1. Introduction
Excessive body fat causing overweightness and obesity is a major pandemic this century, with over a third of adults affected worldwide [
1,
2,
3]. When fat is stored in excess, over time it can cause numerous non-communicable diseases including high blood pressure, type 2 diabetes, fatty liver disease, heart disease, and cancer [
4]. While there are numerous pharmaceuticals to treat obesity, these are not without important side effects. Therefore, discovering natural alternatives and taking preventive measures before the excess body weight progresses to further health complications, is a growing trend [
5].
To this end, plant polyphenols have been found to possess numerous health benefits, including anti-inflammatory, antioxidant, anti-lipogenic and anti-glycemic effects [
6,
7]. For example, plant extracts such as
Hibiscus sabdariffa and
Lippia citriodora have been found to be capable of modulating AMPK activation pathways [
8,
9]. AMPK activation in turn inactivates certain pathways, reducing LDL cholesterol and fatty acid synthesis [
10,
11]. In this sense, there are numerous studies, both preclinical and clinical, that have shown how the combination of these extracts can contribute to reducing body fat, which in turn causes weight loss [
12,
13,
14,
15,
16,
17,
18,
19,
20]. Furthermore, studies have revealed beneficial effects in lowering blood pressure, cholesterol and decreasing appetite, mainly by modulating the satiety-inducing hormone GLP-1.
However, the effects of the ingredient have been clinically tested only with a 500mg daily dose. Therefore, it is unknown if other doses, particularly a lower one, may have different results. To this end, the objective of this study is to assess if this botanical blend may have certain benefits when taken at a lower dose, in this case 300mg. The benefits in using a lower dose reside on reducing the number of capsules to take daily (and thus avoiding pill fatigue and therefore increasing compliance), while also providing a more personalized solution, as depending on the results of the study and the consumer´s expectations with the product, a specific dose may be recommended to the consumer.
2. Materials and Methods
2.1. Dietary Ingredient Description
A double-blind, placebo-controlled, randomized clinical study was conducted. The placebo group took 1 capsule per day, comprising 300 mg each of excipient (microcrystalline cellulose). The experimental product contained a total of 300mg of the botanical blend (which is under the umbrella of the trademark Metabolaid®), comprised of lemon verbena and hibiscus extracts. The composition has been published in previous reports [
12,
14]. The participants were instructed to take the product 30 minutes before breakfast.
2.2. Study Design
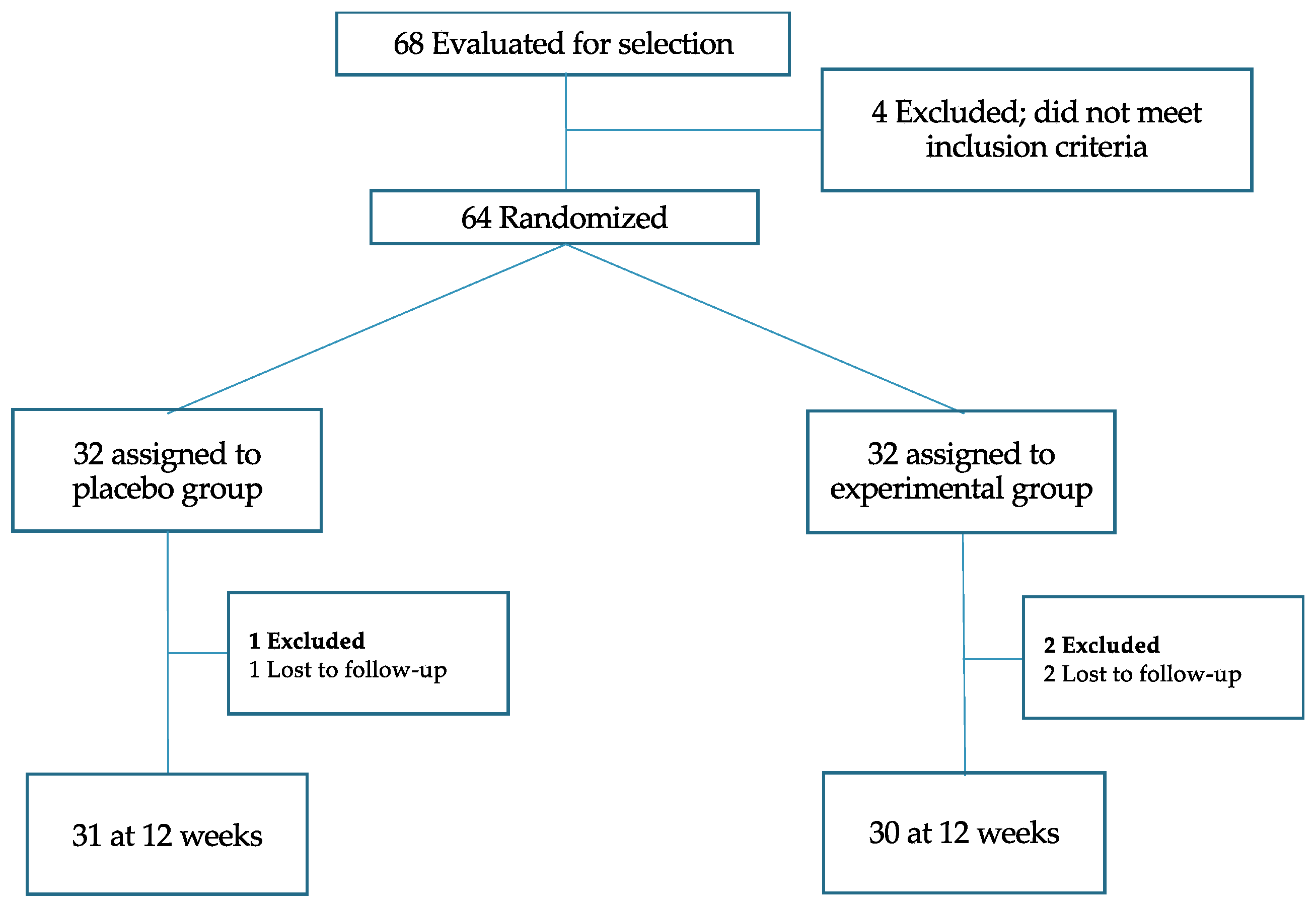
A randomized clinical trial was carried out in which participants were assigned to two groups (placebo and experimental), to determine the effectiveness of a nutraceutical in reducing body weight and improving various health parameters (registration number NCT05906771 in
www.clinicaltrials.gov). Following published recommendations, subjects were electronically randomized using a two-arm block design (
Figure 1).
2.3. Participants
A total of 61 participants, average age 41.7±9.78 years, were recruited at a clinic in Elche, Alicante (Spain) during 2023. Of the total sample, 33 were men (42.6±8.34 years) and 28 were women (41.1±11.0 years). All were volunteers and were required to meet the following inclusion criteria: 1) Adults, 2) Overweight (body mass index 25-29.9 kg/m2), 3) non-medicated, and overall healthy. Details on the participant’s biological parameters can be found in
Table 1.
2.4. Declarations: Ethical Approval, Consent to Participate and Consent to Publication
The present study was carried out in accordance with the standards of the Declaration of Helsinki. The Human Research Ethics Committee of the University of Alicante (Spain) granted approval to conduct a randomized trial (UA-2023-05-08) and all study participants gave written consent prior to participation. Additionally, the researchers maintained the confidentiality of all participants' personal data, coding personal information for this purpose.
2.5. Intervention
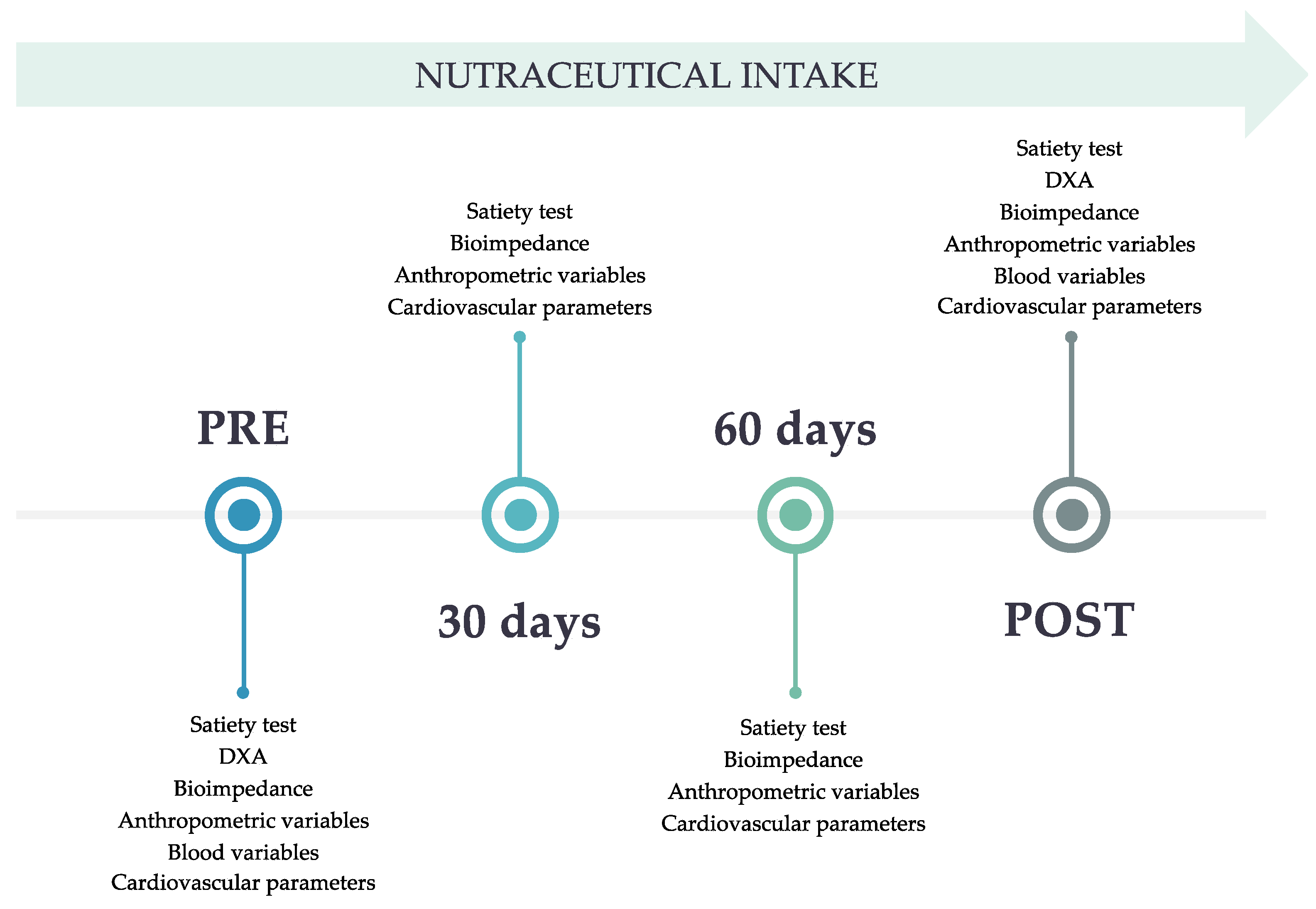
The study was divided into several phases, where specific variables were measured (
Figure 2).
