Submitted:
05 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Extracts Obtained from O. ficus-indica
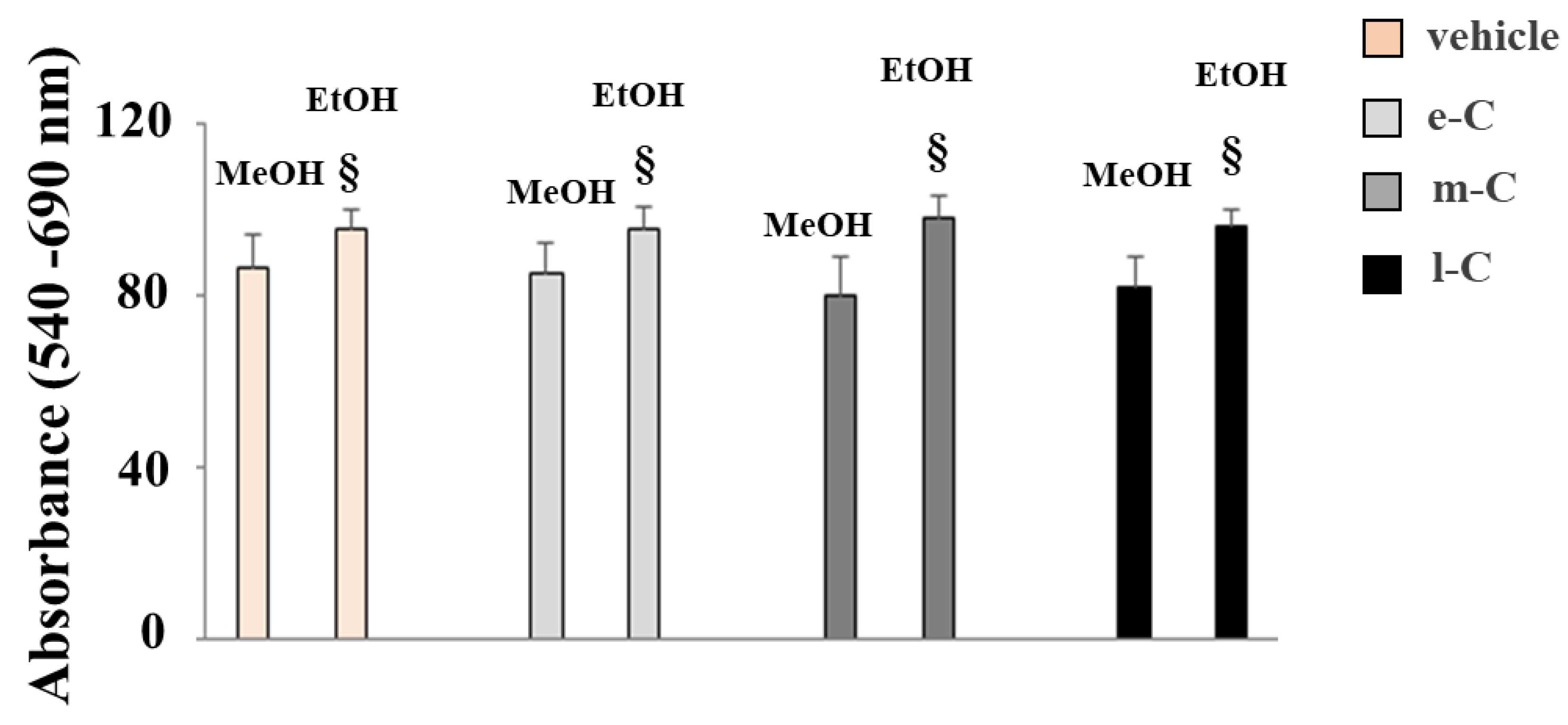
2.2. Use of Ethanol Instead of Methanol
2.3. Antioxidant Activity
2.3.1. ORAC Test
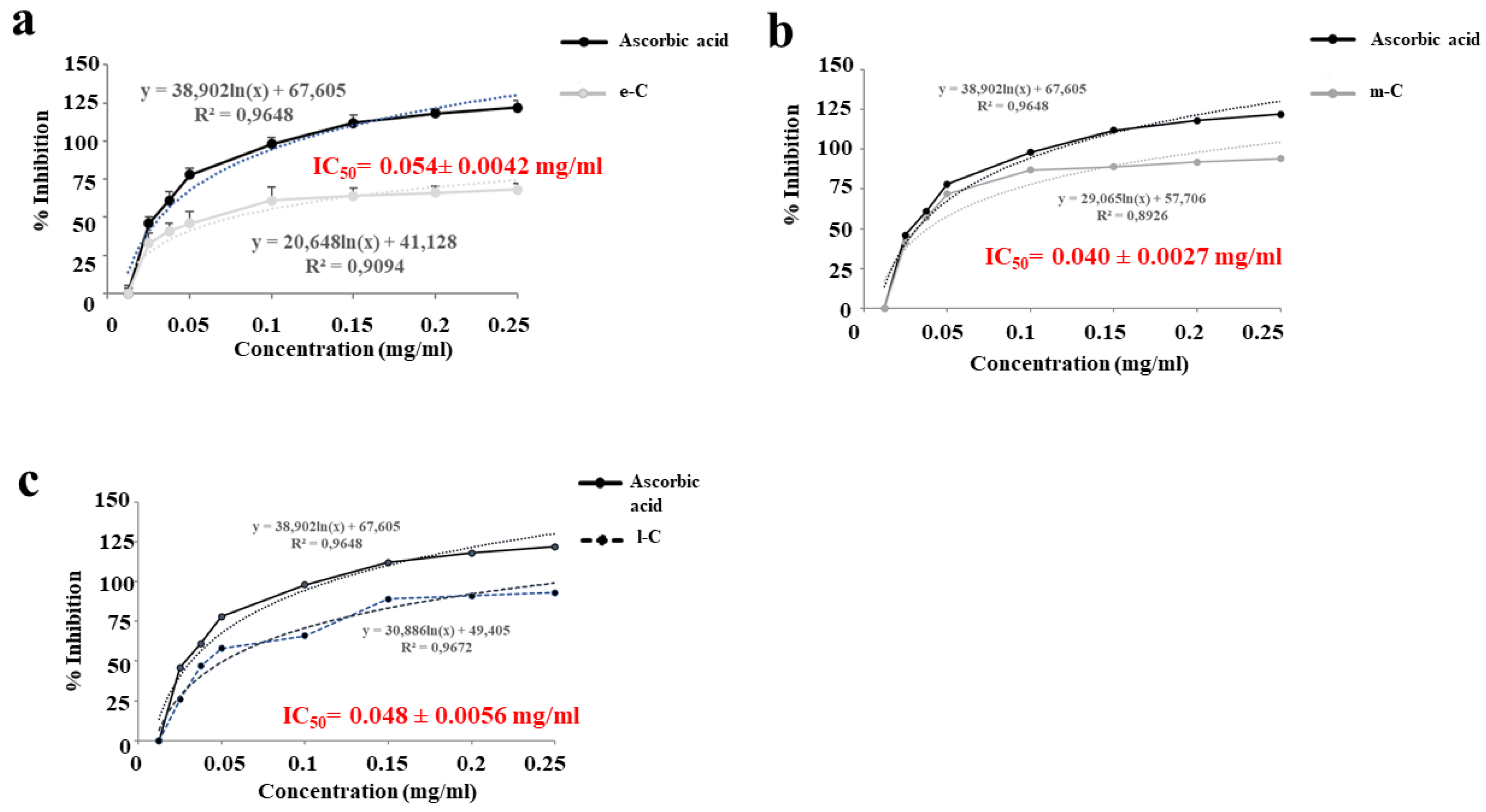
2.3.2. DPPH Test
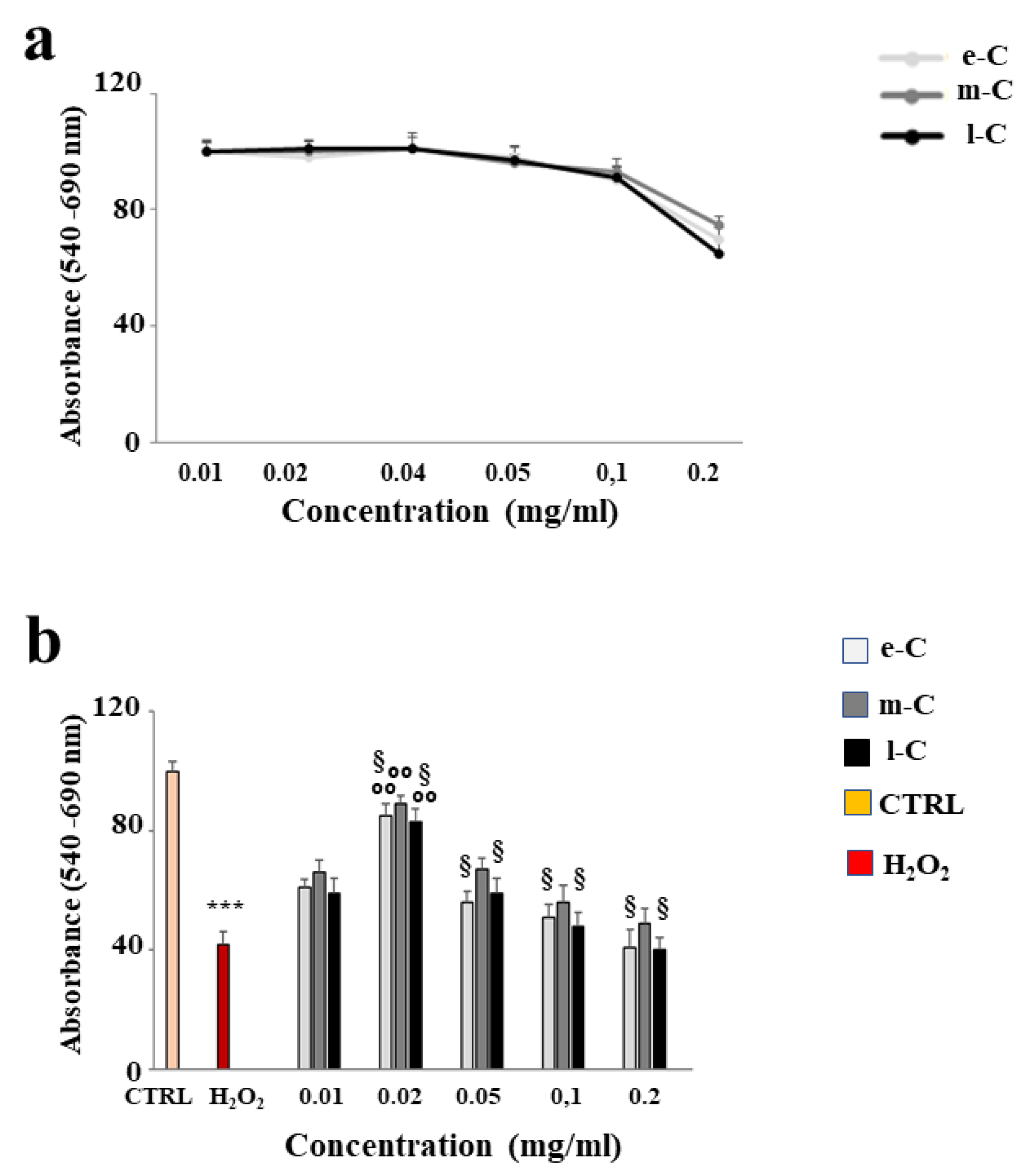
2.4. Results on Cell Viability
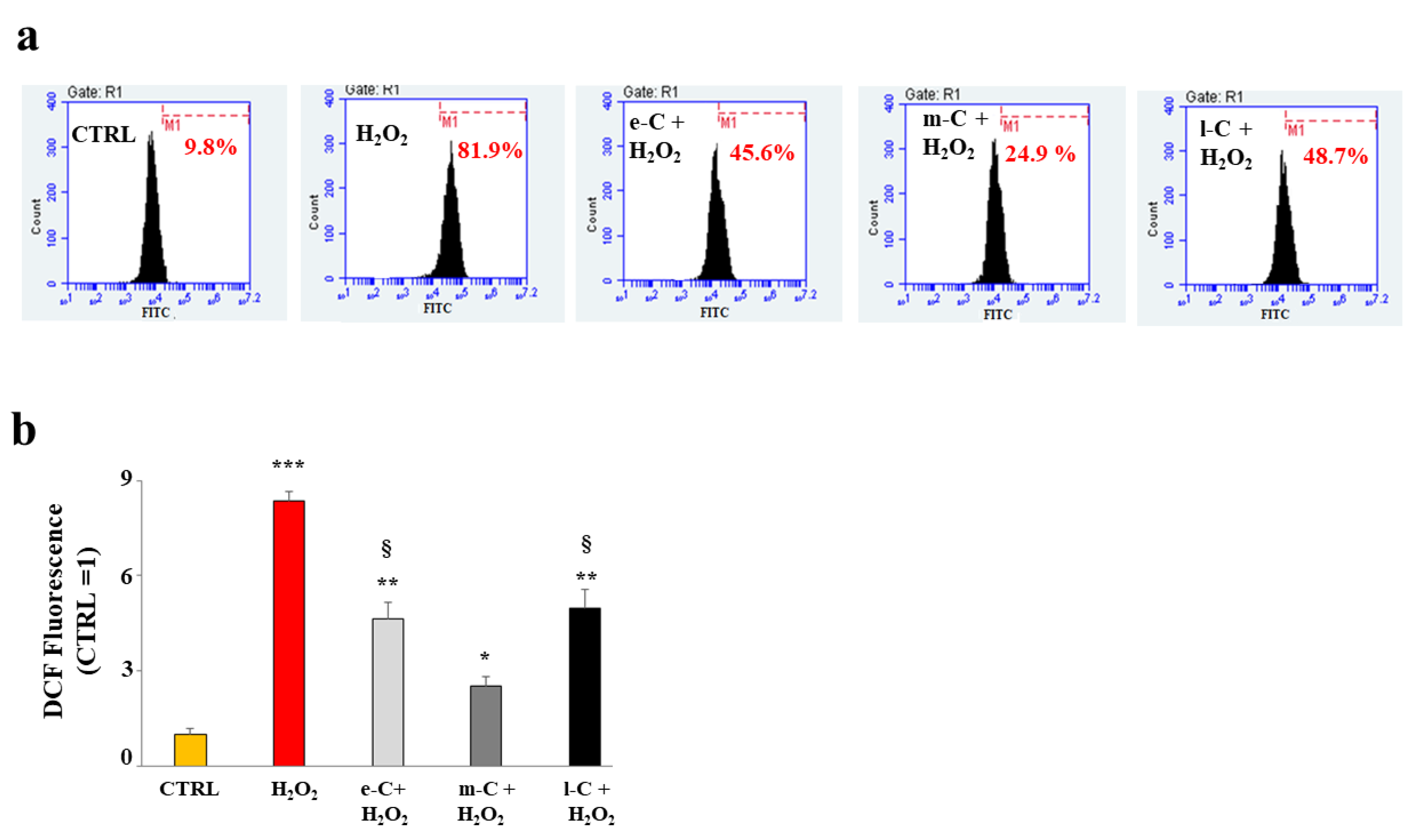
2.5. O. ficus-indica Extracts Reduce the Accumulation of ROS in the Huvec Cell Line
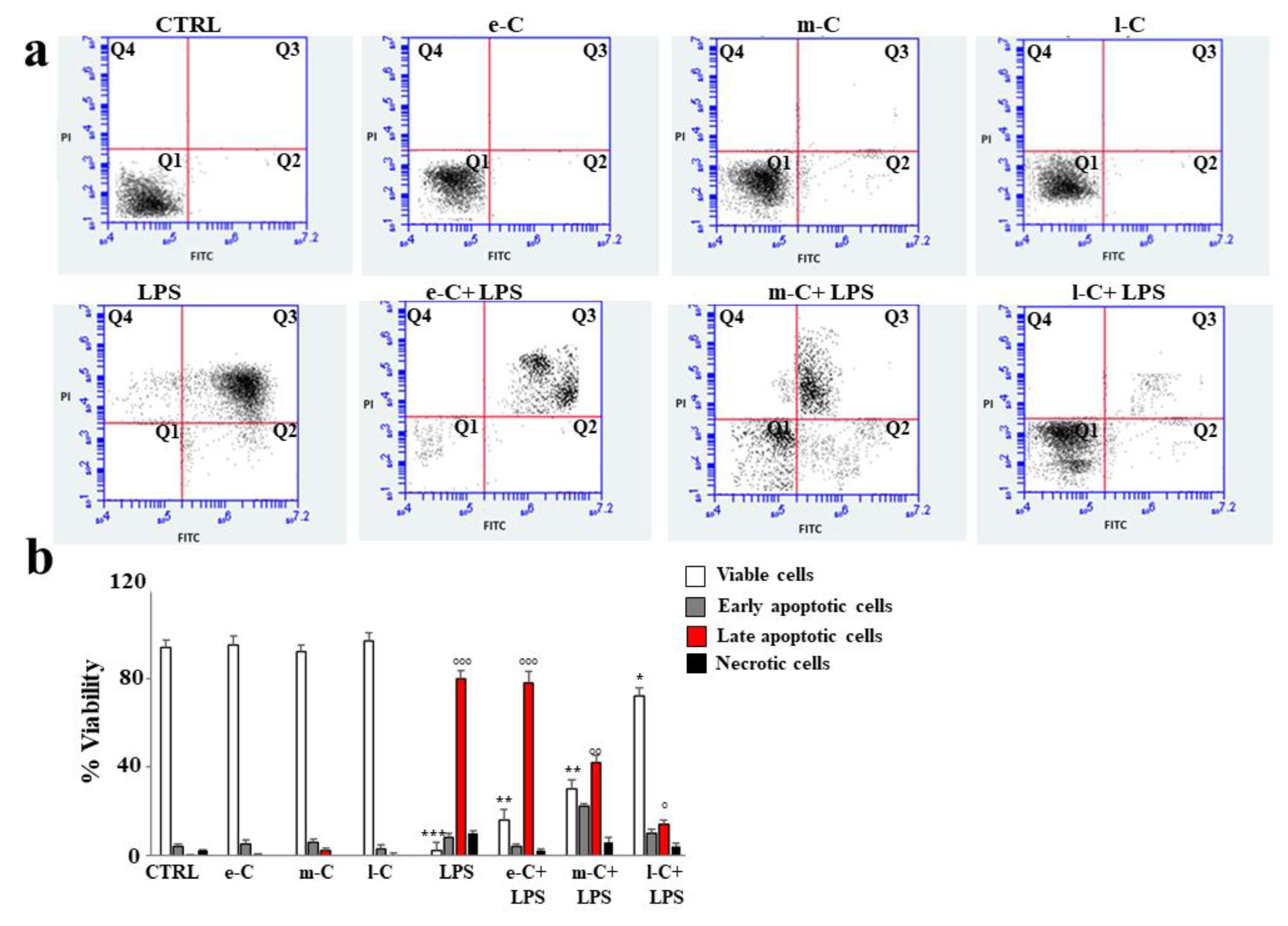
2.6. O. ficus-indica Extracts and Apoptosis
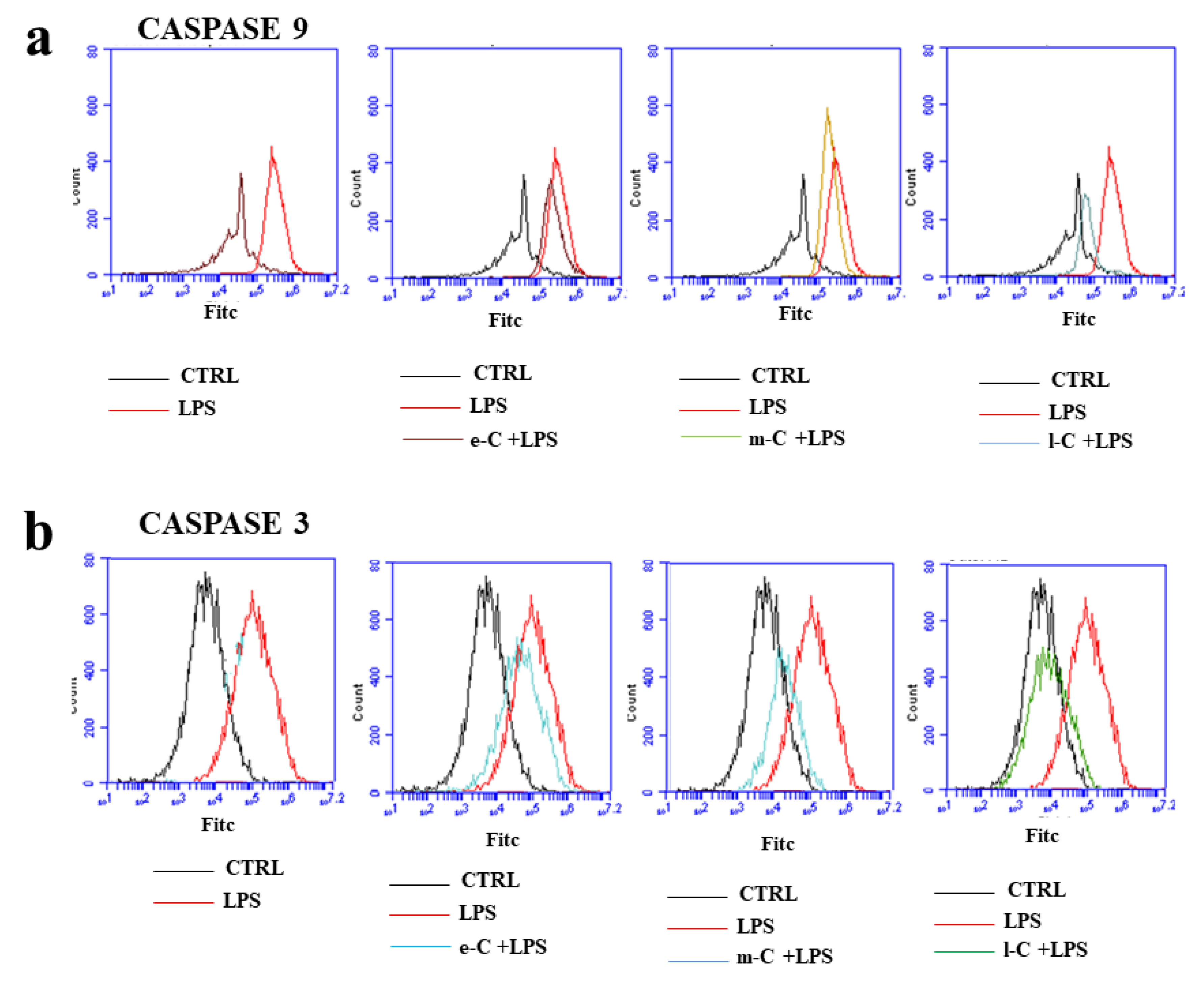
2.7. O. ficus-indica Extracts and Caspases 9 and 3
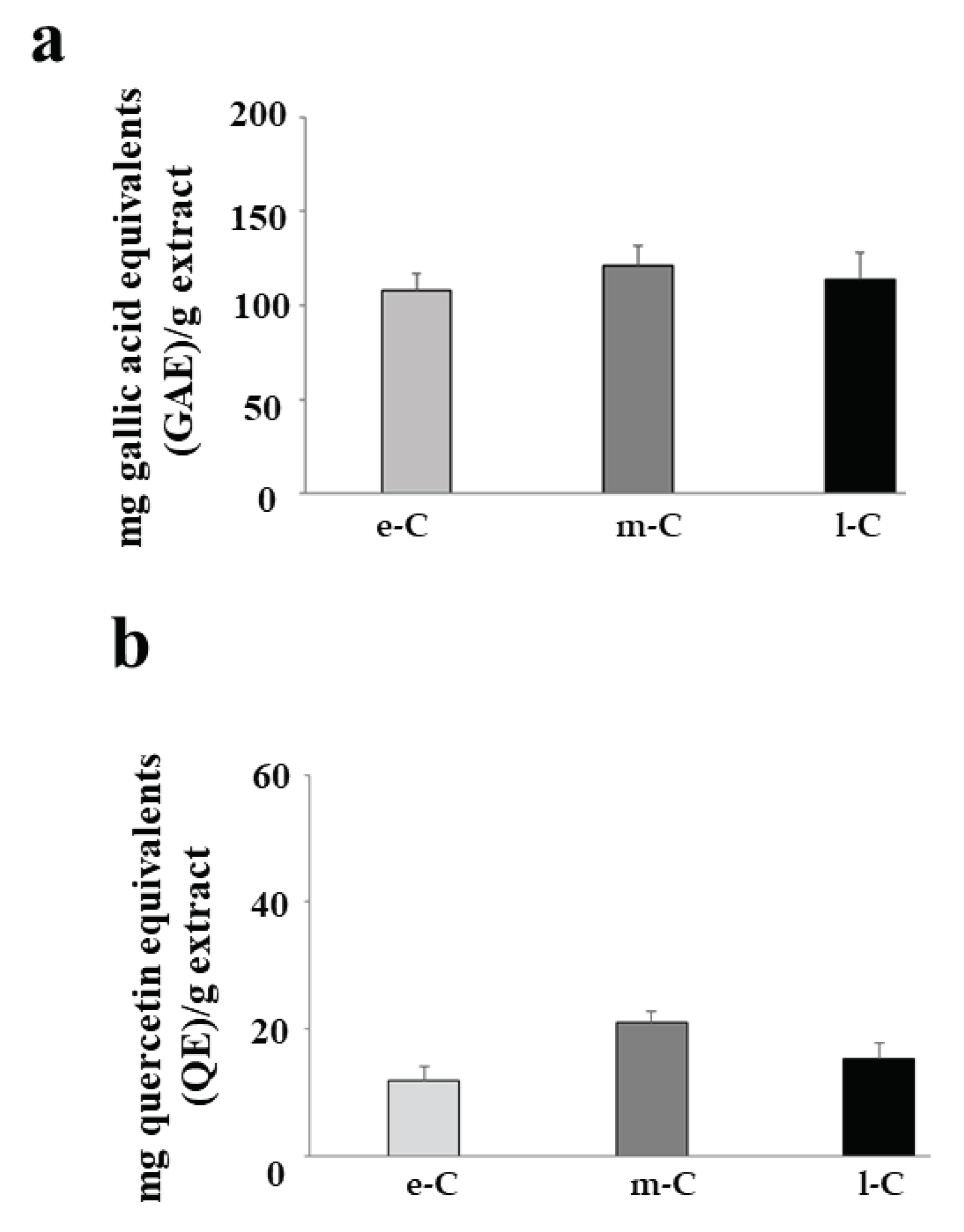
2.8. Determination of Total Phenolic and Flavonoid Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Preparation
4.2. Antioxidant Activity
4.2.1. The ORAC Assay
4.2.2. DPPH Test
4.3. Cell Cultures
4.4. Proliferation Assay
4.5. ROS Measurement in the Huvec Cell Line
4.6. Annexin V/PI Staining
