1. Introduction
Obesity, which is associated with an excessive accumulation or improper distribution of adipose tissue, exerts a significant impact on health. Body adiposity serves as an atherogenic factor, correlating with the occurrence of insulin and leptin resistance, aberrant lipid metabolism, thereby leading to the onset of numerous civilization-related diseases and increasing the risk of mortality [
1]. According to the World Health Organization (WHO), in 2016, overweight affected 1.9 billion individuals (39%), while obesity affected 650 million adults (13%) globally, indicating the emergence of an obesity pandemic [
1].
Individuals with obesity are recommended to modify their lifestyle primarily by increasing physical activity while simultaneously creating an energy deficit through dietary intake, resulting in gradual weight reduction [
2]. One physical activity recommended for this group regardless of age is Nordic walking (NW) training [
3,
4]. Due to the engagement of both upper and lower body muscles, the energy cost of this activity is higher than that of regular walking [
5], with a simultaneous reduction in stress on the knee and intervertebral joints [
6]. This type of training may be recommended for patients with cardiovascular diseases [
7], oncological conditions [
8,
9], or neurological disorders [
10,
11,
12]. Such a wide range underscores the safety of this training modality. The effectiveness of various forms of NW training has been tested so far. In the absence of clear conclusions, this form is combined with other procedures such as cold exposure or time restricted eating [
13,
14].
Time restricted eating (TRE), a temporal pattern of food intake delivery, is increasingly utilized for health purposes, including weight reduction [
15,
16,
17]. Additionally, IF enhances the body's ability to defend against oxidative and metabolic stress [
18]. The human body's adaptation to fasting is crucial for survival and well-being during periods of abstinence, and its implementation carries numerous health benefits, particularly for individuals with excessive adipose tissue content. Under the influence of fasting, instead of relying solely on glucose stored in hepatic glycogen for energy, the body also utilizes energy from ketones derived from adipose tissue [
19]. The increase in blood ketone levels may initiate the activation of various intracellular signaling pathways, which, among other functions, slow down the aging process [
20] and regulate the expression and activity of numerous proteins, including sirtuins [
20,
21,
22].
Increased white adipose tissue (WAT) content, which constitutes the hallmark of obesity, leads to enhanced secretion of adipokines, including leptin, resistin, interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), visfatin, vaspin, and retinol-binding protein 4 (RBP-4).
Leptin is an adipokine involved in both the regulation of body weight and the regulation of hunger and appetite [
23]. Elevated leptin levels are observed in obese individuals [
24], which decrease with weight loss, adjusting the body's metabolism to the energy reserves stored in adipose tissue [
25]. Decreased leptin levels have been observed under the influence of starvation, as well as after following a low-calorie diet [
26], and after physical training [
27].
In obesity, an increase in serum resistin concentration is observed [
28]. Its action is associated with the development of insulin resistance, and inhibition of resistin activity lowers serum glucose levels and increases insulin sensitivity [
29], which has favorable clinical significance. There is scientific evidence of the impact of various forms of physical activity on significant reduction in resistin levels in obese individuals [
30,
31].
Women represent a group with significantly greater constraints stemming from anxiety about engaging in physical activity [
32]. Above the age of 35, a considerable decrease in muscle mass (especially in the legs) along with an increase in the percentage of body fat occurs [
33]. Generally, women tend to have higher BMI values than men [
34]. There are also intensified issues with maintaining a proper diet, often attributed to the phenomenon of emotional eating and stress relief [
35]. This makes overweight and obesity a significant challenge for individuals of this gender. In line with the above, given the high acceptability of physical activity in the form of NW training [
36], this study was undertaken. Its main aim was to assess the impact of two lengths of NW training interventions combined with TRE on improving body composition parameters, lipid profile, and levels of selected adipokines in women with disrupted body composition. It was hypothesized that a 6-week intervention involving a combination of two stimuli: training and dietary, would result in significant changes including: reduction in body weight associated with loss of body fat and fat tissue mass; improvement in serum lipid profile; and reduction in the levels of selected adipokines. Additionally, it was chosen to compare the 6-week intervention with a 12-week one.
2. Materials and Methods
Study Group
Overweight and obese women with a BMI over 25 and no physical activity other than housework were invited to participate in the project. Medical qualification included verification of contraindications to physical exertion (walking with poles) and the presence of glycemic disorders allowing for the diagnosis of diabetes. Exclusion criteria from the study were: dietary changes within 3 months prior to participating in the research project, health problems of neurological or orthopedic origin, participation in other physical activities during the project, and the use of supplementation or medications affecting lipid and carbohydrate metabolism.
The project obtained approval from the Ethics Committee (58/KBL/OLI/2022 dated April 11, 2022). Participants were informed about the purpose and research methods and provided written consent to participate in the study. They were also informed about the possibility of withdrawing from participation at any study stage without providing a reason. Each participant also had the opportunity to access their individual results.