2.6. Study Variables
2.6.1. Satiety
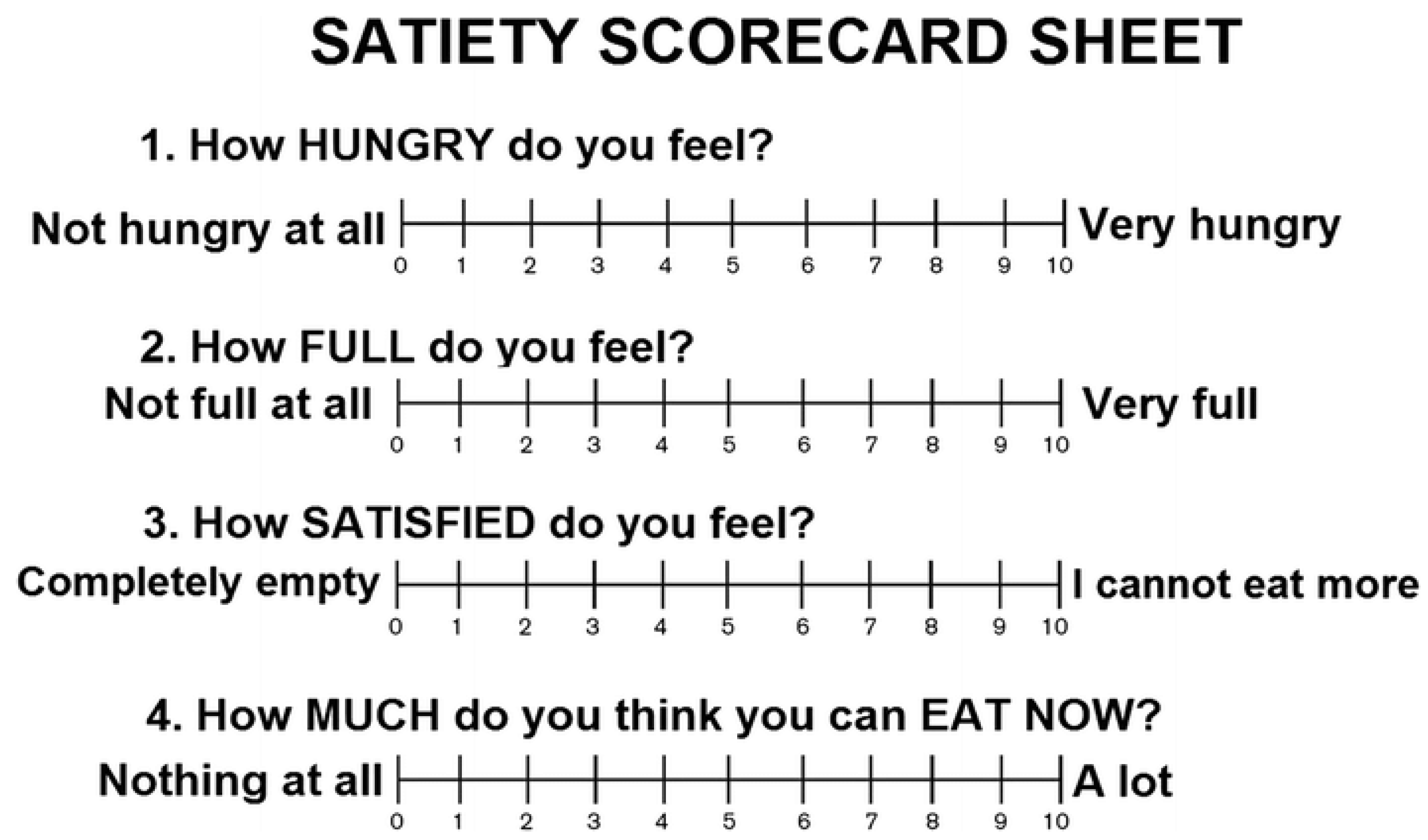
Satiety was assessed using the visual analog scale (VAS), a previously validated questionnaire [
21,
22] that was applied before, immediately after, and at the time of a standardized breakfast [
23]. The satiety test was performed at four different times during the intervention period (initial visit, 30 days, 60 days and 90 days). To perform this test, participants were asked to arrive at the laboratory (in cases where attendance in person was not possible, a video call was performed) in the morning after a 12-h overnight fast, and to refrain from alcohol consumption and intense physical activity for 24 h prior to the testing session. The standardized breakfast had an energy content of 616 kcal (2577,34) for men and 477 kcal (1995,77 kJ) for women [
3]. For men, breakfast consisted of 200mL of semi-skimmed milk or unsweetened soy drink, 100g of whole grain bread with 6g of oil, 50g of chicken or turkey breast, 20g of walnuts, and 200g of apple. Macronutrient content comprised of 82g of carbohydrates, 19g of fat, and 29g of protein. For women, breakfast included 200mL of semi-skimmed milk, 60g of whole grain bread with 3g of oil, 40g of chicken or turkey breast, 15g of walnuts, and 200g of apple. Macronutrient content was 65g of carbohydrates, 15g of fat, and 23g of protein. All participants were instructed to consume the entire meal within 30 minutes. Before, immediately after, and 60 minutes after the standardized breakfast, participants were asked to record their appetite sensations using visual analog scales (VAS), evaluating their
"desire to eat," "hunger," "fullness," and
"prospective food consumption”. Participants were asked to indicate on a scale from 0 to 10 how they felt at the time they answered these questions, for example,
"How strong is your desire to eat?" (very weak—very strong). Subjects were asked to mark the line corresponding to their feelings at each moment.
Figure 3 summarizes the VAS questions and the possible answers and ratings.
2.6.2. Blood Parameters
Blood samples were drawn, and the triglycerides, cholesterol, and glucose levels were assessed using an Accutrend® Plus. The results were obtained in milligrams/deciliter (mg/dL). The Accutrend® Plus test employs capillary serum and is based on the separation of blood cells through filtration with fiberglass when a blood drop is placed on the reactive strip. An enzymatic reaction within the strip occurs upon exposure to oxygen, resulting in a color change. The strip's reflectance is measured at 660 nm, and the concentration of the various circulating parameters is determined using a straightforward algorithm. The accuracy of the Accutrend® Plus, as stated in the product documentation, is 3.4% [
24]. HbA1c were determined using A1cNow®+ (Metrika, Inc., Sunnyvale, CA). It has been determined that HbA1c measured with A1cNow InView is accurate, as shown by the correlation coefficient ("r") of 0.96 [
25].
2.6.3. Cardiovascular Parameters
At each visit, blood pressure was measured on one arm using the Omron RS2 (OMRON Healthcare, Mannheim, Germany) wrist blood pressure monitor. Participants were instructed to sit and unwind for 10 minutes before the measurement was taken. Measurements of both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were conducted during each assessment. The units of measurement are millimeters of mercury (mm Hg).
2.6.4. Assessment of Body Composition and Anthropometry
In this project, the assessment of body composition was carried out using Tanita BC-730F, BIODY XPERTZM and bone densitometry (DXA). Weight was measured with the validated Tanita BC-730F bioimpedance scale (Amsterdam, Netherlands). Participants came to the measurement wearing comfortable, loose clothing, avoiding clothing with zippers, belts or metal buttons. Objects such as keys or wallets that could be found in the area to be examined were removed. They were told that they could eat as usual but not to take calcium supplements in the 24 hours prior to the examination.
Regarding anthropometric measurements, the data obtained from each subject were: height, waist circumference, hip circumference and triceps skinfold. The standard protocol of the International Society for the Advancement of Kinanthropometry (ISAK) was followed [
26]. The height of the subjects was measured with the SECA 123 stadiometer (Hamburg, Germany). From the body mass and height data, BMI (kg/m
2) was calculated. Perimeters were measured with a Lufkin tape measure (accuracy 1 mm) and a Harpenden skinfold caliper (accuracy 0.2 mm) was used for skinfolds. The waist/hip ratio (WHR) was calculated from the waist and hip circumferences. The resulting values may indicate different levels of risk for the health conditions mentioned. In general, a higher WHR indicates a higher proportion of abdominal fat, also known as central or android obesity, which is associated with a higher risk of metabolic and cardiovascular problems.
2.7. Statistical Analysis
The statistical processing and analysis of the data was carried out using the JAMOVI statistical package. Initially, a principal components analysis was applied together with logistic regression and normality analysis, with the objective of identifying variables with the potential to influence the modification of health bio-parameters. Next, comparisons of means were made to evaluate the existence of significant differences between the groups at specific times: before, during and after the intervention. An analysis of variance (ANCOVA) (linear model; time × groups) was used to analyze the effects of the intervention on the results, baseline variables results were included as co-variables, as potential confounding factors. Statistical significance was set at p<0.05 for all tests. The results are reported indicating the mean and standard deviation for each group. Additionally, the effect size was analyzed to provide a quantitative measure of the magnitude of the differences found.
Correlation analysis was performed using Pearson´s coefficient. This value was contrasted with the following hypothesis (using a 95% confidence coefficient): H0 (null hypothesis), there is no correlation between the two variables; H1 (alternative hypothesis), there is correlation between the variables. The p-value is calculated and values below 0.05 were determined to indicate a correlation between the variables. The correlation values ranged from -1 to 1, where negative values indicate an inverse correlation (when one variable increases, the other decreases) and positive values indicate a direct correlation (both variables increase or decrease). The closer the value to -1 or 1, the stronger the correlation.
3. Results
3.1. Satiety
Results of the satiety questionnaires performed can be found in
Table 2. Briefly, no significant differences were reported before breakfast throughout the experimentation process, except for the question
“How full do you feel?”. In this case, the group consuming the experimental product presented a significantly higher value after 90 days of intake, compared to baseline values. Therefore, this result suggests that the product does not reduce hunger in fasting conditions.
However, noticeable differences were observed between the two study groups after the set breakfast. Specifically, the experimental group scored significantly higher compared to baseline values to the questions “How full do you feel?” (at day 60 and 90) and “How satisfied do you feel?” (at day 60), when assessed immediately after breakfast. When the participants filled out the questionnaire 1 hour after breakfast, more significant results appeared, not only with respect to baseline values, but also with respect to the placebo group. To the questions “How hungry do you feel?” and “How much could you eat now?”, the experimental group reported significantly lower values compared to placebo at 90 and 60 days of treatment, respectively. A significantly higher value versus placebo was also detected at day 30 to the questions “How full do you feel?” and “How satisfied do you feel?”.
Overall, these results would suggest that the ingredient has a significant effect on inducing satiety post-meal, but not in fasting conditions. These are similar results to those observed in previous studies, where it was also shown that this effect coincided with a significant increase in the levels of the satiety-inducing hormone GLP-1 in blood [
13,
18].
