1. Introduction
Cyanobacteria, phototrophic prokaryotes that perform oxygenic photosynthesis, are the main contributors to marine primary production [
1] and have a very important ecological impact on global carbon, nitrogen and oxygen cycles. They have evolved sophisticated systems to maintain the homeostasis of carbon/nitrogen assimilation (reviewed by [
2,
3]), the two most abundant elements in all living forms. Cyanobacteria can use different nitrogen sources that are first converted into ammonium and then incorporated into amino acids and other N-containing compounds via the glutamine synthetase-glutamate synthase (GS-GOGAT) pathway using 2-oxoglutarate (2-OG) as a carbon skeleton [
4,
5].
In bacteria and plants 2-OG, a universal indicator of the intracellular carbon-to-nitrogen balance [
6,
7] is sensed by the widely distributed and highly conserved signal transduction protein PII [
8,
9]. Homotrimeric PII proteins are encoded by two paralog genes (
glnB and
glnK) in enterobacteria and by just one representative (
glnB) in cyanobacteria and plants. PII regulates the activity of proteins involved in nitrogen and carbon metabolism by direct protein-protein interactions, perceiving metabolic information through the competitive binding of ATP or ADP and the synergistic binding of ATP and 2-OG [
10,
11,
12]. Despite the remarkable structural conservation of PII proteins across phyla, unique PII targets are found in cyanobacteria.
The yeast two-hybrid system (Y2H), based on fusion proteins that must co-localise to the yeast nucleus to reconstitute the transcriptional activity of GAL4 [
13], have been shown to closely reflect functional interactions mediated by enterobacterial [
14,
15,
16,
17] or cyanobacterial [
18,
19,
20,
21,
22,
23,
24,
25,
26] PII proteins. The first PII receptors identified in cyanobacteria were detected in a Y2H search for proteins of the unicellular strain
Synechococcus elongatus PCC7942 (hereafter
S. elongatus) interacting with PII:
N-Acetyl Glutamate Kinase (NAGK), that form complexes with PII in cyanobacteria and plants [
18,
27,
28] and PipX (
PII
interacting
protein
X), a small protein of 89 amino acids restricted to cyanobacteria [
18,
21,
29,
30,
31]. PipX is composed [
26] of an N-terminal TLD/KOW domain [
32] and a C-terminal domain of two alpha-helices, the first one of which contains a basic arginine-rich patch, whose function remains enigmatic [
33].
PipX was also found as prey in yeast two-hybrid searches with the global transcriptional regulator NtcA, which is involved in nitrogen assimilation in cyanobacteria [
34,
35,
36]. PipX provides a mechanistic link between PII signalling and gene expression in response to nitrogen limitation [
37,
38]. The PipX–NtcA complex consists of one active (2-OG bound) NtcA dimer and two PipX molecules. Each NtcA subunit binds one PipX molecule. PipX stabilizes the conformation of NtcA that is transcriptionally active and probably helps local recruitment of RNA polymerase. PipX uses the same surface of its TLD/KOW domain to bind to either 2-OG-bound NtcA, stimulating DNA binding and transcriptional activity, or to 2-OG-free PII. PII sequestration of PipX at low 2-OG renders PipX unavailable for NtcA binding and activation, reducing the expression of NtcA-dependent gene targets [
19,
20,
24,
26,
39,
40]. In addition, the interaction between PII and PipX is highly sensitive to fluctuations in the ATP/ADP ratio, and thus the energy state of the cells [
41].
The bacterial two-hybrid system (BACTH), a genetic approach to protein-protein interaction (PPI) based on the reconstitution in
E. coli of the adenylate cyclase of
Bordetella pertussis from its T18 and T25 domains fused to the proteins of interest [
42] has been successfully used to identify components and unravel molecular details of interactions networks involved in cell division or heterocyst patterning in cyanobacteria [
43,
44,
45,
46]. We recently used the BACTH system to prove interactions between PipX and the ribosome-assembly GTPase EngA, two proteins whose interactions in cyanobacteria were previously inferred by synteny and
in vivo approaches [
47,
48]. It is worth noting that while BACTH assays gave robust interactions signals between PipX and EngA [
49], the Y2H system did not. However, false negatives are common in both types of two-hybrid systems and often depend on the pair of proteins assayed, although the reasons behind this fact are not always obvious. Since the binding of a given partner to PipX in
