1. Introduction
The study of the human microbiome, which began in the last century, has sparked growing interest given its complexity and its importance in human health.
The main milestone has been the Human Microbiome Project, of the National Institute of Health in the United States (Human Microbiome Project, NIH), with the aim of identifying and characterizing the microorganisms that settle in the different structures of the human body and that can play a role and therefore influence both health and disease. From that moment on, numerous scientific publications and studies link the imbalance of the microbiome with different diseases.
A recent review related to the role of the human microbiome in health and disease in the United Kingdom concluded that the human microbiome plays a fundamental role in health and disease with multiple facets [1]. Various studies have shown that the intricate and complex communities of microorganisms that live inside and on the surface of our body have important and marked effects on several aspects of human physiology, from metabolism or digestive processes to immune function. Furthermore, researchers demonstrated that a balanced and diverse microbiome can contribute to global well-being by protecting against pathogens, assisting in nutrient absorption, and modulating immune responses. In contrast, dysbiosis, that is, alterations or imbalances in the microbiome, has been linked to a wide variety of health conditions, including inflammatory bowel disease, obesity, allergies, and neurological disorders, among others [2]. One of the objectives of current research is to deepen our knowledge of the intricate relationships between the microbiome and human health, in order to develop ways to use the microbiome for therapeutic purposes [1].
The term “human microbiota” has been described as the group of symbiotic microorganisms that co-occur with the human organism in balance and without causing damage. The term “microbiome” refers to the entire microbiota habitat, including microorganisms, their genomes, and the surrounding environment. Likewise, the aim of the use of prebiotics and probiotics in nutritional therapy is to alleviate these imbalances in the microbiota and, in parallel, an important industry linked to these nutritional supplements, also called “nutraceuticals”, has emerged.
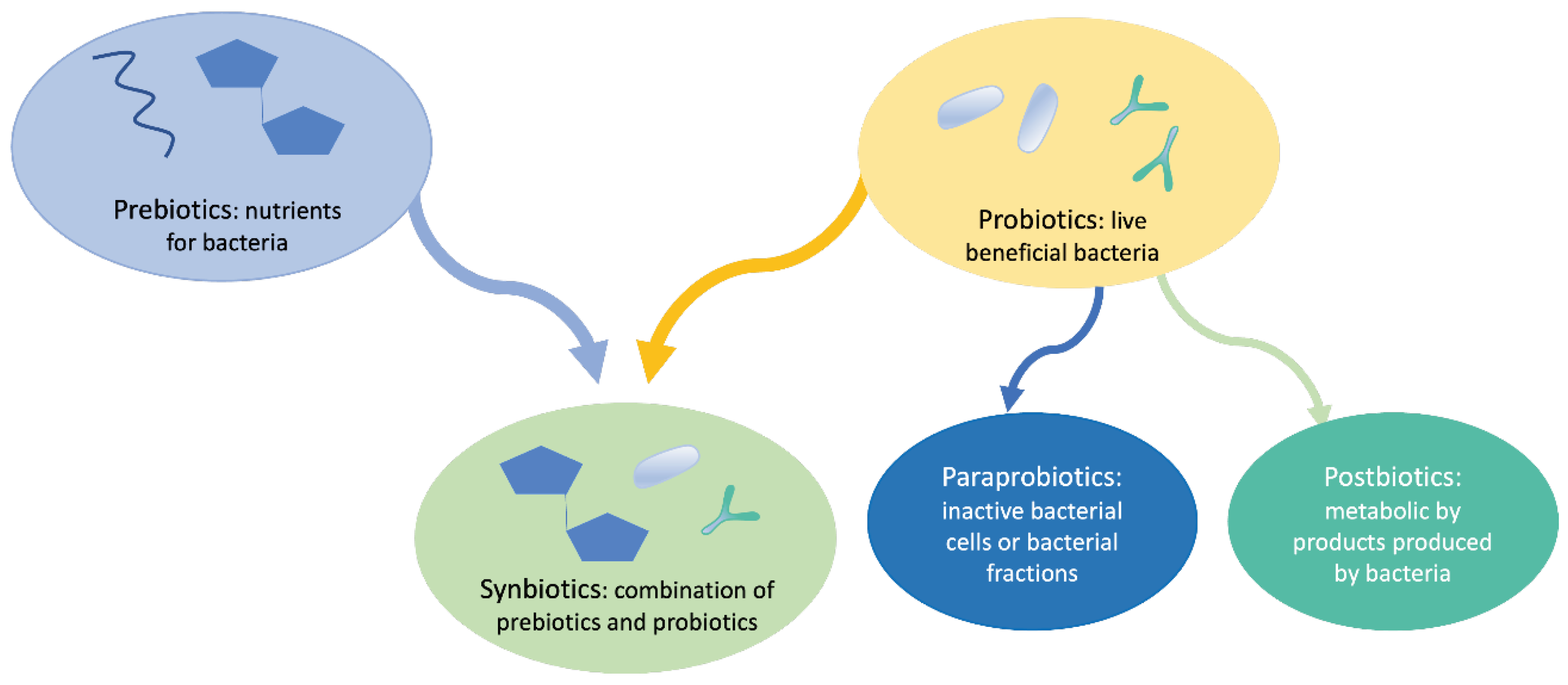
The role of probiotics in regulating intestinal health has been widely studied in the last decades [3]. Besides that, the concept of prebiotics has been developed, and, later on, the concept of synbiotics, postbiotics and paraprobiotics [4–9], in the form of nutraceuticals in the form of oral supplement and for topical application with the aim of repairing or balancing the microbiota. All these concepts are summarized in
Figure 1.
Since the Russian scientist Elie Metchnikoff (1845-1916) coined the concept of probiotic in 1907 [11], numerous studies followed and, finally, a consensus definition was proposed by the International Scientific Association of Probiotics and Prebiotics (ISAPP) in 2014, 2017, and 2021, which includes prebiotics, probiotics and postbiotics definitions. Probiotics have been defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [4]. Prebiotics have been defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [5]. Postbiotics were defined as follows: “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [8]. Later on, the concept of synbiotics emerged, being defined as the combination of both prebiotics and probiotics [8].
The current definition of probiotics does not include inactivated or dead cells, therefore more recently emerges the concept of postbiotic, referring to the use of dead or inactivated cells (non-viable microorganisms), cell extracts, or metabolites of these microorganisms that can provide favorable effects on human health, observing that the action of probiotics depends fundamentally on their metabolites, rather than on living organisms [12,13]. Later on, emerged the concept of para-probiotics consisting of inactivated, dead, or non-viable microbial cells of intact or broken probiotics containing cellular components of probiotic cells after lysis [14]; so that, in the last ten years, several studies have been conducted using inactivated or heat-killed probiotics [15], but the terms ‘postbiotics’, ‘para-probiotics’, and ‘inactivated probiotics’ have been used indistinctively in multiple research studies [16–20]. Postbiotics include the metabolites generated by the microbiota, such as exopolysaccharides, Short Chain Faty Acids (SCFAs), cell wall fragments, enzymes/proteins, and other metabolites [21]; but also as structural, such as teichoic acids, peptides, and plasmalogens, or based on their basic composition (proteins, carbohydrates, lipids, vitamins, etc.) [22]. In this context, several postbiotics have been shown to improve gut health by reinforcing the gut barrier, reducing inflammation, and promoting antimicrobial activity against gut pathogens [23].
According to the most recurrent definition, para-probiotics, also known as non-viable probiotics, inactivated probiotics, tyndallized probiotics, or ghost probiotics, are “non-viable microbial cells (either intact or broken), or crude cell extracts, which, when administered (orally or topically) in adequate amounts, confer a benefit on the human or animal consumer” [7,24]. A recent review by Mehta et al. [25] focused on the ability of different types of para-probiotics and postbiotics to modulate the immune system. The most used strains to develop as para-probiotics are Lactobacillus and Bifidobacterium strains. The postbiotic components that modulate the biological reactions include lipoteichoic acids, bacteriocins, SCFAs, peptidoglycan, and exopolysaccharides [25]. Some studies showed that prescribing live probiotic cells to people with weakened immune systems increases inflammatory responses. In such cases, a combination of dead cells can be a good alternative. Thus, the use of killed or inactive probiotics created a new field and various scientists tried to come up with new terms to describe the mentioned cases [26]. Additionally, Lee et al. [9] described the techniques to obtain para-probiotics, which include thermal treatments, sonication, ionizing radiation, high pressure, ultraviolet rays, and pH modification.
In terms of efficacy, Cuevas-González et al. [6] revised the bioactivities, health-promoting effects, and applications, among other issues related to post and para-probiotics, referring that, in vitro and in vivo studies have shown that some postbiotics and para-probiotics exhibit bioactivities such as immunomodulatory, anti-proliferative, anti-inflammatory, antimicrobial, and antioxidant. The authors postulated that these bioactivities could be involved in the observed health-improving effects, both in clinical trials and in humans, but more investigation is needed, as the mechanisms of action and the signaling pathways involved have not been fully elucidated. They concluded that para-probiotics and postbiotics are of great interest for the development of nutraceutical products, due to their potential for improving health [6].
In the last decade, several studies evaluated the potential uses of pre, pre, syn, post, and para-probiotics mainly focusing on inflammatory bowel diseases [27], other inflammatory diseases, and even in brain dysfunction, oral cavity dysbiosis, and a few of them related to skin diseases [14,9,23,28]. There are also studies in pregnancy, showing that reduced microbiome diversity (dysbiosis) during pregnancy, cesarean delivery, prematurity, and formula feeding can bring on dysbiosis in the newborn; so, microbiota therapy may be a path to restore eubiosis in pregnant women and their babies [29]. Besides, the use of probiotics in the course of antibiotic therapy does not have enough evidence. Éliás et al. [30] conducted a systematic review and meta-analysis of randomized controlled trials that evidenced differences in gut microbiome diversity between patients receiving antibiotic therapy with and without concomitant probiotic supplementation, showing that the results of available randomized controlled trials cannot endorse supplementation with probiotics along antibiotic therapy to avoid decreasing microbiome diversity [30]. On the other hand, other studies showed that probiotics can be used to change the microbiome, but an individual approach should be needed. Patil & Singh [31] suggested that by studying and harnessing individualized microbiota, personalized probiotic therapies could help improve the microbial environment, and aid in improving overall health. But more studies and partnerships between different fields are needed [31].
On the other hand, there is a great field of interest in the use of micro-biotics and nutribiotics in foods, both for animal nutrition and for humans in terms of functional foods [32], and also in the pharmaceutical industry [33].
Finally, it is worth mentioning that multiomics is a useful tool to select probiotics and understand their functions in the host microbiome; so that probiotics and the microbiome can be better understood [34].
This review describes the growing aspects of the use of nutribiotics (general term to refer to the set of microbiotics for human use, also called microbial biotherapy), within the field of nutraceuticals, in skin care health, focusing on the main dermatological diseases and other skin conditions.
2. The Skin Microbiome, a Unique Environment
The skin is a protective organ that performs important barrier functions against external agents in addition to preventing the loss of body fluids. The cells of the epidermis, but also the microorganisms present on its surface, intervene in the barrier function.
The microbiome and the skin are part of a whole that coexists and interrelates with each other. This invisible ecosystem of microorganisms performs important functions in the health of the skin, protecting it against external aggressions and acting as a second genome, interacting with other parts of the body to ensure healthy functioning. Its main role is the defense of the skin and the interrelation with the environment that surrounds it. Furthermore, the skin microbiome has been found to play an important role in pathogen protection, inflammatory regulation, and overall health [35].
The skin microbiome also helps maintain skin homeostasis and the epidermal barrier, aiding in the process of epidermal renewal by the production of protease enzymes. The secretion of lipases by the microorganisms present on the skin surface also plays a regulatory role, since they break down the lipids secreted by the sebaceous gland. In addition, the skin microbiome produces bacteriocins [36]. Also, quorum sensing seems to exert a critical role in the skin barrier function, as a recent study showed that interspecies quorum sensing among bacteria in human skin is considered a necessary defense mechanism to suppress the ability of Staphylococcus aureus to damage the epidermis [37].
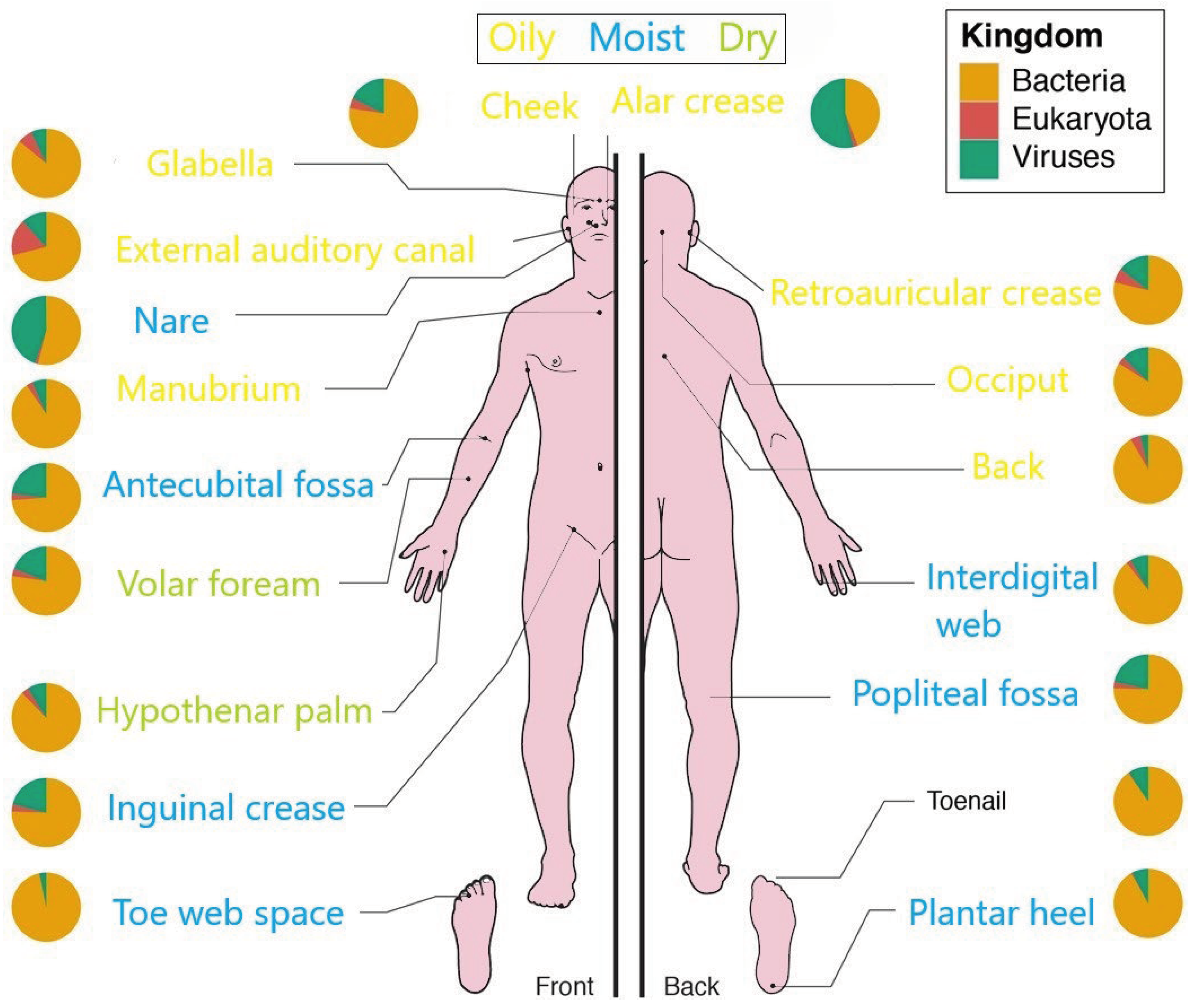
Over the past few years, several studies have focused on the composition of the skin microbiome and how it changes with development or how external factors may affect its diversity. On the other hand, it is well known that the composition of the skin microbiome varies according to the areas of the body that constitute various ecological and physicochemical niches, mainly related to moisture and sebum content on the surface of the skin [38]. These differences influence resident bacteria and fungi; oily surfaces such as the forehead harbor lipid-loving bacteria that differ from dry areas, such as the forearm, in which there is lower microbial density [39]. So that, Cutibacterium spp., Staphylococcus spp., and Streptococcus spp. are the most abundant bacteria on dry sites; Staphylococcus and Corynebacterium spp. prefer moist areas, and on sebaceous sites, lipophilic Cutibacterium species (spp.) are the most abundant [40,41]. Malassezia spp. is the most abundant fungus throughout the body, except in the areas of the foot that present greater diversity [42–44]. On the other hand, it has been observed that two phyla, Bacteroidetes and Firmicutes, tend to predominate in the microbiome of adults, while Actinobacteria and Proteobacteria constitute a smaller portion. Even so, variations can be found in the proportions of these phyla and the species from person to person [45].
