Submitted:
07 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Selection of the Most Prevalent TSAs
2.1.1. Selection of Highly Prevalent TSAs
2.1.2. Evaluation of Cancer Specificity and Sharing of TSA Source Transcripts
2.2. Peripheral Blood Mononuclear Cell Isolation
2.3. Generation of Monocyte-Derived Dendritic Cells
2.4. Peptide Pulsing and Electroporation of moDCs
2.5. MS Experiments
2.5.1. Cell Sample Preparation
2.5.2. Immunoprecipitation of HLA Class I Molecules
2.5.3. Mass Spectrometry Analyses
2.5.4. Bioinformatic Analysis
2.6. Immunogenicity Testing
2.6.1. Coculture of Naïve CD8 T Cells with moDCs
2.6.2. Tetramer Staining
2.6.3. Functional expansion of antigen-specific T cells followed by TCR Vβ CDR3 sequencing (FEST assay)
2.6.4. PRIME 2.0 score for prediction of T-cell recognition
3. Results
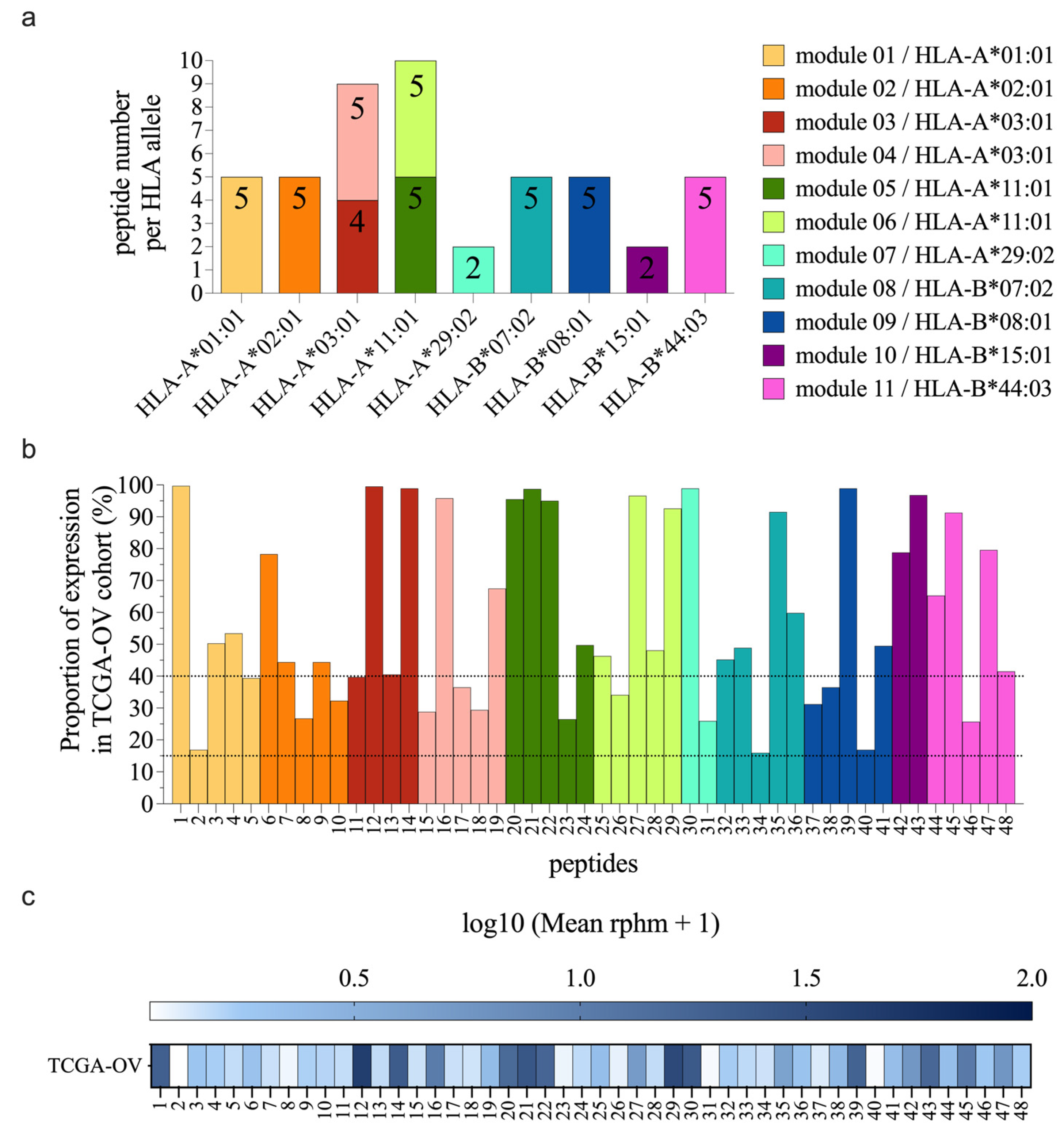