S. elongatus depends on the levels of the effectors favouring the corresponding complexes, we wondered whether the BACTH system is a more appropriated choice than the Y2H to investigate cyanobacterial regulatory networks.
The aim of this work was to gain additional insights into the cyanobacterial nitrogen interaction network and on the advantages and limitations of the two most popular hybrid systems. PipX mutations previously analysed by Y2H assays or in other contexts were now analysed with the BACTH system for self-interactions or cross-interactions with NtcA or PII, its two best known partners, and with GlnB or GlnK, the E. coli homologs of PII. Interactions reported as negative in the Y2H system as well as indirect interactions dependent on host proteins were detected, speaking in favour of a greater biological complexity for the BACTH assays in the present context. Complementary Western assays with relevant PipX variants supported the main inferences.
2. Results and Discussion
2.1. Interactions involving PipX-PII and PipX-NtcA
Multiple factors can affect the results of two-hybrid assays, including the possible occlusion of interaction determinants by the domains added to the tested proteins, or, importantly, biological factors differing between the systems. Thus, comparing results from Y2H and BACTH systems may provide complementary information on promiscuous proteins such as PipX. For instances, we recently found, using multiple PipX/PII or PipX/NtcA pairs of BACTH-based fusion proteins [
49], that while PipX interactions with PII are almost as easy to detect in BACTH as in Y2H assays, interactions between PipX and NtcA were better detected in the BACTH system [
20,
24,
49], perhaps due to lower levels of 2-OG in the yeast nucleus. To get additional insights into the PipX interaction network while comparing outcomes from BACTH and Y2H assays, we performed BACTH assays with representative PipX point mutations previously analysed by Y2H [
20,
24,
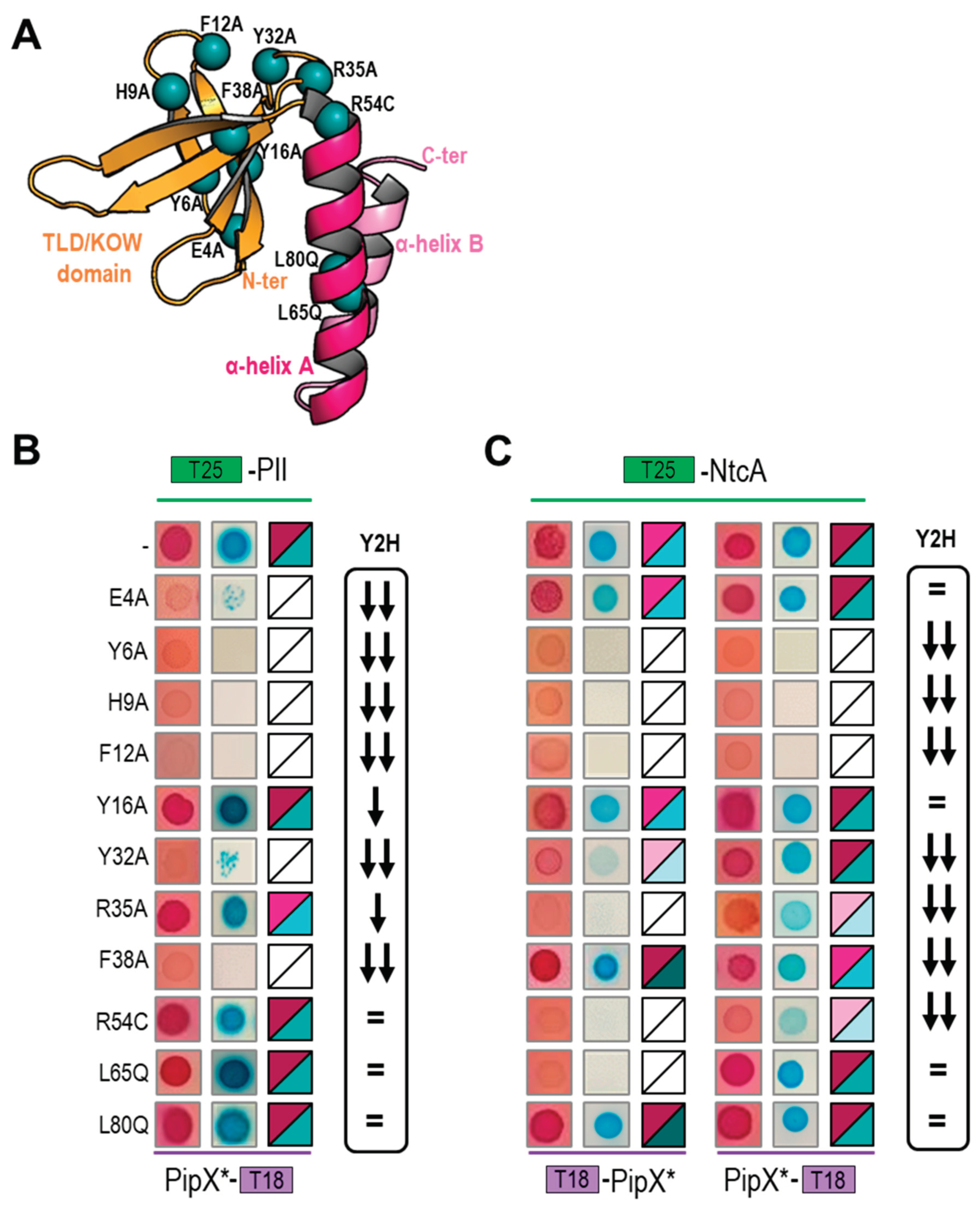
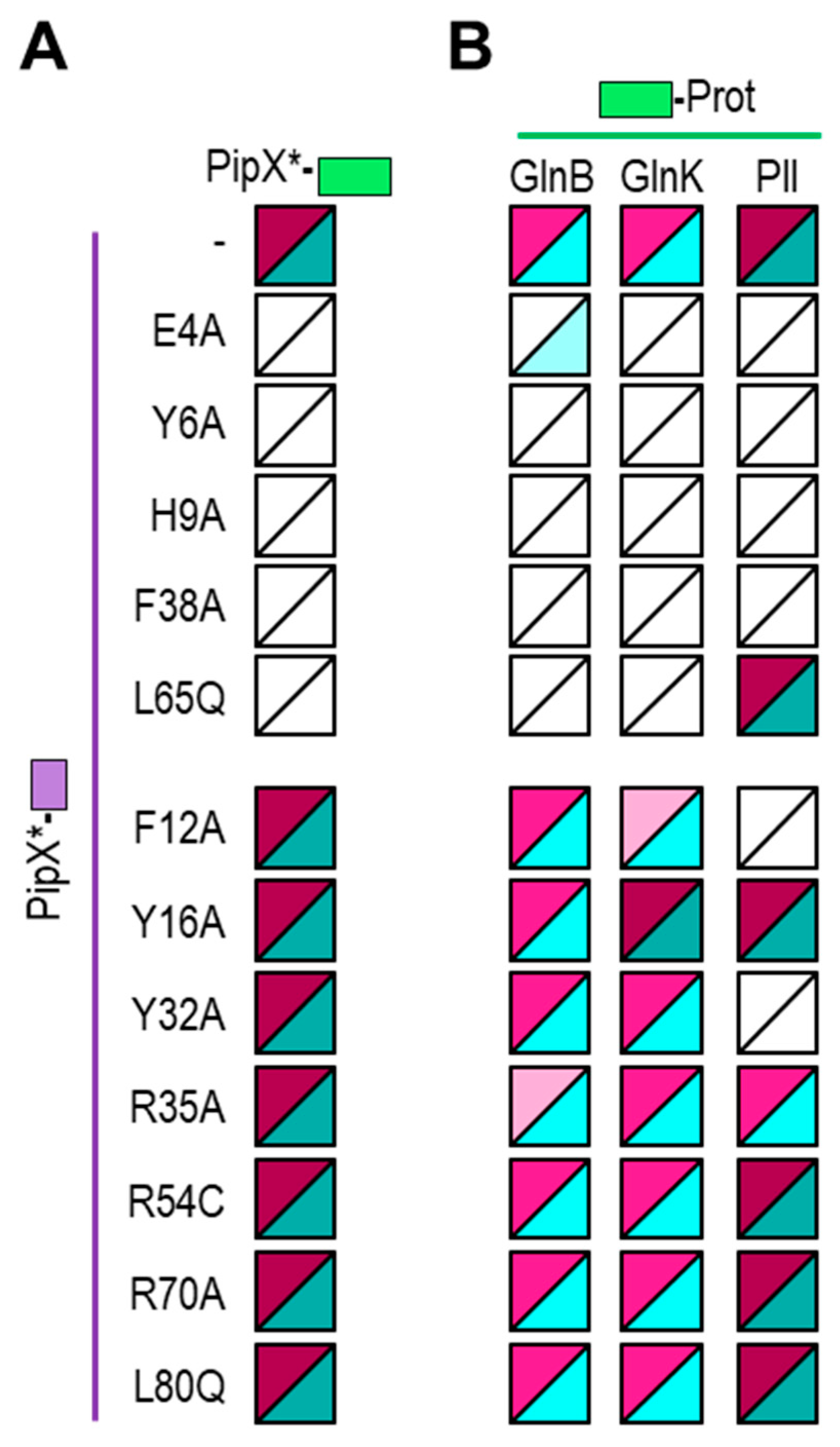
50] The positions of the PipX residues discussed in this work are illustrated in
Figure 1A.
To carry out BACTH assays we first introduced the point mutations of interest into PipX-T18 or T18-PipX constructs to generate the corresponding PipX*-T18 or T18-PipX* derivatives (
Table 1 and
Table S2). PipX*-T18 derivatives were tested against T25-PII or T25-NtcA while T18-PipX* derivatives were only tested against T25-NtcA, since the weak interaction signal produced by the control pair (T18-PipX/T25-PII; [
49]) makes this combination hardly informative. To discriminate amongst different levels of interaction signals with reasonable confidence on plate assays, three independent pools of five or six clones each from two independent transformations were tested in parallel on two different media for each assay. According to the colour (red or blue depending on the indicator media) intensity signals were visually classified into 5 categories ranging from “no-interaction” to “very strong”, exactly as described [
49]. Note that while the strength of the signal is not a direct measure of protein affinity, ranking the different levels of signals from equivalent fusion proteins does allow comparison of protein variants in the BACTH system, assuming that these are equally expressed.
The results of the BACTH analysis are illustrated in Figures 1B and 1C with representative photographs and heatmaps summarising the corresponding information for PipX*/PII and PipX*/NtcA pairs, respectively. For comparison, the impact of the same mutations on Y2H signals corresponding to the same pairs [
20,
24,
26,
50] is represented to the right. For simplicity, Y2H signals are classified into just 3 categories to denote the impact of mutations on the strength of the signal: no effect, significant effect, or very drastic effect.
Highly concordant results from the two types of assays were obtained for most mutations, particularly for PipX*/PII pairs. The concordance was very clear for the most drastic mutations, that is, those abolishing or significantly impairing signals with both partners (Y6A, H9A, F12A), or just with PII (E4A) or with NtcA (R54C) in Y2H assays. Concordance was also observed for two control mutation (Y16A, L80Q) that had no effects on the PipX-PII or PipX-NtcA interactions tested.