3.2. Blood Tests for Glucose, Cholesterol and Triglycerides
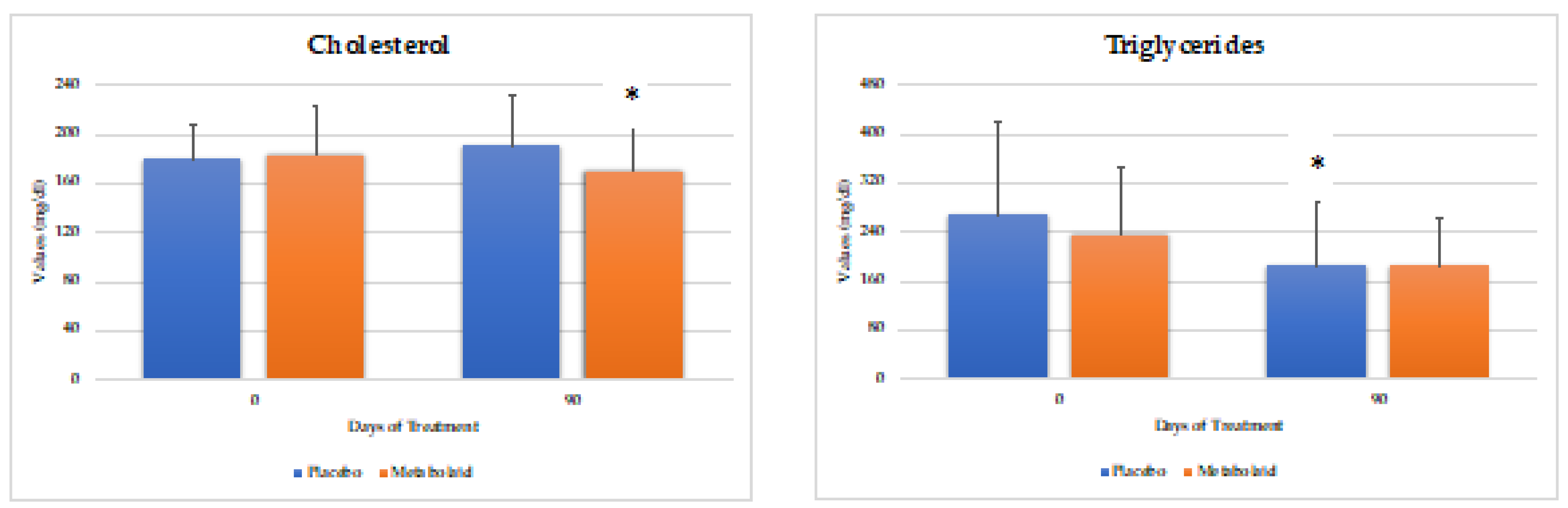
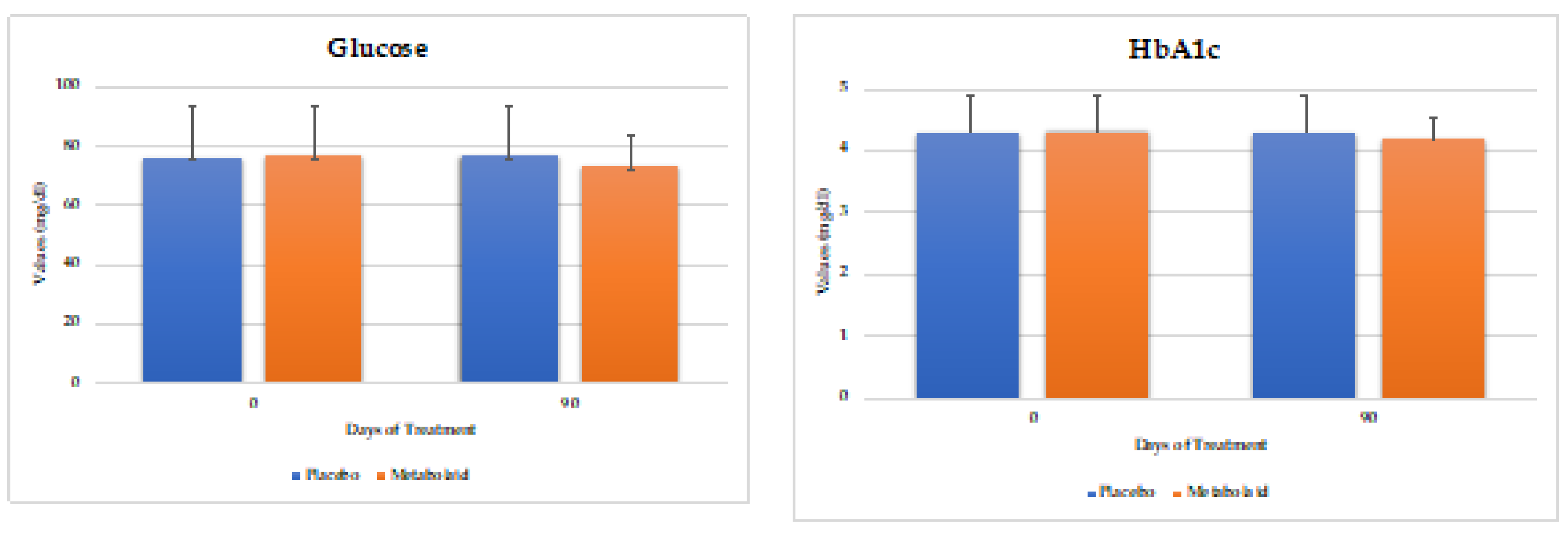
Blood samples were taken from the volunteers at the beginning and end of the study. Cholesterol, triglycerides, and glucose were measured (
Figure 4). As a result, a significant reduction was observed in the experimental group in total cholesterol levels. As for triglyceride levels, both study groups reported a decrease, with the placebo group reaching statistical significance while the experimental group revealing a trend. Finally, blood glucose and HbA1c levels remained unchanged, which was expected as overall the volunteer´s levels were in the healthy range.
Therefore, it can be concluded that the experimental product contributes to lowering cholesterol levels, while glucose levels remain unchanged in normoglycemic individuals.
3.3. Blood Pressure and Heart Rate
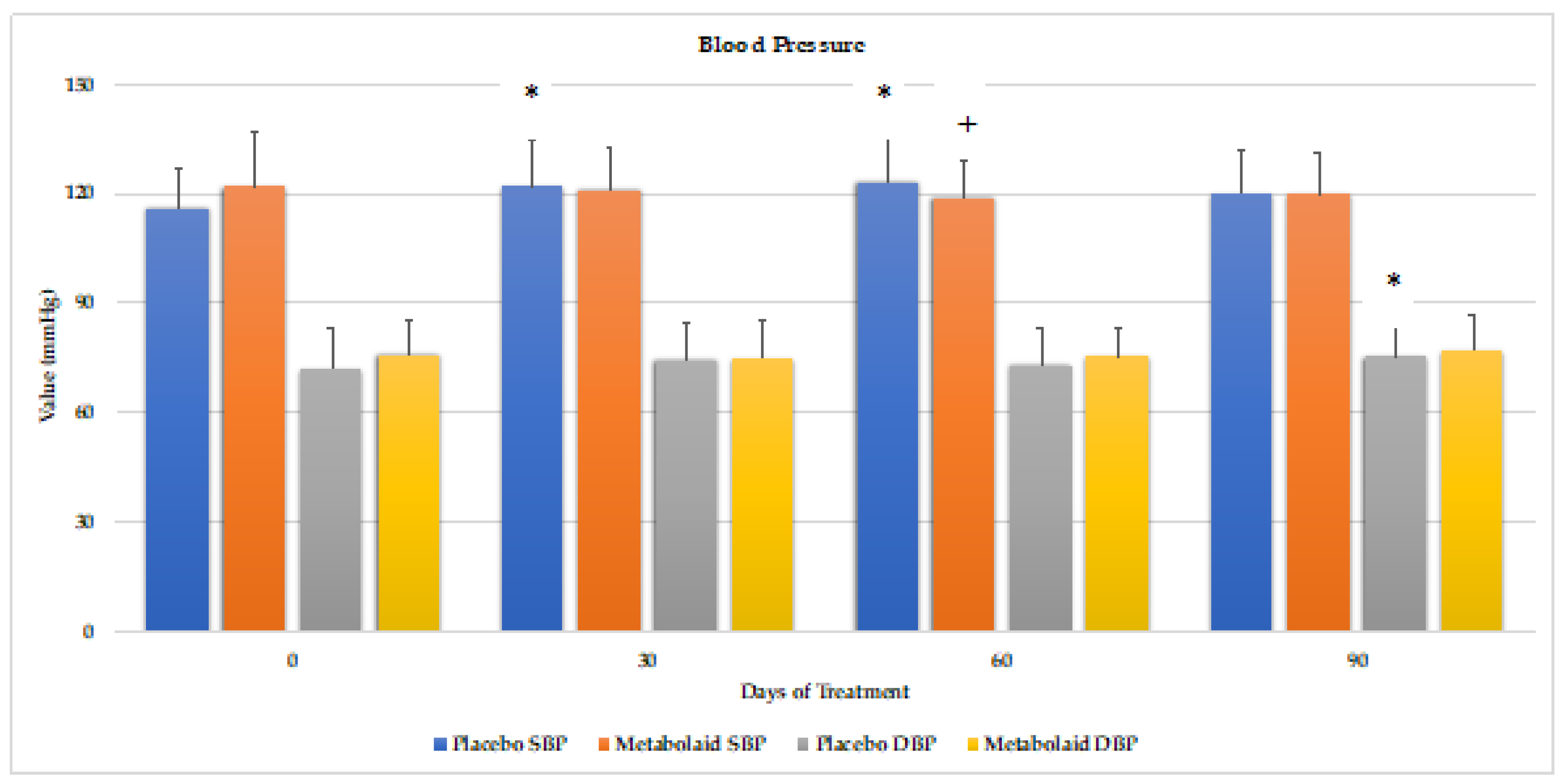
Blood pressure and resting heart rate were analyzed at the beginning of the study and days 30, 60 and 90 (
Figure 5). Interestingly, the placebo group revealed a slight but significant increase in blood pressure levels throughout the study compared to baseline values, at day 30 and 60, with an increase in diastolic blood pressure observed at day 90. The group taking the experimental product, on the other hand, did not reveal such an increase, and this difference was statistically significant at days 30 and 60 with respect to systolic blood pressure. Resting heart rate did not change throughout the study (data not shown). Therefore, these results suggest that the ingredient does not alter the blood pressure levels in normotensive individuals.
