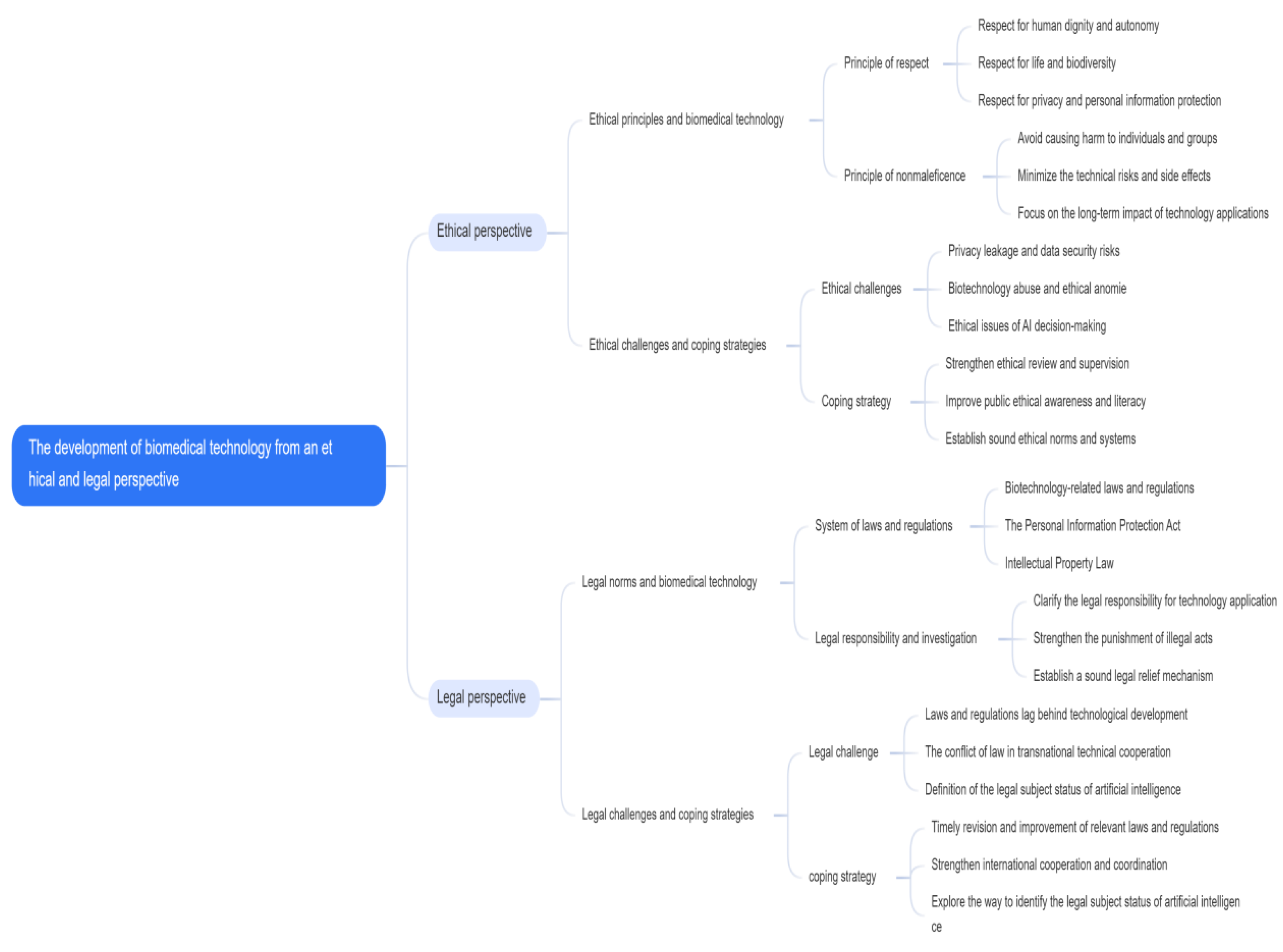
1. The Importance of Ethics and Law in Biomedical Science and Technology
Focuses on the important role of ethics and law in the field of biomedical science and technology (
Table 1). Biomedical science and technology, as a key way for human beings to explore the mystery of life and improve the level of health, every step of its progress affects the nerve of society. The rapid development of technology is often accompanied by ethical and legal challenges. It is these challenges that highlight the indispensable role of ethical principles and laws and regulations in guiding the healthy development of science and technology.
Ethical principles, like the compass of scientific and technological development, provide a clear moral direction for biomedical researchers and practitioners. In the pursuit of scientific and technological innovation, we must always adhere to the respect and protection of life and human welfare. Ethical principles exist precisely to ensure that every step of scientific and technological development does not deviate from this fundamental purpose. It requires us to consider not only the feasibility of the technology, but also its ethical acceptability when conducting biomedical research [
1]. Only in this way can we ensure the rationality and sustainability of scientific and technological development, and win the broad recognition and support of society. Complementary to ethical principles is the regulatory role of laws and regulations. In the field of biomedical science and technology, laws and regulations are like a solid barrier, guarding the compliance and safety of scientific and technological activities. Through the development of strict regulations and standards, we can ensure that the development and application of biomedical technology activities are carried out within the framework of the law, and effectively prevent the occurrence of technological abuse and misconduct. Laws and regulations also provide a strong institutional guarantee for scientific and technological innovation, create a stable and transparent legal environment for researchers and practitioners, and stimulate the vitality and motivation of scientific and technological innovation. It is worth mentioning that ethics and law play a key role in balancing the interests of various parties in biomedical science and technology. In the process of the development of science and technology, the demands and rights of different stakeholders often conflict. At this time, ethical and legal principles and rules become important tools to mediate these conflicts. By clarifying the responsibilities, rights and obligations of all parties, they have established a fair and equitable benefit distribution mechanism to ensure that the fruits of scientific and technological development can benefit a wider range of people and achieve harmonious progress between science and technology and society[
2].