Mites are also found in the skin microbiome.
Demodex spp. are characteristic of sebaceous glands and hair follicles, the most numerous representatives being
D. folliculorum (hair follicles), and
D. brevis (sebaceous and meibomian glands) [45]. The skin virome has also been explored; it is very heterogeneous and complex with various polyomaviruses (Polyomaviridae), circoviruses (Circoviridae), and papillomaviruses (Papillomaviridae) [46,47].
Figure 2 summarizes the relative abundance of bacterial, fungal, and viral components of the microbial community in the different skin microenvironments.
Studies of the skin microbiome (microbial and genomic components) in different age groups have shown that skin microbial communities exhibit dynamics that vary throughout life, developing in the early stages of life after exposure to the maternal microbiome, and following with changes in terms of diversity and community structure until old age [48].
Thus, it has been observed that the microbiome in neonates looks like maternal vaginal communities when delivered vaginally (Lactobacillus and Prevotella spp.), or maternal skin communities if delivered by cesarean section (Staphylococcus, Streptococcus, Corynebacterium, and Propionibacterium spp.). Staphylococcus, Corynebacterium, and Prevotella abound in premature infants, while Brevundimonas, Flavobacterium, and Sphingobacterium predominate in full-term infants [41].
At birth, the pH of the skin is neutral, but in the first hours of life, the development of the cutaneous acid mantle begins, favoring colonization by commensal organisms and inhibiting the growth of pathogens. Breast milk contains microbes, antimicrobial metabolites, IgA antibodies, and cytokines that facilitate the development of the microbiome and the neonatal immune response. The microbiome is influenced by close contacts, and it evolves throughout childhood; thus, Firmicutes (Staphylococcus and Streptococcus) predominate in the skin of babies, followed by Actinobacteria, Proteobacteria, and Bacteroidetes [49,50]. Microbiota diversity increases at least during the first eight years of life, which appears to be related to a reduced dominance of Lactobacillales (especially of the genus Streptococcus) in the skin. In 14-year-olds there is greater interindividual variation in diversity than in younger age groups; the number of Staphylococcus or Streptococcus species decreases, and the amount of Actinobacteria and Proteobacteria species increases [51].
Puberty is another stage of changes in the skin microbiota; thus, Firmicutes (Streptococcus spp.), Bacteroidetes, and Proteobacteria are abundant, while the fungal community becomes more diverse [41]. The hormonal stimulus that occurs in the post-pubertal stage entails the stimulation of the sebaceous glands, with an increase of sebum production, favoring the overgrowth and spread of lipophilic microorganisms, such as Propionibacterium spp; Corynebacterium spp. and Malassezia spp. [41]. At the adult stage Corynebacterium, Propionibacterium, Streptococcus, and Staphylococcus predominate [49]; and finally, at the senile stage, the number of Firmicutes, including S. aureus and Cutibacterium species, decreases [52,53], as well as the production of antimicrobial peptides, increasing susceptibility to bacterial infections [49].
Jo et al. [54] investigated the skin mycobiome, showing that Malassezia predominated on the scalp, trunk, and arm skin of adults (age 20s-30s), children (age < 14) had more diverse fungal communities, for example, Eurotiomycetes which includes common dermatophytes, being M. globose the most predominant in children.
3. Skin Microbiome: Influence of Intrinsic and Extrinsic Factors
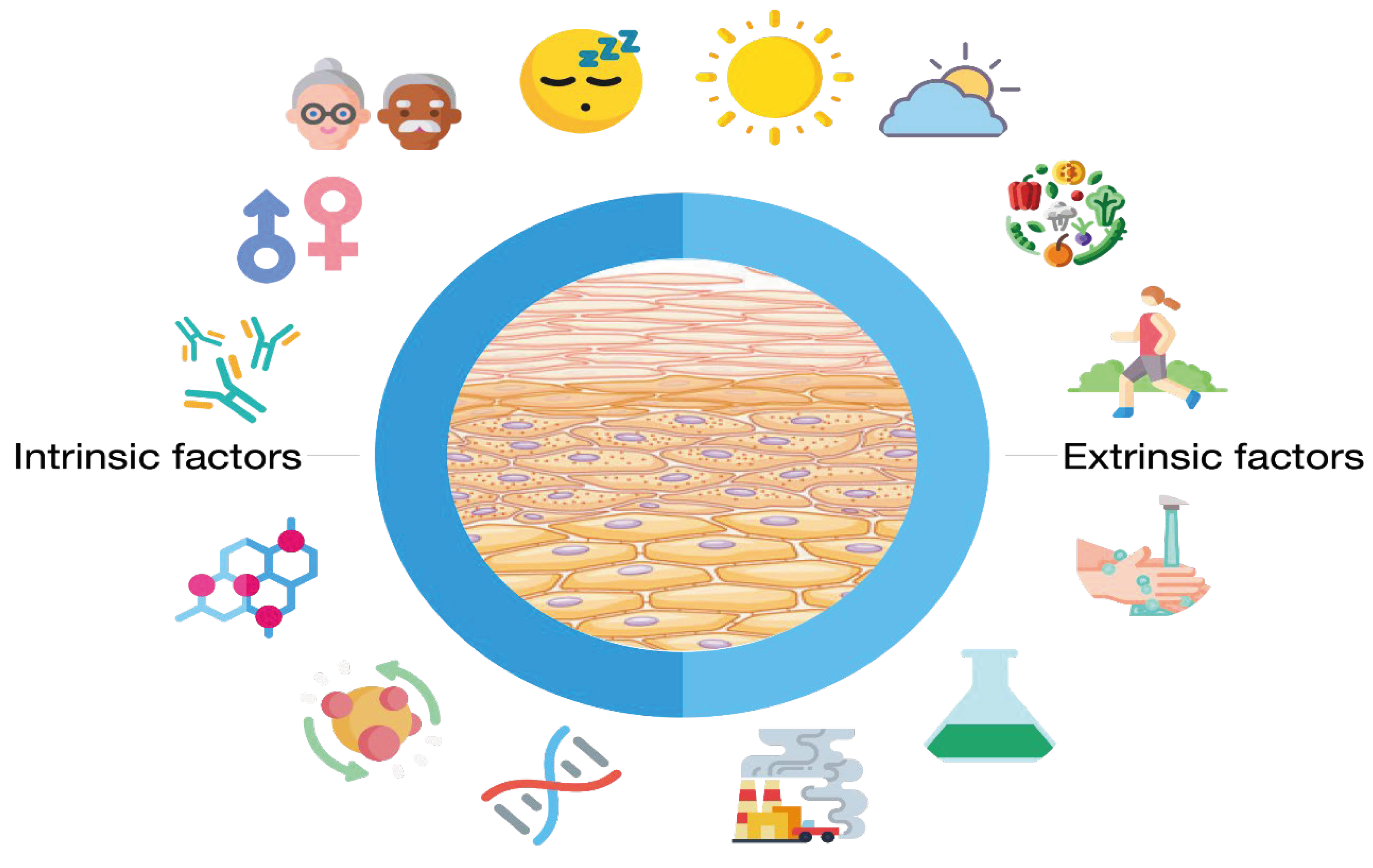
Skin microbiome depends on internal (or intrinsic) and external (or extrinsic) factors. Among the intrinsic factors, genetics, age, gender, hormones, immunity, sleep and stress factors, and metabolism must be mentioned. The exposure of the skin to external factors (UV, pollution, humidity, environmental bacteria, cosmetics, etc.) has also a great influence on the skin microbiome. Skowron et al. [55] reviewed the impact of extrinsic factors (external exposome) on the skin microbiome, and, in short, the most important are climate, sunlight (UV radiation), hygiene and cosmetics routine, environment (air and water pollution, exposure to chemicals), and physical activity and diet can also be added.
Several studies focused on internal factors, finding that among the genetic factors that determine the skin microbiome, ethnicity seems to be secondary, although not insignificant, since some differences have been found; for example, the number of Cutibacterium on the armpits and scalp of males in Africa and Latin America is lower than in other ethnicities (Caucasian, African–American, East Asian, and South Asian), and also differences have been found in the microbiomes of the arms of different ethnicities [56].
Differences linked to gender were also found; the female skin microbiome is characterized by a higher species diversity than that of males, probably due to several factors such as sweat production, and the influence of hormones [57].
The relationship between skin and gut (the so-called gut-skin axis) could explain the influence of the stress factors and metabolism in the skin microbiome [58] as well as nutrition [59]. Furthermore, diet and obesity were found to influence the skin microbiome, such that high-fat diets favor the growth of Corynebacterium, probably because it promote skin inflammation through the expression of mycolic acid. Furthermore, the balance between Firmicutes and Bacteroidetes in obese people is altered, and during weight loss changes occur in the composition of the microbiota, decreasing Firmicutes and increasing Bacteroidetes [60].
The environment of a given individual has also a great influence on the skin microbiome, as well as the profession or the type of daily activity. Some studies suggested that the time children spend outdoors could be relevant, but also other factors (e.g., cultural differences), and the constant and close contact with animals could influence the composition and diversity of the skin microbial communities in healthy people [61,62]. Some authors postulate that differences in the skin microbiome of urban and rural residents may be related to the exposure to microorganisms from the soil, water, and other factors as biomass used in agriculture or livestock [63].
The external environmental conditions have also an important influence on the skin microbiome, including temperature, humidity, and sunlight. When skin is exposed to UV radiation, several impacts may occur. The exposure of the skin to UV rays inhibited the growth of S. aureus and C. acnes; the latest is associated with the decreased production of porphyrins [64]. Furthermore, UV exposure results in a reduction in Lactobacillaceae and Pseudomonadaceae, and an overall increase in Cyanobacteria [65]. Additionally, it has been shown that repetitive and intense exposure to UV radiation may increase skin vulnerability to infections and worsen the associated symptoms, e.g., herpes simplex virus (HSV) [66]; on the contrary, Staphylococcus aureus was reduced by UVB radiation. But there are benefits derived from exposure to light; thus, the antimicrobial effects of photodynamic therapy (APDT) were demonstrated [67]; and some studies suggest that blue light treatment and conventional UV phototherapy may act beneficially in acne vulgaris by reducing Corynebacterium acnes density [68–70]. On the other hand, several studies concluded that skin microbiome has a useful role in protection against UV irradiation, which is linked with immune responses since important roles of TNF and IL-6 were observed [71].
Furthermore, using 16S ribosomal DNA and internal transcribed spacer ribosomal DNA sequencing to profile the microbiomes, Li et al. [72] studied the microbial communities of different ages, and several pathways related to aging (e.g., base excision repair, biosynthesis of amino acids, pantothenate and CoA biosynthesis, D-arginine and D-ornithine metabolism and oxidative phosphorylation, among others), concluding that skin microbiomes may play key roles in skin aging by regulating immune response, UV light resistance, and the biosynthesis of different substances involved in aging.
Other authors postulated that climate change, pollution, and the loss of biodiversity, together with other external factors such as the role of environmental substances (pollen, detergents, tobacco, as well as microplastics and nanoparticles) or the increase in the consumption of fatty acids in the diet, derange the epithelial barrier which causing a leaky epithelium resulting in microbial dysbiosis, including commensals and opportunistic pathogens, and translocation of this content into the interepithelial and sub-epithelial compartments, inducing microinflammation [73].
Finally, the impact of antibiotics on the skin microbiota should be cited. The use of antibiotics in the treatment of skin diseases is effective but may have a great impact on skin microbiota diversity. For example, orally administered doxycycline significantly reduced the number of C. acnes [74]; minocycline decreased the abundance of Cutibacterium, Corynebacterium, Prevotella, Lactobacillus, and Porphyromonas [75]; lymecycline reduced the presence of Cutibacterium and increased the number of Streptococcus, Staphylococcus, Micrococcus, and Corynebacterium [76], and fluoroquinolones (pefloxacin), and macrolides (erythromycin) significantly decreased the number of C. acnes [77].
Despite the lack of studies, some research showed that cosmetics may affect the skin microbiome diversity. For example, Bouslimani et al. [78] reported that antiperspirants and foot powders increased the diversity of the skin microbiome, but the effect disappeared after stopping antiperspirant application, and, in contrast, arm and face lotions had little effect on bacterial communities and archaea.
Other cosmetics as soaps effectively reduce the number of microorganisms but too frequent use of soap or other antiseptics in hand disinfection can alter the microbiome and reduce its diversity due to damage to the barrier function [55]. So, more studies are needed to elucidate the effect of cosmetics on skin microbiota.
Clothing is also of interest when studying skin microbiome. Skin-clothing contact could cause microorganism transference and the formation of the so-called textile and volatile microbiome. Microorganisms that adhere to the fibers can use the lipid components of sebum and dirt as a substrate and produce volatile substances as byproducts that contribute to unpleasant odors [55]. Furthermore, Ferro de Oliveira et al. [79] investigated the role of clothing on the skin microbiome, finding that different textile compositions can lead to the growth or inhibitions of certain microorganisms. For example, Staphylococcus hominis had a high affinity for cotton but did not grow in fleece and viscose; Staphylococcus spp. showed a significant adhesion to textile fibers; and cotton and wool enhanced the growth of different bacteria species, including Staphylococcus epidermidis, Enhydrobacter spp., Cutibacterium spp., and Micrococcus spp. Additionally, cellulose-based fibers exhibited low microbial growth rates for most axillary bacteria, except for Staphylococcus spp., and polyester facilitated greater growth for Cutibacterium spp., Enhydrobacter spp., and Micrococcus spp. So that, authors revised the existing bioactive textiles based on their specificity against microorganisms, i.e., antifungal, antibacterial, and antiviral textiles, and concluded that this knowledge may be an opportunity for the development of microbiota-friendly textiles or antimicrobial textile products capable of targeting specific populations of the skin microbiota with the aim of alleviating skin disorders, allergies or bad odor, preventing growth and the spread of pathogenic microorganisms [79].
Figure 3 summarizes the intrinsic (genetics, age, gender, hormones, immunity, sleep and stress factors, and metabolism), and external factors (climate, sunlight, hygiene, and cosmetics routine, environment (air and water pollution, exposure to chemicals, physical activity, and diet) that influence the skin microbiome.
4. Skin Microbiome and Dermatological Disorders
It is very well known that the skin microbiome plays an important role in developing and maintaining homeostasis and regulation of the host immune system. Belkaid & Segre [39] summarized the “dialogue between skin and immune system” as follows: Microorganisms present on the surface and skin appendages (bacteria, fungi, viruses) can themselves produce antimicrobial peptides and also regulate the production of antimicrobial peptides by keratinocytes, as well as the production of immune mediators such as complement and IL-1. These molecules can directly or indirectly improve skin immunity by improving cellular microbicidal function, promoting cytokine production, and the recruitment of effector cells. Furthermore, IL-17 production by the microbiota may promote the effector function of keratinocytes against invading microbes. Additionally, skin-resident microbes can release defined metabolites that could be captured directly by skin-resident dendritic cells [39].
Skin disorders such as acne, atopic dermatitis, and psoriasis have all been associated with dysbiosis of the skin microbiota. Dysbiosis is the alteration in the composition, activity, or distribution of the cutaneous microbiota. According to Mustari et al. [80], 3 mechanisms can be involved: 1) Overgrowth of a microbiota member (e.g., Cutibacterium acnes in acne); 2) Elimination of a microbiota member, and 3) Invasion by non-member microorganisms (e.g., Staphylococcus aureus in atopic dermatitis) [80]. Dysbiosis is associated with various dermatological conditions such as psoriasis, atopic dermatitis (AD), seborrheic dermatitis, acne, rosacea, vitiligo, hidradenitis suppurative, lepra, and others linked to viruses, but it is not entirely clear if the changes in the microbiota cause diseases or whether certain conditions cause an imbalance in microbial communities [81].
The gut microbiome also plays a role in some skin disorders; there appears to be a bidirectional link between the gut and the skin which in turn is linked to the body's homeostasis, the so-called gut-skin axis. The gut microbiota modulates the functionality and composition of the innate and adaptive immune system, and vice versa. This fact could explain why some skin diseases are linked to intestinal dysbiosis and an imbalance of skin homeostasis, suggesting a role of the intestinal microbiota in the pathogenesis of several inflammatory skin diseases [82]. Multiple studies support a connection between both and several skin diseases associated with gastrointestinal disorders, but more studies are needed to attribute a cause-and-effect relationship between the gut microbiome and dermatological conditions. For example, between 10% and 25% of patients with gastrointestinal diseases, such as Crohn's and celiac disease, and ulcerative colitis, also have associated skin disorders, specifically skin ulcers and psoriasis [83].
Inchingolo et al. [81] postulated that the intestinal microbiota contributes to the allostasis and homeostasis of the integumentary system after any inflammatory process due to the relationship with innate and adaptive immunity. Proinflammatory cytokines could damage the intestinal barrier, and severe intestinal dysbiosis provokes inflammation beyond the intestinal and therefore low-grade systemic inflammation with skin involvement.