3.4. Body Weight, Bioimpedance and Anthropometry Assessment
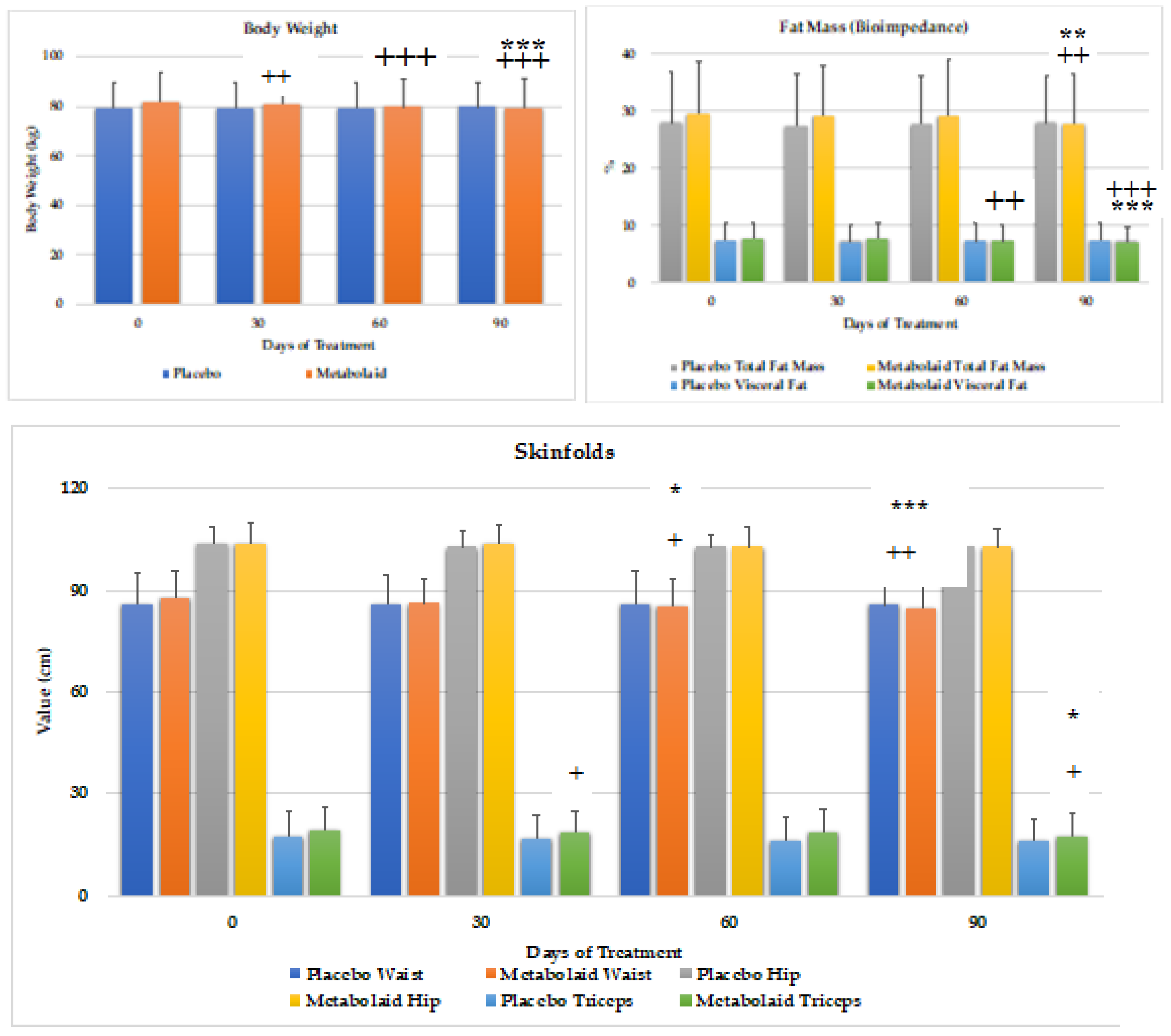
Body weight, bioimpedance (using the TANITA) and anthropometric measurements were performed during each visit (
Figure 6). Regarding body weight, the experimental product reduced body weight (-2.8kg, approximately -3.4% of total body weight) after 90 days of treatment. This result is statistically significant compared to baseline values and placebo throughout the whole study period.
As for fat mass, significant reductions, both compared to baseline and placebo, were observed in total body fat mass (-1.7%, equivalent to -5.7% vs baseline) at 90 days, and visceral fat (-0.65%, equivalent to -8.2% vs baseline) at 60 and 90 days.
Waist circumference analysis revealed a significant reduction at days 60 and 90, whereas no significant changes in hip circumference were detected. Triceps skinfold also revealed a significant reduction after days 30 (vs. placebo) and 90 (vs. baseline and placebo).
Therefore, these studies suggest that the experimental product contributes to lowering body weight, in absence of diet. Furthermore, the main reason behind this weight loss is due to the reduction in fat mass, being the visceral fat the most relevant area of body fat loss, as confirmed through the skinfold analysis.
3.5. Bioimpedance (BIODY XPERTZM) Assay
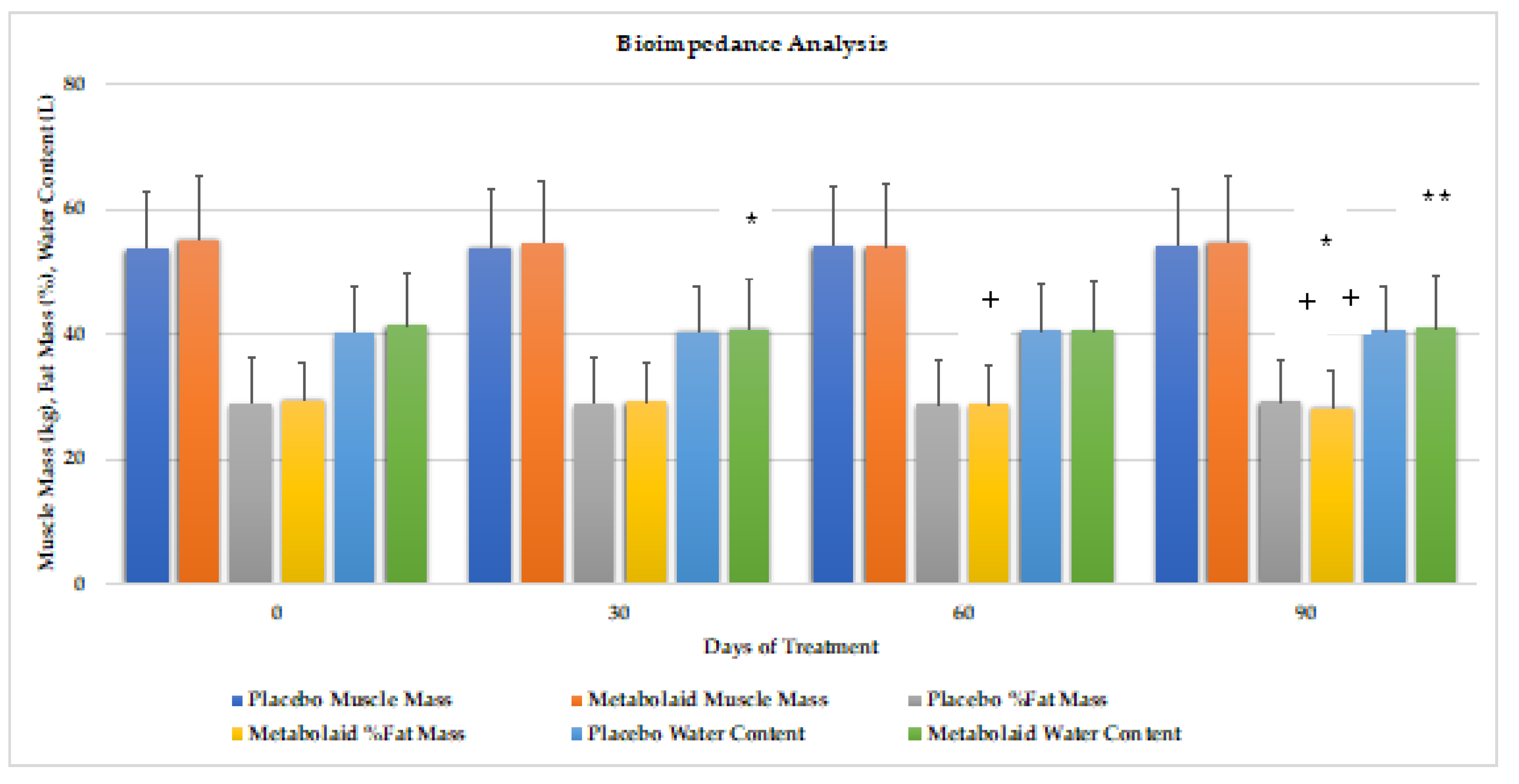
Electrical bioimpedance analysis with the BIODY XPERT
ZM was performed at day 0, 30, 60 and 90 (
Figure 7). Compared to baseline, the experimental group reduced fat mass (-4.1%) throughout the whole study, being statistically significant versus baseline at days 30 and 90, and versus placebo at days 60 and 90. Muscle mass remained unchanged, while slight but significantly lower water mass was observed at day 30 and 90.
Therefore, it can be concluded that the ingredient contributes to lowering body fat mass after 90 days of intake, while maintaining muscle mass, with a slight reduction in water mass observed.
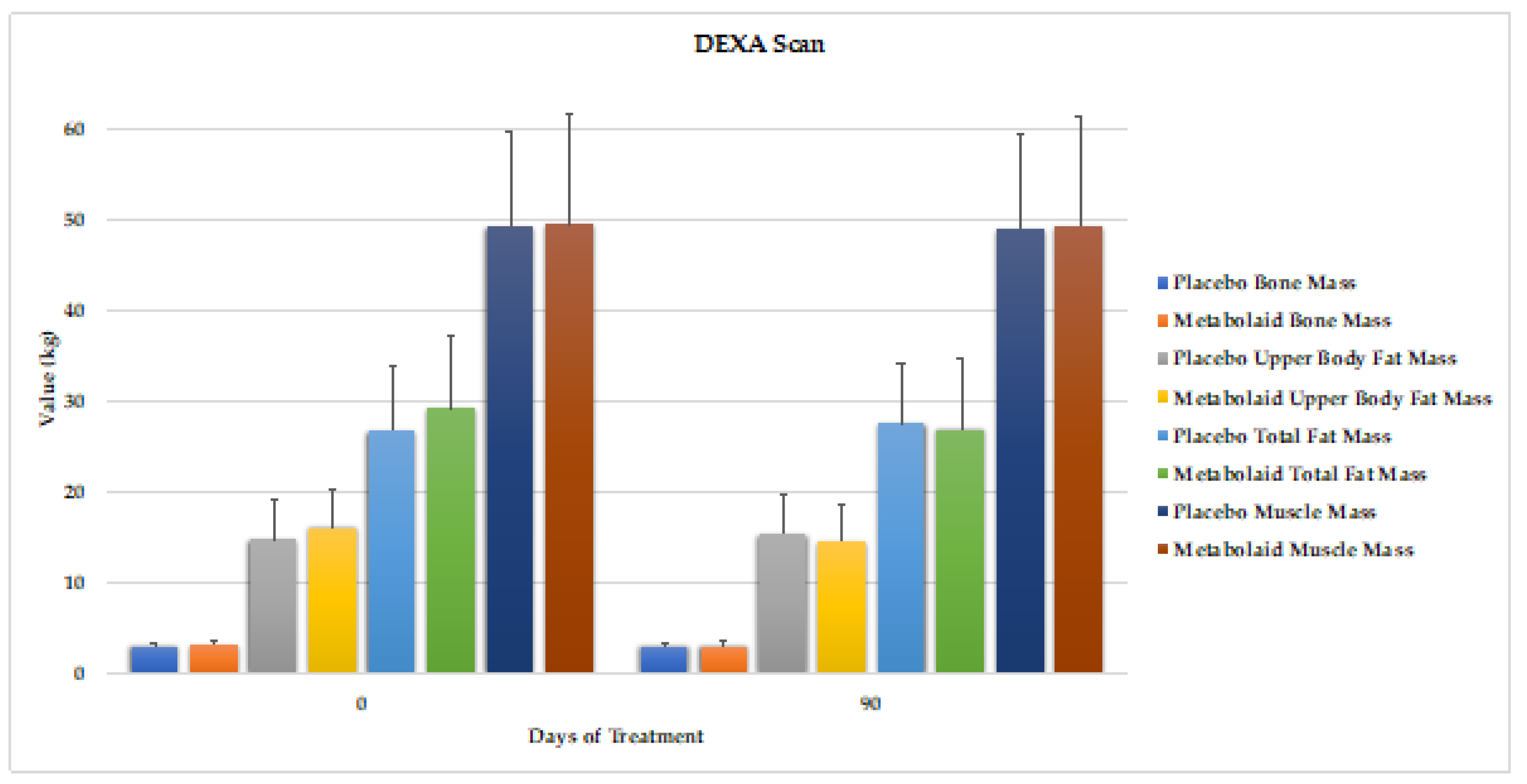
3.6. DEXA Scan
This test was performed at the beginning and end of the study. Bone, upper body fat, total fat and muscle mass were assessed (
Figure 8). Compared to baseline, the placebo group revealed to significantly increase upper body and total fat mass. On the other hand, the experimental group significantly reduced upper body fat mass (-1.6kg, -9.9% vs. baseline values) and total fat mass (-2.4kg, -8.2% vs. baseline values) at day 90. These differences were statistically significant compared to baseline values and placebo. No changes in muscle nor bone mass were observed.
