Submitted:
05 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
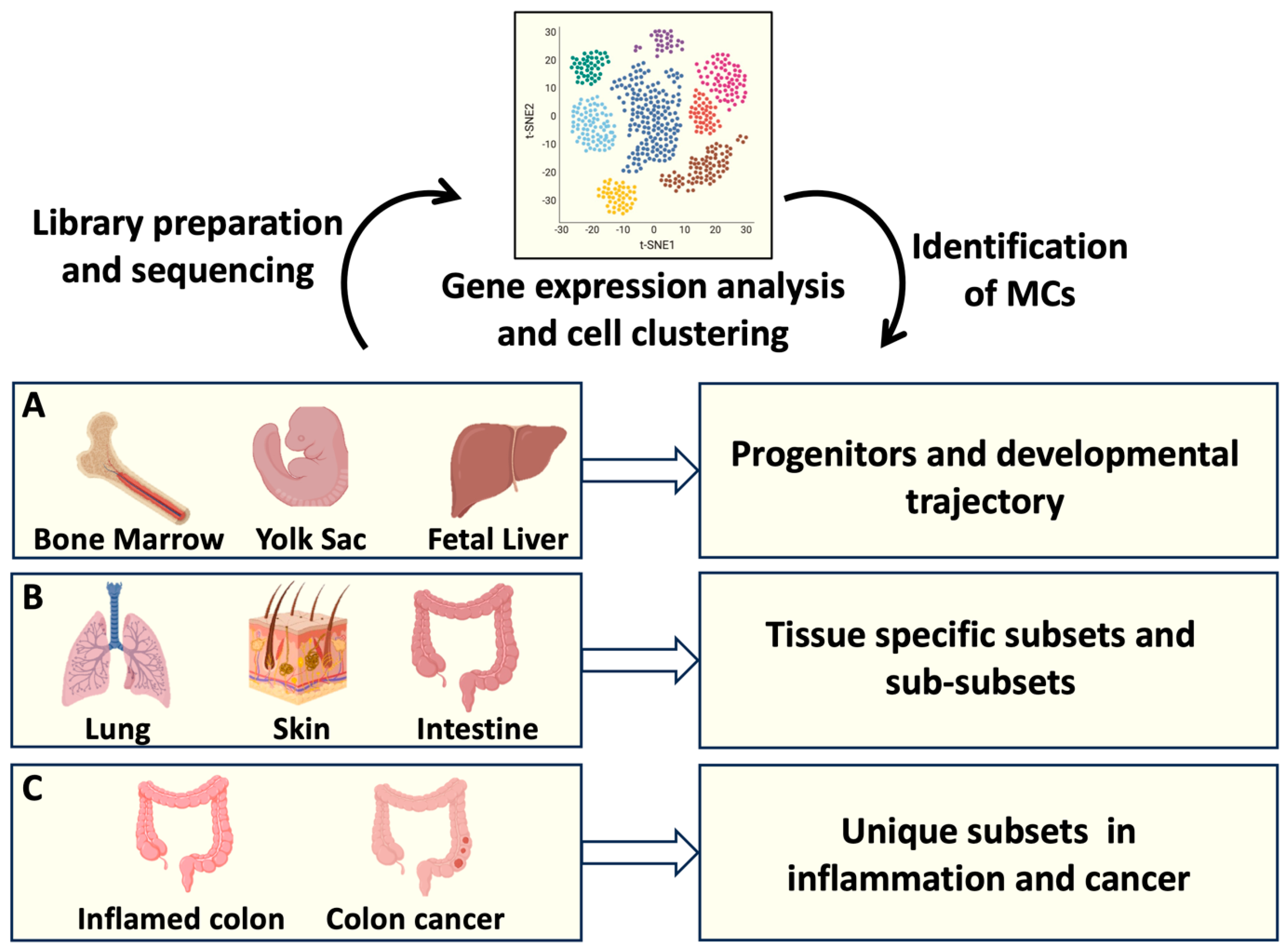
2. Transcriptomic Analysis and MC Development
3. Insights into Intestinal MC Origin and Phenotype through scRNA-Seq
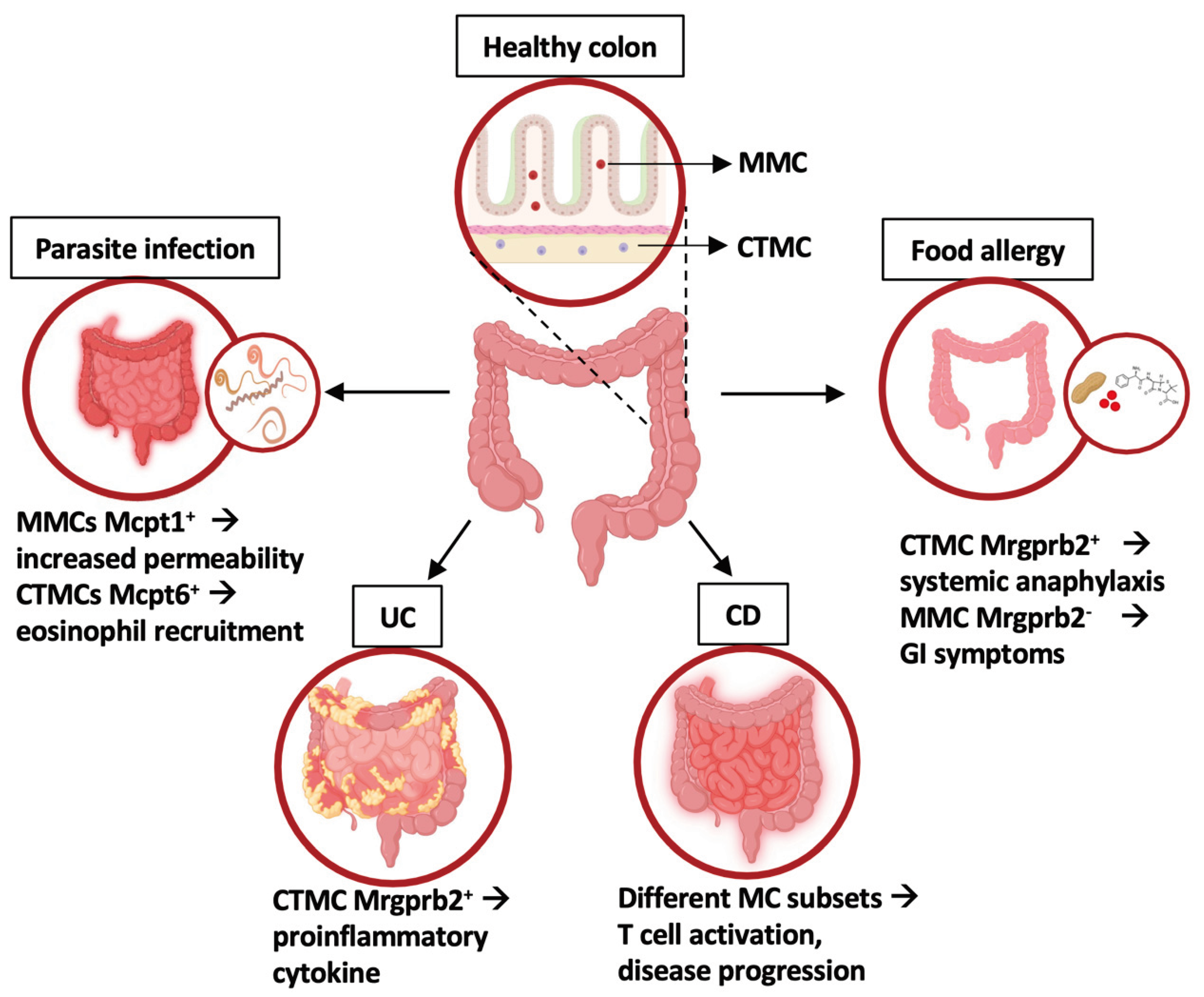
4. Deciphering Intestinal MC Function in Homeostasis and Inflammatory Conditions
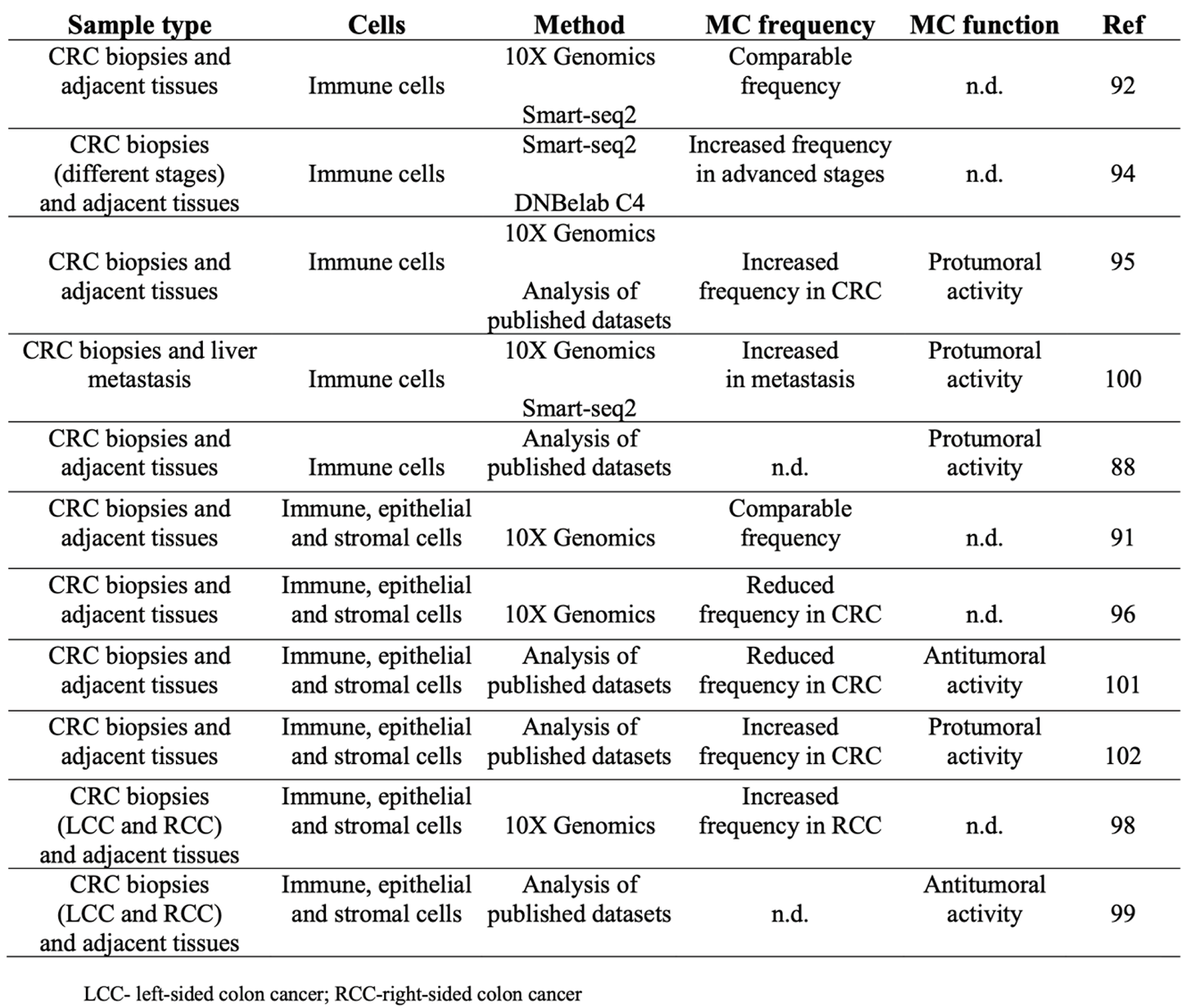
5. Exploring Intestinal Mast Cell Role in Tumor Biology: Colon Cancer under RNA Seq Microscope
 |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11408-13. [CrossRef]
- Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006 Jun;117(6):1285-91. [CrossRef]
- Tsai M, Valent P, Galli SJ. KIT as a master regulator of the mast cell lineage. J Allergy Clin Immunol. 2022 Jun;149(6):1845-1854. [CrossRef]
- Chan CY, St John AL, Abraham SN. Plasticity in mast cell responses during bacterial infections. Curr Opin Microbiol. 2012 Feb;15(1):78-84. [CrossRef]
- Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013 May;13(5):362-75. [CrossRef]
- Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015 May;8(3):444-63. [CrossRef]
- Jiménez M, Cervantes-García D, Córdova-Dávalos LE, Pérez-Rodríguez MJ, Gonzalez-Espinosa C, Salinas E. Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles. Front Immunol. 2021 Jun 15;12:685865. [CrossRef]
- Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010 Jun 17;115(24):4981-90. [CrossRef]
- Elieh Ali Komi D, Wöhrl S, Bielory L. Mast Cell Biology at Molecular Level: a Comprehensive Review. Clin Rev Allergy Immunol. 2020 Jun;58(3):342-365. [CrossRef]
- Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018 Mar;282(1):121-150. [CrossRef]
- Rönnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004 Oct;61(19-20):2535-48. [CrossRef]
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003 Dec;15(6):639-46. [CrossRef]
- Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007 May;7(5):365-78. [CrossRef]
- Nagata Y, Suzuki R. FcεRI: A Master Regulator of Mast Cell Functions. Cells. 2022 Feb 11;11(4):622. [CrossRef]
- Molfetta R, Lecce M, Quatrini L, Caracciolo G, Digiacomo L, Masuelli L, Milito ND, Vulpis E, Zingoni A, Galandrini R, Santoni A, Paolini R. Immune complexes exposed on mast cell-derived nanovesicles amplify allergic inflammation. Allergy. 2020 May;75(5):1260-1263. [CrossRef]
- Shefler I, Salamon P, Mekori YA. Extracellular Vesicles as Emerging Players in Intercellular Communication: Relevance in Mast Cell-Mediated Pathophysiology. Int J Mol Sci. 2021 Aug 25;22(17):9176. [CrossRef]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology (2008) 123:398–410. [CrossRef]