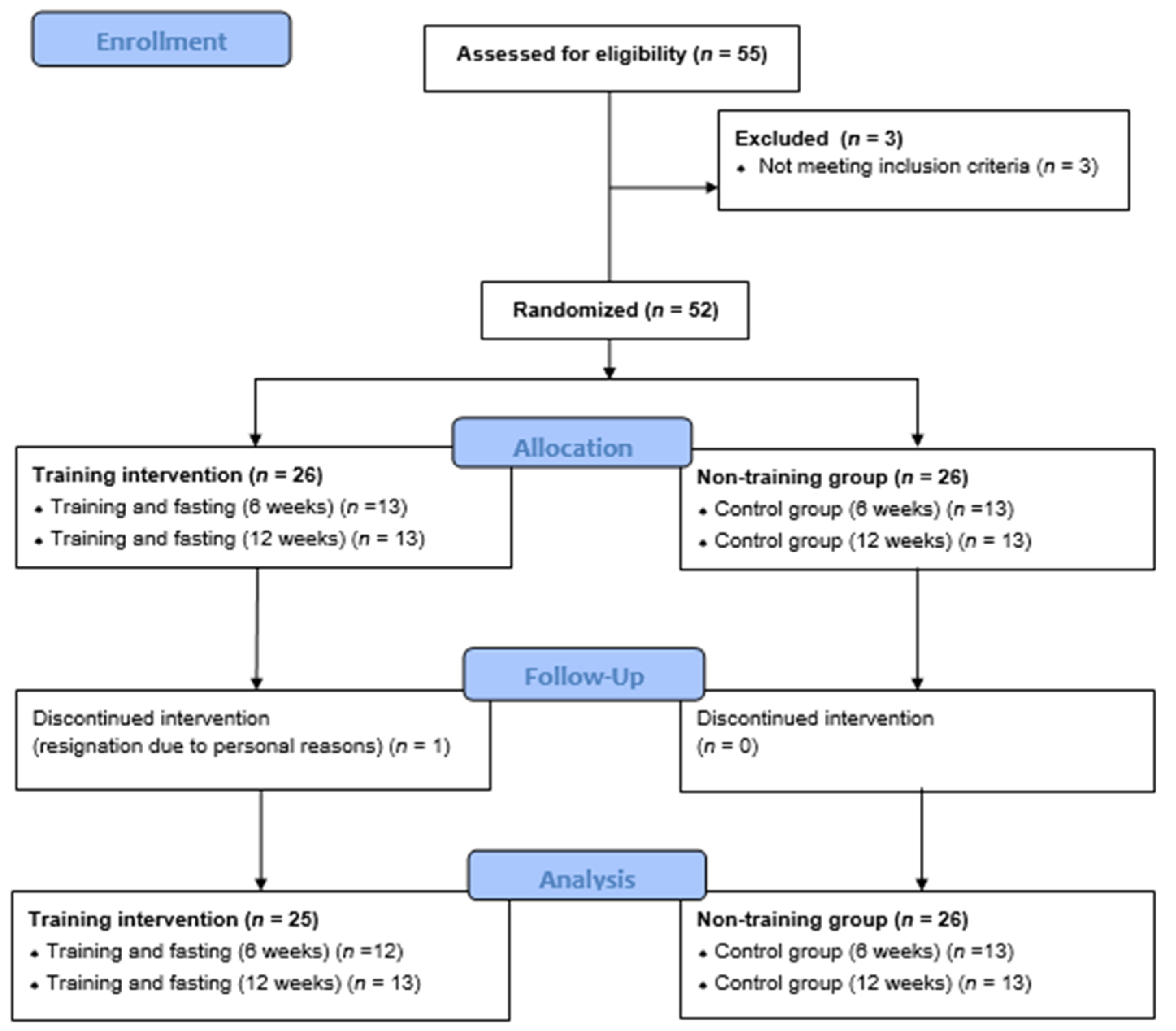
Four groups were selected through randomization: two undergoing interventions: women participating in training sessions and 14-hour fasting for 6 (SG6, n=13) and 12 weeks (SG12, n=13); and two control groups: CON6, n=13; and CON12, n=13. The patient flow diagram is presented in
Figure 1.
Study Protocol
The study on the design of a randomized controlled trial was interventional. Each participant underwent an initial examination by a doctor to assess the presence of the inclusion and exclusion criteria for the project. Subsequently, randomization was conducted to allocate participants to groups. Individual training loads were then determined for the active groups, while the groups practicing fasting were instructed on the principles of time restricted eating. NW (Nordic Walking) training sessions lasted for either 6 weeks or 12 weeks (3 sessions per week).
In all groups, body composition analysis was performed twice (before and after the completion of the training series, in control groups – at the same timepoints). Blood samples were collected before the first training session, as well as before the last training session. In the control groups, blood was drawn twice at corresponding time points.
Time Restricted Eating
Fasting was conducted daily (7 days a week) for 14 hours per day, within individually chosen period intervals by the participants. The feeding window lasted for 10 hours. In addition to this modification, participants were instructed not to change their dietary habits and to continue their self-composed diet as before the project commenced.
NW Training
The Nordic Walking training program was developed by a qualified trainer based on available literature [
37]. The training sessions took place three times a week (Monday, Wednesday, Friday) and lasted 60 minutes each. The sessions were conducted under the supervision of the trainer in green areas. The training intensity was determined using the 2-kilometer walking test (UKK Walk Test) [
38] with the following formula:
Where: Tmin – total minutes of walking; Ts – seconds of walking in the last incomplete minute; HR – heart rate; BMI – body mass index; A – age in years.
Then, the VO2max level was determined individually for each participant, and the training intensity was maintained so as not to exceed 70% of VO2max.
Where: A – age in years; BMI – body mass index; HR – heart rate; T – walking time in minutes converted to decimal system.
The training consisted of 3 stages: the first stage involved familiarizing the participants with the correct Nordic Walking technique. The second stage included strength-resistance and stretching exercises to improve motor skills, flexibility, and joint range of motion, and aerobic exercises preparing for more intense efforts. The third stage involved conditioning training improve endurance capabilities, primarily based on walking with poles for a specified duration (aerobic training and interval training).
The structure of each training unit (60 min) included: warm-up: 10–15 min; main part: 40–45 min with assumed training intensity; cooling down: 5–15 min (stretching exercises and breathing exercises).Training intensity was monitored using sports testers (M400 Polar, Finland) with individually entered user data, including acceptable heart rate levels – if these were exceeded, the device beeped and the instructor adjusted the patient's exercise intensity.
Somatic Measurements and Assessment of Body Composition
Participants were subjected to measurements of body mass (BM) and body composition before and after the training series, as well as a one-time measurement of body height (BH). The indices for analyzing body composition were determined using the Jawon Medical IOI-353 body composition analyzer (certified EC0197-Korea). The following variables were estimated: lean body mass (LBM), soft lean mass (SLM), percent total body water (%TBW), total body water (TBW), percent body fat (%PF), fat mass (FM), visceral fat area (VFA). Body height (BH) was measured using a Martin-type anthropometer (USA) with a measurement accuracy of 0.5 cm. Based on these parameters, the body mass index (BMI) was calculated.
Blood Parameters Measurements
Pre-training blood samples were collected after an overnight fast, followed by standardized breakfast consumption, before the participants engaged in training. Post-training blood collection took place immediately after exercise cessation, no later than 15 minutes thereafter.