Therefore, the results indicate that botanical blend contributes to lower body fat mass in absence of diet. These results also corroborate those observed by the skinfold assessment, where the upper body area is where the most of fat loss was observed.
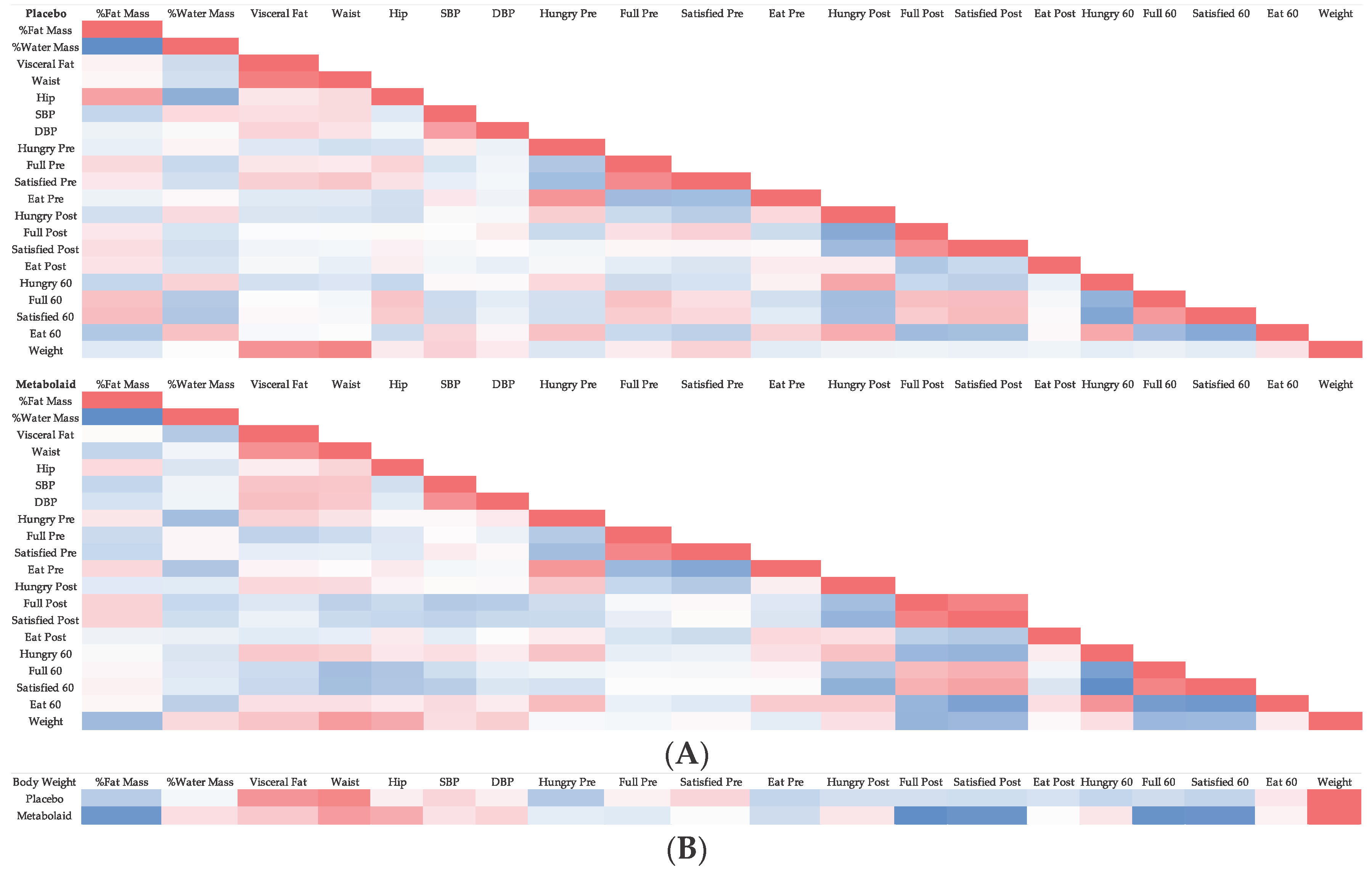
3.7. Correlation Analysis
A correlation analysis was performed to assess how the biological parameters measured behaved with respect to each other (
Figure 9). The most noticeable differences were observed with body weight, where a stronger correlation was detected between body weight and several of the satiety questions. For example, a slightly negative correlation was detected in the placebo group between body weight and the question “how hungry do you feel?” immediately after breakfast as well as 1 hour afterwards, whereas a strong positive correlation was detected in the experimental group. This could be interpreted in the case of the placebo group that less body weight correlated with more hunger after the meal, while in the experimental group, less body weight correlated with less hunger after breakfast. Similar results were observed regarding the questions “how satisfied do you feel?” and “how much could you eat now?”. In these cases, a stronger negative correlation was detected in the experimental group compared to the placebo. Overall, this could suggest that in the experimental group, those losing more weight equally felt less hungry after the meals, and therefore a stronger satiety effect (due to the product´s effect) was perceived by the subjects.
4. Discussion
In the current study, we have demonstrated that a single, daily intake of 300mg of the botanical blend (under the trademark name Metabolaid®) can contribute to help lose body weight through the loss of fat mass especially in the upper body region, after 90 days, with significant results observed as early as during the first month of treatment. Also, the subjects reported higher levels of satiety, especially post-meal. Lastly, a reduction in cholesterol levels was observed.
This evidence is similar to the results observed in previous publications [
12,
13,
14,
15,
16,
17,
18,
19,
20], although in those studies a 500mg daily dose was provided. With respect to the previous publications, the major differences reside on the lack of response in blood pressure and glycemic marker levels, and that overall, the results are not as remarkable (up to twice as much body weight loss was observed in previous studies, for example). Despite this, there are numerous advantages for the use of a lower dose. First of all, a 500mg dose means that the product must be taken in at least 2 capsules, compared to 300mg which fits in a single one. Pill fatigue is a matter of concern, with over half of patients not adhering adequately to long-term treatments [
27]. This is even more pronounced in the case of dietary supplements and can have deleterious effects in cases where there exists a severe condition that can be accompanied by a nutritional deficit [
28,
29,
30,
31]. Regarding weight management treatments, lifestyle interventions have been shown to be beneficial for weight loss [
32], although in most cases the lost weight is eventually regained [
33]. Therefore, finding methods to increase adherence, such as reducing the number of capsules to take, can have longer-lasting benefits.
Similarly, the possibility of using more innovative final product formats, such as gummies, gels or drinks, can also facilitate adherence [
34]. However, these formats have additional technical challenges, such as ingredient stability, solubility, and organoleptic. This is particularly true in the case of botanicals. In this regard, the botanical blend has been tested in gummy formats and drinks, and while the ingredient was found to be stable and soluble, it presented certain organoleptic challenges. Also, a 500mg daily dose requires having to divide the dose into at least 3 gummies, which can be too high. Therefore, the possibility of using a lower dose, while retaining to a certain point its functional benefits, can be advantageous for its use in these end formats.
Besides the differences regarding dose, the current study presents additional insights on the product´s effects that have not been addressed in previous studies. For example, satiety assessment was performed in this case at different intervals of the study, compared to previous reports which were only conducted at the beginning and end of the trial [
13,
18]. As result, it was observed that the product´s effect on satiety can be perceived by the participants as early as 30 days post-meal. This information provides further proof that the product´s effect resides on the signaling pathway that is activated when eating. Previous studies suggested that this was at least partly due to increased GLP-1 expression [
13,
18], which is a known satiety-inducing hormone, and that the gut microbiome plays an important role [
35]. Our investigations have also elucidated on the potential gut microbiome-modulating effects of the ingredient, increasing short-chain fatty acid production, particularly butyrate, while increasing the population of several bacterial populations that are implicated in obesity, such as Akkermansia, Prevotella or Blautia [
20].
In addition, the current study has revealed that under normoglycemic and normotensive conditions, the product does not have any significant effect. This is especially relevant, as there are concerns regarding the effect of certain dietary supplements which may have blood pressure- or glucose-lowering effects, which in healthy individuals could potentially lower their levels to hypotensive or hypoglycemic levels. In the current study, no such effect was observed, proving that the product is safe for these individuals.
Lastly, this is the first study conducted with the ingredient that has provided a complete comparative analysis of body composition using anthropometry, bioimpedance and DEXA scan. While this may seem redundant, it provides proof of the robustness of the evidence observed with the botanical blend on body fat mass loss. Significant reductions were observed in all three assessments, with special emphasis on the reduction in the upper body/visceral fat mass. Therefore, the study provides additional supporting evidence on the effect of the product in reducing body weight mainly by targeting visceral body fat, while muscle, bone and water mass is largely unaffected.
5. Conclusions
The current study reveals that the daily consumption of 300mg of a blend comprised of lemon verbena and hibiscus extracts can help reduce body weight, by promoting body fat loss, especially in the visceral region. Also, increased satiety was perceived, with a more noticeable effect detected after meals. Other additional benefits include lowering blood cholesterol, while glucose and blood pressure, which were initially in the healthy range, remained unchanged. Based on the evidence here presented and that observed in previous studies, it can be concluded that a 300mg daily dose may help reduce appetite and body weight, albeit at a slower rate than the 500mg dose. Further studies are necessary to assess the potential blood pressure, cholesterol and glucose lowering effects of the blend at the lower dose.
Author Contributions
Conceptualization, A.M.R., M.M.O. and J.J.; methodology, A.M.R., M.M.O. and J.J.; software, A.M.R., M.M.O., M.V.M., P.N. and N.A.M.; validation, M.M.O. and J.J.; formal analysis, A.M.R., M.M.O., M.V.M., P.N., N.C. and J.J.; investigation, A.M.R., M.M.O., M.V.M., N.A.M., P.N., N.C. and J.J.; resources, M.M.O. and J.J.; data curation, A.M.R., M.M.O., M.V.M., P.N. and J.J.; writing—original draft preparation, A.M.R., M.M.O. and M.V.M.; writing—review and editing, N.A.M., P.N., N.C. and J.J.; visualization, A.M.R., P.N. and J.J.; supervision, M.M.O. and J.J.; project administration, A.M.R. and J.J.; funding acquisition, J.J.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Alicante University (protocol code UA-2023-05-08).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to personal health information.
Acknowledgments
To the company Monteloeder S.L., which participated in the design of the study protocol and provided the test product samples. The sponsor’s employees did not participate in the data analysis; the investigators were external to the company. To the European Institute of Exercise and Health (EIEH) of Alicante University for their selfless collaboration in this research.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix
Subgroup Analysis Based on Gender
The effects of the ingredient in women or men only were analyzed independently (
Table 3 and
Table 4, respectively). Regarding the satiety questionnaire, overall, the results were more significant in both women and men at 90 days, with respect to the placebo group. However, certain questions, particularly those after breakfast and 1 hour post-meal, were significant at earlier time points.
Regarding the other parameters measured, with respect to the women, significant results versus baseline values were observed for body weight/BMI (-2.7kg, equivalent to -3.2% of total body weight) and body fat starting at 60 days of treatment (30 days in the case of total fat mass). Waist and hip circumference were also reduced, as well as triceps skinfolds. Systolic blood pressure was reduced at 30 days, but then returned to baseline values. Finally, the DEXA scan revealed a significant reduction in body fat mass, particularly in the upper body region (-9.2%). No other parameter, including blood tests, revealed to present significant differences with respect to baseline values.