Many studies have shown that the overgrowth (or decline) of pathogens on the skin is a common occurrence in various skin diseases and conditions. The main changes are summarized in
Table 1.
Acne is a chronic inflammatory skin disease characterized by the presence of comedones, papules, pustules, and sometimes nodules and scars that appear in oily areas s the face and upper trunk. The pathogenesis of acne vulgaris is multifactorial and involves increased production of cutaneous sebum, hyperplasia of sebaceous glands due to the androgenic influence, infra-infundibulum hyper-keratinization which leads to ductal obstruction, proliferation, or presence of certain strains of Cutibacterium acnes, and infiltration of inflammatory cells. In terms of skin microbiota changes, C. acnes is considered the most likely acne pathogen but there are several recognized sub-groups of C. acnes (I, II, and III) and different ribotype [82], so more investigations are needed to clarify its role in the pathogenesis of acne. In addition to its role in inflammation, C. acnes also intervenes in the homeostasis of the skin microbiome by interacting with other skin microorganisms such as Staphylococcus epidermidis, Streptococcus pyogenes, and Pseudomonas species. In the microbiome of healthy skin, S. epidermidis may limit the over-colonization with C. acnes strains and reduce C. acnes-induced IL-6 and TNF-α production by keratinocytes. In turn, C. acnes may limit the proliferation of S. aureus and S. pyogenes by promoting triglyceride hydrolysis and propionic acid secretion, which collaborates in the maintenance of the acidic pH in the pilosebaceous follicle. Furthermore, in the pilosebaceous follicles, C. acnes inhibits the development of S. epidermidis by the same mechanisms, as to say, hydrolyzing sebum triglycerides, secreting propionic acid, and maintaining the acidic pH of the pilosebaceous follicle [87,88]. In acne, a modified profile of C. acnes is observed, as different phylotypes have been shown to differ between patients with and without acne [84–86].
Weng & Cheng [89] carried out a comprehensive review in which studies on the relationships between the skin microbiome and acne vulgaris, rosacea, and skin aging were included. Authors summarized that in acne Firmicutes spp., Proteobacteria spp., Actinobacteria spp., Staphylococcus spp., and Streptococcus spp. they were increased, while S. epidermidis decreased [89].
Atopic Dermatitis (AD) is a chronic inflammatory skin disease affecting approximately 20% of children. In 95% of cases, AD’s first manifestation appears within the first 5 years of life, and, in 25% of the cases, AD continues during adulthood [106]. Genetic and epigenetic factors modulate AD: exposure to indoor and outdoor allergens and pollutants, nutrition, and microbiome are considered to influence and contribute to the development and severity of AD [90]. AD is characterized by an abnormal immune response; high levels of pro-inflammatory cytokines (e.g., IL-4, IL-13, IL-22) promote skin inflammation and contribute to barrier derangement and dysfunction. Due to inflammation, the skin may produce antimicrobial peptides (AMPs) such as defensins and cathelicidins, which can disbalance the skin microbiome [107].
AD has long been linked with Staphylococcus aureus skin colonization; disease outbreaks are associated with a spread of S. aureus in injured areas of the skin and a substantial loss of biodiversity in the skin microbiome. Staphylococcal exoproteins and superantigens cause inflammatory reactions in the host [90]. Fyhrquist et al. [91] also reported a significant increase in colonization by S. aureus and a loss of anaerobic species in AD. Koh et al. [108] appointed that S. aureus isolated from AD patients also express higher levels of virulence factors and a propensity to develop biofilms to promote its colonization. So, the therapies aim to reduce S. aureus (with antimicrobials) but also to balance the diversity of the skin microbiome.
Psoriasis is an immune-mediated inflammatory skin disease, the development of which is linked to both genetic factors and external triggers [109,110]. However, its pathogenesis is still not fully understood, and the influence of gut and skin microbiota is being investigated. Psoriasis is characterized by multiple erythematous lesions with scaly plaques that arise mainly on the elbows, knees, scalp, navel, and lower back, but, in some cases, the disease spreads throughout the body in the form of erythroderma. Increased vascularization could be also found, which allows the accumulation of inflammatory subpopulations of neutrophils, dendritic cells, and T lymphocytes.
Psoriasis is frequently associated with inflammation in other organ systems. Thus, 7%–11% of patients with inflammatory bowel disease (IBD) are also diagnosed with psoriasis, reflecting a strong association with gastrointestinal inflammation. Changes in the gut microbiome in psoriasis are similar to those observed in patients with IBD; in both diseases, Faecalibacterium prausnitzii, Bifidobacterium spp., Lactobacillus spp., Parabacteroides and Coprobacillus were underrepresented, while the abundance of Salmonella sp., Campylobacter sp., Helicobacter sp., Escherichia coli, Alcaligenes sp., and Mycobacterium sp. was increased [111]. Other studies showed that psoriatic exacerbation was considered to be associated with increased colonization of Staphylococcus aureus, Candida albicans, and Malassezia in the skin and gut [112]. Another similarity between psoriasis and IBD is the reduced abundance of two beneficial bacteria species (Parabacteroides and Coprobacillus) observed in patients with psoriasis and psoriatic arthritis and in those with IBD [113]. Thus, it is generally accepted that the inflammatory and immune mechanisms of psoriasis are based on the dysregulation of the gut-brain-skin axis [114].
Additionally, a decrease in Bacteroidetes and an increase in Firmicutes in the intestines of psoriatic patients compared to control patients were also found [116]. Similar findings were reported by other authors who found an increased abundance of Firmicutes, Proteobacteria, and Actinobacteria, together with a decrease in Bacteroidetes in the gut microbiome of psoriatic patients [116,117].
Other recent studies confirmed this relationship between gut microbiota and psoriasis; Zang et al. [118] identified nominal protective roles of Bacteroidetes and Prevotella in psoriasis risks; and some bacterial taxa were recognized as risk factors, including Lactococcus, Ruminiclostridium 5, and Eubacterium fissicatena; but Odoribacter demonstrated a protective effect against psoriasis [119].
When revising the role of skin microbiome in psoriasis, studies have shown relative increases in Streptococcus and Staphylococcus and decreases in Malassezia and Cutibacterium [92].
On the other hand, Alekseyenko et al. [93], comparing swap samples of patients with psoriasis and healthy controls, demonstrated that the microbiome of psoriatic lesions is characterized by an increase of Firmicutes and Actinobacteria, and a general taxonomic diversity reduction. Additionally, Chang et al. [94] found that the microbiome of psoriatic skin has reduced stability compared to the microbiome of healthy skin; and loss of community stability and decline of immunoregulatory bacteria such as Staphylococcus epidermidis and Propionibacterium acnes may result in increased colonization with pathogens such as Staphylococcus aureus, which could exacerbate skin inflammation along the Th17 axis [94].
Rosacea is an inflammatory chronic skin disease that appears exclusively on the central area of the face, such as cheeks, nose, and chin, symmetrically, and also in the central forehead, characterized by flushing, papules and pustules, telangiectasia, and sometimes phymatous alterations accompanied by stinging or itching [120]. Generally, rosacea is classified into four morphologic subtypes: phymatous rosacea, papulopustular rosacea, erythematotelangiectatic rosacea, and ocular rosacea [121].
The clinical manifestations of rosacea are multifactorial and are linked to abnormal neurovascular activation, dysregulated production and release of inflammatory molecules, and overgrowth of microorganisms that naturally inhabit the skin [122].
Demodex folliculorum is found to been implicated in rosacea; still, Demodex is unlikely to be the only cutaneous microorganism that contributes to the disease, since Demodex mites are suspected of carrying Bacillus oleronius, a pro-inflammatory gram-negative bacterium that is receptive to many antibiotics recurrently used to treat rosacea [82], resulting the amelioration of the disease when treating with antibiotics.
The origin of rosacea development is unclear, but several factors are involved including genetic factors, local skin immune imbalance, disorders of neuroimmune function, skin barrier dysfunction, and skin microbiota dysbiosis, as well as alterations of neurovascular circuitry [123]. The role of the microbiota in the rosacea pathogenesis is supported by several evidence. Studies conducted by different authors have pointed to the implication of Staphylococcus epidermidis, Demodex folliculorum, Helicobacter pylori, Bacillus oleronius, and Chlamydia pneumonia in the pathogenesis of rosacea [95–99]. However, there are discrepancies between the results of the investigations, and the specific mechanisms by which the microorganisms have been involved in the pathogenesis of rosacea are not clear, as they are commensal microorganisms. More specifically, the distribution, relative abundance, mechanisms involved, and thus the role of Cutibacterium acnes and S. epidermidis in rosacea need further investigation to provide evidence for future probiotic therapy [124].
Finally, the aforementioned review by Weng & Chen [89] described that, in the papulopustular rosacea, the proportions of Firmicutes and Proteobacteria are higher, and Actinobacteria proportions been lower.
Seborrheic Dermatitis (SD) is an inflammatory rash that appears on sebaceous areas of skin such as the scalp, face, and trunk [102]. The incidence of SD reaches the highest point at three ages: infancy, puberty, and adults over 50 years old, suggesting the role of hormones in sebum production in its pathogenesis [45]. SD is generally associated with Malassezia, however, its role in the development of SD is still poorly understood [100]. Some studies suggest that there are other microorganisms involved. For example, Tanaka et al. [101] found that Acinetobacter, Staphylococcus, and Streptococcus are the dominant genera on the skin microbiome of lesional areas affected with SD compared to healthy skin; and An et al. [102] found that patients with SD had a significant over-colonization of Staphylococcus epidermidis, concluding that this high colonization along with alteration of the skin barrier function, which is more permeable and contributes to the appearance of SD.
Dandruff, considered a form of middle seborrheic dermatitis, has been also found to be linked to Malassezia. A study performed by Wang et al. [103], using molecular techniques, showed an increased colonization with Malassezia restricta, and also of Staphylococcus species when compared to healthy scalps.
Other skin disorders have also been associated with skin microbiome dysbiosis. Hidradenitis suppurativa was found to be linked to the dermal microbiota as the microbial composition differs significantly from that of healthy individuals. In total, the following types of 5 microbes were identified: Corynebacterium spp. (type I), Acinetobacter and Moraxella spp. (type II), Staphylococcus epidermidis (type III), Peptoniphilus spp. and Porphyromonas (type IV), and Propionibacterium acnes (type V), suggesting that Propionibacterium may be involved in its pathogenesis [104].
Tinea pedis is a dermatophyte infection that especially affects the interdigital network and/or the sides of the feet. Different factors have been found that may be related to its appearance as sweating, occlusive footwear, trauma, and an immunocompromised state, among others. When studying the skin microbiota, epidermal samples from patients with tinea pedis have been shown to exhibit decreased bacterial diversity and increased fungal diversity compared to healthy controls; an increase in Trichophyton rubrum was observed in patients with tinea pedis as compared to healthy controls, been the most prevalent bacterial phyla Firmicutes, Actinobacteria, and Proteobacteria, while Staphylococcus constituted more than 30% of the bacterial genera [105].
Finally, it is worth mentioning the relationship between microbiome and melanoma. Fortman et al. [125] revised the studies related to the microbiome and cancer, showing that there is evidence that the gut microbiome can alter responses to chemotherapy and immune checkpoint inhibitors (ICIs). Authors concluded that preclinical and clinical studies have demonstrated the effects of the gut microbiome modulation upon ICI response and immune-related adverse event development in advanced melanoma, with significative evidence supporting the ability of the gut microbiome to improve ICI response in advanced melanoma through increased intake of dietary fiber, and fecal microbiome transplant.
5. Nutribiotics: An Opportunity to Improve Skin Health
The history of probiotics can be tracked back to ancient times, nearly 10,000 years ago, since probiotic microorganisms, and fermented products, such as kefir, kumis, bread beer, and wine had been very frequently used for nutritional and therapeutic purposes [126]. Knowing that the composition of the human microbiota is directly linked with the development and functioning of the immune system, prebiotics and probiotics oral supplementation could be a tool for improving overall human health but more research is needed to better understand the interactions between diet, the microbiome and the immune system to design specific diets with the aim of improving diseases [127]. Gao et al. [128] explain the immunological pathway of oral probiotics as follows: when the probiotics enter the intestinal tract can interact with the host improving intestinal homeostasis, and take part in immunomodulation, gut microbiota homeostasis, digestion, and absorption of nutrients, and also improving the intestinal mucosal barrier.
The use of probiotics in skin care is more recent since the gut-skin axis was investigated. Polak et al. [129] revised the use of prebiotics and probiotics in chronic skin diseases, finding studies mainly in atopic dermatitis (children and adults), but also in acne, chronic ulcers, seborrheic dermatitis, and burns. Later on, in a similar revision, Kianmehr et al. [130] showed that the administration of prebiotics, probiotics, and synbiotics has auspicious effects on preventing and treating various inflammatory skin disorders, such as atopic dermatitis and acne [131,132]. Orally administration of probiotics affects the intestinal microbiome and can improve skin conditions such as atopic dermatitis, acne, or rosacea [133,134], and also other studies showed that using probiotics during gestation and early life could reduce the incidence and severity of atopic dermatitis, by immune modulation and promoting the maturation of the gut barrier function [135,130,136]. Additionally, probiotics are investigated to treat different allergy illnesses, including atopic dermatitis, asthma, allergic rhinitis, and food allergy [137]. Despite of that, Małolepsza & Dembowski [138], after reviewing several studies, concluded that alterations in the intestinal microbiome play an important role not only in the development and aggravation of many skin diseases, but also influence skin aging, although it is necessary more research to evaluate the impact of probiotics.
On the other hand, Pimentel et al. [139] revised the health effect of post-biotics, including skin conditions both in vitro and in vivo, and Mehta et al. [25] discussed the potential of para-probiotics and postbiotics to modulate the immune system.
The following sections describe the use of pre, pro, syn, post, and para-probiotics in skin care.
Table 2 summarizes the clinical studies.
* Clinical studies. **In prevention of polymorphic light eruption.
4.1. Acne
As mentioned above, the gut and skin microbiomes influence each other and contribute to skin health through immune modulation. The preservation of skin homeostasis and the reinforcement of the skin’s barrier function is one of the major objectives in skin care, and the axis gut-skin could take part in it [186].
Probiotics aim to modify the skin environment to prevent over-colonization of C. acnes and other bacteria linked to acne. It has been shown that probiotics directly inhibit C. acnes via the synthesis of antibacterial proteins and organic acids by certain bacterial strains. Additionally, a large-scale review on acne vulgaris concluded that oral probiotic administration was associated with a decrease in acne breakouts [187].
One of the first studies was performed by Robert H. Sawyer in 1961, who reported on the potential benefits of probiotic Lactobacillus. He followed up 300 patients who consumed commercial probiotics, that is, Lactinex® tablets comprising a mixture of L. acidophilus and L. bulgaricus. The protocol consisted of 8 days of oral probiotic, a 2-week washout, and another 8 days of treatment. An improvement of 80% was found, being more notable in cases of inflammatory acne [140]. Later on, similar results were found in studies performed in patients under antibiotic therapy with supplementation of oral probiotics L. acidophilus and Bifidobacterium bifidum as adjuvant therapy [141]. In 2018, Mottin et al. [188] carried out a review of the main strains used in the treatment of acne and atopic dermatitis, finding that those that showed the highest potential to control acne were Staphylococcus, Streptococcus, Lactococcus, Lactobacillus, and Enterococcus, and Vitreoscilla filiformis, Staphylococcus epidermidis, and species of Lactobacillus and Bifidobacterium in the treatment of atopic dermatitis.
In addition, other studies demonstrated that antibiotics and oral probiotic can provide a synergistic effect, especially in inflammatory acne. A randomized, prospective open-label trial demonstrated that consumption of Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, and B. bifidum was as effective as minocycline in the treatment of acne, with a 67% reduction in lesions after twelve weeks of oral treatment, finding fewer side effects [133].
Another randomized-controlled study with twenty subjects showed that oral administration of Lactobacillus rhamnosus SP1 concluded with an improvement or marked improvement of adult acne compared to placebo [142]. Researchers also measured gene expression on the skin of IGF-1, a hormone involved in acne development, and FOXO1, a transcription factor whose deficiency is associated with acne pathogenesis. The intervention also showed a 32% reduction in IGF-1 and a 65% increase in FOXO1 [142].
Other studies focused on interleukin-10 serum levels in acne vulgaris before and after 30 days of oral probiotics, a sachet containing B. lactis W51, B. lactis W52, L. acidophilus W55, L. casei W56, L. salivarius W57, L. lactis W58 with total bacterial cells > 108 CFU. Results showed a significant increase in IL-10 levels after this therapy [143].
