1. Introduction
Body condition is an indicator of ewe health and feeding status. Body condition score (BCS) is a management tool to evaluate ewe condition on a scale between one and five; one represents poor, and five represents obese. BCS is defined to the nearest 0.5 increments with an optimal score is between 2.5 and 3.5 [
1,
2,
3]. BCS can provide an indication of the amount of fat by well-trained evaluators; however, it is a subjective measure [
4,
5,
6,
7].
BCS was used to describe the condition and estimate fatness of ewes [
8] and BCF [
9]. In contrast, BCS has a limitation of estimating ewes fatness if they have a greater proportion of its body fat around the internal organs and require dissection to get a better estimation [
3]. BCS can provide a good indication of BCF by well-trained evaluators, however, these are subjective measures [
4]. One of the difficulties of BCS is that, for example, an ewe that might be considered by a lowlands flock master to be in only moderate condition (condition 2) could be judged as very good (condition 3.5) by someone accustomed to handling hill stock. BCS was found to be unsuitable for evaluating ewes condition and the development of a new technique is required to provide a more accurate estimation of percentage of fat for ewes [
7]. BCS and BCF were measured at two times (fattening and fasting) from four different breeds; Chios (n=80), Lacaune (n=70), Frizarta (n=75), Assaf (n=70). The results showed a weak to moderate correlation between BCS with fat, with a different fat among ewes at the same BCS with R
2=0.69 for Chios, R
2=0.38 for Lacaune, R
2=0.72 for Frizarta and R
2=0.01 for Assaf [
7].
Medical methods such as ultrasound [
10,
11,
12], DEXA [
13] and CT [
14] are used as research methods for BCF. The accuracy of CT, compared with dissection for determining body composition, achieved r
2 values of 0.99 for fat, [
15,
16,
17,
18]. However, these methods are time-consuming, expensive, and require expertise, equipment, and special medical procedures [
12,
13,
19].
1.1. Background
Visual evaluations, BCS and Live Weight (LW) are used to monitor the animal's condition and estimate the level of feeding needed and the nutritional well-being of the flock. When LW and BCS relationships of ewes were investigated, it was found that LW reflects ewes' body size, internal organ size and foetal development at the time of weighing whereas BCS indicates body fat only [
20]. Therefore, BCS is used to evaluate the condition of animal. LW is a combination of body condition and size it is used as an indication of ewe condition [
2]. Some studies discussed the advantages of using BCS in predicting total fat carcass [
8,
9].
BCS is a useful measurement for estimating body fat where the assessment of body condition in ewes is a valuable tool for good flock management [
21]. BCS is used to optimize nutrition by adjusting feeding according to the body condition of ewes, particularly during early lactation and in late pregnancy as the most appropriate alternative to LW [
5,
21,
22]. BCS and LW changes are related; if BCS needs to be increased by one unit (e.g. score 2.0 to score 3.0), ewes need to gain 7–9 kg for Merino ewes. Another study argued per unit change in BCS for mature Churra ewes, an expected change of 5.57 kg of LW [
23].
Determining the BCS at mating is important to evaluate ewe performance [
21]. It is also useful to check BCS during pregnancy as an alternative tool management for evaluating ewe condition [
5] and pre-lambing to ensure lamb and ewe survival [
24]. Changes in body condition in the production cycle affect ovulation rate and lambing percentage: having a BCS of 3.0 the higher the ovulation rate [
3]. A higher BCS score means a sheep has a large amount of fat (i.e. obese) and a lower score means less fat (emaciated).
There is increasing demand for understanding BCF distribution and prediction in sheep as it has links to many production outcomes. Capturing such data requires the use of full-body dissection data collected after slaughter or the use of computerized tomography (CT) scanning. CT scanning is used in the New Zealand sheep industry to estimate the BCF of live animals [
25]. The best way to estimate animal condition is by calculating the amount of BCF using CT [
16]. Because of a direct relationship between X-ray attenuation and tissue density, CT scanning provides an accurate prediction of BCF [
26].
Body parameters have been used to evaluate the characteristics of sheep that may vary due to the influence of breed evolution, environment and nutrition and have been studied to predict body size and LW [
27]. Body parameters can be measured by hand, measuring stick or tape measure where the sheep must stand in a place with correct posture (the sheep body is straight and the head is not turned to the left or the right) [
28,
29].
Body parameters such as body length, height and heart girth have been used to estimate the LW of sheep instead of using scales [
30]. The feasibility of using image processing to estimate the LW of sheep was investigated, and the research indicated that image analysis using body parameters can be used as a reliable guide for estimating the LW and body size of sheep. The sheep were divided into five age groups between 2-6 years in mating time with the highest R
2 of 0.79 found in the LW estimation using body length [
29]. Body parameters of heart depth, height at wither, chest width, heart girth and rump width were used to estimate sheep body weight. The highest R
2 of 0.75 was found using the heart girth to estimate the body weight [
31]. Body parameters have been used for the determination of yak and ewe LWs using image measurements [
27,
32,
33] and using physical measurements [
29,
31,
34,
35]. Body parameters determined by image analysis have been used as a reliable guide for estimating the body size of sheep [
30,
36,
37,
38], newborn lamb size [
39,
40], predicting fat for pigs [
41], and carcass characteristics estimation of sheep [
42].