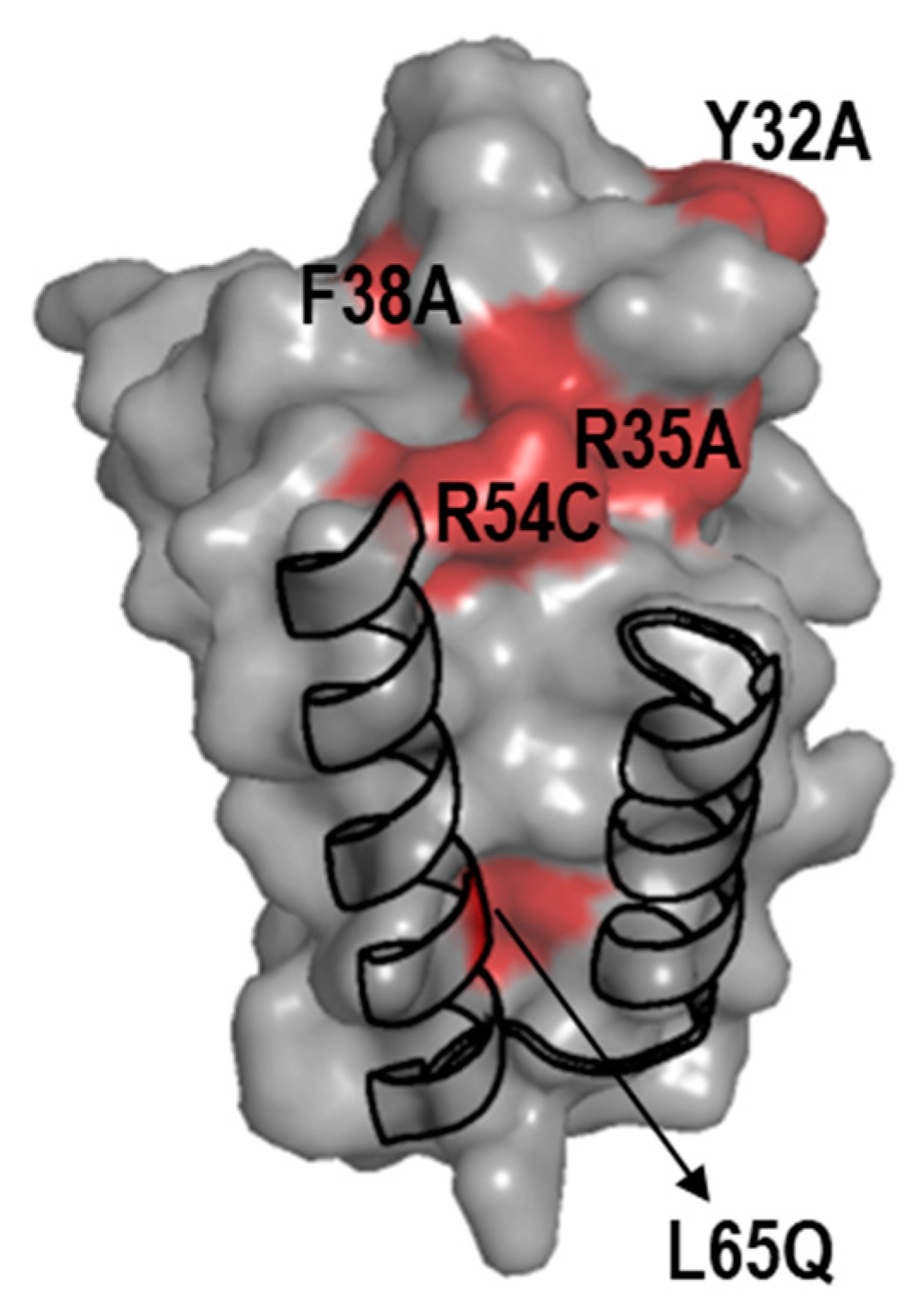
PipX*/NtcA comparisons resulted in discrepancies for several residues whose location on the PipX surface is illustrated in
Figure 2.
Relatively weak but significant BACTH signals with NtcA were produced by variants (Y32A, R35A, F38A and R54C) that gave no signals in Y2H analysis [
20,
24,
26], suggesting that the previous Y2H assays overestimated the impact of mutations on interactions with NtcA. Importantly, the results with those four PipX variants, showing their ability to interact with NtcA in the BACTH system, is easily reconciled with phenotypes observed in
S. elongatus, where mutations R35A, F38A or R54C did not abolish NtcA coactivation and mutation Y32A increased it [
20,
24,
37].
On the other hand, L65Q, a mutation having no impact in the Y2H system, decreased BACTH interaction signals specifically with NtcA. Since L65 is outside the TLD/KOW domain providing the contacts with NtcA, the reason for the rather drastic effect of L65Q on interactions with NtcA in the BACTH system is unclear. However, L65Q was identified as a spontaneous mutation suppressing PipX toxicity and has been recently shown to decrease PipX levels in
S. elongatus [
20,
55], which suggests reduced formation of
in vivo complexes between PipX
L65Q and PII. Thus, interaction or functional assays indicated that the L65Q mutation did have phenotypic consequences that differed for PipX-PII and PipX-NtcA complexes. Our interpretation of the discrepancies between the different assays is that unknown bacterial factors, absent in Y2H assays, are differentially affecting PipX-PII and PipX-NtcA complexes in the corresponding
in vivo assays. In this context, it is tempting to propose that PipX-NtcA complexes may be stabilised in
E. coli by a relatively abundant factor and that the C-terminal helices are involved in that interaction. Based on recent structural data on complexes between NtcA and RNApol from
Anabaena that do not include PipX [
56], it is tempting to propose that an RNApol subunit may provide such interacting partner in
E. coli.
In summary, the BACTH assays performed here in the context of PipX interactions with PII or NtcA support the usefulness of both PPI methods for mutational analyses, further expanding the inferences and conclusions derived from previous Y2H studies. For PipX-NtcA complexes the BACTH approach appears to be the most informative of the two approaches.
2.2. PipX interacts with E. coli GlnB and GlnK proteins in the BACTH system
The
E. coli cytoplasm apparently provides a more biologically informative context than the yeast nucleus for cyanobacterial proteins, but it would also provide a comparatively more complex environment due to the higher abundance of more closely related proteins and thus of potential interacting partners of the protein(s) being assayed. Since those bacterial proteins may affect the BACTH interactions by either competing for binding or by acting as a bridge, they would respectively contribute to false negatives or false positives. It is worth noting that while in the Y2H system false positives are common due to the “stickiness” of the activation domain of GAL4, this does not happen in the BACTH system, where there are no reports of false positives [
57,
58]. Because of this, interactions giving positive in the BACTH system must be considered informative and worth investigated even if they cannot be confirmed by
in vitro assays.
An intriguing “false positive” obtained by us in previous BACTH assays was the strong self-interaction of PipX [
49], given that gel filtration assays [
26,
50] and lack of self-interaction in Y2H analyses [
21,
26,
50] indicated that PipX is monomeric. However, PipX self-interaction was observed in yeast three-hybrid (Y3H) assays when PII was used as a bridge protein [
26] and we reasoned that the
E. coli proteins GlnB and GlnK, by providing a bridge, could be responsible for the PipX self-interaction observed in
E. coli. However, Y2H assays did not support interactions between PipX and
E. coli GlnB or GlnK [
21] and thus the mechanism behind PipX-self interactions in the BACTH system remained enigmatic.
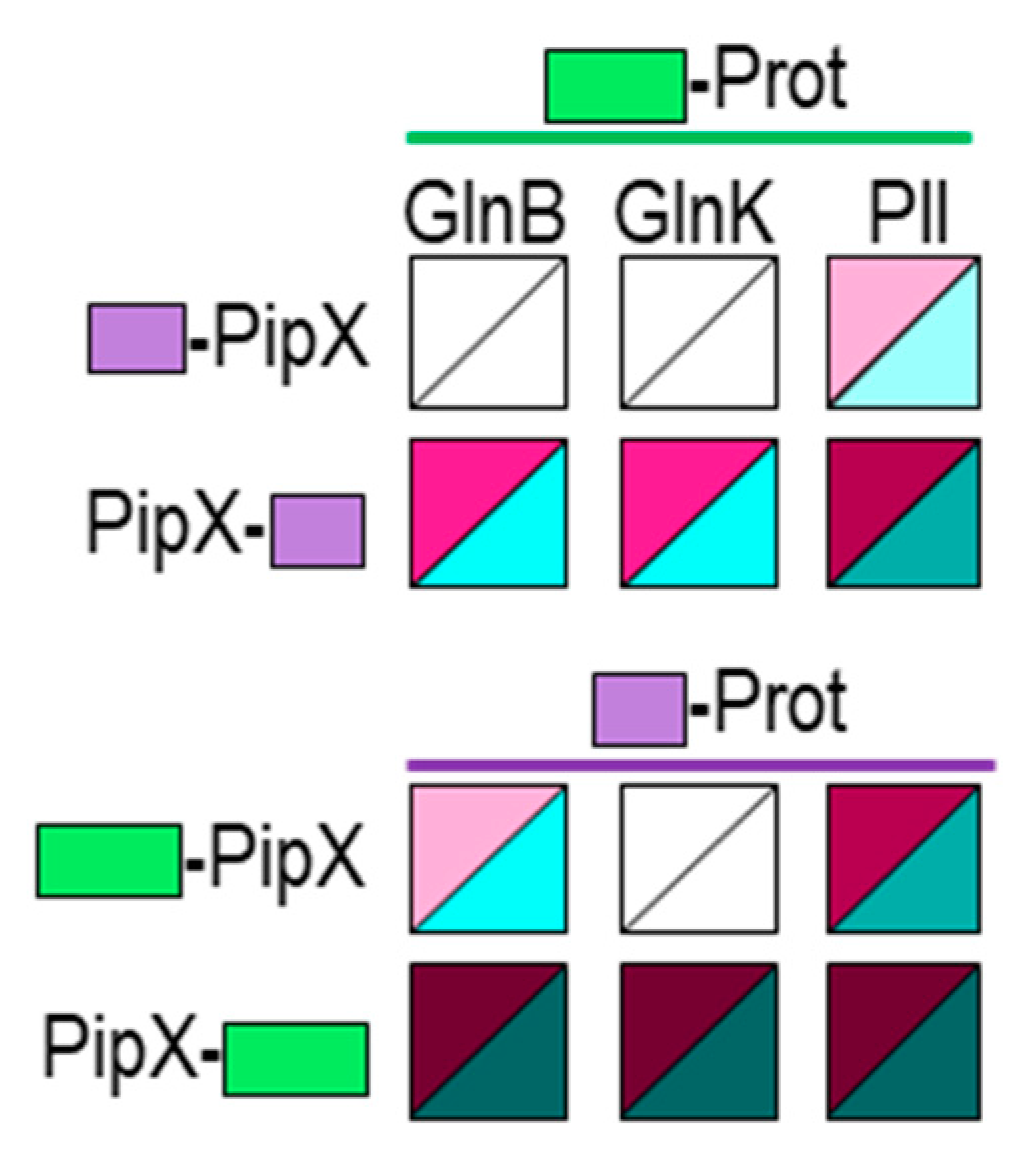
To investigate whether PipX-self interaction is facilitated by E. coli GlnB and/or GlnK proteins in BACTH assays we first determined whether we could detect cross-interaction between PipX and GlnB and/or GlnK proteins in the BACTH system. To this end, we produced N-terminal fusions of each of the T18 and T25 domains to GlnB or GlnK to test interactions between PipX and GlnB and/or GlnK against the corresponding PipX derivatives. For comparison S. elongatus PII fusions were also tested in parallel, giving a total of 12 pairs of fusion derivatives to be assayed for cross-interactions.
