Submitted:
08 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacterial isolation and culture condition
2.3. Experimental Fish and Feeding Trial
2.4. Experimental DIETS
2.5. Sample Collection and Analysis
2.6. Antioxidant Capacity and Non-Specific Immune Response Analyses
2.7. Real-Time PCR
2.8. Challenge Test
2.9. Histology
2.10. Statistical Analysis
3. Results
3.1. Growth Performances
3.2. Whole-Body Proximate Composition
3.3. Hematological Parameters
3.4. Non-Specific Immune Responses
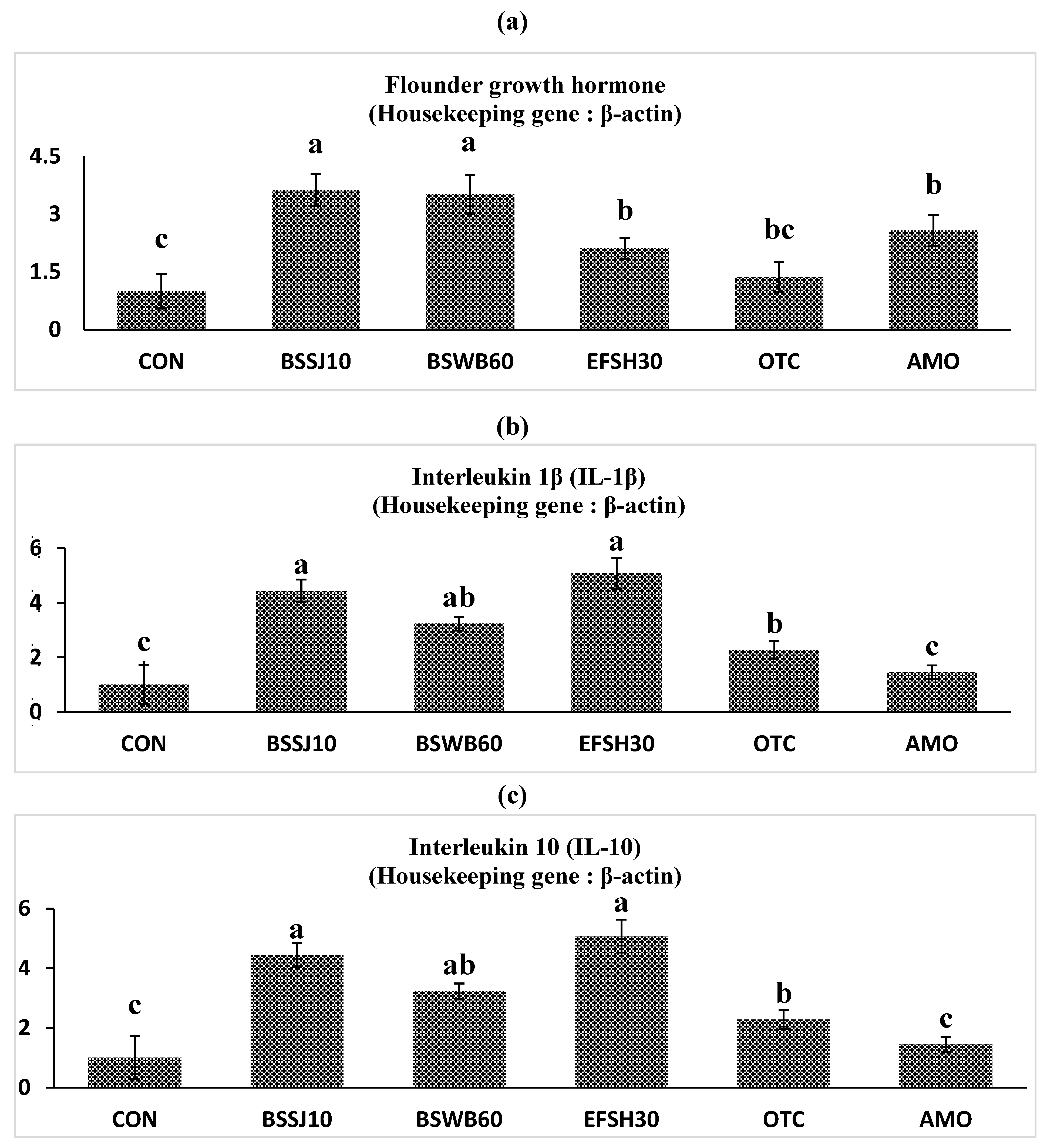
3.5. Growth and Immune Related Gene Expressions
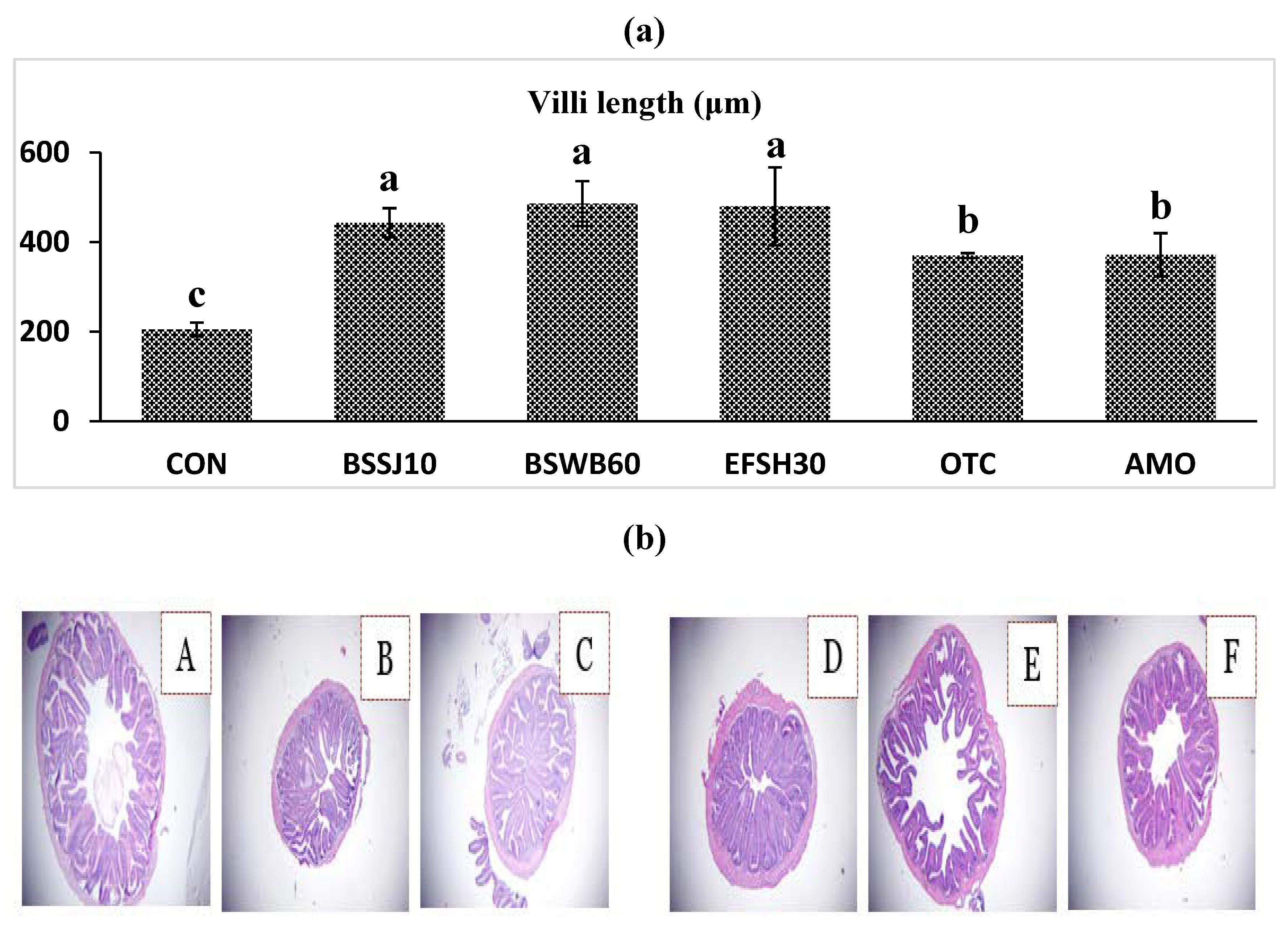
3.6. Histology
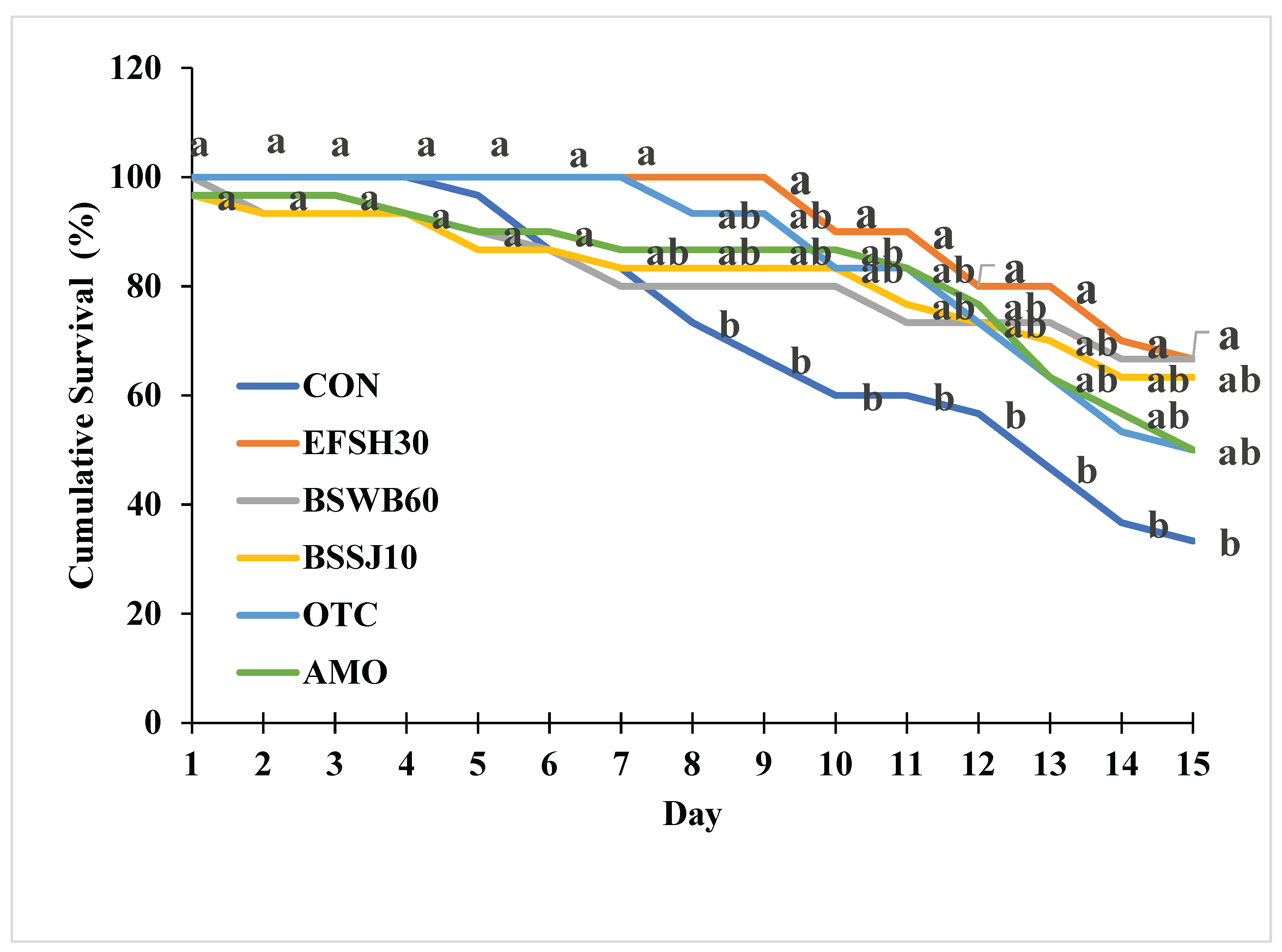
3.7. Challenge Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuji, K; Kobayashi, K. ; Hasegawa, O.; Coimbra, M.R.M.; Sakamoto, T.; Okamoto, N. Identification of a single major genetic locus controlling the resistance to lymphocystis disease in Japanese flounder (Paralichthys olivaceus). Aquaculture, 2006, 254, 203–210. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Won, S.; Lee, S.; Lee, S.; Farris, N.W.; Bai, S.C. Nutrition and feeding of olive flounder Paralichthys olivaceus: A Review. Rev. Fish. Sci. Aquac. 2020, 28, 340–357. [Google Scholar] [CrossRef]
- Statistics Korea. Results of the year 2022 fish farming trend survey. Text by Department of Agriculture and Fisheries Trends Department, In: Fish farming trend survey. Daejeon., 2023.https://kostat.go.kr/board.es?mid=a10301080400&bid=225&act=view&list_no=424510 Accessed 20 February 2024.