Although the relationship between BCS and BCF was investigated at mating [
21], at pregnancy [
5] and at pre-lambing [
24] but it has not been investigated at other important production cycle stages. Therefore, this study investigated the relationship between BCS and BCF at weaning and pre-mating using ANN.
2. Materials and Methods
2.1. Data Collection
BCS and BCF data were collected from 88 ewes aged 2–4 years at Lincoln University sheep farm at weaning and pre-mating. The wool length of the wool was tested and taken into account [
43]. BCS was evaluated by a farm manager and BCF was determined by CT licensed operator. LW was measured using electronic scale manufactured by Prattley.
Ewes were standing in a metal crate while taking body measurements and capturing top and side images. The ewes were fasted with the water removed for 12 hours.
The body parameters were measured using a custom-made ruler that has measurements in millimeters, with one side fixed and the other movable to accurately measure body parameters. Top body parameters of body length, chest width, width and rump width where the side body parameters were front height, back height, depth, body length and side length. The side camera used was Cannon DSLR 750D with 24.2 megapixel sensor, the top camera was GoPro 7 with 12 megapixel sensor.
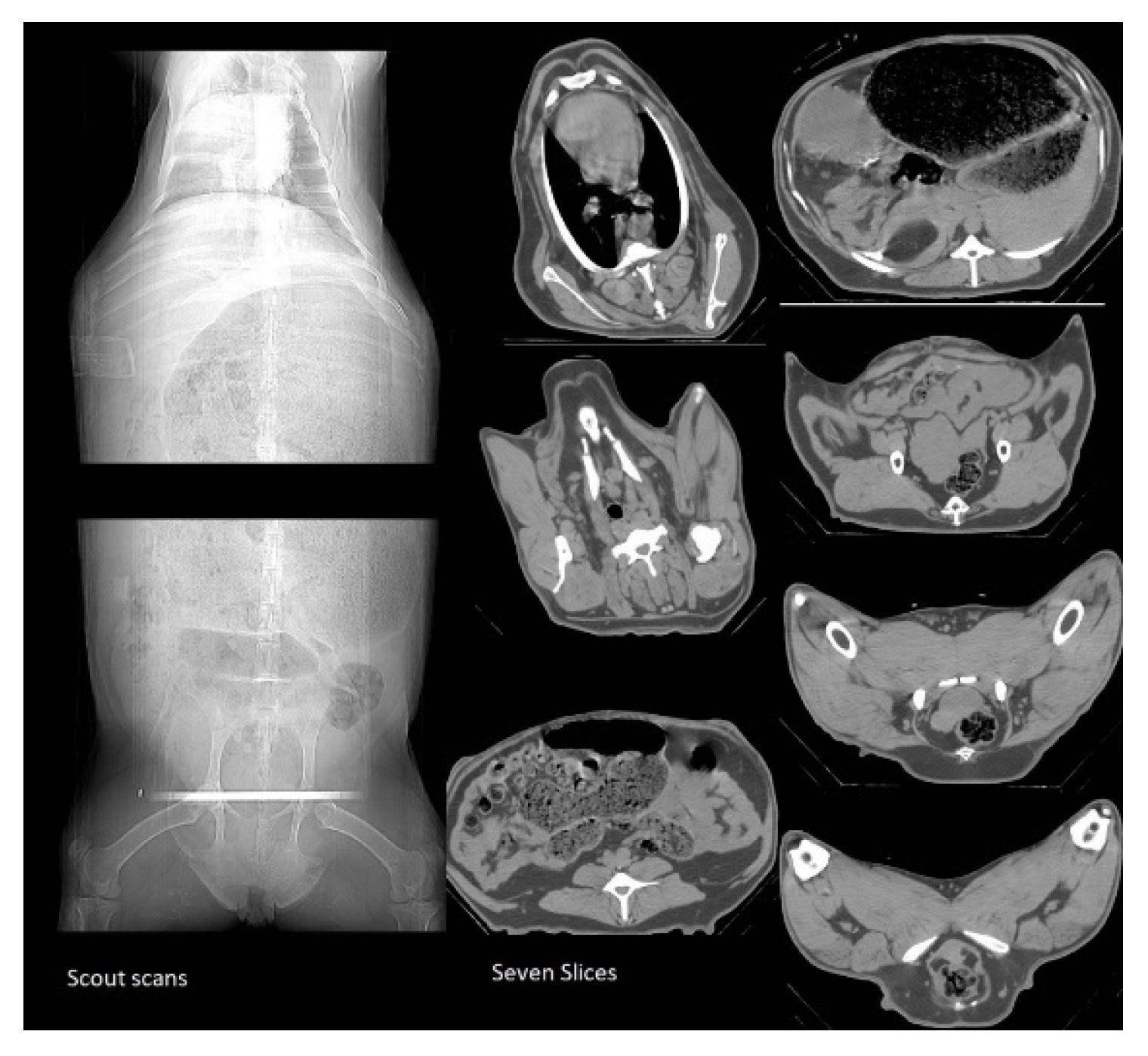
Two CT scout scans were taken for each ewe: one from the top half of the body and another from the bottom half Error! Reference source not found. The first scout scan captured three slices of the ribs. Slice 1 was taken at the first rib, slice 2 at the fifth rib and slice 3 at the last rib. The second scout scan captured four slices: one at the second to last vertebra in the spine; the second slice at the fifth rib in the middle of the pelvis; the third slice at the end of the pelvis; and the last slice was taken at the ischium. Once the scanning procedure was completed, the wooden stretcher was carried out and the ewe was released to the recovery room. The ewe was placed in the sternal recumbency recovery position and ensured that it was kept warm. Ewes were CT-scanned using a CT750 HD machine manufactured by GE Healthcare. The CT slice measurements were measured by the CT operator using the STAR 6.15 software. CT images were then analyzed by an experienced CT operator to determine the amount of fat, lean and bone for each slice based on the color (body carcass fat in dark grey areas, lean in light grey areas, bone in white and in air black areas) by drawing around these areas. These areas were measured using linear measurements or irregular drawings traced manually by the operator. The CT scanning operator held a current license to use ionizing radiation (National Radiation Laboratory) and was an accredited person (as approved for competency by the Lincoln University Animal Ethics Committee).
Figure 1.
CT scout scans and the seven slices.
Figure 1.
CT scout scans and the seven slices.
ANN works by determining the input layers based on the weights. Once the weights are assigned, the input variables that contribute significantly to the output will be determined [
