Introduction
Enterococcus enterica Typhoid fever is a result of infection by the Gram-negative bacteria known as serotype Typhi (Ashurst et al, 2022). Ashurst et al. attribute the naming of "typhoid fever" to Pierre Louis in 1829. Louis saw lesions in the abdominal lymph nodes of a patient who had succumbed to "gastric fever." The term "typhus" originates from the Greek word meaning "smoky," and it was employed to describe the state of madness that afflicted the sufferer. Nevertheless, in 1880, a German pathologist named Karl Eberth discovered Salmonella enterica, the microorganism responsible for causing typhoid fever. In 1884, Georg Gaffky achieved the successful cultivation of S. enterica for the first time. Consequently, Almorth Wright developed a vaccine few years later (Ashurst et al, 2022).
S. enterica serotype typhi encompasses Salmonella typhimurium. The term "serotype" pertains to a categorization framework for bacteria that relies on the antigens or other compounds found on their surface (CDC, 2022). The presence of S. enterica serotype typhi is typically observed when individuals consume water or food that has been contaminated with the excretions of infected humans. Once it has attached itself to the small intestines, it must endure the gastric pH barrier, which is the mucus-bicarbonate barrier in the stomach, in order to survive. The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) facilitates either direct penetration into the epithelial cells or transit through the M-cell, a distinct lymphoid epithelial cell (Ashurst et al., 2022). The development of biofilms by S. typhimurium has been documented in studies conducted by Yahya et al. in 2017 and 2018, as well as by Yaacob et al. in 2021.
Biofilms are stationary aggregations of microorganisms that inhabit and proliferate on the surfaces of medical devices, such as catheters, through the secretion of extracellular polymeric compounds. These substances have the potential to induce infection. Biofilms are formed by densely populated communities of bacteria held together by an extracellular matrix (ECM). This matrix contains several substances released by the bacteria, including exopolysaccharides (EPS), extracellular DNA (eDNA), proteins, and amyloidogenic proteins (Sharma et al., 2019; Kamaruzzaman et al., 2022). In addition, they synthesise a variety of functional proteins that aid in their metabolism during periods of dormancy (Othman and Yahya 2019; Isa et al. 2022). A fraction of the bacteria in the natural environment survives by floating, however the majority of bacteria thrive by forming biofilms in order to adapt to the challenging conditions. After the formation of the biofilm, its susceptibility to damage from several chemicals and antibiotics is much diminished (Zakaria et al. 2023). Hence, it is arduous to entirely eliminate it. As a result, high concentrations of antibiotics are ineffective in eliminating pathogenic biofilms due to the bacteria's effective protection within the biofilm structure. According to Zhao et. al. (2017), biofilms exhibit greater resistance to antimicrobials compared to planktonic cells. As a result, there has been ongoing investigation into the discovery of novel antimicrobial agents derived from natural sources (Zawawi et al., 2020; Man et al., 2022).
Benzoic acid is a colourless compound with an aromatic structure and a carboxylic acid functional group. It occurs naturally in plants, fungi, and animal tissues (Joye, 2019). Benzoic acid is a chemical composed of a benzene ring core with a carboxylic substituent attached to it. Derivatives of this substance are frequently used as antibacterial and antifungal agents or flavour enhancers in the food sector (Del Olmo et. al., 2017). Benzoic acid is frequently employed as an antimicrobial preservative in food and beverages due to its potent antibacterial properties, which are most effective within a pH range of 2.5 - 4.0. According to Kalpana and Rajeswari (2019), benzoic acid has the ability to hinder the growth of bacteria and yeasts, which are significant contributors to food spoiling. A reassessment of the effectiveness of benzoic acid against S. typhimurium biofilm at various incubation durations is necessary. The aim of this study was to investigate the impact of varying concentrations of benzoic acid on the overall biomass and surface coverage of S. typhimurium biofilm after 48 and 72 hours.
Methodology
Preparation of Test Microorganism
The S. typhimurium ATCC 14028 culture was transferred onto a Nutrient Agar (NA) plate using a sterile inoculating loop and then incubated at a temperature of 37 ºC for a duration of 24 hours. The colonies that are likely to be present and their physical characteristics that develop on the NA plate were observed and documented. Following the incubation time, a little amount of the uncontaminated colony was subjected to Gramm staining and examined using a microscope.
