Introduction
Infertility is defined by the World Health Organization (WHO) as a disease of the male or the female reproductive system characterized by the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse. Globally, it affects approximately 70 million people and it is estimated that 50% of all infertility cases result from problems with the male reproductive system. Numerous factors, such as genetic mutations or comorbidities, influence male fertility, with studies also pointing to lifestyle choices as causes of correct or incorrect results of male fertility tests [
1].
Sperm quality analysis is regarded as the basic method of evaluating male fertility [
2]. It comprises quantitative and qualitative measurements in relation to reference ranges [
3]. According to the latest WHO standards, semen volume should be at least 1.4 ml, while the optimal total sperm number should be 39x10
6 per ejaculate. In the case of the other parameters, the norms are expressed in percentages, i.e., the optimal total motility should be 42%, progressive motility – 30%, while normal forms (also known as morphology) – 4% [
4]. In terms of lifestyle, the aforementioned parameters are influenced by a number of factors such as BMI, diet, physical activity, sleep, using drugs such as alcohol or cigarettes, or sexual abstinence. External factors such as environmental pollution, elevated temperature in the testicular area, or radiation [
5].
Due to the fact that, as an element of semen quality analysis, motility is a very important parameter [
1] and considering its association with insulin resistance, it was adopted in the study as the indictor of male fertility. Studies performed on isolated organs show that human sperm travel approx. 19 cm before they meet an oocyte, undergoing several biochemical and physiological changes on the way [
6]. According to the WHO, men whose motility scores are below 40%, or whose progressive motility is lower than 32%, suffer from asthenozoospermia, i.e., sperm motility disorders [
7]. The global trends consisting in progressively worsening numbers and motility of healthy sperm in the ejaculate are associated with an increased risk of infertility [
1]. Studies show that total progressively motile sperm count (TPMSC) may be used as a predictor of positive pregnancy test result in non-donor IUI cycles; in addition, if the level of initial sperm motility is lowered, the process of semen preparation used in IUI, which significantly improves sperm motility, does not guarantee a positive pregnancy outcome [
8,
9]. As far as the relationship between motility and insulin resistance is concerned, glucose metabolism plays an important role in spermatogenesis. Basic cell activities and their specific characteristics such as motility and the activity of a mature sperm that leads to fertilization are maintained by glucose metabolism, with insulin resistance disturbing the process [
10].
Insulin resistance is a condition in which the insulin circulating in the blood is unable to properly stimulate glucose uptake and/or use by insulin-sensitive organs and tissues. In a healthy body, pancreatic β-cells respond to increased blood glucose levels by producing insulin and inhibiting glucose production in the liver. In insulin resistance, not all signals are read correctly and thus the body increases the production both of insulin in the pancreas and glucose in the liver [
6].
Regulation of glucose levels is not the only function of insulin as it also plays an important role in the regulation of lipid metabolism. Increased lipogenesis in the liver is observed in the case of insulin resistance, which ultimately results in non-alcoholic fatty liver disease involving the accumulation of fat in the liver [
6,
7]. Fatty growth of internal organs and the subsequent β-cell hyperplasia leads to abnormal insulin secretion, exacerbating insulin resistance, with the process repeating in a circular pattern [
11].
The HOMA-IR index, i.e., the homeostasis model of assessment of insulin resistance, is the most frequently used index for measuring insulin resistance. It is calculated on the basis of fasting insulin and glucose levels and interpreted in the following manner: if the score is >1, it is considered abnormal, if the score is >2, it indicates insulin resistance [
12]. In addition, one of the best-known indicators determining the opposite relationship, i.e., tissue insulin sensitivity, is the Matsuda index, calculated on the basis of plasma glucose concentration and insulin concentration obtained from fasting blood samples after oral consumption of 75 g of glucose during the OGTT, i.e., oral glucose tolerance test [
13].
Although insulin resistance is more commonly diagnosed in women, some studies suggest that men in fact have lower peripheral insulin sensitivity than women [
14,
15]. This may result from differences in the levels of sex hormones in the blood, as well as the distribution of adipose tissue, which as far as insulin resistance is concerned is less favorable in men. The results of a study performed by Li et al. demonstrated a greater insulin resistance of adipose tissue in men compared to women with similar BMI levels. Additionally, testosterone levels were inversely correlated with the level of insulin resistance of adipose tissue, i.e., among men, the higher the testosterone level, the lower the level of insulin resistance; among women the relationship was reversed [
16]. Some studies also suggest a protective role of estrogens in the context of insulin resistance; however, most of them have so far been conducted only on animal models [
17].
Insulin resistance in men is often associated with obesity and the metabolic syndrome. These conditions are associated with a deterioration in fertility, in addition to decreased levels of general health. The impact of insulin resistance on male fertility specifically has not been widely studied; nevertheless, indirect associations can be found suggesting that it affects the quality of semen and the levels of sex hormones in men [
18]. Among the studied relationships between male fertility and semen quality, the impact of insulin resistance on the concentration and production of testosterone [
19,
20,
21], the reduction in semen volume, or the percentage of progressive sperm [
19] can be mentioned, among others.
An indirect relationship between insulin resistance and sperm quality can be further linked to lifestyle due to the fact that many of its components influence both insulin resistance and male fertility. These include diet, physical activity, sleep, stress, environmental pollution, and the duration of sexual abstinence [
18]. Generally speaking, sperm quality is lower nowadays compared to past decades, which suggests that the problem is a result of various civilization factors [
22], thus making it necessary to study their influence on fertility, including the modern lifestyle.
A modifiable element of the modern lifestyle that has been changing in previous decades is diet. The popular Western-style diet, low in vitamins and high in highly processed products, has been shown to pose numerous health risks [
23], whereas the most effective diets in terms of the treatment and prevention of various conditions, including insulin resistance appear to be the Mediterranean and the DASH diets. Both are based on antioxidant ingredients, i.e., vitamins and minerals, which reduce inflammation in the body [
23,
24,
25]. It has been proven that the diets in question have a positive effect on semen quality, improving parameters such as the total sperm count, its concentration, and the number of progressive sperms [
26,
27,
28].
Another factor that has an impact on male fertility are vitamins. Regardless of whether men adhere to a special diet or not, antioxidant vitamins appear to be extremely important as far as the improvement of semen parameters is concerned, motility in particular. Vitamin E acts as an antioxidant that breaks the chains of peroxide radicals, while vitamin C prevents lipid peroxidation, thus reducing the amount of free oxygen radicals [
29].
Research focused on moderate physical activity confirms its positive effect on both tissue insulin sensitivity [
30] and the improvement of semen quality [
31]. However, high-intensity physical activity may lead to abnormalities. A meta-analysis performed by Ibanez-Perez et al. showed that too intense training may lead to a decreased progressive movement of sperm, as well as reduced parameters such as sperm concentration, volume, and morphology [
32].
Stress is another important factor in the context of male fertility. Physiological stress triggers a cascade of free radicals and pro-inflammatory cytokines that have the ability to damage sperm. It is also a factor that plays a role in insulin resistance as the condition is intensified by it, i.e., insulin resistance can be induced by oxidative stress via the interference with insulin signal transmission and deregulation of adipokines, as well as inflammation – occurring in the course of both insulin resistance and oxidative stress [
33].