44]. All inputs are then multiplied by their respective weights and summed. Afterwards, an activation function is used to determine the correct output. If that output exceeds a given threshold, it activates the node, transforming data to the next layer. This results in the output of one node becoming the input of the next node. To get the best results, ANN relies on training and validating the data to improve accuracy. This process of passing data between layers defines this as a feedforward neural network [
45].
2.2. Statistical Analysis
Over the two weaning and pre-mating experiments, data from 88 ewes were used; 74 ewes were scanned in both experiments and 14 ewes were scanned only once. For the ewes that were scanned in both experiments, each animal has two records. The data of these two experiments were combined, generating 162 records or observations to cover different body compositions during weaning and pre-mating. The percentages and number of observations were 162 observations, which were divided into three groups: 70% for training (114 observations), 15% for validation (24 observations) and 15% for testing (24 observations).ANN is divided into two groups: first, where the network structure does not have loops called feed-forward and secondly, called recursive network, which has loops through feedback connection [
46]. A statistical method called a two-layer feed-forward neural network was used using MATLAB 9.10 and utilizing the Levenberg-Marquardt back-propagation algorithm trained to predict the dependent variable BCF [
47]. The statistical methods were applied to all data, ANN statistical models were compared to find the best model to estimate BCF in terms of R
2 value and error percentage.
3. Result
3.1. Weaning and Pre-Mating Scans Data Changes
CT scan results showed the amount of BCF increased for ewes at pre-mating by an average of 823g, where 64 ewes gained BCF and only 10 ewes lost BCF in the pre-mating scan as shown in the data in Error! Reference source not found. The ewes lost LW by an average of 2877g, out of 74 ewes, 46 ewes lost LW and 28 ewes gained LW due to physiological changes and the size of the gastrointestinal tract size is known to change, this could substantially change the LW of ewes. Therefore, a drop in LW was expected.
Table 1.
Data of body changes in pre-mating scan compared to weaning scan.
Table 1.
Data of body changes in pre-mating scan compared to weaning scan.
| Body parameter |
Mean changes |
| BCF |
+823 g |
| BCS |
-0.18 |
| LW |
-2877 g |
| Angle length |
+52 mm |
| Body length |
+66 mm |
| Side length |
+59 mm |
| Height |
+13 mm |
| Back height |
+24 mm |
| Depth |
+25 mm |
| Top length |
+9 mm |
| Abdominal width |
+14 mm |
| Rump width |
+36 mm |
| Chest width |
+34 mm |
3.2. Application Uncertainty Test
The results showed that top image parameters were better than side image parameters to estimate BFC. An average mean difference of 1% for application measurements of two top images for the same animal of 12 ewes is shown in Error! Reference source not found. The results show a variance of less than +/-21 mm for all top body measurements, with more than 95% of the results having a variance of +/-10 mm. The maximum variance was +20.7 mm on top length. The results show uncertainty of -9.43 to 9.22 mm for chest width, which was used to predict the BFC.
Table 2.
Variance test of multiple images for each ewe (mm) for top measurements.
Table 2.