In the case of men, the results were similar to the women, except the effects were more noticeable earlier on (starting at 30 days of treatment). Interestingly, the men in the placebo group significantly increased their body fat (+7.8%), measured by the DEXA scan, while those taking the experimental product significantly reduced their levels (-8.9%). As in the case of women, no significant results were observed in the blood tests, nor blood pressure.
References
- Wyatt SB, Winters KP, Dubbert PM (2006) Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci 331:166–174. [CrossRef]
- Williams EP, Mesidor M, Winters K et al (2015) Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep 4:363–370. [CrossRef]
- Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet 384:766–781. [CrossRef]
- Guh DP, Zhang W, Bansback N et al (2009) The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9:1–20. [CrossRef]
- Siddiqui SA, Azmy Harahap I, Suthar P, Wu YS, Ghosh N, Castro-Muñoz R. A Comprehensive Review of Phytonutrients as a Dietary Therapy for Obesity. Foods. 2023 Sep 28;12(19):3610. [CrossRef]
- Vendrame S, Klimis-zacas D (2015) Anti-infammatory efect of anthocyanins via modulation of nuclear factor-κB and mitogen activated protein kinase signaling cascades. Nutr Rev 73:348–358. [CrossRef]
- Li A, Li S, Zhang Y et al (2014) Resources and biological activities of natural polyphenols. Nutrients 6:6020–6047. [CrossRef]
- Herranz-López M, Barrajón-Catalán E, Segura-Carretero A, Menéndez JA, Joven J, Micol V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through AMPK-dependent mechanisms. Phytomedicine. 2015 Jun 1;22(6):605-14. [CrossRef]
- Yang MY, Peng CH, Chan KC, Yang YS, Huang CN, Wang CJ. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J Agric Food Chem. 2010 Jan 27;58(2):850-9. [CrossRef]
- Carling D (2017) AMPK signalling in health and disease. Curr Opin Cell Biol 45:31–37. [CrossRef]
- Carlson CA, Kim K-H (1973) Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem 248:378–380. [CrossRef]
- Herranz-López, M. et al. Differential effects of a combination of Hibiscus sabdariffa and Lippia citriodora polyphenols in overweight/obese subjects: A randomized controlled trial. Sci Rep 9, 2999 (2019). [CrossRef]
- Boix-Castejón M. et al. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: a randomized controlled trial. Food Funct. 2018 Jun 20;9(6):3173-3184. Erratum in: Food Funct. 2018 Jul 17;9(7):4037.
- Lee, Y.-S. et al. Metabolaid® Combination of Lemon Verbena and Hibiscus Flower Extract Prevents High-Fat Diet-Induced Obesity through AMP-Activated Protein Kinase Activation. Nutrients 2018, 10, 1204. [CrossRef]
- Marhuenda, J. et al. A Randomized, Double-Blind, Placebo Controlled Trial to Determine the Effectiveness a Polyphenolic Extract (Hibiscus sabdariffa and Lippia citriodora) in the Reduction of Body Fat Mass in Healthy Subjects. Foods 2020, 9, 55. Correction: Foods 2020, 9(1), 55. Foods 2020, 9, 279. [CrossRef]
- Marhuenda, J. et al. A Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Effectiveness of a Polyphenolic Extract (Hibiscus sabdariffa and Lippia citriodora) for Reducing Blood Pressure in Prehypertensive and Type 1 Hypertensive Subjects. Molecules 2021, 26, 1783. [CrossRef]
- Boix-Castejón M. et al. Effect of metabolaid® on pre- and stage 1 hypertensive patients: A randomized controlled trial. Journal of Functional Foods, Volume 84, 2021, 104583, ISSN 1756-4646. [CrossRef]
- Serna A. et al. Effectiveness of a polyphenolic extract (Lippia citriodora and Hibiscus sabdariffa) on appetite regulation in overweight and obese grade I population: an 8-week randomized, double-blind, cross-over, placebo-controlled trial. Eur J Nutr. 2021 Sep 30. [CrossRef]
- Martínez-Rodríguez, A. et al. New App-Based Dietary and Lifestyle Intervention on Weight Loss and Cardiovascular Health. Sensors 2022, 22, 768. [CrossRef]
- Bartolomé et al. Gut microbiome-modulating properties of a polyphenol-enriched dietary supplement comprised of hibiscus and lemon verbena extracts. Monitoring of phenolic metabolites. J Funct Foods 2022 Volume 91, 105016. [CrossRef]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2000 Jan;24(1):38–48. [CrossRef]
- De Graaf C. The validity of appetite ratings. Appetite. 1993 Oct;21(2):156–60. [CrossRef]
- Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, et al. Effects of a Diet-Based Weight-Reducing Program with Probiotic Supplementation on Satiety Efficiency, Eating Behaviour Traits, and Psychosocial Behaviours in Obese Individuals. Nutrients. 2017 Mar;9(3). [CrossRef]
- Scafoglieri A, Tresignie J, Provyn S, Clarys JP, Bautmans I. Reproducibility, accuracy and concordance of Accutrend Plus for measuring circulating lipid concentration in adults. Biochem Med (Zagreb). 2012;22(1):100-8.
- Mattewal A, Aldasouqi S, Solomon D, Gossain V, Koller A. A1cNow InView: a new simple method for office-based glycohemoglobin measurement. J Diabetes Sci Technol. 2007 Nov;1(6):879-84. [CrossRef]
- Borges S. Rev Bras Cineantropom Hum artigo original. 1980;103–13.
- Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011 Apr;86(4):304-14. Epub 2011 Mar 9. [CrossRef]
- Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. [In Press]. Clin Nutr. 2018. [CrossRef]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11-48. [CrossRef]
- Isenring E, Zabel R, Bannister M, et al. Updated evidence-based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy. Nutr Diet. 2013;70(4):312-324. [CrossRef]
- Tappenden KA, Quatrara B, Parkhurst ML, Malone AM, Fanjiang G, Ziegler TR. Critical role of nutrition in improving quality of care: an interdisciplinary call to action to address adult hospital malnutrition. JPEN J Parenter Enteral Nutr. 2013;37(4):482-497. [CrossRef]
- Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020 Feb-Mar;75(2):235-251. [CrossRef]
- Sanaya N, Janusaite M, Dalamaga M, Magkos F. The Physiological Effects of Weight-Cycling: A Review of Current Evidence. Curr Obes Rep. 2024 Jan 3. [CrossRef]
- Tarahi M, Tahmouzi S, Kianiani MR, Ezzati S, Hedayati S, Niakousari M. Current Innovations in the Development of Functional Gummy Candies. Foods. 2023 Dec 25;13(1):76. [CrossRef]
- Hamamah S, Amin A, Al-Kassir AL, Chuang J, Covasa M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients. 2023 Jul 28;15(15):3365. [CrossRef]
Figure 1.
Flowchart of the double-blind trial.
Figure 1.
Flowchart of the double-blind trial.
Figure 2.
Variables evaluated in each of the visits.
Figure 2.
Variables evaluated in each of the visits.
Figure 3.
Visual analogue scale (VAS) for the evaluation of satiety.
Figure 3.
Visual analogue scale (VAS) for the evaluation of satiety.
Figure 4.
Parameters measured in blood. * = p<0.05 vs baseline.
Figure 4.
Parameters measured in blood. * = p<0.05 vs baseline.
Figure 5.
Blood pressure values. SBP means systolic blood pressure, DBP means diastolic blood pressure. * = p<0.05 vs. baseline, + = p<0.05 vs. placebo.
Figure 5.
Blood pressure values. SBP means systolic blood pressure, DBP means diastolic blood pressure. * = p<0.05 vs. baseline, + = p<0.05 vs. placebo.
Figure 6.
Body weight, fat mass and skinfold analysis. * = p<0.05 vs. baseline, ** = p<0.005 vs. baseline, *** = p<0.001 vs. baseline, + = p<0.05 vs. placebo, ++ = p<0.005 vs. placebo, +++ = p<0.001 vs. placebo.
Figure 6.
Body weight, fat mass and skinfold analysis. * = p<0.05 vs. baseline, ** = p<0.005 vs. baseline, *** = p<0.001 vs. baseline, + = p<0.05 vs. placebo, ++ = p<0.005 vs. placebo, +++ = p<0.001 vs. placebo.
Figure 7.
Bioimpedance measurements for fat, muscle and water mass. * = p<0.05 vs. baseline, ** = p<0.01 vs. baseline, + = p<0.05 vs. placebo, ++ = p<0.01 vs. placebo.
Figure 7.
Bioimpedance measurements for fat, muscle and water mass. * = p<0.05 vs. baseline, ** = p<0.01 vs. baseline, + = p<0.05 vs. placebo, ++ = p<0.01 vs. placebo.
Figure 8.
Fat, bone, and muscle mass analysis by DEXA scan. * = p<0.05 vs. baseline, ** = p<0.01 vs. baseline, *** = p<0.001 vs. baseline, +++ = p<0.001 vs. placebo.
Figure 8.
Fat, bone, and muscle mass analysis by DEXA scan. * = p<0.05 vs. baseline, ** = p<0.01 vs. baseline, *** = p<0.001 vs. baseline, +++ = p<0.001 vs. placebo.
Figure 9.
Heatmap depicting the correlation analysis of the bioparameters measured. A) Heatmap of Placebo and Experimental groups. B) Heatmap regarding body weight of Placebo and Experimental groups.
Figure 9.
Heatmap depicting the correlation analysis of the bioparameters measured. A) Heatmap of Placebo and Experimental groups. B) Heatmap regarding body weight of Placebo and Experimental groups.
Table 1.
Average values of the study groups at the beginning of the study.
Table 1.
Average values of the study groups at the beginning of the study.
| |
Placebo |
Experimental |
| Age (years) |
40.99 ± 7.48 |
42.83 ± 7.39 |
| Height (cm) |
170.36 ± 7.60 |
171.99 ± 8.43 |
| Weight (kg) |
79.30 ± 7.21 |
81.06 ± 7.21 |
| BMI (kg/m2) |
27.16 ± 1.49 |
27.60 ± 1.35 |
| Men/Women Ratio |
17/14 |
16/14 |
Table 2.
Satiety questionnaire results. Data are presented as mean±SD.
Table 2.