As far as diagnostic aspects are concerned, research on semen quality shows that the duration of sexual abstinence needs to be factored in in order to obtain reliable fertility results, i.e., it should range from approx. 2 to 7 days prior to the test [
34]. The most beneficial duration of sexual abstinence varies for individual semen parameters, with some of them improving and others weakening depending on its length. Sperm motility, its morphology, and the percentage of DNA fragmentation tend to improve during a shorter period of abstinence, i.e., lasting up to 4 days, while volume and the total sperm count reach the highest values after the 5th day of sexual abstinence, increasing in a directly proportional manner [
35,
36].
Due to the considerable number of literature reports linking the factors described above with male fertility, the aim of the study was to experimentally confirm or disprove the relationship between lifestyle factors such as physical activity, diet, perceived stress, sleep quality, and the duration of sexual abstinence with semen quality in the context of insulin resistance. A further aim was to select those lifestyle factors that would make it possible to predict the level of male fertility, especially when insulin resistance is concerned.
Materials and Methods
The clinical-control study was conducted in 73 men with and without fertility impairment, divided according to the presence or absence of insulin resistance. The participants were patients of the KRIOBANK Infertility Treatment Clinic and the Department of Endocrinology, Diabetology and Internal Medicine of the Medical University of Bialystok. All participants were obliged to complete their personal 3-day food diaries.
The study focused on the following lifestyle factors: physical activity, diet, perceived stress, sleep quality, libido level, and the duration of sexual abstinence. Additionally, in order to make it possible to quantitatively assess and compare the quality of the participant’s lifestyles with other parameters, original indices were created based on the recommendations proposed by the World Health Organization and the Polish Institute of Food and Nutrition [
37]. This solution made it possible to determine the subjects’ adherence to healthy eating habits and the appropriate level of physical activity for healthy men. The following indices were used:
- 1)
Vitamin Consumption Index (VCI) (0-10 pts) – each vitamin consumed in amounts specified in the recommendations was awarded 1 pt. The following ten vitamins were considered in the analysis: vitamin E, vitamin A, thiamine, riboflavin, niacin, vitamin B6, vitamin C, folic acid, vitamin D, and vitamin B12;
- 2)
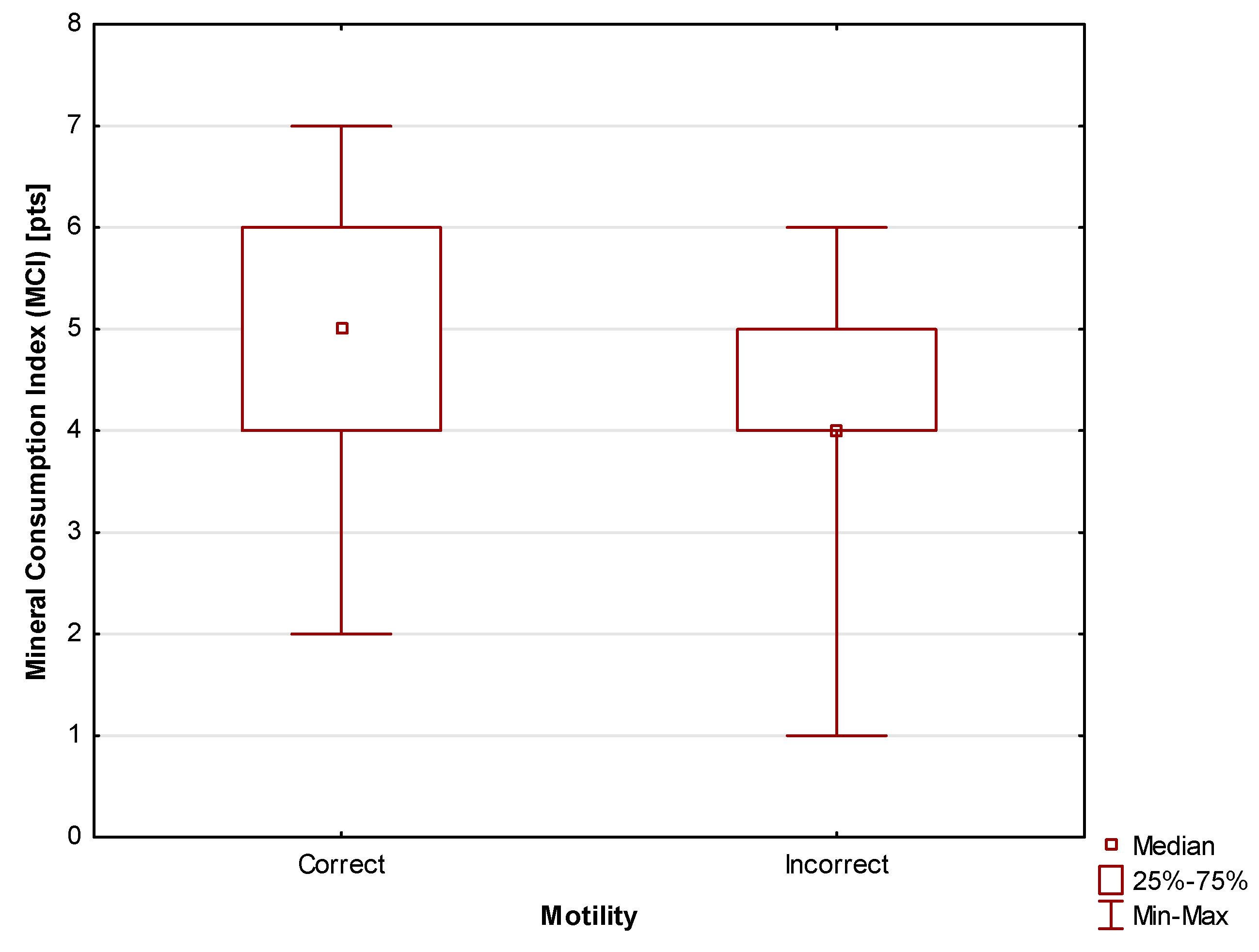