Variance test of multiple images for each ewe (mm) for top measurements.
| Top length |
Rump width |
Chest width |
Width |
| 1st |
2nd |
Δ |
1st |
2nd |
Δ |
1st |
2nd |
Δ |
1st |
2nd |
Δ |
| 770.08 |
770.08 |
0 |
300.92 |
300.81 |
0.11 |
290.83 |
300.05 |
-9.22 |
310.58 |
310.47 |
0.11 |
| 790.25 |
800.88 |
-10.63 |
300.70 |
310.68 |
-9.98 |
290.94 |
300.37 |
-9.43 |
320.78 |
330.10 |
-9.32 |
| 820.62 |
820.62 |
0 |
320.98 |
330.20 |
-9.22 |
300.16 |
300.16 |
0 |
330.43 |
330.32 |
0.11 |
| 840.90 |
850.01 |
-9.11 |
310.68 |
310.68 |
0 |
290.07 |
290.18 |
-0.11 |
320.23 |
320.23 |
0 |
| 850.34 |
850.34 |
0 |
320.76 |
320.87 |
-0.11 |
290.07 |
280.85 |
9.22 |
330 |
320.67 |
9.33 |
| 790.58 |
800.23 |
-9.65 |
280.09 |
280.53 |
-0.44 |
250.59 |
250.81 |
-0.22 |
290.19 |
290.41 |
-0.22 |
| 850.01 |
850.88 |
-0.87 |
320.22 |
320.66 |
-0.44 |
280.63 |
280.42 |
0.21 |
320.02 |
320.13 |
-0.11 |
| 790.68 |
780.82 |
9.86 |
330.42 |
330.42 |
0 |
270.87 |
280.20 |
-9.33 |
310.58 |
310.69 |
-0.11 |
| 880.92 |
860.32 |
20.6 |
330.96 |
350.37 |
-19.41 |
320.00 |
320.44 |
-0.44 |
350.71 |
360.15 |
-9.44 |
| 870.29 |
870.62 |
-0.33 |
350.92 |
360.03 |
-9.11 |
320.11 |
320.22 |
-0.11 |
350.28 |
350.39 |
-0.11 |
| 830.27 |
830.27 |
0 |
340.29 |
340.50 |
-0.21 |
320.98 |
330.31 |
-9.33 |
350.82 |
350.93 |
-0.11 |
| 830.16 |
830.49 |
-0.33 |
320.33 |
320.33 |
0 |
270.98 |
280.20 |
-9.22 |
310.58 |
310.69 |
-0.11 |
| Max, min Δ |
+20, -10.63 |
+0.11, -19.98 |
+9.22, -9.43 |
+9.33, -9.44 |
| Mean diff. |
1% |
1% |
2% |
1% |
3.3. BCS and BCF Ranges
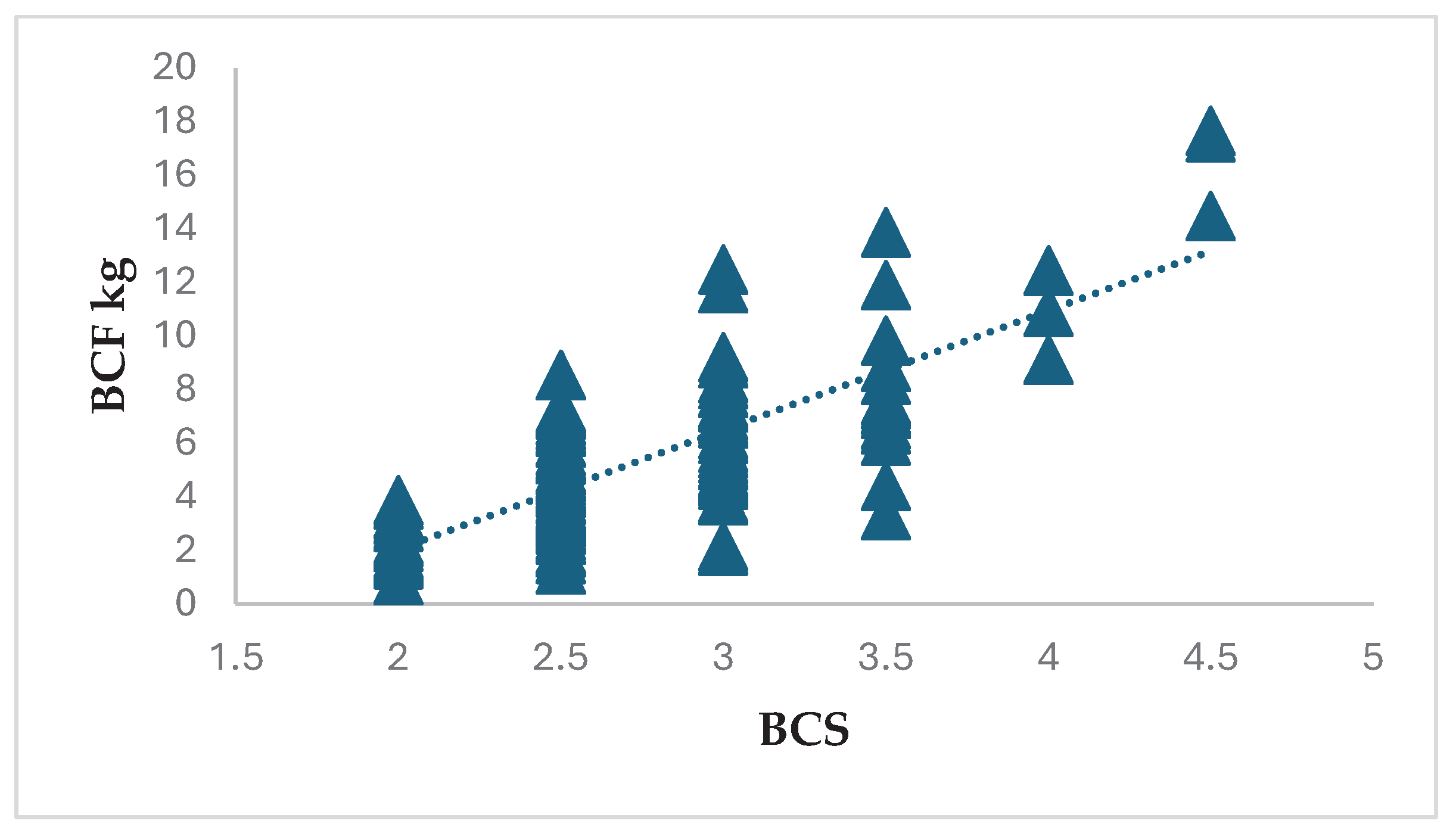
The purpose of this analysis was to quantify how the same BCS reflected the range of fat amount. The BCS results of 88 ewes showed a range between 2.0 and 4.5, while the amount of fat showed a minimum amount of 0.88 kg and a maximum amount of 17.65 kg. The results show a range of fat of 0.88-3.86 kg for condition 2.0, 1.29-8.54 kg for condition 2.5, 1.98-12.47 kg for condition 3.0, 3.31-13.86 for condition 3.5, 9.11-12.44 kg for condition 4.0 and 14.47-17.65 kg for condition 4.5 Error! Reference source not found.
Table 3.
BCS and BCF range.
Table 3.
BCS and BCF range.
| BCS |
Fat range - kg |
Average fat |
Number of ewes |
| 2.0 |
0.88-3.86 |
2.25 |
18 |
| 2.5 |
1.29-8.54 |