As shown in
Figure 3 and S1, and in close agreement with previous results [
49], PII/PipX pairs gave positive in all combinations tested (4/4). In addition, and at odds with previous Y2H assays [
21], positive results were also obtained for some of the GlnB/PipX (3/4) or GlnK/PipX (2/4) pairs, indicating that PipX have affinity for each of these two
E. coli proteins. Since the levels of the interaction signals for GlnB/PipX or GlnK/PipX positive pairs were always lower than for PII/PipX pairs, altogether the results indicate that the affinity of PipX for the
E. coli proteins may be significantly lower than for
S. elongatus PII.
It is worth noting that the intensity of the interaction signals and the levels to which protein fusions accumulate are mutually interdependent in the BACTH system, due to the positive role of cAMP in the expression of the fusion proteins [
57]. This implies that initial differences of expression between fusion proteins will be magnified when interacting pairs reconstitute the adenylate cyclase activity. Therefore, differences in expression between the studied proteins due to differences in codon usage and/or stability between the yeast and
E. coli hosts would contribute to the discrepancies between the two systems. In this context, it appears reasonable that expression of the particularly abundant proteins GlnB and GlnK would be highly optimized in
E. coli, resulting in higher levels of BACTH fusion derivatives in comparison with PII derivatives, in turn overestimating the strength of the GlnB-PipX or GlnK-PipX interactions.
2.3. Self and cross-interactions involving PII, GlnB and GlnK proteins, differences between two-hybrid systems
An additional complication for our BACTH interaction assays is the possibility of heterotrimerization between the endogenous host proteins GlnB or GlnK and
S. elongatus PII derivatives. Heterotrimerisation between
S. elongatus PII and GlnB or GlnK, first suggested from
in vivo studies [
59], has been demonstrated by
in vitro [
60] and Y2H assays [
18]. While it is expected that heterotrimerization with the endogenous proteins could interfere with the self-interactions from each of the three homologs in the BACTH system, the impact of interference would be greater for the less abundant fusion protein, which is likely to be
S. elongatus PII.
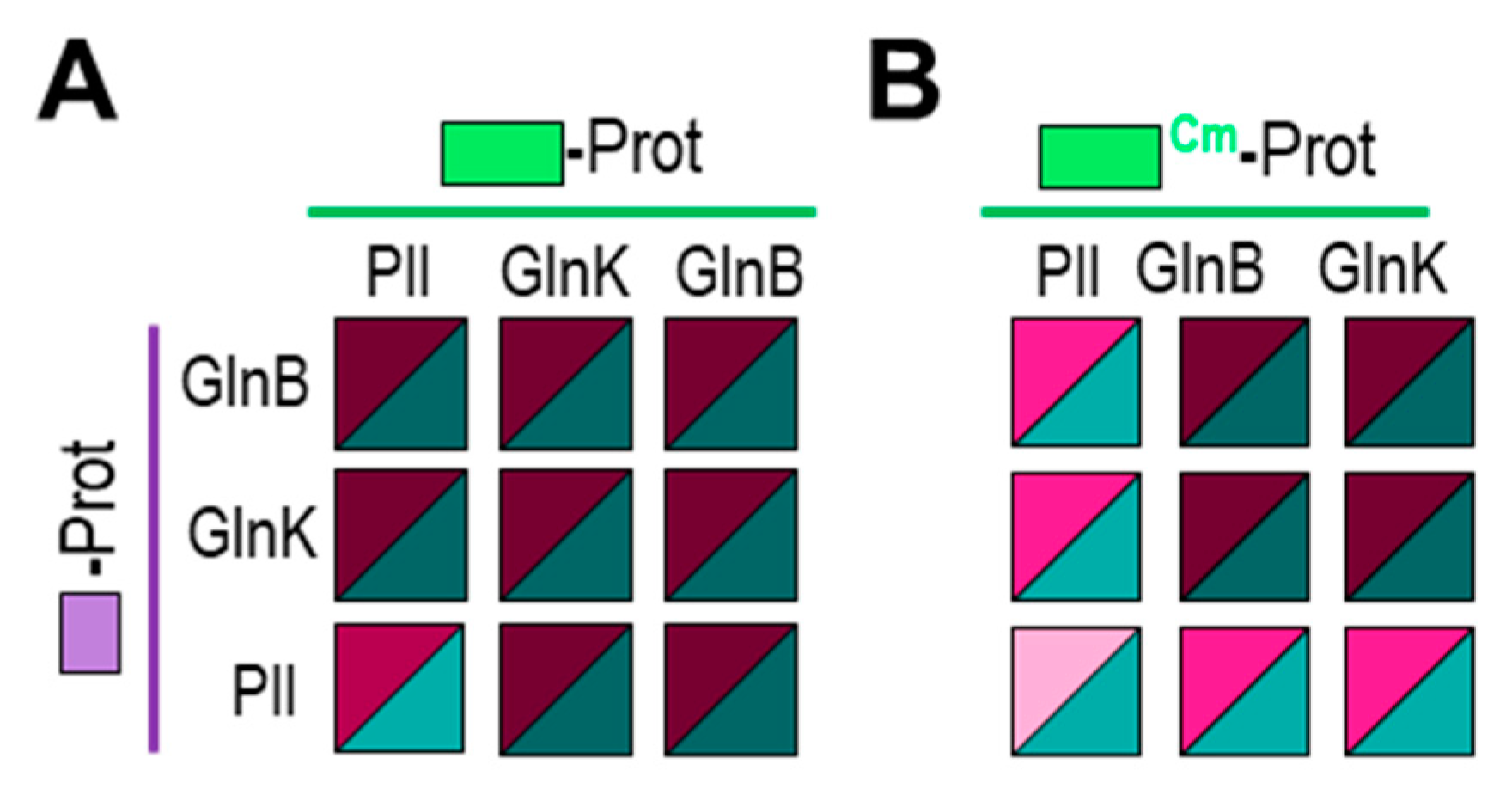
To explore this issue, we first compared interactions involving the three proteins in all 9 compatible pair combinations (
Figure 4A and S1). As expected, all of them were positive, in line with their ability to form homo and heterotrimers. In addition, the weakest signals corresponded to PII self-interaction. This is at odds with previous Y2H assays, that gave very strong self-interaction signals for PII, signals that were in fact stronger than self-interaction signals for GlnB or GlnK and stronger than cross-interactions between PII and GlnB or GlnK [
18,
21]. These discrepancies are in complete agreement with the idea that BACTH interaction signals reflect comparatively lower levels of expression of
S. elongatus PII derivatives, rather than differences in binding affinities, that are not expected to be lower for PII/PII than for PII/GlnB or PII/GlnK interacting pairs.
2.4. PII gives weaker interaction signals than GlnB and GlnK proteins in the BACTH system
Detection of relatively small differences between interacting pairs appear to be prevented by the saturation of the signal, presumably due to the very high levels of expression obtained for GlnB or GlnK derivatives. To test this idea, we next expressed T25-GlnB or T25-GlnK fusion proteins from an essentially identical BACTH vector from which fusion proteins are less efficiently expressed. For simplicity, we refer to the corresponding protein fusions as T25
Cm-GlnB or T25
Cm-GlnK, making reference to the selection marker (Cm instead of Km, see Methods and
Table 1).