Mineral Consumption Index (MCI) (0-9 pts) – each mineral consumed in amounts specified in the recommendations was awarded 1 pt. The following nine minerals were considered in the analysis: sodium, potassium, magnesium, phosphorus, calcium, iron, zinc, copper, and manganese;
- 3)
Macronutrient Consumption Index (MacCI) (0-5 pts) – each of the four macronutrients consumed in amounts specified in the recommendations was awarded 1 pt.; 1 pt. was also awarded for the appropriate caloric intake. The following five parameters were considered in the analysis: fat, carbohydrate, fiber, dietary fiber, and caloric intake;
- 4)
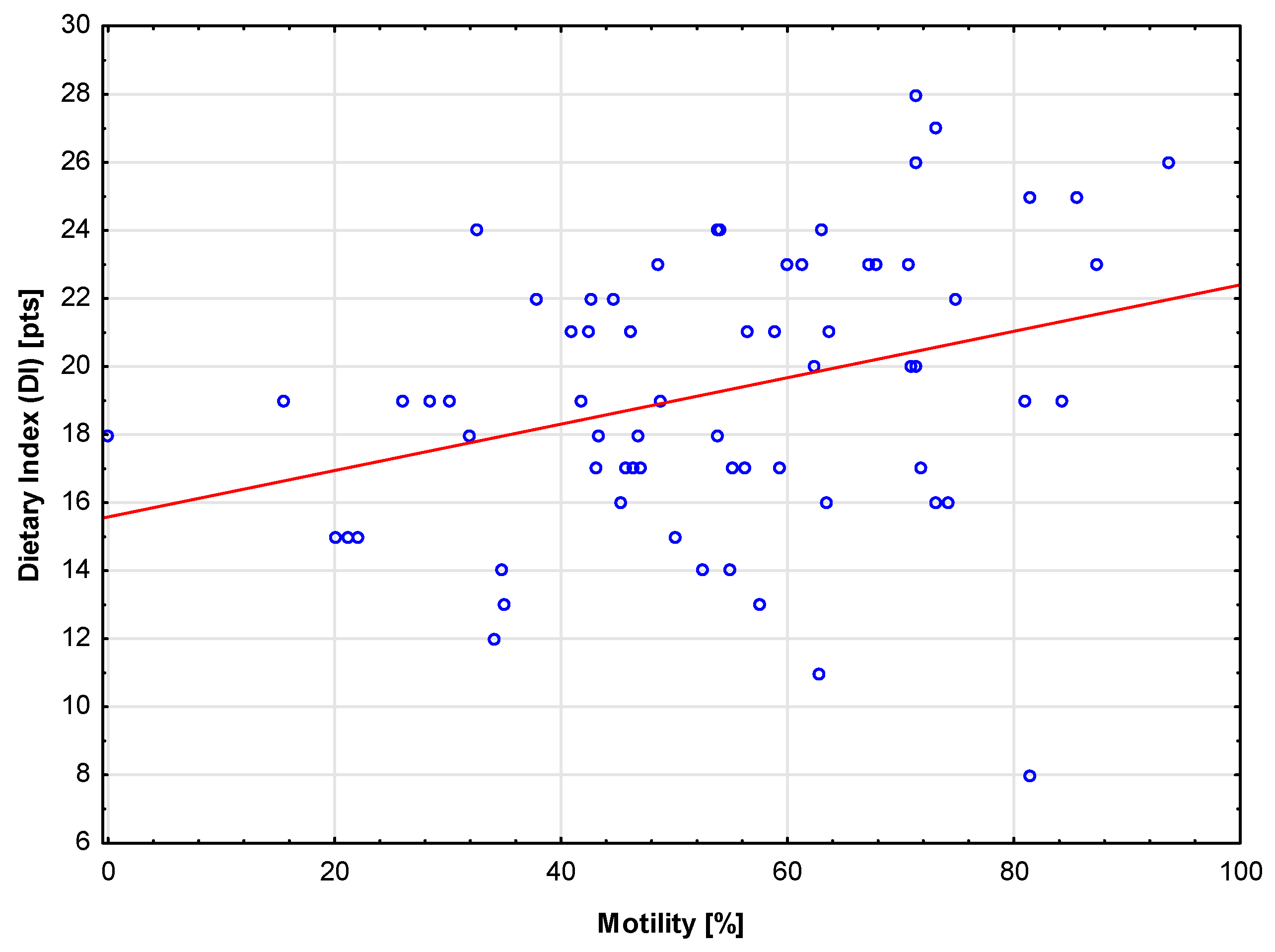
Dietary Index (DI) (0-24 pts) – the index is calculated as VCI + MCI + MacCI;
- 5)
Body Composition Index (BCI) (0-3 pts) – each instance of a body composition norm met by the participant was awarded 1 pt. The following three macronutrients were considered in the analysis: Total Body Water (TBW), Body Fat Mass (BFM), and Skeletal Muscle Index (SMI).
The indices described above, related to the consumption of nutrients, were calculated on the basis of data supplied by the participants in their 3-day food diaries referred to norms and recommendations for the general population. BCI was determined by performing body composition analyses and comparing the results with the applicable norms [
37].
Furthermore, anthropometric measurements of height, body weight, and waist and hip circumferences were taken. In addition, tests of fasting glucose and insulin concentrations at 30, 60, 90, and 120 minutes of the oral glucose tolerance test performed. Based on the glycemia and insulinemia values, the following indicators of insulin resistance were calculated: the HOMA1-IR and the Matsuda index (insulin sensitivity).
Semen quality tests were performed and analyzed, with the following five basic parameters taken into account: volume per milliliter, volume per ejaculate, morphology, motility, and progressive motility. Due to its association with carbohydrate metabolism and because incorrect vales of the parameter signify problems with fertility, special attention was paid to motility.
Due to the lack of normality of the distribution of the analyzed numerical parameters, non-parametric tests were used. The Spearman’s rank-order correlation coefficient was also determined in order to determine the relationship between two numerical or ordinal variables. The non-parametric Mann-Whitney U test was used for comparisons between two independent groups. Pearson’s Chi2 test was used to test the relationships between two nominal variables. Univariate and multivariate logistic regression analysis was conducted for the dependent variable describing correct sperm motility. Results at p<0.05 were considered as statistically significant. The results were prepared in Statistica 13.3 and Stata 18.0 software.