Additionally, in an in vitro cell culture skin model, the probiotic strain Lactobacillus paracasei NCC2461 demonstrated dose-dependent inhibition of CD-4+ T cell activation and induction of the anti-inflammatory cytokines IL-10 and TGF-b [144].
And in a review performed by Goodarzi et al. [189] authors concluded that probiotics can be effective as an adjunct therapy both topical or oral administrations, by preventing the growth of opportunistic bacteria or by controlling inflammation. They suggested that, despite numerous in vitro and in vivo studies, interventional studies are needed using more samples and long-term follow-ups to demonstrate the effectiveness of these type of probiotics and determine potential advantages and disadvantages.
Other studies combine probiotics and plants in oral formulations. For example, Tolino et al. [190] conducted a double-blind clinical trial in men with mild to moderate acne treated with an oral supplement containing probiotics, biotin, vitamin E, zinc, nicotinamide, beta-sitosterol, and Boswellia serrata extract. After 12 weeks of treatment, these patients presented clinical improvement being shown by the reduction of the Global Acne Grading System (GAGS) score [190].
Oral synbiotics were also investigated. In 2010, Al-Ghazzewi et al. [145] studied the capacity of konjac glucomannan hydrolysates and probiotics (L. casei, L. plantarum, L. gasseri, L. lactis) to inhibit C. acnes, finding that significantly inhibited the growth of bacteria, suggesting further research to confirm the use of this type of synbiotics as therapeutics or prophylactics [145].
The Escherichia coli Nissle 1917 strain has also been used in clinical trials in acne patients. Manzhalii et al. [146] performed a study in which this strain was orally administered to 82 patients with intestinal-borne dermatoses (some of them were diagnosed with acne, and others with papular-pustular rosacea and seborrheic dermatitis). They compared two groups of patients; intervention first was treated with conventional topical therapy, and the second with the probiotic E. coli Nissle 1917 strain administered orally for one month. A total of 89% of the patients treated with E. coli Nissle 1917 improved significantly, while 56% improved in the group treated with the conventional therapy. After studying the composition of gut microbiota and other parameters, the authors concluded that the E. coli Nissle 1917 strain was able to restore the intestinal microbiota, protect the intestinal barrier, and so ameliorate the mentioned diseases [146].
Rinaldi et al. [147] evaluated the efficacy of a mixture of the probiotic strains Bifidobacterium breve BR03 DSM 16604, Lacticaseibacillus casei LC03 DSM 27537, and Ligilactobacillus salivarius LS03 DSM 22776 combined with a botanical extract of Solanum melongena and Echinacea in subjects with mild to moderate acne over an 8-week study period through a randomized, placebo-controlled clinical trial. Results showed a decreased presence of C. acnes, the number of acne lesions, the rate of sebum secretion, and the rate of desquamation in patients who were treated with the probiotic mixture and the botanical extract, as well as the mixture of both, concerning placebo treatment. The most notable effects were observed with the probiotic mix plus the botanical extract [147].
The ammonia-oxidizing bacteria Nitrosomonas eutropha had also been used to treat adult patients with mild or moderate acne, finding that, after 12 weeks of treatment, a significant reduction in overall severity, as well as a tendency in the reduction in the number of inflammatory lesions compared to the control group [148].
Topic probiotics could be also useful in treating acne. The production of short-chain fatty acids (such as succinic acid) on the skin can inhibit C. acnes growth [191]. Lactic acid [192] and ceramide [193] produced after topical probiotic administration, showed direct antimicrobial activity against C. acnes.
Additionally, Kang et al. [194] used a cell-free culture supernatant from E. faecalis SL-5 in patients with mild to moderate acne in topical application. The study concluded that this bacteriocin was able to reduce inflammation, so that, researchers suggested that E. faecalis could be an alternative option in future acne therapy [194].
Additionally, a bacteriocin produced by Lactococcus sp. HY499 exerted an inhibitory effect on inflammatory and pathogenic bacteria in the skin such as S. epidermidis, Staphylococcus aureus, S. pyogenes, and P. acnes without affecting the growth and proliferation of fibroblasts. The authors recommended this bacteriocin as an antimicrobial in cosmetic formulations [195].
4.2. Atopic Dermatitis
There are few studies about the use of oral prebiotics to prevent atopic dermatitis. A meta-analysis by Osborn and Sinn [196] analyzed 4 studies (1,218 infants) exploring the effect of specific prebiotics in the prevention of allergy. They found a significant reduction in eczema when using a fructooligosaccharide and galactooligosaccharide combination [196]. Additionally, another research showed that infants at risk of atopy who were fed with an oligosaccharide prebiotic-supplemented formula during the first 6 months of life had a significantly lower cumulative incidence of AD in 5 years [149]. Furthermore, a formula containing a specific mixture of neutral oligosaccharides and pectin-derived acidic oligosaccharides was effective as the primary prevention of AD in low atopy risk infants [150]. Additionally, Kim et al. [197] demonstrated that AD-like skin lesions induced in NC/Nga mice were reduced by oral administration of a prebiotic diet (long-chain fructooligosaccharides, inulin or β-glucan), and intestinal microbiota richness and diversity were also increased with this prebiotic treatment.
Several studies confirm the positive effects of oral probiotics supplementation in AD. Fanfaret et al. [198] reviewed the most relevant articles related to the use of probiotics or prebiotics alone and a combination of the two, finding that the most studied probiotics strains were Lactobacilli and Bifidobacteria. However, the authors conclude that the results are difficult to interpret as in many studies the authors suggest that the disease may tend to improve over time in some groups of patients [198].
Oral supplementation with Lactobacillus rhamnosus GG (LRGG) for 1 month caused a significant improvement in AD severity scoring of atopic dermatitis (SCORAD) index with decreased levels of inflammatory markers such as tumor necrosis factor (TNF-α) and fecal α1-antitrypsin [151]. LRGG also demonstrated anti-inflammatory activity with significantly increased levels of IL-10 and transforming growth factor-β2 (TGF-β2) in AD patients [152,153]. And administration of probiotic Lactobacillus strains (a mixture of Lactobacillus rhamnosus 19070-2 and Lactobacillus reuteri DSM 12246) to children with AD has been shown to result in a moderate improvement in the clinical severity [154].
Other studies in vitro and in vivo confirmed the potential use of probiotics in AD. Lactobacillus casei (LC) is one of the most studied species. Several studies in vivo and in vitro showed that LC may exert an immunomodulatory effect, and the active component has been identified as a protein P14 which has been shown to selectively downregulate serum IgE and interleukin-4 cytokine levels, as well as the AD index and scratching score in AD-like NC/Nga mice [199].
Kim et al. [200,201] investigated the immunomodulatory capacity of Duolac ATP, a mixed formulation of probiotics, composed of four different strains of probiotics: L. casei CBT LC5 (KCTC12398BP), L. plantarum CBT LP3 (KCTC10782BP), L. rhamnosus CBT LR5 (KCTC12202BP), and B. lactis CBT BL3 (KCTC11904BP), both in vitro and in vivo [200,201]. Results showed that Duolac ATP regulated IL-10 and TGF-beta expression and allowed DCs to become functionally tolerant and potentially induce Treg differentiation. Additionally, this formulation regulated transcription factors and cytokines to drive naïve T cell differentiation toward Th1 lineages. The authors concluded that this formula could be a good ally in the management of AD symptoms and serve as an immunomodulatory agent for AD [201].
In another study, the probiotic strain Lactobacillus rhamnosus GG decreased the proportions of IgA and IgM-secreting cells in babies with AD. There were no significant differences in the species composition of intestinal bifidobacteria between the studied group and the control group. On the skin, bacterial counts of the genus Bifidobacterium versus Clostridium coccoides in treated and untreated infants were similar (155). Additionally, oral administration of probiotic bacteria Bifidobacterium lactis HN019 and Lactobacillus rhamnosus HN001 has been observed to improve natural killer cells and phagocytic activity [156].
Later studies showed that AD symptoms can be improved using Lactobacillus paracasei KBL382 isolated from the feces of healthy Koreans. In this study, mice with Dermatophagoides farinae extract (DFE)-induced AD were fed with L. paracasei KBL382 for 4 weeks, demonstrating that oral administration of L. paracasei KBL382 significantly reduced AD-associated skin lesions, the epidermal thickening and serum levels of immunoglobulin E, as well as immune cell infiltration. Furthermore, the administration of L. paracasei KBL382 was able to change the gut microbiota composition in AD mice [202].
Several studies focus on oral probiotics supplementation during pregnancy and breastfeeding. D´Elios et al. [203] revised the efficacy of the most commonly studied probiotic strains for the prevention and treatment of AD, concluding that probiotic supplementation during the prenatal and postnatal periods seems to reduce the incidence of AD in infants and children who are at high risk, especially beginning in gestation through the first 6 months of life. The revised studies included monostrain probiotics as Bifidobacterium dentium [204], Lactobacillus rhamnosus MP108 [205], and heat-treated Lactobacillus paracasei [206]; multistrain probiotics as Lactobacillus acidophilus La-5, and Bifidobacterium animalis subsp. lactis Bb-12 [207], Lactobacillus paracasei and Lactobacillus fermentum [208]; Lactobacillus rhamnosus and Bifidobacterium animalis subsp lactis [209]; and multistrain Bifidobacterium lactis CECT 8145, B. longum CECT 7347, and Lactobacillus casei CECT 9104 [210].
A systematic review and meta-analysis of randomized controlled trials performed by Cuello-Garcia et al. [211] concluded that probiotic supplementation during the last trimester of pregnancy or breastfeeding could reduce the risk of eczema in infants, although the certainty of the evidence was low. Li et al. [212] achieved similar findings, concluding that the use of probiotics during both the prenatal and the postnatal period significantly reduced the incidence of AD; however, analysis of studies of probiotics administered prenatally only or postnatally only did not reach statistical significance. Similarly, Tan-Lim et al. [213] revised the randomized clinical trials related to the use of oral probiotics to prevent AD, finding that the top 3 probiotic preparations in terms of efficacy in reducing the risk of AD are multi-strain: Lactobacillus paracasei ST11, Bifidobacterium longum BL999, and Lactobacillus paracasei ssp. paracasei F19, and multi-strain: Lactobacillus rhamnosus GG, and Bifidobacterium animalis ssp. lactis Bb-12.
Recently, a meta-analysis and systematic review performed by Chen et al. [214] evaluated the efficacy of probiotic supplementation for the prevention of AD in infants, showing that both mothers and infants oral probiotics supplementation were effective in preventing AD in infants.
Synbiotics seem to be also useful in AD. Children with mild to moderate AD, aged 1 to 10 years, were treated with one sachet daily of a novel synbiotics formula containing a mixture of 6 types of gastro-resistant probiotics (not less than 1.5 x 1010 CFU/sachet at the time of production), and triple prebiotics containing inulin, isomalto-oligosaccharides, and fructo-oligosaccharides for 8 weeks. The probiotic mixture was composed of Lactobacillus rhamnosus GG, Lactobacillus acidophilus GKA7, Bifidobacterium longum GKL7, Lactobacillus plantarum GKM3, Bifidobacterium bifidum GKB2, and Lactobacillus paracasei GKS6. Results showed an important improvement in Eczema Area and Severity Index (EASI) without any adverse effects. The presence of key microbial drivers including Bacteroides fragilis and Lactobacillus acidophilus were significantly increased at week 8. The authors also found that high responsiveness to an 8-week probiotic treatment was associated with improvements in the gut microbiome profile with greater relative abundance of probiotic species [157].
Post and parabiotics have also been studied. In 2016, Choi et al. [215] assessed the effect of heat-killed Enterococcus faecalis EF-2001 (EF-2001) on AD in in vivo AD model by repeated local exposure of Dermatophagoides farinae extract, finding that the symptoms and pathological sings were attenuated as well as the production of Ig, and the expression of various pathogenic cytokines in the ears, lymph nodes, and splenocytes. Considering previous studies in allergic diseases which reported that heat-killed Lactobacillus casei Shirota suppressed pro-inflammatory, Th1, and Th2 cytokines in splenocytes [216], authors suggested that EF-2001 is able to significantly inhibit the inflammatory response by blocking both Th1 and Th2 in AD lesions of the ear tissue as well as in the cervical lymph nodes and splenocytes [215].
Formulas including a mixture of pre, probiotics, and postbiotics have also been tested. Patients with a diagnosis of AD were treated for 8 weeks with an oral formula containing 7 types of gastro-resistant probiotics (mixture of Lactobacillus rhamnosus GG, Lactobacillus acidophilus GKA7, Lactococcus lactis GKL2, Lactobacillus casei GKC1, Lactobacillus paracasei GKS6, Bifidobacterium bifidum GKB2, and Bifidobacterium lactis GKK2, not less than 2 x 1010 CFU/capsule), a postbiotic heat-killed Lactobacillus plantarum (10 mg/capsule), and triple prebiotics containing inulin (22 mg/capsule), galactooligosaccharides (8.1 mg/capsule), and fructooligosaccharides (0.9 mg/capsule). Results showed an improvement in the diversity of gut microbiome and significant improvement in AD severity [182].
Colombo et al. [183], performed a real-life, multicenter, retrospective observational investigation designed to evaluate the efficacy and tolerability of a commercial pre- and postbiotic supplement. Patients consumed a daily sachet containing a concentration exceeding 2.5 x 109 AFU (active fluorescent units) of three patented probiotic species: Bifidobacterium animalis subsp. lactis BS01 (LMG P-21384), Lacticaseibacillus rhamnosus LR05 (DSM 19739), and Lactiplantibacillus plantarum LP14 (DSM 33401). Results showed a significant overall and even intra-individual reduction in all severity scores: erythema, edema/papules, excoriation, TIS (Three Item Severity score), and PRURISCORE [183].
While the use of orally administered probiotics for the prevention and treatment of AD has been largely studied, only a small number of studies have focused on the topical application of probiotics, which may be due to the difficulty of delivering viable bacteria to the skin, given that creams and lotions typically have to be preserved [217].
The most used probiotics and postbiotics for topical application in AD are heat-inactivated Lactobacillus johnsonii NCC533 [218], Aquaphilus dolomiae and Vitreoscilla filiformis, which was able to reduce S. aureus colonization [219], and Lactobacillus reuteri DSM 17938, which showed a statistically and clinically significant improvement of the SCORAD index and local SCORAD in adults suffering from AD after 4 and 8 weeks of continuous use [220]. Previous studies showed that the production of the anti-inflammatory molecule IL-10 by dendritic cells was increased after the local application of Vitreoscilla filiformis extracts on AD [221,222].
In a study performed by Nakatsuji et al. [223] a strain of Staphylococcus hominis A9 (ShA9) was selected and applied to AD patients, showing that ShA9 can inhibit skin inflammation by inhibiting quorum sensing. Phase II of clinical trials is ongoing, and results are not still available.
As has been mentioned before, Nitrosomonas eutropha (B244) is a bacterium that produces nitric oxide, a potential anti-inflammatory molecule. In phase II a randomized controlled trial (RCT) in adults, B244 administered as a spray induced a significant improvement of the pruritus. Additionally, an open-label phase Ib pediatric trial showed a similar effect on itching [224].
Finally, it is also worth citing the studies of Myles et al. [225,226] about topical microbiome transplantation with Roseomonas mucosa, which was able to reduce S. aureus colonization.
4.3. Psoriasis
Chen et al. [227] conducted an in vivo study where oral administration of Lactobacillus pentosus GMNL-77 was found to significantly decrease erythematous scaling lesions. Real-time polymerase chain reaction showed that treatment with L. pentosus GMNL-77 significantly decreased the mRNA levels of proinflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor-alpha, and the IL-23/IL-17A axis-associated cytokines (IL-23, IL-17A/F, and IL-22) in the skin of imiquimod-treated mice.
Buhas et al. [158] performed a 12-week open-label, single-center clinical trial with the aim to evaluate the efficacy of probiotics: Bacillus clausii (SC109), Bacillus coagulans (SC208), Bacillus indicus (HU36), Bacillus subtilis (HU58), and Bacillus licheniformis (SL307), and prebiotics such as xylooligosaccharides, fructooligosaccharides, and galactooligosaccharides in patients with psoriasis under topical therapy. Results showed that patients with psoriasis receiving anti-psoriatic local therapy and probiotic and prebiotic supplementation performed better results in Psoriasis Area and Severity Index (PASI), Dermatology Life Quality Index (DLQI), inflammatory biomarkers, and skin thickness compared to those who did not receive supplementation [158].
Finally, it is worth mentioning a case report related to the treatment of a case of pustular psoriasis resistant to steroids, dapsone, and methotrexate responded well to Lactobacillus sporogenes. The patient was administered one sachet thrice daily with biotin 10 mg, and all other drugs were stopped immediately. Within fifteen days the fever decreased, the lesions began to regress, and no new lesions appeared in two weeks. Therefore, the authors concluded that future research should be conducted in this field [159].
