1. Introduction
Endometriosis is a chronic, complex estrogen-driven disorder, with genetic and immunologic driven variation where endometrial tissue is found in extra-uterine sites, eliciting local and systemic inflammation, fibrosis, and pain, [
1,
2,
3] affects 6%–10% of premenopausal women and teens, 60% of those with chronic pelvic pain, 80% of patients with dysmenorrhea, and 30%–50% of women with infertility [
2]. Disease prevalence is likely underestimated and misdiagnosis common due to a lack of patient and health care provider training and awareness, normalization of dysmenorrhea symptoms especially in teens, cultural mores around menstruation and pain in women, and symptoms that are not specific to the disease and its many presentations [
1,
2]. The diagnostic accuracy of the traditional diagnostic standard of laparoscopic surgery and histopathology of only 50% to 75% is problematic [
4]. Women see multiple practitioners over 8–12 years, often hampered by unintended geographic and financial factors, until correctly diagnosed [
5]. As the result of these factors, there is an average of 8.6 years from the time of symptom appearance, until the time of final diagnosis, during which the disease continues to advance exacting an immeasurable toll on the Quality of life while disrupting, educational, and career goals, as well as personal relationships [
5,
6]. The economic impact is underrecognized with US direct medical costs of 26 billion and lost productivity 55 billion annually [
7].
The long-term risks of untreated endometriosis, including infertility, depression, and links to other chronic diseases, such as ovarian cancer, cardiovascular disease, and autoimmune diseases [
11] is driving the need for new methods and technologies for early diagnosis. Diagnostic tests like MRI and transvaginal ultrasound are highly accurate but only in more advanced disease which represents only 15–20% of symptomatic women. However, the diagnostic accuracy is lost after surgical intervention leaving no options for post treatment evaluation of disease [
4]. The remaining 70% of women suffer and average of 8.5 years before diagnosis can be made due to a lack of low cost, accurate, non-invasive, and readily available diagnostic testing. Current non-invasive testing is resource intensive and hampered by variable diagnostic accuracy in addition to requiring the acquisition, storage, transport, complex analysis, and disposal of biological materials [
8,
9,
10,
11,
12,
13]. Questions regarding reproducibility and genetic or ethnic variability have not yet been fully addressed [
14]. New diagnostic testing, shows great promise and awaits multicenter randomized control trials for further validation and broader applications, for example, assessing disease and symptom recurrence across the lifespan [
14].
Concerns regarding the accuracy and expense of surgery, the current diagnostic standard, have led to guideline changes, suggesting the use of non-invasive technology, such as MRI and ultrasound as a diagnostic equivalent [
15]. The publication of the #Enzian classification for non-invasive characterization of endometriosis further emphasizes the need for validated non-invasive, cost effective and accurate diagnostic testing [
16]. This is even more imperative when considering that in a study of 2017 people with endometriosis from 63 countries , patients experienced an average delay of 3.7 years between symptom onset and first presentation of symptoms to a physician (the care-seeking delay) and an average delay of 5.8 years between first presentation of symptoms to a physician and diagnosis of endometriosis (the healthcare-related delay), with an average total diagnostic delay of 9.6 years seeing more than 4–5 providers. This was commonly attributed to being ignored because they were considered unreliable, and participant character attributes (e.g., age, appearance, weight or physical ability) leading to clinician dismissal [
17].
During the past several years, a number of diagnostic tools have evolved and been presented as promising in the setting of endometriosis. These have consisted of blood and saliva borne mRNA fragments, blood borne mutated DNA [
18], endometrial brushings looking at cellular irregularities or chemical parameters such as BCL6 [
19], menstrual fluid analysis for levels of uterine killer cells [
20] or levels of CXCL5 and IL1RN [
21].
The introduction of diagnostic biomarkers , such as salivary mRNA [
10] and others and which could fulfill the need, has not resulted in a change in the current recommendations to use biomarkers [
22]. This has been attributed to reported low accuracy, or in some cases, lack of more extensive validation.
In a prior published study, using transnasal placed motility catheters for 24 h, a unique small bowel motility pattern was observed. This represented a biomarker of gastrointestinal myoelectrical activity (GIMA) showing a unique range of contractile frequencies specific to the diagnosis of endometriosis [
23]. Subsequent evaluation of the GIMA biomarker using non-invasive electroviscerography (EVG) technology confirmed the original study findings and demonstrated an unexpected 100% C-statistic, sensitivity, and specificity from the running spectral analysis display of the GIMA biomarker in an initial small trial demonstration cohort [
24].
The initial robust results of this novel technology to diagnose endometriosis compelled the design of the current multicenter, multi-ethnic study of the novel GIMA Biomarker with AI-derived threshold scoring, using noninvasive Electroviscerography technology, to validate: 1) the diagnostic accuracy of the unique signature of endometriosis utilizing the GIMA biomarker fingerprint, 2) the ability of the test to distinguish between subjects with and without disease, and 3) additional validation of the AI algorithm threshold model based upon the number of variables and the number of patients required to satisfy performance thresholds to maintain high diagnostic accuracy. The current data represents the results of this validation trial of the EVG detected GIMA biomarker in subjects with and without endometriosis.
2. Materials and Methods
2.1. Study Design and Disease Overview
2.1.1. Ethics Statement
The study information and data used in the analysis was obtained as part of a prospective study protocol which was reviewed and approved by the human investigational review board IRB - C.H.C.A. Woman's Hospital, L.P., 00004260, Houston, TX, for a non-randomized open-label prospective comparative study to investigate the detection of a novel gastrointestinal myoelectrical activity biomarker for endometriosis by comparing participants with known and suspected endometriosis, normal asymptomatic women, and subjects with abdominal/pelvic symptoms from other diseases without a diagnosis of endometriosis. Informed consent was obtained from all subjects as a condition to study inclusion. STARD reporting guidelines were observed for study and data analysis [
25].
2.1.2. Study Population
The full study population was composed of 165 women, age 18 or older. Subjects were recruited from a woman’s specialty clinic and gastroenterology practice into one of three cohorts depending on if the criteria for inclusion was satisfied: Cohort 1 consisted of asymptomatic subjects without signs or symptoms of endometriosis, or participants with other documented disease-associated abdominal pain not diagnosed as endometriosis. Cohort 2 was subjects with histologically documented endometriosis without total excision at laparoscopy, and Cohort 3, included women complaining of abdominal or pelvic discomfort, with negative diagnostic testing suspected to have endometriosis who were scheduled for diagnostic laparoscopy. Participating clinicians were blinded to EVG and/or surgical results and EVG technicians were blinded to surgical results. Subjects found to have endometriosis had assigned stage based upon revised American Society for Reproductive Medicine classification (rASRM) [
26]. No changes were made to existing treatment modalities, including birth control medication, medicated IUD’s, other hormonal therapy or GNRH modulators.
Exclusion criteria included: ASA physical status classification ≥III, gastrointestinal tumor or ulcers, stenosis or mechanical bowel or urinary obstruction, prior gastrectomy, extensive small bowel resection or pelvic surgery, or malignancy.
2.1.3. Physiological Concept of Disease Response
Over 31 different cytokines are produced by the female reproductive system, as are large quantities of prostaglandin E2, F-alpha, and I2, with a half-life of ≤30 s [
27]. PGF-alpha causes simultaneous contraction of longitudinal and circular smooth muscle resulting in spasm. PGE2 promotes peristalsis in the small bowel, fallopian tubes and uterus, essential for egg transport, initiating menstruation, and delivery. Endometriosis is associated with elevated prostaglandin PGE2 and PGF2alpha secretion in peritoneal explants, fluid, and serum, [
28,
29,
30], with resultant non-propulsive small bowel motility seizure-like activity and high-frequency bowel patterns [
23]. PGE2 and PGF-alpha, not normally produced in simultaneously elevated quantities except by endometriosis tissue, disables small bowel smooth muscle motor control resulting in high-frequency spasm, detected as GIMA biomarkers. No other diseases are known to produce simultaneously elevated PGE2 and PGF-alpha. The effect occurs in a drug-dose-response curve manner. Studies of over 500 subjects with other gynecological, urological, and gastrointestinal disease failed to demonstrate endometriosis-associated GIMA biomarkers (Noar-Unpublished Data-2/10/2023).
2.1.4. Study Procedures and Protocol
Participants underwent complete history and physical examinations, and a completed standardized pain questionnaire, and electroviscerography (EVG) with water load satiety test (WLST). Participants who satisfied exclusion and inclusion criteria were stratified into one of three main cohorts. Cohort 1, Subgroup 1A asymptomatic subjects without signs or symptoms of endometriosis, and Subgroup 1B participants with other documented disease-associated abdominal pain not diagnosed as endometriosis. Cohort 2 had histologically documented endometriosis without total excision at laparoscopy, and Cohort 3 included participants having abdominal or pelvic discomfort, suspected to have endometriosis scheduled for planned laparoscopy.
2.1.5. Electroviscerogram with Water Load Satiety Test (WLST)
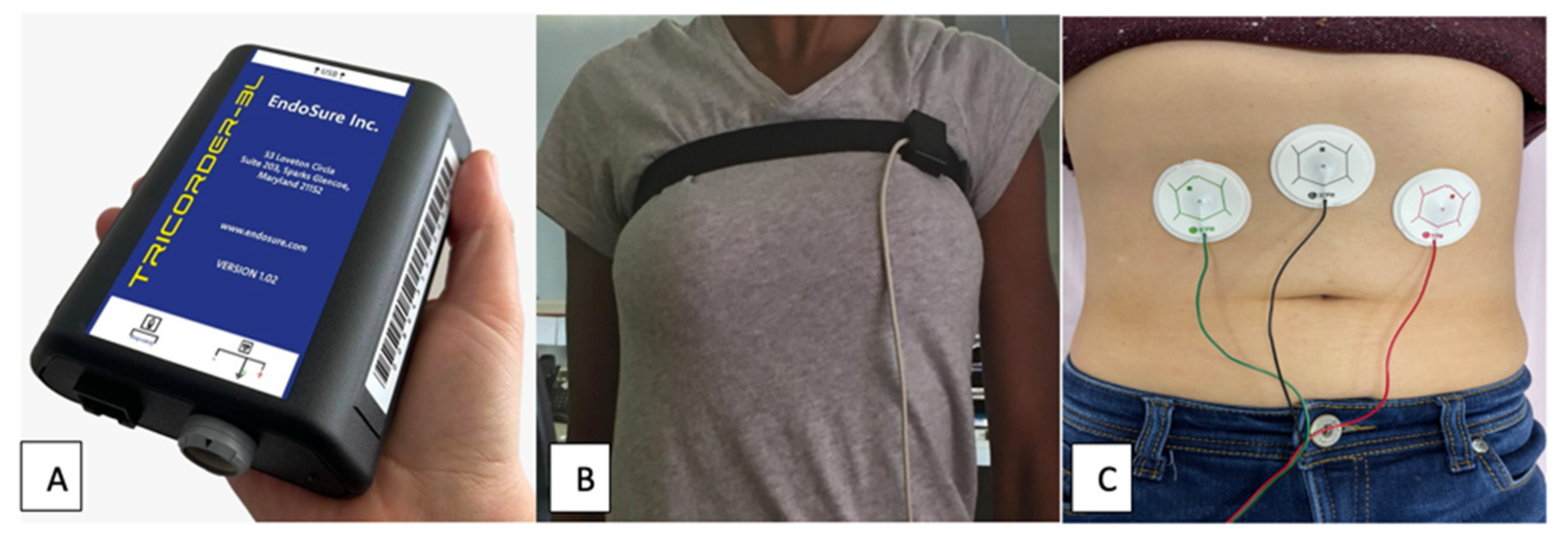
Following an overnight fast of 6–8 h, subjects underwent a standardized EVG with WLST study. Subjects were placed in a 30 to 45° reclining position, wearing loose fitting clothing. Following standard protocol, three dry gel, electrode pads were applied to the anterior, abdomen halfway between the umbilicus and xiphoid process, and 5 cm below the costal border at the midclavicular line on both left and right sides. A respiratory sensor belt was placed across the upper chest to distinguish respirations from bowel activity. (
Figure 1) Initial equipment and calibration testing was conducted over a 3-to-5-min period of time after which the subject remained quietly in the same position for a baseline study, lasting 10 min.
