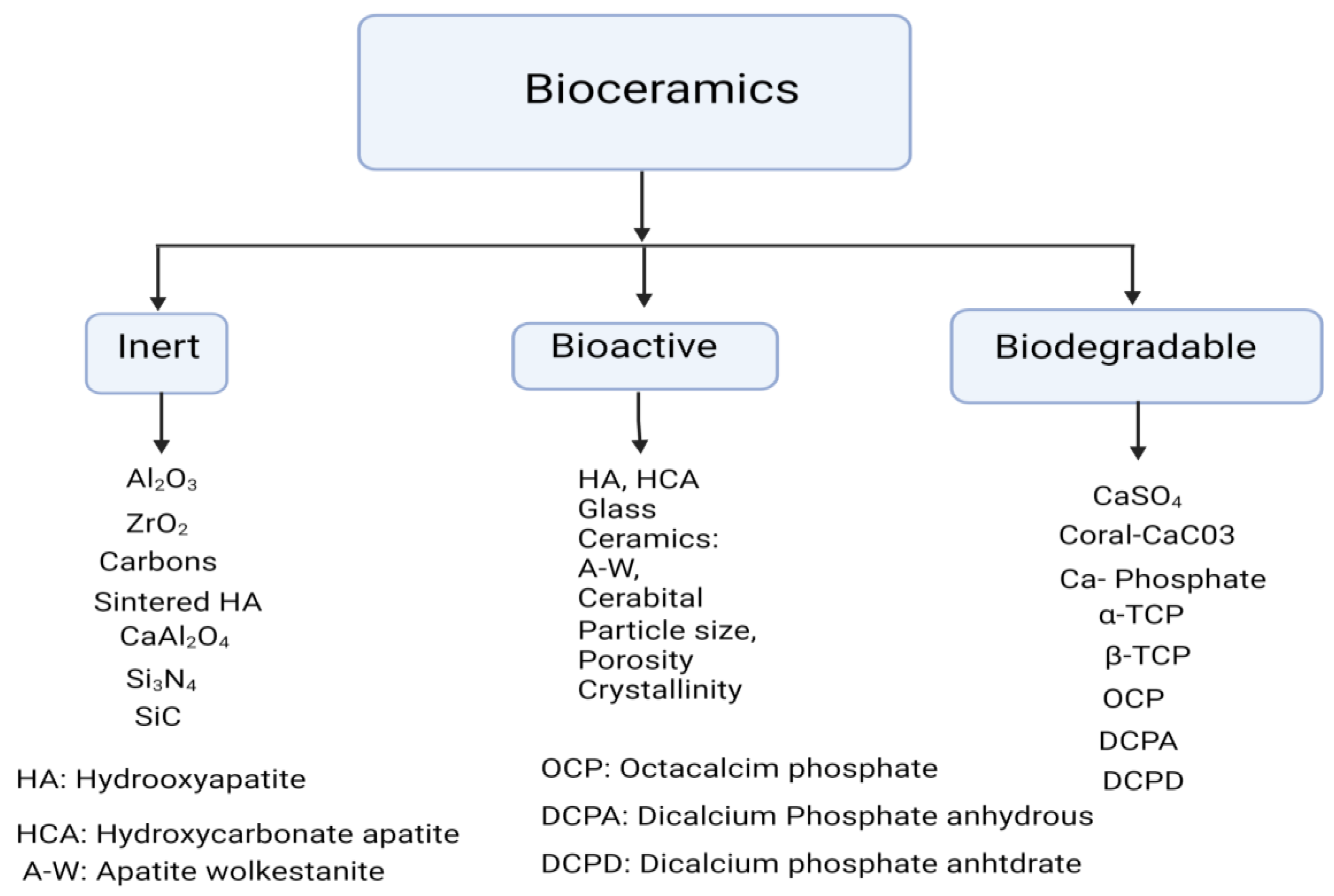
The first generation of inert ceramics aimed to substitute natural bone, hence the research was only focused on inert materials, (e.g. titanium, alumina, polyethylene); the second one was aimed at mimicking some biomineralization-related functions and sol-gel chemistry plays a paramount role in their synthesis and properties (e.g. hydroxyapatite or bioactive glasses). Finally, the purpose of third-generation bioceramics is basically to provide an adequate scaffolding system that helps the bone cells perform their natural processes such as biodegradable ceramics (e.g. peptide or protein-modified, degradable polymers). Tissue engineering attempts to develop artificial materials able to replace biological tissues in situations where the human body cannot perform said replacement by itself (
Figure 2) and (
Table 1) [
38].
4. Relationship of Different Types of Bioceramics
First-generation biomaterials include alumina and zirconia, which are exemplary for their inert bioceramic properties. These materials are still utilized in certain prosthesis components, particularly when low friction coefficients are necessary. However, these components can lead to micro-movements at the bone-to-implant interface, which can escalate over time and ultimately result in prosthesis failure. In general, ceramics are inorganic materials that have a combination of ionic and covalent bonding. Bioceramics can be derived from various substances such as alumina, zirconia, magnesia, carbon, silica-containing compounds, calcium-containing compounds, and several other chemicals. Hence, compounds like calcium phosphates, calcium sulfates, specific glasses, and glass ceramics are recognized as authentic examples of bioceramics. Despite carbon being an element and exhibiting electrical conductivity in its graphite state, it is also classified as a ceramic due to its numerous ceramic-like properties. Presently, research is focused on developing new advanced bioceramics, including ordered mesoporous silica materials and specific compositions of organic-inorganic hybrids. These compounds can be manufactured in dense or porous forms, either in bulk or as crystals, powders, particles, granules, scaffolds, and/or coatings [
50,
51,
52]
5. Mechanism of Action
5.1. The Signaling Pathway of MAPK
The signaling pathways involved in osteogenic differentiation and bone regeneration, focus on MAPK pathways and the effects of biomaterials. [
53].It highlights the role of extracellular stimulation in activating MAPK cascades, with (extracellular signal-regulated kinase) ERK, (c-Jun N-terminal kinase) JNK, and p38 pathways playing essential parts in inflammation, apoptosis, and growth [
54,
55,
56]. Nano-HA accelerates osteogenic gene expression via ERK signaling, while Mg2+ promotes bone regeneration by activating ERK1/2 and p38 pathways [
57,
58,
59]. (Calcium silicate) CS releases ions that stimulate osteoblast proliferation through MAPK pathways, with silicon ions enhancing ERK and p38 activity [
60,
61,
62,
63]. Additionally, CS induces a pro-inflammatory response through the (toll-like receptor 2) TLR2-mediated JNK pathway [
64]. Overall, the interaction between biomaterials and MAPK signaling pathways plays a key role in bone tissue engineering and regeneration [
65].
5.2. The Signaling Pathway of BMP
BMPs, part of the (transforming growth factor-β) T_G_F_-beta superfamily, are essential for bone formation and stem cell differentiation [
66]. They activate signaling pathways by binding to (Bone Morphogenetic Protein Receptor-I) BMPR-I and (Bone Morphogenetic Protein Receptor-II) BMPR-II receptors, leading to phosphorylation events in the( small mother against decapentaplegic) SMAD complex [
67]. This activation ultimately stimulates the expression of (runt-related transcription factor 2) Runx2 a critical transcription factor controlling osteogenesis-related genes [
68,
69]. Bioceramics like HA and CS activate the BMP signaling pathway, promoting osteogenic differentiation of (Bone Marrow Stem Cells) BMSCs. This is evidenced by increased expression of BMP2 and BMP4, along with key molecules in the SMAD pathway [
70]. These pathways play a key role in osteoblast migration and differentiation. Inhibition of BMP2 activity leads to the downregulation of downstream cascades involving bone morphogenetic proteins [
71]. On the other hand, a bioinert ceramic known as zirconium, when utilized at the nanoscale, was discovered to boost the formation of apatite when combined with polymeric scaffolds. [
72] This interaction triggers BMP2 signaling by facilitating the movement of phosphorylated SMADs into the nucleus, thereby promoting osteogenesis [
73].
5.3. The Signaling Pathway of (Wingless/Integrated) Wnt/β-Catenin
The Wnt signaling pathway is crucial for osteoblast differentiation and bone development in (mesenchymal stem cells) MSCs. In the classical pathway, Wnt ligands bind to Frizzled receptors and (Low-density lipoprotein receptor-related protein 5) LRP5 and (Low-density lipoprotein receptor-related protein 6) LRP6, preventing β-catenin degradation and promoting osteogenic gene expression [
74]. Bioceramics release ions like calcium, phosphate, and silicate to activate this pathway, aiding bone repair [
75,
76]. Strontium-substituted bioactive glass increases β-catenin levels in hBMSCs, while Wnt pathway inhibitors block β-catenin expression [
77]. Boron-containing BG (Bioactive Glass) scaffolds enhance osteogenesis via transcription factor 7-like 2, mediated upregulation of lipocalin-2. Overall, activating the Wnt/β-catenin pathway promotes osteogenic differentiation, offering therapeutic potential in bone regeneration [
78].
Furthermore, the heightened surface area facilitated by the nano ceramic trigger facilitated a greater release of calcium ions, leading to elevated expressions of (Angiopoietin-1) Ang-1 and (Angiopoietin-2) Ang-2. These proteins play crucial roles in maintaining the structural integrity of blood vessels and exert antagonistic effects on each other. Ang-1 also plays a role in influencing bone formation, the production and mineralization of (osteocalcin) OC, and the activity of (alkaline phosphatase) ALP (
Figure 3) [
79].