Satiety questionnaire results. Data are presented as mean±SD.
| |
Placebo |
Experimental |
| |
Day 0 |
Day 30 |
Day 60 |
Day 90 |
Day 0 |
Day 30 |
Day 60 |
Day 90 |
| Pre-Breakfast |
How hungry do you feel? |
5.71 |
± |
2.22 |
5.42 |
± |
2.53 |
4.83 |
± |
2.49 |
6.06 |
± |
2.29 |
5.8 |
± |
2.5 |
5.7 |
± |
2.22 |
5.89 |
± |
1.95 |
5.6 |
± |
2.11 |
| How full do you feel? |
2.9 |
± |
1.83 |
3.03 |
± |
1.85 |
4.00 |
± |
2.26 |
3.32 |
± |
2.12 |
3.13 |
± |
2.05 |
3.83 |
± |
1.66 |
3.74 |
± |
2.05 |
4.17 |
± |
1.91* |
| How satisfied do you feel? |
3.29 |
± |
1.88 |
3.65 |
± |
1.72 |
4.33 |
± |
2.25 |
3.58 |
± |
2.08 |
3.3 |
± |
2.05 |
3.83 |
± |
1.74 |
3.85 |
± |
2.05 |
4.23 |
± |
1.91 |
| How much could you eat now? |
6.48 |
± |
2.14 |
6.35 |
± |
2.09 |
6.1 |
± |
2.34 |
6.55 |
± |
2.39 |
6.37 |
± |
2.13 |
6.17 |
± |
1.9 |
6.22 |
± |
1.95 |
5.67 |
± |
2.11 |
| Post-Breakfast |
How hungry do you feel? |
0.52 |
± |
0.89 |
0.77 |
± |
1.09 |
0.4 |
± |
0.86 |
0.74 |
± |
1.12 |
1.1 |
± |
1.58 |
0.9 |
± |
1.4 |
0.59 |
± |
1.08 |
0.53 |
± |
1.01++ |
| How full do you feel? |
9.06 |
± |
1.29 |
8.42 |
± |
1.84 |
8.83 |
± |
1.21 |
8.81 |
± |
1.22 |
8.33 |
± |
1.65 |
8.57 |
± |
1.28+ |
9.15 |
± |
0.99*++ |
9.07 |
± |
1.46*+ |
| How satisfied do you feel? |
9.19 |
± |
1.14 |
8.58 |
± |
2.08 |
8.83 |
± |
1.18 |
8.94 |
± |
1.12 |
8.67 |
± |
1.35 |
8.97 |
± |
1.25+ |
9.41 |
± |
0.89*+ |
9.13 |
± |
1.41 |
| How much could you eat now? |
1.19 |
± |
2.2 |
1.45 |
± |
2.32 |
2.00 |
± |
2.64 |
1.84 |
± |
2.98 |
2.13 |
± |
2.34 |
1.87 |
± |
2.22 |
1.44 |
± |
2.19++ |
1.43 |
± |
2.51 |
| 1 Hour Post Breakfast |
How hungry do you feel? |
1.35 |
± |
1.5 |
1.42 |
± |
1.36 |
1.43 |
± |
1.22 |
1.81 |
± |
1.85 |
1.97 |
± |
1.71 |
1.43 |
± |
1.38 |
1.7 |
± |
2.03 |
0.83 |
± |
1.09***+ |
| How full do you feel? |
7.52 |
± |
2.45 |
6.77 |
± |
2.35 |
7.63 |
± |
1.77 |
7.35 |
± |
1.84 |
7.9 |
± |
1.58 |
8.27 |
± |
1.64++ |
8.3 |
± |
1.51 |
8.47 |
± |
2.13+ |
| How satisfied do you feel? |
8.16 |
± |
1.68 |
7.65 |
± |
1.58 |
7.63 |
± |
1.79 |
7.52 |
± |
1.98 |
7.83 |
± |
1.66 |
8.30 |
± |
1.58+ |
8.48 |
± |
1.72+ |
8.8 |
± |
1.67*+++ |
| How much could you eat now? |
2.1 |
± |
1.89 |
2.68 |
± |
2.21 |
2.5 |
± |
1.78 |
2.77 |
± |
1.96 |
2.83 |
± |
2.26 |
2.43 |
± |
2.1 |
1.93 |
± |
1.52++ |
1.63 |
± |
1.56*++ |
Table 3.
Results observed in women only. Data are presented as Mean±SD.
Table 3.
Results observed in women only. Data are presented as Mean±SD.
| |
|
Women Placebo (n=14) |
Women Experimental (n=14) |
| |
|
0 |
30 |
60 |
90 |
0 |
30 |
60 |
90 |
| How hungry do you feel? |
Pre-breakfast |
5.86 |
± |
1.08 |
5.86 |
± |
1.14 |
5.69 |
± |
1.72 |
6.14 |
± |
1.43 |
5.64 |
± |
1.84 |
6.29 |
± |
1.43 |
6.00 |
± |
1.23 |
5.64 |
± |
1.69 |
| Post-breakfast |
0.50 |
± |
0.64 |
0.64 |
± |
0.83 |
0.15 |
± |
0.26 |
0.57 |
± |
0.82 |
0.64 |
± |
0.92 |
0.50 |
± |
0.71 |
0.15 |
± |
0.28 |
0.21 |
± |
0.37 |
| 1-hour post |
1.29 |
± |
1.37 |
0.79 |
± |
1.01 |
1.08 |
± |
1.18 |
1.36 |
± |
1.80 |
1.79 |
± |
1.61 |
1.50 |
± |
1.21 |
1.85 |
± |
1.68* |
0.50 |
± |
0.71*+ |
| How full do you feel? |
Pre-breakfast |
3.36 |
± |
1.55 |
3.36 |
± |
1.17 |
3.62 |
± |
1.62 |
3.50 |
± |
1.43 |
3.43 |
± |
1.57 |
3.43 |
± |
1.12 |
3.23 |
± |
1.24 |
3.57 |
± |
1.22 |
| Post-breakfast |
9.21 |
± |
1.01 |
8.86 |
± |
0.80 |
9.46 |
± |
0.75 |
9.07 |
± |
0.93 |
8.93 |
± |
0.93 |
9.00 |
± |
0.71 |
9.62 |
± |
0.53* |
9.79 |
± |
0.37*+ |
| 1-hour post |
8.29 |
± |
1.63 |
7.43 |
± |
1.65 |
8.23 |
± |
1.41 |
8.07 |
± |
1.36 |
8.43 |
± |
1.43 |
8.36 |
± |
1.36 |
8.46 |
± |
1.07 |
8.93 |
± |
1.27 |
| How satisfied do you feel? |
Pre-breakfast |
3.43 |
± |
1.29 |
3.71 |
± |
1.29* |
3.77 |
± |
1.60 |
3.36 |
± |
1.55 |
3.29 |
± |
1.43 |
3.86 |
± |
1.31 |
3.62 |
± |
1.28* |
3.43 |
± |
1.29* |
| Post-breakfast |
9.43 |
± |
0.65 |
8.79 |
± |
1.13 |
9.38 |
± |
0.66 |
9.21 |
± |
0.79 |
9.14 |
± |
0.86 |
9.36 |
± |
0.73++ |
9.77 |
± |
0.36+ |
9.79 |
± |
0.37+ |
| 1-hour post |
8.64 |
± |
0.93 |
7.86 |
± |
1.29 |
8.54 |
± |
1.11 |
8.50 |
± |
1.21 |
8.43 |
± |
1.35 |
8.29 |
± |
1.29 |
8.92 |
± |
0.75 |
9.29 |
± |
0.92 |
| How much could you eat now? |
Pre-breakfast |
6.43 |
± |
1.57 |
6.64 |
± |
0.93 |
6.62 |
± |
1.40 |
6.79 |
± |
1.39 |
6.36 |
± |
1.64 |
6.86 |
± |
1.02 |
7.08 |
± |
0.72 |
6.36 |
± |
1.02 |
| Post-breakfast |
1.36 |
± |
1.80 |
1.79 |
± |
2.12 |
1.77 |
± |
2.41 |
2.79 |
± |
3.27 |
1.71 |
± |
2.02 |
1.64 |
± |
1.83 |
1.23 |
± |
1.62* |
1.86 |
± |
2.37 |
| 1-hour post |
1.36 |
± |
1.03 |
2.36 |
± |
1.60 |
1.62 |
± |
0.97 |
1.86 |
± |
1.55 |
2.71 |
± |
2.24 |
2.79 |
± |
1.79 |
1.85 |
± |
1.53**+ |
1.36 |
± |
1.46**+ |
| Body composition antrhopometry and bioimpedance |
Weight (kg) |
73.20 |
± |
7.96 |
73.20 |
± |
7.71 |
72.40 |
± |
7.02 |
73.00 |
± |
7.57 |
74.10 |
± |
8.99 |
73.40 |
± |
9.47 |
72.90 |
± |
10.40* |
71.70 |
± |
10.10** |
| BMI (kg/m2) |
27.50 |
± |
1.92 |
27.60 |
± |
1.92 |
27.50 |
± |
1.99 |
27.50 |
± |
1.94 |
27.70 |
± |
1.89 |
27.40 |
± |
2.10 |
27.30 |
± |
2.18* |
26.80 |
± |
2.24** |
| % FM |
35.80 |
± |
4.97 |
35.20 |
± |
4.37 |
35.40 |
± |
3.91 |
35.10 |
± |
5.03 |
38.00 |
± |
3.31 |
37.30* |
± |
3.36 |
37.50 |
± |
5.47* |
35.80 |
± |
3.85** |
| VF |
6.00 |
± |
2.42 |
5.93 |
± |
2.29 |
5.81 |
± |
2.13 |
5.82 |
± |
2.27 |
6.93 |
± |
1.50 |
6.70 |
± |
1.38 |
6.75 |
± |
1.66* |
6.18 |
± |
1.50** |
| Waist (cm) |
82.20 |
± |
7.82 |
81.60 |
± |
7.23 |
81.50 |
± |
8.99 |
81.50 |
± |
6.79 |
83.00 |
± |
6.20 |
81.80 |
± |
6.00 |
81.80 |
± |
6.45 |
80.80 |
± |
5.74* |
| Hip (cm) |
106.0 |
± |
4.66 |
105.00 |
± |
4.53 |
105.00 |
± |
3.87 |
105.00 |
± |
3.75 |
105.00 |
± |
6.69 |
105.00 |
± |
6.70 |
104.00 |
± |
6.86* |
103.00 |
± |
6.88* |
| Triceps skinfold |
23.80 |
± |
4.98 |
22.60 |
± |
4.74 |
22.10 |
± |
3.86 |
21.30 |
± |
3.56 |
24.50 |
± |
5.09 |
23.80 |
± |
4.79 |
24.00 |
± |
4.30 |
22.80 |
± |
3.68** |
| SBP |
111.00 |
± |
7.18 |
120.00 |
± |
9.01 |
117.00 |
± |
13.70 |
117.00 |
± |
12.40 |
116.00 |
± |
12.70 |
115.00* |
± |
12.80 |
116.00 |
± |
12.10 |
115.00 |
± |
11.00 |
| DBP |
71.40 |
± |
6.99 |
73.30 |
± |
9.69 |
72.80 |
± |
9.93 |
73.70 |
± |
8.04 |
72.00 |
± |
9.76 |
72.00 |
± |
9.34 |
73.40 |
± |
8.15 |
73.10 |
± |
8.64 |
| Total, FM % |
35.20 |
± |
3.53 |
35.20 |
± |
3.31 |
35.10 |
± |
3.27 |
34.60 |
± |
2.93 |
34.80 |
± |
2.94 |
34.60 |
± |
3.04 |
34.00* |
± |
3.39 |
33.50 |
± |
3.51* |
| WM (L) |
33.10 |
± |
3.02 |
33.30 |
± |
2.32 |
32.80 |
± |
2.82 |
33.50 |
± |
3.14 |
33.50 |
± |
3.89 |
33.20 |
± |
3.98 |
33.40 |
± |
3.92 |
33.20 |
± |
3.84 |
| MM (kg) |
45.20 |
± |
3.94 |
45.10 |
± |
3.63 |
44.70 |
± |
3.86 |
45.40 |
± |
4.44 |
45.70 |
± |
5.36 |
45.30 |
± |
5.38 |
45.10 |
± |
5.43 |
45.00 |
± |
5.41 |
| Blood samples |
CHO (mg/dl) |
177.00 |
± |
14.50 |
|
|
|
|
|
|
191.00 |
± |
30.80 |
174.00 |
± |
30.30 |
|
|
|
|
|
|
167.00 |
± |
45.30 |
| TG (mg/dl) |
256.00 |
± |
163.00 |
|
|
|
|
|
|
224.00 |
± |
137.00 |
226.00 |
± |
138.00 |
|
|
|
|
|
|
216.00 |
± |
74.00 |
| Glucose (mg/dl) |
71.50 |
± |
14.30 |
|
|
|
|
|
|
72.70 |
± |
18.10 |
74.40 |
± |
18.40 |
|
|
|
|
|
|
73.00 |
± |
11.10 |
| HbA1c (mg/dl) |
4.12 |
± |
0.50 |
|
|
|
|
|
|
4.16 |
± |
0.63 |
4.22 |
± |
0.64 |
|
|
|
|
|
|
4.17 |
± |
0.39 |
| DEXA |
BM (kg) |
2.73 |
± |
0.37 |
|
|
|
|
|
|
2.77 |
± |
0.39 |
2.82 |
± |
0.40 |
|
|
|
|
|
|
2.73 |
± |
0.50 |
| Upper FM (kg) |
15.70 |
± |
3.53 |
|
|
|
|
|
|
15.90 |
± |
3.67 |
18.10 |
± |
3.27 |
|
|
|
|
|
|
16.50 |
± |
3.44* |
| Total FM (kg) |
30.60 |
± |
5.04 |
|
|
|
|
|
|
30.70 |
± |
5.23 |
34.50 |
± |
5.41 |
|
|
|
|
|
|
32.20 |
± |
5.81* |
| MM (kg) |
39.70 |
± |
4.02 |
|
|
|
|
|
|
39.50 |
± |
4.02 |
37.70 |
± |
5.17 |
|
|
|
|
|
|
37.80 |
± |
5.48 |
Table 4.
