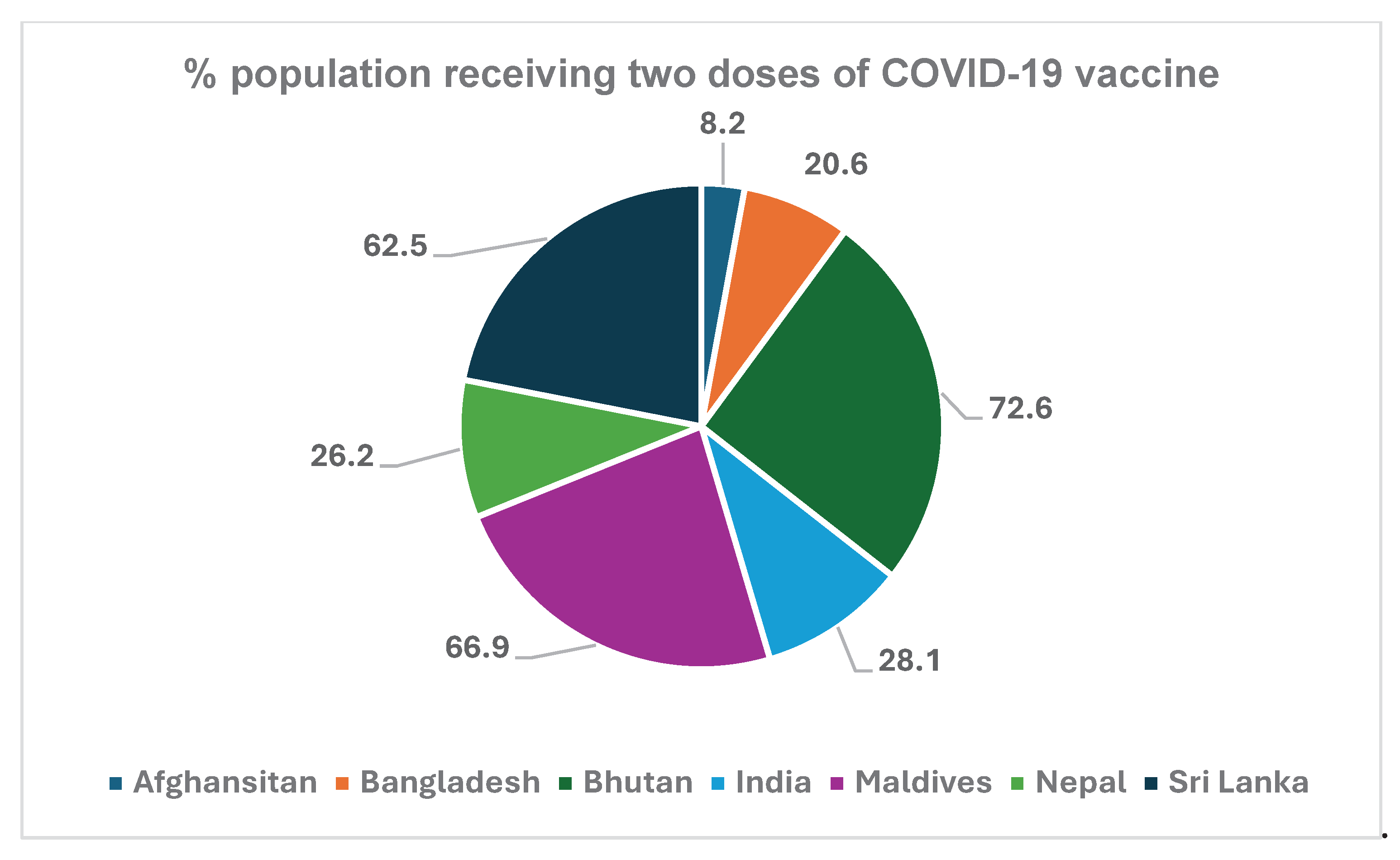South Asia Region
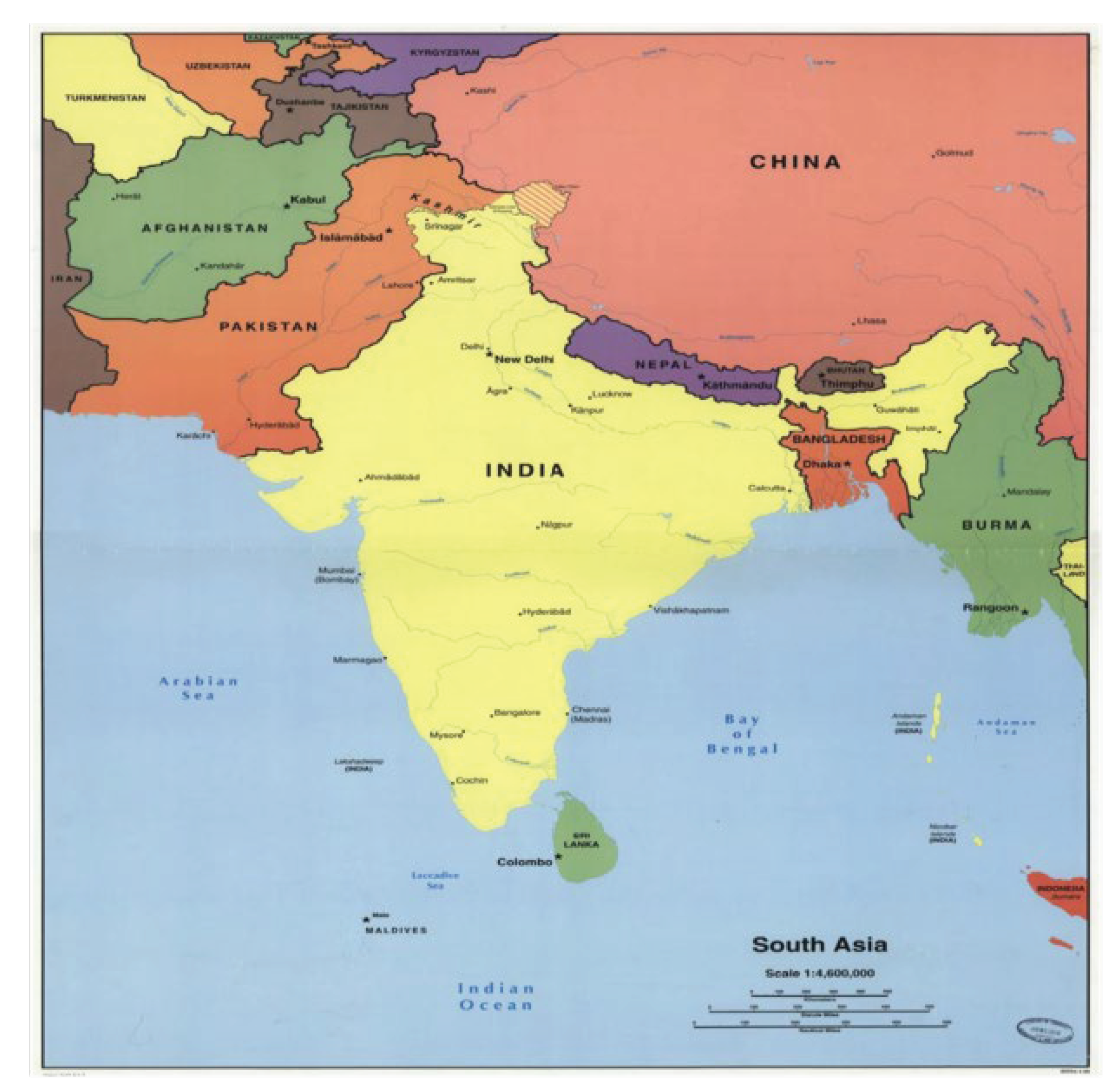
Afghanistan, Bangladesh, Bhutan, India, Maldives, Pakistan, Nepal, and Sri Lanka collectively constitute South Asia. Indian Ocean, Arabian Sea, and Bay of Bengal along with mountain ranges of the Himalayas, Hindu Kush, and the Karakoram are part of South Asia’s geographical landmarks that extend to alluvial plains of the Indus, Ganga, and Brahmaputra rivers (Map 1).