6. Uses of Porous Bioceramics
Porosity plays a key role in the field of scaffold and biomaterial engineering [
80]. The dimensions, configurations, and arrangement of pores influence the behavior of implanted materials through biological mechanisms such as colonization, angiogenesis, vascularization, complete resorption, and replacement of implants by newly formed tissues. Therefore, cellular activities necessitate adequate space for proliferation, multiplication, removal of toxic byproducts, and regeneration of normal body tissues. Achieving this entails, taking care to select materials that can support the desired cells, along with appropriately designed voids to facilitate penetration into biomaterials implanted in vivo [
81]. However, in cases of pathological fractures or extensive bone defects, the process of bone healing and repair may be compromised. Factors such as inadequate blood supply, bone or tissue infections, and systemic diseases can adversely affect bone healing, leading to delays in union or non-unions [
82,
83]. Calcium phosphates and bioactive glasses are excellent materials for constructing scaffolds intended to serve as a three-dimensional porous framework, facilitating new bone formation within their pore structure [
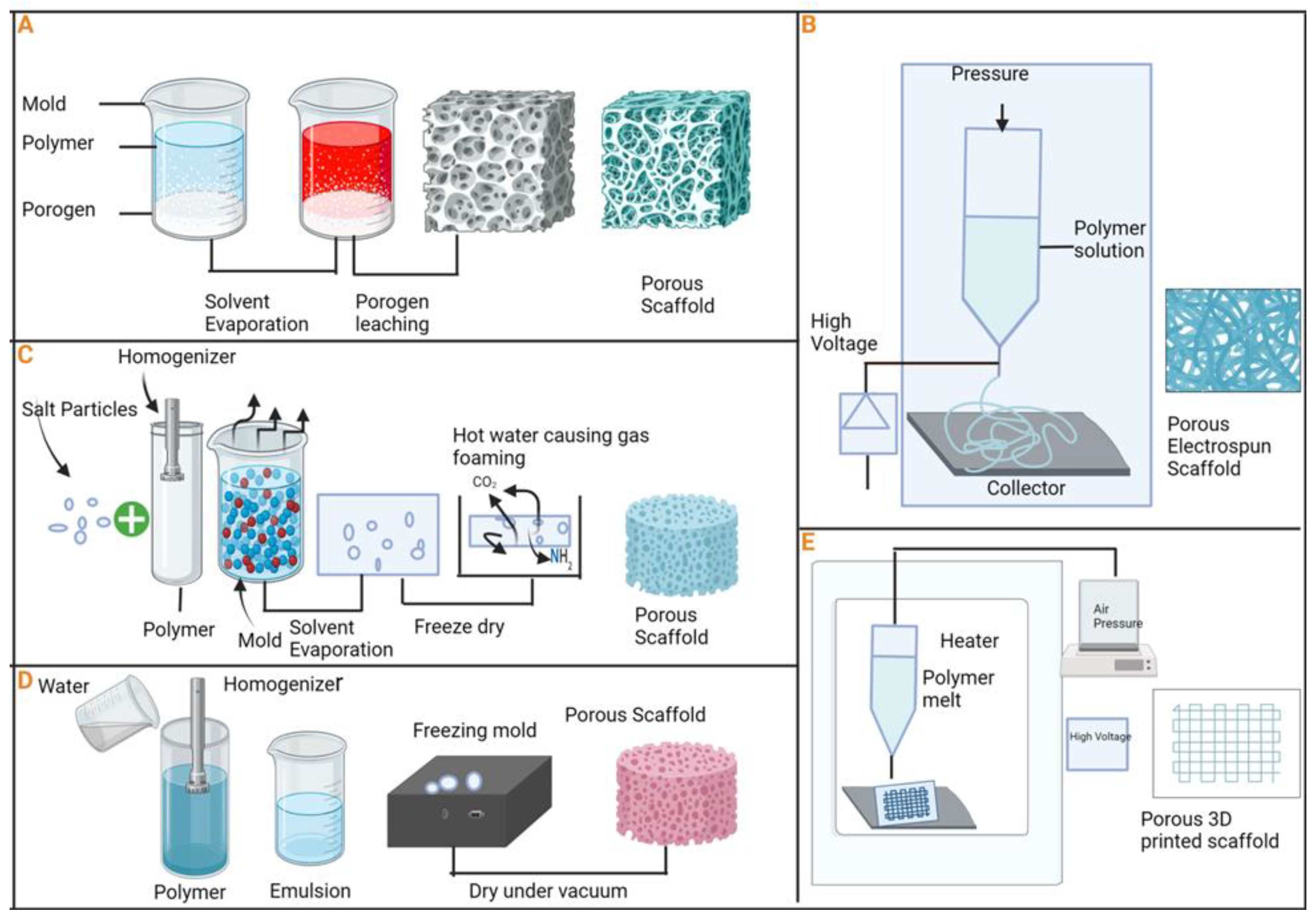
84]. Several techniques have been employed to regulate the porosity of scaffolds (
Figure 4). One effective approach involves combining freeze-drying with leaching template techniques to produce porous structures. In this method, pore size is adjustable by controlling parameters such as the gap space of the leaching template, temperature fluctuations, and altering the density or viscosity of the polymer solution concentration during the freeze-drying process [
85,
86].
The dimensions of osteoblasts typically range from approximately 10 to 50 micrometers (µm) [
87]. Indeed, osteoblasts exhibit a preference for larger pores, typically ranging from 100 to 200 micrometers (µm), when regenerating mineralized bone post-implantation. This size preference facilitates the infiltration of macrophages, which play a crucial role in eliminating bacteria, as well as inducing the infiltration of other cells involved in colonization, migration, and vascularization in vivo [
88]. On the other hand, smaller pore sizes, typically less than 100 micrometers (µm), are associated with the formation of non-mineralized osteoid or fibrous tissue instead of mineralized bone [
89] E. Cheng et al. asserted that the utilization of magnesium scaffolds featuring two pore sizes, 250 and 400 µm, revealed that the larger pore size fosters the enhanced formation of mature bone by facilitating vascularization. This is attributed to newly formed blood vessels, which deliver ample oxygen and nutrients necessary for osteoblastic activity within the larger pores of the implanted scaffolds. Thus, this upregulates the expression of (osteopontin) OPN and collagen type I, leading to the subsequent generation of bone mass [
90]. Shrestha, S., et al. demonstrated that phosphate-enriched nanocomposite comprising titanium and zinc exhibited remarkable potential for bone regeneration in-vitro. This composite induces robust activation of MC3T3-E1 and hBM-MSCs cells, thereby instigating a profound enhancement in cell viability and fostering osteogenic differentiation. This is substantiated by the notable upsurge in the expression levels of critical bone-related markers such as ALP, Col1a1, RUNX2, OPN/Spp1, and OCN. Moreover, in vivo assessments further accentuate the significant augmentation of fresh bone formation observed within critical-sized calvarial defects in a rat model, thus underscoring the unprecedented efficacy of this bioceramic composite in facilitating bone regeneration [
91]. In another study, Cao et al. aforementioned that porous composite scaffolds, comprising (polyglycolic acid/beta-tricalcium phosphate) PGA/β-TCP in weight ratios of 1:1 and 1:3, were created using a sophisticated blend of the solvent forming and particulate leaching method. After their insertion into rats, these scaffolds underwent rigorous evaluation through quantitative imaging analysis and qualitative histological assessments. Results unveiled that bone regeneration commenced within 14 days post-surgery and progressed exceptionally well, with significant healing apparent by the 30-day mark. By the 90-day milestone, bone replacement was nearing completion, showcasing a healthy bone appearance. Remarkably, the scaffold with a ratio of (polyglycolic acid) PGA to (beta-tricalcium phosphate) β-TCP 1:3 demonstrated remarkable osteogenic, mineralization, and biodegradation characteristics [
92]. Huang et al. proclaimed that fabricated (porous poly(lactide-co-glycolide/nano-hydroxyapatite) PLGA/NHA composite scaffolds using the thermally induced phase separation technique. They examined the impacts of solvent composition, polymer concentration, coarsening temperature, coarsening time, and nano-HA content on the micro-morphology and mechanical properties of the scaffolds. Their findings demonstrated that the inclusion of nano-HA significantly enhanced the mechanical properties and water absorption capacity of the scaffolds. Additionally, the PLGA/nano-HA scaffolds exhibited substantially greater cell growth and ALP activity compared to scaffolds without nano-HA [
93]. Lee, U.L. et al. mentioned that at the 6-month follow-up, a significant fusion rate and positive clinical outcomes were observed in the reconstruction of malar defects using patient-specific 3D printed BGS-7 implants. Additionally, all participants in the trial expressed satisfaction with both the aesthetic and functional outcomes following the operation with BGS-7 implants. Consequently, this study underscores the safety and potential efficacy of utilizing patient-specific 3D-printed BGS-7 implants for facial bone reconstruction, suggesting promising value in this approach [
94].