2. Research Purpose and Significance
In the vast field of biomedical science and technology, ethics and law play a crucial role. These two are not only the forces that constrain and guide the development of science and technology, but also the key factors that determine whether science and technology can truly serve society and benefit mankind. Ethical principles serve as a compass for science and technology to ensure that research does not deviate from the values of humanity and justice; Laws and regulations are a solid barrier to ensure the security of science and technology and prevent the risk of abuse. In-depth study of the role of ethics and law in biomedical science and technology, it is not difficult to find that their internal connection and interaction constitute the cornerstone of the healthy development of science and technology. Ethical principles provide the conceptual basis and value orientation for the formulation of laws and regulations, making legal provisions not only a pile of words, but full of respect for human dignity and the value of life. The strict implementation and continuous improvement of laws and regulations also provide a strong guarantee for the implementation of ethical principles in practice. This positive interaction between ethics and law is the key to the continuous innovation and steady development of biomedical science and technology.
In the process of rapid development of biomedical science and technology, it is also faced with a series of practical challenges and problems. Among them, the complex issues involving ethics and law are particularly interesting. The advent of gene-editing technology, for example, has allowed unprecedented intervention in life, but it has also sparked heated debates about deep ethical issues such as the origin of life and human dignity [
3]. For another example, in the process of commercialization of biomedical technology, how to protect patients' rights and interests, prevent conflicts of interest, ensure fair competition and other issues have become increasingly prominent, requiring timely follow-up and effective regulation of laws and regulations. In the face of these challenges and problems, we cannot evade them, still less simplify them. On the contrary, a more open and inclusive mind should be adopted to deeply explore the nature and root causes of these ethical and legal issues and seek practical solutions and suggestions. For example, when it comes to the ethics of gene editing technology, extensive public discussion and expert consultation can be carried out to form social consensus and ethical guidelines, so as to draw boundaries for the rational application of the technology. In the commercialization of biomedical technology, it is necessary to establish a sound legal and regulatory system and strengthen supervision to ensure that the healthy development of science and technology is not kidnapped by commercial interests. Of course, solving these problems requires the joint efforts and wisdom of the whole society. The government, scientific research institutions, enterprises, the public and other parties should assume their respective responsibilities and obligations to form a joint force to promote the healthy development of biomedical science and technology [
4]. The government should strengthen top-level design, formulate scientific and reasonable policies, regulations and development plans, and provide strong support for the innovation and application of science and technology. Scientific research institutions and enterprises should adhere to the right direction of scientific and technological innovation, strengthen self-discipline management, and ensure the safety and effectiveness of research results. The public should improve their scientific literacy and ethical awareness, rationally view the development and application of science and technology, and not blindly follow the trend or reject it. In this process, it is particularly important to strengthen the guidance and norms of ethics and law. It is necessary to constantly improve the system of ethical review and laws and regulations to ensure that the innovation and application of science and technology are always carried out within the framework of ethics and law. It is also necessary to strengthen the ethical education and legal training of scientific researchers, improve their ethical literacy and legal awareness, and ensure that they can consciously abide by ethical principles and laws and regulations in scientific research activities [
5].
Through such efforts, the harmonious development of biomedical science and technology and society can be achieved, so that scientific and technological progress can truly benefit mankind. The power of science and technology is infinite, but the application of science and technology must be measured. Only under the guidance of ethics and law can biomedical science and technology move along the right track and make greater contributions to human health and well-being. As a member of the society, everyone should also actively participate in this process, and use their own actions and wisdom to promote the common progress of science and technology and society.
3. The Application of Ethics in Biomedical Science and Technology
3.1. The Embodiment of Ethical Principles in Biomedical Technology
In the broad field of biomedical science and technology, the application of ethics is particularly critical. Ethical principles not only provide guidelines for the behavior of researchers, but also ensure that the rights and interests of subjects or patients are not infringed. As one of the cornerstones of ethics, the principle of respect has been deeply reflected in biomedical science and technology. It emphasizes respect for individual autonomy, ensuring that each person can make a choice based on their own wishes when deciding whether to participate in research or receive treatment. This respect is not only reflected in the recognition of the autonomy of the subjects or patients, but also in the respect of the researchers for their personality and dignity [
6].
The do no harm principle is another indispensable ethical principle in biomedical technology. During research or treatment, the safety of the subject or patient is always the first priority. Researchers must always be alert to any risk of harm to subjects or patients and take steps to prevent it. This is not only a demonstration of responsibility to the subjects or patients, but also a reflection of the professional ethics of the researchers. The principle of no harm also requires researchers to take responsibility when facing potential risks and take timely measures to solve them, rather than shirking or evading responsibility.
The principle of impartiality is equally important in biomedical technology. The fair distribution of resources is key to ensuring that everyone has equal access to research or treatment. In the field of biomedical science and technology, because of the scarcity of resources and the unlimited demand, how to allocate resources fairly has become an urgent problem to be solved. The principle of justice requires researchers to follow the principle of fairness, justice and openness when allocating resources to ensure that everyone has an equal opportunity to get the resources they need. This is not only a matter of respect for the subject or patient, but also a matter of social justice.
Confidentiality and privacy protection are also important manifestations of ethical principles in biomedical technology. The subject's or patient's personal information and medical records are their privacy and must be strictly protected. When processing such information, researchers should follow relevant laws, regulations and ethical norms to ensure that the privacy of subjects or patients is not disclosed. This is not only to protect the rights and interests of subjects or patients, but also to test the professional ethics of scientific researchers [
7]. Confidentiality and privacy protection also require researchers to adhere to the bottom line when facing the temptation of interests, and not to harm the rights and interests of subjects or patients for personal gain.