Pellicle Assay
The pellicle assay was conducted using the following steps: (i) the medium in the test tube was drained and dried for 10 minutes, (ii) the inner surface of the test tube was rinsed with distilled water, (iii) the test tube was then stained with 0.5% (w/v) crystal violet, (iv) the stain was washed off with distilled water and allowed to dry, (v) the pellicle biofilm was observed by examining the stained surface and looking for ring formation in the test tube.
Biofilm Surface Coverage Assay
The biofilm surface coverage assay was employed to assess the antibiofilm efficacy of benzoic acid against S. typhimurium biofilm. Initially, glass cover slips were inserted into each well of a 6-well microplate. The wells of the test group were filled with a 2 mL mixture of overnight bacterial inoculum and a 2 mL solution of benzoic acid ranging from 3.125% (v/v) to 50% (v/v). Two mL of fresh medium and two mL of bacterial inoculum were added to the negative control well. Following a 48-hour incubation period at a temperature of 37 ºC, the glass cover slips were cautiously removed and washed three times using sterile PBS. Following a 15-minute staining period using a 0.5% (w/v) crystal violet solution, the glass cover slips were delicately rinsed with water to eliminate any residual dye. Analysed with a light microscope, the stained biofilm on glass cover slips was examined. The images were captured at a magnification of 100x. The experiment was repeated for an incubation period of 72 hours.
Biofilm Biomass Assay