A. L. Torres et al. have reported that three types of scaffolds are made from a combination of β-TCP and HA nanopowder at different ratios (80/20%, 90/10%, and 99/1%). Alginate coating was applied to enhance mechanical strength and mimic native bone matrix. The 80/20 scaffold had the highest porosity and mechanical properties. Coating improved mechanical strength, with the 80/20/A scaffold showing the best performance. All scaffolds demonstrated high biocompatibility with human osteoblast cells, with the 80/20 formulation being the most compatible. SEM analysis confirmed cell migration within coated scaffolds. Overall, the 80/20 scaffold, especially when coated with alginate, showed promising properties for bone tissue engineering [
95]. A. Fukuda et al. have reported that implanted cylinders with longitudinal square channels of varying diagonal widths (500, 600, 900, and 1200 μm) scaffold into the dorsal muscles of beagle dogs. These implants were sterilized using ethylene oxide gas and observed over 16, 26, or 52 weeks. Results showed excellent bone formation along the channels, with more bone quantity in implants with smaller diagonal widths (p500 and p600). Significant osteoinduction was observed in p500 and p600, with the greatest effect seen 5 mm from both ends of the implants. Thicker laminated bone formation was noted at later time points (26 and 52 weeks), indicating a time-dependent increase in bone formation [
96]. Nevertheless, second-generation biomaterials present promising forecasts for scaffold fabrication. Specifically, bioceramic scaffolds must adhere to specific porosity standards, aligning closely with those found in natural bone tissue [
97].
7. Application of Adipose-Derived MSCs
Adipose-derived mesenchymal Stem Cells (ADMSC), possess an undifferentiated immunophenotype and exhibit characteristics such as self-renewal and multipotency. This means they express specific surface markers typical of MSCs and can undergo numerous cell divisions while retaining their undifferentiated state, with the ability to differentiate into various mesodermal cell types like adipocytes, osteoblasts, and chondrocytes. These properties make ADMSCs promising candidates for applications in regenerative medicine and tissue engineering, owing to their abundant presence in adipose tissue, ease of isolation, and potent regenerative capabilities [
98,
99,
100,
101,
102,
103,
104]. Furthermore, ADMSCs can release numerous anti-inflammatory proteins in response to inflammatory stimuli, including (tumor necrosis factor-α) TNF-α, (interleukin-4) IL-4, IL-6, IL-10, and IL-1 receptor antagonists. Additionally, these cells can produce a diverse array of growth factors such as T_G_F_-β1, (hepatocyte growth factor) HGF, (vascular endothelial growth factor) V_E_G_F_, and (stromal cell-derived factor-1) S_D_F_-1. These factors play pivotal roles in tissue remodeling, angiogenesis, and antiapoptotic processes [
103,
104,
105].
Research on ADMSCs in the field of tissue engineering has shown significant promise. In recent studies by Rivera-Izquierdo, M. et al. it has been demonstrated that ADMSCs hold potential applications in addressing various musculoskeletal disorders and cartilage-related conditions. These include but are not limited to osteoporosis, osteonecrosis, fractures, osteoarthritis, and cartilage lesions. [
106] McCullen et al. their results suggest that adding 10% (tricalcium phosphate) TCP to electrospun poly(L-lactic acid) (PLA) enhances the proliferation of human adipose-derived stem cells (hASCs). Conversely, incorporating 20% TCP into electrospun PLA promotes osteogenic differentiation and boosts calcium accumulation by hASCs when compared to pure electrospun PLA scaffolds. [
107]. School T et al. demonstrated that after 8 weeks, histological examination revealed substantial tissue regeneration in hypothyroid rats treated with (ADMSC-conditioned media) ADMSC-CM compared to untreated rats. Specifically, the newly formed tissue almost completely covered the defect caused by hypothyroidism in the group treated with ADMSC-CM. Their findings suggest that combining ADMSC-CM with bioceramic collagen could effectively promote bone repair in hypothyroid patients with compromised bone-regeneration capabilities [
108].L. Xia et al. demonstrated that highly interconnected macroporous HAp scaffolds, characterized by diverse surface topographies such as nanosheets, nanorods, and micro-nano-hybrids, effectively promoted the attachment, spreading, proliferation, and osteogenic differentiation of rat adipose-derived stem cells (ASCs). Additionally, these scaffolds were found to upregulate the expression of angiogenic factors, indicating their potential to enhance angiogenesis alongside osteogenesis. Subsequently, in vivo, bone regeneration assessments using rat critical-sized calvarial defect models validated that the combination of the micro-nano-hybrid surface and ASCs significantly augmented both osteogenesis and angiogenesis compared to the control HAp bioceramic scaffold possessing a traditional smooth surface [
109]. Daei-Farshbaf, N., et al. investigated the efficacy of combining Bio-Oss and type 1 collagen gel as a scaffold with human adipose tissue-derived mesenchymal stem cells (AT-MSCs) for bone regeneration in rat critical-size defects. After 8 weeks, no adverse effects were observed, and imaging analysis revealed enhanced bone regeneration in rats treated with Bio-Oss-Gel compared to untreated rats. Additionally, MSC-seeded Bio-Oss-Gel showed the highest level of bone reconstruction, with histological staining confirming an impressive osseointegration. Overall, the combination of AT-MSCs, Bio-Oss, and gel demonstrated synergistic effects in promoting bone regeneration, suggesting potential applications in bone regenerative medicine and tissue engineering [
110]. Sandor, G.K., et al. mentioned that in 13 consecutive cases of cranio-maxillofacial hard-tissue defects across various anatomical sites, autologous adipose tissue was used to harvest ASCs. These ASCs were cultured, expanded, and seeded onto resorbable scaffold materials for implantation into the defects. The scaffolds were either bioactive glass or β-TCP, with some cases supplemented by recombinant human bone morphogenetic protein-2 and follow-up periods ranged from 12 to 52 months. Ultimately, ten patients were successfully treated [
111].
Prins, H.J., et al. stated that in a clinical study, the efficacy of stromal vascular fraction (SVF) without in-vitro cell culture was investigated. 10 patients requiring maxillary sinus floor elevation received treatment with freshly isolated SVF extracted from autograft fat tissue using a Celution 800/CRS device. The SVF was transplanted with ceramics to increase vertical bone height in the posterior maxilla. Panoramic radiographs showed no significant difference between the control and study sides. However, microcomputed tomography and biopsy evaluations revealed significantly greater osteogenesis on the stem cell-transplanted side [
112].
In summary, ASCs offer the advantages of easy access and abundant supply [
113]. While ASCs offer benefits such as easy access and ample supply, ex vivo expansion is typically necessary to minimize contamination with other cell types. However, it's important to note that ASCs cultivated in vitro have demonstrated reduced stemness, self-renewal, or multipotency compared to their native state [
114]. Furthermore, the safety of ASCs has not been definitively established. Chromosomal abnormalities have been detected in cultured ASCs, which raises concerns regarding their safety for therapeutic use [
115]. When compared to (bone marrow-derived mesenchymal stem cells) BMSCs, ASCs have exhibited inferior osteogenicity in laboratory settings (in vitro). However, their superiority in vivo (in living organisms) is still uncertain and requires further investigation [
116]. Despite that the clinical strategy for regeneration, incorporating cell therapy and tissue engineering, relies on the utilization of third-generation materials [
97].
7.1. Application of Bone Marrow-Derived MSCs
BM-MSCs have been thoroughly examined and understood regarding their phenotype and biological characteristics. They have attracted considerable attention due to their abundance in bone marrow and their well-documented capacity to differentiate into multiple cell types, including osteoblasts, adipocytes, and chondrocytes. Scientists have extensively studied the cell surface markers and immunomodulatory properties of BM-MSCs, as well as their secretion of trophic factors. These findings have contributed to understanding their potential therapeutic applications in various diseases and tissue regeneration approaches. Overall, the detailed investigation of BM-MSCs has provided a strong basis for leveraging their therapeutic potential in regenerative medicine and tissue engineering [
117,
118].
Neen, D., et al. demonstrated that in a prospective case-control study, the efficacy of a collagen/hydroxyapatite matrix infused with BMSCs was compared to that of conventional iliac autograft. The BMSC-infused matrix demonstrated similar effectiveness in promoting posterolateral fusion but exhibited comparatively lower efficacy in facilitating interbody and 360° fusion. Despite potential drawbacks in fusion outcomes, the use of BM-MSCs offered a significant advantage by reducing the risk of donor site complications such as pain and neuroma formation [
119]. Quarto, R., et al. mentioned that initial documentation of utilizing culture-expanded BM-MSCs in conjunction with hydroxyapatite biomaterial for addressing substantial bone defects arising from traumatic fractures and unsuccessful lengthening was reported. In this study, 3 patients were treated, and notable outcomes included significant callus formation surrounding the implants and favorable integration observed at the interfaces with the host bones within two months post-surgery. Furthermore, there were no reported adverse reactions to the implants, and all three patients successfully regained full limb functionality. ([
120]. Numerous registered clinical trials have employed autologous (bone marrow mononuclear cells) BM-MNCs, MSCs, or pre-osteoblasts, either administered via injection or co-applied with bone auxiliary materials as transporters, to address delayed unions and non-unions of extended bones. Among these studies, Emadedin, M., et al. documented the safety of injecting cultured MSCs, with evidence of bone union observed in 3 out of 5 treated patients [
121]. Moreover, Gomez-Barrena, E., et al. documented that the surgical administration of culture-expanded bone marrow MSCs in conjunction with bioceramic granules for addressing delayed unions and non-unions was both safe and viable. Their study revealed that 26 out of 28 patients showed radiological evidence of healing one year post-treatment [
122].