In the development of biomedical science and technology, the application of ethical principles is not only a constraint on the behavior of researchers, but also an important guarantee to promote the healthy and sustainable development of science and technology. Only by internalizing ethical principles into their own code of conduct can researchers always adhere to the moral bottom line in the process of research or treatment and ensure that the rights and interests of subjects or patients are not infringed. The application of ethical principles also helps to improve the social recognition of biomedical science and technology and promote the harmonious development of science and technology and society. With the continuous development of biomedical science and technology, new ethical issues are emerging. This requires researchers not only to have solid professional knowledge, but also to have a keen sense of ethics and rich practical experience. In the face of complex ethical issues [
8], they can make correct judgments and decisions. Researchers should also actively participate in the formulation and improvement of ethical norms to contribute to the healthy development of biomedical science and technology. The public's attention and participation in biomedical technology is also increasing. This requires researchers to pay more attention to communication and exchange with the public when carrying out research or treatment, timely answer the public's doubts and confusion, and strive for public understanding and support. The public should also actively participate in the supervision and management of biomedical science and technology to provide strong social support for the healthy development of science and technology. The application of ethics in biomedical science and technology is an important guarantee to ensure the healthy and sustainable development of science and technology. Researchers must always adhere to ethical principles, respect the rights and dignity of subjects or patients, and ensure the safety and effectiveness of research or treatment. The public should also actively participate in the supervision and management of biomedical technology, and contribute their own strength to the healthy development of science and technology. To jointly promote biomedical science and technology to a better future [
9].
3.2. Ethical Review and Supervision Mechanism
In such a sophisticated and uncharted field as biomedical science and technology, the importance of ethics is self-evident. It is like a beacon, guiding researchers and doctors to explore the mystery of life, to overcome the problem of disease not to lose direction, and always adhere to the bottom line of humanity and dignity. The practice and application of ethics in biomedical science and technology is not only related to the compliance of research activities, but also involves the ethical quality of each participant and the healthy development of the whole industry.
In order to ensure that the ethical standards of biomedical research and treatment activities are strictly followed, the establishment of independent ethical review committees is particularly important. Such an institution, like an impartial judge, monitors every step of the research process to ensure that it is carried out within an ethical framework. From the preliminary design of the research protocol, to the specific operation of the experiment, to the collection and analysis of data, the ethics review Committee plays an indispensable role. Its existence is not only a constraint for researchers, but also a protection for them to ensure that they do not deviate from the track of ethics in the pursuit of scientific truth. Of course, ethics review committees alone are not enough. In order to ensure that ethical behavior in the field of biomedical science and technology is fully regulated, a series of strict regulatory regulations and guidance documents are also needed. These regulatory documents, like the "code of ethics" in biomedical technology [
10], provide clear guidelines for practitioners to conduct themselves. Not only do they specify what is and is not allowed, but they also detail how decisions should be made in the event of an ethical dilemma. The formulation of these regulatory documents needs to fully consider the characteristics and development trends of biomedical science and technology to ensure that it is forward-looking and can solve practical problems. In order to ensure that the ethical behavior in the field of biomedical science and technology can be truly effective regulation. Whether it is ethics review boards or regulatory regulations, they are only external restraining forces. In order to truly improve the ethical level in the field of biomedical science and technology, it is also necessary to improve the ethical awareness and literacy of practitioners. This requires greater ethical training and education for researchers, doctors and patients. Through systematic training courses and rich educational activities, we can help them deeply understand the importance of ethical principles and master the methods and skills of applying ethical principles in practical work. In this way, they will be able to make more informed and responsible decisions when faced with complex ethical issues [
11].
The content of ethics training and education should include but not be limited to the following aspects: First, the basic concept and connotation of ethical principles, to help practitioners establish a clear understanding of ethical principles; Secondly, the methods and skills of ethical decision-making, which teach them how to use ethical principles to make decisions in the face of practical problems; Thirdly, it is to identify and deal with ethical dilemmas to help them improve their ability to deal with complex ethical issues in practical work. Through these training and education activities, practitioners' ethical awareness and literacy can be effectively enhanced, providing a strong guarantee for the healthy development of biomedical science and technology. In addition to the aspects mentioned above, there are other measures that can promote ethical practice and application in the field of biomedical science and technology. For example, establish a sound ethical supervision mechanism to track and supervise the whole research process to ensure the compliance of research activities and the satisfaction of ethical standards; Strengthen international cooperation and exchanges, learn from the advanced experience and practices of other countries and regions, and jointly promote the development of ethics in the field of biomedical science and technology; Encourage public participation and supervision by public opinion, and enhance the public's attention to and supervision of ethical issues in the field of biomedical science and technology [
12].
The practice and application of ethics in the field of biomedical science and technology is a complex and important subject. Through the implementation of measures such as the establishment of an independent ethics review committee, the formulation of strict regulatory regulations and guidance documents, and the strengthening of ethics training and education, the ethical level in the field of biomedical science and technology can be effectively improved, the rights and interests of researchers and patients can be protected, and the healthy development of the entire industry can be promoted. We should also recognize that the practice and application of ethics is an ongoing process that requires joint efforts and continuous exploration by all parties. Only in this way can we achieve more brilliant achievements in the field of biomedical science and technology and make greater contributions to human health and well-being [
13].
4. The Regulation of Law in Biomedical Science and Technology
4.1. Supervision and Restriction of Biomedical Science and Technology Activities by Law
In the field of biomedical science and technology, the importance of legal supervision is self-evident. Scientific and technological activities in this field are closely monitored by multiple agencies, such as drug regulatory authorities and health administration departments, which work together to ensure compliance and safety of scientific and technological activities within their respective areas of responsibility. In order to ensure that scientific and technological activities meet the requirements of laws and regulations and prevent potential risks, regulators have adopted a series of specific regulatory measures and means. These measures, including administrative approval, supervision and inspection, and administrative penalties, are aimed at controlling the quality and safety of scientific and technological activities at the source.