4.4. Rosacea
According to the ROSacea International Expert Group (ROSIE), composed by European and US rosacea experts, treatment for rosacea aims to reduce symptoms such as facial flushing and telangiectasias, and eruption of papules and pustules, to prevent or delay exacerbation of the disease manifestations as well as to maintain remission [228]. Treatments are based on topical and systemic therapies (antibiotics, retinoids, etc.), light therapies (e.g., laser), and dermocosmetics [228]. In the literature, there is a lack of studies focused on nutribiotics, although some are promising.
The study mentioned above by Manzhalii et al. [146] in patients with papulopustular exanthema (including 36% with rosacea) who received the bacteria Escherichia coli Nissle 1917 as an oral probiotic as well as a standard topical therapy, demonstrated that oral probiotics therapy had better results than patients who only received standard treatment, improving quality of life and the clinical signs of dermatosis. Clinical improvement was associated with a suppression of the proinflammatory cytokine IL-8 and a significant increase of IgA levels to normal values in serum. Fortuna et al. [160] also reported a case of rosacea with scalp involvement that was treated with a combination of low-dose doxycycline (40 mg/day) and oral probiotics (Bifidobacterium breve BR03 and Lactobacillus salivarius LS01) for 8 weeks, followed by probiotics alone. No relapse or worsening of the disease was observed during the 6 months of follow-up.
4.5. Seborrheic Dermatitis
Dandruff, seborrheic dermatitis, and scalp-associated disorders showed significant improvements after oral supplementation of Lactobacillus paracasei NCC 2461 ST11, observing that free and adherent dandruff, erythema and the global clinical score improved significantly after 56 days of oral intake of a sachet containing ST11 (1×109 CFU) compared to placebo [161].
Additionally, Di Domenico et al. [229] assessed the impact of a topical oily suspension containing Lactobacillus crispatus P17631 and Lacticaseibacillus paracasei I1688 in patients affected by severe to moderate seborrheic dermatitis, finding that this mixture was able to reduce symptoms and modulate the microbiome composition, showing that topical administration of probiotics could also be useful in seborrheic dermatitis.
4.6. Wound Healing
It has been shown that the absence of microbiota can decrease healing time; furthermore, wound infections appear when exogenous bacteria become dominant over systemic and local host resistance factors, and only when a balance is achieved between bacteria and the host can healing processes develop [230].
The most effective wound management strategy is to prevent infections, promote healing, and prevent excess scarring, and probiotics may aid in skin repair by exerting antagonistic effects against pathogens and stimulating the production of immune cells [231]. In a comprehensive review performed by Fijan et al. [162], authors found that the most commonly used probiotics against pathogens of wound infections were well-known strains of the species Lactobacillus plantarum, Lactobacillus casei, Lactobacillus acidophilus, and Lactobacillus rhamnosus. All in vitro studies showed effective inhibition of wound pathogens by selected probiotics. In all in vivo studies, probiotics showed strong activities in counteracting wound infections. Most clinical studies showed a mild or statistically significant lower incidence of surgical site infection, foot ulcers, or burn infections, in patients using probiotics [162].
Tagliari et al. [232] investigated the effect of perioperative oral administration of probiotics on the healing of skin wounds in rats. The probiotic group was supplemented with Lactobacillus paracasei LPC-37, Bifidobacterium lactis HN0019, Lactobacillus rhamnosus HN001, and Lactobacillus acidophilus NCFM® at a dose of 250 mg/day, and the control group was supplemented with oral maltodextrin 250 mg/day, both daily for 15 days. In the intervention group, a faster reduction of the wound area was observed, and authors postulated that this may probably be attributed to a reduction of the inflammatory phase, an acceleration of the fibrosis process, and collagen deposition.
On the other hand, Togo et al. [233] conducted a systematic review focused on the currently available evidence on the effect of enteral or oral probiotic therapy on wound healing both of the skin and oral mucosa, which included seven studies involving 348 people. The results showed that four studies reported positive results for better healing after probiotic therapy, and none of the studies reported adverse effects or increasing in wound healing time. The authors concluded that the results do not generate strong evidence regarding the effectiveness of probiotics for wound healing.
Later on, Tembhre et al. [234] revised the role of probiotics in chronic wounds, finding 14 articles and concluding that probiotics help eliminate pathogenic bacteria and restore normal wound flora when applied topically. The main probiotic strains were from the Lactobacillus species: L. plantarum, L. acidophilus, L. rhamnosus, but also Saccharomyces cerevisiae [234]. S. cerevisiae was shown to achieve an overall improvement in the healing process; specifically provoking an increase in the expression levels of collagen type 1 and transcription growth factor beta 1 (TGF-β1) as well as an improvement of the morphological and biomechanical characteristics of the healing wounds [235]. In vitro study L. acidophilus and L. casei were shown antibacterial activity against Methicillin-resistant Staphylococcus aureus [236]; and L. reuteri and L. rhamnosus reduced the ability of the pathogen to induce keratinocyte cell death [237]. Additionally, L. fermentum was shown an increased wound closure concurrent with production of nitric oxide (gNO) [238].
Recently, Canchy et al. [239] revised the relationship between the skin microbiome and the wound healing process. Most of the studies (as in the previous revision by Tembhre et al. [234]) are related to probiotics topical administration, and the main probiotics strains were again from the Lactobacillus species; authors suggested that probiotics mainly affect the inflammation phase, which plays an important role in wound healing impairment, and the suspected mechanism of action is through the regulation of AMPs, and thus control microbial proliferation [239]. In the same revision, the use of prebiotics and postbiotics in wound healing was revised, finding very little research. Vitreoscilla filiformis has been shown to increase keratinocyte proliferation, epidermal regeneration in vitro, and stratum corneum renewal rate in vivo, as well as to stimulate the expression of collagen I and IV. These results may indicate that this strain could be useful for increasing re-epithelization in wound healing applications [239,222,240].
Another field of interest is phage-therapy. Bacteriophages are viruses that infect and replicate within bacteria which have long been used to treat human bacterial infections. Phages are specific to the species and often the strain level in targeting and infecting bacteria. Topical phage therapies have been reported for the treatment of several types of refractory chronic skin infections, such as diabetic ulcer, venous stasis, or burn-mediated [241], and other investigations focused on the benefits of using phages to reduce S. aureus biofilm mass, and to treat S. aureus infections [242,243].
4.7. Aging and Photoaging
Since Elia Metchnikoff proposed that all microorganisms are not harmful and that several intestinal bacteria “produce useful substances against a premature aging,” favoring instead a “healthy aging” [11], several studies were carried out to investigate the effects of oral probiotics supplementation on skin aging and photoaging [244]. Thus, protection and recovery from sunburn have been one of the first research objectives of probiotics for the skin. These first studies were carried out with fractions of bifidobacteria applied to the skin, with contradictory results [245].
Oral administration of Lactobacillus johnsonii (La1) at 108 CFU/day for 10 days protected against the UVR-induced suppression of contact hypersensitivity, increasing IL-10 serum levels, and decreasing epidermal Langerhans cell density [246]. Additionally, another study demonstrated that Lactobacillus sakei lipoteichoic acid inhibited MMP-1 induced by UVA in normal human dermal fibroblasts [247].
Kim et al. [248] evaluated the effect of Lactobacillus plantarum HY7714 against UVB-induced photoaging in human dermal fibroblasts and hairless mice. The results showed that treatment with L. plantarum HY7714 effectively recovered UVB-reduced procollagen expression by inhibiting UVB-induced matrix metalloproteinase (MMP)-1 expression in human dermal fibroblasts. Furthermore, oral supplementation of L. plantarum HY7714 showed an inhibition of the number and depth of wrinkles in hairless mouse skin and also was able to inhibit UVB-induced epidermal thickness in mice. In addition, zymography western blot data also demonstrated that L. plantarum HY7714 effectively inhibited MMP-13 expression as well as MMP-2 and -9 activities in dermal tissue [248].
Additionally, a randomized double-blind clinical trial demonstrated the antiaging effect of oral administration of L. plantarum HY7714 (1010 CFU/day for 12 weeks), with a significant improvement in the hydration, shine, and elasticity of the skin and also in the reduction of the depth of wrinkles [163].
Gueniche et al. [246] showed that oral supplementation with Lactobacillus johnsonii at 108 CFU/day for 10 days was able to protect against the UVR-induced suppression of contact hypersensitivity, decreased epidermal Langerhans cell density and increased IL-10 serum levels. Furthermore, in a randomized, double-blind controlled trial, oral administration of Lactobacillus johnsonii La-1 demonstrated restoration of CD1a Langerhans cell markers compared to placebo on day 4 after UV irradiation [164].
Weill et al. [249] investigated the effect of lipoteichoic acid (LTA) from Lactobacillus rhamnosus GG against UV-induced carcinogenesis in hairless mice. The results showed that T-cells in the inguinal lymph node of LTA-treated mice produced higher levels of interferon-gamma in lymph nodes and numbers of total, helper, and cytotoxic T-cells compared to controls. A delay in tumors induced by ultraviolet radiation was also found.
Other studies confirmed the use of Lactobacillus to prevent aging. Tyndallized Lactobacillus acidophilus was shown to suppress matrix metalloproteinases (MMPs) for wrinkle prevention in photoaged skin through inhibition of elastase activity [250,251], and also exerted anti-melanogenesis activity by inhibiting the cAMP pathway and suppressing melanin secretion [251].
Bifidobacterium breve strain Yakult (BBY) was also investigated in hairless mice and was shown to be able to suppress UV-induced elastase and IL-1beta production and prevent the loss of elasticity associated with exposure to UV [252]. Similar studies demonstrated that administration of Bifidobacterium breve B-3 to hairless mice suppressed changes in transepidermal water loss, skin hydration, and epidermal thickening and reduced damage to the basement membrane and tight junction structure induced by chronic UVB irradiation, showing a protective effect on skin photoaging [253].
Several studies performed by Kimoto-Nira (2018) focused on Lactococcus lactis H61, finding that oral intake of heat-killed or live cells improved skin status in Japanese women; in addition, heat-killed cells of strain H61 demonstrated an antioxidant effect [184].
Prevention of polymorphic light eruption has also been studied. Marini et al. [165], in a study with light-sensitive patients, investigated the administrations of a combination of Lactobacillus johnsonii La-1, β-carotene, and lycopene; the results showed that this mixture was able to improve the alteration and an increase in intercellular adhesion molecule-1 (ICAM-1) was also observed, suggesting an immunological response.
Prebiotics seem also to be useful for preventing aging. A diet rich in SCFA-producing dietary fibers may also help age-related microbial dysbiosis and in turn, suppress the senescent phenotype. That is the case of butyrate supplementation which demonstrated to be able to counteract age-related microbiota dysbiosis [166].
Recently, the term “gerobiotics” has been proposed by Tsai et al. [254] to define those probiotic strains and their derived postbiotics and para-probiotics that have been shown to reduce physiological aging processes by attenuating the mechanisms of aging, thus improving the health span of the host. In an extensive review, the authors highlight the importance of the new field of gerobiotics, research, and updating, biomarkers for potential targets, and provide recommendations for the development of gerobiotic products, highlighting its potential to improve health and longevity in the future. In this review, several strains were highlight. B. longum BB68, L. gasseri SBT2055, L. fermentum MBC2, B. infantis ATCC15697, and B. subtilis PXN21 (in C. elegans model) were able to increase lifespan. L. brevis OW38, L. paracasei PS23, L. paracasei K71 (in mice model) were able to increase memory and/or cognition; L. plantarum AR501 (in mice model) reduced the liver damage; L. helveticus KLDS1.8701 improved the gut microbiota, and also the memory. In rat models, L. plantarum NDC 75017 increased learning and memory, and L. fermentum DR9 improved exercise capacity [254]. Bifidobacterium animalis ssp. lactis HN019 and L. casei Shirota improved innate immunity in humans [167,168]. Additionally, supplementation with a mixture of Bifidobacterium longum subsp. longum BB536, B. longum subsp. infantis M-63, Bifidobacterium breve M-16V, and B. breve was able to improve mental condition, and decrease body mass index in humans [169].
Teng et al. [255] revised the mechanisms of action of probiotics in photoaging, concluding that oral and topical probiotics, by modulating the skin microbiome and gut-skin microbial interactions, could be useful in preventing and treating skin photoaging through multiple pathways, including reducing oxidative stress, inhibition of ECM remodeling, inhibition of inflammatory cascade reaction and maintenance of immune homeostasis [255]. Most of the studies are in vitro and in vivo, and a few of them clinical studies. An example is the investigation performed by Bouilly-Gauthier (2010) to assess an oral supplement containing Lactobacillus johnsonii and nutritional carotenoids on early UVR-induced skin damage, finding that intake of this mixture for 10 weeks prevented UV-DL-induced decrease in Langerhans cell density and increase in factor XIIIa+ type I dermal dendrocytes, and reducing dermal inflammatory cells [170].
4.8. Other Uses of Nutribiotics
One of the main issues in skin care is the maintenance of skin barrier and hydration. A double-blind trial involving combinations of probiotic and prebiotic (B. breve strain Yakult + galactooligosaccharides) supplemented with fermented milk (100 mL/day for 4 weeks) resulted in the maintenance of an optimal level of skin hydration, a decrease in the activity of cathepsin L-type endopeptidase and the phenol content in serum and urine, exerting beneficial effects on both the intestine and the skin [171]. Additionally, a trial with L. casei (1x1011 bacteria/day for 8 weeks) conducted by Saito et al. [185] demonstrated a significant reduction in TEWL (trans-epidermal water loss) and skin flakiness [172]. Furthermore, oral administration of heat-killed L. lactis (60 mg/day for 8 weeks) significantly modulated various skin properties, such as skin elasticity, melanin content, and increasing sebum content, with a notable effect in the younger age groups. And a para-probiotic prepared from Kimchi-derived Lactobacillus plantarum K8 improved the skin hydration in human keratinocyte [256].
Taking into account that skin immune conditions, such as acne, rosacea, and atopy are associated with skin barrier disruption, and the restoration of this barrier is associated with an amelioration of the conditions [257], topical application of probiotics is also considered. For example, Gueniche et al. [258] found Lactobacillus paracasei CNCM I-2116 (ST11) to inhibit P-substance-induced skin inflammation and accelerate the regeneration and contributing to the epidermal barrier repairment; finding that significantly eliminates all the effects of P-substance, including vasodilatation, edema, mast cell degranulation, and TNF-α release, compared to the controls. Moreover, the ST11-associated skin barrier recovery was found to be accelerated in an ex-vivo skin culture [258]. In a review performed by Benyacoub et al. [144] related to the immune modulation properties of Lactobacillus paracasei NCC2461 (ST11) strain, authors concluded that this strain contributes to the reinforcement of the skin barrier function, and modulates the skin's immune system, reducing skin sensitivity, which leads to an improvement in defenses and the preservation of skin homeostasis.
Melasma could be also a target for nutribiotics. Piyavatin et al. [173] performed an experimental study employing a prospective, double-blind, randomized controlled trial in patients suffering from facial melasma. Participants were randomly treated with oral synbiotics or placebo, 1 sachet daily for 12 weeks; melasma severity and skin health were evaluated at baseline, weeks 4, 8, and 12 weeks. Synbiotics consisted of a combination of 50 billion CFUs of 6 probiotics strains: Lactobacillus acidophilus, Lactococcus lactis, Lactobacillus casei, Bifidobacterium bifidum, Bifidobacterium longum, and Bifidobacterium infantis, with prebiotic fructo-oligosaccharide, skim milk powder, lactose, maltodextrin, and citric acid. Results showed that melasma score in the synbiotics supplement group was significantly lower than that in the placebo group [173].
The use of probiotics in systemic lupus erythematosus (SLE). A systematic review performed by Mirfeizi et al. [259] identified 22 articles examining the effects of probiotics on SLE. These studies, which include in vivo tests, in vitro research, and clinical trials, indicated that probiotics may be effective against inflammation, improving immunological responses as well as the metabolic profiles in SLE patients. The main strains were Lactobacillus delbrueckii and Lactobacillus rhamnosus [260–263]; a mixture of Lactobacillus rhamnosus, Lactobacillus reuteri, Lactobacillus johnsonii, Lactobacillus oris, and Lactobacillus gasseri [264]; and Lactobacillus plantarum [264]. In a double-blind randomized clinical trial, Widhani et al. [174] investigated the effect of a synbiotic formula comprising L. helicus, B. infantis, B. bifidum and fructo-oligosaccharides, finding that this formulation may be effective in decreasing systemic inflammation, reducing SLE disease activity, and inducing changes in both the composition and functions of the intestinal microbiota.