Map 1.
Geographical location of South Asian countries.
Map 1.
Geographical location of South Asian countries.
With a total land area of 6.4 million km
2 inhabited by 2.04 billion people, South Asia has a density of 317 individuals per km
2. Every fourth person in this world inhabits South Asia. Women constitute 48.85% of the entire population.
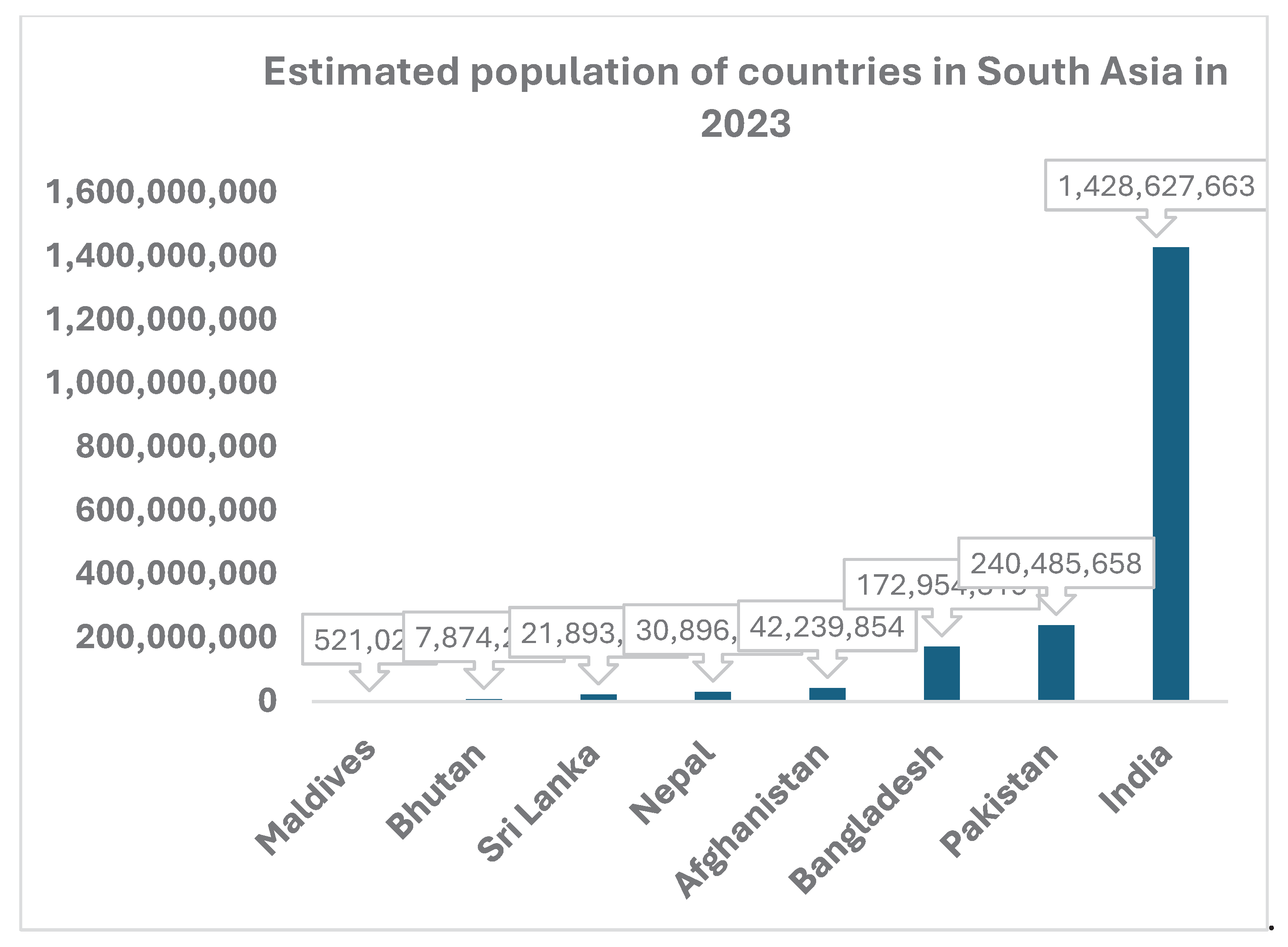
Figure 1 shows the estimated population of South Asian countries in 2023. This indicates a huge population base of adults and children that requires vaccination for various diseases. With a fertility rate of 2.04, this population is likely to increase to 26.5% of the global population by 2030 [
1].
The World Bank has categorized South Asia countries based upon their gross national income (GNI). All South Asia countries, except Afghanistan and Maldives have been classified as belonging to low middle income country, Afghanistan continues to be a low income category, while Maldives is categorized as upper middle income country [
2]. The human development index (HDI) values for seven countries ranges from 0.478 (Afghanistan) to 0.782 (Sri Lanka). Maldives has a value of 0.747 and remaining four countries are with HDI value of little more than 0.600 [
3].
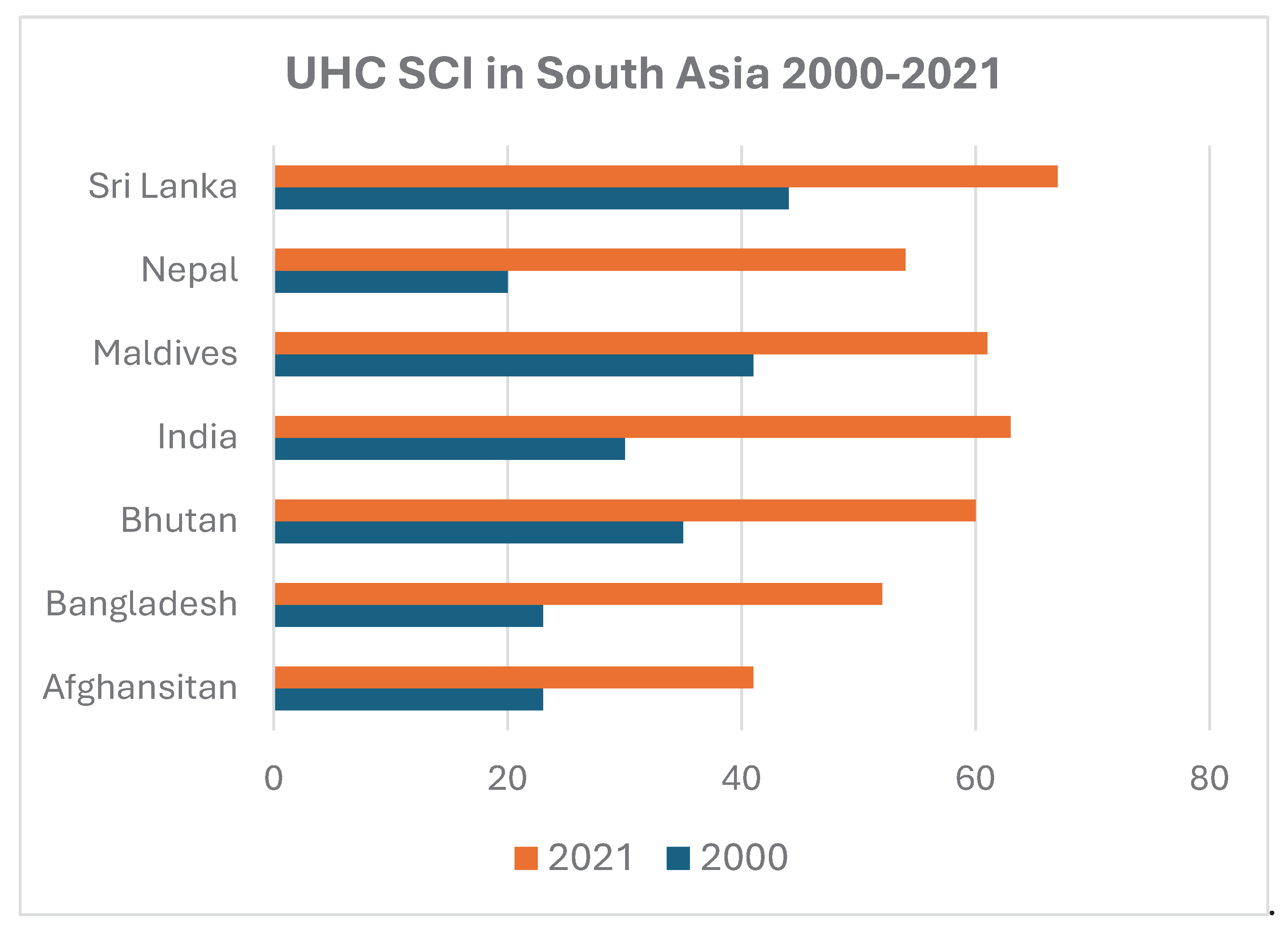
Vaccination is greatly influenced by the extent and outreach of the health system especially efficiency of its universal health coverage (UHC). In recent past, South Asian countries have made considerable progress (
Figure 2) in improving their respective UHC Service Coverage Index in accordance with the UN Sustainable Development Goal 3.8 [
4].