The susceptibility of S. typhimurium biofilm was evaluated at five distinct concentrations, ranging from 3.125% (v/v) to 50% (v/v), using a sterile 96-well microplate. The test groups were administered with 100 μL of benzoic acid solution and 100 μL of bacterial inoculum. The negative control wells were filled with 100 μL of fresh medium and 100 μL of bacterial inoculum. Following a 48-hour incubation at a temperature of 37 ºC, the liquid containing freely floating cells was removed from the 96-well microplate and washed with a PBS solution two times. The biofilm wells, which were attached, were subjected to heat fixation at a temperature of 60 ºC for a duration of 30 minutes. This was followed by staining with crystal violet solution at a concentration of 0.5% for a period of 5 minutes at room temperature. The soiled well was meticulously disposed of and cleansed with a PBS solution. Stained-biofilm wells were filled with a solution of absolute ethanol. The quantification of biofilm biomass was conducted using a BioTek Synergy H1 Hybrid microplate reader (Yahya et al., 2018) at a wavelength of 600nm, with slight modifications. The experiment was repeated for an incubation period of 72 hours.
Data Analysis
The mean, standard deviation, and percent inhibition of all data from the antibiofilm screening were calculated using n=5. An independent T-test was used to assess the level of significance of the difference between control and test groups, with p-value of <0.05 being deemed significant.
Results and Discussion
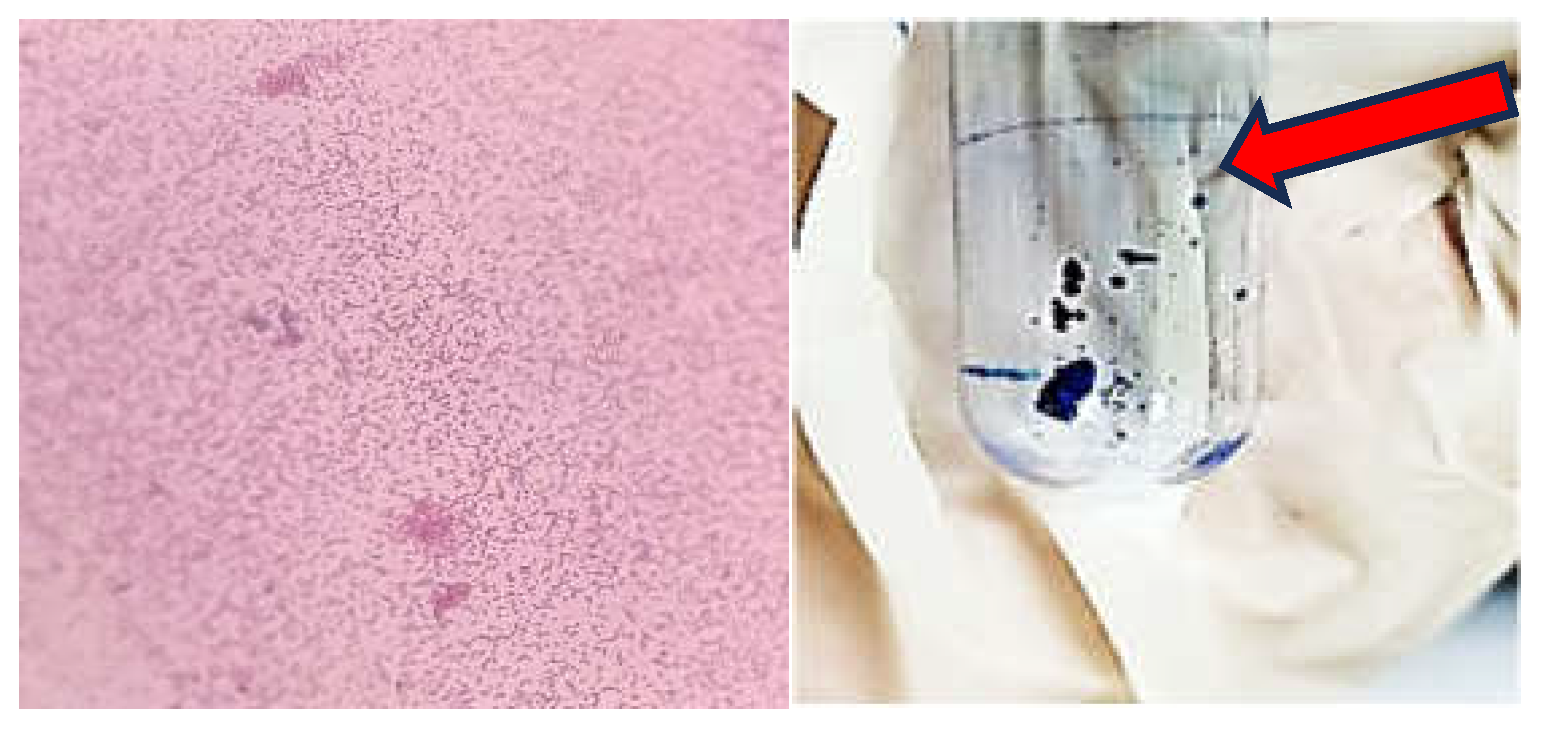
The Gram staining revealed the morphology of S. Typhimurium (
Figure 1, left panel). The pellicle assay demonstrated the formation of a ring at the air-liquid interface (
Figure 1, right panel). This confirms that S. Typhimurium is capable of forming biofilms, particularly at the liquid-air interface.