Xu et al. mentioned that initially, MSCs were cultured for two weeks before being introduced into the damaged area. Subsequently, these cells were placed onto a bioactive glass-collagen-hyaluronic acid phosphatidylserine scaffold. The presence of MSCs notably boosted the generation of new bone tissue. [
123]. Zhang, M., et al. have reported that following a 7-day culture period, (Sr2ZnSi2O7) SZS and (Sr2MgSi2O7) SMS were found to significantly enhance the osteogenic differentiation of BMSCs compared to conventional β-TCP. Furthermore, in terms of their overall effectiveness, SZS and SMS exhibited comparable abilities to akermanite (Ca2MgSi2O7), and CMS in promoting cell growth and stimulating cell differentiation. Their results collectively suggested that SZS and SMS hold promise for use in bone regeneration applications. [
124]
Sun, H., et al. demonstrate that, in addition to the enhancement of cell proliferation, akermanite promoted osteoblastic differentiation of hBMSC in vitro by upregulating osteogenic gene expression. Akermanite bioactive ceramics not only simply boosted the osteoblastic differentiation of hBMSC in osteogenic media (a-MEM supplemented with ascorbic acid, glycerophosphate, and dexamethasone) but also improved cell proliferation in normal growth medium without osteogenic reagents. These results suggested that akermanite may be used as a more promising bioactive ceramic for bone regeneration and tissue engineering applications. [
125]. Xia, L., et al. have reported that akermanite bioceramics have several benefits for bone regeneration in osteoporosis. They enhance cell growth and differentiation, increase angiogenic factor expression, and suppress osteoclast activity. In animal models, they outperform β-TCP bioceramics in promoting bone formation, angiogenesis, and inhibiting osteoclastogenesis. The presence of magnesium and silicon ions in akermanite bioceramics contributes to these positive effects, making them promising for osteoporotic bone regeneration [
126]. Maiti, S.K., et al. have elaborated that, on live rabbits using a (critical size defect) CSD model confirming the application of BMSCs whether obtained from the same individual (autogenous), a genetically distinct individual of the same species (allogenous), or even from a different species (xenogenous) with seeding onto bioscaffolds, accelerates the healing process of critical-size defects. Therefore, it can be inferred that BMSCs show potential for promoting bone formation in situations like fracture healing and non-union [
127]. Lin, K., et al. studies involved creating macroporous scaffolds using (strontium-containing calcium silicate) SrCS bioceramics. Findings showed that SrCS materials, which release bioactive strontium and silicon ions, created an environment conducive to directing rBMSCs-OVX toward becoming osteoblasts and promoted angiogenesis in (endothelial cells) ECs. Both pure CS and SrCS inhibited osteoclastogenesis by stimulating (osteoprotegerin) OPG and suppressing receptor activator of (nuclear factor kappa-Β ligand) RANKL. However, the inhibitory effect was stronger and longer-lasting with SrCS compared to CS, which only showed early-stage inhibition [
128]. Maiti, S.K., et al. demonstrate that cultivating autologous (Rabbit bone marrow-derived mesenchymal stem cell) rBMSCs on a bioscaffold made of (silica-coated calcium hydroxyapatite) HASi could expedite the scaffold's osteoconductive properties, presenting a potential substitute for autogenous bone grafts in addressing substantial bone defects and non-healing fractures. Additionally, introducing growth proteins, specifically ( recombinant human bone morphogenetic protein ) rhBMP-2, to the autogenous MSC-seeded HASi bio-ceramic framework could accelerate bone regeneration in a rigorously controlled study using a large critical-size bone defect model [
129].
Gomez-Barrena, E., et al. demonstrated that effective bone consolidation utilizing expanded hBM-MSCs in combination with biomaterials, as assessed through clinical and radiological examinations, was particularly emphasized during the 12-month assessment. Bone regeneration was additionally confirmed via bone biopsies. There were no notable disparities observed in the consolidation of affected bones, although tibial non-unions displayed slower consolidation rates. Individuals who smoked exhibited reduced consolidation scores at both the 6 and 12-month intervals, while no noticeable impact was identified based on gender or the duration since the initial fracture [
130].
7.2. Application of Extracellular Vesicle-Derived MSCs
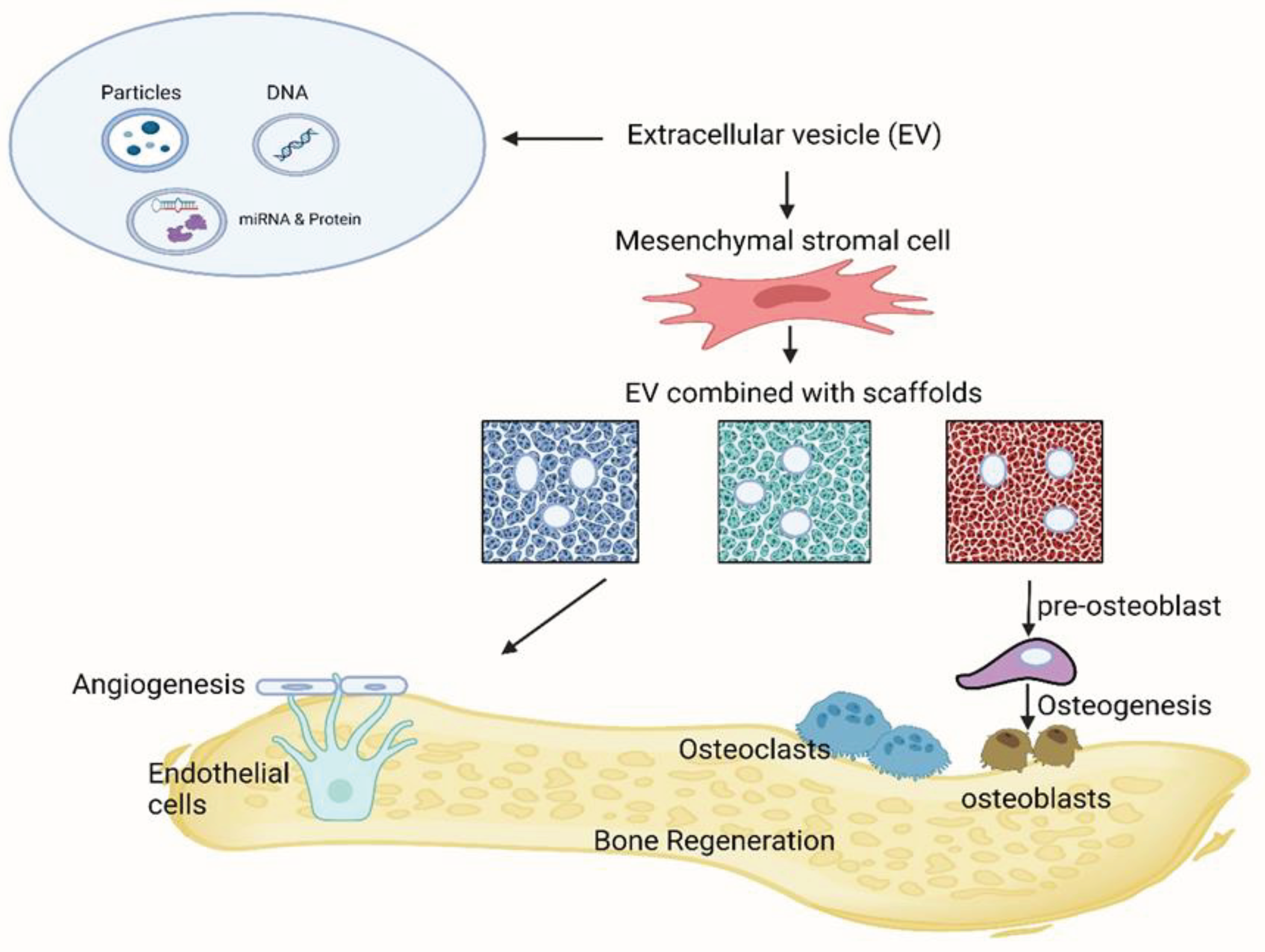
Intercellular communication, crucial for multicellular organisms, traditionally occurs through direct cell-cell contact or the transfer of secreted molecules. However, a third mode of communication has emerged in the last two decades, involving the exchange of EVs between cells. These EVs, encompassing exosomes and microvesicles, have gained prominence for their roles in liquid biopsy as biomarkers and their potential therapeutic applications. EVs, by ferrying a diverse array of biomolecules, can influence the behavior and function of recipient cells, impacting various physiological and pathological processes. Their presence in bodily fluids offers opportunities for non-invasive disease diagnosis and monitoring. Moreover, EV-based therapies hold promise in regenerative medicine, drug delivery, and immunotherapy. In essence, the discovery and understanding of EVs have broadened our insights into intercellular communication and opened new avenues for medical diagnostics and treatments [
131]. Particularly, the therapeutic application of EVs is involved in bone regeneration, thanks to the regulation of immune environments, enhancement of angiogenesis, differentiation of osteoblasts and osteoclasts, and promotion of bone mineralization. Since naked EVs are vulnerable when transplanted in vivo and difficult to target at bone defect sites, the approach of loading EVs with biomaterial systems possesses tremendous advantages, as shown in the schematic illustration in (
Figure 5).
Indeed, EVs delivered via biomaterials show great promise in the field of bone regeneration. These EVs can be immobilized within gels, actively linked to molecular binders, or affixed onto the surfaces of biomaterials, enabling precise control over their release. This controlled release mechanism holds the potential for enhancing bone healing and regeneration processes. [
132] Zhang, J., et al. mentioned that the integration of exosome/β-TCP combination systems enhanced osteogenesis ability compared to the utilization of β-TCP scaffolds alone in a rat critical-sized calvarial bone defect model [
133]. Wiklander, O.P.B., et al. also illustrated that attained alveolar bone regeneration using an exosome/β-TCP system [
134].