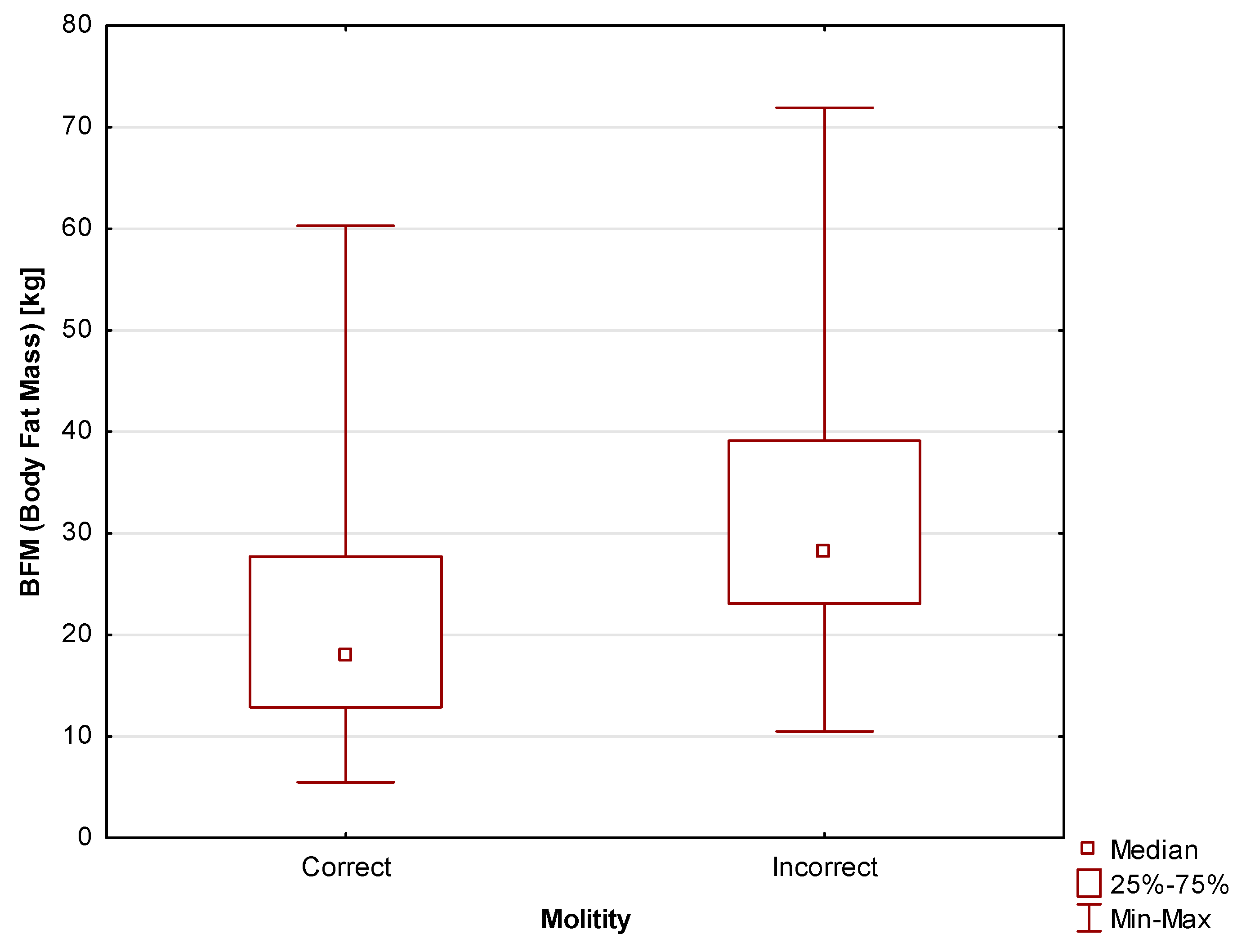
Body Composition
In terms of body composition, it was found that two important parameters, i.e., Body Fat Mass (BFM) and Body Composition Index (BCI), correlated with motility, indicating better scores in the case of men with a healthier body composition. Body Fat Mass (BFM) differed between participants with correct and incorrect motility scores at a level of
statistical significance of p=0.006. The median for the study participants with correct motility scores was Me=17.9 kg (Q
1=12.9 kg; Q
3=27.7 kg), while for those with lower motility scores the median was Me=28.2 kg (Q
1=23.1 kg; Q
3=39.1 kg). The differences are shown in
Figure 4.
Similarly to the case of BFM, a
statistically significant (p=0.04) relationship was found between body composition (BCI) and motility. A clear tendency for motility to improve together with increasing BCI was observed. At a low BCI value (BCI=0 pts), participants with correct and incorrect sperm motility comprised 50% of the total number each. As BCI increased, the proportion of participants with correct motility increased to 75% for BCI=1 pt. Among those men for whom almost all of the body composition parameters were correct (BCI=2 pts), participants with correct motility comprised 85.71%. As far as the participants whose body composition was completely healthy, the proportion of those with correct motility was 91.67%. The results are presented in
Table 1.
Insulin Resistance
The results presented above, which consistently show a relationship between motility and the indices connected with micronutrients intake and body composition are further expanded with those results of the study that are related to insulin resistance.
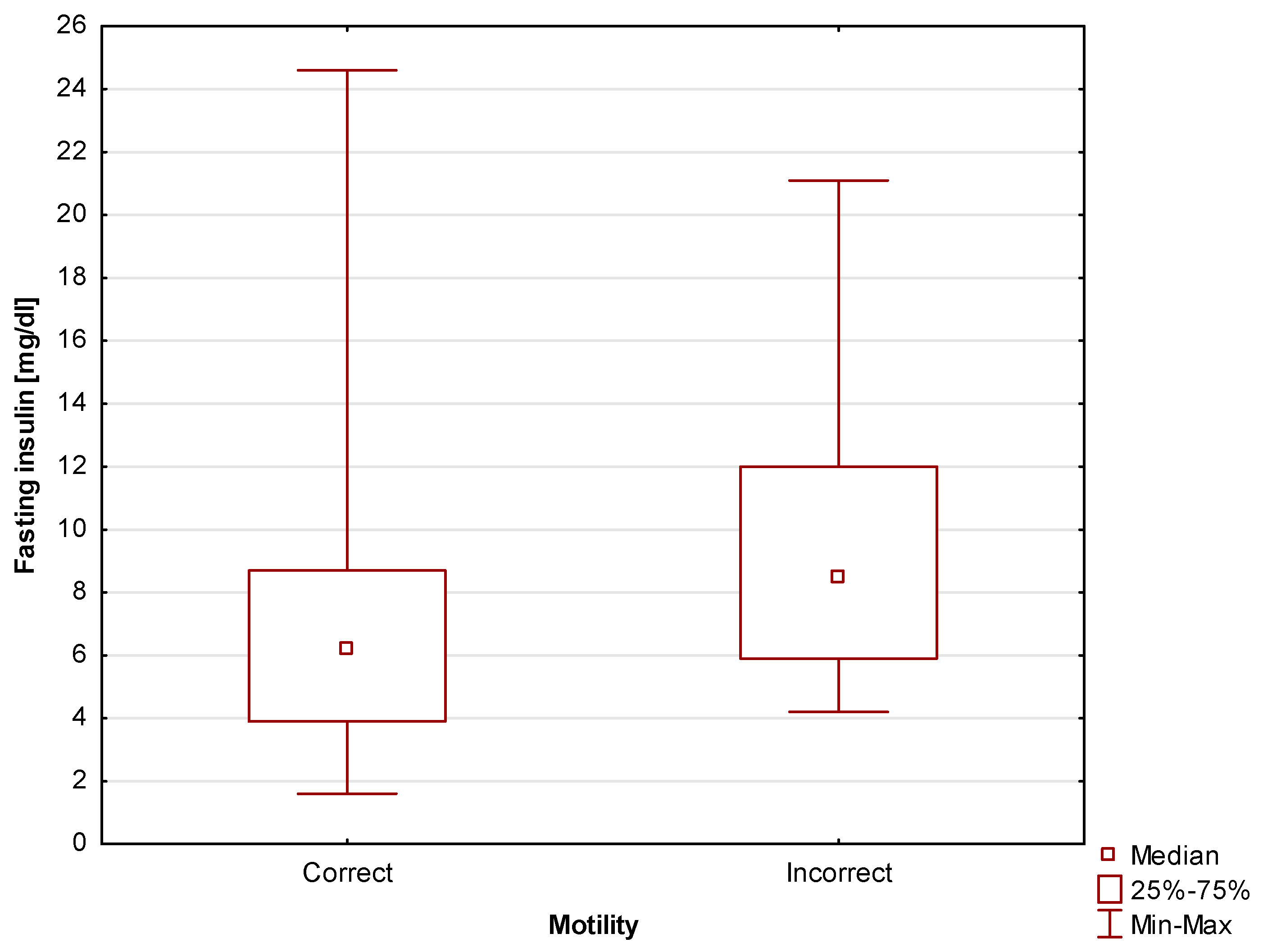
First of all,
statistically significant (p=0.03)
differences in fasting insulin level between men with correct and incorrect motility scores were found. For study participants with correct motility scores the median was Me=6.2 mg/dl (Q
1=3.9 mg/dl; Q
3=8.7 mg/dl), while for those with incorrect motility scores, the median was Me=8.5 mg/dl (Q
1=5.9 mg/dl; Q
3=12 mg/dl). Due to the fact that the study also involved calculating the HOMA-IR index, fasting insulin level can be seen as an intermediate parameter. Nevertheless, the fact that taken in isolation this basic parameter also shows a relationship with motility scores is also worth emphasizing. The differences are shown in
Figure 5.