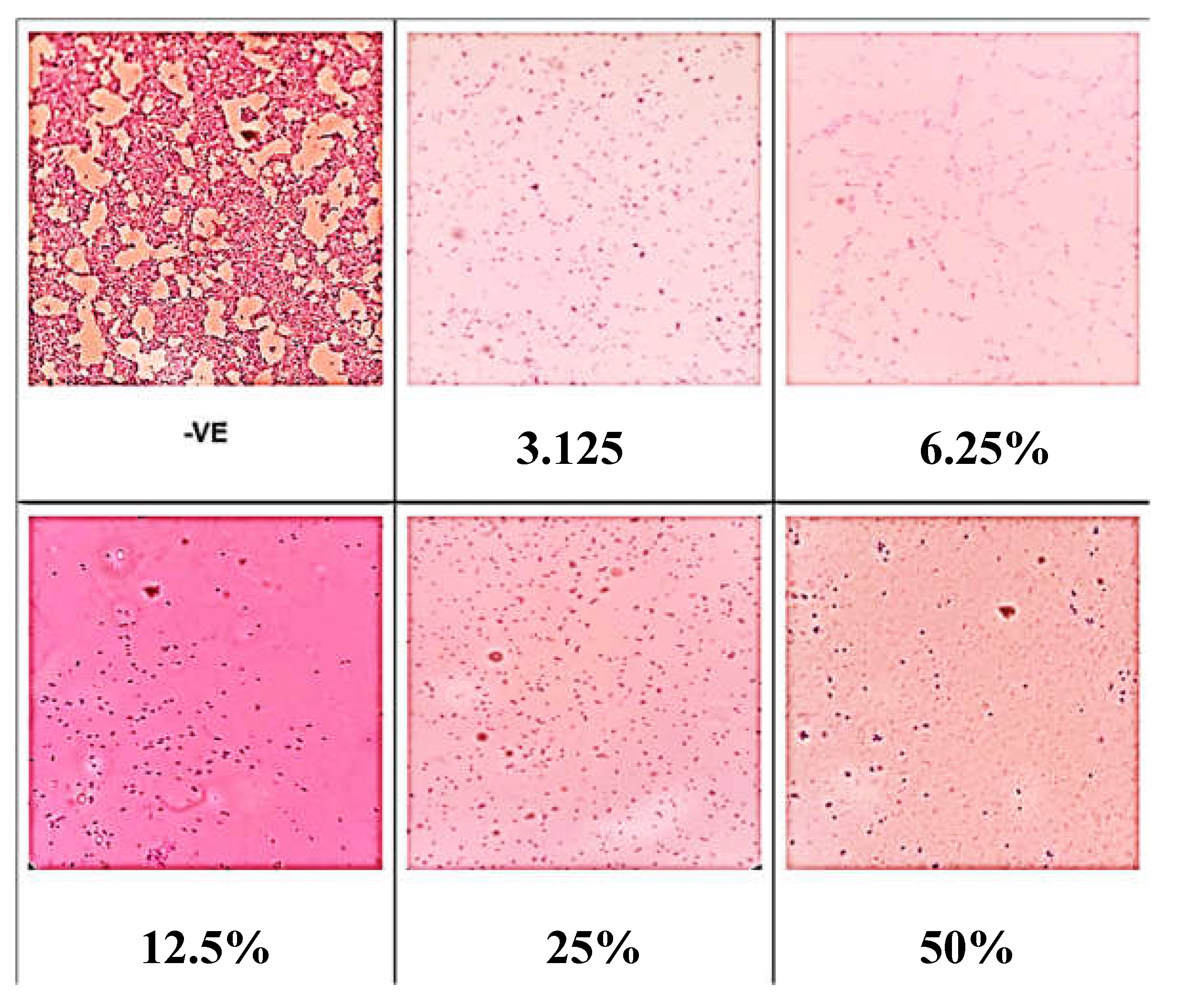
The negative control indicating conditions without any treatment or intervention, exhibited the highest surface coverage by biofilm (
Figure 2). This shows that under normal conditions, without any inhibitory agent, the biofilm formation was most extensive. At 48 h, all tested concentrations of benzoic acid resulted in a reduction in biofilm surface coverage. This demonstrates the effectiveness of benzoic acid in inhibiting biofilm formation across various concentrations.
Similarly, the negative control, which represents conditions without any treatment or intervention, showed the maximum amount of biofilm covering on the surface after 72 hours (
Figure 3). This demonstrates that in the absence of any inhibitory agent, the biofilm growth was the most widespread under typical conditions. Every concentration of benzoic acid that was tested led to a decrease in the extent to which the biofilm covered the surface. This study showcases the efficacy of benzoic acid in suppressing the growth of biofilms at different doses.
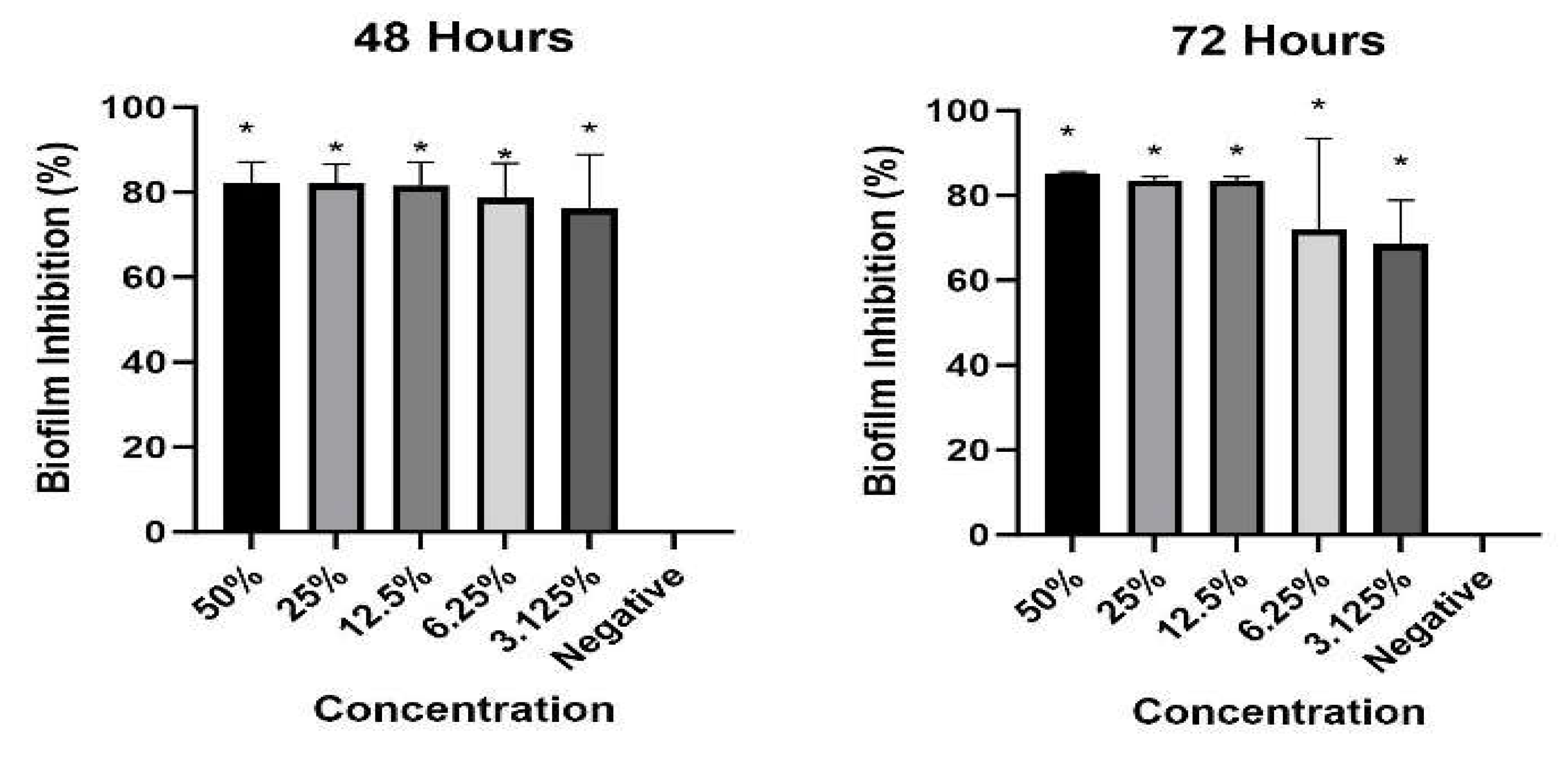
Across all tested concentrations, benzoic acid significantly inhibited biofilm biomass (
Figure 4). Higher concentrations of benzoic acid resulted in lower biofilm biomass. This concentration-dependent response indicates that the effectiveness of benzoic acid in inhibiting biofilm formation increases with higher concentrations. The inhibitory effects of benzoic acid on biofilm biomass were observed at both 48 hours and 72 hours.