In accordance with prevailing standards, a laboratory diagnostician collected venous blood from the elbow bend into Vacumed® vacuum system tubes (F.L. Medical, Italy). Blood was collected into 3 tubes – 1 containing EDTA K2 and 2 containing coagulation activator. Tubes with coagulation activator were left to clot for 15 minutes, then centrifuged (10 minutes; 5˚C).
Blood samples collected in EDTA and 1 sample with a coagulation activator were sent for analysis to a medical diagnostic laboratory following generally accepted methodology. Blood collected into EDTA tubes was used for determining morphological blood parameters. Serum obtained from blood samples collected on the clot was used for lipid profile determinations (total cholesterol concentration (TC), high-density lipoprotein cholesterol concentration (HDL-C), triglyceride concentration (TG)), and glucose.
Based on these parameters, subsequent indices were calculated: atherogenic index (AIP) and LDL/HDL ratio as predictors of cardiovascular diseases.
The material from the last of the tubes containing the coagulation activator was centrifuged for 10 minutes at 4°C and 2500 rpm, and the resulting supernatant, showing no signs of hemolysis, was transferred to Eppendorf-type microtubes. In this form, they were frozen in a low-temperature freezer (-80°C) until biochemical assays were performed. The determination of leptin and resistin concentrations in serum was carried out using an immunoenzymatic method (ELISA test) utilizing the multiplex method (Magpix Luminex, Xmap instrument; Luminex Corporation, USA).
Statistical Analysis
All statistical analyses were conducted using JASP 0.16.4 software (University of Amsterdam, Netherlands). The obtained results were presented using descriptive statistics (mean ± standard deviation, SD). For biochemical variables, as well as body composition parameters, repeated measures ANOVA (RMANOVA) analysis was performed, preceded by an assessment of assumptions (normality, variance, sphericity). If the sphericity assumption was violated (Mauchly's test), Greenhouse-Geisser or Huynh-Feldt corrections were applied. In the case of non-normal distribution assumption (Shapiro-Wilk test) and/or heterogeneity of variances (Levene's test), the non-parametric Friedman test was used. Statistically significant RMANOVA results for group, time, and group*time interaction parameters were subjected to post hoc Holm-Bonferroni tests. The effect size was assessed using the Eta squared coefficient (η²) and interpreted as follows: 0.1 – small; 0.25 – medium; 0.37 large size effect.
Correlation analysis was also performed, depending on the normality of the variable’s distribution, using the Pearson correlation coefficient or Spearman's rho.
3. Results
The results of body composition measurements are presented in
Table 1. Significant differences were indicated for BM (p=0.018). The differences were observed between measurements before and after the training series in the SG12 group (p=0.010), where the mean weight loss was 1.96 (±1.59) kg. For the group training for 6 weeks, the difference was 1.59 (±1.11) kg but did not reach statistical significance (p=0.081). Both control groups did not significantly change their body weight. The proposed interventions did not affect LBM, TBW [kg], and VFA.
For the variable BF [kg], the differentiating factor was time (p=0.021). Differences for the parameters group and interaction (time x group) were within the range of statistical trend (p=0.074 and 0.060, respectively). Further analyses indicated significantly lower results for the SG12 group after the training series (p=0.05). The mean fat tissue loss was 1.64 kg. Assessing the variable BFP showed significance for the time parameter (<0.001), while the group parameter and interaction were insignificant. Post hoc tests revealed significant differences for the SG12 group after the training series compared to baseline and compared to the control (mean BFP loss: 1.44%). During this analysis, it was also revealed that the CON12 group significantly increased the percentage of body fat content over 12 weeks.
Statistically significant differences were indicated for TBW [%], and these differences pertained to pre- and post-intervention results for the CON12 group (p=0.017), SG6 group (p=0.005), and SG12 group (p<0.001). In all indicated groups, an increase in TBW was observed. BMI significantly differed for the group x time interaction (p=0.012). Significant results were shown for the SG12 group post-intervention compared to the control group.
The results of biochemical tests are presented in
Table 2. Statistical analysis indicated that the proposed interventions did not affect the levels of TC, LDL, and TG in the serum of the examined women. For HDL, significance in terms of statistical trend (p=0.057) for the combined effect of factors: time and group, can be indicated. Significant differences were obtained for the LDL/HDL ratio, where the results before the project differed significantly from the results obtained after the intervention (p=0.011, ƞ²=0.023). An interaction between time and group was also indicated (p=0.048, ƞ²=0.034). However, post hoc tests did not reveal significant differences in comparisons between the two groups. The calculated AIP index did not differentiate the examined groups at the assumed significance level. The conducted analysis also did not show significant differences in blood glucose levels.
Another group of examined parameters is blood morphological markers. The results obtained in the project are presented in
Table 3. Significant differences were indicated for the quantity of leukocytes, erythrocytes, blood platelets, percentage of neutrophils, and monocytes. Significant differences were also noted in the results of HCT in relation to time – with lower values after interventions but a low effect size (p=0.042, ƞ²=0.012).
For the leukocyte count, the differentiating factors ware time (p=0.030, ƞ²=0.009) and group (p=0.017, ƞ²=0.209). Differences were noted in measurements before and after the training series (p=0.030), and the post hoc test indicated differences between the SG6 group and CON6 group (p=0.039).
The erythrocyte count was differentiated by time but the effect size was small (p=0.038, ƞ²=0.007). Differences were also noted in measurements before and after the training series (p=0.030). However, post hoc tests did not reveal significant differences. Characteristics of erythrocytes also significantly differed. For the variable MCV (ƞ²=0.253), significant differences were indicated between SG12 and the control (p=0.050), as well as between SG6 and SG12 (p=0.038). The examined groups also differed regarding the MCHC variable (ƞ²=0.187). Significant differences were detected between SG12 and its corresponding control group (p=0.017). A difference was indicated between the two intervention groups in terms of statistical trend (p=0.065). For the variable HCT, the differentiating factor was time (p=0.042, ƞ²=0.012).