As could already be suspected from the fasting insulin level scores, a
statistically significant (p=0.04) relationship was found between the presence of insulin resistance (measured using the HOMA-IR index) and sperm motility. Correct motility scores were observed in the vast majority (82.98%) of non-insulin-resistant participants, while in the group of insulin resistant participants, correct sperm motility was observed in only 61.54%. These results indicate a clear connection between these two parameters. The results are shown in
Table 2.
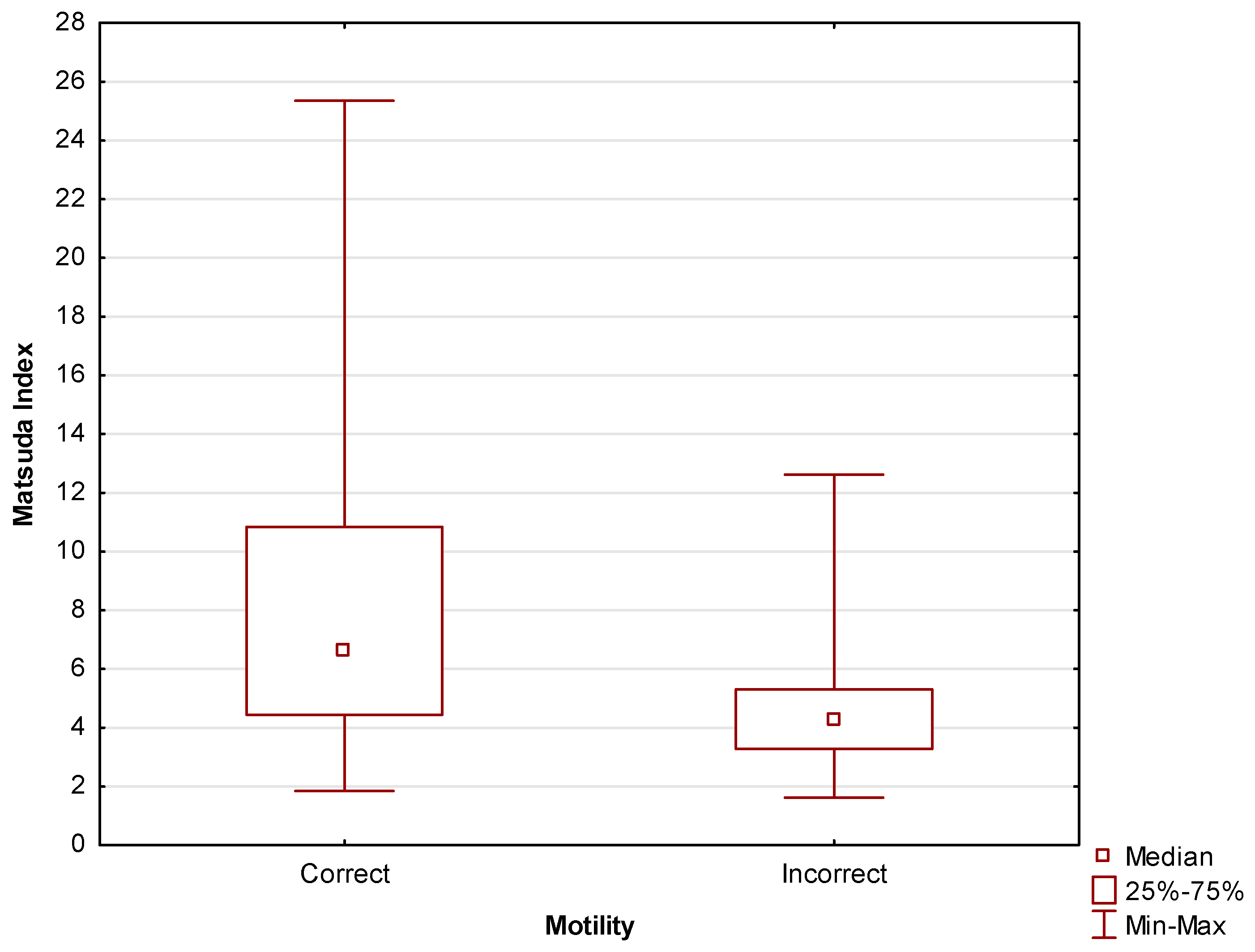
The relationship between insulin resistance and motility discussed above is further corroborated by Matsuda index scores. A relationship between insulin resistance measured using the Matsuda index and motility at a level of
statistical significance of p=0.03. Among non-insulin-resistant participants, those with correct motility comprised as much as 89.29% of the total number of participants. Among the patients diagnosed with insulin resistance, correct motility was observed in only 66.67% of patients. The results of tests are shown in
Table 3.
Figure 6 shows the same relationship, with the Matsuda index values expressed numerically.
Statistically significant (p=0.004)
differences in Matsuda index values were found between men with correct and incorrect motility scores. For the former, the median was Me=6.6 (Q
1=4.4; Q
3=10.8), for the latter, the median was Me=4.2 (Q
1=3.3; Q
3=5.3).
Due to the fact that it is based on insulin sensitivity, Matsuda is a more sensitive index than HOMA-IR, which is reflected in the greater number of patients that are diagnosed with insulin resistance when the former is used.
The results presented above clearly show the existence of a relationship between infertility, determined based on sperm motility; insulin resistance, measured using HOMA-IR and Matsuda indices; and dietary indices. The results show that worse motility scores were observed in cases of micronutrient deficiencies and unhealthy body composition, associating these with the presence of insulin resistance.
Sexual Abstinence
Sexual abstinence is another analyzed lifestyle-related factor, whose importance is diagnostic rather than connected with modifiability, although its analyses are informative as far as intercourse planning is concerned. Among the participants, the duration of sexual abstinence and sperm motility were found to be related at a level of
statistical significance of p=0.006. Among those who declared a duration of sexual abstinence of up to 4 days, correct motility was observed in 83.33%. In those who declared a duration of sexual abstinence of 4 days or more, correct and incorrect motility was reported in 50% each. The results are shown in
Table 4.
In the performed univariate logistic regression analysis, a statistically significant influence on sperm motility was found in the case of the following parameters: Matsuda index, HOMA-IR index, Mineral Consumption Index (MCI), Vitamin Consumption Index (VCI), and the duration of sexual abstinence. On the other hand, Macronutrient Consumption Index (MacCI), tobacco smoking, physical activity, BMI, and age were not found to be statistically significant. The results are shown in
Table 5.
On the basis of the results of the univariate logistic regression analysis, the
construction of a multivariate model was attempted. Despite the fact that a statistically significant influence on sperm motility was found in the case of some of the parameters was found in the univariate analysis, they were not included in any of the attempted models. Eventually, a model based on the following three parameters was proposed, i.e. Matsuda index, VCI, and the duration of sexual abstinence [
Table 6].