Administrative approval is the primary link in the supervision of biomedical science and technology activities. Before carrying out any scientific and technological activities, the relevant agencies must submit detailed application information, and pass the strict review of the drug regulatory department and other agencies in order to obtain the corresponding approval. This process ensures that scientific and technological activities are carried out in accordance with the requirements of laws and regulations, and lays the foundation for subsequent regulatory work. Supervision and inspection is an important means for regulatory agencies to continuously supervise biomedical science and technology activities [
14]. Through the on-site inspection of scientific and technological activities, the review of relevant records and data, and the questioning of relevant personnel, the regulatory authorities can keep abreast of the progress of scientific and technological activities, and identify and correct existing problems. This dynamic regulatory approach helps ensure that science and technology activities always operate on the track of laws and regulations. Administrative punishment is a powerful means for regulators to punish scientific and technological activities that violate laws and regulations. Once violations of laws and regulations are found in scientific and technological activities, the regulator will punish relevant institutions and individuals in accordance with the law, including warnings, fines, revocation of licenses and other measures. These penalties not only play a disciplinary role, but also warn other institutions and individuals to strictly abide by laws and regulations, and jointly maintain good order in the field of biomedical science and technology. In addition to the above specific regulatory measures and means, regulatory agencies also focus on strengthening cooperation and exchanges with other countries and regions, learning from advanced international regulatory experience and practices, and constantly improving their own regulatory level and capacity. Regulators also actively advocate industry self-discipline, encourage relevant institutions and individuals to consciously comply with laws and regulations, and jointly promote the healthy development of the biomedical science and technology field.At present, there are still some problems and challenges in the supervision of biomedical science and technology activities. Among them, insufficient supervision is a more prominent problem. Due to limited regulatory resources, regulators are often powerless in the face of numerous scientific and technological activities, resulting in some illegal activities to escape supervision. The single regulatory means is also an important factor restricting the improvement of regulatory efficiency. At present, regulators mainly rely on traditional means such as administrative approval, supervision and inspection, and administrative penalties for supervision, and lack more flexible and diversified supervision methods and methods. In order to solve these problems and further improve the efficiency of supervision, regulators need to take a series of measures. Strengthen the construction of the supervisory team, improve the professional quality and ability of the supervisory personnel, so that they can better perform their supervisory duties. We will make innovations in regulatory means and methods, and use modern scientific and technological means such as big data and artificial intelligence to improve the intelligence and precision of regulation, reduce regulatory costs and improve regulatory efficiency. We will strengthen social supervision and co-governance, encourage the public to participate in the supervision process, give play to the supervisory role of social forces, and form a co-governance pattern that combines government supervision and social supervision [
15].
In the future development, the field of biomedical science and technology will continue to maintain a momentum of rapid development and make greater contributions to the cause of human health. As an important means to ensure the healthy development of this field, legal supervision will continue to adapt to the new situation and needs, and provide a more solid and powerful guarantee for the progress and development of biomedical science and technology.
4.2. Legal Liability and Risk Prevention and Control
In this era full of challenges and opportunities, it must be clearly recognized that biomedical science and technology activities are not only related to the academic pursuit of researchers, but also directly related to the health and safety of the public. Clarifying legal responsibilities and establishing and improving risk prevention and control mechanisms have become an inevitable choice to promote the healthy and orderly development of biomedical science and technology. In the activities of biomedical science and technology, the types of legal liability are diverse and interrelated. Civil liability, administrative liability, criminal liability and other forms of legal liability together constitute the legal liability system in the field of biomedical science and technology. These forms of responsibility exist to ensure that researchers fully respect and protect the legitimate rights and interests of others in the pursuit of scientific breakthroughs.
The bearing of civil liability means that in the activities of biomedical science and technology, if the damage is caused to others due to negligence or illegal acts, the relevant liable subject must bear the compensation liability according to law. The existence of this form of responsibility effectively protects the rights and interests of the subjects, but also encourages researchers to conduct research activities more carefully.
The assumption of administrative responsibility reflects the government's regulatory responsibility for biomedical science and technology activities. When relevant institutions or individuals violate administrative regulations, they will face legal consequences such as administrative penalties. The existence of this form of responsibility ensures the compliance of biomedical science and technology activities and maintains the fair competition order of the market.
The bearing of criminal responsibility is the most severe form of legal responsibility. In the field of biomedical science and technology, if the relevant acts violate the criminal law, such as involving fraud, serious harm to public security and other criminal acts, the relevant responsible subjects will bear criminal responsibility according to law. The existence of this form of responsibility has effectively cracked down on illegal and criminal acts and maintained the legal order in the field of biomedical science and technology [
16].
Clarifying legal liability alone is not sufficient to address all challenges in the field of biomedical science and technology. The establishment and improvement of risk prevention and control mechanisms are equally important. As the primary link of risk prevention and control, risk assessment provides an important basis for subsequent risk warning and response through scientific assessment of possible risks in biomedical science and technology activities. The establishment of risk early warning mechanism can respond in time when risks emerge and prevent the expansion and spread of risks. The improvement of the risk response mechanism ensures that the risk can be quickly and effectively taken when the risk occurs, and the risk is controlled within the tolerable range. In the construction of risk prevention and control mechanism, we should pay attention to the role of the government, enterprises, scientific research institutions and other multi-entities. The government should strengthen supervision, improve the legal and regulatory system, and provide a strong institutional guarantee for risk prevention and control. Enterprises should actively fulfill their social responsibilities, strengthen internal management, and ensure product safety and effectiveness [
17]. Scientific research institutions should strengthen the construction of scientific research ethics, improve the risk awareness and prevention ability of scientific research personnel.