4.57 |
67 |
| 3.0 |
1.98-12.47 |
6.45 |
33 |
| 3.5 |
3.31-13.86 |
8.25 |
13 |
| 4.0 |
9.11-12.44 |
11.89 |
3 |
| 4.5 |
14.47-17.65 |
16.51 |
3 |
Most of ewes were given a BCS between 2.5 and 3.0, which is the optimal condition score, but had higher BCS and less fat or lower BCS and more BCF. For example, one ewe had a BCS of 2.5 with an 8.54 kg amount of BCF, but another had a 3.0 score but 1.98 kg of BCF. Furthermore, one ewe had a score of 4.0 with 12.44 kg BCF but another ewe had 12.47 kg BCF with a score of 3.0 or 13.86 BCF with a score of 3.5 Error! Reference source not found. This result confirms that there is a high range of fat for a given BCS in this experiment.
Figure 2.
Relationship between BCS and BCF – training data.
Figure 2.
Relationship between BCS and BCF – training data.
3.4. BCF Prediction
The predicted model of BCF with highest R2 value was the model using all variable with an r2 = 0.95 and RMSE = 1.19 for the training data. This model had 12 inputs which are chest width, angle length, body length, side length, front height, back height, depth, top length, width, rump width, top area and side area with one hidden layer and one output (BCF).
The known amount of BCF was tested, and a relationship was found to validate the BCF prediction model using the testing data, with an r2 = 0.96 and an RMSE = 1.01 Error! Reference source not found.
Table 4.
Relationships between LW and body parameters to estimate BCF—ANN.
Table 4.
Relationships between LW and body parameters to estimate BCF—ANN.
| Independent Variables |
r2
|
RMSE |
| BCS |
0.72 |
3.33 |
| LW, Chest width |
0.89 |
1.12 |
| LW, Angle length |
0.85 |
1.55 |
| LW, Body length |
0.84 |
1.29 |
| LW, Side length |
0.83 |
1.29 |
| LW, Front height |
0.82 |
1.61 |
| LW, Back height |
0.83 |
1.53 |
| LW, Depth |
0.86 |
1.34 |
| LW, Top length |
0.86 |
1.31 |
| LW, Width |
0.85 |
1.16 |
| LW, Rump width |
0.85 |
1.15 |
| LW, Top area |
0.86 |
1.13 |
| LW, Side area |
0.87 |
1.23 |
| All variables |
0.95 |
1.19 |
The average body parameter changes at pre-mating scans are shown in Error! Reference source not found. The increase in BCF amount explains the increase in body parameters, while the changes on the gastrointestinal tract could substantially change the LW of ewes.