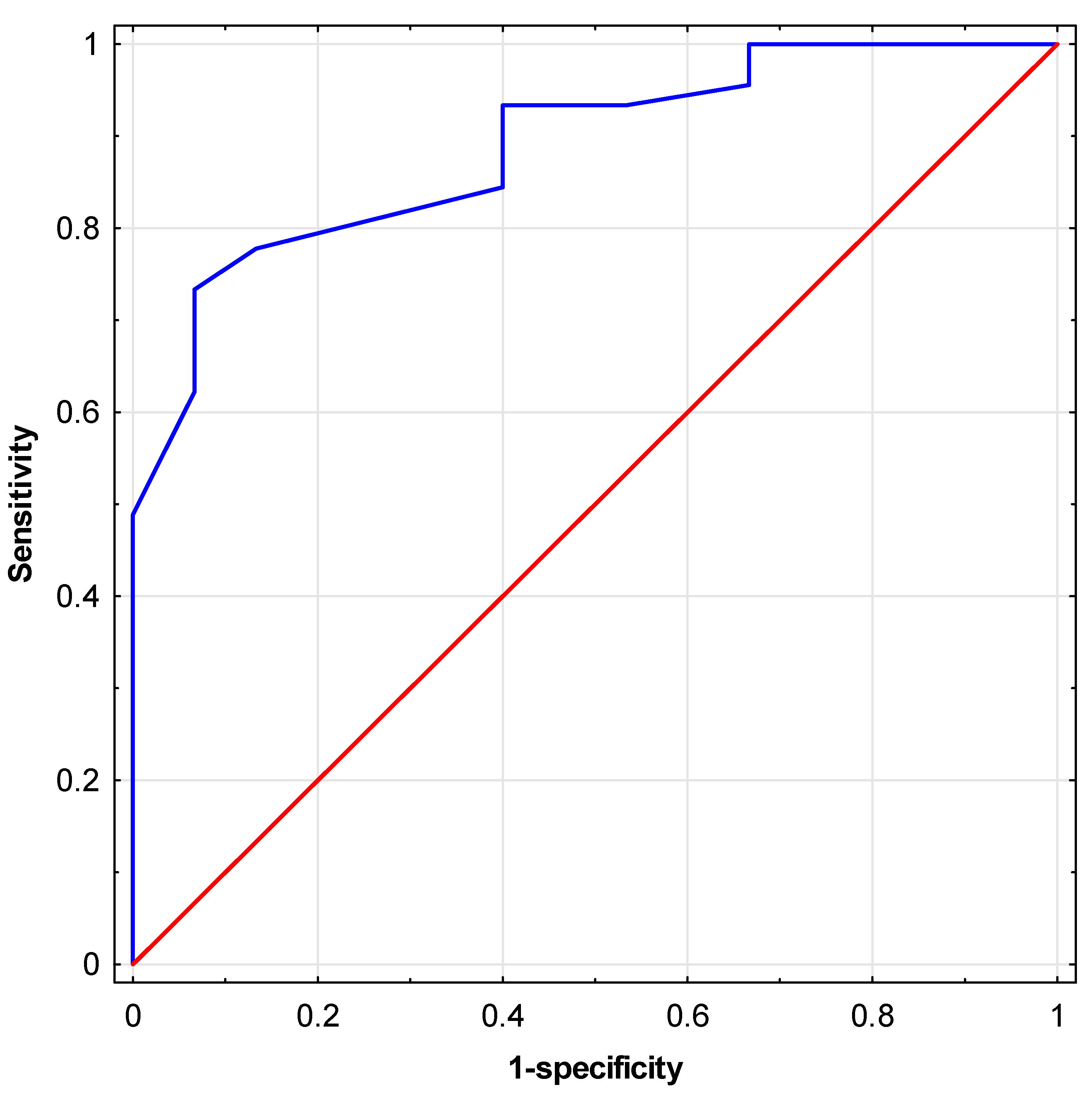
The conformity of fit of the created multivariate logistic regression model was assessed using the Hosmer-Lemeshow goodness-of-fit test, resulting in a high quality of fit (p=0.96). Subsequently, an ROC analysis was conducted, and the area under the curve (AUC) was determined for the predictor created based on the proposed logistic regression model [
Figure 7]. A high AUC value of 0.89 (95% CI: 0.80; 0.97) was obtained. The OR values obtained in the model for individual independent variables indicate that individuals with insulin resistance diagnosed using the Matsuda index have over 37 times (1/0.0271) lower odds of obtaining semen with correct motility than men without diagnosed insulin resistance. Individuals with less than 4 days of sexual abstinence before semen collection had over 20 times (1/0.0477) greater odds of obtaining semen with correct motility than those with 4 days or more of abstinence. On the other hand, patients who consumed vitamin quantities
according to established norms showed over 72% greater odds (OR=1.7243) of obtaining semen with correct motility than men not meeting the required vitamin intake [
Table 6].
Discussion
Sperm motility depends on complex mechanisms, both structural and molecular [
1]. In mammalian sperm, the axoneme is covered with additional structures: a fibrous sheath, a mitochondrial sheath, and dense fibers located at various points. In humans, the mitochondrial sheath has a spiral shape and wraps around the axoneme, supplying ATP, i.e., the energy necessary for sperm motility. The main component of the axoneme has receptors for signaling proteins that influence motility regulation, as well as proteins involved in hyperactivation and capacitation [
11]. These essential structures for semen production are unique to the sperm tail. They provide fertility to semen by offering additional stiffness and the energy necessary for sperm to move within the female reproductive tract – ultimately leading to a higher probability of pregnancy [
38]. This shows that the axoneme is a factor that has a bearing on both sperm motility and fertility and, at a structural level, may explain the importance of sperm motility as a key parameter to be used in fertility analyses.
There are numerous studies on the influence of lifestyle components on motility [
12,
13,
14,
15,
16,
17], including alcohol consumption [
12], tobacco product use [
13], obesity [
14], sleep deficiency or disorders [
15], high consumption of meat products [
16], stress [
17], and intense physical activity [
18]. These studies also focus on the significance of the balance between antioxidants and free radicals in the testicular microenvironment [
19,
20]. As a result of an imbalance in the testicular microenvironment, sperm damage can occur through sperm agglutination, inhibition of capacitation, reduced motility and cervical mucus penetration ability, or faulty sperm interaction with the egg cell [
39]. Therefore, imbalance in the testicular microenvironment is one of the mechanisms linking oxidative stress with motility, and thus also with fertility.