- West PW, Bahri R, Garcia-Rodriguez KM, Sweetland G, Wileman G, Shah R, et al.. Interleukin-33 amplifies human mast cell activities induced by complement anaphylatoxins. Front Immunol (2021) 11:615236. [CrossRef]
- Agier J, Pastwińska J, Brzezińska-Błaszczyk E. An overview of mast cell pattern recognition receptors. Inflamm Res. 2018 Sep;67(9):737-746. [CrossRef]
- Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016 Sep;138(3):700-710. [CrossRef]
- Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. 2012 Jan;1822(1):85-92. [CrossRef]
- Bischoff SC. Mast cells in gastrointestinal disorders. Eur J Pharmacol. 2016 May 5;778:139-45. [CrossRef]
- Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, Granata F. Are Mast Cells MASTers in Cancer? Front Immunol. 2017 Apr 12;8:424. [CrossRef]
- Rigoni A, Colombo MP, Pucillo C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin Immunol. 2018 Feb;35:29-34. [CrossRef]
- Komi DEA, Redegeld FA. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin Rev Allergy Immunol. 2020 Jun;58(3):313-325. [CrossRef]
- Dwyer DF, Barrett NA, Austen KF; Immunological Genome Project Consortium. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol. 2016 Jul;17(7):878-87. [CrossRef]
- Dahlin JS, Malinovschi A, Öhrvik H, Sandelin M, Janson C, Alving K, Hallgren J. Lin- CD34hi CD117int/hi FcεRI+ cells in human blood constitute a rare population of mast cell progenitors. Blood. 2016 Jan 28;127(4):383-91. [CrossRef]
- Huang H, Li Y, Liu B. Transcriptional regulation of mast cell and basophil lineage commitment. Semin Immunopathol. 2016 Sep;38(5):539-48. [CrossRef]
- Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human mast cells and basophils-How are they similar how are they different? Immunol Rev. 2018 Mar;282(1):8-34. [CrossRef]
- Iuliano C, Absmaier-Kijak M, Sinnberg T, Hoffard N, Hils M, Köberle M, Wölbing F, Shumilina E, Heise N, Fehrenbacher B, Schaller M, Lang F, Kaesler S, Biedermann T. Fetal Tissue-Derived Mast Cells (MC) as Experimental Surrogate for In Vivo Connective Tissue MC. Cells. 2022 Mar 8;11(6):928. [CrossRef]
- Cildir G, Yip KH, Pant H, Tergaonkar V, Lopez AF, Tumes DJ. Understanding mast cell heterogeneity at single cell resolution. Trends Immunol. 2021 Jun;42(6):523-535. [CrossRef]
- Derakhshan T, Boyce JA, Dwyer DF. Defining mast cell differentiation and heterogeneity through single-cell transcriptomics analysis. J Allergy Clin Immunol. 2022 Oct;150(4):739-747. [CrossRef]