COVID-19 Pandemic in South Asia
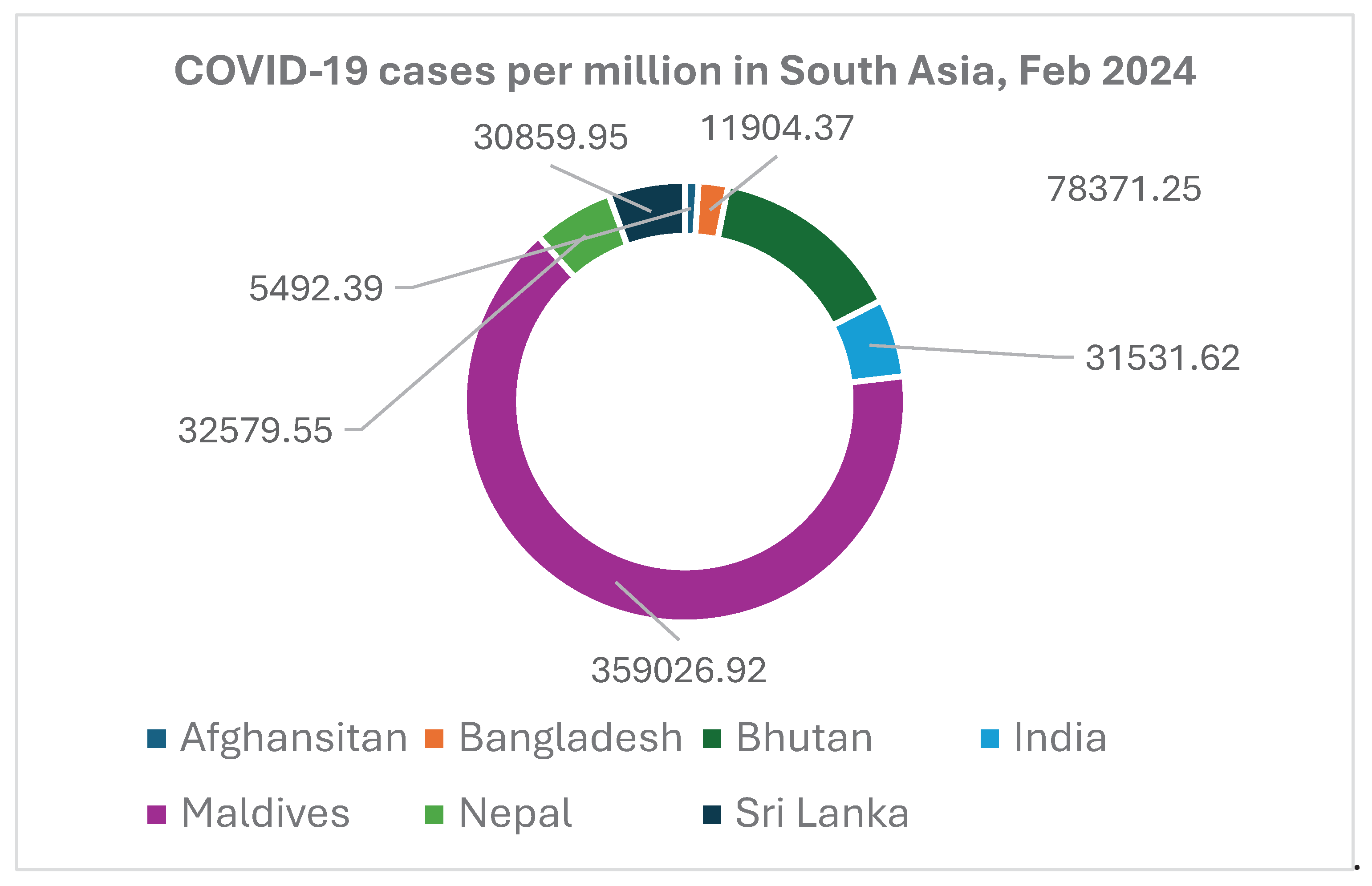
The World Health Organization (WHO) has reported, till February 2024, 49.23 million cases of COVID-19 and more than 600,000 deaths from South Asia. India accounts for almost 92% of cases and 89% of all deaths in South Asia [
5].
Figure 3 shows the number of COVID-19 cases per million population in South Asia.
Role of COVID-19 vaccines
Vaccines have been recognized as the most cost-effective public health intervention including in any health emergency. Despite a novel virus, vaccines against SARS CoV 2 were rapidly developed and mass produced by several vaccine manufacturers across the world. The national regulatory authorities and the World Health Organization (WHO) also fast-tracked the approval processes and granted emergency use authorization to a large number of vaccines.
Till March 2024, more than 13 billion doses of COVID-19 vaccine doses have been administered globally, thereby vaccinating > 70% of world population with at least one dose. The disparities between developed and developing countries becomes obvious in access and utilization of COVID-19 vaccine with only 32.7% of people in low income countries having received only one dose during this period [
6].
Despite challenges in access and deployment of COVID-19 vaccine, it became the mainstay of the pandemic control in South Asia as soon as their efficacy in reducing severity, hospitalization and mortality became obvious based upon studies elsewhere [
7].
An Indian study had demonstrated that vaccination with even a single dose reduces deaths, duration of hospitalization, and disease severity [
8]. Among Indian armed forces, vaccination with two doses showed significant reduction in mortality [
9]. Similar results were observed with two doses in the general community [
10]. It is estimated that almost 14.4 million deaths from COVID-19 infection could be averted globally during December 2020 and 2021[
11].