Still robust, but significantly weaker signals were obtained for PII/GlnB, PII/GlnK and to a greater extent for PII/PII pairs when T25
Cm-PII, T25
Cm-GlnB or T25
Cm-GlnK were involved (
Figure 4B and S1), further supporting our inference of
S. elongatus PII derivatives being expressed at lower levels in the BACTH system. On the other hand, signals were still maximal for either self-interactions or cross-interaction mediated by GlnB and/or GlnK, a result in line with the comparatively higher levels of the
E. coli fusion proteins in the system.
Differences in codon usage or susceptibility to E. coli proteases may contribute to the comparatively low levels of expression of S. elongatus PII, and presumably of the other cyanobacterial proteins analysed in this work. The implication is that when assaying proteins from phylogenetically distant bacteria weak interaction signals and false negative would still be compatible with strong binding affinity between the corresponding proteins in their natural environment.
2.5. Mutations impairing PipX self-interactions also impair binding to GlnK or GlnB in E. coli
BACTH analysis with deletions eliminating the C-terminal domain or just the last α-helix (helix B) of PipX indicated that the TLD/KOW domain and the helix A are required for maximal self-interaction signals [
49]. Since we hypothesised that self-interaction could be facilitated by PipX trimerization over GlnB and/or GlnK in
E. coli, it was important to determine whether determinants for PipX self-interactions coincided with PipX determinants for binding to GlnB and/or GlnK. To this end PipX*-T25 derivatives (Tables 1 and S2) were next tested against their cognate PipX*-T18 derivatives to determine the impact of each of the PipX point mutations on PipX self-interaction. The results of self-interaction assays for each of the mutant versions are summarised in
Figure 5A and S2.
5 out of the 12 tested point mutations impaired interaction signals and all except one (L65Q at helix A) of the mutations impairing self-interaction also target the N-terminal domain, thus supporting the importance of the TLD/KOW domain and helix A of PipX for self-interaction.
Once we had identified residues whose mutations are either neutral or impair PipX self-interaction in
E. coli, we next determined whether these PipX mutations have similar effects on interactions with GlnB or GlnK. To this end we tested the set of PipX*-T18 derivatives against T25-GlnB or T25-GlnK (
Figure 5B and S2). To facilitate discussion and comparisons, we grouped separately results corresponding to mutations impairing (top) or not (botton) self-interactions and include the heatmaps previously obtained with PipX*/PII at the right.
The mutations impairing PipX self-interactions could be classed into two interaction patterns: those impairing signals in all three cases (E4A, Y6A, H9A, F38A) and those impairing signals just with GlnB and GlnK (L65Q). Importantly, none of them were neutral or increased interaction signals with GlnB. On the other hand, the mutations having no effect on self-interactions (F12A, Y16A, Y32A, R35A, R54C, R70A, L80Q) did not abolish signals with either GlnB or GlnK. One of them (R35A) slightly decreased interaction signals with GlnB while another one (Y16A) slightly increased them with GlnK. Last but not least, two of the neutral mutations in the context of PipX self-interactions (F12A, Y32A) impaired signals just with PII.
Therefore, the results strongly support the hypothesis that PipX self-interaction in the BACTH assays is due to presence of GlnB and/or GlnK proteins and call attention to the ability of the system to detect indirect interactions, particularly from abundant host proteins such GlnB or GlnK.
2.6. Mutations impairing PipX binding to GlnK or GlnB in E. coli also impair PipX levels in E. coli
Because PipX mutations impairing binding to PII also impair PipX in levels
S. elongatus [
24,
55], it was relevant to know whether GlnB and/or GlnK proteins can perform the same chaperon role in
E. coli, in which case the mutations affecting binding to GlnB and/or GlnK and PipX self-interaction should also impair protein levels.
To provide independent evidence of the link between PipX self-interaction and binding to GlnB and/or GlnK proteins, we next analysed the impact of selected
pipX mutations on PipX levels in
E. coli BTH101, the host for BACTH assays. To strengthen the analysis with additional mutations while testing the effect of co-expression of the downstream gene
pipY [
54,
61] in PipX levels in
E. coli we used previously generated shuttle plasmids (
Table 1) encoding
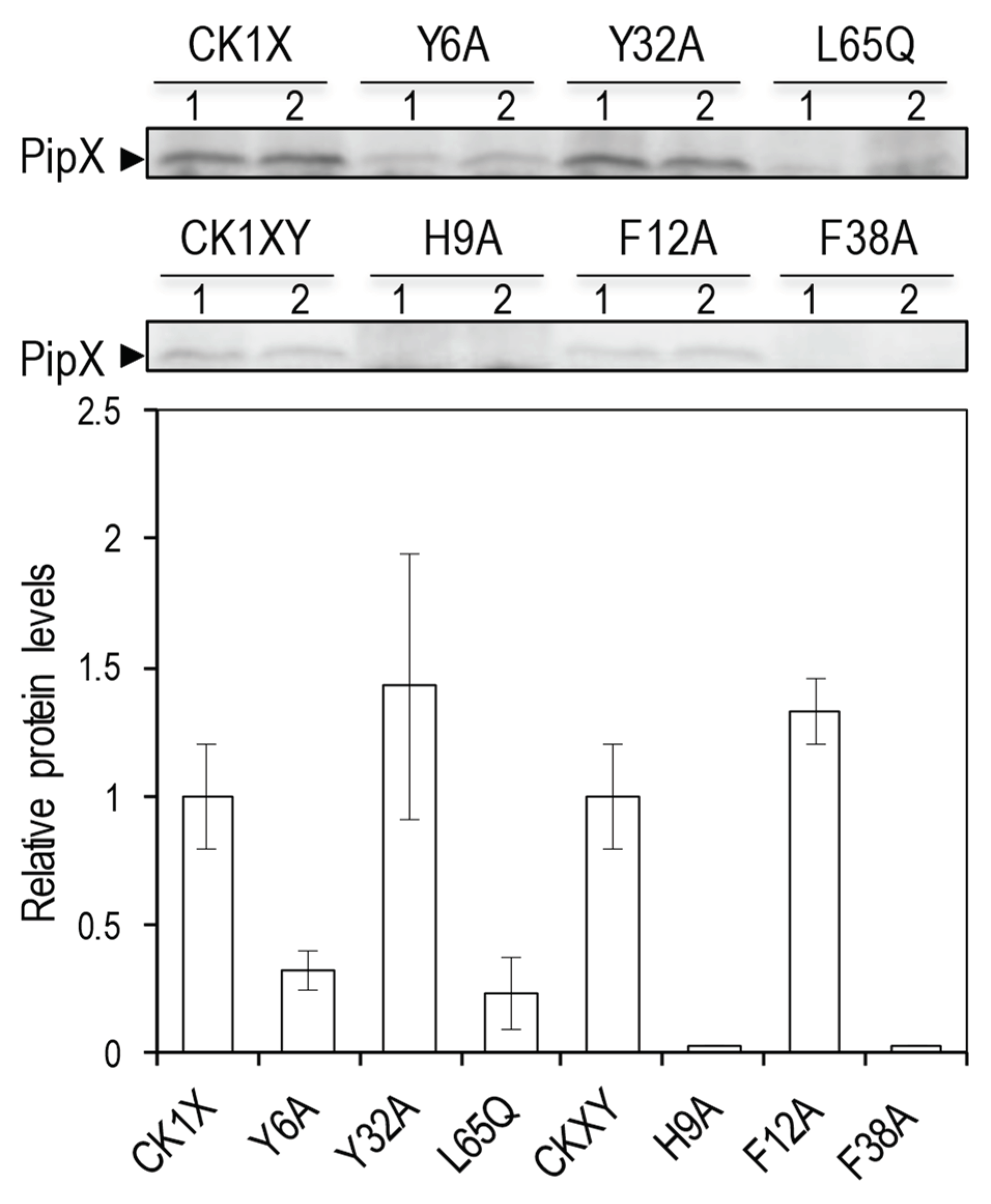
pipX* (Y6A, Y32A or L65Q in CKX constructs) and constructed additional ones (H9A, F12A or F38A in CKXY constructs).
In all cases a strong constitutive promoter provided by the C.K1 cassette drives expression of pipX*. In total, four PipX variants impairing self-interactions (Y6A, H9A, F38A, L65Q) and two not impairing them (F12A, Y32A) were analysed alongside their corresponding wild type controls by Western blot with anti-PipX antibodies.