As reported in a recent review and research paper indicate that EVs derived from osteoblasts, osteoclasts, osteocytes, monocytes, macrophages, and dendritic cells contain various miRNAs and proteins. These components are implicated in either enhancing or inhibiting osteogenic activity. [
135] Research has indicated that extracellular vesicle (EV)-borne miRNA can be targeted by high mobility group AT-hook 2 (HMGA2) [
136], glycogen synthase kinase-3β/β-catenin [
137], or the Wnt/β-catenin pathway [
138], all of which are known to contribute to osteogenic differentiation. Furthermore, EVs also carry abundant proteins such as (tissue non-specific alkaline phosphatase) TNAP6 and matrix metalloproteinase (MMPs), which are essential for bone remodeling.
8. Impact of Environmental Factors on Bioceramics
In recent eras, a superfluity of groundbreaking biomaterials with the capacity for remote activation has emerged, presenting an inimitable prospect to precisely and locally treat diseases by modulating cell signaling pathways in vitro [
139,
140,
141,
142]. Ophthalmic, electrical, ultrasound, and magnetic methodologies have been widely deployed to encourage synthetic biomaterials to modulate cell signaling, leveraging their non-invasive attributes and precise aptitude to regulate biological functions. These modalities reveal significant potential for translation into in vivo surroundings [
143,
144,
145,
146,
147]. Amid the assortment of approaches, near-infrared (NIR) light stimulation stands out for its expedient features, as it imposes minimal detriment upon cells and organs.
Fu, J., et al. have elaborated that, utilizing near-infrared (NIR) light to activate photoelectrons within a bismuth sulfide/hydroxyapatite (BS/HAp) film, effectively guides cellular fate towards osteogenic differentiation in vitro and promotes improved bone regeneration in vivo. The engineered Ti-BS/HAp composite demonstrates elevated photocurrent levels, credited to the reduction of h+ ions and the transfer of interfacial charges facilitated by HAp. This mechanism empowers the regulation of biological processes within deep tissues using NIR light [
148].
The physical and chemical features of bioceramics are subject to amendment in environments categorized by varying pH levels [
149]
. The instigation of hydration, formation of hydrate phases, and reactions of cement clinkers can be obstructed by the concentration of hydrogen ions and the pH level [
150]. Acute mechanical and chemical features of a biomaterial, such as setting reaction, compressive and tensile strength, hardness, and microleakage, could be conceded in acidic or alkaline environments [
151,
152,
153]
. It appears that the pace of pH fluctuations during the initial stages of hydration might influence the setting procedure and crystallization of bioceramics [
154].
9. Drug Delivery
(Poly(methyl methacrylate), PMMA the primary material used for local antibiotic carriers in clinical settings, has several disadvantages. Firstly, heat generated during scaffold synthesis can lead to the degradation of the antibiotic and surrounding tissues. Secondly, PMMA is non-absorbable, requiring surgical removal, which increases the risk of infection. Additionally, PMMA may hinder bone regeneration and conduction, adding complexity to clinical outcomes [
155,
156]. Hence, there is a growing interest in utilizing absorbable and osteogenic novel materials for fabricating antibiotic-loaded carriers [
157].
For that reason, the incorporation of antibiotics into osteoconductive materials such as calcium sulfate, hydroxyapatite, and tricalcium phosphate for localized osteomyelitis treatment has shown promise, effectively addressing the issue of dead space, while simultaneously eradicating the infection [
158,
159,
160,
161,
162,
163]. Ongoing research aims to develop bioceramics capable of releasing antibiotics over a sufficient duration to effectively treat the infection. However, these bioceramics are designed to cease drug delivery at a specific time point to prevent low antibiotic concentrations and the development of bacterial resistance. In in vivo experiments using animal models with infected proximal tibial defects, implantation of the (vancomycin-loaded bone-like hydroxyapatite/poly(amino acid) V-BHA/PAA scaffold after surgical debridement revealed several positive outcomes. Not only the infection effectively was cleared, but the V-BHA/PAA scaffold was also closely integrated with the host bone. Additionally, there was observed ingrowth of new vessels and trabecular bone around the scaffold. As the scaffold degraded over time, new bone formation gradually replaced it, demonstrating excellent osteoconductive properties. Moreover, the absence of necrosis in the surrounding bone indicated good osteogenic properties of the scaffold, with simultaneous new bone formation occurring at the defect site [
164]. The vancomycin released from the V-BHA/PAA scaffold, a novel vancomycin-loaded bone repair material, demonstrated potent antibacterial activity both in vitro and in vivo against both regular S. aureus and MRSA (Methicillin-resistant Staphylococcus aureus) strains. Furthermore, the antibacterial efficacy of the V-BHA/PAA scaffold surpassed that of V-PMMA significantly. Given its biodegradability and ability to induce osteogenesis, V-BHA/PAA stands out as a promising option for the treatment of chronic bone infections [
165,
166]. HAC is a bone cement used in vivo that fully converts to hydroxyapatite and exhibits excellent biocompatibility. By incorporating antibiotic solutions, such as gentamicin, HAC can serve as a carrier for various antibiotics. Mixing gentamicin into HAC does not compromise the effectiveness of the antibiotic or the mechanical properties of the cement within the concentration range typically used. This delivery system provides sustained release of high levels of antimicrobial agents, resulting in long-term local antibacterial efficacy. Our in vivo study demonstrates a beneficial impact on bone infection resolution and regeneration. [
167]. In a rat model of debrided osteomyelitis, ceramic void filler containing gentamicin (CERAMENT G) demonstrated a decreased rate of persistent infection and enhanced new bone growth compared to the same void filler lacking antibiotics (CERAMENT) and an empty defect. Additionally, tobramycin-incorporated (calcium phosphate bone substitute) CPB effectively facilitated soft tissue and bone healing while preventing early bacterial colonization by
Staphylococcus aureus in infected tibia [
168].
10. Limitations and Future Potential Development of Bioceramics
The primary drawback of bioceramics lies in its suboptimal mechanical properties, particularly its limited reliability when subjected to tensile loads. Bioceramics exhibits low toughness, inadequate fatigue resistance, brittleness, and impact resistance, which significantly restricts its suitability for serving as a bulk material capable of withstanding tension or impact forces [
169,
170,
171]. Incorporating bioceramics into composite materials with other reinforcements enhances mechanical properties. Techniques such as nanoparticle doping and nanofiber reinforcement improve strength, toughness, and ductility at the nanoscale. In addition, introducing dopant elements such as magnesium or strontium enhances mechanical properties and bioactivity. Sintering process control during fabrication influences microstructure and mechanical properties. Optimization of parameters including temperature and time enhances density, strength, and fatigue resistance.
The drawback of scaffold research lies in the absence of specific recommendations regarding which type of scaffold—whether macro, meso, or micro—is best suited for different types of bones. Nevertheless, the choice of scaffold for bone fracture healing depends on factors such as bone position, mechanical properties, and biological requirements, as well as scaffold material features. Long bone fractures may require stable macroporous scaffolds, while flat bones could benefit from microporous scaffolds with controlled pore sizes. Irregular bones might need mesoporous scaffolds accommodating different cell types. Customized scaffold designs tailored to specific pore structures and material properties may be necessary for optimal healing. Researchers should carefully consider these factors and conduct comprehensive studies to assess scaffold effectiveness across different bone types and anatomical locations.
Additionally, a promising avenue for advancing bioceramics for bone regeneration involves combining bioactive ceramics with drug delivery and biodegradable materials, along with the use of extracellular vesicles. This innovative approach holds significant potential for developing future generations of effective bioceramics for bone healing.
11. Conclusions
Our review thoroughly scans the role of bioceramics in bone regeneration, covering their history, classifications, and how they work. We explore how bioceramics combined with scaffold, cells, and drugs as well as how they can aid regeneration, especially when used in porous forms. Despite progress, research in this area is still there are so many gaps as well as many questions are unanswered. So it is crucial to keep investigating to unlock bioceramics' full potential. Recent studies have shown promise to utilize porous bioceramic scaffolds for bone regeneration, but we need to figure out which type of porous scaffold is best fit for different parts of the body. Although bioceramics hold great promise for bone tissue engineering, they still have weaknesses like being too brittle, techniques like nanoparticle doping and nanofiber reinforcement offer hope for making bioceramics stronger for clinical use. ongoing research is crucial for improving bioceramics and making them better at helping bone healing. In conclusion, gaining a more profound comprehension of the involvement of bioceramics would significantly enhance the development of more potent and efficacious therapeutic approaches for bone regeneration.
Author Contributions
Md Amit Hasan Tanvir: manuscript writing, review, conceptualization, and final manuscript preparation. Md Abdul Khaleque: manuscript writing, conceptualization, manuscript review, and project administration. Ga-Hyun Kim: manuscript review, and funding acquisition. Whang-Yong Yoo: manuscript review, project administration, and funding acquisition. Young -Yul Kim: conceptualization, manuscript review, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Basic Research Laboratory Program of NRF (National Research Foundation of Korea) of Republic of Korea (NRF-2022R1A4A1028747).
Institutional Review Board Statement
The experimental protocol was approved by the Institutional Review Board of the Animal Experimentation Committee (IRB number CMCDJ-P-2024-014).