Though indigenous development and production of COVID-19 vaccines were limited to India in South Asia, this made it possible for other countries to obtain vaccines from India. Till May 2022, India had produced 2.4 billion doses of COVID-19 vaccines of which almost 94% were utilized domestically [
12] for immunizing 1.02 billion people with at least one dose [
13]. Six Indian vaccines have obtained Emergency Use Authorization (EUA) by the national regulatory agency of India. These are COVISHIELD (AstraZeneca: ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant),CoVaxin (whole cell vaccine indigenously produced), ZyCoV-D- (DNA vaccine), CORBEVAX
TM- (protein subunit vaccine.), GEMCOVAC™-19 – (mRNA vaccine) and iNCOVACC-(intranasal COVID-19 Vaccine)[
14]
During the COVID-19 pandemic, the South Asian countries had access to WHO Emergency Use Listing (EUL) approved seven COVID-19 vaccines that included Sinovac, Sinopharm, Oxford/AstraZeneca, Covishield, Moderna, Pfizer/BioNTech, and Janssen [
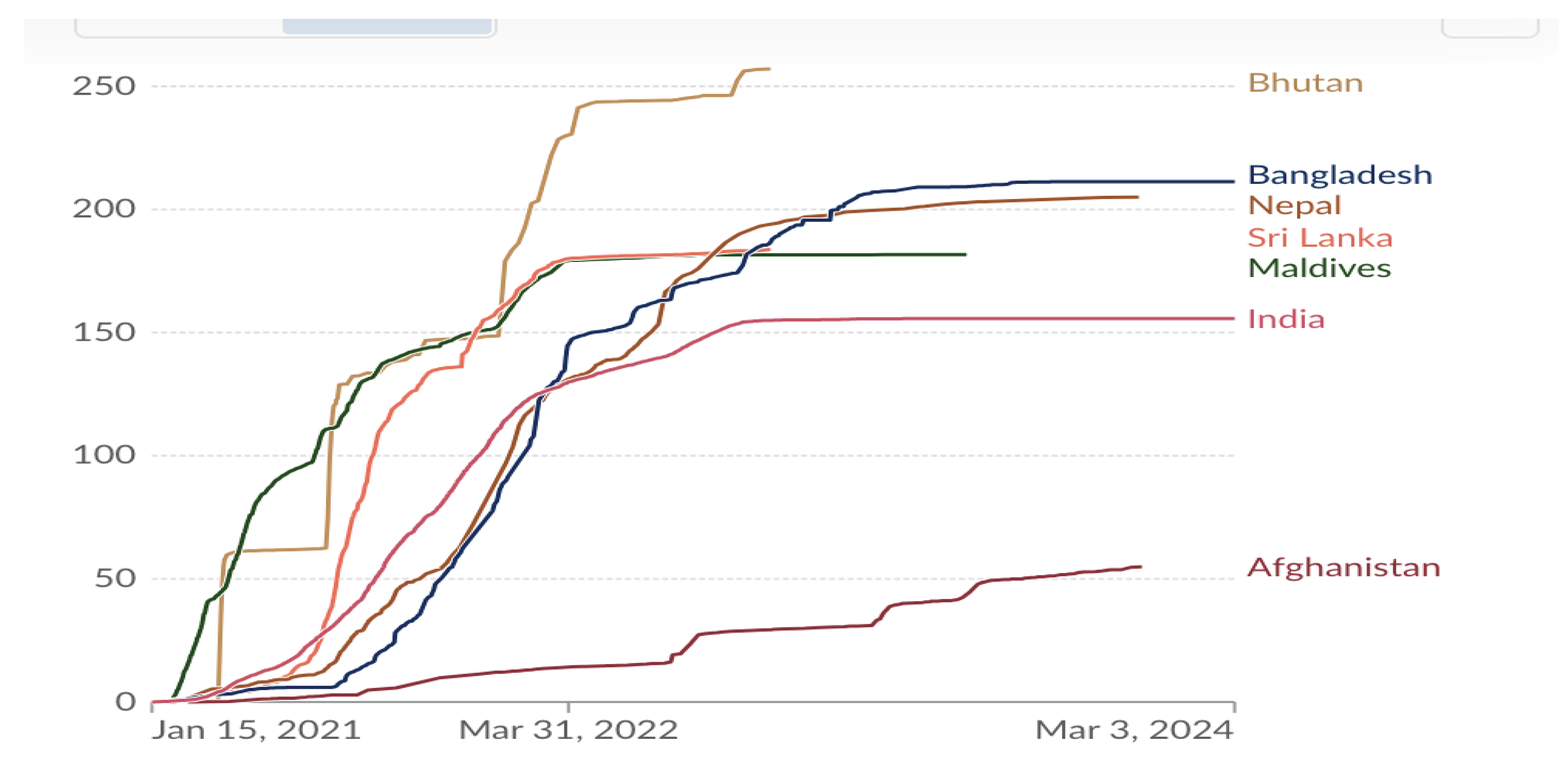
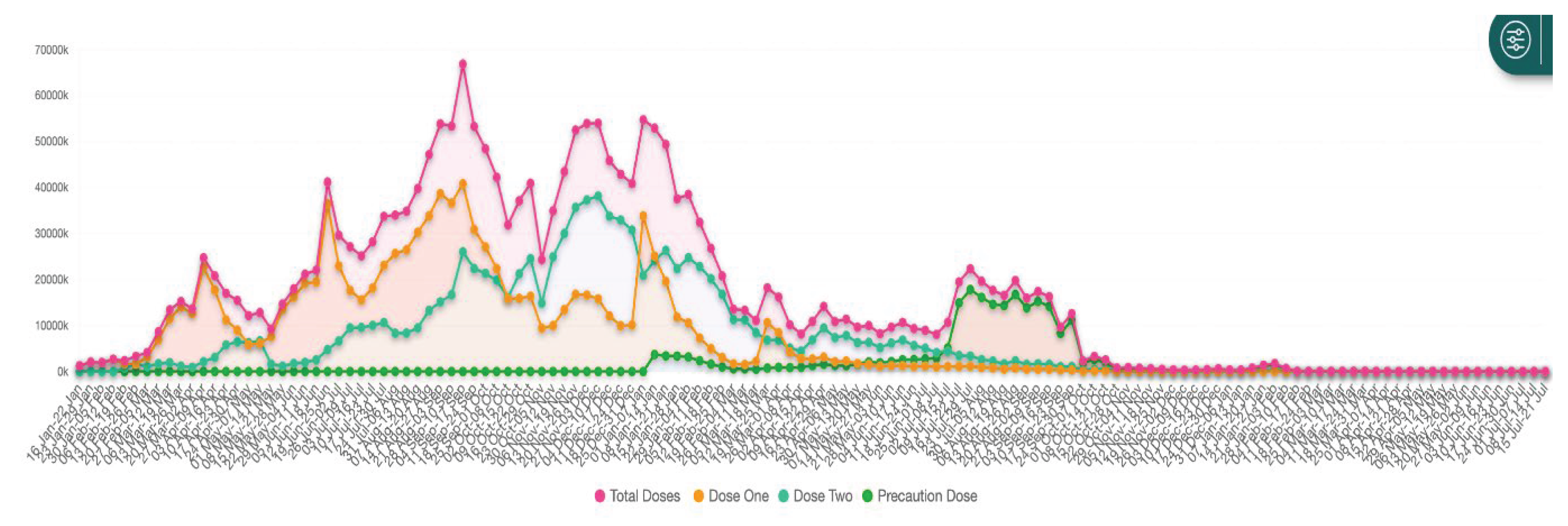
15]. WHO, in alliance with the Global Alliance for Vaccines and Immunization (GAVI), the Coalition for Epidemic Preparedness Innovations (CEPI), and UNICEF, launched COVAX programme with the goal of providing equitable access to COVID-19 vaccines across the world. The number of doses of COVID-19 vaccine administered in South Asia till March 2024 is shown in
Figure 4. The variations in numbers are due to financial, logistical, access and deployment factors apart from vaccine hesitancy that was shown by different segments of population and described subsequently.
The COVAX programme was complemented by the Asian Development Bank (ADB) by launching a
$9 billion Asia Pacific Vaccine Access Facility (APVAX) to provide support to ADB member countries to procure and deploy safe and effective vaccines and appropriately strengthening the health systems to efficiently utilize the COVID-19 vaccines [
16]. With the help of ADB, and under its APVAX facility, Bangladesh shall be establishing a vaccine manufacturing unit and strengthening its regulatory mechanism in the next few years [
17].
Political Commitment and National Initiatives
Despite having a history of frequent public health emergencies due to infectious agents, South Asia countries have not been fully prepared to combat the onslaught of COVID-19 pandemic that began its fury in this region in 2020. The Global Health Security index for South Asian countries has, in 2021, varied from 28.8 for Afghanistan to 42.8 for India [
2] with Bangladesh (35.5), Bhutan (39.8), Maldives (32.0), Nepal (34) and Sri Lanka (34.1) having an index of between 30-40 [
18].
Yet, soon after the onset of COVID-19 pandemic in February-March 2020, South Asian countries initiated public health interventions. These included international travel restrictions, quarantine, proactive lockdown measures and vaccination, as soon as it became available. Recognizing the importance of intercountry cooperation, a ‘COVID-19 Emergency Fund’ was established to implement a regional strategy to fight the pandemic [
19].
A State of Emergency was declared in Maldives and Sri Lanka under the available legal instruments. Other countries utilized existing laws. As an example, India invoked Epidemic Diseases Act [
20] and National Disaster management Act [
21] that provided additional sweeping powers to the executive to contain spread of pandemic and management of cased. Similarly Nepal and Bangladesh invoked respective existing laws and ordinances [
22].