Additionally, the age of the ewes would affect LW as an increase in body parameters would normally expected for younger ewes whereas the increase is less for older ewes.
Table 5.
Body composition changes in pre-mating compared with weaning.
Table 5.
Body composition changes in pre-mating compared with weaning.
| Body parameter |
Mean changes |
| BCF |
+823 g |
| BCS |
-0.18 score |
| LW |
-2877 g |
| Angle length |
+52 mm |
| Body length |
+66 mm |
| Side length |
+59 mm |
| Height |
+13 mm |
| Back height |
+24 mm |
| Depth |
+25 mm |
| Top length |
+9 mm |
| Abdominal width |
+14 mm |
| Rump width |
+36 mm |
| Chest width |
+34 mm |
| Top area |
+69000 mm2 |
| Side area |
+81600 mm2 |
The maximum difference between BCF determined by CT and estimated by ANN data was 1.352 kg where the minimum difference was 0.013 kg. The BCF has a minimum BCF of 3.742 kg and a maximum BCF of 13.867 kg Error! Reference source not found.
Table 6.
Relationship between BCF, ANN and BCS test data in (kg).
Table 6.
Relationship between BCF, ANN and BCS test data in (kg).
| CT BCF |
ANN |
BCS |
| 13.867 |
13.951 |
3 |
| 12.479 |
11.931 |
2.5 |
| 7.249 |
7.236 |
3 |
| 4.390 |
3.895 |
4 |
| 6.674 |
6.342 |
3 |
| 9.066 |
8.168 |
2.5 |
| 6.209 |
6.139 |
2.5 |
| 7.919 |
6.869 |
3.5 |
| 12.329 |
11.937 |
2.5 |
| 7.242 |
6.736 |
2.5 |
| 5.117 |
4.932 |
3 |
| 5.943 |
5.835 |
3 |
| 13.034 |
12.673 |
2.5 |
| 4.451 |
4.243 |
2.5 |
| 3.742 |
3.664 |
2.5 |
| 4.513 |
4.461 |
2.5 |
| 10.940 |
11.015 |
3 |
| 7.403 |
6.051 |
2.5 |
| 6.301 |
6.563 |
2.5 |
| 5.148 |
5.135 |
3.5 |
| 5.595 |
5.456 |
3 |
| 7.114 |
6.620 |
2.5 |
| 6.616 |
6.411 |
2.5 |
| 3.525 |
3.666 |
2.5 |
4. Discussion
An alternative methodology has been explored (with the potential to create an automated approach) further to predict the BCF of ewes during their production life cycle. This method provided an accurate estimation of BCF during the weaning stage when ewes had lost body condition. It also showed that body composition changes after pre-mating, which shows that ewes had gained BCF. The ewes showed a slight loss in BCS and LW in the pre-mating scan, but all other body parameters increased. The reason ewes lost BCS could be due to BCS is a manual process that is subjective depending on the operator as well as ewes losing LW. This estimation helps the farmer to make decisions about the appropriate nutrition intake for each animal to make it ready for mating. Condition is used by farmers to describe the amount of fatness a sheep has. BCS is used to evaluate the condition of sheep by farmers [
1,
2,
3]. This study investigated the relationship between BCS and BCF for Coopworth ewes at the pre-mating and weaning stages of production cycle. The correlation between the amount of BCF determined by CT and the amount of BCF predicted by BCS was R
2=0.72. This correlation confirmed that BCS was not a significant predictor of BCF and has inaccuracy when predicting the BCF [
13].
The changes of BCF between pre-mating and weaning are crucial as it describes the condition of ewe during its lowest and best condition. The increase of BCF reflects the improvement of ewes condition. The BCF increased by 823g at pre-mating compared to the BCF at weaning. However, the BCS decreased by 0.18 score and the LW decreased by 2877g. The result also showed an increase for all body parameters with a minimum increase of 9mm for top length and a maximum increase of 66mm for the body length.
The results showed a range of BCF for BCS 2.0 of 0.88 to 3.86 kg, 1.29-8.54 kg for BCS 2.5, 1.98-12.47 kg for condition 3.0, 3.31-13.86 kg for condition 3.5, 9.11-12.44 kg for condition 4.0 and 14.47-17.65 kg for condition 4.5. The results also showed 106 ewes out of 138 ewes had BCS between 2.5-3.0, but other ewes have higher BCS and less BCF or lower condition score and more BCF. For example, one ewe had a BCS of 2.5 with 8.54 kg of BCF, while another had a BCS of 3.0 but the BCF was only 1.98 kg; another ewe had a BCS of 4.0 with 12.44 kg of BCF, compared to another ewe that had more BCF (12.47 kg) but a lower BCS (3.0) and one with 13.86 kg of BCF with a BCS of 3.5.
BCS was found as a good indication of BCF [
4] and as an alternative method to the LW to evaluate ewe condition [
5]. By contrast, the development of a new BCS is essential to assess ewe condition [
7]. Similarly, the findings of this study showed a weak relationship between BCS and BCF were the BCS provided inaccurate results of BCF compared to body parameters which are in accordance with the recommendation of Termatzidou’s study [
7].