- Saito H, Nakajima T, Matsumoto K. Human mast cell transcriptome project. Int Arch Allergy Immunol. 2001 May;125(1):1-8. [CrossRef] [PubMed]
- Wu C, Boey D, Bril O, Grootens J, Vijayabaskar MS, Sorini C, Ekoff M, Wilson NK, Ungerstedt JS, Nilsson G, Dahlin JS. Single-cell transcriptomics reveals the identity and regulators of human mast cell progenitors. Blood Adv. 2022 Aug 9;6(15):4439-4449. [CrossRef]
- Velten L, Haas SF, Raffel S, Blaszkiewicz S, Islam S, Hennig BP, Hirche C, Lutz C, Buss EC, Nowak D, Boch T, Hofmann WK, Ho AD, Huber W, Trumpp A, Essers MA, Steinmetz LM. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol. 2017 Apr;19(4):271-281. [CrossRef]
- Zheng S, Papalexi E, Butler A, Stephenson W, Satija R. Molecular transitions in early progenitors during human cord blood hematopoiesis. Mol Syst Biol. 2018 Mar 15;14(3):e8041. [CrossRef]
- Popescu DM, Botting RA, Stephenson E, Green K, Webb S, Jardine L, Calderbank EF, Polanski K, Goh I, Efremova M, Acres M, Maunder D, Vegh P, Gitton Y, Park JE, Vento-Tormo R, Miao Z, Dixon D, Rowell R, McDonald D, Fletcher J, Poyner E, Reynolds G, Mather M, Moldovan C, Mamanova L, Greig F, Young MD, Meyer KB, Lisgo S, Bacardit J, Fuller A, Millar B, Innes B, Lindsay S, Stubbington MJT, Kowalczyk MS, Li B, Ashenberg O, Tabaka M, Dionne D, Tickle TL, Slyper M, Rozenblatt-Rosen O, Filby A, Carey P, Villani AC, Roy A, Regev A, Chédotal A, Roberts I, Göttgens B, Behjati S, Laurenti E, Teichmann SA, Haniffa M. Decoding human fetal liver haematopoiesis. Nature. 2019 Oct;574(7778):365-371. [CrossRef]
- Hamey FK, Lau WWY, Kucinski I, Wang X, Diamanti E, Wilson NK, Göttgens B, Dahlin JS. Single-cell molecular profiling provides a high-resolution map of basophil and mast cell development. Allergy. 2021 Jun;76(6):1731-1742. [CrossRef]
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T, Forrest AR, Babina M; FANTOM consortium. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood. 2014 Apr 24;123(17):e58-67. [CrossRef] [PubMed] [PubMed Central]
- Tusi BK, Wolock SL, Weinreb C, Hwang Y, Hidalgo D, Zilionis R, Waisman A, Huh JR, Klein AM, Socolovsky M. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature. 2018 Mar 1;555(7694):54-60. [CrossRef]
- Li Z, Liu S, Xu J, Zhang X, Han D, Liu J, Xia M, Yi L, Shen Q, Xu S, Lu L, Cao X. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity. 2018 Oct 16;49(4):640-653.e5. [CrossRef]
- Inclan-Rico JM, Hernandez CM, Henry EK, Federman HG, Sy CB, Ponessa JJ, Lemenze AD, Joseph N, Soteropoulos P, Beaulieu AM, Yap GS, Siracusa MC. Trichinella spiralis-induced mastocytosis and erythropoiesis are simultaneously supported by a bipotent mast cell/erythrocyte precursor cell. PLoS Pathog. 2020 May 18;16(5):e1008579. [CrossRef]
- Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol. 2013 Dec;43(12):3108-15. [CrossRef]
- Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, Koni PA, Gurish MF. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood. 2005 Jun 1;105(11):4308-13. [CrossRef]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005 Sep;167(3):835-48. [CrossRef]
- Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012 Jul 27;37(1):25-33. [CrossRef]
- Xing W, Austen KF, Gurish MF, Jones TG. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):14210-5. [CrossRef]
- Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464-8. [CrossRef]
- da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014 Oct;62(10):698-738. [CrossRef]
- Weidner N, Austen KF. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol Res Pract. 1993 Mar;189(2):156-62. [CrossRef]
- Vogel P, Janke L, Gravano DM, Lu M, Sawant DV, Bush D, Shuyu E, Vignali DAA, Pillai A, Rehg JE. Globule Leukocytes and Other Mast Cells in the Mouse Intestine. Vet Pathol. 2018 Jan;55(1):76-97. [CrossRef]
- Gentek R, Ghigo C, Hoeffel G, Bulle MJ, Msallam R, Gautier G, Launay P, Chen J, Ginhoux F, Bajénoff M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity. 2018 Jun 19;48(6):1160-1171.e5. [CrossRef]
- Tauber M, Basso L, Martin J, Bostan L, Pinto MM, Thierry GR, Houmadi R, Serhan N, Loste A, Blériot C, Kamphuis JBJ, Grujic M, Kjellén L, Pejler G, Paul C, Dong X, Galli SJ, Reber LL, Ginhoux F, Bajenoff M, Gentek R, Gaudenzio N. Landscape of mast cell populations across organs in mice and humans. J Exp Med. 2023 Oct 2;220(10):e20230570. [CrossRef]
- Forsythe P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019 Jan;42(1):43-55. [CrossRef]
- Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, Finkelman FD, Pejler G, Hogan SP. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22381-6. [CrossRef]
- Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000 Dec 18;192(12):1849-56. [CrossRef]
- McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7761-6. [CrossRef]
- Sorobetea D, Holm JB, Henningsson H, Kristiansen K, Svensson-Frej M. Acute infection with the intestinal parasite Trichuris muris has long-term consequences on mucosal mast cell homeostasis and epithelial integrity. Eur J Immunol. 2017 Feb;47(2):257-268. [CrossRef]
- Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008 Apr 1;180(7):4885-91. [CrossRef]