Another field of interest is the treatment of oral mucositis. According to Feng et al. [266] probiotics exerts a significant protective effect against oral mucositis in cancer patients. In recent review, Liu et al. [267] found eight trials on patients who were treated with chemotherapy or/and radiotherapy. The oral probiotics used were: L. rhamnosus GG, 1 capsule, 2 times a day during the whole chemotherapy course [175]; oral lavage with kefir containing Lactobacillus spp., and Bifidobacterium spp., and swallow, 250 mL, 2 times a day after meal, first 5 days of each chemotherapy cycle [176]; L. brevis CD2 lozenges, 1 lozenge, 6 times a day to be dissolved in the mouth and then swallowed for 8 weeks [177]; oral rinse containing L. lactis, 15 mL, 3 or 6 times a day [268]; combination of B. longum, L. lactis, and Enterococcus faecium on capsules 2 times a day for 7 weeks [178]; L. brevis CD2 lozenges, 1 lozenge 6 times/day to be dissolved in the mouth and then swallowed up to one week after the end of cancer treatment [179]; probiotic combination of L. rhamnosus LGG-18, L. plantarum MH-301, B. animalis subsp. Lactis LPL-RH, and L. acidophilus, 1 capsule, 2 times a day for 7 weeks [180]; Bacillus clausii oral suspension, 5 mL, 2 times a day, until the completion of radiotherapy course [181]. Therefore, nutribiotics may be useful in this type of sequelae of cancer therapies.
In the scientific literature, oral probiotics in post-surgery can be also found. Trone et al. [269] scrutinized how prebiotics, probiotics, and synbiotics may play a role in modulating the immune response in the perioperative period, and the degree to which they may affect surgical outcomes. The authors suggested that even short-term gut microbiome pre-habilitation could significantly alter surgical outcomes, and future studies should consider evidence-based formulations comprising specific strains and also study the optimal treatment duration. In addition, dietary interventions, such as high-fiber diets and fermented foods should be considered in perioperative regimens [269].
And in terms of topical application, research tried to develop a plaster/bandage for the application of inhibitory substances produced by probiotics when applied to diseased skin; Lactobacillales were the most active against Cutibacterium acnes, Staphylococcus aureus, and Pseudomonas aeruginosa. Authors suggested that probiotic-containing pads can be applied topically for the treatment of skin disorders either replacing antibiotic treatment or as adjunctive therapy [270].
6. Conclusions and Future Perspectives
Intense research into the gut microbiome has provided tools to explore the role of the microbiota in other physiological systems, including the skin. Despite the scarce knowledge about the physiological role of skin microbiota in cutaneous biology, several strategies have been implemented to modulate the microbiome and improve skin health. The most recent strategy is the use of pre, pro, syn, and post and para-probiotics, which seems to be beneficial on different skin disorders as atopic dermatitis, psoriasis, acne, seborrheic dermatitis, etc., as well as repairing the skin barrier, preventing aging or promoting wound healing.
Nevertheless, a deeper understanding of the skin microbiome, the distribution of cutaneous microbial communities, as well as the differences between microbiomes in healthy and altered skin is needed. Thus, metagenomic technologies could facilitate functional characterization of the microbiome and perhaps even provide a personalized approach to diagnosing and treating conditions underpinned by microbial dysbiosis [44].
It should also be considered the potential adverse effects of probiotics, such as risk of systemic infections, excessive immune stimulation in susceptible individuals, minor gastrointestinal side effects [271], or disturbances in the abundance in the gut Bifidobacteria [272].
New investigations about the use of nanotechnology in the probiotic’s formulation are also of great interest as its delivery without any effect on gastrointestinal digestion is one of the most important points for their application [273]. Additionally, the genetic engineering of microbiomes has recently become an area of interest for researchers since it provides solutions to a significant health problem. In this context, strategies such as conjugative plasmids, bacteriophage, mating-assisted genetically integrated cloning (MAGIC), and environmental transformation sequencing (ETSeq) could be effective in the genetic modification of the microbiome [2].
In conclusion, skin microbiome has emerged as a new field with high potential to develop innovative solutions to manage skin health and disease. Future advances in this field may facilitate the treatment of skin dysbiosis through means that are more respectful of the physiology of the skin, being nutribiotics a suitable method for skin care.
Author Contributions
MLM: Conceptualization, Methodology, Writing—original draft preparation, Writing—review and editing. CPG: Methodology, Investigation, Writing—review and editing. JLL: Writing—review and editing. LP: Writing—review and editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wesley, A. Role of the Human Microbiome in Health and Disease in United Kingdom. Int J Nat Sci. 2023, 3, 35–47. [Google Scholar] [CrossRef]
- Mousavinasab, F.; Karimi, R.; Taheri, S.; Ahmadvand, F.; Sanaaee, S.; Najafi, S.; et al. Microbiome modulation in inflammatory diseases: Progress to microbiome genetic engineering. Cancer Cell Int. 2023, 23, 271. [Google Scholar] [CrossRef] [PubMed]
- Folign, B.; Daniel, C.; Pot, B. Probiotics from research to market: The possibilities, risks and challenges. Curr Opin Microbiol. 2023, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients. 2021, 13, 1225. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Lee, N.K.; Park, Y.S.; Kang, D.K.; Paik, H.D. Paraprobiotics: Definition, manufacturing methods, and functionality. Food Sci Biotechnol. 2023, 32, 1981–1991. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Hydrobiome of Thermal Waters: Potential Use in Dermocosmetics. Cosmetics 2023, 10, 94. [Google Scholar] [CrossRef]
- Caramia, G.; Atzei, A.; Fanos, V. Probiotics and the skin. Clin Dermatol. 2008, 26, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Dwivedi, M.K. The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Dwivedi, M.K., Amaresan, N., Sankaranarayanan, A., Kemp, E.H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–11. [Google Scholar] [CrossRef]
- Vallejo-Cordoba, B.; Castro-López, C.; García, H.S.; González-Córdova, A.F.; Hernández-Mendoza, A. Postbiotics and paraprobiotics: A review of current evidence and emerging trends. Adv Food Nutr. Res. 2020, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; et al. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Domínguez-Maqueda, M.; Cerezo, I.M.; Tapia-Paniagua, S.T.; De La Banda, I.G.; Moreno-Ventas, X.; Moriñigo, M.Á.; et al. A Tentative Study of the Effects of Heat-Inactivation of the Probiotic Strain Shewanella putrefaciens Ppd11 on Senegalese Sole (Solea senegalensis) Intestinal Microbiota and Immune Response. Microorganisms. 2021, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules. 2021, 11, 1903. [Google Scholar] [CrossRef]
- Patel, R.M.; Denning, P.W. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: What is the current evidence? Clin Perinatol. 2013, 40, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Posadas, G.A.; Broadway, P.R.; Thornton, J.A.; Carroll, J.A.; Lawrence, A.; Corley, J.R.; et al. Yeast Pro- and Paraprobiotics Have the Capability to Bind Pathogenic Bacteria Associated with Animal Disease. Transl Anim Sci. 2017, 1, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods. 2022, 11, 1077. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; et al. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods. 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules. 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, L.; Anglenius, H.; Tiihonen, K.; Ouwehand, A.C. Probiotics and Their Various Forms Supporting Skin Health. In Probiotic Research in Therapeutics: Volume 3: Probiotics and Gut Skin Axis–Inside Out and Outside In; Kaur, I.P., Beri, K., Kaur Deol, P.K., Sandhu, S.K., Eds.; Springer: Singapore, 2022; pp. 57–109. [Google Scholar] [CrossRef]
- Mehta, J.P.; Ayakar, S.; Singhal, R.S. The potential of paraprobiotics and postbiotics to modulate the immune system: A Review. Microbiol Res. 2023, 275, 127449. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomedicine & Pharmacotherapy 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients. 2020, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.B.; Oliveira, C.S.; Tavaria, F.K. Novel Strategies for Preventing Dysbiosis in the Oral Cavity. Front Biosci (Elite Ed) 2023, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Salge, M.M.H. The Importance of a Healthy Microbiome in Pregnancy and Infancy and Microbiota Treatment to Reverse Dysbiosis for Improved Health. Antibiotics 2023, 12, 1617. [Google Scholar] [CrossRef]
- Éliás, A.J.; Barna, V.; Patoni, C.; Demeter, D.; Veres, D.S.; Bunduc, S.; et al. Probiotic supplementation during antibiotic treatment is unjustified in maintaining the gut microbiome diversity: A systematic review and meta-analysis. BMC Med. 2023, 21, 262. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Singh, N. Probiotics Change the Microbiota: From the Lab to the Bedside. Preprints 2023, 2023071028. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Schnorr, C.E.; Pasquali, M.A.B. Paraprobiotics and Postbiotics-Current State of Scientific Research and Future Trends toward the Development of Functional Foods. Foods. 2023, 12, 2394. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; et al. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods. 2022, 11, 3094. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. 'Multi-omics' data integration: Applications in probiotics studies. NPJ Sci Food. 2023, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Athar, A.; Rasool, A.; Muzaffar, H.S.; Mahmood, A.; Abdullah, M.; Ali, Z.; et al. The human microbiome: A critical player in health and disease. World J. Biol. Biotechnol. 2023, 8, 31–37. [Google Scholar] [CrossRef]
- Baldwin, H.E.; Bhatia, N.D.; Friedman, A.; Eng, R.M.; Seite, S. The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier. J Drugs Dermatol. 2017, 16, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Hill, P.; Bonev, B.; Chan, W.C. Quorum-sensing, intra- and inter-species competition in the staphylococci. Microbiology (Reading). 2023, 169, 001381. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science. 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. NISC Comparative Sequencing Program. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; et al. The skin and gut microbiome and its role in common dermatologic conditions. Microorganisms, 2019, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The human skin double-stranded DNA virome: Topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio. 2015, 6, e01578-15. [Google Scholar] [CrossRef] [PubMed]
- Luna, P.C. Skin Microbiome as Years Go By. Am J Clin Dermatol. 2020, 21 (Suppl 1), 12–17. [Google Scholar] [CrossRef] [PubMed]
- Schoch, J.J.; Monir, R.L.; Satcher, K.G.; Harris, J.; Triplett, E.; Neu, J. The infantile cutaneous microbiome: A review. Pediatr Dermatol. 2019, 36, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.N. Infant Skin Microbiome. In Skin Microbiome Handbook: From Basic Research to Product Development, 1st ed.; Dayan, N., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 131–142. [Google Scholar]
- Lehtimäki, J.; Karkman, A.; Laatikainen, T.; Paalanen, L.; von Hertzen, L.; Haahtela, T.; et al. Patterns in the skin microbiota differ in children and teenagers between rural and urban environments. Sci Rep. 2017, 7, 45651. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu, P.A.; Iker, B.; Malik, K.; Leung, H.; Mohn, W.W.; Hillebrand, G.G. New Insights into the Intrinsic and Extrinsic Factors That Shape the Human Skin Microbiome. mBio. 2019, 10, e00839-19. [Google Scholar] [CrossRef]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef]
- Jo, J.H.; Deming, C.; Kennedy, E.A.; Conlan, S.; Polley, E.C.; Ng, W.I. NISC Comparative Sequencing Program; Segre JA, Kong HH. Diverse Human Skin Fungal Communities in Children Converge in Adulthood. J Invest Dermatol. 2016, 136, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; et al. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Perez Perez, G.I.; Gao, Z.; Jourdain, R.; Ramirez, J.; Gany, F.; Clavaud, C.; et al. Body Site Is a More Determinant Factor than Human Population Diversity in the Healthy Skin Microbiome. PLoS ONE 2016, 11, e0151990. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Solt, I. The Human Microbiome and Gender Medicine. Gender Genome 2018, 2, 123. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022, 14, 2096995. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N. Is there a connection between sun exposure, microbiome and skin cancer? A future research perspective. In Skin Microbiome Handbook: From Basic Research to Product Development; Dayan, N., Ed.; Scrivener Publishing LLC: Beverly, USA, 2020; pp. 377–388. [Google Scholar] [CrossRef]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J Investig Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013, 2, e00458. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Shu, M.; Jiang, Y.; Gallo, R.L.; Liu, Y.T.; et al. The response of human skin commensal bacteria as a reflection of UV radiation: UV-B decreases porphyrin production. PLoS ONE. 2012, 7, e47798. [Google Scholar] [CrossRef]
- Burns, E.M.; Ahmed, H.; Isedeh, P.N.; Kohli, I.; Van Der Pol, W.; Shaheen, A.; et al. Ultraviolet radiation, both UVA and UVB, influences the composition of the skin microbiome. Exp Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Patra, V.; Byrne, S.N.; Wolf, P. The skin microbiome: Is it affected by UV-induced immune suppression? Front Microbiol. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Charakida, A.; Seaton, E.D.; Charakida, M.; Mouser, P.; Avgerinos, A.; Chu, A.C. Phototherapy in the treatment of acne vulgaris: What is its role? Am J Clin Dermatol. 2004, 5, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Noborio, R.; Nishida, E.; Kurokawa, M.; Morita, A. A new targeted blue light phototherapy for the treatment of acne. Photodermatol Photoimmunol Photomed. 2007, 23, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Rassai, S.; Rafeie, E.; Ramirez-Fort, M.K.; Feily, A. Adjuvant Narrow Band UVB Improves the Efficacy of Oral Azithromycin for the Treatment of Moderate to Severe Inflammatory Facial Acne Vulgaris. J Cutan Aesthet Surg. 2014, 7, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Rai, G.; Kumar, A. Eco-evolutionary impact of ultraviolet radiation (UVR) exposure on microorganisms, with a special focus on our skin microbiome. Microbiol Res. 2022, 260, 127044. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, X.; Peng, T.; Yi, X.; Luo, L.; Yang, J.; et al. New Insights Into the Skin Microbial Communities and Skin Aging. Front Microbiol. 2020, 11, 565549. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy. 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.S.; Lee, S.H.; Kim, S. Characterization and Analysis of the Skin Microbiota in Acne: Impact of Systemic Antibiotics. J Clin Med. 2020, 9, 168. [Google Scholar] [CrossRef]
- Chien, A.L.; Tsai, J.; Leung, S.; Mongodin, E.F.; Nelson, A.M.; Kang, S.; Garza, L.A. Association of Systemic Antibiotic Treatment of Acne With Skin Microbiota Characteristics. JAMA Dermatol. 2019, 155, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Murillo, N.; Raoult, D. Skin microbiota: Overview and role in the skin diseases acne vulgaris and rosacea. Future Microbiol. 2013, 8, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Ferro de Oliveira, C.S.; Tavaria, K.F. The impact of bioactive textiles on human skin microbiota. Eur J Pharm Biopharm. 2023, 188, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Mustari, A.P.; Agarwal, I.; Das, A.; Vinay, K. Role of Cutaneous Microbiome in Dermatology. Indian J Dermatol. 2023, 68, 303–312. [Google Scholar] [PubMed]
- Inchingolo, A.D.; Cazzolla, A.P.; Di Cosola, M.; Greco Lucchina, A.; Santacroce, L.; Charitos, I.A.; et al. The integumentary system and its microbiota between health and disease. J Biol Regul Homeost Agents. 2021, 35, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Agüera-Santos, J.; Navarro-López, V. Acne, Microbiome, and Probiotics: The Gut-Skin Axis. Microorganisms. 2022, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef]
- Dréno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seite, S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp Dermatol. 2017, 26, 798–803. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am J Clin Dermatol. 2020, 21 (Suppl 1), 18–24. [Google Scholar] [CrossRef] [PubMed]
- Condrò, G.; Guerini, M.; Castello, M.; Perugini, P. Acne Vulgaris, Atopic Dermatitis and Rosacea: The Role of the Skin Microbiota-A Review. Biomedicines. 2022, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, M.A.; Corvec, S.; Timon-David, E.; Khammari, A.; Dréno, B. Cutibacterium acnes and Staphylococcus epidermidis: The unmissable modulators of skin inflammatory response. Exp Dermatol. 2022, 31, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Corvec, S.; Dreno, B. Staphylococcus epidermidis: A potential new player in the physiopathology of acne? Dermatology. 2019, 235, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.C.; Chen, Y.J. Skin microbiome in acne vulgaris, skin aging, and rosacea: An evidence-based review. Dermatologica Sinica 2022, 40, 129. [Google Scholar] [CrossRef]