5. Conclusions
In conclusion, the result of this study showed that BCS is not suitable to evaluate ewe condition at weaning and pre-mating as it provided inaccurate results of BCF estimation. Where the use of body parameters might provide more accurate results of BCF during ewe production cycle. Future work could involve the use of a fully and quick automated method to body parameters and LW from the images then do mathematical models to estimate BCF for the whole flock within minutes. A larger data set representing different breeds (e.g. Merino) will be used to test the effect of wool on the different breeds, ages, and different stages of the production cycle and use new statistical models.
Author Contributions
A.S. led the conception and design of the research. Material preparation, analysis and results were carried out by A.S. Data collection was undertaken by A.S., C.L., and R.J. and they were assisted by S.P., P.A., S.C. and M.S. The first draft of the manuscript was written by A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by Lincoln University SOP 83 and Animal Ethical Committee (AEC) #642—AEC 2020-19 on 22 July 2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Keinprecht, H., et al., Short term repeatability of body fat thickness measurement and body condition scoring in sheep as assessed by a relatively small number of assessors. Small Ruminant Research, 2016. 139: p. 30-38. [CrossRef]
- Kenyon, P.R., S.K. Maloney, and D. Blache, Review of sheep body condition score in relation to production characteristics. New Zealand journal of agricultural research, 2014. 57(1): p. 38-64. [CrossRef]
- Russel, A., Body condition scoring of sheep. In Practice, 1984. 6(3): p. 91.
- Tait, I., et al., Associations of body condition score and change in body condition score with lamb production in New Zealand Romney ewes. New Zealand Journal of Animal Science and Production, 2019. 79: p. 91-94.
- van Burgel, A.J., et al., The merit of condition score and fat score as alternatives to liveweight for managing the nutrition of ewes. Animal Production Science, 2011. 51(9): p. 834-841. [CrossRef]
- McHugh, N., et al., Mean difference in live-weight per incremental difference in body condition score estimated in multiple sheep breeds and crossbreds. Animal, 2019. 13(3): p. 549-553. [CrossRef]
- Termatzidou, S.A., et al., Association of body condition score with ultrasound backfat and longissimus dorsi muscle depth in different breeds of dairy sheep. Livestock Science, 2020. 236: p. 104019. [CrossRef]
- Yates, W. and A. Gleeson, Relationships between condition score and carcass composition of pregnant Merino sheep. Australian Journal of Experimental Agriculture, 1975. 15(75): p. 467-470. [CrossRef]
- Teixeira, A., R. Delfa, and F. Colomer-Rocher, Relationships between fat depots and body condition score or tail fatness in the Rasa Aragonesa breed. Animal Science, 1989. 49(2): p. 275-280. [CrossRef]
- Ribeiro, F., et al., Comparison of real-time ultrasound measurements for body composition traits to carcass and camera data in feedlot steers. The Professional Animal Scientist, 2014. 30: p. 597-601. [CrossRef]
- Dias, L., S. Silva, and A. Teixeira, Simultaneously prediction of sheep and goat carcass composition and body fat depots using in vivo ultrasound measurements and live weight. Research in Veterinary Science, 2020. 133: p. 180-187. [CrossRef]
- Morales-Martinez, M.A., et al., Developing equations for predicting internal body fat in Pelibuey sheep using ultrasound measurements. Small Ruminant Research, 2020. 183: p. 106031. [CrossRef]
- Miller, D., et al., Dual-energy X-ray absorptiometry scans accurately predict differing body fat content in live sheep. Journal of animal science and biotechnology, 2019. 10(1): p. 248-253. [CrossRef]
- Kvame, T. and O. Vangen, Selection for lean weight based on ultrasound and CT in a meat line of sheep. Livestock Science, 2007. 106(2): p. 232-242. [CrossRef]
- Macfarlane, J.M., et al., Predicting carcass composition of terminal sire sheep using X-ray computed tomography. Animal Science, 2007. 82(3): p. 289-300. [CrossRef]
- Macfarlane, J.M., et al., Predicting carcass composition of terminal sire sheep using X-ray computed tomography. British Society of Animal Science, 2006. [CrossRef]
- Johnson, P., J. Juengel, and W. Bain, Predicting internal adipose from selected computed tomography images in sheep. New Zealand Journal of Animal Science and Production, 2020. 80: p. 113-116.
- Lambe, N., et al., Body composition changes in Scottish Blackface ewes during one annual production cycle. Animal Science, 2003. 76: p. 211-219. [CrossRef]
- Bain, W., et al., Estimation of computed tomography (CT) predicted meat yield in New Zealand lamb. 2018.