3.1. Selection of the Best TSA Candidates
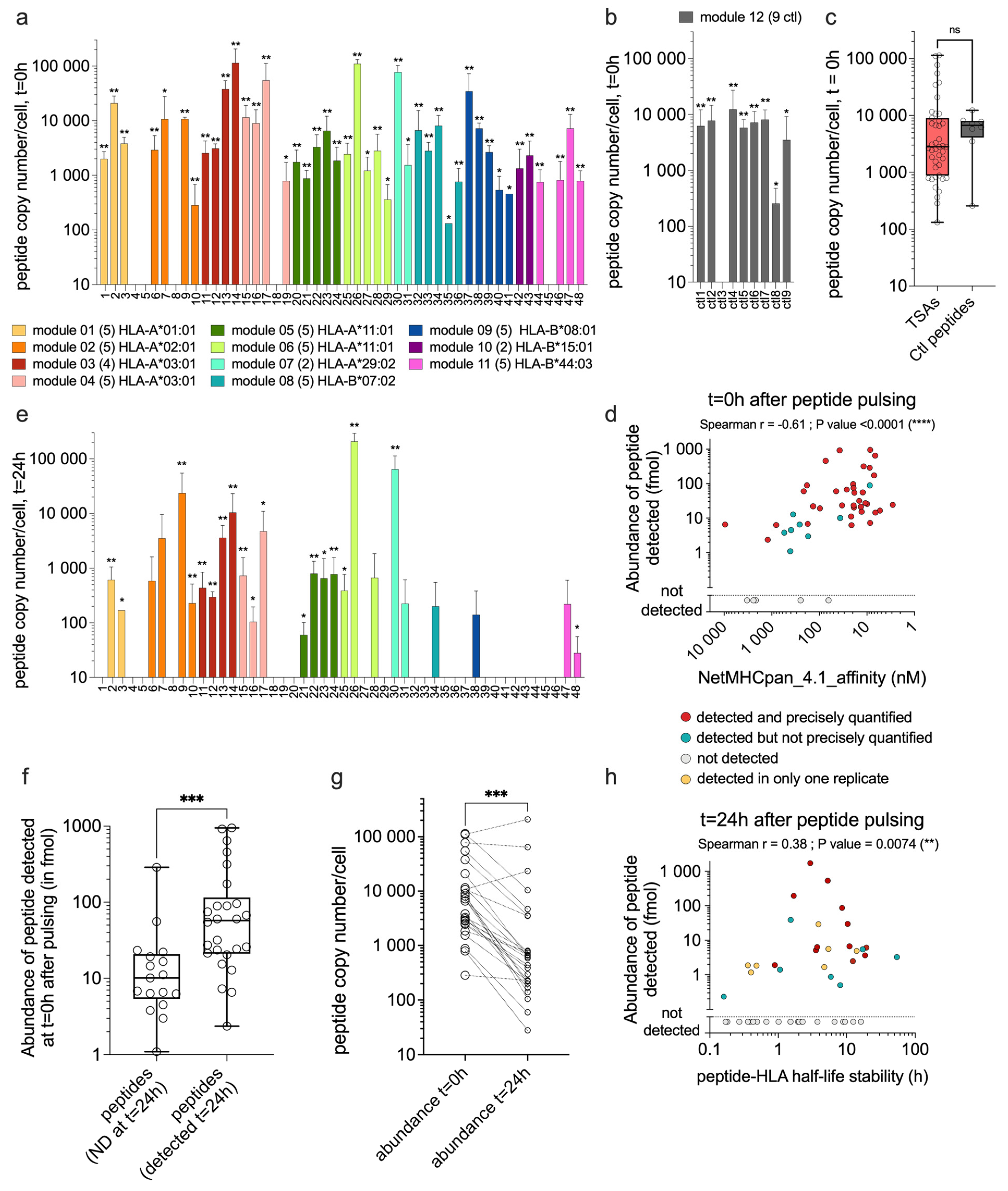
3.2. TSAs are Well Presented by moDCs after Peptide Pulsing
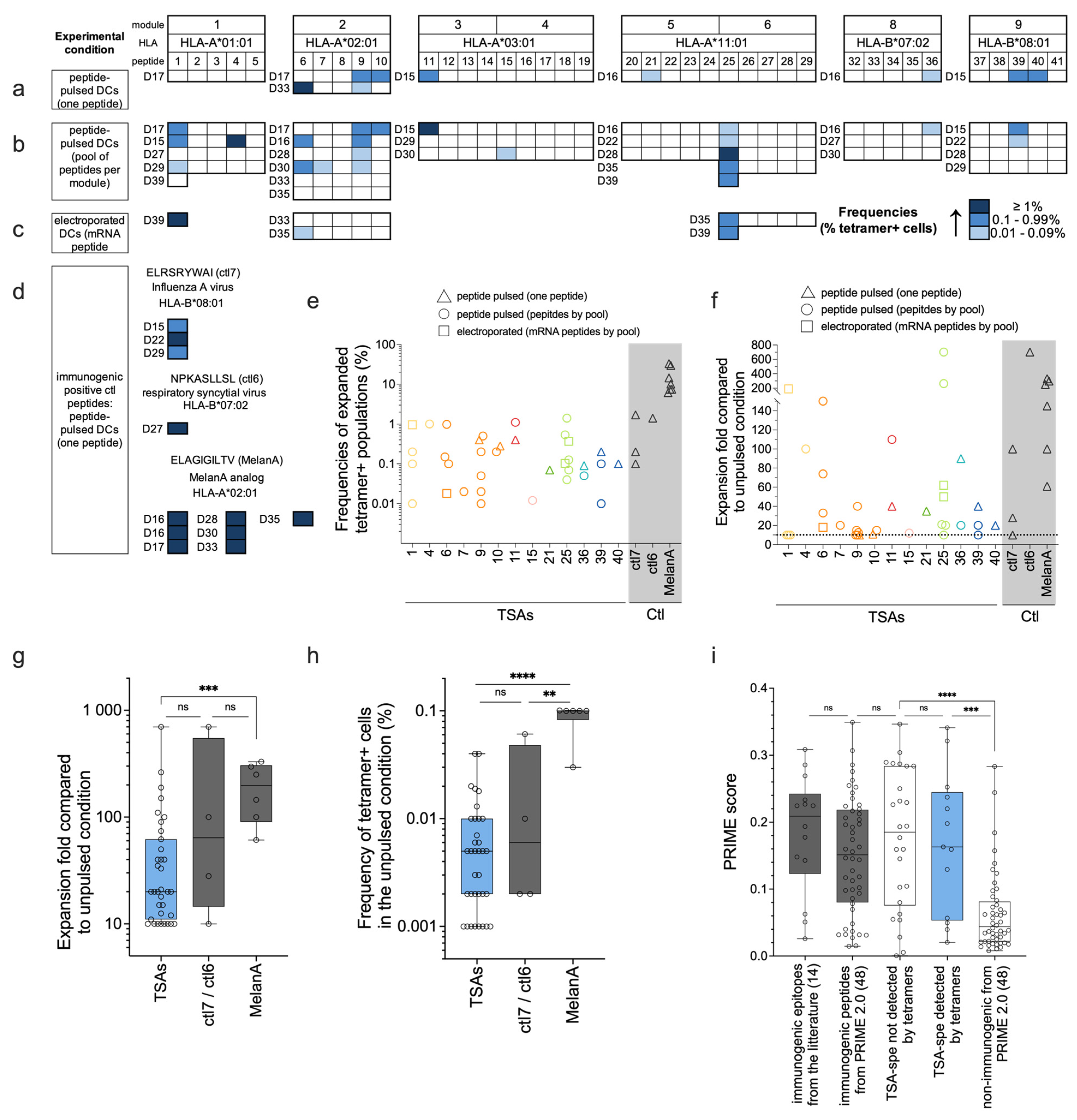
3.3. TCR-Vβ CDR3 Sequencing After Functional Expansion
3.4. Tetramer Staining After Functional Expansion
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lheureux, S.; et al. Epithelial ovarian cancer. Lancet, 2019. 393(10177): P. 1240-1253.