5. Conflict and Coordination between Ethics and Law in the Practice of Biomedical Technology
5.1. Analysis of Conflict Points between Ethics and Law
In the complex and rapidly changing field of biomedical science and technology, the intertwine of ethics and law is particularly striking. This kind of conflict is not simply black and white, but involves multiple dimensions and levels of tradeoffs and choices.The first concern is the delicate balance between privacy protection and data sharing. In a data-driven era, biomedical research has an increasingly urgent need for personal information and biological data. These data are not only vital for advancing scientific research, but also have great potential for disease prevention, diagnosis and treatment. The protection of personal privacy should not be ignored. Everyone has the right not to misuse and violate his or her biometric data. How to realize reasonable sharing and utilization of data under the premise of ensuring personal privacy security has become an urgent problem to be solved. This requires better legal frameworks and ethical guidelines to clarify the boundaries and procedures for data collection, storage, use, and sharing. The development of technologies to enhance data security and privacy protection is also key. Through encryption technology, anonymous processing and other means, can reduce the risk of data leakage and abuse to a certain extent. Public education and awareness-raising are equally important. Only when everyone is aware of the importance of personal information and learns how to protect their privacy in their daily lives can a more secure and reliable data sharing environment be built. Another point of conflict that cannot be ignored is the tension between research ethics and commercial interests. The development of biomedical technology is often accompanied by huge commercial interests. This can not only promote the transformation and application of scientific research results, but also may bring considerable economic returns to investors. The involvement of commercial interests can also bring its own set of problems. For example, the impartiality and objectivity of research may be questioned, the rights and interests of subjects may be violated, and the quality and ethical principles of research may even be sacrificed in the pursuit of profit. In order to meet these challenges, it is necessary to establish a sound regulatory mechanism and conflict of interest management system. This includes strict review and supervision of research projects to ensure scientific and ethical research; Disclosure and management of researchers' conflicts of interest to prevent transfer of benefits and improper influence; And the rights and interests of the subjects should be fully protected to ensure that they are treated fairly and compensated reasonably in the process of participating in the research. The challenge between the dignity of life and scientific and technological progress cannot be ignored. The development of biomedical technology has provided unprecedented opportunities and possibilities. For example, the advent of gene editing technology holds the promise of curing some genetic diseases and may even change the future of humanity. These technological advances also raise a series of profound ethical questions. Is there a right to intervene and transform life? Where is the line between such intervention and transformation? How to ensure that scientific and technological progress does not violate the dignity of human life? There are no easy answers to these questions. They require deep reflection and discussion to find a way to balance technological progress with respect for the dignity of life. This may require reexamining and defining the meaning and extension of the dignity of life, as well as the goals and boundaries of scientific and technological progress. It is also necessary to establish better ethical review mechanisms and public participation channels to ensure scientific, fair and transparent scientific and technological decision-making [
18].
5.2. Conflict Resolution Mechanism and Policy
In the field of biomedical science and technology, the conflict and coordination between ethics and law are particularly important and complicated. In order to properly address the challenges in this frontier area, a variety of conflict resolution mechanisms and strategies need to be explored and implemented in depth. This is not just a legislative issue, but also involves the intersection of technology, society, ethics and law.Fundamentally speaking, the perfection of law is the cornerstone to solve this problem. The ethical bottom line and code of conduct in biomedical science and technology research must be established through precise legislation, so that all relevant research activities can be carried out within a clear legal framework. This is not only a constraint on researchers, but also a kind of protection for them, but also a solemn commitment to the public interest of society. The explicit provisions of law can provide a direct and powerful basis for resolving the conflict between ethics and law, so that any behavior that violates ethical principles can be punished by law in time. Laws alone are not enough. The law is often backward, but the development of technology is rapid. This requires the establishment of a flexible and effective ethical review mechanism outside the rigid framework of the law. This mechanism should be independent of governments and scientific institutions, and should be composed of professional ethicists, jurists, and scientific experts, whose responsibility is to conduct strict ethical checks on all biomedical research. In this way, even in areas not yet covered by law, this ethical review mechanism can be relied on to detect and stop possible ethical risks in a timely manner. Of course, ethical education for researchers should not be neglected. Ethical principles are not only a set of norms imposed on researchers from the outside, but also their inner conscious pursuit. It is necessary to strengthen the ethical training of researchers, so that they always adhere to the ethical bottom line in scientific research activities, even in the face of temptation and pressure. Such education can not only reduce the occurrence of ethical and legal conflicts, but also improve the overall quality of researchers and promote the healthy development of biomedical science and technology. Public participation and supervision is also an indispensable part of resolving ethical and legal conflicts. Biomedical science and technology research is closely related to the life of the public, and its research results often have a direct impact on public health and well-being. It is necessary to establish an open and transparent information disclosure platform to let the public know about the progress and risks of relevant research, while also encouraging the public to submit comments and suggestions [
19]. In this way, a pluralistic co-governance structure can be constructed, so that ethical and legal conflicts can be discussed and resolved on a broader level. At the operational level, we also need to focus on international cooperation and exchanges. Biomedical science and technology is a global field, and researchers in all countries are working for the health and well-being of mankind. We should actively participate in international ethical and legal discussions, learn from the successful experiences and practices of other countries, and jointly address ethical and legal challenges. This not only helps to improve their own ethical and legal level, but also helps to promote the common progress of global biomedical science and technology. It is a systematic project to solve the conflict between ethics and law in the field of biomedical science and technology, which needs to form a comprehensive and effective solution strategy from many aspects, such as legislation, ethical review, scientific research ethics education, public participation and international cooperation. Only in safeguarding scientific and technological innovation can we also maintain the ethical order and legal dignity of society. This is not only a challenge for researchers, but also a test for the whole society.