- Borg, R.C., D.R. Notter, and R.W. Kott, Phenotypic and genetic associations between lamb growth traits and adult ewe body weights in western range sheep. Journal of Animal Science, 2009. 87(11): p. 3506-14. [CrossRef]
- Cam, M.A., A.V. Garipoglu, and K. Koray, Body condition status at mating affects gestation length, offspring yield and return rate in ewes. Archiv für Tierzucht, 2018. 61(2): p. 221-228. [CrossRef]
- Brozos, C., V.S. Mavrogianni, and G.C. Fthenakis, Treatment and Control of Peri-Parturient Metabolic Diseases: Pregnancy Toxemia, Hypocalcemia, Hypomagnesemia. Veterinary Clinics: Food Animal Practice, 2011. 27(1): p. 105-113. [CrossRef]
- Frutos, P., A.R. Mantecón, and F.J. Giráldez, Relationship of body condition score and live weight with body composition in mature Churra ewes. Animal Science, 2010. 64(3): p. 447-452. [CrossRef]
- Corner-Thomas, R.A., et al., Ewe lamb live weight and body condition scores affect reproductive rates in commercial flocks. New Zealand Journal of Agricultural Research, 2015. 58(1): p. 26-34. [CrossRef]
- Scholz, A.M., et al., Non-invasive methods for the determination of body and carcass composition in livestock: dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: invited review. Animal, 2015. 9(7): p. 1250-64. [CrossRef]
- Bünger, L., et al., Use of X-Ray Computed Tomography (CT) in UK Sheep Production and Breeding. 2011.
- Riva, J., et al., Body measurements in Bergamasca sheep. Small Ruminant Research, 2004. 55(1): p. 221-227. [CrossRef]
- Zhang, L., et al., Advances in body size measurement and conformation appraisal for sheep. 2016. 32: p. 190-197.
- Yilmaz, O., I. Cemal, and O. Karaca, Estimation of mature live weight using some body measurements in Karya sheep. Tropical Animal Health and Production, 2013. 45(2): p. 397-403. [CrossRef]
- Burke, J., P.L. Nuthall, and A.E. McKinnon, An Analysis of the Feasibility Of Using Image Processing To Estimate the Live Weight of Sheep. Farm and Horticultural Management Group Applied Management and Computing Division Lincoln University 2004.
- Topai, M. and M. Macit, Prediction of Body Weight from Body Measurements in Morkaraman Sheep. Journal of Applied Animal Research, 2004. 25(2): p. 97-100. [CrossRef]
- Yan, Q., et al., Body weight estimation of yaks using body measurements from image analysis. Measurement, 2019. 140. [CrossRef]
- Zhang, et al., Body Weight Estimation of Yak Based on Cloud Edge Computing. 2020.
- Iqbal, F., et al., PREDICTING LIVE BODY WEIGHT OF HARNAI SHEEP THROUGH PENALIZED REGRESSION MODELS. Journal of Animal and Plant Sciences, 2019. 29: p. 1541-1548.
- Sabbioni, A., et al., Body weight estimation from body measures in Cornigliese sheep breed. Italian Journal of Animal Science, 2020. 19(1): p. 25-30. [CrossRef]
- Zhang, A.L.N., et al., Development and validation of a visual image analysis for monitoring the body size of sheep. Journal of Applied Animal Research, 2018. 46(1): p. 1004-1015. [CrossRef]
- Abdelhady, A., et al., Automatic Sheep Weight Estimation Based on K-Means Clustering and Multiple Linear Regression. 2019. p. 546-555. [CrossRef]
- Zhang, A., et al., Algorithm of sheep body dimension measurement and its applications based on image analysis. Computers and Electronics in Agriculture, 2018. 153: p. 33-45. [CrossRef]
- Puth, M.-T., M. Neuhäuser, and G.D. Ruxton, Effective use of Spearman's and Kendall's correlation coefficients for association between two measured traits. Animal Behaviour, 2015. 102: p. 77-84. [CrossRef]
- Khojastehkey, M., et al., Body size estimation of new born lambs using image processing and its effect on the genetic gain of a simulated population. Journal of Applied Animal Research, 2016. 44(1): p. 326-330. [CrossRef]
- Doeschl, A.B., et al., The relationship between the body shape of living pigs and their carcass morphology and composition. Animal Science, 2004. 79(1): p. 73-83. [CrossRef]
- Bautista-Díaz, E., et al. Prediction of Carcass Traits of Hair Sheep Lambs Using Body Measurements. Animals, 2020. 10. [CrossRef]
- Cottle, D., International Sheep and Wool Handbook. 1 ed. 2010: Nottingham University Press. 766.
- Kayakutlu, G. and E. Güreşen, Artificial Neural Networks: Definition, Properties and Misuses. 2011. p. 171-189.
- Samarasinghe, S., Neural networks for applied sciences and engineering. From fundamentals to complex pattern recognition. 2007.
- Li, M. and J. Wang, An Empirical Comparison of Multiple Linear Regression and Artificial Neural Network for Concrete Dam Deformation Modelling. Mathematical Problems in Engineering, 2019. 2019: p. 7620948. [CrossRef]
- Rajab, S. and V. Sharma, Performance Evaluation of ANN and Neuro-Fuzzy System in Business Forecasting. 2015.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).