Ethics Statements
Not applicable.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
We would like to acknowledge the financial support from the Basic Research Laboratory Program of NRF (National Research Foundation of Korea) of Republic of Korea (NRF-2022R1A4A1028747 and the Catholic University of Korea Daejeon St. Mary’s Hospital, Clinical Research Institute).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front Cell Dev Biol 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. BIOMECHANICAL AND MOLECULAR REGULATION OF BONE REMODELING. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int J Biol Macromol 2016, 93, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Khaleque, M.A.; Kim, J.-H.; Lee, H.-H.; Kim, G.-H.; You, W.-Y.; Lee, W.-J.; Kim, Y.-Y. Comparative Analysis of Autophagy and Apoptosis in Disc Degeneration: Understanding the Dynamics of Temporary-Compression-Induced Early Autophagy and Sustained-Compression-Triggered Apoptosis. Int. J. Mol. Sci. 2024, 25, 2352. [Google Scholar] [CrossRef] [PubMed]
- Fairag, R.; Rosenzweig, D.H.; Ramirez-Garcialuna, J.L.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Appl Mater Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Kong, Y.; Yu, L.; Li, Y.; Gao, C.; Peng, S.; Pan, H.; Zhao, Z.; Shuai, C. Molybdenum disulfide nanosheets embedded with nanodiamond particles: co-dispersion nanostructures as reinforcements for polymer scaffolds. Appl. Mater. Today 2019, 17, 216–226. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact Mater 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Cathro, P.; Ivanovski, S.; Muhammad, N. Biomimetic bone grafts and substitutes: A review of recent advancements and applications. Biomed. Eng. Adv. 2023, 6. [Google Scholar] [CrossRef]
- Implants, f.t.C.o.B.; Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-Graft Substitutes: Facts, Fictions, and Applications. JBJS 2001, 83, S98–S103. [Google Scholar]
- Abbasi, N.; Hamlet, S.; Love, R.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. 2020. [CrossRef]
- Garcia-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Shuai, C.; Peng, B.; Feng, P.; Yu, L.; Lai, R.; Min, A. In situ synthesis of hydroxyapatite nanorods on graphene oxide nanosheets and their reinforcement in biopolymer scaffold. J Adv Res 2022, 35, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 2020, 74. [Google Scholar] [CrossRef]
- Bayazit, V.; Bayazit, M.; Bayazit, E. Evaluation of bioceramic materials in biology and medicine. Dig. J. Nanomater. Biostructures 2010, 7, 211–222. [Google Scholar]
- Bavya Devi, K.; Nandi, S.K.; Roy, M. Magnesium Silicate Bioceramics for Bone Regeneration: A Review. J. Indian Inst. Sci. 2019, 99, 261–288. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; Montanheiro, T.L.d.A.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Bioactive ceramics: from bone grafts to tissue engineering. RSC Adv. 2013, 3. [Google Scholar] [CrossRef]
- Pina, S.; Rebelo, R.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Bioceramics for Osteochondral Tissue Engineering and Regeneration. Adv Exp Med Biol 2018, 1058, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M.; Lee, M.W. Comparison of drying methods in the fabrication of collagen scaffold via indirect rapid prototyping. J Biomed Mater Res B Appl Biomater 2007, 82, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front Bioeng Biotechnol 2019, 7, 352. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int J Env. Res Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Robey, P.G.; Kuznetsov, S.A.; Ren, J.; Klein, H.G.; Sabatino, M.; Stroncek, D.F. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone 2015, 70, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Saxer, F.; Scotti, C.; Schreiner, S.; Studer, P.; Scherberich, A.; Heberer, M.; Martin, I. Perspective on the evolution of cell-based bone tissue engineering strategies. Eur Surg Res 2012, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Ishii, Y. Apatite cement containing cis-diamminedichloroplatinum implanted in rabbit femur for sustained release of the anticancer drug and bone formation. J Orthop Sci 2001, 6, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishiguchi, S.; Neo, M.; Tamura, J.; Kawanabe, K.; Nakamura, T. Bone Bonding in Bioactive Glass Ceramics Combined with a New Synthesized Agent TAK-778. Key Eng. Mater. 2000, 192-195, 417–420. [Google Scholar] [CrossRef]
- de Groot, K. Ceramics of calcium phosphates: preparation and properties. Bioceram. Calcium Phosphate 2018, 99–114. [Google Scholar]
- Vallet-Regi, M.; Ruiz-Hernandez, E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Castner, D.; Ratner, B. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Revisiting ceramics for medical applications. Dalton Trans 2006, 5211–5220. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J Mater Sci Mater Med 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, M.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and Mechanical Properties of Polydimethylsiloxane (PDMS)-CaO-SiO2 Hybrids with Different PDMS Contents. J. Sol-Gel Sci. Technol. 2001, 21, 75–81. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Nanostructured mesoporous silica matrices in nanomedicine. J Intern Med 2010, 267, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Salinas, A.J. Sol–Gel Silica-Based Biomaterials and Bone Tissue Regeneration. In Handbook of Sol-Gel Science and Technology, 2016; pp. 1-22. [CrossRef]
- Tsuru, K.; Aburatani, Y.; Yabuta, T.; Hayakawa, S.; Ohtsuki, C.; Osaka, A. Synthesis and In Vitro Behavior of Organically Modified Silicate Containing Ca Ions. J. Sol-Gel Sci. Technol. 2001, 21, 89–96. [Google Scholar] [CrossRef]
- JARCHO, M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clin. Orthop. Relat. Res. ® 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Damia, C.; Marchat, D.; Lemoine, C.; Douard, N.; Chaleix, V.; Sol, V.; Larochette, N.; Logeart-Avramoglou, D.; Brie, J.; Champion, E. Functionalization of phosphocalcic bioceramics for bone repair applications. Mater Sci Eng C Mater Biol Appl 2019, 95, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Heness, G.; Ben-Nissan, B. Innovative bioceramics. Mater. Forum 2004, 27, 104–114. [Google Scholar]
- Heimann, R.B. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Olofsson, J.; Pettersson, M.; Teuscher, N.; Heilmann, A.; Larsson, K.; Grandfield, K.; Persson, C.; Jacobson, S.; Engqvist, H. Fabrication and evaluation of SixNy coatings for total joint replacements. J Mater Sci Mater Med 2012, 23, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, M.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part I: processing, microstructure, mechanical properties, cytotoxicity. J Mater Sci Mater Med 2008, 19, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Lobel, K.; Hench, L. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J. Biomed. Mater. Res. : Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 39, 575–579. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis--a review. Acta Biomater 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Evolution of bioceramics within the field of biomaterials. Comptes Rendus Chim. 2010, 13, 174–185. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. Ceramics as bone repair materials. In Bone repair biomaterials, Elsevier: 2019; pp. 141–178.
- Dorozhkin, S. Current state of bioceramics. J. Ceram. Sci. Technol 2018, 9, 353–370. [Google Scholar]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J Cell Mol Med 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Voisin, L.; Saba-El-Leil, M.K.; Julien, C.; Fremin, C.; Meloche, S. Genetic demonstration of a redundant role of extracellular signal-regulated kinase 1 (ERK1) and ERK2 mitogen-activated protein kinases in promoting fibroblast proliferation. Mol Cell Biol 2010, 30, 2918–2932. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory effects of the degradation products from Mg-Ca-Sr alloy on the osteogenesis through regulating ERK signaling pathway. Sci Rep 2016, 6, 32323. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A Magnesium-Enriched 3D Culture System that Mimics the Bone Development Microenvironment for Vascularized Bone Regeneration. Adv Sci (Weinh) 2019, 6, 1900209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Liu, C.; Tan, W.; Tang, M.; Zhou, X.; Sun, T.; Deng, Y. Mg(2+) in beta-TCP/Mg-Zn composite enhances the differentiation of human bone marrow stromal cells into osteoblasts through MAPK-regulated Runx2/Osx. J Cell Physiol 2020, 235, 5182–5191. [Google Scholar] [CrossRef]
- Wu, B.C.; Kao, C.T.; Huang, T.H.; Hung, C.J.; Shie, M.Y.; Chung, H.Y. Effect of verapamil, a calcium channel blocker, on the odontogenic activity of human dental pulp cells cultured with silicate-based materials. J Endod 2014, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Hung, C.J.; Huang, T.H.; Lin, C.C.; Kao, C.T.; Shie, M.Y. Odontogenic differentiation of human dental pulp cells by calcium silicate materials stimulating via FGFR/ERK signaling pathway. Mater Sci Eng C Mater Biol Appl 2014, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Declercq, H.; Martens, L.; De Coster, P. Gene Expression Profiling and Molecular Signaling of Various Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J Endod 2016, 42, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Srinath, P.; Abdul Azeem, P.; Venugopal Reddy, K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020, 17, 2450–2464. [Google Scholar] [CrossRef]
- Palakurthy, S.; Azeem, A.; Reddy, K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020, 17. [Google Scholar] [CrossRef]
- Lai, S.; Chen, L.; Cao, W.; Cui, S.; Li, X.; Zhong, W.; Ma, M.; Zhang, Q. Dicalcium Silicate Induced Proinflammatory Responses through TLR2-Mediated NF-kappaB and JNK Pathways in the Murine RAW 264.7 Macrophage Cell Line. Mediat. Inflamm 2018, 2018, 8167932. [Google Scholar] [CrossRef] [PubMed]
- Luther, G.; Wagner, E.; Zhu, G.; Kang, Q.; Luo, Q.; Lamplot, J.; Bi, Y.; Luo, X.; Luo, J.; Teven, C.; et al. BMP-9 Induced Osteogenic Differentiation of Mesenchymal Stem Cells: Molecular Mechanism and Therapeutic Potential. Curr. Gene Ther. 2011, 11, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Wu, J.; Feng, C.; Wang, M.; Wu, H.; Zhu, X.; Li, X.; Chen, X.; Zhang, X. Whisker of biphasic calcium phosphate ceramics: Osteo-immunomodulatory behaviors. Nano Res. 2022, 15, 9169–9182. [Google Scholar] [CrossRef]
- Han, F.; Li, T.; Li, M.; Zhang, B.; Wang, Y.; Zhu, Y.; Wu, C. Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing. Bioact Mater 2023, 20, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Nethala, S.; Tripathi, A.; Saravanan, S.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing silicon dioxide and zirconia nano particles for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Roohani-Esfahani, S.-I.; Lu, Z.; Zreiqat, H.; Dunstan, C.R. Zirconium ions up-regulate the BMP/SMAD signaling pathway and promote the proliferation and differentiation of human osteoblasts. PLoS One 2015, 10, e0113426. [Google Scholar] [CrossRef] [PubMed]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural basis of Wnt recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int J Nanomed. 2015, 10, 7031–7044. [Google Scholar] [CrossRef] [PubMed]
- Ruolan, W.; Liangjiao, C.; Longquan, S. The mTOR/ULK1 signaling pathway mediates the autophagy-promoting and osteogenic effects of dicalcium silicate nanoparticles. J Nanobiotechnology 2020, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, Y.; Wang, J.; Huang, C.; Wang, Y.; Yang, H.; Liu, W.; Wang, T.; Wang, D.; Wang, G.; et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/beta-catenin signaling pathway. Bioact Mater 2020, 5, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, L.; Zhang, Q.; Wang, Q.; Wang, X.; Yan, G. Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair. Biomater Sci 2023, 11, 7034–7050. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, A.; Barradas, A.; de Boer, J.; Moroni, L.; van Blitterswijk, C.; Habibovic, P. Combining technologies to create bioactive hybrid scaffolds for bone tissue engineering. Biomatter 2013, 3, e23705. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, M.; Sharrock, P.; Fiallo, M.; ElFeki, H. Hydroxyapatite bioceramic with large porosity. Mater Sci Eng C Mater Biol Appl 2017, 76, 985–990. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; van Blitterswijk, C.; Thomsen, P.; Hubbell, J.; Cancedda, R.; De Bruijn, J.; Lindahl, A.; Sohier, J.; Williams, D.F. Tissue engineering; Elsevier: 2008.