4.7. Measurement of Protein Expression through Immuno-Cytofluorometry
4.8. Determination of Total Phenolic and Flavonoid Content
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pimienta-Barrios, E. Prickly pear (Opuntia spp.): a valuable fruit crop for the semiarid land of Mexico. J Arid Environ 1994, 28, 1–11. [Google Scholar] [CrossRef]
- de Albuquerque, J.G.; de Souza Aquino, J.; de Albuquerque, J.G.; de Farias, T.G.S.; Escalona-Buendía, H.B.; Bosquez-Molina, E.; Azoubel, P.M. Consumer perception and use of nopal (Opuntia ficus-indica): A cross-cultural study between Mexico and Brazil. Food Res Int. 2019, 124, 101–108. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, H.I.; Elemlegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus indica) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]
- Piga, A. Cactus Pear: A fruit of nutraceutical and functional importance. J. Prof. Assoc. Cactus Dev. 2004, 6, 9–22. [Google Scholar]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Natural product research 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Baldino, N.; Ceramella, J.; Iacopetta, D.; Scali, E.; Basile, G.; Saturnino, C.; Catalano, A. Opuntia ficus indica (L.) Mill. An Ancient Plant Source of Nutraceuticals. Curr Top Med Chem. 2022, 22, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Perucini-Avendaño, M.; Nicolás-García, M.; Jiménez-Martínez, C.; de Jesús Perea-Flores, M.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and structural properties, biological activity, and polyphenols profile. Food Sci Nutr 2021, 9, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications. A review. International Journal of Food Science and Technology, 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- López-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vázquez-Manjarrez, N.; Tovar, A. R.; Pedraza-Chaverri, J.; Torres, N. The effect of nopal (Opuntia ficus indica) on postprandial blood glucose, incretins, and antioxidant activity in Mexican patients with type 2 diabetes after consumption of two different composition breakfasts. Journal of the Academy of Nutrition and Dietetics 2014, 114, 1811–1818. [Google Scholar] [CrossRef]