The COVID-19 pandemic has exposed the vulnerabilities of the South Asian region. The region is fraught with the burgeoning double burden of communicable and non-communicable diseases, limited or low access to quality health care, widespread poverty and malnourishment [
23]. All these contributed to amplifying the impact of the pandemic on human life, social chaos and economic distress.
Engagement with Private Sector
All sectors of society were affected by the COVID-19 pandemic. The response was multisectoral with the private sector, being the dominant health services provider in all South Asian countries, except Bhutan, roped in through governmental orders, policy tweaking, and wilful cooperation of the private sector.
The private health-care sector in LMICs is characterized by a variety of establishments that range from individual practitioners. Chemists, non-for-profit organizations as well as for-profit specialist hospitals [
27]. On availability of COVID-19 vaccine, all segments of the private sector were engaged to deliver vaccines at a nominal change.
Coordination with the private sector was the theme of one of the 11 high powered committees constituted by the Government of India [
24]. Representatives of the private sector were incorporated in India’s National Technical Group on Vaccination against COVID-19 [
25]. Discovery and production of vaccines by the private sector were encouraged, supported and collaborated in India resulting in access to the country, and numerous other countries to quality COVID-19 vaccines.
Sri Lanka established the Presidential Task Force for COVID-19 to steer multisectoral response and designated the National Operations Centre for Prevention of COVID-19 Outbreak to coordinate prevent, control, quarantine, and other pandemic-related operations through all public and private sector entities [
26].
Private sector also provided support to the national authorities through donation of cash and in-kind. Major contributions of the private sector were for supplementing government activities in vaccination including production and administration of vaccines at government approved rates.
Vaccine Utilization in South Asia
Almost all vaccines that were accorded emergency authorization by the World Health Organization, and were available in the global market were utilized in South Asia (
Table 1). Only exception was the Sinovac vaccine. AstraZeneca vaccine produced in India was the mainstay of the vaccination programme in South Asian countries. Few of the vaccines were donated, some came through COVAX while remaining were procured by the countries through their respective internal resources. Substantial funding was provided by the international development partners mainly the World Bank and the ADB [
28]. The vaccination program indeed reduced the COVID-19 impact and eased the lockdowns and other restrictions thus facilitating economic activities.
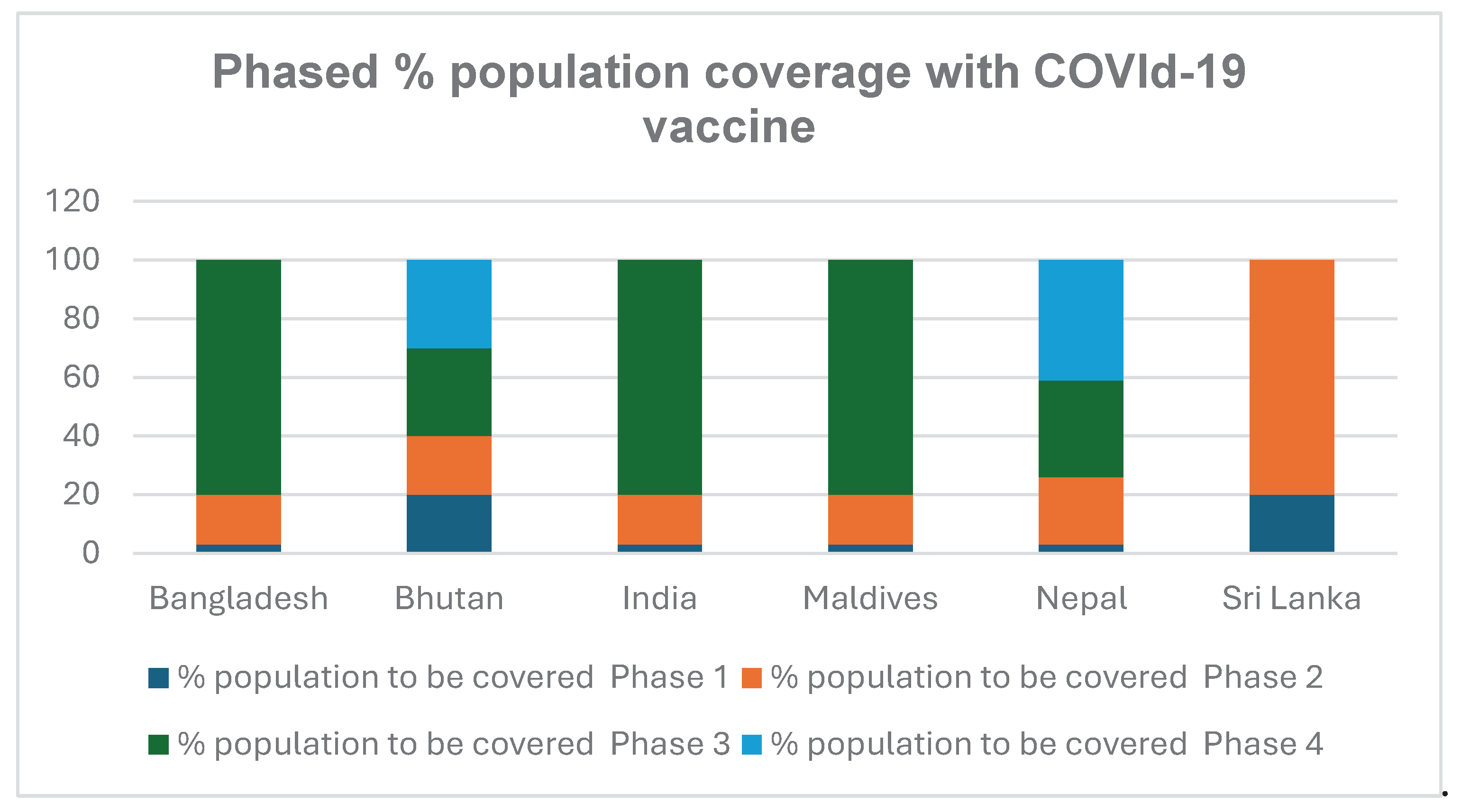
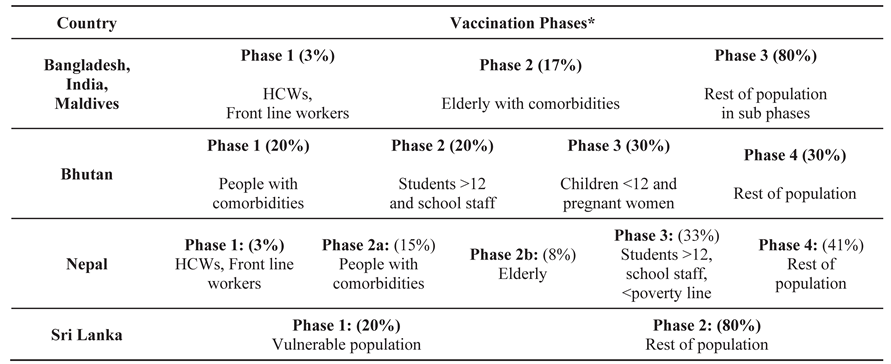
The governments of the South Asian countries spent a good percentage of their health budget to purchase COVID-19 vaccines from various sources including the global market. Vaccine supply was, however,limited in the initial first year. Accordingly, countries decided to phase out vaccination process matching with the risk profile of the population. Different phases were created by the national authorities to make best and rational use of available vaccines as shown in
Table 2.
Phasing of Vaccination
The basic principle of phasing of vaccination was to prioritize the high risk and vulnerable population. Accordingly, all countries offered vaccination to front line workers and health care staff that were in continuous and direct contact with the infectious patients especially in areas with weak infection prevention and control facilities and practices. Based upon epidemiological data of significantly higher mortality and severe clinical outcomes in elderly with co-morbidities [
29], they were categorized priority population and given precedence over otherwise healthy population in all countries.