At the end of the 10-minute baseline period, the subject began a standardized water load stimulation. The purpose of the WLST is to cause gastric distention with activation of the gastric pacemaker and subsequent activation of small bowel contractility. This is considered an essential part of any gastrointestinal motility study. The WLST consists of drinking room temperature water until the subject indicates that they feel completely full. The WLST usually takes approximately 2 to 5 min, with the subject typically ingesting between 300 to 1000 cc of fluid. The amount ingested is recorded. The ingestion of the fluid takes place in the same position as during the baseline and for the remainder of the study. After the WLST, the subject remains motionless, in a reclining position for a 30-minute period of time at which point the study is completed. Results are then immediately available.
The standardized EVGs were recorded using an FDA cleared hand-held EVG device and respiratory belt to distinguish respirations from bowel contractions [
23,
24,
31]. Three silver-chloride electrodes were positioned on the abdomen. EVGSAS custom software (3CPM Company, Sparks Glencoe, MD) performed recording and data analysis of measurements of filtered percent distribution of power at 15–20, 20–30, 30–40, 40–50, and 50–60 cpm ranges during baseline recording and 10, 20, and 30 min after water load. These are the GIMA biomarker frequency ranges specific to endometriosis [
23,
24].
Additionally, a running spectral analysis (RSA) is created, stratifying frequency over time and AUC measurements at specified frequency ranges provided visual recognition of disease-state GIMA biomarker abnormal frequencies versus normal range values. The RSA is a visual representation of biomarker activity over time and provides a visual diagnosis of disease. However, for more precise statistical analysis, it is the AUC that is used. The % frequency distribution of power of the AUC was used to determine sensitivity, specificity, positive and negative predictive values as well as the diagnostic predictability.
2.1.6. Pain/Discomfort Score
Pain was calculated using modified ENDOPAIN 4-D standardized pain questionnaires [
32]. Participants used a 10-point verbal rating scale for categorizing the pain associated with menstruation, urination, sexual intercourse, defecation, and otherwise general levels of abdominal and pelvic pain. The calculated score was the highest single score of reported items.
2.1.7. Statistical Methods
Baseline characteristics of Cohorts 1–3 were compared using rank-sum test and categorical variables using Fisher's exact test. Baseline GIMA biomarker characteristics of cohort 1 subgroups 1A and 1B were assessed for similarity.
GIMA biomarkers were measured at baseline, 10, 20, and 30 mins for frequencies 10–60 cpm. Unadjusted median and inter-quartile range (IQR) were calculated for each combination of time and frequency and compared within cohort 1 by presence and absence of symptoms and across cohorts using a rank-sum test. Box plots were used to demonstrate the difference between cohorts for the distribution of frequencies 10.0–60.0 cpm at each time.
A univariable and multivariable mixed effects linear regression model assessed differences over time by cohort separately per biomarker frequency. Multivariable models were adjusted for age, BMI, symptom score, and water load quantity. AUC for frequencies 10.0–60.0 used trapezoidal rule calculation. Median AUC per frequency was compared between cases and controls by rank-sum test. Kernel density plots were used to assess and exhibit AUC differences between the cohorts. Distribution of AUC by cohorts was explored using a box plot. Univariable and adjusted mean AUC differences by cohorts were estimated using linear regression models. Univariable and adjusted mean AUC differences by cohorts were estimated using linear regression models. Multivariable models were adjusted for age, BMI, symptom score, and water load using logistic regression analysis for GIMA biomarker predictive modeling and calculating sensitivity, specificity, negative and positive predictive values, and C-statistic of the diagnostic test. The cut off level for significance used to conduct the statistical analysis for the study was 5%. All statistical analysis utilized R 4.2.2.
3. Results
3.1. Description of the GIMA Biomarker Cohorts
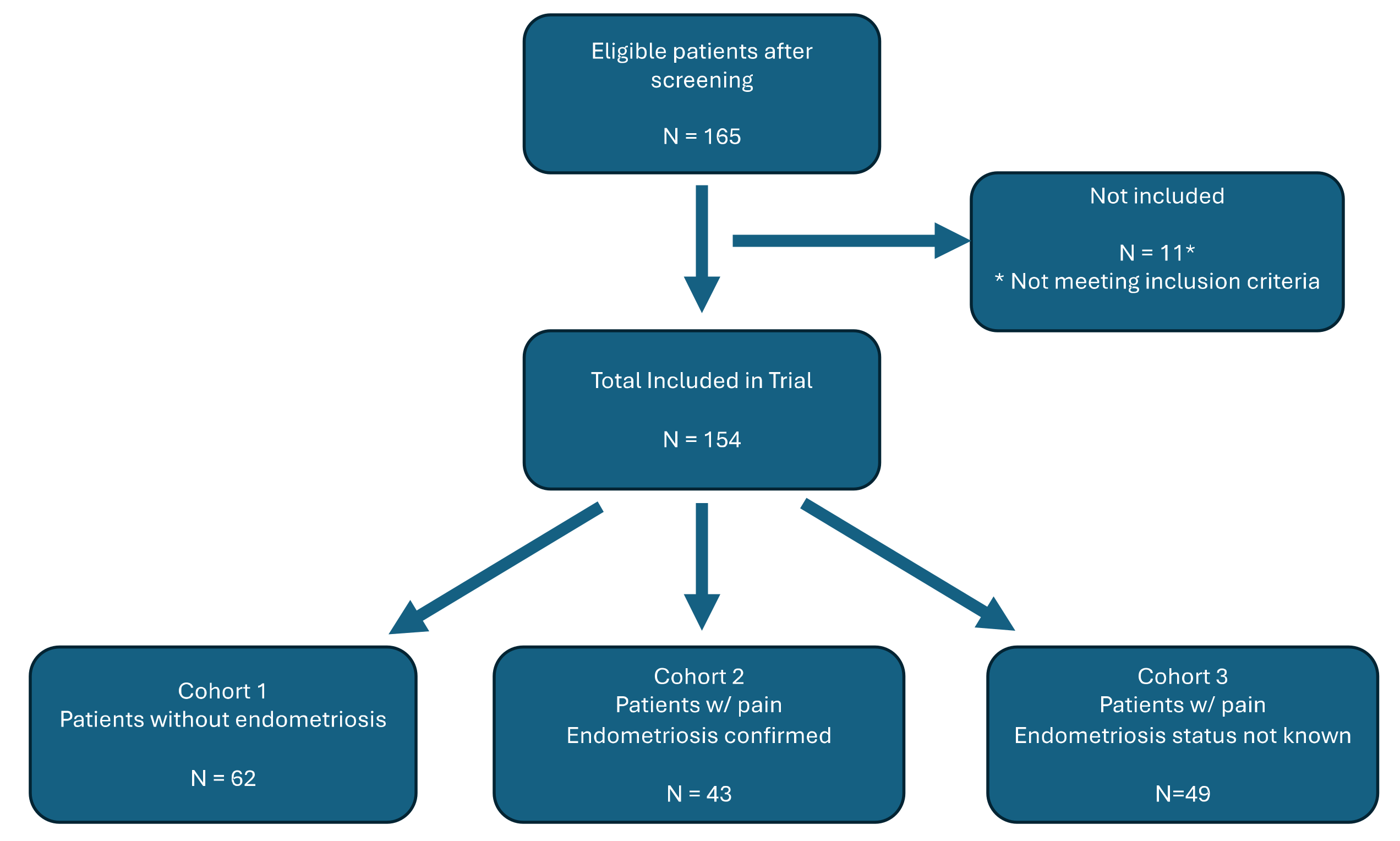
There were 165 subjects initially recruited into the study of which 65 were in Cohort 1, 50 were in Cohort 2 and 50 in Cohort 3. Three of the subjects in Cohort 1, seven from Cohort 2, and 1 from Cohort 3 did not meet inclusion criteria due to failure to obtain informed consent or complete pain scores (
Figure 2).
All remaining subjects were able to successfully undergo an EVG with WLST after completing pain questionnaires and informed consent. It was possible to obtain adequate running spectral analyses and sufficient data to be able to calculate the AUC. Artificial intelligence derived GIMA threshold scores were calculated for each subject using the AUC data at 30–40 cpm and 40–50 cpm at time points 10-, 20-, and 30-minutes post water load.
The clinical and demographic characteristics of the 154 patients in the study are presented in
Table 1 and
Table 2. Ethnicity did not differ significantly in cohorts 3 vs 1 (p 0.49) but statistical differences were seen between cohorts 1 vs 2 (p<0.001) and cohorts 2 vs 3 (p 0.04). Women were more likely to be Asians (0% vs 21%) and less likely to Caucasian (82% vs 67%) while comparing cohort 1 and 2. While women were more likely to be Asians in cohort 2(21%) but more likely to be Caucasians (85%) in cohort 3. BMI did not differ in the three cohorts and was statistically similar. Expected statistically significant (p<0.001) difference was observed in symptom scores between the healthy controls and EM positive cohort 2 and 3 (p<0.001). But no statistical difference was observed between cohort 2 vs 3 (p 0.07). Pain was highly associated with EM positive cohorts, with statistically significant difference (p<0.001) observed between cohort 1 and EM positive cohorts 2 and 3. Abdominal pain was reported by 89% cohort 3 participants, 98% in cohort 2 versus 69% in cohort 1. Bloating noted in 70% and 60% of cohorts 3 and 2 respectively, compared to 45% in cohort 1. Endo4D pain scores, used a VAS ranging from 0–10, with 10 being the highest level of pain, rating the pain score using median IQR.(
Table 1)
Medians were compared using a rank-sum test & percentages were compared using a Fischer’s exact test. A p-value of less than 0.05 was considered to be statistically significant.
Clinical conditions and comorbidities, medication use, and surgical staging results are shown in
Table 2.
Among the 154 subjects in the study, 90 had histologically documented endometriosis. The 62 subjects not documented as having endometriosis were divided between 25 asymptomatic and 37 symptomatic non-endometriosis controls. Of those with endometriosis, ASRM stages 1–4 were reported to be represented equally, without significant difference between staging.
Comorbidities in the non-EM control group were diabetes and insulin sensitivity in 5/62 (8%), constipation/IBS (9/62 (15%), and PCOS, fibroids, simple ovarian cysts, inflammatory bowel disease, collagen vascular disease, interstitial cystitis in the 2–5/62 or 3–8% range. The absence of comorbidities in endometriosis positive cohorts 2 and 3 were not significant (60% vs. 65%) but was significantly higher than the non-endometriosis Cohort 1 of 53%.
Hormonal therapy was noted in 16 (26%) in the non-endometriosis Cohort 1 and 24 (56%) in Endometriosis Cohort 2, and 36 (64%) in Cohort 3. Cohort 1 hormonal treatment consisted of 9 (14%) combined oral contraceptive pill, 4 (6%) estrogen only, 2 (3%) progestin, 1 (2%) medicated IUD and no GNRH agonist, while subjects in Cohort 2 reported 11 (26%) oral contraceptive pill, 3 (7%) progestin, 1 (2%) androgen, 1 (2%) medicated IUD, 2 (5%) estrogen only, and 6 (14%) GNRH agonist. Cohort 3 hormonal treatment consisted of 14 (29%) combined oral contraceptive pill, 6 (12%) progestin, 3 6%) GNRH agonist, 7 (14%) estrogen only, and no medicated IUD.