- Avila-Nava, A.; Calderón-Oliver, M.; Medina-Campos, O.N.; Zou, T.; Gu, L.; Torres, N.; Tovar, A.R.; Pedraza-Chaverri, J. Extract of cactus (Opuntia ficus indica) cladodes scavenges reactive oxygen species in vitro and enhances plasma antioxidant capacity in humans. Journal of Functional Foods 2014, 10, 13–24. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, H.; Lou, H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Salzano, S.; Checconi, P.; Hanschmann, E-M. ; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S. et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A 2014, 111, 12157–12162. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaeidh, N.; Bouoni, Z.; Sakly, M.; Rhouma, K.B. Ameliorative effect of Opuntia ficus indica juice on ethanol-induced oxidative stress in rat erythrocytes. Experimental and Toxicologic Pathology 2013, 65, 391–396. [Google Scholar] [CrossRef]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg Med Chem Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An overview and update on the chemical composition and potential health benefits of Opuntia ficus-indica (L.) Miller. J Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J Biol Sci 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Jordan, J.S.; Linder, R.; Woods, E.T.; Sun, X.; Kemme, N.; Manning, K.C.; Cherry, B.R.; Yarger, J.L.; Majure, L.C. Microscale Mechanism of Age Dependent Wetting Properties of Prickly Pear Cacti (Opuntia). Langmuir 2016, 32, 9335–9341. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; Gutiérrez-Cortez, E.; Del Real, A.; Rojas-Molina, A.; Rodríguez-García, M.; Rubio, E.; Quintero-García, M.; Rojas-Molina, I. Bone Mineral Density, Mechanical, Microstructural Properties and Mineral Content of the Femur in Growing Rats Fed with Cactus Opuntia ficus indica (L.) Mill. (Cactaceae) Cladodes as Calcium Source in Diet. Nutrients 