- Nakano N, Kitaura J. Mucosal Mast Cells as Key Effector Cells in Food Allergies. Cells. 2022 Jan 19;11(3):329. [CrossRef]
- Oettgen HC. Mast cells in food allergy: Inducing immediate reactions and shaping long-term immunity. J Allergy Clin Immunol. 2023 Jan;151(1):21-25. [CrossRef] [PubMed]
- Benedé S, Berin MC. Mast cell heterogeneity underlies different manifestations of food allergy in mice. PLoS One. 2018 Jan 25;13(1):e0190453. [CrossRef]
- Zhang L., Song J., Hou X. Mast cells and irritable bowel syndrome: From the bench to the bedside. J. Neurogastroenterol. Motil. 2016;22:181–192. [CrossRef]
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005 Dec 8;353(23):2462-76. Erratum in: N Engl J Med. 2006 May 18;354(20):2200. [CrossRef]
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013 Aug 22;369(8):699-710. [CrossRef]
- Atlasy N, Bujko A, Bækkevold ES, Brazda P, Janssen-Megens E, Lundin KEA, Jahnsen J, Jahnsen FL, Stunnenberg HG. Single cell transcriptomic analysis of the immune cell compartment in the human small intestine and in Celiac disease. Nat Commun. 2022 Aug 22;13(1):4920. [CrossRef]
- Smillie CS, Biton M, Ordovas-Montanes J, Sullivan KM, Burgin G, Graham DB, Herbst RH, Rogel N, Slyper M, Waldman J, Sud M, Andrews E, Velonias G, Haber AL, Jagadeesh K, Vickovic S, Yao J, Stevens C, Dionne D, Nguyen LT, Villani AC, Hofree M, Creasey EA, Huang H, Rozenblatt-Rosen O, Garber JJ, Khalili H, Desch AN, Daly MJ, Ananthakrishnan AN, Shalek AK, Xavier RJ, Regev A. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell. 2019 Jul 25;178(3):714-730.e22. [CrossRef]
- Chen E, Chuang LS, Giri M, Villaverde N, Hsu NY, Sabic K, Joshowitz S, Gettler K, Nayar S, Chai Z, Alter IL, Chasteau CC, Korie UM, Dzedzik S, Thin TH, Jain A, Moscati A, Bongers G, Duerr RH, Silverberg MS, Brant SR, Rioux JD, Peter I, Schumm LP, Haritunians T, McGovern DP, Itan Y, Cho JH. Inflamed Ulcerative Colitis Regions Associated With MRGPRX2-Mediated Mast Cell Degranulation and Cell Activation Modules, Defining a New Therapeutic Target. Gastroenterology. 2021 Apr;160(5):1709-1724. [CrossRef]
- Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42(1):48-52. [CrossRef] [PubMed]
- Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett. 2008;269(1):1-6. [CrossRef]
- Liu J, Zhang Y, Zhao J, Yang Z, Li D, Katirai F, Huang B. Mast cell: Insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011;30(2):177-84. [CrossRef]
- Marichal T, Tsai M, Galli SJ. Mast cells: Potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1(5):269-79. [CrossRef]