- Lheureux, S., M. Braunstein, and A.M. Oza, Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin, 2019. 69(4): P. 280-304.
- Siegel, R.L.; et al. Cancer statistics, 2023. CA Cancer J Clin, 2023. 73(1): P. 17-48.
- Zhang, L.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med, 2003. 348(3): P. 203-13.
- Hwang, W.T.; et al., Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol Oncol, 2012. 124(2): P. 192-8.
- Kroeger, D.R., K. Milne, and B.H. Nelson, Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res, 2016. 22(12): P. 3005-15.
- Kandalaft, L.E., K. Odunsi, and G. Coukos, Immunotherapy in Ovarian Cancer: Are We There Yet? J Clin Oncol, 2019. 37(27): P. 2460-2471.
- Le Page, C.; et al., Exploring the Clinical Impact of Predictive Biomarkers in Serous Ovarian Carcinomas. Curr Drug Targets, 2020. 21(10): P. 974-995.
- Clouthier, D.L.; et al., An interim report on the investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (INSPIRE). J Immunother Cancer, 2019. 7(1): P. 72.
- Scheper, W.; et al., Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med, 2019. 25(1): P. 89-94.
- Schumacher, T.N., W. Scheper, and P. Kvistborg, Cancer Neoantigens. Annu Rev Immunol, 2019. 37: P. 173-200.
- Tran, E., P. F. Robbins, and S.A. Rosenberg, 'Final common pathway' of human cancer immunotherapy: Targeting random somatic mutations. Nat Immunol, 2017. 18(3): P. 255-262.
- Smith, C.C.; et al. Alternative tumour-specific antigens. Nat Rev Cancer, 2019. 19(8): P. 465-478.
- Laumont, C.M.; et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci Transl Med, 2018. 10(470).
- Laumont, C.M. and C. Perreault, Exploiting non-canonical translation to identify new targets for T cell-based cancer immunotherapy. Cell Mol Life Sci, 2018. 75(4): P. 607-621.
- Frankiw, L., D. Baltimore, and G. Li, Alternative mRNA splicing in cancer immunotherapy. Nat Rev Immunol, 2019. 19(11): P. 675-687.
- Ehx, G.; et al. Atypical acute myeloid leukemia-specific transcripts generate shared and immunogenic MHC class-I-associated epitopes. Immunity, 2021. 54(4): P. 737-752.e10.
- Bassani-Sternberg, M.; et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun, 2016. 7: P. 13404.
- Löffler, M.W.; et al. Multi-omics discovery of exome-derived neoantigens in hepatocellular carcinoma. Genome Med, 2019. 11(1): P. 28.
- Newey, A.; et al. Immunopeptidomics of colorectal cancer organoids reveals a sparse HLA class I neoantigen landscape and no increase in neoantigens with interferon or MEK-inhibitor treatment. J Immunother Cancer, 2019. 7(1): P. 309.
- Kraemer, A.I.; et al. The immunopeptidome landscape associated with T cell infiltration, inflammation and immune editing in lung cancer. Nat Cancer, 2023. 4(5): P. 608-628.
- Marty, R.; et al. MHC-I Genotype Restricts the Oncogenic Mutational Landscape. Cell, 2017. 171(6): P. 1272-1283.e15.