Patra et al. (2021) reported varying frequencies for prevalence of Salmonella Typhimurium as well as subtle evidence on resistance of this pathogen to multiple antibiotics in different countries in South East Asia. Vietnam ranked top in terms of reports for prevalence and antimicrobial resistance. Although the pathogen was not found as dominant serovar in South East Asia in last 20 years unlike sub-Saharan Africa, it may be still considered as a major threat in this region due to available evidences for infection in humans as well as contamination in several animal and food sources. On the other hand, Moghadam et al. (2023) demonstrated that most of the antibiotics commonly used in the livestock and poultry industries are ineffective against most Salmonella isolates. This finding underscores the need for alternative approaches to combat Salmonella infections. The antibiofilm activity of benzoic acid against S. typhimurium reported herein corroborates a recent study conducted by Rohatgi and Gupta (2023) demonstrating its efficacy against Klebsiella pneumoniae biofilm. Antibiofilm activity of benzoic acid against Pseudomonas aeruginosa has also been reported (Kınaytürk et al. 2023).
Conclusion
All concentrations of benzoic acid led to reduced biofilm surface coverage. Benzoic acid also significantly inhibited biofilm biomass across all concentrations, with higher concentrations resulting in lower biomass, indicating a concentration-dependent response. This finding suggests that benzoic acid is useful for controlling Salmonella infections.
References
- Ashurst, J. V., Truong, J., & Woodbury, B. (2022). Salmonella typhi - statpearls - NCBI bookshelf. Retrieved January 14, 2023, from https://www.ncbi.nlm.nih.gov/books/NBK519002/.