The platelet count differed between groups (p=0.037, ƞ²=0.179), however, in seeking groups that elicited this effect, only a trend between SG6 and SG12 was noted (p=0.088). Differences were also found for the percentages of neutrophils (time: p=0.043, ƞ²=0.010; group: p=0.043, ƞ²=0.166) where the values measured after intervention were higher in SG6 and CON6 groups. However, this was not confirmed in post hoc analysis. For monocytes significant differences were also found (time: p=0.038, ƞ²=0.010; group: p=0.004, ƞ²=0.253). Monocyte counts were higher in the second measurement in all groups except CON12. Statistically significant differences were found between groups SG6 (in which the counts were highest) and CON 12 (p=0.011), as well as between SG6 and SG12 (in which the counts were lowest) (p=0.009) This result was primarily driven by the difference between the SG6 group and the control group. Percentages of lymphocytes, eosinophils, and basophils did not differ between the examined groups.
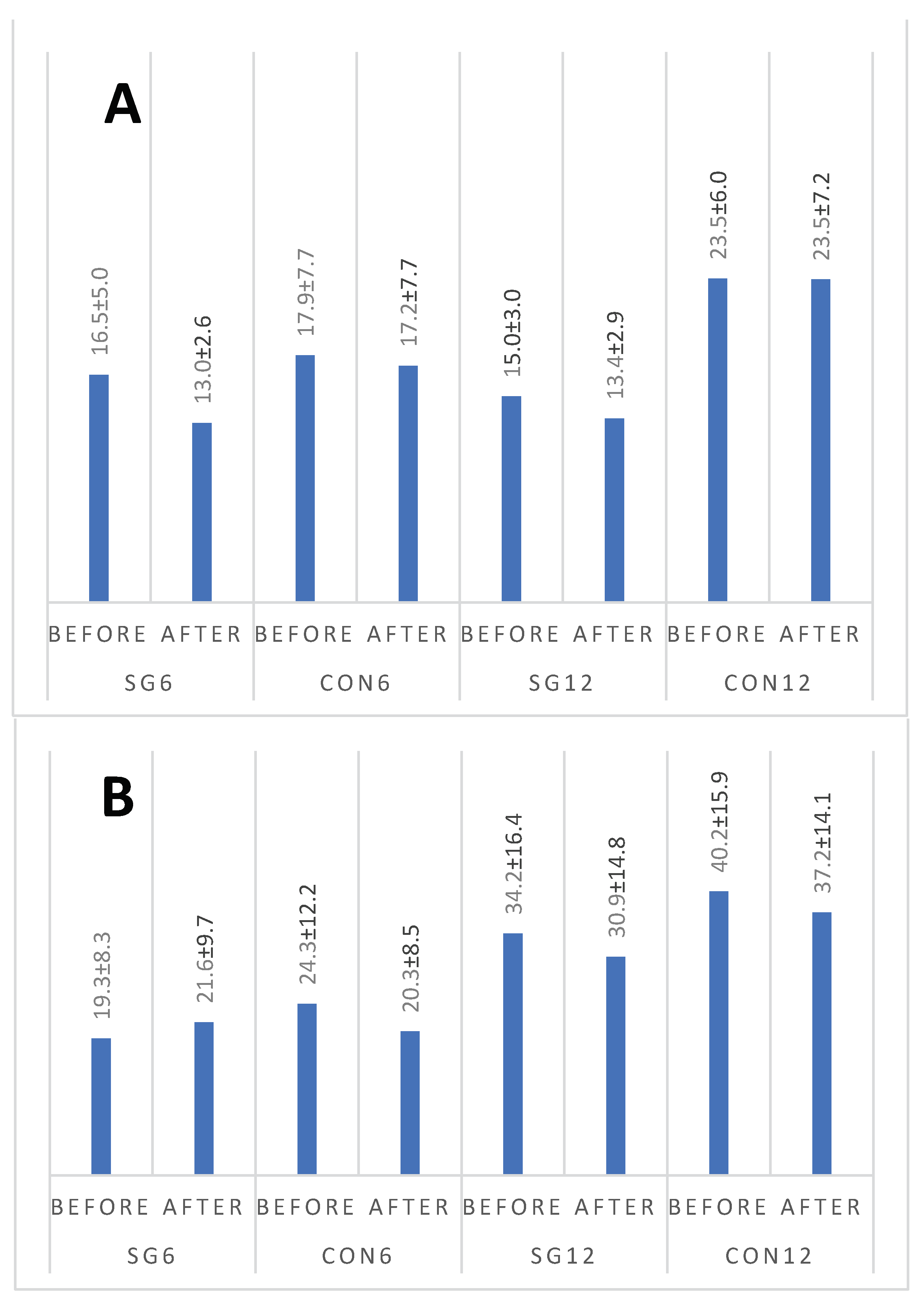
Figure 2 A,B present changes in leptin and resistin concentrations. Statistical analysis indicated that leptin concentration differed between groups (p=0.006). However, results for neither group changed over time. For resistin, the differentiating factors were time (p=0.019, with lower results observed after the intervention) and group (p=0.001, with higher results observed for the CON12 group). No significant interaction was found (p=0.231).
In searching for correlations between the examined adipokines and other variables, significant associations were identified only for leptin. An inverse correlation was demonstrated between leptin and SLM (r=0.424, p=0.006), as well as direct proportional relationships with the percentage of body fat (r=0.52, p<0.001), Visceral Fat Area (VFA) (r=0.446, p=0.004), and body mass (r=0.363, p=0.021). Moreover, the magnitude of the change in leptin concentration obtained after the interventions was found to be dependent on SLM (r=319, p=0.045). The results of significant correlations between the examined adipokines and body composition are presented in
Table 4.
4. Discussion
The results presented in this study indicate the effectiveness of the intervention in the form of NW training combined with TRE (14/24) only in certain areas examined. Beneficial effects were observed with the extended intervention (12 weeks), primarily concerning improvements in body composition and changes in blood morphology. However, neither the lipid profile results nor the calculated indices (AIP and LDL/HDL ratio) showed improvement. There was also no significant improvement in fasting glucose levels in response to both programs. On the other hand Kortas et al recently observed that NW supported by IF ameliorated glycated hemoglobin [
13].