- Hegde, C.; Shetty, V.; Wasnik, S.; Ahammed, I.; Shetty, V. Use of bone graft substitute in the treatment for distal radius fractures in elderly. Eur J Orthop Surg Traumatol 2013, 23, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.R.; Bhagat, S.B.; Shukla, D.D. Bovine cancellous xenograft in the treatment of tibial plateau fractures in elderly patients. Int Orthop 2009, 33, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Hench, L.L. Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J Biomed Mater Res B Appl Biomater 2004, 68, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Nasri-Nasrabadi, B.; Ghaedi, K.; Salehi, H.; Dolatshahi-Pirouz, A.; Arpanaei, A. Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater Sci Eng C Mater Biol Appl 2016, 66, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bodenberger, N.; Kubiczek, D.; Abrosimova, I.; Scharm, A.; Kipper, F.; Walther, P.; Rosenau, F. Evaluation of methods for pore generation and their influence on physio-chemical properties of a protein based hydrogel. Biotechnol Rep (Amst) 2016, 12, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Kamioka, H.; Honjo, T.; Tezuka, K.-i.; Takano-Yamamoto, T. Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 2005, 36, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Vehof, J.W.; Dean, D.; van der Waerden, J.P.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res 2002, 59, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, G.; Xu, H.; Hu, K.; Sun, J.; Liu, M.; Zhang, F.; Gu, N. Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration. NPG Asia Mater. 2018, 10, 827–839. [Google Scholar] [CrossRef]
- Cheng, M.Q.; Wahafu, T.; Jiang, G.F.; Liu, W.; Qiao, Y.Q.; Peng, X.C.; Cheng, T.; Zhang, X.L.; He, G.; Liu, X.Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci Rep 2016, 6, 24134. [Google Scholar] [CrossRef]
- Shrestha, S.; Yeon Lee, S.; Shrestha, D.; Kandel, R.; Yoo, Y.-J.; Tae, H.-J.; Kumar Shrestha, B.; Hee Park, C.; Sang Kim, C. Micro/nanometer-sized porous structure of zinc phosphate incorporated Ti(HPO4)2 hydrate bioceramic induces osteogenic gene expression and enhances osteoporotic bone regeneration. Chem. Eng. J. 2022, 450, 138360. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Ren, J.; Chen, C.; Ren, T.B.; Zhou, X.Y. Preparation and properties of poly(lactide-co-glycolide) (PLGA)/ nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J Biomater Appl 2008, 22, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.L.; Lim, J.Y.; Park, S.N.; Choi, B.H.; Kang, H.; Choi, W.C. A Clinical Trial to Evaluate the Efficacy and Safety of 3D Printed Bioceramic Implants for the Reconstruction of Zygomatic Bone Defects. Mater. (Basel) 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.L.; Gaspar, V.M.; Serra, I.R.; Diogo, G.S.; Fradique, R.; Silva, A.P.; Correia, I.J. Bioactive polymeric-ceramic hybrid 3D scaffold for application in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl 2013, 33, 4460–4469. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater 2011, 7, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Barbanti Brodano, G.; Mazzoni, E.; Tognon, M.; Griffoni, C.; Manfrini, M. Human mesenchymal stem cells and biomaterials interaction: a promising synergy to improve spine fusion. Eur Spine J 2012, 21 Suppl 1, S3–9. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, D. Adipose-Derived Mesenchymal Stem Cells: A New Tool for the Treatment of Renal Fibrosis. Stem Cells Dev 2018, 27, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci Rep 2017, 7, 7110. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res 2019, 37, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, J.; Chen, H.; Shi, X.; Wang, X.; Zhu, Y.; Tan, Z. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater Sci 2019, 7, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.M.; Mozdzen, L.C.; Harley, B.A.; Bailey, R.C. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials 2014, 35, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int J Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Cabeza, L.; Lainez-Ramos-Bossini, A.; Quesada, R.; Perazzoli, G.; Alvarez, P.; Prados, J.; Melguizo, C. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin Biol Ther 2019, 19, 233–248. [Google Scholar] [CrossRef]
- McCullen, S.D.; Zhu, Y.; Bernacki, S.H.; Narayan, R.J.; Pourdeyhimi, B.; Gorga, R.E.; Loboa, E.G. Electrospun composite poly(L-lactic acid)/tricalcium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomed Mater 2009, 4, 035002. [Google Scholar] [CrossRef] [PubMed]
- Sanchooli, T.; Norouzian, M.; Ardeshirylajimi, A.; Ghoreishi, S.K.; Abdollahifar, M.A.; Nazarian, H.; Piryaei, A. Adipose Derived Stem Cells Conditioned Media in Combination with Bioceramic-Collagen Scaffolds Improved Calvarial Bone Healing in Hypothyroid Rats. Iran. Red Crescent Med. J. 2017, 19. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Fang, B.; Xu, Y.; Liu, J.; Zeng, D.; Zhang, M.; Zhang, X.; Chang, J.; et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials 2014, 35, 8514–8527. [Google Scholar] [CrossRef] [PubMed]
- Daei-Farshbaf, N.; Ardeshirylajimi, A.; Seyedjafari, E.; Piryaei, A.; Fadaei Fathabady, F.; Hedayati, M.; Salehi, M.; Soleimani, M.; Nazarian, H.; Moradi, S.L.; et al. Bioceramic-collagen scaffolds loaded with human adipose-tissue derived stem cells for bone tissue engineering. Mol Biol Rep 2014, 41, 741–749. [Google Scholar] [CrossRef]
- Sandor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerstrom, B.; Patrikoski, M.; Seppanen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med 2014, 3, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.J.; Schulten, E.A.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Helder, M.N. Bone Regeneration Using the Freshly Isolated Autologous Stromal Vascular Fraction of Adipose Tissue in Combination With Calcium Phosphate Ceramics. Stem Cells Transl Med 2016, 5, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.M.; Azevedo, J.M.; Reis, R.L.; Gomes, M.E. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev Rep 2012, 8, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Meza-Zepeda, L.A.; Noer, A.; Dahl, J.A.; Micci, F.; Myklebost, O.; Collas, P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med 2008, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells 2014, 6, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Amati, E.; Perbellini, O.; Rotta, G.; Bernardi, M.; Chieregato, K.; Sella, S.; Rodeghiero, F.; Ruggeri, M.; Astori, G. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res Ther 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Neen, D.; Noyes, D.; Shaw, M.; Gwilym, S.; Fairlie, N.; Birch, N. Healos and Bone Marrow Aspirate Used for Lumbar Spine Fusion: A Case Controlled Study Comparing Healos With Autograft. Spine 2006, 31, E636–E640. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. New Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Emadedin, M.; Labibzadeh, N.; Fazeli, R.; Mohseni, F.; Hosseini, S.E.; Moghadasali, R.; Mardpour, S.; Azimian, V.; Goodarzi, A.; Ghorbani Liastani, M.; et al. Percutaneous Autologous Bone Marrow-Derived Mesenchymal Stromal Cell Implantation Is Safe for Reconstruction of Human Lower Limb Long Bone Atrophic Nonunion. Cell J 2017, 19, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, P.; Wang, Y.; Chen, X.; Meng, Y.; Liu, C.; Yu, X.; Yang, X.; Yu, W.; Zhang, X.; et al. A novel biomimetic composite scaffold hybridized with mesenchymal stem cells in repair of rat bone defects models. J. Biomed. Mater. Res. Part A 2010, 95A, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C.; Lin, K.; Fan, W.; Chen, L.; Xiao, Y.; Chang, J. Biological responses of human bone marrow mesenchymal stem cells to Sr-M-Si (M = Zn, Mg) silicate bioceramics. J Biomed Mater Res A 2012, 100, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, C.; Dai, K.; Chang, J.; Tang, T. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials 2006, 27, 5651–5657. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yin, Z.; Mao, L.; Wang, X.; Liu, J.; Jiang, X.; Zhang, Z.; Lin, K.; Chang, J.; Fang, B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci. Rep. 2016, 6, 22005. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Shivakumar, M.U.; Mohan, D.; Kumar, N.; Singh, K.P. Mesenchymal Stem Cells of Different Origin-Seeded Bioceramic Construct in Regeneration of Bone Defect in Rabbit. Tissue Eng. Regen. Med. 2018, 15, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Ninu, A.R.; Sangeetha, P.; Mathew, D.D.; Tamilmahan, P.; Kritaniya, D.; Kumar, N.; Hescheler, J. Mesenchymal stem cells-seeded bio-ceramic construct for bone regeneration in large critical-size bone defect in rabbit. J Stem Cells Regen Med 2016, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Padilla-Eguiluz, N.