3.2. RSA–Qualitative Analysis–GIMA Biomarker Fingerprint Pattern Recognition
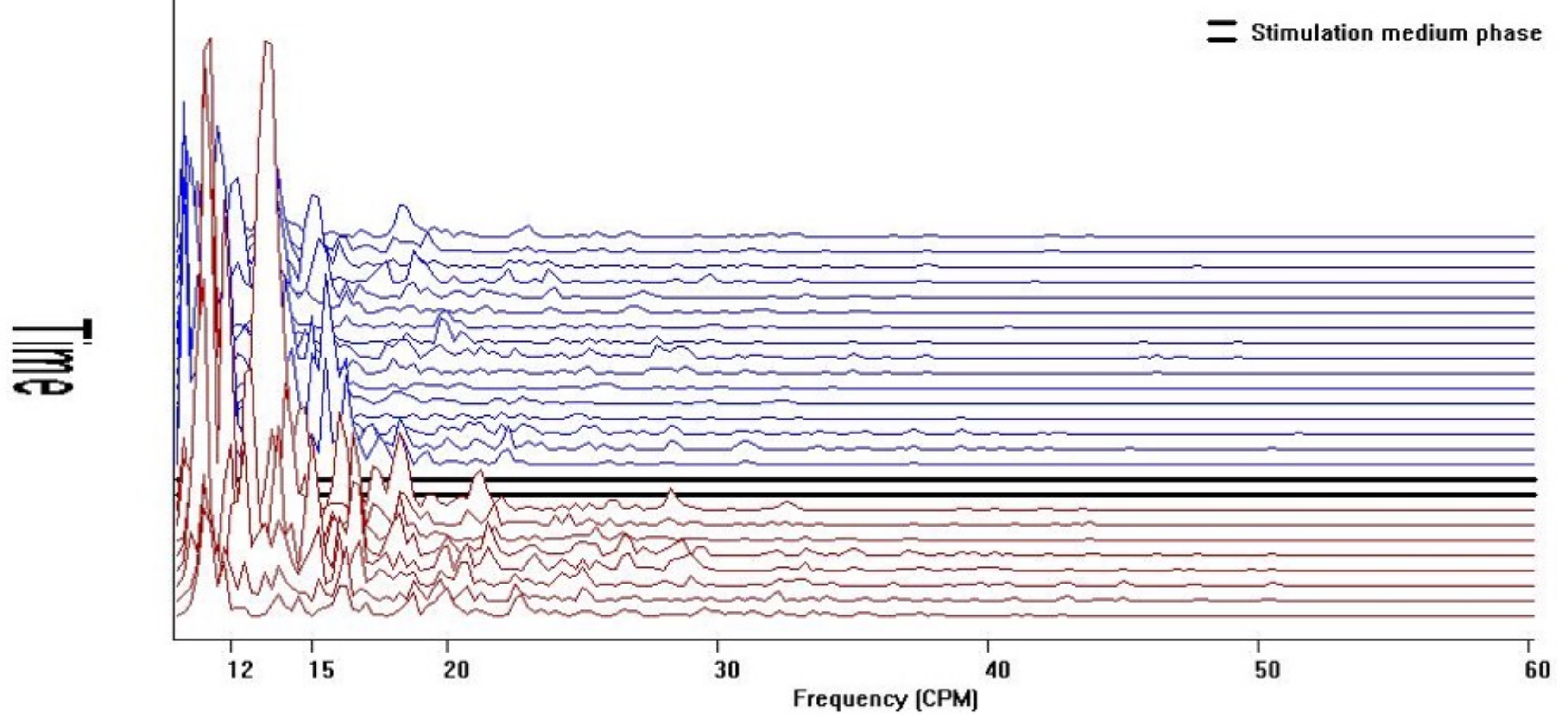
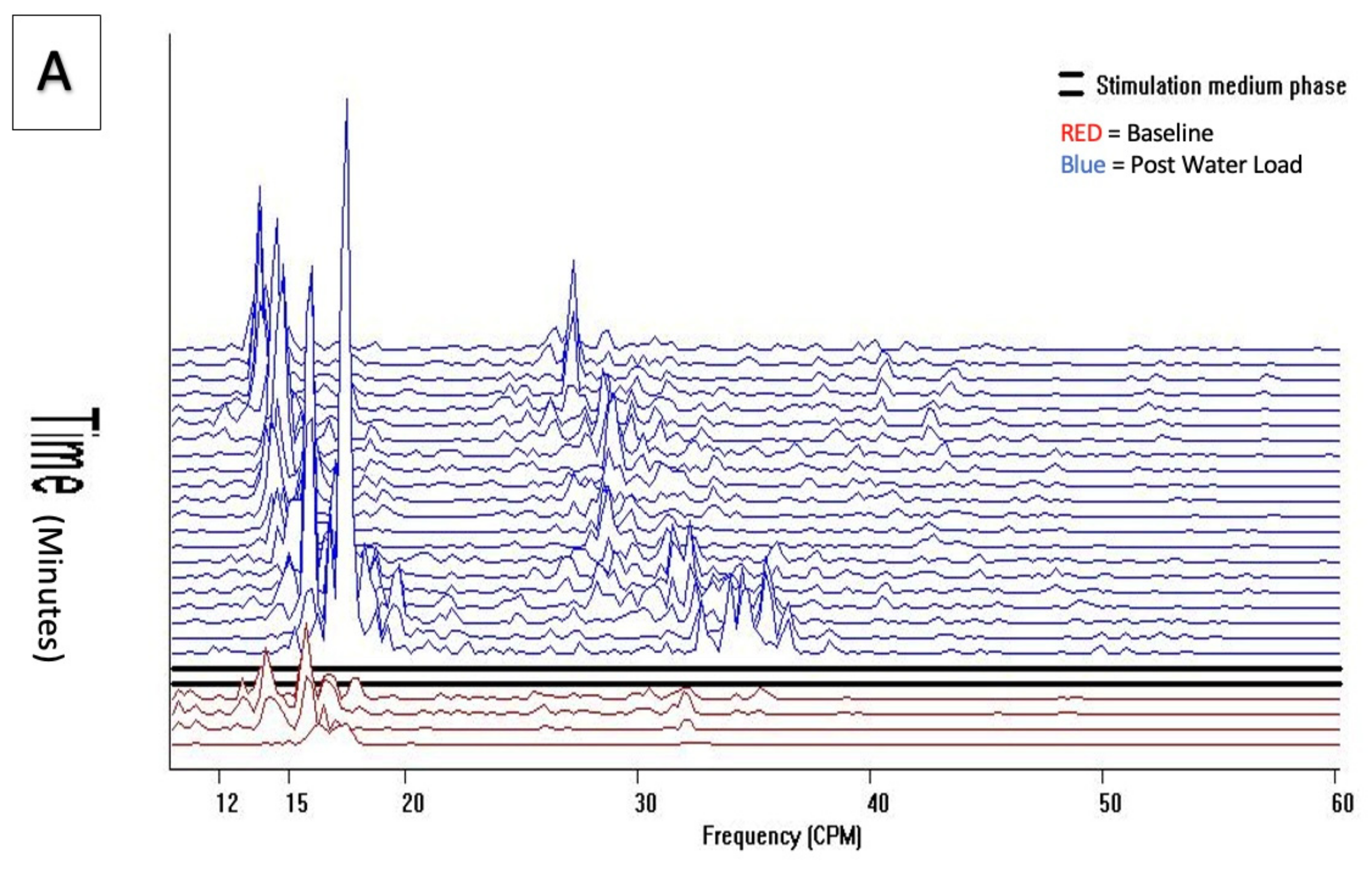
Cohort 1 qualitative visual pattern was flat both at baseline and after water load, without the unique diagnostic 157–60 cpm GIMA biomarker pattern. (
Figure 3).
Power of frequency distribution of GIMA characteristics at frequencies (10–60 cpm) among non-endometriosis participants, who were with or without symptoms at baseline and 10 min, 20 min, and 30 min post water load.
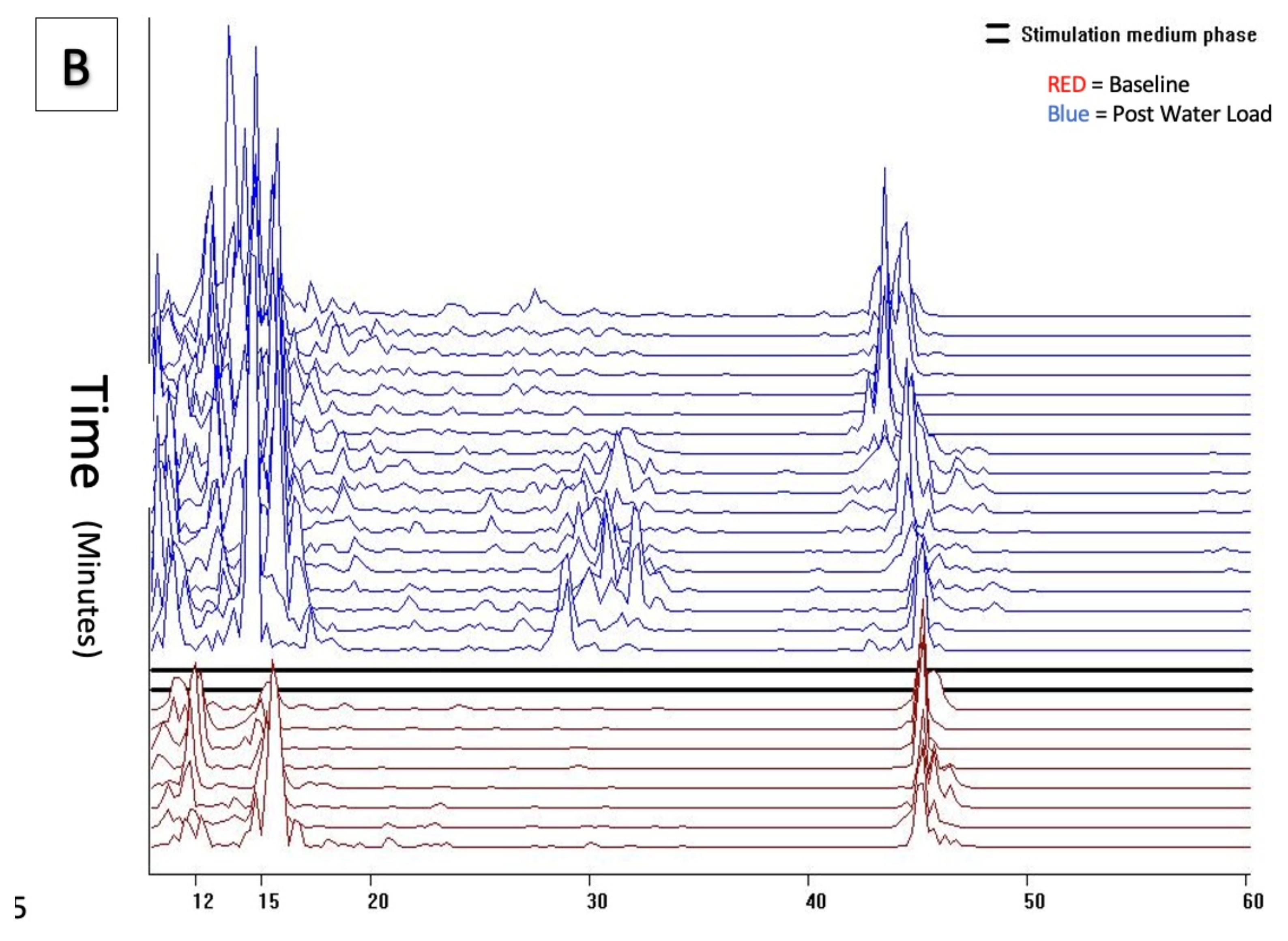
Comparatively, cohorts 2 and 3 demonstrated significant and visually distinct patterns with increased activity in both the baseline and post water load periods with increased GIMA biomarker activity in the 15–20 cpm, 30–40 cpm and 40–50 cpm frequency ranges representing known endometriosis-associated GIMA biomarker activity. (
Figure 4a,b) These differences were noted in 43/43 of cohort 2 subjects, 47/49 Cohort 3 subjects, but were absent in all 62 Cohort 1 subjects as well as absent in 2/49 (4%) cohort 3 subjects who did not have endometriosis at the time of surgery. These qualitative findings were confirmed by histopathological positivity and/or absence of endometriosis.
Power of frequency distribution of GIMA characteristics at frequencies (10–60 cpm) among subjects with endometriosis Cohort 2 and Cohort 3, at Baseline and 10 min, 20 min and 30 min post water load.
3.3. EVG GIMA Biomarker Predictive Modeling
The comparison of EVG GIMA biomarkers was derived as the percent frequency distribution of power for median (IQR) between non-endometriosis controls (cohort1) versus endometriosis positive women (cohort 2 & cohort 3). Frequencies 15–60 cpm at baseline, 10, 20, and 30 min after water load were found to be significantly different (p < 0.05) as seen in (
Table 3 Figure 5). Cohort 2 and cohort 3 were similar for all frequencies and time combinations except for 15–20 cpm at 10 min and 20 min (
Table 3). The GIMA biomarker positive endometriosis participants in cohort 3 had the same quantitative GIMA biomarker findings as histologically positive endometriosis cohort 2 subjects.