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Mounir, B.; Younes, E.G.; Asmaa, M.; Abdeljalil, Z.; Abdellah, A. Physico-chemical changes in cladodes of Opuntia ficus-indica as a function of the growth stage and harvesting areas. J Plant Physiol. 2020, 251, 153196. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, R.; Serra, M.; Nucera, S.; Ruga, S.; Oppedisano, F.; Scarano, F.; Macrì, R.; Muscoli, C.; Palma, E.; Musolino, V.; Statti, G.; Mollace, V.-; Maiuolo, J. Antioxidant Activity and Seasonal Variations in the Composition of Insoluble Fiber from the Cladodes of Opuntia ficus-indica (L.) Miller: Development of New Extraction Procedures to Improve Fiber Yield. Plants 2024, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Kotikova, K.; Klepis, P.; Ridzon, P.; Hlusicka, J.; Navratil, T.; Rulisek, J.; Zak, I.; Zakharov, S. Peripheral polyneuropathy after acute methanol poisoning: Six-year prospective cohort study. Neurotoxicology. 2020, 79, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Toxicology and the biological role of methanol and ethanol: Current view. Biomed Pap. Med Fac Univ Palacky Olomouc Czech Repub 2016, 160, 54–63. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Chemical composition, antioxidant, anti-inflammatory, and cytotoxic activities of Opuntia stricta cladodes. PLoS One. 2019, 14, e0209682. [Google Scholar] [CrossRef] [PubMed]
- Perucini-Avendaño, M.; Perea-Flores, M.J.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and structural properties, biological activity, and polyphenols profile. Food Sci Nutr. 2021, 9, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Banaszczak, E.; Radzikowska, D.; Ratajczak, K. Chemical profile and antioxidant activity of Trollius europaeus under the influence of feeding aphids. Open Life Sci. 2018, 13, 312–318. [Google Scholar] [CrossRef]
- Vanacker, H.; Guichard, M.; Bohrer, A.-S.; Issakidis-Bourguet, E. Redox Regulation of Monodehydroascorbate Reductase by Thioredoxin y in Plastids Revealed in the Context of Water Stress. Antioxidants 2018, 7, 183. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int J Mol Sci 2020, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Fejér, J.; Gruľová, D.; Eliašová, A.; Kron, I. Seasonal Variability of Juniperus communis L. Berry Ethanol Extracts: 2. In Vitro Ferric Reducing Ability of Plasma (FRAP) Assay. Molecules. 2022, 27, 9027. [Google Scholar] [CrossRef]
- Scioneaux, A.N.; Schmidt, M.A.; Moore, M.A.; Lindroth, R.L.; Wooley, S.C.; Hagerman, A.E. Qualitative variation in proanthocyanidin composition of Populus species and hybrids: genetics is the key. J Chem Ecol 2011 37, 57–70. [CrossRef]
- Murai, Y.; Setoguchi, H.; Ono, E.; Iwashina, T. Flavonoids and their qualitative variation in Calystegia soldanella and related species (Convolvulaceae). Nat Prod Commun 2015, 10, 429–432. [Google Scholar] [CrossRef]
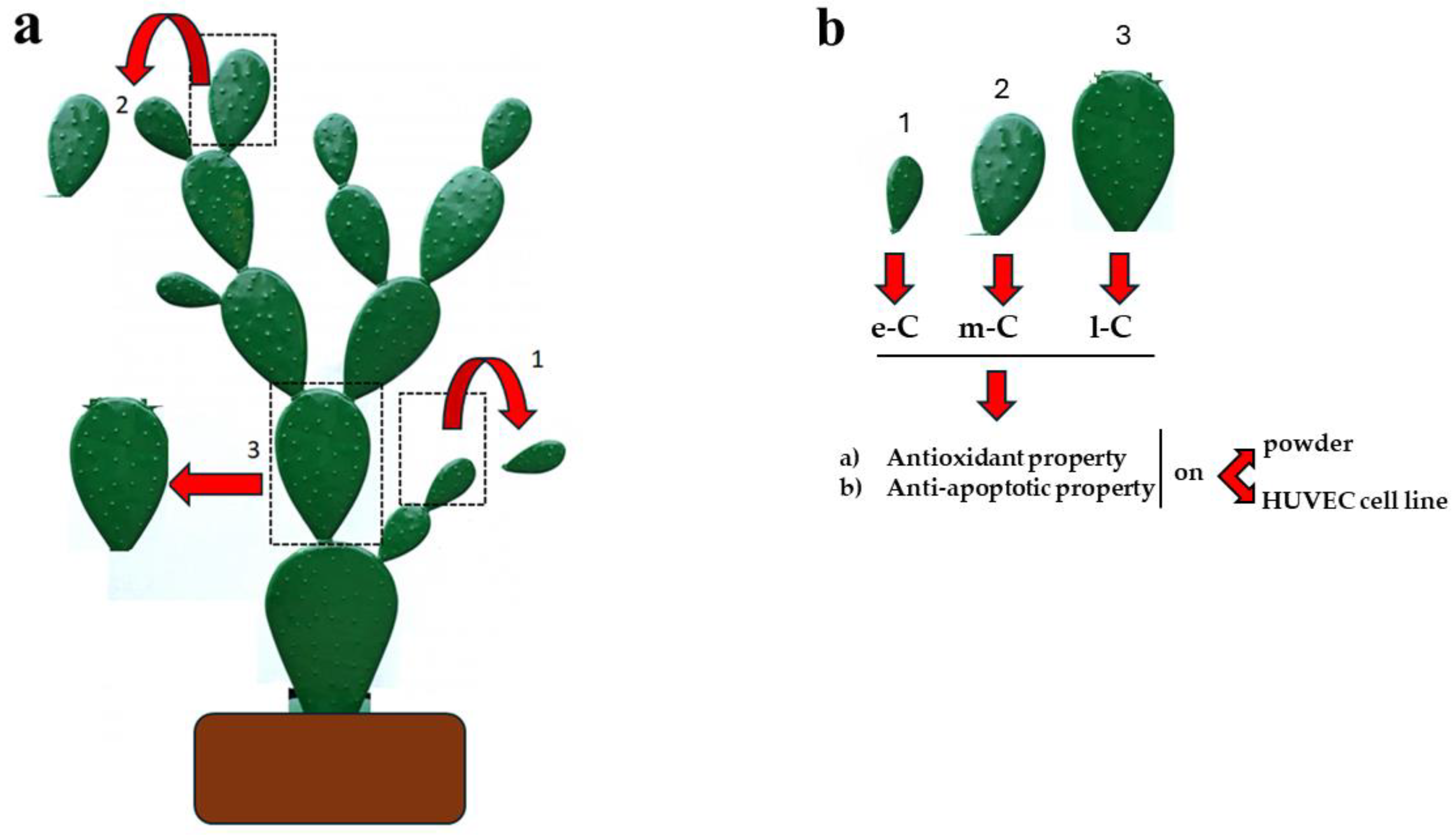

| Early cladodes | Medium cladodes | Late cladodes | |
|---|---|---|---|
| Days | 40 | 100 | 150 |
| Grams | 150-220 | 280 | 415 |
| cm | 3-10 cm | 11-25cm | 26-50 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