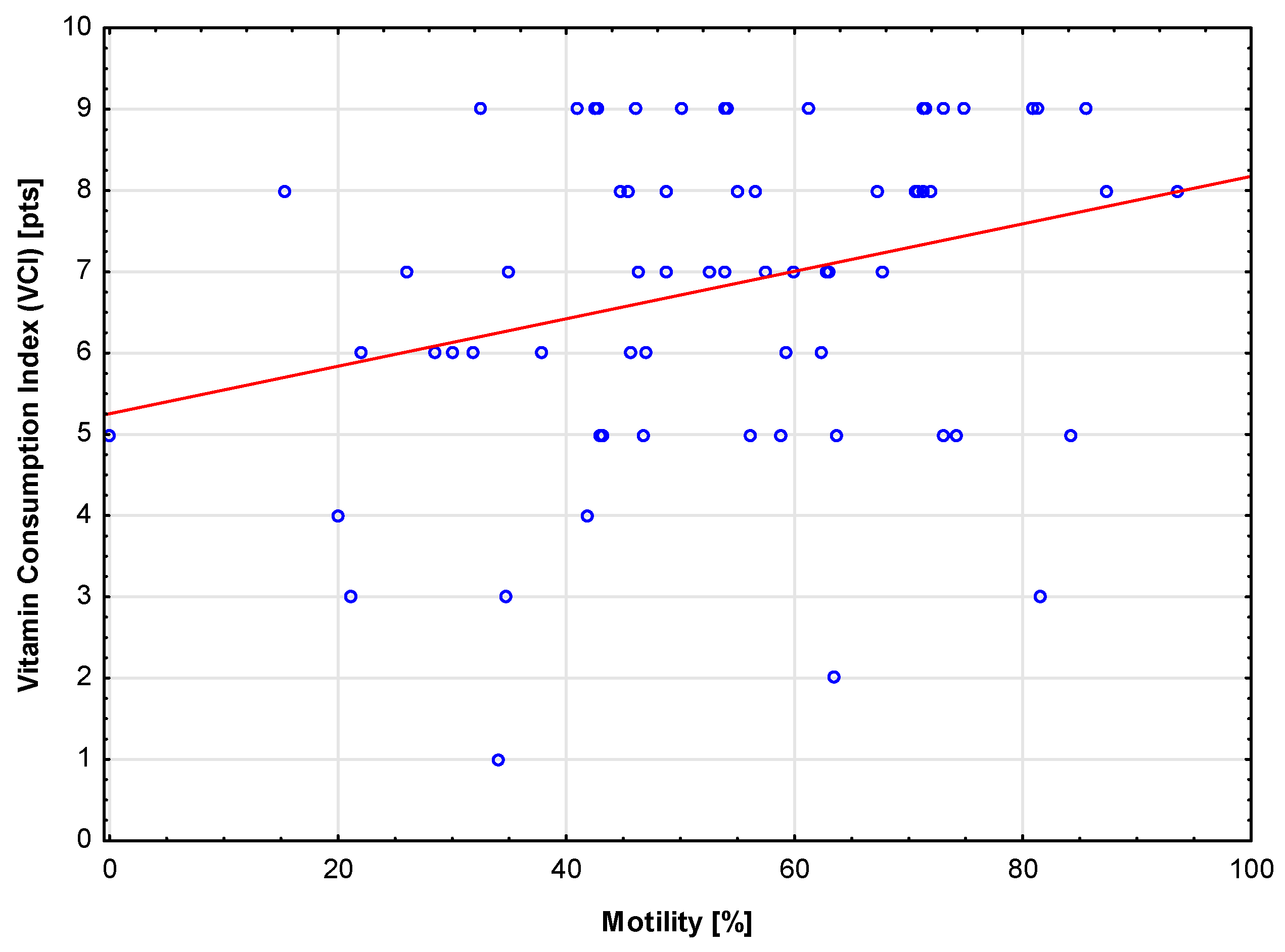
This study showed a positive correlation between vitamin intake (VCI), diet quality (DI), and sperm motility. It was also found that men with correct motility scores were characterized by higher mineral consumption levels (MCI) compared to those with incorrect scores. This suggests that a well-balanced diet meeting the requirements for v,itamins and mineral components may have a positive impact on fertility. This result is consistent with the study performed by Oostingh et al., who showed a positive correlation between sperm motility and healthy dietary habits [
21]. Similarly, Agarwal et al. demonstrated that antioxidant supplementation among men undergoing infertility treatment improves motility [
24], while Salas-Huetos et al. showed that consumption of pro-inflammatory products, low intake of antioxidants found in food products, in addition to a high glycemic index and load in the diet, lead to increased oxidative stress, i.e., increased production of pro-inflammatory cytokines in the body [
25]. The effectiveness of supplementation of antioxidant vitamins and minerals in improving fertility is also supported by numerous studies [
26,
27], while research on male fertility suggests that vitamin C combined with L-carnitine and tocopherol should be used in the diagnosis of oxidative stress in men planning to have children. Another effective combination is selenium supplementation with N-acetylcysteine, which improves hormonal balance. Moreover, it is beneficial to combine selenium with vitamin E and zinc as used in combination, they rebuild sperm damaged by oxidative stress and improve sperm motility and endurance [
28].
Due to their associations with obesity and, consequently, with the possibility of insulin resistance, body composition and weight are often considered in the context of male fertility. In this study, it was found that the more healthy the body composition of a participant, the more likely is he to achieve correct motility scores. Differences in Body Fat Mass levels were also observed between men with correct and incorrect sperm motility scores; i.e., in the former group, the proportion of body fat was significantly lower. This is an unsurprising result as excess adipocytes have the ability to release adipokines and inflammatory mediators, which disrupt cell function and increase inflammation in the body [
30]. Additionally, excess adipose tissue impairs heat circulation, contributing to increased testicular temperature and thus potentially worsening the quality of the produced sperm [
31].
Hyperglycemia, which can result from an improper diet – and is thus associated with obesity – negatively affects sperm motility due to the fact that glucose metabolism significantly influences spermatogenesis [
25]. In order to access the testes, glucose must penetrate the blood-testis barrier. This is possible through facilitated diffusion via glucose transporters (GLUT), dependent on the redistribution of GLUT in the cell membrane and the overall level of GLUT. Research results indicate that glucose transporter 1 (GLUT1) and glucose transporter 3 (GLUT3) play a synergistic role in Sertoli cells in maintaining glucose uptake to ensure lactate production, and subsequently ATP utilized by sperm for movement [
32]. Scientists from Guangzhou attempted to link lifestyle and its individual components to various semen parameters. They demonstrated that a “sweet diet” positively correlated with sperm motility (p=0.017), which may be associated with obtaining energy from simple sugars [
40]. Studies on fertility problems in diabetes, where hyperglycemia and insulin resistance often occur, are already well-known in the scientific world [
32,
33,
34,
35], however, the number of studies directly linking sperm quality with insulin resistance is limited.
In this study, it was observed that non-insulin-resistant men were more likely to have correct sperm motility scores compared to men with insulin resistance. Additionally, lower fasting insulin levels and higher Matsuda index scores were found in men with correct sperm motility scores compared to those with incorrect motility scores. In the study performed by Ma et al. it was found that the HOMA-IR index was an independent factor influencing progressive motile sperm [
36]. Similarly, Andlib et al. reported that insulin resistance may impair the function of the hypothalamus, pituitary gland, and gonads, resulting in reduced secretion of reproductive hormones such as GnRH (gonadotropin-releasing hormone), FSH (follicle-stimulating hormone), LH (luteinizing hormone), and testosterone. These findings reflect those obtained for insulin deficiency. Researchers also emphasize that insulin resistance affects glucose metabolism, which is responsible for the motility and fertilizing activity of mature sperm [
10]. Based on these findings, it can be concluded that insulin resistance, not necessarily in conjunction with diabetes, affects sperm motility and, consequently, semen quality and male fertility in general.