Moreover, AUC calculated for all frequency cycles was significantly higher (p<0.001) for cohort 2 (
Table 4a) and cohort 3, (
Table 4b) versus non-endometriosis cohort 1. (
Figure 6,
Figure S7a-f)
AUC values for higher frequencies were similar between cohort 2 and cohort 3 with the exception being the frequency 15–20 cpm demonstrated a significant difference (p 0.005), (
Table 4c).
Additionally, linear regression analysis of AUC for differences between non-endometriosis controls (cohort 1) versus women with endometriosis (cohort 2 and cohort 3) was significant (p<0.001) at 15–20 cpm, 30–40 cpm and 40–50 cpm frequency ranges (
Table 5a,
Table 5b).
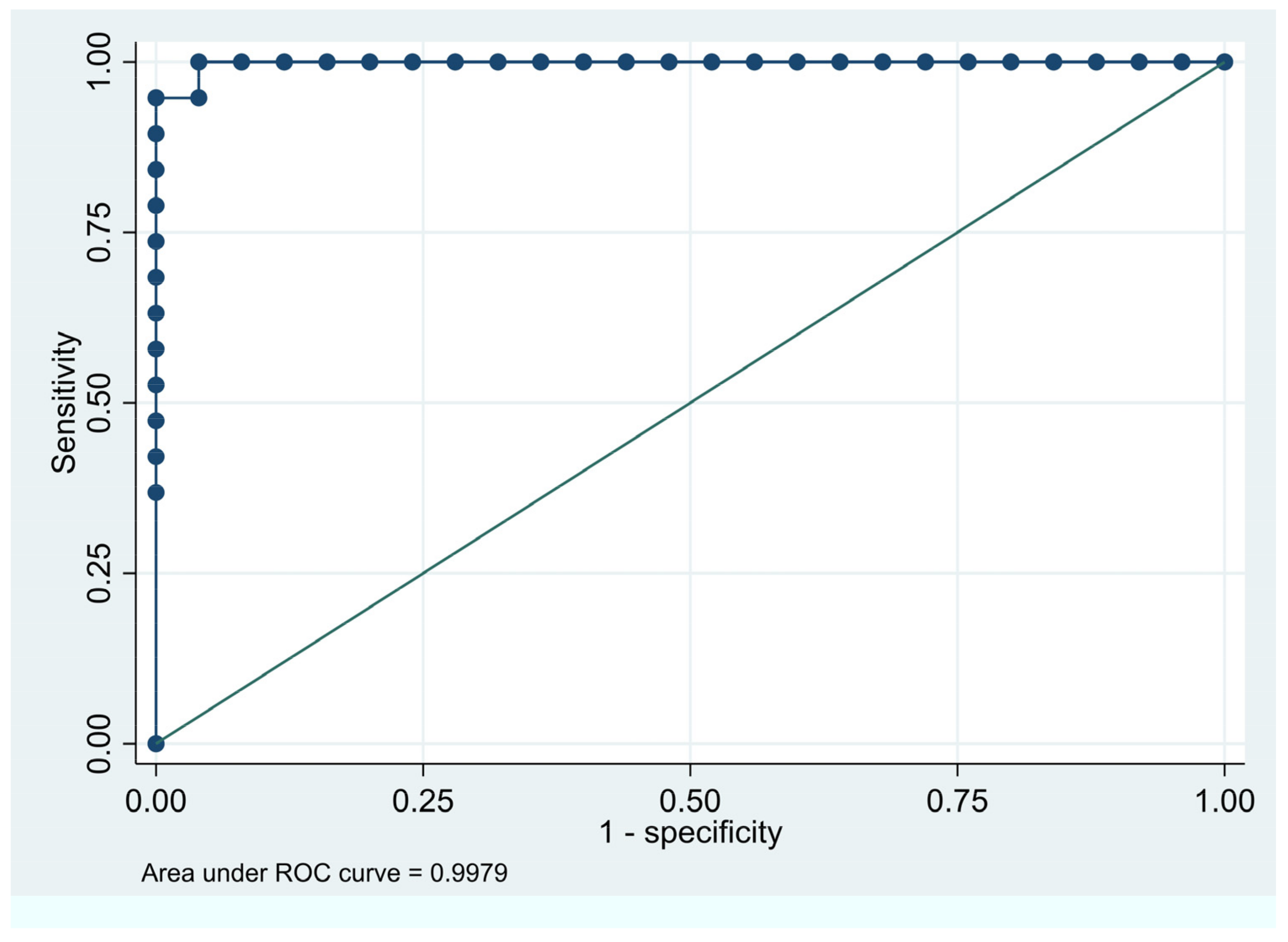
More specifically the ROC of the GIMA biomarker AUC graphs (
Figure 8a,b) affirm that women with higher GIMA biomarker AUC are more likely to have a diagnosis of endometriosis, correlating with the high specificity and predictability attributed to the GIMA biomarker.
Figure 8a.
ROC Curve for Disease Predictability Age ≤ 35yrs. Area under the ROC curve of GIMA biomarker AUC is 0.9979. The closer to a value of 1.0 the higher the predictive value of the test.
Figure 8a.
ROC Curve for Disease Predictability Age ≤ 35yrs. Area under the ROC curve of GIMA biomarker AUC is 0.9979. The closer to a value of 1.0 the higher the predictive value of the test.
Figure 6b.
ROC Curve for disease predictability Age ≥36yrs. Area under the ROC curve of GIMA biomarker AUC is 0.9847. The closer to a value of 1.0 the higher the predictive value of the test.
Figure 6b.
ROC Curve for disease predictability Age ≥36yrs. Area under the ROC curve of GIMA biomarker AUC is 0.9847. The closer to a value of 1.0 the higher the predictive value of the test.
In addition, EVG identifies not only participants with endometriosis, but also those without disease despite other concomitant illness present, as evidenced by absence of the distinct fingerprint or GIMA biomarker derived electronic signature of endometriosis in both cohort 1 subgroups which were statistically similar. (
Table 6). The data validates the GIMA biomarker as a means to non-invasively predict endometriosis in asymptomatic and symptomatic women with suspected endometriosis.
3.4. EVG Ai Derived GIMA Biomarker Algorithm for Predicting Endometriosis
Area under the curve (AUC) for each GIMA frequencies per participant was measured at baseline, 10, 20, and 30 mins was calculated. Multivariable logistic regression models were used to assess the effect of AUC GIMA frequencies and confounding variables age, and symptom score. Analysis was stratified by participants older than 35, or 35 and younger. Permutations of AUC frequencies were used in the model (
Table 7 and
Table 8). Ai methods revealed the most parsimonious model with highest C-Statistic values and lowest rate of misclassification to estimate the probability of disease.
Notable is the purposeful decision to exclude the 15–20 cpm frequency AUC from the final ideal Ai-derived calculation, due to the previously noted statistically significant difference between cohorts 2 and 3 at that frequency level. This is most likely accounted for by the presence of gallbladder disease disproportionally noted between cohorts 2 and 3 (see
Table 2). Gallbladder disease is known to induce duodenal wall spasm in the 12–20 cpm range [
33]. Therefore, elimination of this frequency band avoids the potential to have gallbladder disease as a confounding factor in the diagnosis of endometriosis.
Predicted probability of endometriosis was calculated from the logistic regression equation. Women were classified as having endometriosis if the predicted probability was > 50%. This supervised predictive model diagnosed endometriosis if the estimated probability was higher or equal to the threshold value of ≥0.5 and excluded disease if the estimated probability was less than the threshold value of <0.5.
3.5. GIMA Biomarker Model Performance
In Cohort 2, the model displayed a 95% sensitivity, 96% specificity, 95% positive predictive value (PPV), 96% negative predictive value (NPV) and >99% C-statistic for women ≤ 35 years.
In women ≥36 years the model displayed 91%, sensitivity, 95% specificity, 91% PPV, 95% NPV and 98% C-statistic, with a marginal drop of 4% in sensitivity and PPV (
Table 7 and
Table 8).
When applied to validation cohort (cohort 3) to confirm the proportion of women who could be identified correctly using the prediction model, 91% were correctly classified, with a 91% C-statistic and 96% specificity noted. (
Table 9) Variance from cohort 2 is accounted for by the previously noted variance in the AUC
15–20 between cohorts 3 and 3. (
Table 4c)
4. Discussion
At this time, this analysis of GIMA Biomarkers in subjects both with endometriosis, and without endometriosis with and without other non-endometriosis pain-associated disease, appears to be the first prospective study to report a unique GIMA biomarker signature or fingerprint for endometriosis. The impact of early diagnosis, in the current healthcare environment, of an accurate, non-invasive test for endometriosis with immediate results and not requiring the usual infrastructural time and costs of current testing methods would be profound. The combination of a non-invasive EVG device which detects the novel GIMA biomarker and Ai-derived diagnostic threshold modeling represents its intrinsic value. In a disease known for complexity and lack of predictability and homogeneity, the stability of this mature technology married with modern analytical methodology and a uniform unique myoelectrical reaction in response to endometriosis provides accuracy and reproducibility.
Implicit to the development of the EVG system to take advantage of the GIMA biomarker association with endometriosis was that it would result in a low cost, resource efficient system that could be easily deployed by anyone, anywhere in the world that there was access to a computing device, water to drink and a place to recline. The overriding goal was to eliminate the unintended financial or geographic discrimination of delayed diagnosis, stemming from current bottlenecks such as facility/provider access, infrastructural costs of laboratory-based testing, transport and processing, pressure on costly resources such as room and personnel time, and equipment availability.
In the diverse milieu of endometriosis, this prospective multicenter validation trial confirms the diagnostic capabilities as well as the accuracy and performance of the non-invasive EVG technology detection of the GIMA biomarker unique to endometriosis. The stability and reproducibility of the GIMA biomarker coupled with the Ai-derived diagnostic threshold level for detecting endometriosis and distinguishing the disease from non-disease status is confirmed by the data. In addition, the accuracy regardless of ethnicity suggests potential applicability across international populations. The two centers where the study was performed represent two different medical models spanning from the expert tertiary care center to the routine outpatient setting, with differing levels of severity of disease presentation.
The study demonstrated the ability to detect and differentiate between endometriosis confirmed subjects and non-endometriosis controls, with 95% sensitivity and PPV, 96% specificity and NPV, and 99% and 98% C-statistic predictability in women ≤35 y/o and ≥36 y/o women, respectively. The results are predicated upon GIMA biomarker activity driven by PGE2 and PGFα mediated 15–60 cpm smooth muscle GIMA activity, unique to endometriosis. Variations related to age, concurrent hormonal therapy, or stage of the disease had no impact, nor did the presence of confounding illnesses like inflammatory bowel diseases, irritable bowel syndrome, urinary or pelvic infection, chronic interstitial cystitis, biliary or ulcer disease, and polycystic ovary disease.
The application of this technology will likely be influenced by the disease severity. Advanced stage disease noted in 15% of women is easily diagnosed with transvaginal or transanal ultrasound, MRI, or physical exam [
9]. In the advanced stage subgroup, GIMA biomarker detection will be useful in providing long-sought-after post-therapeutic monitoring, currently limited by diagnostic accuracy and differentiation between postoperative changes and recurrent disease. The most compelling subgroup is the over 70% of women with early-stage disease who remain symptomatic and suffer from lack of diagnosis or inadequate response to medical therapy. GIMA biomarker threshold scoring could reduce delayed diagnosis of endometriosis of those remaining undiagnosed for decades with an average of 8.3 years between symptom onset and diagnosis in patients with pain, contrasted with 1.8 years in those with infertility [
34,
35]. Cultural complexities, sexual discrimination, geographic and financial barriers influence delay [
36].