The phased vaccination was implemented vigorously by all South Asian countries (
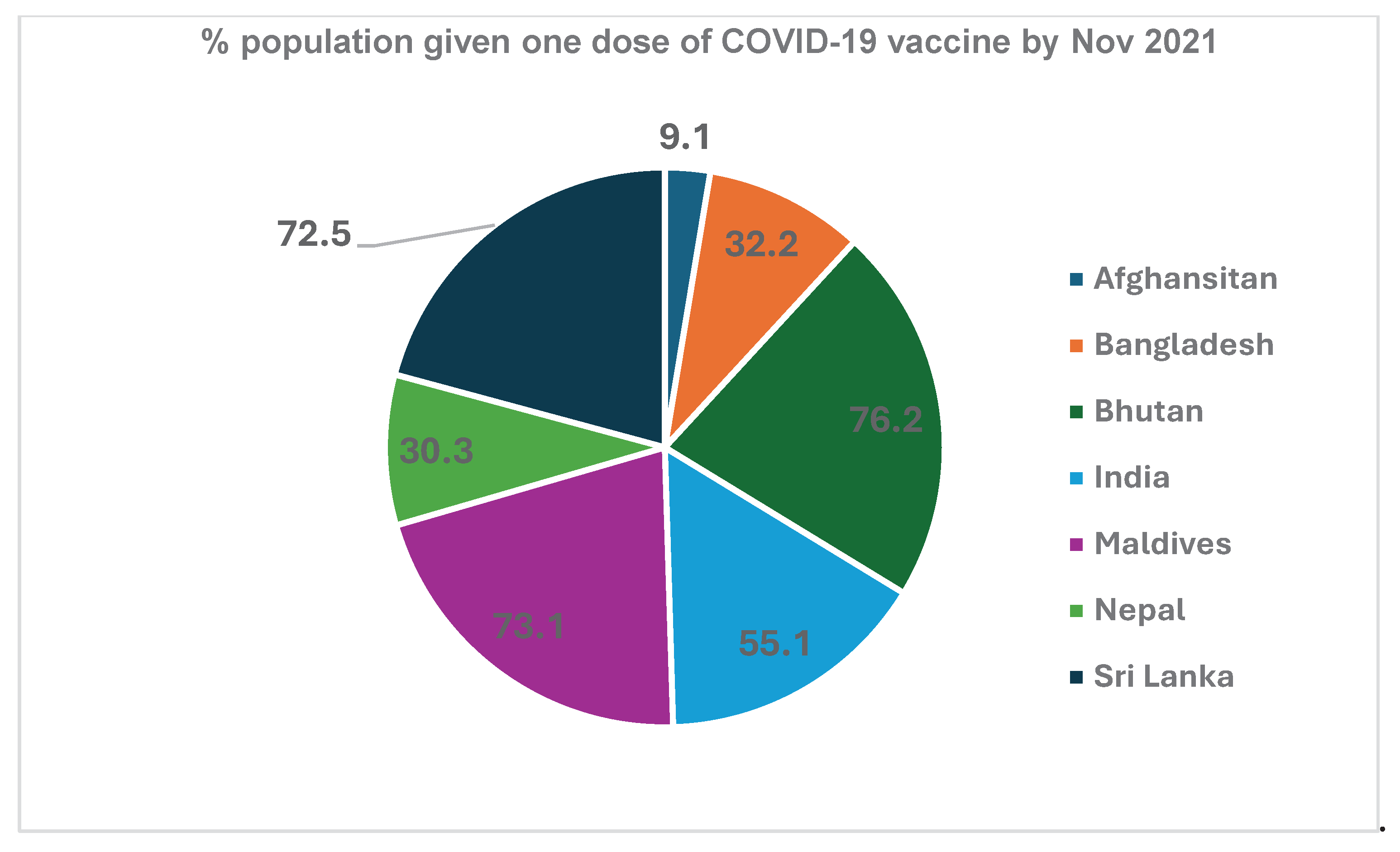
Figure 5). This resulted in vaccination of a significant population with at least a single dose of COVID-19 vaccine. Vaccination of various segments of populations, as per the phasing pattern, resulted in partial vaccination with one dose of a large number of people (
Figure 6).
Subsequent vaccination for the second dose lagged because of short supply of the vaccine and vaccine hesitancy amongst the people. The percent of population with two-dose vaccination by the end of November 2021 is shown in
Figure 7.
COVID-19 Vaccination Data Management
Data management is critical for any efficient vaccine programme. India developed and implemented an exemplary and swift tool, CoWIN which became the digital backbone of India’s vaccination programmes. This open platform for universal vaccination was citizen-centric and provided end-to-end solutions from registration to obtaining certificate of vaccination [
30]. It provided information on the next dose, location of vaccination centre, and fixed appointment for vaccination. It generated actionable data for use by the programme managers at all levels. Graphic presentation (
Figure 8) of all data are available in public domain [
31]
Support by International Development Partners
USAID provided around
$500 million to South Asia, part of this funding was activities related to vaccine procurement and deployment [
32]. China and India provided substantial donations or supply of vaccines to countries in South Asia. China was the first to donate a vaccine to Sri Lanka. The latter procured additional doses with funding support from the World Bank and ADB and quickly vaccinated half of its population with a single dose each. By March 2022, adult vaccination coverage in Sri Lanka with two doses had reached 95% [
23] .
The World Bank and the ADB have been the major donors to South Asia countries for responding to COVID-19 pandemic including vaccine procurement and deployment as well as technical support in designing vaccine related strategies [
33].
The Asia Pacific Vaccine Access Facility (APVAX) is ADB’s
$9 billion vaccine initiative offering rapid and equitable support to its developing member countries as they procure and deliver effective and safe COVID-19 vaccines [
34]. The APVAX support was extended to Bangladesh, Bhutan, India, Maldives, Nepal and Sri Lanka.
Vaccine Hesitancy
Vaccine hesitancy for all vaccines has been defined by the WHO as the reluctance or refusal to vaccinate despite the availability of vaccines [
35]. WHO considers it as of the ten threats to improve global health. It became true during the COVID-19. A study conducted before the onset of the pandemic and availability of the COVID-19 vaccine had predicted the uptake of COVID-19 vaccine to be 55-90% [
36]. The European Centre for Disease Control estimated that by 2022, only 75% of the European population had been vaccinated [
37].
WHO SAGE committee has identified the ‘3 Cs’ model of determinants of vaccine hesitancy —confidence, complacency and convenience [
38]. Vaccine hesitancy in South Asia has a complex genesis with several globally recognized factors influencing it [
39] and compounded by the lack of trust in the public health system and rapid misinformation spread through social media [
40].
Vaccine hesitancy in Afghanistan was characterized by the belief amongst the public that the vaccine provided to the low-income countries, is of low quality, unsafe and sufficient immunity to combat the SARS CoV virus pre-existed in the local community [
41]. Unwillingness to accept vaccines in Bangladesh mainly centred around misinformation on poor efficacy of vaccines since it was produced in India [
42]. High coverage of vaccines in Bhutan (72%,
Figure 7) was indicative of its overall acceptance by the citizens [
43]. Nepal, because of its low literacy and superstitions posed a public health challenge in vaccinating the target number of people [
44]. Sri Lankans were concerned with the vaccine brand, its side effects, or allergies, and raised the question of how long they would be protected from COVID-19 [
45].
In a multicentric study in four urban slums in India leading major predictors for vaccine hesitancy were found to be [
1] complications/futility of getting vaccinated, [
2] lack of understanding of safety of vaccine and adverse events following immunization, [
3] uncertainty of getting vaccine, [
4] dependence on others or family to make decision to vaccine and subsequent registration, [
5] fear of getting vaccinated and [
6] unable to register [
46]. All these factors are prevalent in developing countries.
Challenges
Vaccinating an epidemiologically significant number of people during a pandemic is an enormous task requiring substantial financial, technical, logistical and deployment strength which is invariably lacking in almost all the developing countries. Vaccine development with an infrastructure for scaling up production is extremely cost intensive. UNICEF has highlighted the plight of low-income countries in timely accessing quality COVID-19 vaccine [
47]. Several challenges have been experienced in other Asian countries too, making these generic for all developing countries [
48].
COVID-19 pandemic demonstrated the unethical advantage that resource rich countries have over the rest of the world – all of which was affected by the pandemic. Preorder of more than 4.2 billion doses of COVID-19 vaccine obviated access to this product to developing countries even if the countries had mobilized the resources for vaccine procurement [
49].