- The problem with neoantigen prediction. Nature Biotechnology, 2017. 35(2): P. 97-97.
- Zhao, Q.; et al. Proteogenomics Uncovers a Vast Repertoire of Shared Tumor-Specific Antigens in Ovarian Cancer. Cancer Immunol Res, 2020. 8(4): P. 544-555.
- Kina, E.; et al. Breast cancer immunopeptidomes contain numerous shared tumor antigens. J Clin Invest, 2024. 134(1).
- Ehx, G. and C. Perreault, Discovery and characterization of actionable tumor antigens. Genome Med, 2019. 11(1): P. 29.
- Hardy, M.P., K. Vincent, and C. Perreault, The Genomic Landscape of Antigenic Targets for T Cell-Based Leukemia Immunotherapy. Front Immunol, 2019. 10: P. 2934.
- Reynisson, B.; et al. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Research, 2020. 48(W1): P. W449-W454.
- Jørgensen, K.W.; et al. NetMHCstab - predicting stability of peptide-MHC-I complexes; impacts for cytotoxic T lymphocyte epitope discovery. Immunology, 2014. 141(1): P. 18-26.
- Maiers, M., L. Gragert, and W. Klitz, High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol, 2007. 68(9): P. 779-88.
- Cuevas, M.V.R.; et al. BamQuery: A proteogenomic tool to explore the immunopeptidome and prioritize actionable tumor antigens. Genome Biology, 2023. 24(1): P. 188.
- The Genotype-Tissue Expression (GTEx) project. Nat Genet, 2013. 45(6): P. 580-5.
- Larouche, J.D.; et al. Widespread and tissue-specific expression of endogenous retroelements in human somatic tissues. Genome Med, 2020. 12(1): P. 40.
- Fergusson, J.R.; et al. Maturing Human CD127+ CCR7+ PDL1+ Dendritic Cells Express AIRE in the Absence of Tissue Restricted Antigens. Front Immunol, 2018. 9: P. 2902.
- Ali, M.; et al. Induction of neoantigen-reactive T cells from healthy donors. Nat Protoc, 2019. 14(6): P. 1926-1943.
- Sirois, I.; et al. Immunopeptidomics: Isolation of Mouse and Human MHC Class I- and II-Associated Peptides for Mass Spectrometry Analysis. J Vis Exp, 2021(176).
- Lanoix, J.; et al. Comparison of the MHC I Immunopeptidome Repertoire of B-Cell Lymphoblasts Using Two Isolation Methods. Proteomics, 2018. 18(12): P. e1700251.
- Danilova, L.; et al. The Mutation-Associated Neoantigen Functional Expansion of Specific T Cells (MANAFEST) Assay: A Sensitive Platform for Monitoring Antitumor Immunity. Cancer Immunol Res, 2018. 6(8): P. 888-899.
- Gfeller, D.; et al. Improved predictions of antigen presentation and TCR recognition with MixMHCpred2.2 and PRIME2.0 reveal potent SARS-CoV-2 CD8+ T-cell epitopes. Cell Systems, 2023. 14(1): P. 72-83.e5.
- Snary, D.; et al. Molecular structure of human histocompatibility antigens: The HLA-C series. Eur J Immunol, 1977. 7(8): P. 580-5.
- Perez, C.R. and M. De Palma, Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun, 2019. 10(1): P. 5408.
- Ebrahimi-Nik, H.; et al. Mass spectrometry driven exploration reveals nuances of neoepitope-driven tumor rejection. JCI Insight, 2019. 5(14).
- Campillo-Davo, D., D. Flumens, and E. Lion, The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses. Cells, 2020. 9(7).
- Burrows, S.R.; et al. Peptide-MHC class I tetrameric complexes display exquisite ligand specificity. J Immunol, 2000. 165(11): P. 6229-34.
- Goulder, P.J.R.; et al. Characterization of a Novel Respiratory Syncytial Virus-Specific Human Cytotoxic T-Lymphocyte Epitope. Journal of Virology, 2000. 74(16): P. 7694-7697.
- Pittet, M.J.; et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med, 1999. 190(5): P. 705-15.