5.3. The Mutual Promotion and Coordinated Development of Ethics and Law
In the vast field of biomedical science and technology, the interweaving of ethics and law constitutes a powerful driving force to promote its development. The two do not exist in isolation, but in constant interaction and dialogue, jointly maintain the boundary and bottom line of scientific research. Ethics, as the cornerstone of human moral judgment, provides a necessary moral guide for the exploration of biomedical science and technology. The law, as the concrete embodiment of social norms, delineates an insurmountable red line for scientific research activities. The interaction of ethics and law in the field of biomedical science and technology reflects a delicate balance. As ethical principles evolve, they are always trying to respond to the moral challenges posed by new technologies to ensure that human values are not eroded by the rapid development of technology. Under the guidance of ethical principles, the law is constantly improved and adjusted to meet the new requirements of scientific and technological development [
20]. This dynamic balancing process not only reflects the moral consciousness of human society, but also demonstrates the far-reaching influence of the spirit of rule of law on the development of science and technology. In the practice of biomedical science and technology, the legalization of ethical principles is a phenomenon that cannot be ignored. With the progress of science and technology, some issues that originally belong to the category of ethics have gradually risen to legal issues, which need to be regulated through the force of law. For example, in cutting-edge technologies such as gene editing and human embryo research, the line between ethics and law has become increasingly blurred, requiring lawmakers, ethicists and researchers to work together to find the right balance. Legal provisions also strengthen and safeguard the implementation of ethical principles. The law provides strong support for the implementation of ethical principles in scientific research activities through clear provisions and strict sanctions. When conducting research, researchers should not only abide by the laws of science, but also abide by the norms of law to ensure that the research results meet the moral expectations of society. The mutual promotion and coordinated development of ethics and law is of great significance to the healthy development of biomedical science and technology [
21]. It helps to solve the ethical conflicts and legal problems that may occur in scientific research activities, and guarantees the compliance and morality of scientific research activities. In addition, it also creates a more relaxed and favorable environment for scientific and technological progress, encouraging researchers to innovate and explore boldly under the premise of following ethical and legal principles.
It is worth noting that the synergy between ethics and law in the field of biomedical science and technology does not happen overnight. It requires constant effort and dialogue on all sides to find its proper place in the ever-changing world of science and technology. Legislators need to pay close attention to the latest developments in science and technology, and timely adjust and improve relevant laws and regulations; Ethicists need to deeply study the moral challenges brought by new technologies to provide strong ethical support for scientific research activities. On the other hand, researchers need to actively promote scientific and technological progress and contribute wisdom and strength to human health and well-being under the premise of following ethical and legal principles. In the future development, we expect to see a closer integration and more effective collaboration between ethics and law in the field of biomedical science and technology. Only in ensuring that scientific and technological progress is in line with human moral values can we fully release the great potential of science and technology and make greater contributions to human health and development. This process will also be a comprehensive test and improvement of human wisdom and morality [
22].
6. Role of Ethics and Law in Promoting the Development of Biomedical Science and Technology
6.1. Guiding and Motivating Role of Ethics and Law
Ethical principles, as the guiding light of biomedical research, always shine on the road of scientific research. It is not only a set of abstract moral rules, but also the bottom line that researchers must adhere to when facing complex scientific problems. Under the guidance of ethics, biomedical research can avoid those pitfalls that may harm human well-being, and ensure that every experimental advance and every technological breakthrough can contribute to human health and happiness. The existence of this legal system provides a solid guarantee for the development of biomedical science and technology [
23]. Through the protection of intellectual property rights, the law ensures that the legitimate rights and interests of innovators are not infringed, thus stimulating the enthusiasm of researchers for innovation. Tax incentives and other policy measures are like showers, moistening the soil of scientific and technological innovation, so that the research in the field of biomedicine can thrive in a more superior environment. The interaction between ethics and law has injected a strong impetus into the development of biomedical science and technology. Under the guidance of ethics, the legal system can play a more precise role to protect scientific and technological innovation; The perfection of laws also provides a strong support for the implementation of ethical principles and ensures the moral sustainability of scientific and technological development [
24]. This synergy can be seen in all areas of biomedical technology. In the process of drug development, ethical principles ensure the welfare of experimental animals and the rights and interests of subjects are not infringed, and laws ensure the safety and effectiveness of drugs through strict approval procedures and monitoring mechanisms. In the field of cutting-edge technology such as gene editing, the double constraints of ethics and law ensure the healthy development of technology and avoid potential risks and abuse. Ethics and law also play an important role in the commercialization of biomedical technologies. In today's increasingly competitive market, some enterprises may ignore the principles of ethics and law in pursuit of profit. It is the existence of ethics and law that reminds these companies of their social responsibility to ensure that their products are not only safe and effective, but also meet the moral expectations of society [
25].