Both NW training and modified, low-calorie dietary intervention contribute to weight loss and improvement in lipid profiles in obese men [
40]. NW training of varying duration and intensity tailored to the fitness levels of participants leads to improvements in body composition, reduction in waist circumference, improvement in lipid profile and adipokines, carbohydrate metabolism, reduction in oxidative stress, and inflammatory state in women with various degrees of obesity [
41,
42,
43,
44,
45,
46]. Physical activity, through increased cellular metabolism affecting adipose tissue, indirectly influences its metabolites.
It is indicated that NW training, used as an intervention to modify body composition, demonstrates greater effectiveness when supervised and conducted by qualified trainers [
47]. Therefore, in this project, supervised NW training led by a qualified trainer was implemented. The training sessions were conducted on soft surfaces to allow for full utilization of NW technique and to protect the joints of the participants. This is particularly important for individuals with obesity, where joint overload may be more pronounced. The same group of authors cited earlier also pointed out the greater effectiveness of NW compared to walking [
47]. NW training is also highly popular among various groups of patients requiring rehabilitation-focused training interventions, as evidenced by high adherence to training regimens, which was also observed in this project (unpublished data).
Analyzing the impact of the proposed intervention on body composition, a beneficial effect of combined training with TRE conducted over 12 weeks was observed on body mass (small effect size) as well as a reduction in fat tissue content (both expressed in kg - small effect size; and as a percentage of total body mass: large effect size). These data are highly significant for the selected group, which is characterized by abnormal body composition indicative of overweight or obesity. For CON12, it was indicated that after 12 weeks of observation, BFP was significantly higher than before starting the project, which is a characteristic manifestation of the progression of obesity as a disease. Similar effects have been observed earlier [
48], indicating the necessity of introducing interventions for obese individuals, as leaving them without any actions leads to disease progression.
On the other hand, it is not surprising that the combination of fasting with NW yielded favorable effects on body composition, considering that beneficial effects on body mass and BMI had already been observed for isolated interventions in the form of intermittent fasting [
49]. However, in these studies, fasting encompassed 10 hours, which for some patients (especially those with obesity) may pose a greater challenge.
NW workouts without dietary intervention in some of the published studies do not affect body weight, thus the reduction in BM in the SG12 group indicated in this study shows the beneficial effect of combining workouts with TRE. The results indicate that extending the intervention for an additional 12-24 weeks is likely to achieve even better results, but this needs to be confirmed in further studies. It is also worth noting that the proposed interventions led to an increase in TBW in the trained individuals. As early as 6 weeks into the intervention, water content began to increase, and this effect was also observed after 12 weeks of intervention. Although the effect was be evaluated as low, it is still beneficial from a clinical perspective.
In this study, a beneficial trend of decreasing concentrations of TC, TG, and LDL was observed in the fasting group for 6 weeks. However, in the fasting group for 12 weeks, these concentrations slightly increased. Opposite changes were observed for HDL in both groups. However, these differences were statistically insignificant. Similar favorable changes in the lipid profile after 6 weeks, as those obtained in this study in the SG6 group, but statistically significant, were observed in the study by Naseer et al. [
50]. This may indicate that a shorter intervention may have a more beneficial effect. In the study by Kortas et al. [
13], these parameters were also evaluated in combination with TRE and NW, but in an older age group. Similar to our study, no statistically significant differences were obtained. Therefore, it can be suggested that physical activity in groups with high burden should be used as an adjunctive method in the treatment of dyslipidemia rather than as a form of monotherapy.
It is indicated that TRE may affect blood morphological parameters. In a study conducted on patients with sickle cell anemia, a monthly TRE regimen during Ramadan influenced, in both women and men, a non-significant decrease followed by an increase in leukocyte count [
51]. Similarly, in both fasting groups in this study, there was an increase in leukocyte count, although it was statistically insignificant. The cited study also reported a significant increase in platelet count after a 4-week period of TRE, whereas in the groups in this study, the changes were not statistically significant. In the group undergoing fasting with training for 6 weeks, there was a decrease in platelet count, while in the SG12 group, there was a slight increase. However, the magnitude of changes in both groups was not clinically significant.
In the study by Gasmi et al. [
52], it was demonstrated that a 12-week fasting regimen can influence changes in blood morphology parameters in both older and younger men. Significant decreases in hematocrit, white blood cell count, lymphocytes, and neutrophils were observed in the participants. In our study, a 12-week fasting group also showed a decrease in hematocrit values; however, it was not statistically significant. Meanwhile, in both fasting groups, the leukocyte count increased, which may be related to the training effect, similar to the observed increases in neutrophil and lymphocyte counts. A similar effect was observed in patients with multiple myeloma participating in a 6-week cycle of Nordic walking training, where an increase in leukocyte count was also noted [
8].
The combination of dietary intervention and Nordic walking training was also studied in the context of peripheral blood morphology. In the study by Kortas et al. [
13], blood morphology parameters were evaluated after 12 weeks of TRE and NW training. Similar changes were observed in the present study regarding the white blood cell count, both after 6 and 12 weeks of intervention. Similarly, the number of erythrocytes changed in a similar manner after 6 and 12 weeks, although in this study, these changes were not statistically significant. The increase in leukocyte count may indicate stimulation of the immune system due to training combined with fasting. Training combined with fasting led to a significant decrease in HCT level in SG12 which could related with an increase in blood volume. In this context, a slight decrease in RBC count observed should not be associated with impaired erythropoiesis. Nevertheless, it is worth emphasizing that all observed results fell within the range of reference values, and further directions of changes associated with longer-term TRE would require additional research.