The developing countries of South Asia inherently lacked pandemic preparedness and response competence including their programmatic capacity for planning, need assessment, procurement, quality assessment, logistics, allocation to different areas or populations, and deployment decisions. Many countries (Afghanistan, Bhutan, Maldives, India, Nepal) have difficult terrain and unreachable areas [
50]. The cold chain especially for mRNA vaccines was inadequate outside big towns. Though all countries had basic infrastructure available for storage and shipment of children’s vaccines, suddenly expanded vaccine needs overwhelmed the system which required subsequent expansion and building new capacity to respond efficiently in future.
Several COVID-19 vaccines were provided emergency use authorization by the WHO but national regulatory authorities in most of the countries in South Asia have weak national regulatory authority and ill equipped national regulatory laboratories. A strong regulatory process instills confidence in the people and assures access to quality biological products.
Vaccine hesitancy proved to be a hurdle in expanding vaccination and achieving the targets. Misinformation and myths perpetuated by social media diminished confidence of the communities in utility, safety and efficacy of the vaccines. Mass media and workers at the field level could not fully mitigate hesitancy for vaccines.
Lessons Learnt
COVID-19 vaccine development was accelerated swiftly during the pandemic itself to respond to unprecedented global crisis. The traditional vaccine development period used to be 10-12 years. It was significantly reduced to 12 months for COVID-19 vaccine. Several reliable technologies viz whole cell, recombinant, mRNA, DNA etc were explored and utilized to develop COVID-19 vaccines. Using these technologies, especially mRNA platform, it is now possible that a vaccine against a novel pathogen can be developed within 100 days [
51].
The technologies developed for COVID-19 vaccines, especially mRNA vaccines, can easily be utilized for production of other novel vaccines. This opens up avenues for production of vaccines against viruses such as HIV. Repurposing a manufacturing plant for producing a new vaccine is tedious and complicated but can be done as has been shown during the pandemic. This was demonstrated by rapidly scaling up the production of new vaccines. More than 13 billion doses of various COVID-19 vaccines were produced globally and administered in almost two years [
54]. However, the protocols need to be developed for the production platforms to comply with swift switch-over to production of new vaccines.
Bridging public and private sector expertise and infrastructure can accelerate development of vaccine and scaling up production. In India, Indian Council of Medical Research and the Department of Biotechnology (DBT), Government of India worked and supported several private sector vaccine manufacturers to develop new COVID-19 vaccines. During pandemic, one vaccine was indigenously developed in India. Subsequently with support from DBT, four more vaccines have been indigenously developed in India [
52]. This highlights the role government encouragement can play to stimulate private sector to innovate and discover new products.
International technological advances and cooperation can yield swift results in developing countries. Cooperation between AstraZenaca and Serum Institute of India (SII) resulted in production by SII of more than 2 billion doses of COVID-19 vaccine which benefitted 146 countries [
53]. The foundations for such collaboration must be laid in advance.
Fast tracking regulatory approvals is possible and can be employed in future for quickly deploying new vaccines and medicines especially to mitigate public health emergencies. The National Regulatory Authorities (including national regulatory laboratories) need to be strengthened to be effective using the WHO Global Benchmarking Tool that evaluates the overarching regulatory framework and various regulatory functions that comprise it viz. national regulatory system, registration and marketing authorization, vigilance, market surveillance and control, licensing establishments, regulatory inspection, laboratory testing, clinical trials oversight, and NRA lot release. A special component of quickly responding to public health emergency needs should be built into it.
COVAX started with a noble intent of the equitable global vaccine distribution. This project was co-led by CEPI, Gavi and WHO, with logistical support from UNICEF. However, it fell short of funding and could not meet the vaccine procurement and distribution target of 2 billion doses by end 2021 [
55]. The manufacturers who had committed to supply to COVAX (including SII) faulted since either they had cut lucrative deals with rich countries or their national governments forced them for preferential domestic supply because of raging pandemic. Global cooperation became futile talk when it came to the national interest. Nationalism preceded pandemic containment. Resource rich countries and those with indigenous production capacity were more worried about their own citizens and not for the pandemic containment or the protection of people in other countries. Of 13.57 billion doses produced, around 71% of the world population received at least one dose. However, only one out of three persons living in low income countries received a single dose of the vaccine.
Inequity was grossly visible during the pandemic despite the fact that in this interconnected world, in pandemic scenario, either everyone is protected or none. Pandemics are contained only when a majority of people across the world, irrespective of status and location, are vaccinated. But that was not the case in the COVID-19 pandemic. According to latest data (March 2024) from Worldindata.org, the number of doses given per 100 population in high income countries was 227; for upper middle income countries the number was 216; for lower middle income countries 145 and for low income countries it was a meagre 45 per 100 population [
55]. It was unethical and contrary to the global public health principles. This disparity can be eliminated ONLY if the developing countries have their own R&D and production facilities within the country or within the Region. In Asia, this capacity is available in China, India, Indonesia, and Thailand. ADB is supporting Bangladesh to set up a vaccine manufacturing plant. At least Asia has adequate manufacturing capacity. Development of new vaccine, scale production, rapid deployment and timely need-based access remain challenges.
Organizations such as Gavi, the World Bank, JICA, ADB and other development partners should strengthen and make COVAX and APVAX arrangements more strong, robust, effective, binding, equitable, ethical and feasible for playing a greater role in next public health emergency.
References
- Worldometer. Population of South Asia. Available at https://www.worldometers.info/world-population/southern-asia-population/#:~:text=Countries%20in%20Southern%20Asia&text=The%20current%20population%20of%20Southern,of%20the%20total%20world%20population. Accessed on 16 March 2024.
- The World Bank. Available at https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed on 20 March 2024.
- UNDP Human Development Report 2021. Available at https://www.security-risks.com/post/human-development-index-2022-south-asia-a-review Accessed on 10 March 2024.
- WHO. Available at https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/sdg-target-3.8-achieve-universal-health-coverage-(uhc)-including-financial-risk-protection, accessed on 19 March 2024.
- WHO. Available at https://data.who.int/dashboards/covid19/deaths?m49=144&n=c accessed on 17 March 2024.
- Our World in Data. COVID-19 vaccination. Available at https://ourworldindata.org/covid-vaccinations accessed on 11 March 2024.
- Marrone G, Nicolay N, Bundle N, Karki T, Spiteri G, Suija H, Kärblane KG, et al Risk reduction of severe outcomes in vaccinated COVID-19 cases: an analysis of surveillance data from Estonia, Ireland, Luxembourg and Slovakia, January to November 2021. Euro Surveill. 7(2022):2200060.
- De S, Sahu D, Mahilang D, Ganga RT, Behera AK. Effectiveness of partial COVID-19 vaccination on the outcome of hospitalized COVID-19 patients during the second pandemic in India. Perspect Clin Res. 15 (2024):46-47 . [CrossRef]
- Muthukrishnan J, Vardhan V, Mangalesh S, Koley M, Shankar S, Yadav AK, Khera A. Vaccination status and COVID-19 related mortality: A hospital based cross sectional study. Med J Armed Forces India. 77(2021):S278-S282 . [CrossRef]
- Verma M, Sharma S, Kumar A, et al. Comorbidities and Vaccination Status of COVID-19 All-Cause Mortality at a Tertiary Care Center of Western India. Cureus 14(2022): e21721. [CrossRef]
- Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022 Sep;22(9):1293-1302 . [CrossRef]
- WHO. Available at https://www.wto.org/english/tratop_e/covid19_e/vaccine_trade_tracker_e.htm accessed on 20 March 2024.
- Ministry of Health & Family Welfare, Government of India. Available at https://www.mohfw.gov.in/ accessed on 7 March 2024.
- Press Information Bureau. Government of India. Available at https://www.pib.gov.in/PressReleasePage.aspx?PRID=1894167 accessed on 25 March 2024.
- WHO. Available at https://covid19.trackvaccines.org/agency/who/ accessed on 21 March 2024.
- Asian Development Bank. Available at https://www.state.gov/wp-content/uploads/2021/09/IO-Asian-Development-Bank-COVID-19-Summit-Participant-Statement.pdf accessed on 20 March 2024.