- Wollina, U. Microbiome in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017, 10, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat Commun. 2019, 10, 4703. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Chan, W.H.; Hinojosa, T.; Hsu, S.; Feldman, S.R. Mechanisms of microbial pathogenesis and the role of the skin microbiome in psoriasis: A review. Clin Dermatol. 2019, 37, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013, 1, 31. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018, 6, 154. [Google Scholar] [CrossRef]
- Zhu, W.; Hamblin, M.R.; Wen, X. Role of the skin microbiota and intestinal microbiome in rosacea. Front Microbiol. 2023, 14, 1108661. [Google Scholar] [CrossRef]
- Lacey, N.; Delaney, S.; Kavanagh, K.; Powell, F.C. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007, 157, 474–481. [Google Scholar] [CrossRef]
- Murillo, N.; Mediannikov, O.; Aubert, J.; Raoult, D. Bartonella quintana detection in Demodex from erythematotelangiectatic rosacea patients. Int J Infect Dis. 2014, 29, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Microbiota in Rosacea. Am J Clin Dermatol. 2020, 21, 5–35. [Google Scholar] [CrossRef]
- Yuan, C.; Ma, Y.; Wang, Y.; Wang, X.; Qian, C.; Hocquet, D.; et al. Rosacea is associated with conjoined interactions between physical barrier of the skin and microorganisms: A pilot study. J Clin Lab Anal. 2020, 34, e23363. [Google Scholar] [CrossRef]
- Paulino, L.C. New perspectives on dandruff and seborrheic dermatitis: Lessons we learned from bacterial and fungal skin microbiota. Eur J Dermatol. 2017, 27, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Cho, O.; Saito, C.; Saito, M.; Tsuboi, R.; Sugita, T. Comprehensive pyrosequencing analysis of the bacterial microbiota of the skin of patients with seborrheic dermatitis. Microbiol. Immunol. 2016, 60, 521–526. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Sun, M.; Qi, R.Q.; Zhang, L.; Zhai, J.L.; Hong, Y.X.; et al. High Staphylococcus epidermidis Colonization and Impaired Permeability Barrier in Facial Seborrheic Dermatitis. Chin Med J (Engl). 2017, 130, 1662–1669. [Google Scholar] [CrossRef]
- Wang, L.; Clavaud, C.; Bar-Hen, A.; Cui, M.; Gao, J.; Liu, Y.; et al. Characterization of the major bacterial-fungal populations colonizing dandruff scalps in Shanghai, China, shows microbial disequilibrium. Exp Dermatol. 2015, 24, 398–400. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Riis, P.T.; et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol 2017, 153, 897–905. [Google Scholar] [CrossRef]
- McLoughlin, I.J.; Wright, E.M.; Tagg, J.R.; Jain, R.; Hale, J.D.F. Skin Microbiome-The Next Frontier for Probiotic Intervention. Probiotics Antimicrob Proteins. 2022, 14, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C. Clinical practice. Atopic dermatitis. N Engl J Med. 2005, 352, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. An Overview of the Relevance of Human Gut and Skin Microbiome in Disease: The Influence on Atopic Dermatitis. Appl Sci. 2023, 13, 10540. [Google Scholar] [CrossRef]
- Koh, L.F.; Ong, R.Y.; Common, J.E. Skin microbiome of atopic dermatitis. Allergol Int. 2022, 71, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Fontanez, N.; Soler, D.C.; McCormick, T.S. Current knowledge on psoriasis and autoimmune diseases. Psoriasis 2016, 6, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Capon, F. The genetic basis of psoriasis. Int J Mol Sci 2017, 18, 2526. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int J Mol Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Fry, L.; Baker, B.S. Triggering psoriasis: The role of infections and medications. Clin Dermatol 2007, 25, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wu, L.; Xiao, S.; Ji, Y.; Tan, Y.; et al. Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed Pharmacother. 2021, 137, 111065. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhu, W.; Kuang, Y.; Liu, T.; Zhang, W.; et al. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front Microbiol. 2020, 11, 589726. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gao, R.; Yu, N.; Zhu, Y.; Ding, Y.; Qin, H. Dysbiosis of gut microbiota was closely associated with psoriasis. Sci China Life Sci. 2019, 62, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Stec, A.; Chrabaszcz, M.; Knot, A.; Waskiel-Burnat, A.; Rakowska, A.; et al. Gut Microbiome in Psoriasis: An Updated Review. Pathogens. 2020, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Liu, J.; Mao, M.; Zhu, W.; Chen, W.; Wei, B. Causal Associations Between Gut Microbiota and Psoriasis: A Mendelian Randomization Study. Dermatol Ther (Heidelb). 2023, 13, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wang, J.; Liu, Y.; Guo, Y. Investigating the gut microbiota's influence on psoriasis and psoriatic arthritis risk: A Mendelian randomization analysis. Precis Clin Med. 2023, 6, pbad023. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Santos, A.C.; Gonçalves, T.; Peixoto, D.; Pires, P.C.; Velsankar, K.; Jha, N.K.; et al. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol Pharm. 2023, 20, 3804–3828. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Feinstein, A.; Odom, R.; et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002, 46, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea. J Am Acad Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Cao, P.; Liu, Q.; Yao, W.; Nie, Z.; Zhang, L. Analysis and Characterization of the Facial Skin Microbiota in Rosacea. Jundishapur J. Microbiol. 2003, 16. [Google Scholar] [CrossRef]
- Fortman, D.D.; Hurd, D.; Davar, D. The Microbiome in Advanced Melanoma: Where Are We Now? Curr Oncol Rep. 2023, 25, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef Microbes. 2015, 6, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Mukherjee, S.K.; Parai, D. Association of Probiotics and Prebiotics with Human Microbiome and the Functioning of Immune System. In Probiotics, Prebiotics, Synbiotics, and Postbiotics: Human Microbiome and Human Health; Khotari, V., Kumar, P., Ray, S., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 101–115. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients. 2023, 15, 3123. [Google Scholar] [CrossRef] [PubMed]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome-Current State of Art. Int J Mol Sci. 2021, 22, 4529. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, S.; Jahani, M.; Moazzen, N.; Ahanchian, H.; Khameneh, B. The Potential of Probiotics for Treating Skin Disorders: A Concise Review. Curr Pharm Biotechnol. 2022, 23, 1851–1863. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, N.; Ahanchian, H.; Jabbari Azad, F.; Mohammadi, M.; Farid, R.; Nikpoor, A.R.; et al. Subcutaneous immunotherapy and synbiotic combination shift T-helper 1 and cytotoxic T Cells in allergic rhinitis. Int J Pediatr. 2020, 8, 10731–10742. [Google Scholar] [CrossRef]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, Randomized, Open-Label Trial Comparing the Safety, Efficacy, and Tolerability of an Acne Treatment Regimen with and without a Probiotic Supplement and Minocycline in Subjects with Mild to Moderate Acne. J Cutan Med Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics in skin conditions: A systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020, 29, 15–21. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Steinhoff, M. Meta-analysis on preventive and therapeutic effects of probiotic supplementation in infant atopic dermatitis. J Dtsch Dermatol Ges. 2023, 21, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Fard, N.A.; Mazhary, Z.; Javanshir, N. Probiotic Bacteria in Microbiome against Allergy. In: Natalia V. Beloborodova, Andrey V. Grechko, editors. Human Microbiome. IntechOpen 2020. [Google Scholar] [CrossRef]
- Małolepsza, A.; Dembowski, T. Probiotics and gut-skin axis-new look on factors affecting skin condition. J Edu. Health Sport. 2023, 31, 55–60. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.; da Costa, W.K.A.; da Silva Rocha, R.; de Souza Pedrosa, G.T.; et al. Postbiotics: An overview of concepts, inactivation technologies, health effects, and driver trends. Trends Food Sci. 2023, 138, 199–214. [Google Scholar] [CrossRef]
- Siver, R. Lactobacillus for the control of acne. Journal of the Medical Society of New Jersey 1961, 59, 52–53. [Google Scholar]
- Marchetti, F.; Capizzi, R.; Tulli, A. Efficacy of regulators of the intestinal bacterial flora in the therapy of acne vulgaris. Clin Ter. 1987, 122, 339–343. [Google Scholar] [PubMed]
- Fabbrocini, G.; Bertona, M.; Picazo, Ó.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 2016, 7, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Rahmayani, T.; Putra, I.B.; Jusuf, N.K. The Effect of Oral Probiotic on the Interleukin-10 Serum Levels of Acne Vulgaris. Open Access Maced J Med Sci. 2019, 7, 3249–3252. [Google Scholar] [CrossRef] [PubMed]
- Benyacoub, J.; Bosco, N.; Blanchard, C.; Demont, A.; Philippe, D.; Castiel-Higounenc, I.; Guéniche, A. Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defenses. Benef Microbes. 2014, 5, 129–136. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Effect of konjac glucomannan hydrolysates and probiotics on the growth of the skin bacterium Propionibacterium acnes in vitro. Int J Cosmet Sci. 2010, 32, 139–142. [Google Scholar] [CrossRef]
- Manzhalii, E.; Hornuss, D.; Stremmel, W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016, 22, 5415–5421. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Marotta, L.; Mascolo, A.; Amoruso, A.; Pane, M.; Giuliani, G.; et al. Facial Acne: A Randomized, Double-Blind, Placebo-Controlled Study on the Clinical Efficacy of a Symbiotic Dietary Supplement. Dermatol Ther (Heidelb). 2022, 12, 577–589. [Google Scholar] [CrossRef] [PubMed]
- AOBiome Therapeutics. Available online: https://www.aobiome.com/pressreleases/aobiome-therapeutics-reports-positive-efficacy-results-from-phase-2b-clinical-trial-of-ammonia-oxidizing-bacteria-aob-for-the-treatment-of-acne-vulgaris/ (accessed on 4 January 2024).
- Arslanoglu, S.; Moro, G.E.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first years of life. J Biol Regul Homeost Agents. 2012, 26, 49–59. [Google Scholar] [PubMed]
- Grüber, C.; van Stuijvenberg, M.; Mosca, F.; Moro, G.; Chirico, G.; Braegger, C.P.; et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010, 126, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Arvola, T.; Sütas, Y.; Moilanen, E.; Salminen, S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000, 30, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Pessi, T.; Sütas, Y.; Hurme, M.; Isolauri, E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy 2000, 30, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Kalliomäki, M.; Isolauri, E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002, 109, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, V.; Benfeldt, E.; Nielsen, S.D.; Michaelsen, K.F.; Jeppesen, D.L.; Valerius, N.H.; et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003, 111, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Nermes, M.; Kantele, J.M.; Atosuo, T.J.; Salminen, S.; Isolauri, E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin Exp Allergy. 2011, 41, 370–377. [Google Scholar] [CrossRef]
- Ouwehand, A.; Lahtinen, S.; Nurminen, P. Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019. In Handbook of probiotics and prebiotics; Lee, Y.K., Salminen, S., Eds.; Wiley: Hoboken, 2009; pp. 473–477. [Google Scholar]
- Choy, C.T.; Siu, P.L.K.; Zhou, J.; Wong, C.H.; Lee, Y.W.; Chan, H.W.; et al. Improvements in Gut Microbiome Composition Predict the Clinical Efficacy of a Novel Synbiotics Formula in Children with Mild to Moderate Atopic Dermatitis. Microorganisms. 2023, 11, 2175. [Google Scholar] [CrossRef]
- Buhaș, M.C.; Candrea, R.; Gavrilaș, L.I.; Miere, D.; Tătaru, A.; Boca, A.; et al. Transforming Psoriasis Care: Probiotics and Prebiotics as Novel Therapeutic Approaches. Int J Mol Sci. 2023, 24, 11225. [Google Scholar] [CrossRef] [PubMed]
- Vijayashankar, M.; Raghunath, N. Pustular psoriasis responding to probiotics–A new insight. Our Dermatol Online 2012, 3, 326–329. [Google Scholar] [CrossRef]
- Fortuna, M.C.; Garelli, V.; Pranteda, G.; Romaniello, F.; Cardone, M.; Carlesimo, M.; et al. A case of scalp rosacea treated with low dose doxycycline and probiotic therapy and literature review on therapeutic options. Dermatol Ther. 2016, 29, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Reygagne, P.; Bastien, P.; Couavoux, M.P.; Philippe, D.; Renouf, M.; Castiel-Higounenc, I.; et al. The positive benefit of Lactobacillus paracasei NCC2461 ST11 in healthy volunteers with moderate to severe dandruff. Benef Microbes 2017, 8, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; Ter Haar Née Younes, J.A.; Heschl, A.; et al. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed Res Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J Microbiol Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Peguet-Navarro, J.; Dezutter-Dambuyant, C.; Buetler, T.; Leclaire, J.; Smola, H.; Blum, S.; et al. Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double blind, randomized placebo controlled clinical trial. Eur J Dermatol. 2008, 18, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Jaenicke, T.; Grether-Beck, S.; Le Floc'h, C.; Cheniti, A.; Piccardi, N.; et al. Prevention of polymorphic light eruption by oral administration of a nutritional supplement containing lycopene, beta-carotene, and Lactobacillus johnsonii: Results from a randomized, placebo-controlled, double-blinded study. Photodermatol Photoimmunol Photomed. 2014, 30, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients. 2021, 13, 4550. [Google Scholar] [CrossRef]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur J Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef]
- Miller, L.E.; Lehtoranta, L.; Lehtinen, M.J. The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis. Nutrients. 2017, 9, 191. [Google Scholar] [CrossRef]
- Inoue, T.; Kobayashi, Y.; Mori, N.; Sakagawa, M.; Xiao, J.Z.; Moritani, T.; et al. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018, 9, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Bouilly-Gauthier, D.; Jeannes, C.; Maubert, Y.; Duteil, L.; Queille-Roussel, C.; Piccardi, N.; et al. Clinical evidence of benefits of a dietary supplement containing probiotic and carotenoids on ultraviolet-induced skin damage. Br J Dermatol. 2010, 163, 536–543. [Google Scholar] [CrossRef]
- Kano, M.; Masuoka, N.; Kaga, C.; Sugimoto, S.; Iizuka, R.; Manabe, K.; et al. Consecutive Intake of Fermented Milk Containing Bifidobacterium breve Strain Yakult and Galacto-oligosaccharides Benefits Skin Condition in Healthy Adult Women. Biosci Microbiota Food Health. 2013, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Mihara, T.; Maruyama, K.; Saito, J.; Ikeda, M.; Tomonaga, A.; et al. Effects of intake of Lactobacillus casei subsp. casei 327 on skin conditions: A randomized, double-blind, placebo-controlled, parallel-group study in women. Biosci Microb Food Health 2017, 36, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Piyavatin, P.; Chaichalotornkul, S.; Nararatwanchai, T.; Bumrungpert, A.; Saiwichai, T. Synbiotics supplement is effective for Melasma improvement. J Cosmet Dermatol. 2021, 20, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Widhani, A.; Djauzi, S.; Suyatna, F.D.; Dewi, B.E. Changes in gut microbiota and systemic inflammation after synbiotic supplementation in patients with systemic lupus erythematosus: A randomized, double-blind, placebo-controlled trial. Cells. 2022, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br J Cancer. 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Topuz, E.; Derin, D.; Can, G.; Kürklü, E.; Cinar, S.; Aykan, F.; et al. Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Invest New Drugs. 2008, 26, 567–572. [Google Scholar] [CrossRef]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Belgioia, L.; Cante, D.; LA Porta, M.R.; Caspiani, O.; Guarnaccia, R.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients With Head and Neck Tumors: A Multicentric Randomized Study. Anticancer Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; et al. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Aruna, D.; Irukulla, M. Efficacy of Bacillus clausii UBBC—07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treat. Res. Commun. 2022, 31, 100523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Choy, C.T.; Lin, Y.; Wang, L.; Hou, J.; Tsui, J.C.C.; et al. Effect of a Novel E3 Probiotics Formula on the Gut Microbiome in Atopic Dermatitis Patients: A Pilot Study. Biomedicines. 2022, 10, 2904. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Rigoni, C.; Cantù, A.; Carnevali, A.; Filippetti, R.; Franco, T.; et al. Probiotics and Prebiotics Orally Assumed as Disease Modifiers for Stable Mild Atopic Dermatitis: An Italian Real-Life, Multicenter, Retrospective, Observational Study. Medicina (Kaunas). 2023, 59, 2080. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H. New lactic acid bacteria for skin health via oral intake of heat-killed or live cells. Anim Sci J. 2018, 89, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J Nutr Sci. 2012, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays. 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Bowe, W.; Patel, N.B.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis: From anecdote to translational medicine. Benef Microbes. 2014, 5, 185–199. [Google Scholar] [CrossRef]