In this project, changes in the concentration of two selected adipokines under the influence of interventions were assessed. The mean concentration of leptin in the studied population was 29.0 ± 15.9 ng/ml, which, despite higher values of this adipokine in women than in men [
53], still indicates exceeding reference values and informs about pathologically high values. As previous observations indicate, training durations below 12 weeks do not affect the concentration of this adipokine (except in patients with diabetes) [
54]. It is also indicated that training has a stronger impact on leptin concentration in women than in men [
55]. The results of this study will complement these data by showing that the combined effect of 12 weeks of training and TRE is still not a strong enough stimulus to induce a change in leptin concentration. However, exercise protocols that result in fat mass reduction (as was the case in this project) lower leptin concentration. Therefore, most researchers report decreased leptin concentration after achieving fat tissue loss [
54]. However, there are conflicting results regarding long-term (>12 weeks) exercise studies, with many studies showing no effect on leptin concentration. For example, studies by Pasman et al. [
56] showed that the exercise-induced decrease in leptin concentration is independent of fat tissue content and serum glucose concentration. Similarly, in this project, no significant correlations were found between the magnitude of differences obtained before and after the training series and body composition (except for a correlation with SLM r=0.319). However, a relationship between baseline leptin concentration and body composition was indicated: BM, VFA, and the strongest correlation with BF. Negative correlations were observed for SLM. An interesting observation is the indication that the change in leptin concentration was negatively correlated with its baseline concentration, which can be interpreted as indicating that in individuals with pathologically high leptin concentration (leptin resistance?), changes induced by diet and training will be more challenging to achieve.
The average concentration of resistin in individuals in this project was 17.8 ± 6.4 pg/ml. Resistin concentration in healthy individuals does not differ by age and sex and correlates with BMI [
57]. However, in individuals with diabetes, resistin levels are not correlated with BMI. In this project, no correlation was found between resistin concentration and BMI. On the one hand, this may, indicate the presence of glycemic disturbances in the studied population or be a consequence of the group selection, where the minimum BMI was 25. Thus the distribution of this variable did not allow for a correct correlation analysis. Measurements after the specified time (6 or 12 weeks) showed lower resistin concentrations than baseline results. High resistin concentration is associated with insulin resistance [
57], so it can be indicated that the proposed interventions led to favorable changes in a population characterized by metabolic disorders, including carbohydrate metabolism disorders. However, the results obtained in this project do not unequivocally confirm this hypothesis..
5. Conclusions
The combination of Nordic walking training and time restricted eating is a well-tolerated intervention for individuals with disrupted body composition. Extended to 12 weeks, this intervention allows for improvement in body composition with a noticeable, strong effect on reducing fat tissue content. Neither 6 nor 12 weeks of training and fasting affected the lipoprotein profile, indicating the need for dietary modification not only concerning meal timing but also their quantity and quality. To achieve favorable effects, dietary consultations or pharmacological support are recommended. The conducted interventions did not affect leptin concentration; however, they allowed for a decrease in resistin concentration, which may indicate an improvement in carbohydrate homeostasis in the studied women despite no changes in fasting glucose concentration, although this requires confirmation in further studies. An interesting observation is the indication that the change in leptin concentration was negatively correlated with its baseline concentration, suggesting that in individuals with pathologically high leptin concentration, changes induced by diet and training will be more difficult to achieve.
Author Contributions
Conceptualization, A.P.; O.C-L. and T.P.; methodology, M.Ż., A.P., A.B and J.R.; software, Ł.R.; validation, M.MB., O.C-L. and A.D.; formal analysis, O.C-L. and A.P.; investigation, A.P., A.B and J.R.; resources, J.K.; data curation, W.K.; writing—original draft preparation, A.P.; writing—review and editing, E.Z., O.C-L., M.Ż. and A.B.; visualization, A.P.; supervision, M.Ż. and A.P..; project administration, A.P.; funding acquisition, A.P. and T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed as part of the Ministry of Science and Higher Education program titled “Regional Excellence Initiative” in the years 2019–2022 (Project No. 022/RID/2018/19) with a grant amount of 11,919,908 PLN (internal number at University: 47/PB/RID/2022) and the APC was funded by University of Physical Education in Krakow.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of the Regional Medical Chamber of Kraków, Poland (58/KBL/OLI/2022 dated April 11, 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to bioethics committee decision.
Acknowledgments
The authors would like to express their sincere thanks to prof. Wanda Pilch for the support and mentoring we received at every stage of this work: from the design of the experiment to the finalization of the project.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Harborg, S.; Kjærgaard, KA; Thomsen, R. ; Borgquist, S.; Cronin-Fenton, D.; Hjorth C. New Horizons: Epidemiology of Obesity, Diabetes Mellitus, and Cancer Prognosis. J Clin Endocrinol Metab. 2024, 109, 924–935 https://. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, B.; Hill, J.; Gunn, A. Obesity: Scope, Lifestyle Interventions, and Medical Management. Tech Vasc Interv Radiol, 2020, 23, 100653. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lastra, M.; Miller, K.; Martínez-Lemos, R.; Giráldez, A.; Ayán, C. Nordic Walking for Overweight and Obese People: A Systematic Review and Meta-Analysis. J Phys Act Health. 2020, 17, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Runenko, S.; Achkasov, E.; Volodina, K.; Zhukovskaya, A.; Mushkambarov, N.; Butko, D. Nordic Walking as an effective physical activity for weight loss among overweight young adults in high schools. J Sports Med Phys Fitness. 2020, 60, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Cebula, A.; Tyka, A.; Tyka, A.; Pałka, T.; Pilch, W.; Luty, L.; Mucha, D. Physiological response and cardiorespiratory adaptation after a 6-week Nordic Walking training targeted at lipid oxidation in a group of post-menopausal women. PLoS One. 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Morsø, L.; Bendix, T.; Manniche, C. Supervised and non-supervised Nordic walking in the treatment of chronic low back pain: a single blind randomized clinical trial. BMC Musculoskelet Disord. 2010, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Manca, A.; Yeo, T.; Bassareo, P.; Mercuro, G.; Kaski, J. ; Nordic walking for individuals with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2017, 24, 1938–1955. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska-Ledwig, O.; Jurczyszynm, A.; Piotrowska, A.; Pilch, W.; Antosiewicz, J.; Żychowska, M. The Effect of a Six-Week Nordic Walking Training Cycle on Oxidative Damage of Macromolecules and Iron Metabolism in Older Patients with Multiple Myeloma in Remission-Randomized Clinical Trial. Int J Mol Sci. 2023, 24, 15358. 24. [CrossRef]