Results observed in men only. Data are presented as Mean±SD.
Table 4.
Results observed in men only. Data are presented as Mean±SD.
| |
|
Men Placebo (n=14) |
Men Experimental (n=14) |
| |
|
0 |
30 |
60 |
90 |
0 |
30 |
60 |
90 |
| How hungry do you feel? |
Pre-breakfast |
5.59 |
± |
2.65 |
5.06 |
± |
3.15 |
4.18 |
± |
2.60 |
6.00 |
± |
2.67 |
5.94 |
± |
2.64 |
5.19 |
± |
2.51 |
5.79 |
± |
2.22 |
5.56 |
± |
2.10 |
| Post-breakfast |
0.53 |
± |
1.01 |
0.88 |
± |
1.11 |
0.59 |
± |
1.06 |
0.88 |
± |
1.17 |
1.50 |
± |
1.83 |
1.25 |
± |
1.65 |
1.00 |
± |
1.30 |
0.81 |
± |
1.22**+ |
| 1-hour post |
1.41 |
± |
1.42 |
1.94 |
± |
1.30 |
1.71 |
± |
0.99 |
2.18 |
± |
1.51 |
2.13 |
± |
1.71 |
1.38 |
± |
1.41*+ |
1.57 |
± |
1.22 |
1.13 |
± |
1.20*+ |
| How full do you feel? |
Pre-breakfast |
2.53 |
± |
1.62 |
2.76 |
± |
2.05 |
4.29 |
± |
2.39* |
3.18 |
± |
2.38 |
2.88 |
± |
2.19 |
4.19 |
± |
1.80** |
4.21 |
± |
2.29* |
4.69 |
± |
2.18** |
| Post-breakfast |
8.94 |
± |
1.30 |
8.06 |
± |
2.22 |
8.35 |
± |
1.22* |
8.59 |
± |
1.28* |
7.81 |
± |
1.83 |
8.19 |
± |
1.47 |
8.71 |
± |
1.07+ |
8.44 |
± |
1.71 |
| 1-hour post |
6.88 |
± |
2.64 |
6.24 |
± |
2.44 |
7.18 |
± |
1.67 |
6.76 |
± |
1.75 |
7.44 |
± |
1.31 |
8.19 |
± |
1.56+ |
8.14 |
± |
1.46 |
8.06 |
± |
2.21 |
| How satisfied do you feel? |
Pre-breakfast |
3.18 |
± |
2.07 |
3.59 |
± |
1.84 |
4.76 |
± |
2.36* |
3.76 |
± |
2.28 |
3.31 |
± |
2.33 |
3.81 |
± |
1.76 |
4.07 |
± |
2.27 |
4.94 |
± |
1.91* |
| Post-breakfast |
9.00 |
± |
1.37 |
8.41 |
± |
2.50 |
8.41 |
± |
1.28* |
8.71 |
± |
1.21 |
8.25 |
± |
1.48 |
8.63 |
± |
1.45 |
9.07 |
± |
1.07+ |
8.56 |
± |
1.67 |
| 1-hour post |
7.76 |
± |
1.99 |
7.47 |
± |
1.66 |
6.94 |
± |
1.78* |
6.71 |
± |
1.78 |
7.31 |
± |
1.54 |
8.31 |
± |
1.62* |
8.07 |
± |
2.06+ |
8.38 |
± |
1.82*+ |
| How much could you eat now? |
Pre-breakfast |
6.53 |
± |
2.45 |
6.12 |
± |
2.67 |
5.71 |
± |
2.71 |
6.35 |
± |
2.87 |
6.38 |
± |
2.31 |
5.56 |
± |
2.19 |
5.43 |
± |
2.28 |
5.06 |
± |
2.49*+ |
| Post-breakfast |
1.06 |
± |
1.68 |
1.18 |
± |
1.70 |
2.18 |
± |
1.85* |
1.06 |
± |
1.56 |
2.50 |
± |
1.79 |
2.06 |
± |
1.73* |
1.64 |
± |
1.55*++ |
1.06 |
± |
1.06**++ |
| 1-hour post |
2.71 |
± |
2.11 |
2.94 |
± |
2.28 |
3.18 |
± |
1.81 |
3.53 |
± |
1.59 |
2.94 |
± |
1.98 |
2.13 |
± |
1.89* |
2.00 |
± |
1.18* |
1.88 |
± |
1.41*+ |
| Body composition antrhopometry and bioimpedance |
Weight (kg) |
84.10 |
± |
88.90 |
84.30 |
± |
8.53 |
84.70 |
± |
8.68 |
85.10 |
± |
8.46 |
88.90 |
± |
7.83 |
87.80 |
± |
7.74*** |
85.90 |
± |
8.03*** |
85.70 |
± |
8.36*** |
| BMI (kg/m2) |
26.90 |
± |
27.50 |
27.00 |
± |
1.78 |
27.10 |
± |
1.81 |
27.20 |
± |
1.82 |
27.50 |
± |
1.37 |
27.10 |
± |
1.38*** |
26.80 |
± |
1.42*** |
26.50 |
± |
1.48*** |
| % FM |
21.50 |
± |
22.00 |
21.00 |
± |
6.30 |
21.90 |
± |
5.59 |
22.10 |
± |
5.38 |
22.00 |
± |
4.95 |
22.30 |
± |
4.95 |
21.20 |
± |
5.54 |
20.90 |
± |
5.20* |
| VF |
8.47 |
± |
8.75 |
8.26 |
± |
3.23 |
8.50 |
± |
3.38 |
8.75 |
± |
3.07 |
8.75 |
± |
2.96 |
8.94 |
± |
3.11 |
8.11 |
± |
3.32* |
8.19 |
± |
2.93* |
| Waist (cm) |
89.50 |
± |
92.20 |
89.30 |
± |
8.92 |
89.90 |
± |
8.56 |
89.60 |
± |
8.80 |
92.20 |
± |
6.76 |
90.30 |
± |
6.32* |
89.30 |
± |
7.14** |
88.60 |
± |
6.22*** |
| Hip (cm) |
102.00 |
± |
4.21 |
101.00 |
± |
3.86 |
102.00 |
± |
3.69 |
102.00 |
± |
3.65 |
104.00 |
± |
5.67 |
103.00 |
± |
4.70 |
103.00 |
± |
4.95 |
102.00 |
± |
4.08 |
| Triceps skinfold |
12.10 |
± |
14.40 |
12.10 |
± |
4.19 |
12.20 |
± |
4.40 |
12.10 |
± |
4.56 |
14.40 |
± |
4.13 |
13.70 |
± |
3.92 |
13.60 |
± |
3.95 |
13.00 |
± |
3.90** |
| SBP |
121.00 |
± |
128.00 |
123.00 |
± |
15.10 |
128.00 |
± |
18.30 |
123.00 |
± |
11.10 |
128.00 |
± |
15.30 |
125.00 |
± |
9.27 |
123.00 |
± |
5.63 |
125.00 |
± |
9.51 |
| DBP |
72.70 |
± |
79.30 |
75.30 |
± |
11.70 |
73.60 |
± |
10.80 |
77.00 |
± |
10.30 |
79.30 |
± |
8.92 |
77.40 |
± |
11.30 |
77.00 |
± |
7.43 |
80.80 |
± |
9.07 |
| Total, FM % |
23.80 |
± |
24.80 |
23.60 |
± |
5.44 |
23.80 |
± |
5.57 |
24.70 |
± |
5.37 |
24.80 |
± |
4.37 |
24.70 |
± |
3.69 |
23.70 |
± |
3.72 |
23.60 |
± |
3.31* |
| WM (L) |
46.10 |
± |
48.50 |
46.30 |
± |
3.87 |
46.50 |
± |
3.52 |
46.20 |
± |
3.42 |
48.50 |
± |
3.57 |
47.40* |
± |
3.70 |
47.20 |
± |
4.23 |
47.70 |
± |
4.27* |
| MM (kg) |
61.00 |
± |
4.70 |
61.20 |
± |
4.64 |
61.30 |
± |
4.36 |
61.30 |
± |
4.26 |
64.70 |
± |
4.29 |
62.60 |
± |
4.55 |
62.20 |
± |
5.15 |
63.10 |
± |
5.45 |
| Blood samples |
CHO (mg/dl) |
182.00 |
± |
191.00 |
|
|
|
|
|
|
190.00 |
± |
47.10 |
193.70 |
± |
45.00 |
|
|
|
|
|
|
173.00 |
± |
42.10 |
| TG (mg/dl) |
277.00 |
± |
244.00 |
|
|
|
|
|
|
154.00 |
± |
58.60** |
151.00 |
± |
88.10 |
|
|
|
|
|
|
158.00 |
± |
71.70 |
| Glucose (mg/dl) |
80.10 |
± |
79.20 |
|
|
|
|
|
|
79.90 |
± |
16.20 |
79.20 |
± |
15.80 |
|
|
|
|
|
|
73.60 |
± |
9.82 |
| HbA1c (mg/dl) |
4.42 |
± |
0.67 |
|
|
|
|
|
|
4.41 |
± |
0.57 |
4.39 |
± |
0.55 |
|
|
|
|
|
|
4.19 |
± |
0.34 |
| DXA |
BM (kg) |
3.21 |
± |
0.31 |
|
|
|
|
|
|
3.22 |
± |
0.31 |
3.49 |
± |
0.33 |
|
|
|
|
|
|
3.44 |
± |
0.34 |
| Upper FM (kg) |
14.10 |
± |
4.94 |
|
|
|
|
|
|
15.20 |
± |
4.78*** |
14.50 |
± |
4.23 |
|
|
|
|
|
|
12.90 |
± |
4.09*** |
| Total FM (kg) |
23.60 |
± |
7.09 |
|
|
|
|
|
|
25.10 |
± |
6.82** |
24.80 |
± |
7.27 |
|
|
|
|
|
|
22.20 |
± |
6.78*** |
| MM (kg) |
57.40 |
± |
6.44 |
|
|
|
|
|
|
56.90 |
± |
6.63 |
60.10 |
± |
4.19 |
|
|
|
|
|
|
59.50 |
± |
5.37 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).