In the current medical environment, non-invasive diagnostic options are either lacking, expensive, or have limited availability or diagnostic accuracy. [
8] Transvaginal ultrasound and MRI are effective but limited in early disease or post-surgical treatment. [
9] Blood mRNA or mutated DNA elements have varied accuracy, requiring biological material handling and laboratory services [
10,
11]. Menstrual fluid and endometrial scrapings face similar challenges [
12,
13]. The more recent use of salivary mRNA show promise and although there is reported sensitivity and specificity in the 95% to 100% range, AUC diagnostic accuracy is variable ranging from 69%-98%, with further challenges due to logistics and high cost [
10,
37,
38]. In contrast, the GIMA biomarker testing does not require the logistical challenges of biological sampling, laboratory infrastructure, specialized facilities, and cost.
As with any diagnostic test to be introduced into routine clinical practice, significant external validation will be required to answer the necessary questions concerning specificity and applicability across broad, diverse ethnic populations with variable disease. However, once accomplished, immediate clinical benefits of timely non-invasive diagnosis are earlier access to therapy for symptom control and limitation of disease progression. No guarantees exist regarding disease advancement, and randomized clinical trial data, with laparoscopy before and after an interval without treatment, demonstrated 30% short-interval advancement, without means of predicting progression [
39].
Low cost, accurate, and mobile testing would decentralize access, allowing universal rapid deployment, shortening the time between initial presentation, diagnosis, and treatment. With reproducibility in general populations, a positive impact on participants, caregivers, medical costs, and productivity is expected, eliminating unintended geographical or financial discrimination [
40].
Further positive impact includes improved 1) understanding of natural history of disease and differences/similarities between ethnic groups or countries, 2) post-surgical or medical treatment monitoring and detection of recurrence, 3) new medication development, and 4) improved adolescent dysmenorrhea screening. Threshold scores of similarly derived biomarkers may allow differential identification of other presentations of endometriosis, adenomyosis, autoimmune diseases or malignancies like ovarian cancer with unique diagnostic GIMA biomarkers. Employment of GIMA biomarker threshold scoring may play an important decisional role especially in the 47%-71% of patients that have no evidence of residual endometriosis after undergoing repeat surgery following complete excision of all recognized endometriosis [
41,
42]. It may limit unnecessary repeat surgeries and additionally help to refocus on finding either unsuspected palpable nodules missed laparoscopically, or non-visualized unrecognized bowel endometriotic satellites [
43].
4.1. Strengths and Limitations
The EVG device resembles devices including the handheld electrocardiogram making it intuitive, recognizable, and practical [
24,
31] with procedural proficiency after 1–2 procedures. Low procedural costs as well as minimal technical proficiency required to perform the test will translate into greater availability.
At this stage, inherent limitations need to be recognized. These types of studies performed in ideal or tertiary research settings may not translate directly into real-world clinical practice and further testing is needed in larger varied populations of patients. Secondly, as in all studies including a control group, not suspected of having endometriosis, finding ideal disease-free control groups is inherently difficult with many ways to miss endometriosis [
42] even in asymptomatic women and with a recognized surgical diagnostic accuracy of only 50–75% [
4] .In the control group of this analysis, it was encouraging to see the homogeneity of absent GIMA biomarkers compared to those with known disease.
Moreover, while the data is statistically sufficient to justify conclusions, model-building is an evolving process. Continued experience will refine the model, account for potential population variances, improve predictability, confirm GIMA biomarker threshold score variations, predict surgical stage/disease activity, facilitate post-treatment monitoring, and assess hormonal suppression impact. Hormonal suppression did not affect study results, yet newer suppressants may exert unknown effects. Other unidentified coexisting disease may simultaneously secrete PGE2 and PGFα, presenting as false positive results. The potential that adenomyosis could have similar GIMA biomarkers requires further evaluation of the possible impact on GIMA biomarker threshold scoring. Finally, it is not yet possible to make broadly definitive conclusions regarding the potential confounding effects of ethnic variation or other inflammatory diseases, which will require study in larger affected patient cohorts.
5. Conclusions
An unmet need exists for cost-effective, widely available, accurate, non-invasive endometriosis testing. This analysis of a prospective multicenter study provides data on the diagnostic accuracy of the non-invasive EVG detection of the unique GIMA biomarker to distinguish between subjects with or without disease. Further marriage of the EVG biomarker detection with the AI-derived threshold scoring has demonstrated a noninvasive tool with beyond reasonable accuracy to diagnose endometriosis. Testing predicted endometriosis with 98% - >99% accuracy regardless of surgical stage or hormonal therapy. With further validation in larger study cohorts and other confirmatory studies, it is reasonable to expect that this new non-invasive test and others will permit timelier diagnosis of this devastating disease, and may lead to: 1) discovery of additional biomarkers for other benign and malignant disease, 2) improve out of control costs of direct care and lost productivity, 3) increased accuracy of return to surgery decisions, 4) pretesting prior to routine surgery, 5) prenatal screening to detect unexpected endometriosis which is associated with significant post-partum complications [
44], and 5) entry of new therapeutics into the marketplace.
6. Patents
The following patents are related to the work reported in this manuscript:
1) US Patent No. 7,160,254. Intelligent Self-interpreting Electroviscerogram System and Method.
2) US Patent No. 11/369,310. Method and System for Predicting Successful Treatment Methods and Outcomes of Bodily Tissue Disorders Based On Energy Activity of the Tissue.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S7a–f: Figure 7: A–F Distribution of AUC for frequencies 10–60 cpm for controls (no endometriosis) and women with endometriosis.
Author Contributions
Conceptualization, Mark Noar and John Mathias; Data curation, Mark Noar and Ajit Kolatkar; Formal analysis, Mark Noar and Ajit Kolatkar; Investigation, Mark Noar and John Mathias; Methodology, Mark Noar and John Mathias; Project administration, Mark Noar, John Mathias and Ajit Kolatkar; Resources, Mark Noar, John Mathias and Ajit Kolatkar; Software, Mark Noar; Supervision, Mark Noar and Ajit Kolatkar; Validation, Mark Noar and Ajit Kolatkar; Visualization, Mark Noar and John Mathias; Writing–original draft, Mark Noar; Writing–review & editing, Mark Noar, John Mathias and Ajit Kolatkar. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Investigational Review Board IRB - C.H.C.A. Woman's Hospital, L.P., 00004260, Houston.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
Dan Martin, MD provided guidance and advice in analysis, review, and writing. Nelda Fraga, R.N. performed electroviscerograms in control and study subjects. Nikhil Gupte, PhD. gave statistical guidance and review. Written permission has been obtained to include names in acknowledgments.
Conflicts of Interest
MN reports a relationship with Endosure, Inc as a founder and board member. MN has patent #7,160,254 licensed to Endosure, Inc. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362: 2389–98.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244–56.
- Chen S, Liu Y, Zhong Z, Wei C, Liu Y, Zhu X. Peritoneal immune microenvironment of endometriosis: Role and therapeutic perspectives. Front Immunol. 2023 Feb 14;14:1134663. [CrossRef] [PubMed] [PubMed Central]
- Taylor HS, Adamson GD, Diamond MP, Goldstein SR, Horne AW, Missmer SA, Snabes MC, Surrey E, Taylor RN. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018 Aug;142(2):131-142. [CrossRef] [PubMed]
- Nnoaham KE, Hummelshoj L, Webster P, et al. KT; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil Steril 2011;96:366–73.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod Oxf Engl 2012;27:1292–9.
- Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018 Jul 3. pii: S1521-6934(18)30109-3. doi: 10.1016/j.bpobgyn.2018.06.001 PMID: 300175814. Evans MB, DeCherney AH. Fertility and Endometriosis. Clin Obstet Gynecol. 2017 Sep;60(3):497-502. [CrossRef] [PubMed]
- Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, Singh SS, Taylor HS. Clinical diagnosis of endometriosis: A call to action. Am J Obstet Gynecol. 2019 Apr;220(4):354.
- Abrao MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007 Dec;22(12): 3092–7.
- Bendifallah S, Suisse S, Puchar A, Delbos L, Poilblanc M, et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J Clin Med. 2022 Jan 26;11(3):612.
- Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 2016 Jul 13;7.
- Shih, A.J., Adelson, R.P., Vashistha, H. et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med 2022 20, 315.
- DF, Flores I, Waelkens E, D'Hooghe T. Non-invasive diagnosis of endometriosis: Review of current peripheral blood and endometrial biomarkers. Best Pract Res Clin Obstet Gynaecol 2018 Jul;50:72–83.
- Giudice, Linda C. MD, PhD. Advances in approaches to diagnose endometriosis. Global Reproductive Health 9(1):e0074, Spring 2024. [CrossRef]
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, van Hanegem N, Vulliemoz N, Vermeulen N; ESHRE Endometriosis Guideline Group. ESHRE guideline: Endometriosis. Hum Reprod Open. 2022 Feb 26;2022(2):hoac009. [CrossRef] [PubMed] [PubMed Central]
- Keckstein J, Saridogan E, Ulrich UA, Sillem M, Oppelt P, Schweppe KW, Krentel H, Janschek E, Exacoustos C, Malzoni M, Mueller M, Roman H, Condous G, Forman A, Jansen FW, Bokor A, Simedrea V, Hudelist G. The #Enzian classification: A comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand. 2021 Jul;100(7):1165-1175. [CrossRef] [PubMed]
- Requadt E, Nahlik AJ, Jacobsen A, Ross WT. Patient experiences of endometriosis diagnosis: A mixed methods approach. BJOG. 2023 Nov 13. [CrossRef] [PubMed]
- Creed J, Maggrah A, Reguly B, Harbottle A. Mitochondrial DNA deletions accurately detect endometriosis in symptomatic females of child-bearing age. Biomark Med. 2019 Mar;13(4):291-306. [CrossRef] [PubMed]
- Nezhat C, Rambhatla A, Miranda-Silva C, Asiaii A, Nguyen K, Eyvazzadeh A, Tazuke S, Agarwal S, Jun S, Nezhat A, Roman RA. BCL-6 Overexpression as a Predictor for Endometriosis in Patients Undergoing In Vitro Fertilization. JSLS. 2020 Oct-Dec;24(4):e2020.00064. [CrossRef] [PubMed] [PubMed Central]
- Warren, L.A., Shih, A., Renteira, S.M. et al. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol Med 24, 1 (2018). [CrossRef]
- Ji S, Liu Y, Yan L, Zhang Y, Li Y, Zhu Q, Xia W, Ge S, Zhang J. DIA-based analysis of the menstrual blood proteome identifies association between CXCL5 and IL1RN and endometriosis. J Proteomics. 2023 Oct 30;289:104995. [CrossRef] [PubMed]
- Wioletta Dolińska, Hannah Draper, Lara Othman, Chloe Thompson, Samantha Girvan, Keith Cunningham, Jane Allen, Alan Rigby, Kevin Phillips, Barbara-ann Guinn, Accuracy and utility of blood and urine biomarkers for the noninvasive diagnosis of endometriosis: A systematic literature review and meta-analysis, F&S Reviews, Volume 4, Issue 2, 2023, Pages 116-130.
- Mathias JR, Franklin R, Quast DC, Fraga N, Loftin CA, Yates L, Harrison V. Relation of endometriosis and neuromuscular disease of the gastrointestinal tract: New insights. Fertil Steril. 1998 Jul;70(1):81-8.
- Noar, M. AI-Derived Threshold Score of Intraabdominal Myoelectrical Activity Predicts Presence and Stage of Endometriosis with 100% Accuracy, Journal of Minimally Invasive Gynecology, Volume 29, Issue 11, Supplement, 2022, Pages S4-S5.
- Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, Irwig L, Levine D, Reitsma JB, de Vet HC, Bossuyt PM. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open. 2016 Nov 14;6(11):e012799. [CrossRef] [PubMed] [PubMed Central]
- Metzemaekers J, Haazebroek P, Smeets MJGH, English J, Blikkendaal MD, Twijnstra ARH, Adamson GD, Keckstein J, Jansen FW. EQUSUM: Endometriosis QUality and grading instrument for SUrgical performance: Proof of concept study for automatic digital registration and classification scoring for r-ASRM, EFI and Enzian. Hum Reprod Open. 2020 Dec 30;2020(4):hoaa053. [CrossRef] [PubMed] [PubMed Central]
- Clark, K, Myatt, L. Prostaglandins and the Reproductive Cycle Glob. libr. women's med.,(ISSN: 1756-2228) 2008.