6.2. Normative and Safeguarding Role of Ethics and Law
In the course of the development of biomedical science and technology, ethical norms and legal systems have been accompanied by each other, and they complement and work together to provide indispensable support for the continuous progress and healthy development of this field. Since biomedical science and technology involves sensitive areas such as human life and health, the compliance and ethics of its research activities have always been the focus of social attention. In this context, the role of ethical norms and legal systems is particularly prominent. Ethical norms can be said to be the internal binding force for the development of biomedical science and technology. In the pursuit of scientific breakthroughs, researchers must always adhere to the bottom line of ethics, respect human life and dignity, and safeguard social justice and conscience. These ethics include but are not limited to honesty and trustworthiness, fair competition, respect for intellectual property rights, and protection of subjects' rights and interests. They run through all aspects of scientific research activities to ensure that researchers do not lose their way in the process of exploring the unknown, and always maintain a high sense of responsibility for human well-being [
26]. The legal system provides external compulsory protection for biomedical science and technology. Through legislation, the rights and responsibilities of all parties are clearly defined, and red lines are drawn to ensure that all research activities are carried out within the legal framework. In biomedical research, subjects are usually in a vulnerable position, and their rights and interests are the focus of the legal system. From informed consent to privacy protection, from risk assessment to damage compensation, the legal system provides a series of strict protection measures for subjects. For researchers and institutions that violate laws and regulations, the law also stipulates corresponding punishment measures to ensure the authority and deterrence of the legal system [
27]. It is worth emphasizing that ethical norms and legal systems do not exist in isolation, but are interrelated and influence each other. Ethical norms often provide value orientation and basic principles for the formulation of legal systems. In formulating relevant regulations, legislators will fully consider the requirements and expectations of ethical norms to ensure that the legal system is in harmony with the moral values of society. The legal system also provides a strong guarantee for the implementation of ethical norms. Through the enforcement power and universal binding force of laws, ethical norms can be implemented and enforced in a wider scope. In the actual development of biomedical science and technology, close cooperation and interaction between ethical norms and legal systems can be seen. For example, in cutting-edge research such as gene editing, researchers must conduct experiments and explorations in accordance with ethical norms. The legal system will also track and evaluate these emerging technologies in a timely manner, and formulate corresponding regulations and policies as necessary to ensure their healthy development without violating ethical principles and social interests. Ethical norms and legal systems also play an important role in promoting the transformation and application of biomedical technology. The transformation and application of scientific research results often involve many factors such as commercial interests and social responsibility, and should be carried out under the premise of following ethical norms and legal systems. Through strict ethical review and compliance assessment, it can ensure that the application of scientific and technological achievements meets the requirements of social interests and human well-being; The perfection and implementation of the legal system provides a stable legal environment and reliable legal guarantee for the transformation and application of scientific and technological achievements [
28].
6.3. Promoting and Facilitating Role of Ethics and Law
In the magnificent development process of biomedical science and technology, the role of ethics and law is like a solid rock, which not only provides a solid support for the research and application of this field, but also plays an indispensable role in promoting its continuous progress. Ethical review, as an important gateway to safeguard the sanctity of research, has always stood at the forefront of safeguarding the quality and ethical standards of scientific research. Every research, at the beginning of the launch, must go through a strict ethical review to ensure that its research purposes, methods and results are in line with society's ethical expectations and moral bottom line. This kind of review is not only a powerful constraint on researchers, but also a quality guarantee for the entire scientific research process. It is precisely because of such a tight ethical defense line that the research of biomedical science and technology can win the broad trust of the public, and its research results can be better transformed into practical applications to benefit the general public. The continuous improvement of this legal environment also provides a powerful legal guarantee for the vigorous development of biomedical science and technology. Under the protection of the law, researchers can feel more secure and boldly explore the unknown, without worrying that their research results will be stolen or misused by criminals. With the deepening of international cooperation, law has played an increasingly important role in promoting scientific research exchanges and cooperation between countries. Cooperation between countries under a common ethical and legal framework not only ensures research compliance and mutual trust, but also injects a strong impetus into the global development of biomedical science and technology.
7. Case Study
In the vast field of biomedical science and technology, ethics and law are not only beacons to constrain and guide practice, but also catalysts to promote innovation and development. This is vividly demonstrated in two remarkable practices (Flow chart of the study). Witness a biopharmaceutical company's strict adherence to ethical principles in every step of drug development. The company understands that every study carries with it human expectations for health and life, and that every subject is a light of hope for future treatments. During the design phase of clinical trials, they work closely with ethical review committees to ensure that every research decision is based on the utmost respect for subjects' rights. This is not only for ethical reasons, but also for a deep understanding of science and human nature. Because of this, they have not only harvested satisfactory research and development results, but more importantly, set up a new ethical flag for the entire biomedical industry. The practice of this company fully demonstrates that in the journey of exploration of biomedical science and technology, ethics is not only a stumbling block, but a guiding light leading mankind to a higher realm [
29]. Putting the safety and dignity of subjects in the first place and integrating ethical principles into every link of research can not only reap the fruits of science, but also win the trust and respect of society. Another government has shown great leadership and forward-thinking in the global tide of biomedical technology. They know that the law is a powerful tool for invigorating innovation and fostering international cooperation. Therefore, they are committed to building a set of legal framework that conforms to international norms and conforms to national realities, aiming to provide a solid institutional guarantee for all kinds of cooperation in the field of biomedicine. Under the guidance of this set of laws, enterprises and scientific research institutions at home and abroad have found a bridge and bond for cooperation, and various innovative resources can be efficiently converged and fully collided here. Soon, a series of epoch-making new drugs and technologies came into being in this hot land. This case once again confirms the irreplaceable role of law in the development of biomedical science and technology [
30]. A sound legal environment can not only give full protection and support to innovators, but also become a powerful magnetic field to attract top talents and resources in the world. Under the support of this legal framework, the development of biomedical science and technology is no longer an isolated process, but a global collaboration and win-win.
Figure 1.
Flow chart of the study.
Figure 1.
Flow chart of the study.
8. Conclusions
In the rapid development of biomedical science and technology, new challenges and problems will continue to emerge. But as long as we stick to the bottom line of ethics and constantly improve the legal system, we will certainly be able to go further and more stable on the road of exploring the unknown. Because ethics and law are not only the constraints and guidance of human beings, but also the strength and guarantee. Under their guidance and protection, biomedical science and technology will surely usher in another brilliant tomorrow. It is also hoped that these successful practices can stimulate more thinking and discussion, and promote more new ideas and new methods to promote the development of biomedical science and technology at the intersection of ethics and law. After all, in this era full of challenges and opportunities, only by continuous learning and continuous progress can we go wider and wider on the road of biomedical technology.
References
- hao-dong wang. Edited by gene technology ethics risk legal governance [J]. Journal of jinzhou medical university (social science edition), 2021 (05) : 10-14.
- cantona. Strong pharmaceutical across to "keep the bottom line, and track line" [J]. Chinese health, 2021, (6) : 74-76.