- Hanuszkiewicz, J.; Woźniewski, M.; Malicka, I. The Influence of Nordic Walking on Isokinetic Trunk Muscle Endurance and Sagittal Spinal Curvatures in Women after Breast Cancer Treatment: Age-Specific Indicators. Int J Environ Res Public Health. 2021, 18, 2409. [Google Scholar] [CrossRef]
- Santoyo-Medina, C.; Janer Cabo, M.; Xaudaró, D.; Sanmillan, G.; Sanchez Pous, S.; Cartaña, I.; Meza Murillo, E.; Sastre-Garriga, J.; Montalban, X. Effect of Nordic Walking Training on Walking Capacity and Quality of Life for People With Multiple Sclerosis. Int J MS Care. 2023, 25, 118–123. [Google Scholar] [CrossRef]
- Angiolillo, A.; Leccese, D.; Ciccotelli, S.; Di Cesare, G.; D'Elia, K.; Aurisano, N.; Matrone, C.; Dentizzi, C.; Di Costanzo, A. Effects of Nordic walking in Alzheimer's disease: A single-blind randomized controlled clinical trial. Heliyon. 2023, 9, e15865. [Google Scholar] [CrossRef]
- Harro, C.; Horak, I.; Valley, K.; Wagner, D. Nordic walking training in persons with Parkinson's disease: Individualized prescription-A case series. Physiother Theory Pract. 2023, 39, 2208–2222. [Google Scholar] [CrossRef] [PubMed]
- Kortas, J.; Reczkowicz, J.; Juhas, U.; Ziemann, E.; Świątczak, A.; Prusik, K.; Olszewski, S.; Soltani, N.; Rodziewicz-Flis, E.; Flis, D.; Żychowska, M.; Gałęzowska, G.; Antosiewicz, J. Iron status determined changes in health measures induced by Nordic walking with time-restricted eating in older adults- a randomised trial. BMC Geriatr, /: 29, 300. https; 29. [CrossRef]
- Lubkowska, A.; Dudzińska, W.; Bryczkowska, I.; Dołęgowska, B. Body composition, lipid profile, adipokine concentration, and antioxidant capacity changes during interventions to treat overweight with exercise programme and whole-body cryostimulation. Oxid Med Cell Longev, 2015. [Google Scholar] [CrossRef]
- Hutchison, A.; Liu, B.; Wood, R.; Vincent, A.; Thompson, C.; O'Callaghan, N.; Wittert, G.; Heilbronn, L. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity (Silver Spring). 2019, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Drăgoi, C. Intermittent Fasting on Human Health and Disease. Nutrients. 2023, 15, 4491. [Google Scholar] [CrossRef] [PubMed]
- Uher, I.; Kűchelová, Z.; Icimboláková, I.; Pivovarník, J. Intermittent fasting and its influence on health. Physical Activity Review. 2016, 4, 184–191. [Google Scholar] [CrossRef]
- Stockman, M.; Thomas, D.; Burke, J.; Apovian, C. Intermittent Fasting: Is the Wait Worth the Weight? Curr Obes Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Hong, N.; Kim, K.; Cho, S.; Lee, M.; Lee, Y.; Lee, Y.; Kang, E.; Cha, B.; Lee, B. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J Clin Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.; Bradshaw, P.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017, 69, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Dzidek, A.; Czerwińska-Ledwig, O.; Żychowska, M.; Pilch, W.; Piotrowska, A. The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms. Int J Mol Sci. 2023, 24, 9655. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Gonzalez-Gay, A.; Mera, A.; Lago, F.; Gómez, R.; Mobasheri. A.; Gualillo, O. Adipokines and inflammation: is it a question of weight? Br J Pharmacol. 2018, 175, 1569–1579. [Google Scholar] [CrossRef]
- Zheng, S.; Du, D.; Li, X. Leptin Levels in Women With Polycystic Ovary Syndrome: A Systematic Review and a Meta-Analysis. Reprod Sci. 2017, 24, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Gogga, P.; Karbowska, J.; Meissner, W.; Kochan, Z. [Role of leptin in the regulation of lipid and carbohydrate metabolism]. Postepy Hig Med Dosw (Online). 2011, 65, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Giovannetti, E.; Aquilano, K. Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging (Albany NY). 2016, 8, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; Fernando, F.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Letizia, C.; Jimenez, A.; Fallucca, F.; Pugliese, G. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010, 20, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Janowska, J.; Zahorska-Markiewicz, B.; Olszanecka-Glinianowicz, M. Relationship between serum resistin concentration and proinflammatory cytokines in obese women with impaired and normal glucose tolerance. Metabolism. 2006, 55, 1495–1149. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Li, Y.; Zhang, D.; Yuan, J.; Zhang, C.; Liu, Y.; Song, L.; Lin, Q.; Li, M.; Dong, J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front Physiol. 2019, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J Sport Health Sci, /: 12, 147-157. https; 12. [CrossRef]
- Vuolteenaho, K.; Leppänen, T.; Kekkonen, R.; Korpela, R.; Moilanen, E. Running a marathon induces changes in adipokine levels and in markers of cartilage degradation--novel role for resistin. PLoS One. 2014, 9, e110481. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, L. , Parker, A. Gender, physical activity and fear: women, exercise and the great outdoors. Qual Res Sport Exerc Health. 2013, 5, 43–57. [Google Scholar] [CrossRef]
- Hunter, G.; Weinsier, R.; Gower, B.; Wetzstein, C. Age-related decrease in resting energy expenditure in sedentary white women: effects of regional differences in lean and fat mass. Am J Clin Nutr, /: 73, 333-337. https; 73. [CrossRef]
- Anekwe, C.; Jarrell, A.; Townsend, M.; Gaudier, G.; Hiserodt, J.; Stanford, F. Socioeconomics of Obesity. Curr Obes Rep. 2020, 9, 272–279. [Google Scholar] [CrossRef]
- Konttinen, H.; van Strien, T.; Männistö, S.; Jousilahti, P.; Haukkala, A. Depression, emotional eating and long-term weight changes: a population-based prospective study. Int J Behav Nutr Phys Act. 2019, 16, 28. [Google Scholar] [CrossRef]
- Piotrowicz, E.; Zieliński, T.; Bodalski, R.; Rywik, T.; Dobraszkiewicz-Wasilewska, B.; Sobieszczańska-Małek, M.; Stepnowska, M.; Przybylski, A.; Browarek, A.; Szumowski, Ł.; Piotrowski, W.; Piotrowicz, R. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol. 2015, 22, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Kantaneva, M. Sauvakävely – Nordic Walking. 2005 [https://onwf.