- Koite H, Egawa T, Ohtsuka M, et al. Correlation between dysmenorrheic severity and prostaglandin production in women with endometriosis. Prostaglandins, Leukot Essent Fatty Acids 1990;46:133–7.
- Morita M, Yano Y, Otaka K, et al. Minimal and mild endometriosis: Nd:Yag laser treatment and changes in prostaglandin concentrations in peritoneal fluid. J Reprod Med 1990; 35:621– 4.
- Creatsas G, Deligeoroglou E, Zachari A, Loutradis D, Papadimitriou T, Miras K, Aravantinos D. Prostaglandins: PGF2 alpha, PGE2, 6-keto-PGF1 alpha and TXB2 serum levels in dysmenorrheic adolescents before, during and after treatment with oral contraceptives. Eur J Obstet Gynecol Reprod Biol. 1990 Sep;36(3):292-8. [CrossRef] [PubMed]
- Noar, MD, Intelligent Self-Interpreting Electroviscerogram System and Method. US-7160254-B2. USPTO Jan 9, 2007.
- Puchar A, Panel P, Oppenheimer A, Du Cheyron J, Fritel X, Fauconnier A. The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis. J Clin Med. 2021 Jul 21;10(15):3216.
- Koussayer T, Ducker TE, Clench MH, Mathias JR. Ampulla of Vater/duodenal wall spasm diagnosed by antroduodenal manometry. Dig Dis Sci. 1995 Aug;40(8):1710-9. [CrossRef] [PubMed]
- Van der Zanden M, Teunissen DAM, van der Woord IW, Braat DDM, Nelen WLDM, Nap AW. Barriers and facilitators to the timely diagnosis of endometriosis in primary care in the Netherlands. Fam Pract. 2020 Feb 19;37(1):131-136.
- Staal AH, van der Zanden M, Nap AW. Diagnostic Delay of Endometriosis in the Netherlands. Gynecol Obstet Invest. 2016;81(4):321-4.
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D'Hooghe T. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012 May;27(5):1292-9.
- Ferrier C, Bendifallah S, Suisse S, Dabi Y, Touboul C, Puchar A, Zarca K, Durand Zaleski I. Saliva microRNA signature to diagnose endometriosis: A cost-effectiveness evaluation of the Endotest®. BJOG. 2023 Mar;130(4):396-406. [CrossRef] [PubMed]
- Bendifallah S, Dabi Y, Suisse S, Jornea L, Bouteiller D, Touboul C, Puchar A, Daraï E. A Bioinformatics Approach to MicroRNA-Sequencing Analysis Based on Human Saliva Samples of Patients with Endometriosis. Int J Mol Sci. 2022 Jul 21;23(14):8045. [CrossRef] [PubMed] [PubMed Central]
- Hans Evers JL. Is adolescent endometriosis a progressive disease that needs to be diagnosed and treated? Hum Reprod. 2013 Aug;28(8):2023. [CrossRef] [PubMed]
- Surrey E, Soliman AM, Trenz H, Blauer-Peterson C, Sluis A. Impact of Endometriosis Diagnostic Delays on Healthcare Resource Utilization and Costs. Adv Ther. 2020 Mar;37(3):1087-1099.
- Yeung P Jr, Sinervo K, Winer W, Albee RB Jr. Complete laparoscopic excision of endometriosis in teenagers: Is hormonal suppression necessary? Fertil Steril. 2011 May;95(6):1909-12.
- Redwine DB. Conservative laparoscopic excision of endometriosis by sharp dissection: Life table analysis of reoperation and persistent or recurrent disease. Fertil Steril. 1991 Oct;56(4):628-34.
- Roman H, Merlot B, Forestier D, Noailles M, Magne E, Carteret T, Tuech JJ, Martin DC. Nonvisualized palpable bowel endometriotic satellites. Hum Reprod. 2021, 36(3):656-665.
- Nagase Y, Matsuzaki S, Ueda Y, Kakuda M, Kakuda S, Sakaguchi H, Maeda M, Hisa T, Kamiura S. Association between Endometriosis and Delivery Outcomes: A Systematic Review and Meta-Analysis. Biomedicines. 2022 Feb 17;10(2):478. [CrossRef] [PubMed] [PubMed Central]
Figure 1.
EVG system with A: Tricorder-3l, B: Respiratory belt and C: Dry gel electrodes.
Figure 1.
EVG system with A: Tricorder-3l, B: Respiratory belt and C: Dry gel electrodes.
Figure 2.
Cohort Stratification.
Figure 2.
Cohort Stratification.
Figure 3.
Running spectral analysis of Cohort 1.
Figure 3.
Running spectral analysis of Cohort 1.
Figure 4.
Running spectral analysis of Cohort 2 and Cohort 3.
Figure 4.
Running spectral analysis of Cohort 2 and Cohort 3.
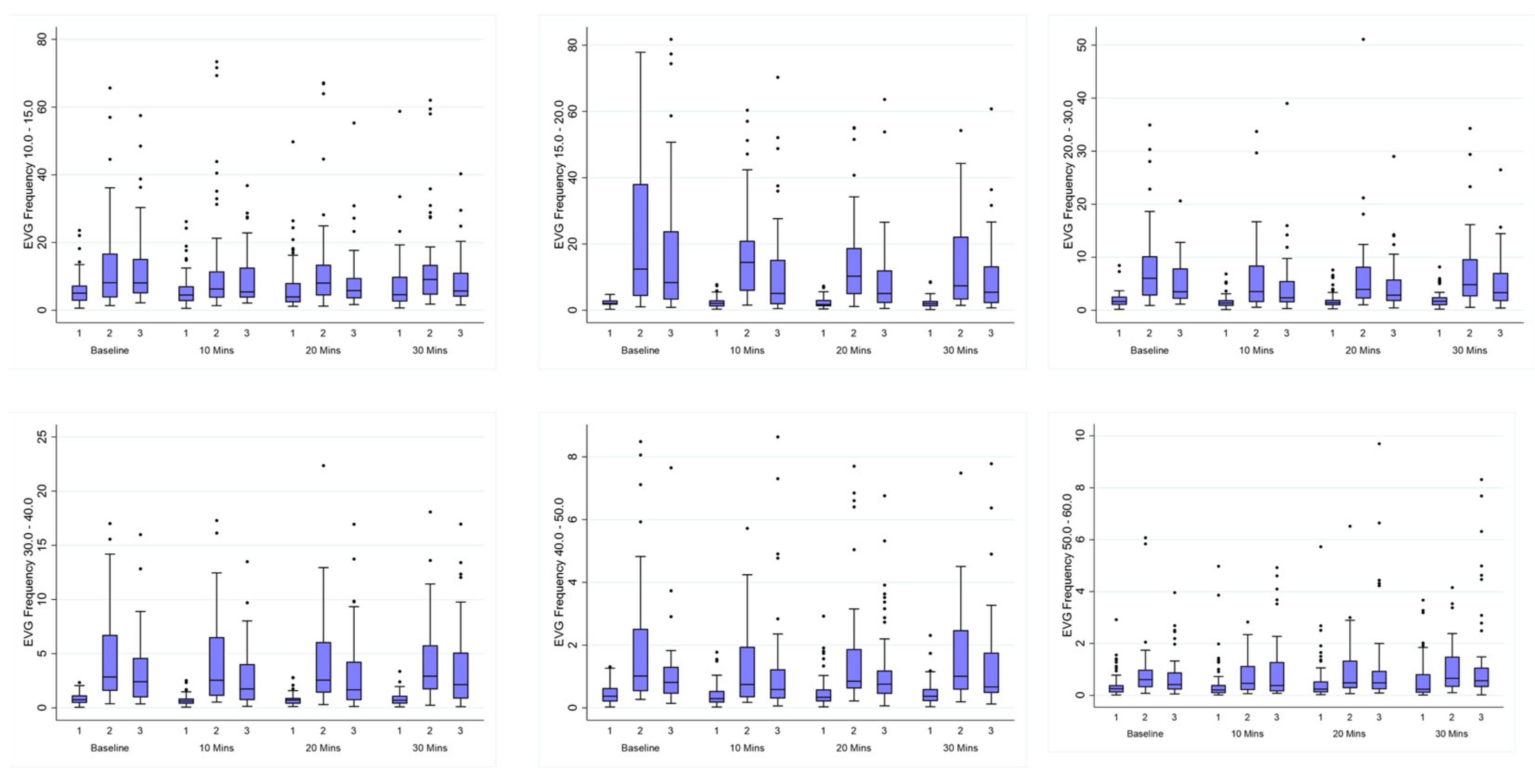
Figure 5.
Box plots depict comparison of distribution of GIMA characteristics using Median (IQR). Unadjusted median and interquartile ranges for frequencies (10–60 cpm) among healthy controls (Cohort 1) versus subjects with endometriosis (Cohorts 2 and 3) at BL, 10 min, 20 min and 30 min.
Figure 5.
Box plots depict comparison of distribution of GIMA characteristics using Median (IQR). Unadjusted median and interquartile ranges for frequencies (10–60 cpm) among healthy controls (Cohort 1) versus subjects with endometriosis (Cohorts 2 and 3) at BL, 10 min, 20 min and 30 min.
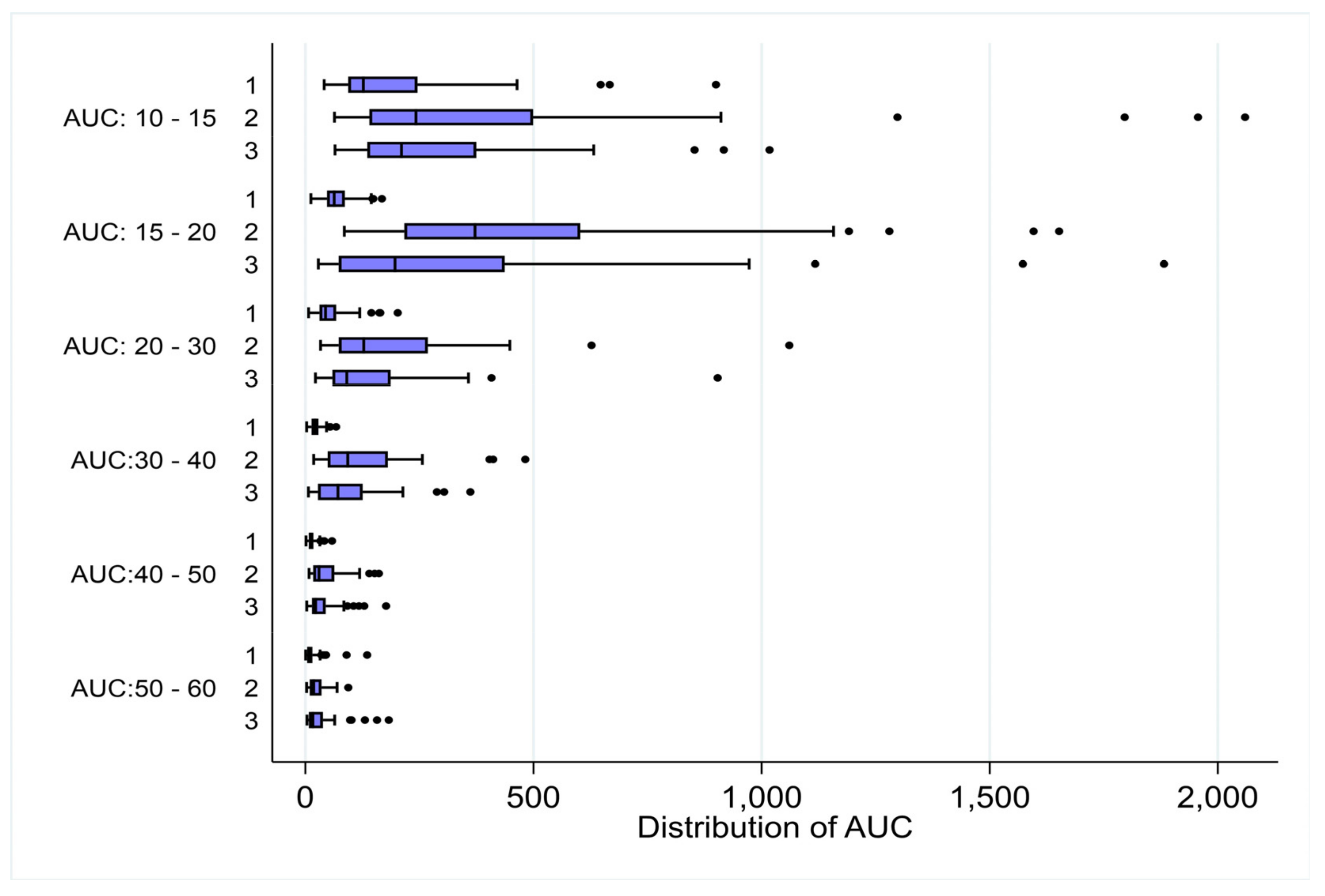
Figure 6.