- Mottin, V.H.M.; Suyenaga, E.S. An approach on the potential use of probiotics in the treatment of skin conditions: Acne and atopic dermatitis. Int J Dermatol. 2018, 57, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, A.; Mozafarpoor, S.; Bodaghabadi, M.; Mohamadi, M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatol Ther. 2020, 33, e13279. [Google Scholar] [CrossRef] [PubMed]
- Tolino, E.; Skroza, N.; Mambrin, A.; Bernardini, N.; Zuber, S.; Balduzzi, V.; et al. Novel combination for the treatment of acne differentiated based on gender: A new step towards personalized treatment. G. Ital. Dermatol. Venereol. 2018, 153, 866–871. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef]
- Lebeer, S.; Oerlemans, E.; Claes, I.; Wuyts, S.; Henkens, T.; Spacova, I.; et al. Topical cream with live lactobacilli modulates the skin microbiome and reduce acne symptoms. bioRxiv 2018, 463307. [Google Scholar] [CrossRef]
- Di Marzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of Skin-Ceramide Levels in Aged Subjects following a Short-Term Topical Application of Bacterial Sphingomyelinase from Streptococcus Thermophilus. Int J Immunopathol Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.H.; Ko, Y.; Sim, J.H.; Kim, K.S.; Lee, S.H.; et al. Effect of bacteriocin produced by Lactococcus sp. HY 449 on skin-inflammatory bacteria. Food Chem Toxicol. 2006, 44, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev 2013, 3, CD006474. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.H.; Kim, I.S.; Yu, D.Y.; Kim, G.I.; Moon, Y.S.; et al. Galectin-9 Induced by Dietary Prebiotics Regulates Immunomodulation to Reduce Atopic Dermatitis Symptoms in 1-Chloro-2,4-Dinitrobenzene (DNCB)-Treated NC/Nga Mice. J Microbiol Biotechnol. 2020, 30, 1343–1354. [Google Scholar] [CrossRef]
- Fanfaret, I.S.; Boda, D.; Ion, L.M.; Hosseyni, D.; Leru, P.; Ali, S.; et al. Probiotics and prebiotics in atopic dermatitis: Pros and cons (Review). Exp Ther Med. 2021, 22, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.E.; Yoon, Y.S.; Kim, T.; Seo, J.G.; Chung, M.J.; et al. Improvement of atopic dermatitis-like skin lesions by IL-4 inhibition of P14 protein isolated from Lactobacillus casei in NC/Nga mice. Appl. Microbiol. Biotechnol. 2015, 99, 7089–7099. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.E.; Yoon, Y.S.; Seo, J.G.; Chung, M.J.; Yum, D.Y. A probiotic preparation alleviates atopic dermatitis-like skin lesions in murine models. Toxicol Res. 2016, 32, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Hong, R.; Choi, E.Y.; Yu, K.; Kim, N.; Hyeon, J.Y.; et al. A Probiotic Mixture Regulates T Cell Balance and Reduces Atopic Dermatitis Symptoms in Mice. Front Microbiol. 2018, 9, 2414. [Google Scholar] [CrossRef]
- Kim, W.K.; Jang, Y.J.; Han, D.H.; Jeon, K.; Lee, C.; Han, H.S.; et al. Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes. 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- D’Elios, S.; Trambusti, I.; Verduci, E.; Ferrante, G.; Rosati, S.; Marseglia, G.L.; et al. Probiotics in the prevention and treatment of atopic dermatitis. Pediatr Allergy Immunol. 2020, 31, 43–45. [Google Scholar] [CrossRef]
- Avershina, E.; Cabrera Rubio, R.; Lundgård, K.; Perez Martinez, G.; Collado, M.C.; et al. Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J Allergy Clin Immunol. 2017, 139, 1399–1402.e8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wu, W.F.; Hung, C.W.; Ku, M.S.; Liao, P.F.; Sun, H.L.; et al. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect. 2017, 50, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.C.; Hung, C.H.; Sy, L.B.; Lue, K.H.; Shih, I.H.; Yang, C.Y.; et al. A randomized, double-blind, placebo-controlled trial assessing the oral administration of a heat-treated Lactobacillus paracasei supplement in infants with atopic dermatitis receiving topical corticosteroid therapy. Skin Pharmacol Physiol. 2019, 32, 201–211. [Google Scholar] [CrossRef]
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015, 15, 13. [Google Scholar] [CrossRef]
- Wang, I.J.; Wang, J.Y. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin Exp Allergy. 2015, 45, 779–787. [Google Scholar] [CrossRef]
- Schmidt, R.M.; Pilmann Laursen, R.; Bruun, S.; Larnkjaer, A.; Mølgaard, C.; Michaelsen, K.F.; et al. Probiotics in late infancy reduce the incidence of eczema: A randomized controlled trial. Pediatr Allergy Immunol. 2019, 30, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; et al. Effect of oral administration of a mixture of probiotic strains on SCORAD Index and use of topical steroids in young patients with moderate atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Brozek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Terracciano, L.; et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, Z.; Niu, X.; Zhang, G.; Jia, Y.; Zhang, S.; et al. Probiotic supplementation for prevention of atopic dermatitis in infants and children: A systematic review and meta-analysis. Am J Clin Dermatol. 2019, 20, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef]
- Chen, L.; Ni, Y.; Wu, X.; Chen, G. Probiotics for the prevention of atopic dermatitis in infants from different geographic regions: A systematic review and Meta-analysis. J Dermatolog Treat. 2022, 33, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Iwasa, M.; Han, K.I.; Kim, W.J.; Tang, Y.; Hwang, Y.J.; et al. Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model. Nutrients. 2016, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.H.; Li, H.Y.; Huang, C.H.; Lee, B.W.; Lee, Y.K.; Chua, K.Y. The effects of heat-killed wild-type Lactobacillus casei Shirota on allergic immune responses in an allergy mouse model. Int. Arch. Allergy Immunol. 2009, 148, 297–304. [Google Scholar] [CrossRef]
- Fölster-Holst, R. Probiotics in the treatment and prevention of atopic dermatitis. Ann Nutr Metab. 2010, 57, 16–19. [Google Scholar] [CrossRef]
- Blanchet-Réthoré, S.; Bourdès, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jaros, J.; Shi, V.Y. Staphylococcus aureus in atopic dermatitis: Past, present, and future. Dermatitis. 2020, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Butler, É.; Lundqvist, C.; Axelsson, J. Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis. Microorganisms. 2020, 8, 1026. [Google Scholar] [CrossRef]
- Guéniche, A.; Cathelineau, A.C.; Bastien, P.; Esdaile, J.; Martin, R.; Queille Roussel, C.; et al. Vitreoscilla filiformis biomass improves seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2008, 22, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Volz, T.; Skabytska, Y.; Guenova, E.; Chen, K.M.; Frick, J.S.; Kirschning, C.J.; et al. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol. 2014, 134, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Hata, T.R.; Tong, Y.; Cheng, J.Y.; Shafiq, F.; Butcher, A.M.; et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021, 27, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Lio, P.A.; Simpson, E.L.; Li, C.; Brownell, D.R.; Gryllos, I.; et al. Efficacy and safety of topically applied therapeutic ammonia oxidising bacteria in adults with mild-to-moderate atopic dermatitis and moderate-to-severe pruritus: A randomised, double-blind, placebo-controlled, dose-ranging, phase 2b trial. EClinicalMedicine. 2023, 60, 102002. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Castillo, C.R.; Barbian, K.D.; Kanakabandi, K.; Virtaneva, K.; Fitzmeyer, E.; et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020, 12. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, C.S.; Chao, Y.H.; Lin, C.C.; Tsai, H.Y.; Li, Y.R.; et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017, 25, 559–566. [Google Scholar] [CrossRef]
- Elewski, B.E.; Draelos, Z.; Dréno, B.; Jansen, T.; Layton, A.; Picardo, M. Rosacea-global diversity and optimized outcome: Proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011, 25, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Truglio, M.; Sivori, F.; Cavallo, I.; Abril, E.; Licursi, V.; et al. Probiotic-enriched oily suspension in modulating skin microbiome and treating seborrheic dermatitis. Research Square.com 2023. [Google Scholar] [CrossRef]
- Lolou, V.; Panayiotidis, M.I. Functional role of probiotics and prebiotics on skin health and disease. Fermentation. 2019, 5, 41. [Google Scholar] [CrossRef]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair-Modulation of Microbial Colonization and Biofilm Formation. Int J Mol Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed]
- Tagliari, E.; Campos, L.F.; Campos, A.C.; Costa-Casagrande, T.A.; Noronha, L. Effect of probiotic oral administration on skin wound healing in rats. Arq Bras Cir Dig. 2019, 32, e1457. [Google Scholar] [CrossRef] [PubMed]
- Togo, C.; Zidorio, A.P.; Gonçalves, V.; Botelho, P.; de Carvalho, K.; Dutra, E. Does Probiotic Consumption Enhance Wound Healing? A Systematic Review. Nutrients. 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Tembhre, M.K.; Chawla, M.K.; Berthiaume, F.; Kumar, S. Relationship Between Probiotics and Gut-Skin Axis in Skin Wound Healing: A Recent Update. In Probiotic Research in Therapeutics: Volume 3: Probiotics and Gut Skin Axis–Inside Out and Outside In; Kaur, I.P., Beri, K., Kaur Deol, P.K., Sandhu, S.K., Eds.; Springer: Singapore, 2022; pp. 173–196-109. [Google Scholar] [CrossRef]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The concurrent use of probiotic microorganism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Karska-Wysocki, B.; Bazo, M.; Smoragiewicz, W. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol Res. 2010, 165, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Prince, T.; Mcbain, A.J.; O’Neill, C.A. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl Environ Microbiol. 2012, 78, 5119–5126. [Google Scholar] [CrossRef]
- Jones, M.; Ganopolsky, J.G.; Labbe, A.; Gilardino, M.; Wahl, C.; Martoni, C.; et al. Novel nitric oxide producing probiotic wound healing patch: Preparation and in vivo analysis in a New Zealand whiterabbit model of ischaemic and infected wounds. Int Wound J. 2012, 9, 330–343. [Google Scholar] [CrossRef]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.M. Wound healing and microbiome, an unexpected relationship. J Eur Acad Dermatol Venereol. 2023, 37, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Liboutet, M.; Cheilian, S.; Fagot, D.; Juchaux, F.; Breton, L. Vitreoscilla filiformis extract for topical skin care: A review. Front Cell Infect Microbiol. 2021, 11, 747663. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, C.A.; Biswas, B. A review of topical phage therapy for chronically infected wounds and preparations for a randomized adaptive clinical trial evaluating topical phage therapy in chronically infected diabetic foot ulcers. Antibiotics 2020, 9, 377. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Perez Esteban, P.; Arnot, T.C.; Harper, D.R.; et al. Combined use of bacteriophage K and a novel bacterio- phage to reduce Staphylococcus aureus biofilm formation. Appl Environ Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Kiousi, D.E.; Karapetsas, A.; Karolidou, K.; Panayiotidis, M.I.; Pappa, A.; Galanis, A. Probiotics in Extraintestinal Diseases: Current Trends and New Directions. Nutrients. 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Lahtinen, S.; Tiihonen, K. The potential of probiotics and prebiotics for skin health. In Textbook of Aging Skin; M.A. Farage, M., Miller, K.W., Maibact, H.I., Eds.; Springer-Verlag: Berlin, Heidelberg, 2015; pp. 799–809. [Google Scholar] [CrossRef]
- Guéniche, A.; Benyacoub, J.; Buetler, T.M.; Smola, H.; Blum, S. Supplementation with oral probiotic bacteria maintains cutaneous immune homeostasis after UV exposure. Eur J Dermatol. 2006, 16, 511–517. [Google Scholar] [CrossRef] [PubMed]
- You, G.E.; Jung, B.J.; Kim, H.; Kim, H.G.; Kim, T.R.; Chung, D.K. Lactobacillus sakeilipoteichoic acid inhibits MMP-1 induced by UVA in normal dermal fibroblasts of human. J Microbiol Biotechnol. 2013, 23, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J Microbiol Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Weill, F.S.; Cela, E.M.; Paz, M.L.; Ferrari, A.; Leoni, J.; Gonzalez Magilo, D.H. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br J Nutr. 2013, 109, 457–466. [Google Scholar] [CrossRef]
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Protective effects of tyndallized Lactobacillus acidophilus IDCC 3302 against UVB-induced photodamage to epidermal keratinocytes cells. Int J Mol Med. 2019, 43, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; et al. Antiwrinkle and Antimelanogenesis Effects of Tyndallized Lactobacillus acidophilus KCCM12625P. Int J Mol Sci. 2020, 21, 1620. [Google Scholar] [CrossRef]
- Sugimoto, S.; Ishii, Y.; Izawa, N.; Masuoka, N.; Kano, M.; Sone, T.; et al. Photoprotective effects of Bifidobacterium breve supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Photodermatol Photoimmunol Photomed. 2012, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; et al. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benefic Microbes. 2015, 6, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Cheng, L.H.; Liu, Y.W.; Jeng, O.J.; Lee, Y.K. Gerobiotics: Probiotics targeting fundamental aging processes. Biosci Microbiota Food Health. 2021, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The Role of Probiotics in Skin Photoaging and Related Mechanisms: A Review. Clin Cosmet Investig Dermatol. 2022, 15, 2455–2464. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.; Kim, W.J.; Chung, D.K. Effect of paraprobiotic prepared from Kimchi-derived Lactobacillus plantarum K8 on skin moisturizing activity in human keratinocyte. J Func Foods. 2020, 75, 104244. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Xie, H.; Jian, D.; Xu, S.; Peng, Q.; et al. Claudin reduction may relate to an impaired skin barrier in rosacea. J Dermatol. 2019, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Benyacoub, J.; Philippe, D.; Bastien, P.; Kusy, N.; Breton, L.; et al. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur J Dermatol 2010, 20, 731–737. [Google Scholar]
- Mirfeizi, Z.; Mahmoudi, M.; Faridzadeh, A. Probiotics as a complementary treatment in systemic lupus erythematosus: A systematic review. Health Sci Rep. 2023, 6, e1640. [Google Scholar] [CrossRef]
- Esmaeili, S.A.; Mahmoudi, M.; Rezaieyazdi, Z.; Sahebari, M.; Tabasi, N.; Sahebkar, A.; et al. Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J Cell Biochem. 2018, 119, 7865–7872. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.A.; Taheri, R.A.; Mahmoudi, M.; Momtazi-Borojeni, A.A.; Morshedi, M.; Bahramifar, A.; et al. Inhibitory effects of tolerogenic probiotics on migratory potential of lupus patient-derived DCs. Iran J Basic Med Sci. 2021, 24, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, Z.; Samadi, M.; Mahmoudi, M.; RezaieYazdi, Z.; Sahebari, M.; Tabasi, N.; et al. Lactobacillus rhamnosus and Lactobacillus delbrueckii ameliorate the expression of miR-155 and miR-181a in SLE patients. J Funct Foods. 2018, 48, 228–233. [Google Scholar] [CrossRef]
- Khorasani, S.; Mahmoudi, M.; Kalantari, M.R.; Lavi Arab, F.; Esmaeili, S.A; Mardani, F.; et al. Amelioration of regulatory T cells by Lactobacillus delbrueckii and Lactobacillus rhamnosus in pristane-induced lupus mice model. J Cell Physiol. 2019, 234, 9778–9786. [Google Scholar] [CrossRef]
- Cabana-Puig, X.; Mu, Q.; Lu, R.; Swartwout, B.; Abdelhamid, L.; Zhu, J.; et al. Lactobacillus spp. act in synergy to attenuate splenomegaly and lymphadenopathy in lupus-prone MRL/lpr mice. Front Immunol. 2022, 13, 923754. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yao, P.; Wang, H.; Yuan, Q.; Wang, X.; Feng, W.; et al. Effects of Lactobacillus plantarum HFY15 on Lupus nephritis in mice by regulation of the TGF-beta 1 signaling pathway. DDDT. 2022, 16, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gao, M.; Zhao, C.; Yang, J.; Gao, H.; Lu, X.; et al. Oral Administration of Probiotics Reduces Chemotherapy-Induced Diarrhea and Oral Mucositis: A Systematic Review and Meta-Analysis. Front Nutr. 2022, 9, 823288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wu, C.R.; Huang, T.W. Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2022, 23, 13268. [Google Scholar] [CrossRef] [PubMed]
- Limaye, S.A.; Haddad, R.I.; Cilli, F.; Sonis, S.T.; Colevas, A.D.; Brennan, M.T.; et al. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer. 2013, 119, 4268–4276. [Google Scholar] [CrossRef]
- Trone, K.; Rahman, S.; Green, C.H.; Venegas, C.; Martindale, R.; Stroud, A. Synbiotics and Surgery: Can Prebiotics and Probiotics Affect Inflammatory Surgical Outcomes? Curr Nutr Rep. 2023, 12, 238–246. [Google Scholar] [CrossRef]
- Khalfallah, G.; Gartzen, R.; Möller, M.; Heine, E.; Lütticken, R. A New Approach to Harness Probiotics Against Common Bacterial Skin Pathogens: Towards Living Antimicrobials. Probiotics Antimicrob Proteins. 2021, 13, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Bindurani, S. Probiotics in dermatology. J. Skin Sex. Transmitted Dis. 2019, 1, 66–71. [Google Scholar]
- Jordan, D.; Andreas, P.; Brad, B.; Sabine, H. Impact of probiotics on gut microbiome Bifidobacterium relative abundance: First do no harm. J. Clin. Trials. 2021, 11, 1–4. [Google Scholar]
- Al-Hazmi, N.E.; Naguib, D.M. Antioxidant and Antibacterial Activities of Nano-probiotics Versus Free Probiotics Against Gastrointestinal Pathogenic Bacteria. Indian J Microbiol. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).