- Oja, P.; Laukkanen, R.; Pasanen, M.; Tyry, T.; Vuori, I. A 2-km walking test for assessing the cardiorespiratory fitness of healthy adults. Int J Sports Med. 1991, 12, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004, 50, 1113–1115. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Gdańska, A.; Rozpara, M.; Pilch, W.; Přidalová, M.; Bańkowski, S. Effect of 12-Week Interventions Involving Nordic Walking Exercise and a Modified Diet on the Anthropometric Parameters and Blood Lipid Profiles in Overweight and Obese Ex-Coal Miners. Obes Facts. 2020, 13, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Cebula, A. ; Tyka, A,; Pilch, W. Effects of 6-week Nordic walking training on body composition and antioxidant status for women 55 years of age Int J Occup Med Environ Health. 2017, 30, 445–454. [Google Scholar] [PubMed]
- Pellegrini, B.; Peyré-Tartaruga, L.; Zoppirolli, C.; Bortolan, L.; Bacchi, E.; Figard-Fabre, H.; Schena, F. Exploring Muscle Activation during Nordic Walking: A Comparison between Conventional and Uphill Walking. PLoS One. 2015, 10, e0138906. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Tota, Ł.; Piotrowska, A.; Śliwicka, E.; Czerwińska-Ledwig, O.; Zuziak, R.; Pilaczyńska-Szcześniak, Ł. Effects of Nordic Walking on Oxidant and Antioxidant Status: Levels of Calcidiol and Proinflammatory Cytokines in Middle-Aged Women. Oxid Med Cell Longev. 2018, 2018, 6468234. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Tota, Ł.; Sadowska-Krępa, E.; Piotrowska, A.; Kępińska, M.; Pałka, T.; Maszczyk, A. The Effect of a 12-Week Health Training Program on Selected Anthropometric and Biochemical Variables in Middle-Aged Women. Biomed Res Int. 2017, 2017, 9569513. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Li, Q.; Wang, J.; Tan, S. Exercise Training at Maximal Fat Oxidation Intensity for Overweight or Obese Older Women: A Randomized Study. J Sports Sci Med. 2019, 18, 413–418. [Google Scholar] [PubMed]
- Kantorowicz, M.; Szymura, J.; Szygula, Z.; Kusmierczyk, J.; Maciejczyk, M.; Wiecek, M. Nordic walking at maximal fat oxidation intensity decreases circulating asprosin and visceral obesity in women with metabolic disorders. Front Physiol. 2021, 12, 726783. [Google Scholar] [CrossRef]
- Muollo, V.; Rossi, A.; Milanese, C.; Zamboni, M.; Rosa, R.; Schena, F.; Pellegrini, B. Prolonged unsupervised Nordic walking and walking exercise following six months of supervision in adults with overweight and obesity: A randomised clinical trial. Nutr Metab Cardiovasc Dis. 2021, 31, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Grodecka, A.; Czerwińska-Ledwig, O.; Dzidek, A.; Lis, W.; Cwalińska, D.; Kozioł, W.; Teległów, A.; Pałka, T.; Piotrowska, A. Effect of the hypoxic chamber training series on skin characteristics of overweight and obese women. Cosmetics 2023, 10, 128. [Google Scholar] [CrossRef]
- Manoogian, E.; Zadourian, A.; Lo, H.; Gutierrez, N.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.; Wang, X.; Sui, J.; Hou, Z.; Fleischer, J.; Golshan, S.; Taub, P.; Panda, S. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Farooq, J.; Siddiqi, H.; Meo, S.; Kulsoom, B.; Laghari, A.; Jamshed, H.; Pasha, F. Impact of Intermittent Fasting on Lipid Profile-A Quasi-Randomized Clinical Trial. Front Nutr. 2021, 7, 596787. [Google Scholar] [CrossRef]
- Ahmed, K.; Abdu, Y.; Khasawneh, S.; Shukri, A.; Adam, E.; Mustafa, S.; Affas, M.; Mohamed Ibrahim, M.; Al Zayed, A.; Yassin, M. The effect of intermittent fasting on the clinical and hematological parameters of patients with sickle cell disease: A preliminary study. Front Med (Lausanne). 2023, 10, 1097466. [Google Scholar] [CrossRef]
- Gasmi, M.; Sellami, M.; Denham, J.; Padulo, J.; Kuvacic, G.; Selmi, W.; Khalifa, R. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition. 2018, 51-52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Luo, Y.; Li, Y.; Zhang, F.; Zhang, X.; Zhou, X.; Ji, L. Sex- and body mass index-specific reference intervals for serum leptin: a population based study in China. Nutr Metab (Lond). 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.; Chu, H.; Castracane, V. Leptin and exercise. Exp Biol Med (Maywood). 2002, 227, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.; Houmard, J.; Considine, R.; Tyndall, G.; Midgette, J.; Gavigan, K.; Weidner, M.; McCammon, M.; Israel, R.; Caro, J. Gender-dependent effects of exercise training on serum leptin levels in humans. Am J Physiol. 1997, 272, E562–566. [Google Scholar] [CrossRef]
- Pasman, W.; Westerterp-Plantenga, M.; Saris, W. The effect of exercise training on leptin levels in obese males. Am J Physiol. 1998, 274, E280–286. [Google Scholar] [CrossRef]
- Schäffler, A.; Büchler, C.; Müller-Ladner, U.; Herfarth, H.; Ehling, A.; Paul, G.; Schölmerich, J.; Zietz, B. Identification of variables influencing resistin serum levels in patients with type 1 and type 2 diabetes mellitus. Horm Metab Res. 2004, 36, 702–707. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).