- Centers for Disease Control and Prevention (2019). Drug-resistant salmonella serotype typhi - centers for disease control and prevention. Retrieved January 14, 2023, from https://www.cdc.gov/drugresistance/pdf/threats-report/salmonella-typhi-508.pdf.
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2015, 57, 3084–3103. [CrossRef]
- Isa, S.F.M.; Hamid, U.M.A.; Yahya, M.F.Z.R. Treatment with the combined antimicrobials triggers proteomic changes in P. aeruginosa-C. albicans polyspecies biofilms. ScienceAsia 2022, 48, 215–222. [CrossRef]
- Joye, I. J. (2019). Acids and bases in food. Encyclopedia of Food Chemistry, 1–9. [CrossRef]
- Kalpana, V. N., & Rajeswari, V. D. (2019). Preservatives in beverages: Perception and needs. Preservatives and Preservation Approaches in Beverages, 1–30. [CrossRef]
- Kamaruzzaman, A.N.A.; Mulok, T.E.T.Z.; Nor, N.H.M.; Yahya, M.F.Z.R. FTIR SPECTRAL CHANGES IN Candida albicans BIOFILM FOLLOWING EXPOSURE TO ANTIFUNGALS. Malays. Appl. Biol. 2022, 51, 57–66. [CrossRef]
- Kınaytürk, N.K.; Önem, E.; Oturak, H. Benzoic acid derivatives: Anti-biofilm activity in Pseudomonas aeruginosa PAO1, quantum chemical calculations by DFT and molecular docking study. Bull. Chem. Soc. Ethiop. 2022, 37, 171–181. [CrossRef]
- Man, C.A.I.C.; Razak, W.R.W.A.; Yahya, M.F.Z.R. ANTIBACTERIAL AND ANTIBIOFILM ACTIVITIES OF Swietenia macrophylla King ETHANOLIC EXTRACT AGAINST FOODBORNE PATHOGENS. Malays. Appl. Biol. 2022, 51, 45–56. [CrossRef]
- Moghadam, M.N.; Rahimi, E.; Shakerian, A.; Momtaz, H. Prevalence of Salmonella Typhimurium and Salmonella Enteritidis isolated from poultry meat: virulence and antimicrobial-resistant genes. BMC Microbiol. 2023, 23, 1–8. [CrossRef]
- Othman, N.A. & Yahya; F.Z.R. In silico analysis of essential gene and non-homologous proteins in Salmonella typhimurium biofilm. Journal of Physics: Conference Series, 2019, 1349, 012133. [Google Scholar]
- Patra, S.D.; Mohakud, N.K.; Panda, R.K.; Sahu, B.R.; Suar, M. Prevalence and multidrug resistance in Salmonella enterica Typhimurium: an overview in South East Asia. World J. Microbiol. Biotechnol. 2021, 37, 1–17. [CrossRef]
- Rohatgi, A.; Gupta, P. Benzoic acid derivatives as potent antibiofilm agents against Klebsiella pneumoniae biofilm. J. Biosci. Bioeng. 2023, 136, 190–197. [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [CrossRef]
- Yaacob, M.F.; Murata, A.; Nor, N.H.M.; Jesse, F.F.A.; Yahya, M.F.Z.R. Biochemical composition, morphology and antimicrobial susceptibility pattern of Corynebacterium pseudotuberculosis biofilm. J. King Saud Univ. - Sci. 2020, 33, 101225. [CrossRef]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Antibiofilm activity and mode of action of DMSO alone and its combination with afatinib against Gram-negative pathogens. Folia Microbiol. 2017, 63, 23–30. [CrossRef]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Subtractive Protein Profiling of Salmonella typhimurium Biofilm Treated with DMSO. Protein J. 2017, 36, 286–298. [CrossRef]
- Zakaria, N.F.S., Yahya, M.F.Z.R., & Jamil, N.M. (2023). Multiple Bacterial Strategies to Survive Antibiotic Pressure: A Review. Preprints 2023, 2023040591.
- Zawawi, W.M.A.W.M.; Ibrahim, M.S.A.; Rahmad, N.; Hamid, U.M.A.; Yahya, M.F.Z.R. Proteomic analysis of Pseudomonas aeruginosa biofilm treated with Chromolaena odorata extracts. Malays. J. Microbiol. 2020, 16, 124–133. [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).