- Boyd, C.E. Overview of aquaculture feeds: Global impacts of ingredient use, in: Feed Feed. Pract. Aquac., Elsevier, 2015, pp. 3–25.
- Bharadwaj, A.S.; Brignon, W.R.; Gould, N.L.; Brown, P.B.; Wu, Y.V. Evaluation of meat and bone A.S. meal in practical diets fed to juvenile hybrid striped bass Morone chrysops × M. saxatilis. J. World. Aquac. Soc. 2002, 33, 448–457. [Google Scholar] [CrossRef]
- Markey, J.C.; Amaya, E.A.; Davis, D.A. Replacement of poultry by-product meal in production diets for the Pacific white shrimp, Litopenaeus vannamei. J. World. Aquac. Soc. 2010, 41, 893–902. [Google Scholar] [CrossRef]
- Kang, Y.J.; Lee, S.M.; Yang, S.G.; Bai, S.C. Effects of meat meal, blood meal or soybean meal as a dietary protein source replacing fish meal in parrot fish, Oplegnathus fasciatus. J. Aquacult. 1999, 12, 205–212. [Google Scholar]
- McGoogan, B.B.; Gatlin, III D. M. Effects of replacing fish meal with soybean meal in diets for red drum Sciaenops ocellatus and potential for palatability enhancement. J World Aquac Soc. 1997, 28, 374–385. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Feed ingredients for warmwater fish, fish meal and other processed feedstuffs., FAO Fish. Circ. (FAO). No. 856.
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Done, H.Y.; Venkatesan, A.K.; Halden, R.U. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J 2015, 17, 513–524. [Google Scholar] [CrossRef]
- Balcázar, J.L.; De Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Múzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006, 114, 173–186. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; De Blas, I.; Ruiz-Zarzuela, I.; Muzquiz, J.L. Probiotics: a tool for the future of fish and shellfish health management. J. Aquac. Trop. 2004, 19, 239–242. [Google Scholar]
- Garriques, D. An evaluation of the production and use of a live bacterial isolate to manipulate the microbial flora in the commercial production of Penaeus vennamei postlarvae in Ecuador., Swim. through Troubl. Water. Proc. Spec. Sess. Shrimp Farming, Aquac. World Aquac Soc 1995, pp 53–59.
- Direkbusarakom, S.; Yoshimizu, M.; Ezura, Y.; Ruangpan, L.; Danayadol, Y. Vibrio spp., the dominant flora in shrimp hatchery against some fish pathogenic viruses. J. Mar. Biotechnol. 1998, 6, 266–267. [Google Scholar] [PubMed]
- Lee, S.; Katya, K.; Hamidoghli, A.; Hong, J.; Kim, D.J.; Bai, S.C. Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 2018, 83, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Jang, W.J.; Kim, H.; Lee, B.J.; Kim, K.W.; Hur, S.W.; Lim, S.G.; Bai, S.C.; Kong, I.S. Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 82, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, M.; Gao, F.; Lu, M.; Chen, G. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim. Nutri. 2020, 6, 69–79. [Google Scholar] [CrossRef] [PubMed]
- AOAC, Official Methods of Analysis, Aquac. Res. sixteenth (1995).