Distribution of AUC for frequencies between healthy controls (Cohort 1) and subjects with endometriosis (Cohorts 2 and 3). Box plots were used to compare distribution of AUC between controls and cases for a given frequency. For all frequencies p value was less than 0.001 while comparing cohort 1 vs cohort 2 and cohort 1 vs cohort 3 from 15 cpm to 60 cpm.
Figure 6.
Distribution of AUC for frequencies between healthy controls (Cohort 1) and subjects with endometriosis (Cohorts 2 and 3). Box plots were used to compare distribution of AUC between controls and cases for a given frequency. For all frequencies p value was less than 0.001 while comparing cohort 1 vs cohort 2 and cohort 1 vs cohort 3 from 15 cpm to 60 cpm.
Table 1.
Comparison of baseline demographic characteristics of all cohorts.
Table 1.
Comparison of baseline demographic characteristics of all cohorts.
| Baseline characteristics |
Cohort 1
N = 62 |
Cohort 2
N = 43 |
Cohort 3
N = 49 |
Cohort 2 vs. Cohort 1 |
Cohort 3 vs. Cohort 1 |
Cohort 3 v/s Cohort 2 |
| Age, Median (IQR) |
40 (30–49) |
32 (27–38) |
36 (27–38) |
0.003 |
0.01 |
0.42 |
| BMI, Median (IQR) |
25.1
(20.6–29.1) |
24.4
(21.1–28.6) |
23.6
(19.9–27.4) |
0.90 |
0.18 |
0.14 |
Ethnicity, n (%)
Asian
Black
Caucasian
Hispanic
|
0
3 (5%)
51 (82%)
8 (13%) |
9 (21%)
2 (5%)
29 (67%)
3 (7%) |
1 (2%)
3 (7%)
39 (85%)
3 (7%) |
0.001 |
0.49 |
0.04 |
| ENDO-4D Pain Score, Median (IQR) range 0–10 |
1.5 (0–3.0) |
4.0 (3.0–5.5) |
5.0 (4.0–6.0) |
p < 0.001 |
p < 0.001 |
0.07 |
Pain
No
Yes
|
19 (31%)
43 (69%) |
1 (2%)
42 (98%) |
5 (11%)
42 (89%) |
p < 0.001 |
0.02 |
0.62 |
Bloating
No
Yes
|
34 (55%)
28 (45%) |
17 (40%)
26 (60%) |
14 (30%)
33 (70%) |
0.17 |
0.01 |
0.34 |
Table 2.
Clinical Characteristics of the GIMA Biomarkers Cohorts.
Table 2.
Clinical Characteristics of the GIMA Biomarkers Cohorts.
| Characteristic |
Cohort 1
Non-endometriosis Patients
(n=62) |
Cohort 2 Endometriosis Surgically Confirmed
(n=43) |
Cohort 3
Post-Surgical Confirmation Endometriosis
(n=49) |
| Mode of diagnosis |
|
|
|
| Surgically Confirmed |
- |
43/43 |
47/49 |
| Surgically Excluded |
- |
0 |
2/49 |
| ASRM Classification |
|
| I–II |
- |
23/43 |
25/47 |
| III-IV |
- |
20/43 |
22/47 |
| MEDICATIONS, n(%) |
16 (26%) |
24 (56%) |
30 (64%) |
| Oral Dual Contraceptive |
9 (14%) |
11 (26%) |
14 (29%) |
| Progestins |
2 (3%) |
3 (7%) |
6 (12%) |
| Androgens |
0 |
1 (2%) |
0 |
| Medicated IUD |
1 (2%) |
1 (2%) |
0 |
| GNRH agents |
0 |
6 (14%) |
3 (6%) |
| Estrogen only |
4 (6%) |
2 (5%) |
7 (14%) |
| Control diagnoses (not endometriosis) n(%) |
|
|
|
| No abnormality |
33 (53%) |
26 (60%) |
32 (65%) |
| Polycythemia vera |
1 (2%) |
0 |
0 |
| Microscopic/Ulcerative Colitis/Crohns |
3 (5%) |
0 |
0 |
| Ehlers Danlos |
1 (2%) |
0 |
0 |
| Thyroid Disease |
2 (3%) |
1 (2%) |
2 (4%) |
| PCOS |
2 (3%) |
1 (2%) |
1 (2%) |
| Collagen Vascular Disease |
3 (5%) |
3 (7%) |
2 (4%) |
| Interstitial Cystitis |
3 (5%) |
4 (9%) |
3 (6%) |
| Fibroids |
5 (8%) |
3 (7%) |
1 (2%) |
| Simple Ovarian Cysts |
2 (3%) |
2 (5%) |
4 (8%) |
| IBS |
9 (15%) |
3 (7%) |
3 (6%) |
| Diabetes |
5 (8%) |
4 (9%) |
7 (14%) |
| Migraines |
1 (2%) |
1 (2%) |
0 |
| Gallbladder Disease |
0 |
2 (5%) |
0 |
Table 3.
Comparison of GIMA Biomarker characteristics using Median (IQR). Unadjusted median and interquartile ranges calculated for each frequency ranging from 10cpm to 60 cpm at times BL, 10, 20, and 30 min comparing non-endometriosis controls (Cohort 1) and subjects with endometriosis (Cohort 2 & Cohort 3) using rank-sum test. P value of less than 0.05 is statistically significant. * p-value > 0.05 for comparing Cohort 2 versus Cohort 3; ** p-value > 0.05 for comparing Cohort 1 v/s Cohort 3. All other p-values are < 0.05.
Table 3.
Comparison of GIMA Biomarker characteristics using Median (IQR). Unadjusted median and interquartile ranges calculated for each frequency ranging from 10cpm to 60 cpm at times BL, 10, 20, and 30 min comparing non-endometriosis controls (Cohort 1) and subjects with endometriosis (Cohort 2 & Cohort 3) using rank-sum test. P value of less than 0.05 is statistically significant. * p-value > 0.05 for comparing Cohort 2 versus Cohort 3; ** p-value > 0.05 for comparing Cohort 1 v/s Cohort 3. All other p-values are < 0.05.
Frequency
(cycles/Min) |
Cohort 1
(n = 62) |
Cohort 2
(n = 43) |
Cohort 3
(n = 49) |
10.0–15.0
Baseline
10 min
20 min
30 min |
5.1 (2.8–7.3)
4.5 (2.7–7.1)
4.0 (2.3–8.1)
4.6 (2.5–9.9) |
8.1 (3.8–16.7)
6.3 (3.7–11.4)
8.0 (4.4–13.5)
9.1 (4.6–13.4) |
8.1 (5.0–15.2)*
5.4 (3.7–12.6)*
5.8 (3.5–9.5)*
5.7 (4.0–11.1)*,**
|
15.0–20.0
Baseline
10 min
20 min
30 min |
2.1 (1.6–3.0)
2.1 (1.1–3.0)
1.7 (1.2–3.1)
2.0 (1.2–2.8) |
12.4 (4.3–38.1)
14.4 (5.8–21.0)
10.3 (4.9–18.8)
7.4 (3.2–22.2) |
8.4 (3.2–23.8)*
5.1 (1.8–15.2)
5.0 (2.2–12.0)
5.4 (2.2–13.3)*
|
20.0–30.0
Baseline
10 min
20 min
30 min |
1.6 (1.0 - 2.6)
1.4 (0.8–1.9)
1.4 (0.8–1.9)
1.7 (0.9–2.5) |
6.0 (2.8 -10.2)
3.5 (1.5–8.4)
3.9 (2.2–8.2)
4.8 (2.6 -9.6) |
3.5 (2.1 - 7.9) *
2.4 (1.5–5.5) *
2.8 (1.8 -5.8) *
3.3 (1.7–7.0) *
|
30.0–40.0
Baseline
10 min
20 min
30 min |
0.7 (0.4–1.2)
0.6 (0.4 - 0.9)
0.6 (0.4 -0.9)
0.7 (0.4–1.2) |
2.9 1.6–6.7)
2.5 (1.1 -6.5)
2.6 (1.4 -6.1)
2.9 (1.7–5.8) |
2.4 (1.0–4.6) *
1.7 (0.7–4.0) *
1.7 (0.7–4.3) *
2.1 (0.9–5.1) *
|
40.0–50.0
Baseline
10 min
20 min
30 min |
0.4 (0.2–0.6)
0.3 (0.2–0.5)
0.3 (0.2–0.6)
0.4 (0.2–0.6) |
1.0 (0.5–2.5)
0.7 (0.3–1.9)
0.8 (0.6–1.9)
1.0 (0.6–2.5) |
0.8 (0.4–1.3) *
0.6 (0.3–1.2) *
0.8 (0.6–1.2) *
0.7 (0.5–1.8) *
|
50.0–60.0
Baseline
10 min
20 min
30 min |
0.3 (0.1–0.4)
0.2 (0.1–0.4)
0.2 (0.1–0.5)
0.2 (0.1 - 0.8) |
0.6 (0.3–1.0)
0.5 (0.2–1.1)
0.5 (0.3 -1.3)
0.7 (0.3–1.5) |
0.4 (0.2 -0.9) *
0.4 (0.2–1.3) *
0.5 (0.2–0.9) *
0.6 (0.3–1.1) *
|
Table 4a.
Summary of AUC of healthy controls (Cohort 1) and subjects with endometriosis (Cohort 2). Area under the curve (AUC) calculated for each woman for a given frequency. Median AUC’s were estimated and compared using the rank-sum test.
Table 4a.
Summary of AUC of healthy controls (Cohort 1) and subjects with endometriosis (Cohort 2). Area under the curve (AUC) calculated for each woman for a given frequency. Median AUC’s were estimated and compared using the rank-sum test.
| AUC Frequency |
Cohort 1 |
Cohort 2 |
p-value |
| 10–15 cpm |
127.0 (93.9–245.7) |
242.3 (140.2–499.1) |
p < 0.001 |
| 15–20 cpm |
63.1 (47.4–86.5) |
371.8 (217.0–602.6) |
p < 0.001 |
| 20–30 cpm |
44.1 (40.0–67.4) |
128.0 (73.2–268.4) |
p < 0.001 |
| 30–40 cpm |
21.2 (13.6–28.5) |
92.8 (48.8–180.8) |
p < 0.001 |
| 40–50 cpm |
11.5 (7.4–17.6) |
29.8 (17.2–63.3) |
p < 0.001 |
| 50–60 cpm |
7.3 (3.9–15.3) |
18.1 (9.4–35.3) |
p < 0.001 |
Table 4b.
Summary of GIMA Biomarker AUC of non-endometriosis controls (Cohort 1) and subjects with endometriosis (Cohort 3). Area under the curve calculated for each woman for a given frequency. Median AUCs were estimated and compared using the rank-sum test.
Table 4b.
Summary of GIMA Biomarker AUC of non-endometriosis controls (Cohort 1) and subjects with endometriosis (Cohort 3). Area under the curve calculated for each woman for a given frequency. Median AUCs were estimated and compared using the rank-sum test.
| AUC Frequency |
Cohort 1 |
Cohort 3 |
p-value |
| 10–15 cpm |
127.0 (93.9–245.7) |
210.6 (135.7–374.6) |
0.003 |
| 15–20 cpm |
63.1 (47.4–86.5) |
196.4 (73.0–436.8) |
p < 0.001 |
| 20–30 cpm |
44.1 (40.0–67.4) |
90.8 (59.3–186.8) |
p < 0.001 |
| 30–40 cpm |
21.2 (13.6–28.5) |
71.3 (27.5–125.5) |
p < 0.001 |
| 40–50 cpm |
11.5 (7.4–17.6) |
22.5 (14.5–44.5) |
p < 0.001 |
| 50–60 cpm |
7.3 (3.9–15.3) |
16.3 (7.5–38.4) |
p < 0.001 |
Table 4c.