As shown in
Figure 6, all four variants impairing self-interactions impaired PipX levels, while the other two did not. It is worth noting that Y32A, that did impair PipX levels in
S. elongatus [
24], allowed maximal levels of expression in
E. coli, thus indicating that the stability of PipX is differentially affected by mutations in
E. coli and
S. elongatus and further agreeing with the different effect of mutation Y32A on interactions with PII and GlnB or GlnK. In addition, we detected significantly higher levels of PipX from CK1X than from CK1XY constructs (
Figure S3), a result at odds with that obtained in
S. elongatus, where PipY had a positive effect on PipX levels [
54].
Altogether the results show the role of GlnB and/or GlnK in the self-interaction of PipX in BACTH assays. This is, to our knowledge, the first elucidation of the molecular basis of a “false positive” in this bacterial system for protein-protein interactions.
3. Materials and Methods
3.1. Strains, oligonucleotides, and plasmid construction.
Strains and plasmids used in this work are listed in
Table 1, oligonucleotides in
Table S1. Cloning procedures were carried out with
Escherichia coli XL1-Blue, using standard techniques [
62]. Antibiotics used were ampicillin (50 µg ml
-1), kanamycin (40 µg ml
-1) or chloramphenicol (34 µg ml
-1). All constructs were analyzed by automated dideoxy DNA sequencing.
QuickChange Mutagenesis was performed using pUAGC934 (to generate for PipX*-T18 derivatives), pUAGC444 (T18-PipX* derivatives) or pUAGC1045 (PipX*-T25 derivatives) as templates.
Table S2 summarizes the pair primers and template used, with indication of the resulting plasmids and protein fusions obtained in each case.
E. coli glnK sequences were PCR-amplified from E. coli MG1655 total genomic DNA wih primers GLNK-BYTH-1F and GLNK-BYTH-1R, digested with BamHI and SmaI and cloned into pUT18c, pT25 and pKT25, giving plasmids pUAG660, pUAG661 and pUAG665, respectively. E. coli glnB sequences from pUAG652 were digested with BamHI and KpnI and cloned into pKT25, giving plasmid pUAG663.
To generate plasmid pUAGC408, pipXpipY sequences from pUAGC125 were digested with SalI + KspAI and cloned into plasmid pUAGC410 cut with SalI + SmaI. QuickChange mutagenesis with pUAGC408 as template and PipX-H9A-1F/1R, PipX-F12A-1F/1R or PipX-F38A-1F/1R as primers pairs were used to generate plasmids pUAGC948, pUAGC939 and pUAGC940.
To generate E. coli strains expressing PipX derivatives, BTH101 was transformed with pUAGC410, pUAGC685, pUAGC686, pUAGC682, pUAGC408, pUAGC948, pUAGC939, or pUAGC940.
3.2. BACTH assays
E. coli BTH101 was transformed with pairwise combination of plasmids (50 ng). Five transformant clones from each plate were inoculated into 0.5 mL of LB containing the corresponding pair of antibiotics and 0.5 mM IPTG (Isopropyl β-D-1-thiogalactopyranoside), and incubated at 30 ºC for 24 hours.
Interactions were assayed by dropping 3 μl of each saturated culture onto M63 (containing 0.3 % maltose, 0.0001 % thiamine, 1 mM magnesium sulfate, 0.5 mM IPTG, and 40 µg ml-1 X-gal) and MacConkey (containing 1% lactose and 0.5 mM IPTG) reporter plates. Reporter plates were incubated for 24 (MacConkey) or 48 hours (M63) at 30 ºC and photographs were taken at 24 h intervals.
3.3. Protein extraction and immunodetection assays
For immunodetection assays, cultures were grown in 5 ml LB with Ap (50 µg ml
-1) overnight at 37ºC, diluted to a OD
600nm of 0.05 and grown at 30ºC until they reach an OD
600nm of 0.5. Cells were harvested by centrifugation at 7300g for 6 min at 4ºC. The pellets were resuspended in 60 μl of lysis buffer (25 mM Tris/HCl pH 7.5, 0.5 mM EDTA, 1 mM beta-mercaptoethanol, 1mM PMSF) and cells were disrupted using a spoon of 0.1 µm glass beads, as described [
50]. Mixtures were subjected to three cycles of 60 s at a speed of 5 m/s in a high-speed homogenizer Minibeadbeater always followed by a 60 s at 4 ºC during each cycle. Samples were centrifuged (5500g for 5 min) and the supernatant fractions (crude protein extracts) were transferred to a new tube and stored at -20ºC until needed.
Protein concentrations were estimated by the Bradford method using the Pierce
TM detergent compatible Bradford assay kit (ThermoScientific) in a VICTOR3
TM 1420 Multilabel Plate Reader (PerkinElmer). Immunodetection was performed by loading 60 µg of total protein extract in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tricine-SDS-PAGE; 18% polyacrylamide). Tricine–SDS–PAGE was conducted according to the method described by [
63]. The samples were electrophoresed at a constant voltage of 30 mV until all samples entered the stacking gel and then at a constant voltage of 100 mV until the end.
The gel electrophoresis was followed by wet immunoblotting with 0.1 μm polyvinylidene fluoride membranes (from GE Healthcare). The membranes were blocked with Tris-Buffered Saline (TBS-tween; 20 mM Tris/HCl pH 7.5, 500 mM NaCl, tween 20 0.1%) solution containing 5% non-fat dried milk for 30 min at room temperature and then incubated overnight in TBS plus 0.1% Tween 20 solution containing 2% non-fat dried milk and the primary antibody. Then, the membranes were incubated at room temperature for 1.5 hours with a 1:150000 dilution of ECL rabbit IgG, HRP-linked F(ab')2 fragment (from donkey; GE Healthcare). The signal was detected with the addition of the SuperSignal WestFemto reagent (ThermoScientific) in a Biorad ChemiDoc Imager using the automatic exposure mode and avoiding pixel saturation or using X-ray and scanning the films. All the membranes were treated first with a 1:5000 dilution of primary anti-PipX antibody. Antiserum against PipX (Pineda Antikörper Service, Berlin, Germany) and PlmA (Genosphere Biotechnologies, Paris) were produced in rabbits.
3.4. Computational methods
Protein intensity levels were quantified from the western blot images using the ImageJ software. Bands were picked up using the “rectangle” function and the area plot corresponding to the intensity was measured with the “wand” tool. Each area from the PipX immunodetection was normalized using the corresponding area of an unspecific inner band and referred to the control strain.
Graphical representations of PipX structure were generated with PyMOL (The PyMOL Molecular Graphics System, Version 1.7.1.7 Schrödinger, LLC).
4. Conclusions
In this work we have used the bacterial two-hybrid system to perform interaction analyses involving PipX-NtcA or PipX-PII complexes from cyanobacteria as well as heterologous complexes between PipX and GlnB or GlnK, the two PII homologues from E. coli. The results obtained here, discussed alongside those from several Y2H studies, provided further insights into the PipX interaction network and allowed us to compare outcomes between the two most popular genetic systems to study protein-protein interactions.
Interactions previously recorded as negative in the yeast system as well as indirect interactions dependent on host proteins were detected and explained on the bases of differential features of the Y2H and BACTH systems. To show that PipX self-interaction is due to oligomerisation over GlnB and/or GlnK proteins in E. coli, we performed comprehensive mutational analysis, identifying determinants involved on PipX self- or cross-interactions with GlnB or GlnK and further corroborating results by Western analysis showing that complex formation with GlnB and/or GlnK from E. coli stabilises PipX. The discussions and considerations made here, calling attention to the complexities, advantages and limitations of these two universal biological approaches to study protein interactions, would help researchers from different fields to take the most of their two-hybrid analyses.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: Representative photographs of BACTH assays used to produce the heatmaps of
Figure 3 and
Figure 4, Figure S2: Representative photographs of BACTH assays used to produce the heatmaps of
Figure 5, Figure S3: Comparison of PipX protein levels in
E. coli expressed by CKX or CKXY constructions. Table S1: Oligonucleotides, Table S2: Quick-change mutagenesis of PipX derivatives for BACTH analysis.
Author Contributions
Conceptualization, funding acquisition, project administration, and writing—original draft preparation, AC; investigation, data curation and writing—review and editing, PS, SB, RC, CJ, LT and TM. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant PID2020-118816GB-I00 funded by MCIN/AEI/10.13039/501100011033 from the Spanish Government, grants VIGROB23-126 and GRE20-04-C from the University of Alicante to AC. CJ was the recipient of a Ph.D. fellowship (ACIF/2019/045) from Conselleria d’Innovació, Universitats, Ciència i Societat Digital of the Generalitat Valenciana. SB was supported by a National Grant from the Algerian Ministry of Higher Education and Scientific Research.