- Quade, M.J. , Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Cha, J.H.; Yang, S.Y.; Woo, S.H.; Song, J.W.; Oh, D.H.; Lee, K.J. Effects of dietary supplementation with Bacillus sp. on growth performance, feed utilization, innate immunity and disease resistance against Streptococcus iniae in olive flounder Paralichthys olivaceus. Korean J. Fish. Aquat. Sci. 2012, 45, 35–42. [Google Scholar]
- Park, Y.; Kim, H.; Won, S.; Hamidoghli, A.; Hasan, M.T.; Kong, I.S.; Bai, S.C. Effects of two dietary probiotics (Bacillus subtilis or licheniformis) with two prebiotics (mannan or fructo oligosaccharide) in Japanese eel, Anguilla japonica. Aquac. Nutr. 2020, 26, 316–327. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Roosta, H.; Masoumi, H.; Farhadi, A.; Jeffs, A. Long-term effects of three probiotics, singular or combined, on serum innate immune parameters and expressions of cytokine genes in rainbow trout during grow-out. Fish Shellfish Immunol. 2020, 98, 748–757. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, E.Y.; Lee, J.M.; Kim, J.K.; Kong, I.S. Characterisation of a novel Bacillus sp. SJ-10 β-1, 3--1, 4-glucanase isolated from jeotgal, a traditional Korean fermented fish. Bioprocess Biosyst. Eng. 2013, 36, 721–727. [Google Scholar] [CrossRef] [PubMed]
- da Costa, Sousa, N. et al. Effects of an Enterococcus faecium-based probiotic on growth performance and health of Pirarucu, Arapaima gigas. Aquac. Res. 2019, 50, 3720–3728. [Google Scholar] [CrossRef]
- Abumourad, I.M.K.; et al. Enterococcus faecium probiotic as a growth promoter and its impact on the expression of the host innate immune in cultured Oreochromis niloticus. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1747–1761. [Google Scholar]
- Lara-Flores, M.M.; Olvera-Novoa, A. The use of lactic acid bacteria isolated from intestinal tract of Nile tilapia (Oreochromis niloticus), as growth promoters in fish fed low protein diets. Lat. Am. J. Aquat. Res. 2013, 41, 490–497. [Google Scholar] [CrossRef]
- Wang, Y.B.; Tian, Z.Q.; Yao, J.T.; Li, W. Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 2008, 277, 203–207. [Google Scholar] [CrossRef]
- Aly, S.M.; Mohamed, M.F.; John, G. Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquac. Res. 2008, 39, 647–656. [Google Scholar] [CrossRef]
- Helal, R.; Melzig, M.F. In vitro effects of selected saponins on the production and release of lysozyme activity of human monocytic and epithelial cell lines. Sci. Pharm. 2011, 79, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, F.C.; Landon, J. Growth hormone secretion in response to stress in man. Nature 1966, 210, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Bornfeldt, K.E.; Arnqvist, H.J.; Dahlkvist, H.H.; Skottner, A.; Wikberg, J.E.S. Receptors for insulin-like growth factor-I in plasma membranes isolated from bovine mesenteric arteries. Eur. J. Endocrinol. 1988, 117, 428–434. [Google Scholar] [CrossRef]