Some studies suggest shortening the duration of sexual abstinence before semen collection for Assisted Reproduction Techniques (ART) to prevent a decline in semen quality. Shorter abstinence significantly improves motility and correlates positively with ART outcomes in couples with infertility issues due to the male factor. Recent research also indicates semen freshness as a possible determinant of a higher number of live births [
41]. Sauer et al. found that the duration of sexual abstinence did not affect sperm motility, but it is worth noting that in the studied groups, the maximum duration of sexual abstinence was only 120 hours [
42]. However, most studies suggest that the duration of sexual abstinence does affect sperm motility, with the ideal duration that would lead to obtaining the highest values of this parameter is up to 4 days [
43,
44,
45]. This conclusion was confirmed in the present study, where men reporting sexual abstinence of less than 4 days achieved positive sperm motility results in around 83%, compared to those reporting sexual abstinence of 4 days or longer (only 50%). In itself, sexual abstinence is not a parameter strictly related to lifestyle; however, it would seem necessary to use the findings discussed above to establish standards in sample collection for fertility tests. Nonetheless, the duration of sexual abstinence appears to have a significant impact on semen quality in men, particularly motility [
41]. This relationship suggests that making clinical decisions based on data from samples collected at an inappropriate time may prove to be incorrect.
As part of the study, a multivariate logistic regression model was created, demonstrating the simultaneous influence of three parameters, i.e., the Matsuda index, VCI (Vitamin Consumption Index), and the duration of sexual abstinence on motility scores. The model indicates that the presence of insulin resistance and excessively long duration of sexual abstinence will negatively impact sperm motility, while adequate vitamin intake will have a positive effect on the parameter. The very high area under the ROC curve obtained for the predictor developed based on the model indicates its high effectiveness in predicting correct or incorrect sperm motility scores. It is also worth noting that each of the parameters included in the model has a different nature, which results in various diagnostic and clinical implications. Considering the above, the multivariate model can be considered an effective and practical tool to be utilized in infertility research, especially in the context of the presence or absence of insulin resistance.
The inclusion of insulin resistance in the model confirms the association between this condition and male fertility. As the condition is modifiable by lifestyle factors to a minimal degree, its presence or absence should primarily be regarded as a clinical feature that increases the likelihood of fertility problems in the studied men. It would also be interesting to relate the prevalence of insulin resistance in today’s society and its status as a civilization disease to the similar features of infertility, i.e., its growing incidence and civilization-related development. The ever-lowering norms for parameters describing fertility also signify that the progress of civilization constitutes an overreaching cause of the two issues [
46]. In this context, the inclusion of insulin resistance in the logistic regression model developed in this study may be interpreted as a reflection of the connections between various civilization diseases and their consequences.
The presence of vitamin supplementation in the model has the greatest clinical implications due to the fact that the factor is easily modifiable and adequate doses of vitamins can have a tangible effect on improving semen parameters, and thus male fertility. It should be emphasized that although various modifiable parameters impact fertility, a multivariate model (characterized by a high AUC) that would include insulin resistance could only be successfully constructed for vitamins. The antioxidant potential of vitamins has protective effects on both sperm DNA and motility. Vitamin C has the ability to neutralize damage caused by free radicals in semen, thus increasing motility, and reducing DNA fragmentation and lipid peroxidation levels [
47]. Vitamin E, found in vegetable oils, has the ability to repair oxidative radicals – preventing increased lipid peroxidation and mitigating the negative effects of oxidative stress on male fertility [
48]. In addition to antioxidant vitamins, adequate intake of B-group vitamins and vitamin D may also play a significant role in male fertility. Vitamin B12 enhances the functionality of reproductive organs, reduces the production of free radicals and homocysteine, which are toxic to sperm DNA, and decreases inflammation [
49]. Both reduced and elevated serum levels of vitamin D increase the incidence of abnormalities in sperm morphology and negatively affect progressive motility and total sperm count [
50].
Salas-Huetos et al. created six different multidimensional linear regression models to assess the relationship between adherence to the Mediterranean diet and semen parameters. The data was adjusted for age, energy intake, and BMI. It was shown that men who adhered to the Mediterranean diet rigorously exhibited total sperm motility scores compared to those adhering to the Mediterranean diet to a lesser extent [
51]. Although the researchers focused on adherence to a specific diet, knowledge concerning the high content of antioxidant vitamins in the Mediterranean diet makes it possible to conclude that these results are consistent with the implications of the multivariate model designed in this study.
Conclusion
The results of the study point to the existence of an association between certain lifestyle factors and semen quality, as measured using sperm motility as the key indicator. Additionally, a significant relationship between the presence or absence of insulin resistance and male fertility has been demonstrated. Most importantly, the study led to the construction of a multivariate model which makes it possible to predict the outcome regarding correct or incorrect motility scores on the basis of three independent parameters, i.e., the Matsuda index, vitamin intake (VCI), and the duration of sexual abstinence.
In general, the lifestyle choices and habits typical for the modern civilization often have a negative impact on male fertility [
22]. This can be counteracted by implementing lifestyle modifications, including dietary changes, which may ultimately halt or even reverse this unfavorable trend. The fact that the norms regarding semen quality are being systematically reduced further confirms the existence of a deepening trend of declining semen quality. Coupled with a lifestyle increasingly reliant on the achievements of civilization, the process will remain an increasingly concerning global issue for the foreseeable future.
The study results indicate that the influence of lifestyle factors on male fertility is of a key importance, particularly in the context of insulin resistance, whose presence or absence is an indicator of potential fertility issues. What this finding signifies is that diagnostics towards insulin resistance should be routinely included in fertility-related interventions and procedures.
Modifiable lifestyle factors that influence fertility primarily include vitamin and mineral consumption, in connection with dietary modifications in line with WHO recommendations, with a particular emphasis on diets containing large amounts of antioxidant-rich foods, especially the Mediterranean diet. The results of this study show that vitamin intake levels correlate with motility scores. In addition, the presented multivariate model indicates the considerable influence of vitamin intake on fertility. The results of this study show that the issue of appropriate micronutrient supplementation targeted on fertility outcomes requires further research, particularly studies that would focus on designing vitamin consumption regimens tailored to the needs of men who want to improve their fertility scores.
As far as the association between the duration of sexual abstinence and semen quality is concerned, the reduced motility after the 4th day of abstinence should be considered in the process of semen collection for diagnostic purposes in order to avoid false-negative results and erroneous clinical decisions.
In view of the findings of this study, research on the impact of insulin resistance and modifiable lifestyle factors appears to be a promising direction as far as semen quality studies are concerned. Moreover, to validate the strength of the proposed multivariate model, further prospective studies should be conducted using new, independent, and larger datasets – based on introducing lifestyle modifications – to assess whether increasing micronutrient (particularly vitamin) intake to the adequate levels and improving selected lifestyle factors in men with insulin resistance could enhance their fertility. This will be evaluated in the authors’ next planned study, where additional parameters will be examined, particularly concerning carbohydrate metabolism disorders and the determination of vitamin levels in the blood.