- Asian Development Bank. Available at https://www.adb.org/news/adb-help-develop-vaccine-production-bangladesh accessed on 21 March 2024.
- Global Health Security Index. Available at https://ghsindex.org/ accessed on 26 March 2024.
- Bhatnagar T, P. Kaur, V. Kumaraswami. Links between the epidemiology and control of noncommunicable diseases and neglected tropical diseases in Asia Neglected tropical diseases – East Asia, Cham: Springer (2019), pp. 149-173,.
- Indiacode. Available at https://www.indiacode.nic.in/bitstream/123456789/15942/1/epidemic_diseases_act%2C1897.pdf accessed on 22 March 2024.
- National Disaster Management Authority, India. Available at https://ndma.gov.in/Reference_Material/DMAct2005 accessed on 26 March 2024.
- Asia Centre. Moving Beyond COVID-19 Restrictions in South Asia: Available at https://asiacentre.org/wp-content/uploads/Moving-Beyond-COVID-19-Restrictions-in-South-Asia.pdf accessed on 23 March 2024.
- Giridhara R. Babu, Sonalini Khetrapal, Daisy A. John, R. Deepa, K.M. Venkat Narayan, Pandemic preparedness and response to COVID-19 in South Asian countries. International Journal of Infectious Diseases, 104 (2021) 169-174. [CrossRef]
- Press Information Bureau, Government of India. Available at https://static.pib.gov.in/WriteReadData/specificdocs/documents/2021/dec/doc2021122421.pdf accessed on 21 March 2024.
- Press Information Bureau, Government of India. Available at https://pib.gov.in/PressReleasePage.aspx?PRID=1645363# accessed on 21 March 2024.
- Rannan-Eliya RP, Ghaffoor A, Amarasinghe S, Nirmani MD, Wijemunige N, Perera S,et al. Sri Lanka’s COVID-19 response and maintaining health services: implications for future pandemics. BMJ Glob Health. 8(2024) (Suppl 6):e013286.
- Mackintosh M, Channon A, Karan A, Selvaraj S, Cavagnero E, Zhao H. What is the private sector? understanding private provision in the health systems of low-income and middle-income countries. Lancet. (2016) 388:596–605 . [CrossRef]
- Hayat M, Uzair M, Ali Syed R, Arshad M, Bashir S. Status of COVID-19 vaccination around South Asia. Hum Vaccin Immunother. 18(2022):2016010. [CrossRef]
- Wei C , Liu Y , Liu Y , Zhang K , Su D , Zhong M , et al. Clinical characteristics and manifestations in older patients with COVID-19. BMC Geriatr 2020; 20(1): 395.
- UNDP. Available at https://www.undp.org/india/projects/winning-over-covid-cow accessed on 24 March 2024.
- Ministry of Health & FW, Government of India. COWIN. Available at https://dashboard.demo.co-vin.in/ accessed on 12 March 2024.
- USAID. Available at https://www.usaid.gov/sites/default/files/2022-05/Regional_Fact_Sheet_4_-_ASIA.pdf accessed on 12 March 2024.
- The World Bank. Available at https://www.worldbank.org/en/region/sar/coronavirus#:~:text=The%20World%20Bank%20has%20made,hit%20the%20region%20this%20year. Accessed on 20 March 2024.
- Asian Development Bank. Available at https://www.adb.org/where-we-work/south-asia/covid-19-response accessed on 13 March 2024.
- The World Health Organization. Available at https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 accessed on 25 March 2024.
- Lazarus, J. V. et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 27 (2021), 225–228.
- ECDC. COVID - 19 Country overviews - Country overview report, https://covid19-country-overviews.ecdc.europa.eu/ (2022).
- WHO . Report of The Sage Working Group on Vaccine Hesitancy. WHO; Geneva, Switzerland: 2014.
- Gerretsen, P. et al. Individual determinants of COVID-19 vaccine hesitancy. PLoS ONE 16, e0258462. (2021) . [CrossRef]
- Carrieri, V., Guthmuller, S. & Wübker, A. Trust and COVID-19 vaccine hesitancy. Nature Sci Rep 13 (2023), 9245 . [CrossRef]
- Nemat A, Bahez A, Salih M, Raufi N, Noor NAS, Essar MY, Ehsan E, Asady A. Public willingness and hesitancy to take the COVID-19 vaccine in Afghanistan. Am J Trop Med Hyg. 2021;105(3):713–7 . [CrossRef]
- Akiful Haque MM, Rahman ML, Hossian M, Matin KF, Nabi MH, Saha S, Hasan M, Manna RM, Barsha SY, Hasan SMR, et al. Acceptance of COVID-19 vaccine and its determinants: evidence from a large sample study in Bangladesh. Heliyon. 2021;7(6):e07376. [CrossRef]
- Dorji T, Tamang ST. Bhutan’s experience with COVID-19 vaccination in 2021. BMJ Global Health. 2021;6(5):e005977 . [CrossRef]
- Adhikari K. Third wave of COVID-19 in Nepal: challenges and way forward. J Chitwan Med Coll. 2021;11:1–2 . [CrossRef]
- Wijesinghe MSD, Weerasinghe W, Gunawardana I, Perera SNS, Karunapema RPP. Acceptance of COVID-19 vaccine in Sri Lanka: applying the health belief model to an online survey. Asia-Pacific Journal of Public Health. 2021;33(5):598–602 . [CrossRef]
- Tamysetty S, Babu GR, Sahu B, Shapeti S, Ravi D, Lobo E, et al Predictors of COVID-19 Vaccine Confidence: Findings from Slums of Four Major Metro Cities of India. Vaccines (Basel). 2021 Dec 31;10(1):60.
- UNICEF. Costs and predicted financing gap to deliver COVID-19 vaccines in 133 low- and middle-income countries. [Apr; 2022];https://www.unicef.org/media/114216/file/Costs-and-Predicted-Financing-Gap-to-Deliver-COVID-19-Vaccines-in-133-Low-and-Middle-Income-Countries.pdf 2022 accessed on 16 March 2024.
- Fu JYL, Pukhari MH, Dela Cruz KA, Soebandrio A, Tan LV, Jantarabenjakul W, Sawitri AAS, Chantasrisawad N, et al. Association of Southeast Asian Nations (ASEAN) Sero-Surveillance Study on COVID-19 vaccines (ASSeSS) Working Group. Charting the path forward in Southeast Asia: Learning from the COVID-19 vaccination challenges in six ASEAN countries. J Glob Health 2024;14:03016. [CrossRef]
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment.. Lancet. 2021;397:1023–1034.
- Acharya KP, Ghimire TR, Subramanya SH. Access to and equitable distribution of COVID-19 vaccine in low-income countries. NPJ Vaccines. 2021;6:54. [CrossRef]
- CEPI. Available at https://cepi.net/cepi-supports-novel-mrna-vaccine-development-korea-protect-against-future-disease-x#:~:text=mRNA%20technology%20has%20been%20identified,a%20new%20viral%20threat%20is accessed on 20 March 2024.
- Press Information Bureau, Government of India. Available at https://www.pib.gov.in/PressReleasePage.aspx?PRID=1894167#:~:text=The%20four%20vaccines%20are%2D%20ZyCoV%2DD%2D%20World’s%201st,developed%20intranasal%20COVID%2D19%20Vaccine. Accessed on 19 March 2024.
- CEPI. Available at https://cepi.net/news_cepi/new-partnership-aims-to-advance-vaccine-against-mers-coronavirus/ accessed on 21 March 2024.
- The World Health Organization. COVID-19 advice for the public: Getting vaccinated. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice#:~:text=Since%202021%2C%20more%20than%2013,19%20vaccines%20are%20extremely%20rare. Accessed on 22 March 2024.
- Reddy, Sanjay G. and Acharya, Arnab, The Failure of COVAX: A Predictable Outcome (December 22, 2023). Available at SSRN: https://ssrn.com/abstract=4673596 or http://dx.doi.org/10.2139/ssrn.4673596.
- Ourworld in data. Coronavirus vaccinations. Available at https://ourworldindata.org/covid-vaccinations accessed on 3 March 2024.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).