Acknowledgments
The authors thank A. Llop for technical contributions and constructive discussions and V. Rubio for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blank, C.E.; Sánchez-Baracaldo, P. Timing of Morphological and Ecological Innovations in the Cyanobacteria – a Key to Understanding the Rise in Atmospheric Oxygen. Geobiology 2010, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, K.; Selim, K.A. Carbon/Nitrogen Homeostasis Control in Cyanobacteria. FEMS Microbiol Rev 2020, 44, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-C.; Zhou, C.-Z.; Burnap, R.L.; Peng, L. Carbon/Nitrogen Metabolic Balance: Lessons from Cyanobacteria. Trends Plant Sci 2018, 23, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Herrero, A. Nitrogen Assimilation and Nitrogen Control in Cyanobacteria. Biochem Soc Trans 2005, 33, 164–167. [Google Scholar] [CrossRef]
- Muro-Pastor, M.I.; Reyes, J.C.; Florencio, F.J. Ammonium Assimilation in Cyanobacteria. Photosynth Res 2005, 83, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Huergo, L.F.; Dixon, R. The Emergence of 2-Oxoglutarate as a Master Regulator Metabolite. Microbiology and Molecular Biology Reviews 2015, 79, 419–435. [Google Scholar] [CrossRef]
- Senior, P.J. Regulation of Nitrogen Metabolism in Escherichia coli and Klebsiella aerogenes: Studies with the Continuous-Culture Technique. J Bacteriol 1975, 123, 407–418. [Google Scholar] [CrossRef]
- Leigh, J.A.; Dodsworth, J.A. Nitrogen Regulation in Bacteria and Archaea. Annu Rev Microbiol 2007, 61, 349–377. [Google Scholar] [CrossRef]
- Ninfa, A.J.; Jiang, P. PII Signal Transduction Proteins: Sensors of α-Ketoglutarate That Regulate Nitrogen Metabolism. Curr Opin Microbiol 2005, 8, 168–173. [Google Scholar] [CrossRef]
- Forchhammer, K.; Selim, K.A.; Huergo, L.F. New Views on PII Signaling: From Nitrogen Sensing to Global Metabolic Control. Trends Microbiol 2022, 30, 722–735. [Google Scholar] [CrossRef]
- Kamberov, E.S.; Atkinson, M.R.; Ninfa, A.J. The Escherichia coli PII Signal Transduction Protein Is Activated upon Binding 2-Ketoglutarate and ATP. Journal of Biological Chemistry 1995, 270, 17797–17807. [Google Scholar] [CrossRef]
- Selim, K.A.; Ermilova, E.; Forchhammer, K. From Cyanobacteria to Archaeplastida: New Evolutionary Insights into PII Signalling in the Plant Kingdom. New Phytologist 2020, 227, 722–731. [Google Scholar] [CrossRef]
- Fields, S.; Song, O. A Novel Genetic System to Detect Protein–Protein Interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Argudo, I.; Martín-Nieto, J.; Salinas, P.; Maldonado, R.; Drummond, M.; Contreras, A. Two-hybrid Analysis of Domain Interactions Involving NtrB and NtrC Two-component Regulators. Mol Microbiol 2001, 40, 169–178. [Google Scholar] [CrossRef]
- Martínez-Argudo, I.; Contreras, A. PII T-Loop Mutations Affecting Signal Transduction to NtrB Also Abolish Yeast Two-Hybrid Interactions. J Bacteriol 2002, 184, 3746–3748. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Argudo, I.; Salinas, P.; Maldonado, R.; Contreras, A. Domain Interactions on the ntr Signal Transduction Pathway: Two-Hybrid Analysis of Mutant and Truncated Derivatives of Histidine Kinase NtrB. J Bacteriol 2002, 184, 200–206. [Google Scholar] [CrossRef]
- Salinas, P.; Contreras, A. Identification and Analysis of Escherichia coli Proteins That Interact with the Histidine Kinase NtrB in a Yeast Two-Hybrid System. Molecular Genetics and Genomics 2003, 269, 574–581. [Google Scholar] [CrossRef]
- Burillo, S.; Luque, I.; Fuentes, I.; Contreras, A. Interactions between the Nitrogen Signal Transduction Protein PII and N -Acetyl Glutamate Kinase in Organisms That Perform Oxygenic Photosynthesis. J Bacteriol 2004, 186, 3346–3354. [Google Scholar] [CrossRef]
- Espinosa, J.; Castells, M.A.; Laichoubi, K.B.; Contreras, A. Mutations at pipX Suppress Lethality of PII -Deficient Mutants of Synechococcus elongatus PCC 7942. J Bacteriol 2009, 191, 4863–4869. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Castells, M.A.; Laichoubi, K.B.; Forchhammer, K.; Contreras, A. Effects of Spontaneous Mutations in pipX Functions and Regulatory Complexes on the Cyanobacterium Synechococcus elongatus Strain PCC 7942. Microbiology (N Y) 2010, 156, 1517–1526. [Google Scholar] [CrossRef]
- Espinosa, J.; Forchhammer, K.; Burillo, S.; Contreras, A. Interaction Network in Cyanobacterial Nitrogen Regulation: PipX, a Protein That Interacts in a 2-oxoglutarate Dependent Manner with PII and NtcA. Mol Microbiol 2006, 61, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Fuentes, I.; Burillo, S.; Rodrí guez-Mateos, F.; Contreras, A. SipA, a Novel Type of Protein from Synechococcus Sp. PCC 7942, Binds to the Kinase Domain of NblS. FEMS Microbiol Lett 2006, 254, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; Maheswaran, M.; Ruppert, U.; Forchhammer, K. The Synechococcus elongatus PII Signal Transduction Protein Controls Arginine Synthesis by Complex Formation with N -acetyl- <scp>l</Scp> -glutamate Kinase. Mol Microbiol 2004, 52, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Laichoubi, K.B.; Espinosa, J.; Castells, M.A.; Contreras, A. Mutational Analysis of the Cyanobacterial Nitrogen Regulator PipX. PLoS One 2012, 7, e35845. [Google Scholar] [CrossRef] [PubMed]
- Laichoubi, K.B.; Beez, S.; Espinosa, J.; Forchhammer, K.; Contreras, A. The Nitrogen Interaction Network in Synechococcus WH5701, a Cyanobacterium with Two PipX and Two PII-like Proteins. Microbiology (N Y) 2011, 157, 1220–1228. [Google Scholar] [CrossRef]
- Llácer, J.L.; Espinosa, J.; Castells, M.A.; Contreras, A.; Forchhammer, K.; Rubio, V. Structural Basis for the Regulation of NtcA-Dependent Transcription by Proteins PipX and PII. Proceedings of the National Academy of Sciences 2010, 107, 15397–15402. [Google Scholar] [CrossRef] [PubMed]
- Llácer, J.L.; Contreras, A.; Forchhammer, K.; Marco-Marín, C.; Gil-Ortiz, F.; Maldonado, R.; Fita, I.; Rubio, V. The Crystal Structure of the Complex of PII and Acetylglutamate Kinase Reveals How PII Controls the Storage of Nitrogen as Arginine. Proceedings of the National Academy of Sciences 2007, 104, 17644–17649. [Google Scholar] [CrossRef] [PubMed]
- Llácer, J.L.; Fita, I.; Rubio, V. Arginine and Nitrogen Storage. Curr Opin Struct Biol 2008, 18, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Forcada-Nadal, A.; Llácer, J.L.; Contreras, A.; Marco-Marín, C.; Rubio, V. The PII-NAGK-PipX-NtcA Regulatory Axis of Cyanobacteria: A Tale of Changing Partners, Allosteric Effectors and Non-Covalent Interactions. Front Mol Biosci 2018, 5, 91. [Google Scholar] [CrossRef]
- Labella, J.I.; Cantos, R.; Salinas, P.; Espinosa, J.; Contreras, A. Distinctive Features of PipX, a Unique Signaling Protein of Cyanobacteria. Life 2020, 10, 79. [Google Scholar] [CrossRef]
- Selim, K.A.; Haffner, M.; Watzer, B.; Forchhammer, K. Tuning the in vitro Sensing and Signaling Properties of Cyanobacterial PII Protein by Mutation of Key Residues. Sci Rep 2019, 9, 18985. [Google Scholar] [CrossRef] [PubMed]
- Kyrpides, N.C.; Woese, C.R.; Ouzounis, C.A. KOW: A Novel Motif Linking a Bacterial Transcription Factor with Ribosomal Proteins. Trends Biochem Sci 1996, 21, 425–426. [Google Scholar] [CrossRef]