- Maltby S, Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, et al.. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30(1):45-60. [CrossRef]
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019 Dec;16(12):713-732. [CrossRef]
- Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am J Gastroenterol. 2017 Oct;112(10):1509-1525. [CrossRef] [PubMed]
- Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020 Jan;158(2):291-302. [CrossRef]
- Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015 Apr 9;372(15):1441-52. [CrossRef]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006 Sep 29;313(5795):1960-4. [CrossRef]
- Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboüe R, Frebourg T, Pagès F, Valge-Archer V, Latouche JB, Galon J. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016 Mar 15;44(3):698-711. [CrossRef]
- Lanzi A, Pagès F, Lagorce-Pagès C, Galon J. The consensus immunoscore: toward a new classification of colorectal cancer. Oncoimmunology. 2020 Jul 11;9(1):1789032. [CrossRef]
- Fionda C, Scarno G, Stabile H, Molfetta R, Di Censo C, Gismondi A, Paolini R, Sozzani S, Santoni A, Sciumè G. NK Cells and Other Cytotoxic Innate Lymphocytes in Colorectal Cancer Progression and Metastasis. Int J Mol Sci. 2022 Jul 16;23(14):7859. [CrossRef]
- Molfetta R, Paolini R. The Controversial Role of Intestinal Mast Cells in Colon Cancer. Cells. 2023 Jan 31;12(3):459. [CrossRef]
- Liu X, Li X, Wei H, Liu Y, Li N. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front Immunol. 2023 Jul 11;14:1209056. [CrossRef]
- Ko EA, Sanders KM, Zhou T. A transcriptomic insight into the impacts of mast cells in lung, breast, and colon cancers. Oncoimmunology. 2017 Aug 8;6(11):e1360457. [CrossRef]
- Wedemeyer J, Galli SJ. Decreased susceptibility of mast cell-deficient Kit(W)/Kit(W-v) mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Lab Invest. 2005 Mar;85(3):388-96. [CrossRef]
- Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007 Dec 11;104(50):19977-82. [CrossRef]
- Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, et al.. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112(4):1269-79. [CrossRef]
- Rigoni A, Bongiovanni L, Burocchi A, Sangaletti S, Danelli L, Guarnotta C, Lewis A, Rizzo A, Silver AR, Tripodo C, Colombo MP. Mast Cells Infiltrating Inflamed or Transformed Gut Alternatively Sustain Mucosal Healing or Tumor Growth. Cancer Res. 2015 Sep 15;75(18):3760-70. [CrossRef]