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; et al. Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury 2020, 51 (Suppl. 1), S63–S73. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Balbi, C. Extracellular Vesicles: From Biomarkers to Therapeutic Tools. Biol. (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Teixeira, J.H.; Almeida, M.I.; Goncalves, R.M.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration. Eur J Pharm Sci 2017, 98, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; EL Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Yang, G.H.; Pan, X.H.; Zhang, S.J.; Zhao, C.; Qiu, B.S.; Gu, H.F.; Hong, J.F.; Cao, L.; Chen, Y.; et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One 2014, 9, e114627. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, H.; Wang, S.; Li, T.; Fan, J.; Liang, X.; Li, J.; Han, Q.; Zhu, L.; Fan, L.; et al. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev 2014, 23, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, Y.; Lin, Z.; Wan, J.; Xu, C.; Zeng, Y.; Zhu, Y. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3beta/beta-catenin signaling pathway. Biochem Biophys Res Commun 2016, 477, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Maeda, Y.; Taipaleenmaki, H.; Zhang, W.; Jafferji, M.; Gordon, J.A.; Li, Z.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem 2012, 287, 42084–42092. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; McBain, S.; Dobson, J.; El Haj, A.J. Selective activation of mechanosensitive ion channels using magnetic particles. J R Soc Interface 2008, 5, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jung, H.J.; Wong, D.S.H.; Kim, S.K.; Lin, S.; Chan, K.F.; Zhang, L.; Li, G.; Dravid, V.P.; Bian, L. Remote Control of Heterodimeric Magnetic Nanoswitch Regulates the Adhesion and Differentiation of Stem Cells. J Am Chem Soc 2018, 140, 5909–5913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Chen, C. Remote control and modulation of cellular events by plasmonic gold nanoparticles: implications and opportunities for biomedical applications. ACS Nano 2017, 11, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Li, Z.; Ma, G.; Zhou, Y.; Han, G. Illuminating cell signaling with near-infrared light-responsive nanomaterials. ACS Nano 2016, 10, 3881–3885. [Google Scholar] [CrossRef] [PubMed]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: a high-resolution analysis. J R Soc Interface 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.S.; Li, J.; Yan, X.; Wang, B.; Li, R.; Zhang, L.; Bian, L. Magnetically Tuning Tether Mobility of Integrin Ligand Regulates Adhesion, Spreading, and Differentiation of Stem Cells. Nano Lett 2017, 17, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.H.; Yoo, D.; Kim, J.W.; Park, S.W.; Shin, J.S.; Cheon, J. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat Mater 2012, 11, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Deepagan, V.G.; You, D.G.; Um, W.; Ko, H.; Kwon, S.; Choi, K.Y.; Yi, G.R.; Lee, J.Y.; Lee, D.S.; Kim, K.; et al. Long-Circulating Au-TiO(2) Nanocomposite as a Sonosensitizer for ROS-Mediated Eradication of Cancer. Nano Lett 2016, 16, 6257–6264. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, L.; Zhao, B.; Xie, R.; Lin, W.; Li, H.; Li, Y.; Shi, M.; Chen, Y.G.; Springer, T.A.; et al. Carbon nanotube-assisted optical activation of TGF-beta signalling by near-infrared light. Nat Nanotechnol 2015, 10, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, X.; Tan, L.; Cui, Z.; Zheng, Y.; Liang, Y.; Li, Z.; Zhu, S.; Yeung, K.W.K.; Feng, X.; et al. Photoelectric-Responsive Extracellular Matrix for Bone Engineering. ACS Nano 2019, 13, 13581–13594. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Huang, T.H.; Kao, C.T.; Huang, C.H.; Ding, S.J. The effect of a physiologic solution pH on properties of white mineral trioxide aggregate. J Endod 2009, 35, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Stark, J. Early hydration of ordinary Portland cement with an alkaline shotcrete accelerator. Adv. Cem. Res. 2005, 17, 1–8. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Lotfi, M.; Saghiri, A.M.; Vosoughhosseini, S.; Fatemi, A.; Shiezadeh, V.; Ranjkesh, B. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod 2008, 34, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Namazikhah, M.S.; Nekoofar, M.H.; Sheykhrezae, M.S.; Salariyeh, S.; Hayes, S.J.; Bryant, S.T.; Mohammadi, M.M.; Dummer, P.M. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J 2008, 41, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: composition, properties, and clinical applications. J Investig Clin Dent 2017, 8. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Sanati, E. Surface Microstructure of Two Bioceramics: Calcium-Enriched Mixture and Cold Ceramic in Setting Environments with Different pH Values. Int J Dent 2023, 2023, 7130619. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J Control Release 2008, 130, 202–215. [Google Scholar] [CrossRef]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin. Drug Deliv. 2013, 10, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Bertazzoni Minelli, E.; Sartori, M.; Benini, A.; Della Bora, T.; Sambri, V.; Gaibani, P.; Borsari, V.; Salamanna, F.; Martini, L.; et al. Microbiological and pharmacological tests on new antibiotic-loaded PMMA-based composites for the treatment of osteomyelitis. J Orthop Res 2012, 30, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P.; Mateus, A.Y.; Sousa, J.C.; Monteiro, F.J. Nanohydroxyapatite microspheres as delivery system for antibiotics: release kinetics, antimicrobial activity, and interaction with osteoblasts. J Biomed Mater Res A 2007, 81, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; van de Belt, H.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials 2003, 24, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- Trécant, M.; Guicheux, J.m.; Grimandi, G.; Leroy, M.; Daculsi, G. Dynamic compaction: a new process to compact therapeutic agent-loaded calcium phosphates. Biomaterials 1997, 18, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, K.; Okada, Y.; Matsusue, Y.; Iida, H.; Nakamura, T. Treatment of osteomyelitis with antibiotic-soaked porous glass ceramic. J. Bone Jt. Surg. Br. Vol. 1998, 80-B, 527–530. [Google Scholar] [CrossRef]
- Solberg, B.D.; Gutow, A.P.; Baumgaertner, M.R. Efficacy of Gentamycin-Impregnated Resorbable Hydroxyapatite Cement in Treating Osteomyelitis in a Rat Model. J. Orthop. Trauma 1999, 13, 102–106. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.C. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res, 1097; .26. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo osteogenic activity of the novel vancomycin-loaded bone-like hydroxyapatite/poly(amino acid) scaffold. J Biomater Appl 2016, 30, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold. Int J Nanomed. 2017, 12, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Bertazzoni Minelli, E.; Sartori, M.; Benini, A.; Della Bora, T.; Sambri, V.; Gaibani, P.; Borsari, V.; Salamanna, F.; Martini, L.; et al. Microbiological and pharmacological tests on new antibiotic-loaded PMMA-based composites for the treatment of osteomyelitis. J. Orthop. Res. 2012, 30, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Joosten, U.; Joist, A.; Frebel, T.; Brandt, B.; Diederichs, S.; von Eiff, C. Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin in the treatment of chronic osteomyelitis: studies in vitro and in vivo. Biomaterials 2004, 25, 4287–4295. [Google Scholar] [CrossRef] [PubMed]
- Dvorzhinskiy, A.; Perino, G.; Chojnowski, R.; van der Meulen, M.C.H.; Bostrom, M.P.G.; Yang, X. Ceramic composite with gentamicin decreases persistent infection and increases bone formation in a rat model of debrided osteomyelitis. J Bone Jt Infect 2021, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhou, X.; Wang, M. Influence of sintering temperatures on hardness and Young's modulus of tricalcium phosphate bioceramic by nanoindentation technique. Mater. Charact. 2004, 52, 301–307. [Google Scholar] [CrossRef]
- Bouslama, N.; Ben Ayed, F.; Bouaziz, J. Sintering and mechanical properties of tricalcium phosphate–fluorapatite composites. Ceram. Int. 2009, 35, 1909–1917. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).