- Cantos, R.; Labella, J.I.; Espinosa, J.; Contreras, A. The Nitrogen Regulator PipX Acts in Cis to Prevent Operon Polarity. Environ Microbiol Rep 2019, 11, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Martín, M.A.; López-Lozano, A.; Clavería-Gimeno, R.; Velázquez-Campoy, A.; Seidel, G.; Burkovski, A.; Díez, J.; García-Fernández, J.M. Differential NtcA Responsiveness to 2-Oxoglutarate Underlies the Diversity of C/N Balance Regulation in Prochlorococcus. Front Microbiol 2018, 8, 2641. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Ferreira, A.A.; Inaba, M.; Fort, A.; Araújo, W.L.; Sulpice, R. Nitrogen Metabolism in Cyanobacteria: Metabolic and Molecular Control, Growth Consequences and Biotechnological Applications. Crit Rev Microbiol 2018, 44, 541–560. [Google Scholar] [CrossRef]
- Herrero, A.; Muro-Pastor, A.M.; Flores, E. Nitrogen Control in Cyanobacteria. J Bacteriol 2001, 183, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Rodríguez-Mateos, F.; Salinas, P.; Lanza, V.F.; Dixon, R.; de la Cruz, F.; Contreras, A. PipX, the Coactivator of NtcA, Is a Global Regulator in Cyanobacteria. Proceedings of the National Academy of Sciences 2014, 111, 201404030–201404097. [Google Scholar] [CrossRef]
- Giner-Lamia, J.; Robles-Rengel, R.; Hernández-Prieto, M.A.; Muro-Pastor, M.I.; Florencio, F.J.; Futschik, M.E. Identification of the Direct Regulon of NtcA during Early Acclimation to Nitrogen Starvation in the Cyanobacterium Synechocystis Sp. PCC 6803. Nucleic Acids Res 2017, 45, 11800–11820. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Forchhammer, K.; Contreras, A. Role of the Synechococcus PCC 7942 Nitrogen Regulator Protein PipX in NtcA-Controlled Processes. Microbiology (N Y) 2007, 153, 711–718. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Jiang, Y.-L.; He, Y.-X.; Chen, Y.-F.; Teng, Y.-B.; Chen, Y.; Zhang, C.-C.; Zhou, C.-Z. Structural Basis for the Allosteric Control of the Global Transcription Factor NtcA by the Nitrogen Starvation Signal 2-Oxoglutarate. Proceedings of the National Academy of Sciences 2010, 107, 12487–12492. [Google Scholar] [CrossRef]
- Zeth, K.; Fokina, O.; Forchhammer, K. Structural Basis and Target-Specific Modulation of ADP Sensing by the Synechococcus elongatus PII Signaling Protein. Journal of Biological Chemistry 2014, 289, 8960–8972. [Google Scholar] [CrossRef] [PubMed]
- Karimova, G.; Pidoux, J.; Ullmann, A.; Ladant, D. A Bacterial Two-Hybrid System Based on a Reconstituted Signal Transduction Pathway. Proceedings of the National Academy of Sciences 1998, 95, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Guerrero, L.; Camargo, S.; Valladares, A.; Picossi, S.; Luque, I.; Ochoa de Alda, J.A.G.; Herrero, A. FtsZ of Filamentous, Heterocyst-Forming Cyanobacteria Has a Conserved N-Terminal Peptide Required for Normal FtsZ Polymerization and Cell Division. Front Microbiol 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Marbouty, M.; Saguez, C.; Cassier-Chauvat, C.; Chauvat, F. ZipN, an FtsA-like Orchestrator of Divisome Assembly in the Model Cyanobacterium Synechocystis PCC6803. Mol Microbiol 2009, 74, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ramos-León, F.; Mariscal, V.; Frías, J.E.; Flores, E.; Herrero, A. Divisome-dependent Subcellular Localization of Cell–Cell Joining Protein SepJ in the Filamentous Cyanobacterium Anabaena. Mol Microbiol 2015, 96, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rachedi, R.; Foglino, M.; Talla, E.; Latifi, A. Interaction Network among Factors Involved in Heterocyst-Patterning in Cyanobacteria. Molecular Genetics and Genomics 2022, 297, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Labella, J.I.; Llop, A.; Contreras, A. The Default Cyanobacterial Linked Genome: An Interactive Platform Based on Cyanobacterial Linkage Networks to Assist Functional Genomics. FEBS Lett 2020, 594, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Llop, A.; Bibak, S.; Cantos, R.; Salinas, P.; Contreras, A. The Ribosome Assembly GTPase EngA Is Involved in Redox Signaling in Cyanobacteria. Front Microbiol 2023, 14, 1242616-NA. [Google Scholar] [CrossRef] [PubMed]
- Jerez, C.; Salinas, P.; Llop, A.; Cantos, R.; Espinosa, J.; Labella, J.I.; Contreras, A. Regulatory Connections Between the Cyanobacterial Factor PipX and the Ribosome Assembly GTPase EngA. Front Microbiol 2021, 12, 781760-NA. [Google Scholar] [CrossRef]
- Labella, J.I.; Obrebska, A.; Espinosa, J.; Salinas, P.; Forcada-Nadal, A.; Tremiño, L.; Rubio, V.; Contreras, A. Expanding the Cyanobacterial Nitrogen Regulatory Network: The GntR-Like Regulator PlmA Interacts with the PII-PipX Complex. Front Microbiol 2016, 7, 1677. [Google Scholar] [CrossRef]
- Bullock, W.O.; Fernandez, J.M.; Short, J.M. XL1-Blue: A High Efficiency Plasmid-Transforming recA Escherichia coli Strain with β-Galactosidase Selection. Biotechniques 1987, 5, 376–378. [Google Scholar]
- Guyer, M.S.; Reed, R.R.; Steitz, J.A.; Low, K.B. Identification of a Sex-Factor-Affinity Site in E. coli As Gamma Delta. Cold Spring Harb Symp Quant Biol 1981, 45, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Karimova, G.; Ullmann, A.; Ladant, D. Protein-Protein Interaction between Bacillus stearothermophilus Tyrosyl-TRNA Synthetase Subdomains Revealed by a Bacterial Two-Hybrid System. J Mol Microbiol Biotechnol 2001, 3, 73–82. [Google Scholar] [PubMed]
- Labella, J.I.; Cantos, R.; Espinosa, J.; Forcada-Nadal, A.; Rubio, V.; Contreras, A. PipY, a Member of the Conserved COG0325 Family of PLP-Binding Proteins, Expands the Cyanobacterial Nitrogen Regulatory Network. Front Microbiol 2017, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Llop, A.; Tremiño, L.; Cantos, R.; Contreras, A. The Signal Transduction Protein PII Controls the Levels of the Cyanobacterial Protein PipX. Microorganisms 2023, 11, 2379. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-J.; Jiang, Y.-L.; You, L.-L.; Shen, L.-Q.; Wu, X.; Yang, F.; Cui, N.; Kong, W.-W.; Sun, H.; Zhou, K.; et al. DNA Looping Mediates Cooperative Transcription Activation. Nat Struct Mol Biol 2024, 31, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Battesti, A.; Bouveret, E. The Bacterial Two-Hybrid System Based on Adenylate Cyclase Reconstitution in Escherichia coli. Methods 2012, 58, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, S.P.; Karimova, G.; Davi, M.; Ladant, D. Analysis of Membrane Protein Interactions with a Bacterial Adenylate Cyclase–Based Two-Hybrid (BACTH) Technique. Curr Protoc Mol Biol 2017, 118. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, K.; Hedler, A. Phosphoprotein PII from Cyanobacteria. Eur J Biochem 1997, 244, 869–875. [Google Scholar] [CrossRef]
- Forchhammer, K.; Hedler, A.; Strobel, H.; Weiss, V. Heterotrimerization of PII -like Signalling Proteins: Implications for PII -mediated Signal Transduction Systems. Mol Microbiol 1999, 33, 338–349. [Google Scholar] [CrossRef]
- Llop, A.; Labella, J.I.; Borisova, M.; Forchhammer, K.; Selim, K.A.; Contreras, A. Pleiotropic Effects of PipX, PipY, or RelQ Overexpression on Growth, Cell Size, Photosynthesis, and Polyphosphate Accumulation in the Cyanobacterium Synechococcus elongatus PCC7942. Front Microbiol 2023, 14, 1141775-NA. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J., F. E.F., & M.T. Molecular Cloning: A Laboratory Manual; No. Ed. 2.; Cold spring harbor laboratory press, 1989.
- Schägger, H. Tricine–SDS-PAGE. Nat Protoc 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).