- Sakita JY, Elias-Oliveira J, Carlos D, de Souza Santos E, Almeida LY, Malta TM, Brunaldi MO, Albuquerque S, Araújo Silva CL, Andrade MV, Bonato VLD, Garcia SB, Cunha FQ, Cebinelli GCM, Martins RB, Matthews J, Colli L, Martin FL, Uyemura SA, Kannen V. Mast cell-T cell axis alters development of colitis-dependent and colitis-independent colorectal tumours: potential for therapeutically targeting via mast cell inhibition. J Immunother Cancer. 2022 Oct;10(10):e004653. [CrossRef]
- Molfetta R, Lecce M, Milito ND, Putro E, Pietropaolo G, Marangio C, Scarno G, Moretti M, De Smaele E, Santini T, Bernardini G, Sciumè G, Santoni A, Paolini R. SCF and IL-33 regulate mouse mast cell phenotypic and functional plasticity supporting a pro-inflammatory microenvironment. Cell Death Dis. 2023 Sep 20;14(9):616. [CrossRef]
- Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, Wong M, Choi PJ, Wee LJK, Hillmer AM, Tan IB, Robson P, Prabhakar S. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017 May;49(5):708-718. [CrossRef]
- Lee HO, Hong Y, Etlioglu HE, Cho YB, Pomella V, Van den Bosch B, Vanhecke J, Verbandt S, Hong H, Min JW, Kim N, Eum HH, Qian J, Boeckx B, Lambrechts D, Tsantoulis P, De Hertogh G, Chung W, Lee T, An M, Shin HT, Joung JG, Jung MH, Ko G, Wirapati P, Kim SH, Kim HC, Yun SH, Tan IBH, Ranjan B, Lee WY, Kim TY, Choi JK, Kim YJ, Prabhakar S, Tejpar S, Park WY. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat Genet. 2020 Jun;52(6):594-603. [CrossRef]
- Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O'Brien SA, He Y, Wang L, Zhang Q, Kim A, Gao R, Orf J, Wang T, Sawant D, Kang J, Bhatt D, Lu D, Li CM, Rapaport AS, Perez K, Ye Y, Wang S, Hu X, Ren X, Ouyang W, Shen Z, Egen JG, Zhang Z, Yu X. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell. 2020 Apr 16;181(2):442-459.e29. [CrossRef]
- Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, Bejnood A, Dionne D, Ge WH, Xu KH, Chao SX, Zollinger DR, Lieb DJ, Reeves JW, Fuhrman CA, Hoang ML, Delorey T, Nguyen LT, Waldman J, Klapholz M, Wakiro I, Cohen O, Albers J, Smillie CS, Cuoco MS, Wu J, Su MJ, Yeung J, Vijaykumar B, Magnuson AM, Asinovski N, Moll T, Goder-Reiser MN, Applebaum AS, Brais LK, DelloStritto LK, Denning SL, Phillips ST, Hill EK, Meehan JK, Frederick DT, Sharova T, Kanodia A, Todres EZ, Jané-Valbuena J, Biton M, Izar B, Lambden CD, Clancy TE, Bleday R, Melnitchouk N, Irani J, Kunitake H, Berger DL, Srivastava A, Hornick JL, Ogino S, Rotem A, Vigneau S, Johnson BE, Corcoran RB, Sharpe AH, Kuchroo VK, Ng K, Giannakis M, Nieman LT, Boland GM, Aguirre AJ, Anderson AC, Rozenblatt-Rosen O, Regev A, Hacohen N. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021 Sep 2;184(18):4734-4752.e20. [CrossRef]