- Clemmons, D.R.; Busby, W.; Clarks, J.B.; Parker, A.; Duan, C.; Nam, T.J. Modifications of insulin-like growth factor binding proteins and their role in controlling IGF actions. Endocr. J. 1998, 45, S1–S8. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Tang, J.; Cai, J.; Yu, H.; Wang, Z.; Abarike, E.D.; Lu, Y.; Li, Y.; Afriyie, G. In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture 2020, 527, 735440. [Google Scholar] [CrossRef]
- Back, S.J.; et al. The effects of dietary heat-killed probiotics bacteria additives in low-fishmeal feed on growth performance, immune responses, and intestinal morphology in juvenile olive flounder Paralichthys olivaceus. Aquac. Rep. 2020, 18, 100415. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Maisey, K.; Reyes-López, F.; Toro-Ascuy, D.; Sandino, A.M.; Imarai, M. Fish cytokines: current research and application. Fish. Sci. 2021, 87, 1–9. [Google Scholar]
- Dinarello, C.A. Interleukin-1 and its biologically related cytokines. Adv. Immunol. 1989, 44, 153–205. [Google Scholar] [PubMed]
- Pleguezuelos, O.; Zou, J.; Cunningham, C.; Secombes, C.J. Cloning, sequencing, and analysis of expression of a second IL-1 β gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics 2000, 51, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Shin, D.H.; Nakao, M.; Yano, T. Molecular cloning and expression analysis of carp (Cyprinus carpio) interleukin-1β, high affinity immunoglobulin E Fc receptor γ subunit and serum amyloid A. Fish Shell. Immunol. 2000, 10, 229–242. [Google Scholar] [CrossRef]
- Scapigliati, G.; Buonocore, F.; Bird, S.; Zou, J.; Pelegrin, P.; Falasca, C.; Prugnoli, D.; Secombes, C.J. Phylogeny of cytokines: molecular cloning and expression analysis of sea bass Dicentrarchus labrax interleukin-1β. Fish Shell. Immunol. 2001, 11, 711–726. [Google Scholar] [CrossRef]
- Pelegrín, P.; García-Castillo, J.; Mulero, V.; Meseguer, J. Interleukin-1β isolated from a marine fish reveals up-regulated expression in macrophages following activation with lipopolysaccharide and lymphokines. Cytokine 2001, 16, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Corripio-Miyar, Y.; Bird, S.; Tsamopoulos, K.; Secombes, C.J. Cloning and expression analysis of two pro-inflammatory cytokines, IL-1β and IL-8, in haddock (Melanogrammus aeglefinus). Mol. Immunol. 2007, 44, 1361–1373. [Google Scholar] [CrossRef]
- Lee, D.S.; Hong, S.H.; Lee, H.J.; Jun, L.J.; Chung, J.K.; Kim, K.H.; Jeong, H.D. Molecular cDNA cloning and analysis of the organization and expression of the IL-1β gene in the Nile tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 307–314. [Google Scholar] [CrossRef]
- Huo, H.J.; Chen, S.N.; Li, L.; Nie, P. Functional characterization of IL-10 and its receptor subunits in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2019, 97, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Yang, H.J.; Liu, Y.; Chen, S.J.; Guo, D.Q.; Yu, Y.; Tian, L.X. Effects of graded levels of threonine on growth performance, biochemical parameters and intestine morphology of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2014, 424, 113–119. [Google Scholar] [CrossRef]
- Klurfeld, D.M. Nutritional regulation of gastrointestinal growth. Front. Biosci. 1999, 4, D299–302. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | % |
|---|---|
| Anchovy fish meal1 | 45 |
| Soybean meal | 12 |
| Starch2 | 3.8 |
| Wheat flour | 7.0 |
| Blood meal | 4.5 |
| Squid liver powder | 5.6 |
| Meat and bone meal | 8.0 |
| Poultry by product meal | 4.5 |
| Fish oil3 | 4.3 |
| Vitamin premix4 | 1.2 |
| Mineral premix5 | 1.2 |
| etc.6 | 3.0 |
| Proximate analysis (% of DM basis) | |
| Moisture | 8.56 |
| Crude protein | 56.2 |
| Crude lipid | 8.35 |
| Crude ash | 11.4 |
| Parameters | Diets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | BSWB60 | BSSJ10 | EFSH30 | OTC | AMO | ||||||
| Moisture | 8.56 | 9.01 | 8.91 | 8.56 | 8.91 | 8.71 | |||||
| Crude Protein | 56.2 | 55.9 | 54.8 | 57.1 | 55.9 | 56.1 | |||||
| Crude Lipid | 8.35 | 8.29 | 8.34 | 8.23 | 811 | 8.32 | |||||
| Crude Ash | 11.4 | 10.9 | 11.2 | 10.9 | 11.2 | 11.8 | |||||
| Name of gene | Sense | Oligonucleotide Sequence (5’ to 3’) | Base pair (bp) |
Gene bank accession number |
|---|---|---|---|---|
| β-actin | F | CAGCATCATGAAGTGTGACGTG | 107 | HQ386788.1 |
| R | CTTCTGCATACGGTCAGCAATG | |||
| FGH | F | CGCCGTATGGAAACTCTGAACT | 160 | M23439.1 |
| R | GGGTGCAGTTAGCTTCTGGAAA | |||
| IL-1β | F | ATGGAATCCAAGATGGAATGC | 250 | KF025662.1 |
| R | GAGACGAGCTTCTCTCACAC | |||
| IL-10 | F | AGCGAACGATGACCTAGACACG | 114 | KF025662.1 |
| R | ACCGTGCTCAGGTAGAAGTCCA |
| Parameters | Diets | Pooled SEM |
|||||
|---|---|---|---|---|---|---|---|
| CON | BSWB60 | BSSJ10 | EFSH30 | OTC | AMO | ||
| IBW | 12.5ns | 12.4 | 12.2 | 12.4 | 12.4 | 12.4 | 0.04 |
| FBW | 36.7 b | 42.0a | 40.8ab | 40.7ab | 36.9b | 37.0b | 0.98 |
| WG (%) | 194b | 230a | 224ab | 229ab | 202b | 199b | 8.38 |
| SGR (%/day) | 1.95b | 2.24a | 2.16ab | 2.16ab | 2.01b | 1.99b | 0.05 |
| FE (%) | 106b | 118ab | 125a | 115ab | 111b | 110b | 2.79 |
| PER | 0.65b | 0.84a | 0.76ab | 0.76ab | 0.70ab | 0.66b | 0.03 |
| Survival (%) | 93.3ns | 90.7 | 89.3 | 89.3 | 96.0 | 98.7 | 5.57 |
| HSI (%) | 1.24ns | 1.66 | 1.29 | 1.31 | 1.13 | 1.12 | 0.08 |
| VSI (%) 1 | 2.05ns | 1.94 | 1.74 | 1.94 | 1.88 | 1.74 | 0.05 |
| CF | 0.92ns | 0.97 | 0.93 | 0.93 | 0.92 | 0.91 | 0.01 |
| Parameters | Diets | Pooled SEM |
|||||
|---|---|---|---|---|---|---|---|
| CON | BSWB60 | BSSJ10 | EFSH30 | OTC | AMO | ||
| Moisture | 76.3ns | 75.5 | 75.1 | 75.8 | 76.8 | 76.2 | 0.25 |
| Protein | 20.3ns | 21.0 | 20.8 | 21.5 | 22.3 | 20.7 | 0.29 |
| Lipid | 2.42ns | 2.39 | 2.30 | 2.34 | 2.41 | 2.41 | 0.02 |
| Ash | 4.09ns | 4.25 | 4.21 | 4.16 | 4.14 | 4.19 | 0.02 |
| Parameters | Diets | Pooled SEM |
|||||
|---|---|---|---|---|---|---|---|
| CON | BSWB60 | BSSJ10 | EFSH30 | OTC | AMO | ||
| ALT | 4.67ns | 4.00 | 4.67 | 4.33 | 4.00 | 4.00 | 0.13 |
| AST | 16.3bc | 26.7a | 15.3c | 22.0ab | 20.0bc | 21.7ab | 1.69 |
| GLU | 3.1ns | 3.4 | 3.3 | 3.2 | 3.3 | 3.5 | 0.06 |
| TP | 13.3ns | 11.8 | 16.0 | 15.1 | 14.8 | 13.2 | 1.04 |
| Parameters | Diets | Pooled SEM |
|||||
|---|---|---|---|---|---|---|---|
| CON | BSWB60 | BSSJ10 | EFSH30 | OTC | AMO | ||
| Lysozyme2 | 0.22c | 0.69a | 0.65a | 0.67a | 0.30bc | 0.39b | 0.08 |
| MPO3 | 1.36b | 1.81a | 1.55ab | 1.56ab | 1.49ab | 1.45b | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