Summary of GIMA Biomarker AUC of Cohort 2 and Cohort 3. Area under the curve calculated for each woman for a given frequency. Median AUCs were estimated and compared using the rank-sum test.
Table 4c.
Summary of GIMA Biomarker AUC of Cohort 2 and Cohort 3. Area under the curve calculated for each woman for a given frequency. Median AUCs were estimated and compared using the rank-sum test.
| AUC Frequency |
Cohort 2 |
Cohort 3 |
p-value |
| 10–15 cpm |
242.3 (140.2–499.1) |
210.6 (135.7–374.6) |
0.32 |
| 15–20 cpm |
371.8 (217.0–602.6) |
196.4 (73.0–436.8) |
0.005 |
| 20–30 cpm |
128.0 (73.2–268.4) |
90.8 (59.3–186.8) |
0.05 |
| 30–40 cpm |
92.8 (48.8–180.8) |
71.3 (27.5–125.5) |
0.04 |
| 40–50 cpm |
29.8 (17.2–63.3) |
22.5 (14.5–44.5) |
0.16 |
| 50–60 cpm |
18.1 (9.4–35.3) |
16.3 (7.5–38.4) |
0.59 |
Table 5a.
Linear Regression Analysis of GIMA Biomarker AUC by frequencies for differences in non-endometriosis controls (Cohort 1) and subjects with endome triosis (Cohort 2) *Model adjusted for age, symptom score, water load and BMI.
Table 5a.
Linear Regression Analysis of GIMA Biomarker AUC by frequencies for differences in non-endometriosis controls (Cohort 1) and subjects with endome triosis (Cohort 2) *Model adjusted for age, symptom score, water load and BMI.
| Frequency |
Univariable Analysis |
Multivariable Analysis* |
| |
Mean Difference (95% CI) |
p-value |
Mean Difference (95% CI) |
p-value |
| AUC10 - 15 |
227.4
(93.6 – 361.1) |
0.001 |
278.3
(125.6 – 430.9) |
0.001 |
| AUC15 - 20 |
417.8
(321.0 – 514.7) |
p < 0.001 |
402.4
(289.7 – 515.1) |
p < 0.001 |
| AUC20 - 30 |
147.9
(98.9 – 197.0) |
p < 0.001 |
149.2
(93.2 – 205.3) |
p < 0.001 |
| AUC30 - 40 |
105.2
(77.6 – 132.8) |
p < 0.001 |
102.1
(70.0 – 134.2) |
p < 0.001 |
| AUC40 – 50 |
31.9
(21.5 – 42.3) |
p < 0.001 |
28.2
(16.6 – 39.9) |
p < 0.001 |
| AUC50 - 60 |
10.8
(2.3 – 19.4) |
0.01 |
11.9
(2.2 – 21.7) |
0.02 |
Table 5b.
Linear Regression Analysis of GIMA Biomarker AUC by frequencies for differences in non-endometriosis controls (Cohort 1) and subjects with endometriosis (cohort 3) *Model adjusted for age, symptom score, water load and BMI.
Table 5b.
Linear Regression Analysis of GIMA Biomarker AUC by frequencies for differences in non-endometriosis controls (Cohort 1) and subjects with endometriosis (cohort 3) *Model adjusted for age, symptom score, water load and BMI.
| Frequency |
Univariable Analysis |
Multivariable Analysis* |
| |
Mean Difference (95% CI) |
p-value |
Mean Difference (95% CI) |
p-value |
| AUC10 - 15 |
85.4
(-30.4, 201.2) |
0.15 |
113.2
(-23.8 – 250.2) |
0.11 |
| AUC15 - 20 |
272.9
(160.5 – 385.3) |
p < 0.001 |
302.0
(166.7 – 437.4) |
p < 0.001 |
| AUC20 - 30 |
88.6
(39.0 – 138.2) |
0.001 |
95.5
(36.1 – 154.9) |
0.72 |
| AUC30 - 40 |
68.4
(40.5 – 96.2) |
p < 0.001 |
71.2
(37.6 – 104.8) |
p < 0.001 |
| AUC40 – 50 |
23.4
(12.1 – 34.8) |
p < 0.001 |
24.2
(10.7 – 37.7) |
P < 0.001 |
| AUC50 - 60 |
16.7
(5.8 – 27.6) |
0.003 |
18.1
(5.6 – 30.7) |
0.005 |
Table 6.
Comparison of GIMA Biomarker characteristics between Sub-group 1 and Sub-group 2 using Median (IQR). Unadjusted median and interquartile ranges, calculated for each frequency ranging from 10 cpm to 60 cpm at times BL, 10, 20 and 30 min compared within Cohort 1 between sub-group 1 and sub-group 2 using rank-sum test.
Table 6.
Comparison of GIMA Biomarker characteristics between Sub-group 1 and Sub-group 2 using Median (IQR). Unadjusted median and interquartile ranges, calculated for each frequency ranging from 10 cpm to 60 cpm at times BL, 10, 20 and 30 min compared within Cohort 1 between sub-group 1 and sub-group 2 using rank-sum test.
Frequency
(cpm=cycles/Min) |
Sub-group 2
Asymptomatic non-endometriosis Controls w/o symptoms (n =7) |
Sub-group 1
Symptomatic non-endometriosis controls (n = 55) |
p-value |
10.0–15.0 cpm
Baseline
10 min
20 min
30 min |
5.8 (1.7–9.5)
3.0 (1.1–5.1)
2.0 (2.0–5.1)
4.4 (2.9–9.1) |
5.1 (2.8–7.3)
4.8 (2.8–7.5)
4.0 (2.4–10.1)
4.7 (2.4–9.9) |
0.9
0.2
0.1
0.9 |
15.0–20.0 cpm
Baseline
10 min
20 min
30 min |
2.4 (1.0–4.4)
0.8 (0.3–2.9)
1.2 (0.6–1.8)
2.1 (0.9–3.9) |
2.0 (1.7–3.0)
2.1 (1.2–3.1)
1.8 (1.2–3.1)
2.0 (1.2–2.8) |
0.8
0.1
0.3
p > 0.95 |
20.0–30.0 cpm
Baseline
10 min
20 min
30 min |
2.0 (1.0–2.4)
0.8 (0.4–1.9)
1.2 (0.5–1.5)
1.4 (1.3–2.6) |
1.6 (1.0–2.8)
1.5 (0.9–1.9)
1.5 (0.9–2.0)
1.8 (0.9–2.3) |
0.9
0.2
0.1
0.7 |
30.0–40.0 cpm
Baseline
10 min
20 min
30 min |
0.8 (0.2–1.6)
0.4 (0.1–0.9)
0.5 (0.4–0.9)
0.8 (0.4–1.1) |
0.7 (0.5–1.2)
0.6 (0.4–0.9)
0.7 (0.4–0.9)
0.7 (0.4–1.1) |
0.8
0.3
0.8
0.7 |
40.0–50.0 cpm
Baseline
10 min
20 min
30 min |
0.3 (0.1–0.7)
0.1 (0.1 - 0.5)
0.3 (0.2–0.5)
0.4 (0.2–1.0) |
0.4 (0.2–0.6)
0.3 (0.2–0.5)
0.4 (0.2–0.6)
0.4 (0.2–0.6) |
0.5
0.2
0.7
0.3 |
50.0–60.0 cpm
Baseline
10 min
20 min
30 min |
0.3 (0.2–1.1)
0.2 (0.04–1.4)
0.4 (0.1–0.6)
0.4 (0.1–1.0) |
0.3 (0.1–0.4)
0.2 (0.1–0.4)
0.2 (0.1–0.5)
0.2 (0.1–0.8) |
0.2
p> 0.95
0.9
0.7 |
Table 7.
AI derived permutations of Prediction Modeling for subjects age ≤35. The yellow highlighted bold values designate the best predictive model for subjects aged 35 and younger, in cohort 2.
Table 7.
AI derived permutations of Prediction Modeling for subjects age ≤35. The yellow highlighted bold values designate the best predictive model for subjects aged 35 and younger, in cohort 2.
| Age ≤ 35 years (N=24) |
Sensitivity |
Specificity |
PPV |
NPV |
C-Statistic |
Correctly Classified |
| AUC15–20
|
76% |
92% |
89% |
82% |
91% |
85% |
| AUC15–20 + Symptom Score |
95% |
96% |
95% |
96% |
99% |
95% |
| AUC15–20 + Symptom Score + Age |
95% |
96% |
95% |
96% |
99% |
95% |
| AUC30–40 |
71% |
96% |
94% |
80% |
88% |
85% |
| AUC30–40 + Symptom Score |
95% |
92% |
90% |
96% |
99% |
93% |
| AUC30–40 + Symptom Score + Age |
95% |
92% |
90% |
96% |
99% |
93% |
| AUC40–50
|
52% |
88% |
79% |
69% |
79% |
72% |
| AUC40–50 + Symptom Score |
79% |
92% |
88% |
85% |
96% |
86% |
| AUC40–50 + Symptom Score + Age |
89% |
92% |
89% |
92% |
97% |
91% |
| AUC15–20 + AUC30–40 + Symptom Score |
95% |
96% |
95% |
96% |
99% |
95% |
| AUC30–40 + AUC40 - 50 + Symptom Score |
95% |
96% |
95% |
96% |
> 99% |
95% |
| AUC15–20 + AUC30–40 + AUC40–50 + Symptom Score |
95% |
96% |
95% |
96% |
> 99% |
95% |
Table 8.
AI derived permutations of Prediction Modeling for subjects age ≥ 36. The yellow highlighted bold values designate the best predictive model for subjects aged 36 and older, in cohort 2.
Table 8.
AI derived permutations of Prediction Modeling for subjects age ≥ 36. The yellow highlighted bold values designate the best predictive model for subjects aged 36 and older, in cohort 2.
| Age ≥36 years (N=19) |
Sensitivity |
Specificity |
PPV |
NPV |
C-Statistic |
Correctly Classified |
| AUC15–20
|
61% |
95% |
88% |
80% |
83% |
82% |
| AUC15–20 + Symptom Score |
70% |
92% |
84% |
83% |
90% |
83% |
| AUC15–20 + Symptom Score + Age |
78% |
97% |
95% |
88% |
94% |
90% |
| AUC30–40 |
70% |
95% |
89% |
83% |
89% |
85% |
| AUC30–40 + Symptom Score |
83% |
92% |
86% |
89% |
97% |
88% |
| AUC30–40 + Symptom Score + Age |
91% |
95% |
91% |
95% |
99% |
93% |
| AUC40–50
|
48% |
89% |
73% |
73% |
81% |
73% |
| AUC40–50 + Symptom Score |
78% |
89% |
82% |
87% |
92% |
85% |
| AUC40–50 + Symptom Score + Age |
91% |
92% |
88% |
94% |
95% |
92% |
| AUC15–20 + AUC30–40 + Symptom Score |
91% |
95% |
91% |
95% |
99% |
93% |
| AUC30–40 + AUC40–50 + Symptom Score |
91% |
95% |
91% |
95% |
98% |
93% |
| AUC15–20 + AUC30–40 + AUC40 - 50 + Symptom Score |
91% |
95% |
91% |
95% |
98% |
93% |
Table 9.
GIMA Biomarker Performance in Predictive Model on Validation Cohort segregated by age subgroups age ≤35 and ≥ 36 (cohort 3).
Table 9.
GIMA Biomarker Performance in Predictive Model on Validation Cohort segregated by age subgroups age ≤35 and ≥ 36 (cohort 3).
Age Derived Subsets
(N=47) |
Sensitivity |
Specificity |
PPV |
NPV |
C-Statistic |
Correctly Classified |
Age ≤35 years
(N=25) |
86% |
96% |
86% |
96% |
91% |
91% |
Age ≥36 years
(N=22) |
84% |
95% |
84% |
95% |
90% |
91% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).