- Wang W, Zhong Y, Zhuang Z, Xie J, Lu Y, Huang C, Sun Y, Wu L, Yin J, Yu H, Jiang Z, Wang S, Wang C, Zhang Y, Huang Y, Han C, Zhong Z, Hu J, Ouyang Y, Liu H, Yu M, Wei X, Chen D, Huang L, Hou Y, Lin Z, Liu S, Ling F, Yao X. Multiregion single-cell sequencing reveals the transcriptional landscape of the immune microenvironment of colorectal cancer. Clin Transl Med. 2021 Jan;11(1):e253. [CrossRef]
- Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, Qin S, Zhang L, Ouyang H, Du P, Jiang L, Zhang B, Yang Y, Wang X, Ren X, Bei JX, Hu X, Bu Z, Ji J, Zhang Z. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021 Feb 4;184(3):792-809.e23. [CrossRef]
- Becker WR, Nevins SA, Chen DC, Chiu R, Horning AM, Guha TK, Laquindanum R, Mills M, Chaib H, Ladabaum U, Longacre T, Shen J, Esplin ED, Kundaje A, Ford JM, Curtis C, Snyder MP, Greenleaf WJ. Single-cell analyses define a continuum of cell state and composition changes in the malignant transformation of polyps to colorectal cancer. Nat Genet. 2022 Jul;54(7):985-995. [CrossRef]
- Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, Ding X, Bao R, Hong L, Jia W, Fang F, Liu H, Chen L, Zhong J, Zou D, Liu L, Han L, Ginhoux F, Liu Y, Ye Y, Su B. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat Commun. 2022 Apr 1;13(1):1742. [CrossRef]
- Guo W, Zhang C, Wang X, Dou D, Chen D, Li J. Resolving the difference between left-sided and right-sided colorectal cancer by single-cell sequencing. JCI Insight. 2022 Jan 11;7(1):e152616. [CrossRef]
- Guo JN, Chen D, Deng SH, Huang JR, Song JX, Li XY, Cui BB, Liu YL. Identification and quantification of immune infiltration landscape on therapy and prognosis in left- and right-sided colon cancer. Cancer Immunol Immunother. 2022 Jun;71(6):1313-1330. [CrossRef]
- Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu K, Hu X, Bu Z, Peng J, Ren X, Zhang Z. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022 Apr 11;40(4):424-437.e5. [CrossRef]
- Xie Z, Niu L, Zheng G, Du K, Dai S, Li R, Dan H, Duan L, Wu H, Ren G, Dou X, Feng F, Zhang J, Zheng J. Single-cell analysis unveils activation of mast cells in colorectal cancer microenvironment. Cell Biosci. 2023 Nov 29;13(1):217. [CrossRef]
- Wang Q, Zhang YF, Li CL, Wang Y, Wu L, Wang XR, Huang T, Liu GL, Chen X, Yu Q, He PF. Integrating scRNA-seq and bulk RNA-seq to characterize infiltrating cells in the colorectal cancer tumor microenvironment and construct molecular risk models. Aging (Albany NY). 2023 Dec 5;15(23):13799-13821. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