- Geng Wenqian, Qin Dan, Dai Mingjun, et al. Problems and countermeasures of ethical review and regulation of high-risk medical technology: A case study of gene editing technology [J]. Chinese Journal of Medical Ethics, 2019,33(06):695-698.
- Wu Jiashi «, Wu Meiyun, Forensic Science [M]. Sichuan University Press :201307.398.
- Shen Zhixiong, Gao Yangyuxi. Improve national biosafety system and maintain national biosafety [J]. World Knowledge,2020,(10):20-23.
- Sun, J. Introduction to the supervision and management system of medical supplies in Australia. China Pharmaceutical Affairs,1999,(05):76-78. (in Chinese).
- Yuan Jiangfan, Liu Shihui, Zheng Xueqian. Research on the path of constructing harmonious doctor-patient relationship from the legal perspective: Based on the analysis of the case precedent of obstetric medical injury [J/OL]. Medical ethics in China, 2024, (03) : 283-290 [2024-03-29]. http://kns.cnki.net/kcms/detail/61.1203.R.20240321.1508.010.html.
- Shi Jiayou, Xu Jingyi. Medical artificial intelligence application legal challenges and governance [J/OL]. Journal of northwest university (philosophy and social sciences edition), 1-13 [2024-03-29]. [CrossRef]
- Liu Huiming, Pan Junyu. Legal regulation of medical blockchain technology application [J]. Soft Science of Health,2023,37(12):28-33.
- Ge Shuai, Liao Jiazhi, Li Gang, et al. Baseline survey report on legal affairs construction of medical institutions in Hubei Province [J]. Modern Hospital Management,2023,21(05):1-4.
- Lv Yiling. Discussion on relevant conflicts in legal control of medical behavior [J]. Medicine and Law,2023,15(05):20-23. (in Chinese).
- Hu Xinyue, Zhang Hongjiang. Legal dilemma and Relief of minors' medical consent Right [J]. Medicine and Philosophy, 2019,44(15):56-61. (in Chinese).
- Build academic heights of Medical intellectual property to serve pharmaceutical enterprises and medical institutions: Overview of the construction of Medical Law and Intellectual Property Research Center of Shanghai International Intellectual Property College, Tongji University [J]. World Science and technology-Modernization of Traditional Chinese Medicine,2023,25(07):2237-2240.
- Ren Guozheng. The Theory, System and Reconstruction Plan of Trust in Smart Medical Law [C]// Shanghai Law Society. Shanghai Law Research, Vol. 5, 2023 - Proceedings of the Rule of Law Forum of the 2023 World Artificial Intelligence Conference. International Institute of Green Finance, Central University of Finance and Economics; Laboratory of Health Finance, Central University of Finance and Economics;, 2023:10.
- Xie Yan. Cosmetic damage compensation applicable law issues research [D]. Jiangsu university, 2023. [CrossRef]
- Zhao Hengzhe. Revision and supplement of the legal theory of excessive medical tort: Based on 111 judgment documents [J]. Journal of jinzhou medical university (social science edition), 2022, 20 (5) : 14-19.
- Zheng Qiushi. Expert consensus on legal Issues related to end-stage medical decision-making [J]. Chinese Journal of Medical Ethics,2022,35(09):931-932. (in Chinese).
- Li Guilian. Research on the Application of Medical Insurance Law [C]// Intelligent Learning and Innovation Research Committee of China Intelligent Engineering Research Society. Proceedings of the 2022 Social Development Forum (Guiyang Forum) (1). Medical Security Bureau of LAN County, Luliang City, Shanxi Province;, 2022:3.
- Zhao Wei. Medical Algorithm Discrimination and its legal Regulation in Digital Age [J]. Shanghai Law Research,2022,7(01):177-199. (in Chinese).
- Zhao Hong. Biomedical domain gene patent monopoly law regulation [D]. The northern industrial university, 2022. [CrossRef]
- Gong Yimei. The guardian medical decisions by legal system research [D]. Yunnan university of finance and economics, 2022. [CrossRef]
- Zhangsheng Sun. A Study on the Relationship of Macroeconomic Regulation and Its Legal Adjustments. LNEP (2023) Vol. 26:149-157. [CrossRef]
- Kalyuzhny, Rostislav, Olena Makeieva, and Lyudmila Shapenko. "Biomedical ethics and human rights in the context of innovation and information development of society." Journal of History Culture and Art Research 9.1 (2020): 96-106. [CrossRef]
- Zhangsheng Sun, Research on anti-monopoly and unfair competition from the perspective of administrative law. Science of Law Journal (2023), Vol. 2:22-30. [CrossRef]
- Jongsma, Karin R., and Annelien L. Bredenoord. "Ethics parallel research: an approach for (early) ethical guidance of biomedical innovation." BMC Medical Ethics 21.1 (2020): 81. [CrossRef]
- Zhangsheng Sun* and Xinyan Li. Explore the Legal Challenges and Coping Strategies for the Development of New Biomedical Technologies. Res Dev Material Sci., 20 (1). RDMS. 000976. 2024. [CrossRef]
- Bahadur, Sher. "Biomedical Technologies, Eugenics and Human Cloning: Public Health Law and Legal Issues in Health Practice." Journal of Rehman Medical Institute 3.3-4 (2017): 01-04.
- Beauchamp, Tom L. "Morality and the social control of biomedical technology." New knowledge in the biomedical sciences: Some moral implications of its acquisition, possession, and use. Dordrecht: Springer Netherlands, 1982. 55-76. [CrossRef]
- Haldar, Bijayesh, Bishnanand Dubey, and Durg Vijay Rai. "Biomedical Ethics and Legal Perspectives." Ethics in Biology, Engineering and Medicine: An International Journal 5.1 (2014).
- Fox, Renee C. "Advanced medical technology-social and ethical implications." Annual Review of Sociology 2.1 (1976): 231-268. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).