- Heidema, J.; et al. Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes. J Gen Virol, 2004. 85(Pt 8): P. 2365-2374.
- Alanio, C.; et al. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood, 2010. 115(18): P. 3718-25.
- Legoux, F.; et al. Impact of TCR reactivity and HLA phenotype on naive CD8 T cell frequency in humans. J Immunol, 2010. 184(12): P. 6731-8.
- Romero, P.; et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev, 2002. 188: P. 81-96.
- Neller, M.A.; et al. Naive CD8⁺ T-cell precursors display structured TCR repertoires and composite antigen-driven selection dynamics. Immunol Cell Biol, 2015. 93(7): P. 625-33.
- Nguyen, T.H.; et al. Understanding CD8(+) T-cell responses toward the native and alternate HLA-A*02:01-restricted WT1 epitope. Clin Transl Immunology, 2017. 6(3): P. e134.
- Tauber, C.; et al. Inefficient induction of circulating TAA-specific CD8+ T-cell responses in hepatocellular carcinoma. Oncotarget, 2019. 10(50): P. 5194-5206.
- Azoury, M.E.; et al. CD8(+) T Cells Variably Recognize Native Versus Citrullinated GRP78 Epitopes in Type 1 Diabetes. Diabetes, 2021. 70(12): P. 2879-2891.
- Apavaloaei, A.; et al. Induced pluripotent stem cells display a distinct set of MHC I-associated peptides shared by human cancers. Cell Rep, 2022. 40(7): P. 111241.
- Schuster, H.; et al. The immunopeptidomic landscape of ovarian carcinomas. Proc Natl Acad Sci U S A, 2017. 114(46): P. E9942-e9951.
- Nelde, A.; et al. Immune Surveillance of Acute Myeloid Leukemia Is Mediated by HLA-Presented Antigens on Leukemia Progenitor Cells. Blood Cancer Discov, 2023. 4(6): P. 468-489.
- Müller, M.; et al. Machine learning methods and harmonized datasets improve immunogenic neoantigen prediction. Immunity, 2023. 56(11): P. 2650-2663.e6.
- Henrickson, S.E.; et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nature Immunology, 2008. 9(3): P. 282-291.
- Prlic, M., G. Hernandez-Hoyos, and M.J. Bevan, Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med, 2006. 203(9): P. 2135-43.
- Obst, R.; et al. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med, 2005. 201(10): P. 1555-65.
- Itano, A.A.; et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity, 2003. 19(1): P. 47-57.
- Fonteneau, J.F.; et al. Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J Immunol Methods, 2001. 258(1-2): P. 111-26.
- Aspord, C.; et al. pDCs efficiently process synthetic long peptides to induce functional virus- and tumour-specific T-cell responses. Eur J Immunol, 2014. 44(10): P. 2880-92.
- Hoppes, R.; et al. Altered peptide ligands revisited: Vaccine design through chemically modified HLA-A2-restricted T cell epitopes. J Immunol, 2014. 193(10): P. 4803-13.
- Carretero-Iglesia, L.; et al. High Peptide Dose Vaccination Promotes the Early Selection of Tumor Antigen-Specific CD8 T-Cells of Enhanced Functional Competence. Front Immunol, 2019. 10: P. 3016.
- Ichikawa, J.; et al. Rapid Expansion of Highly Functional Antigen-Specific T Cells from Patients with Melanoma by Nanoscale Artificial Antigen-Presenting Cells. Clin Cancer Res, 2020. 26(13): P. 3384-3396.
- Stolk, D.A.; et al. Lipo-Based Vaccines as an Approach to Target Dendritic Cells for Induction of T- and iNKT Cell Responses. Front Immunol, 2020. 11: P. 990.
- Sykulev, Y.; et al. Evidence that a Single Peptide–MHC Complex on a Target Cell Can Elicit a Cytolytic T Cell Response. Immunity, 1996. 4(6): P. 565-571.
- Stutzmann, C.; et al. Unlocking the potential of microfluidics in mass spectrometry-based immunopeptidomics for tumor antigen discovery. Cell Rep Methods, 2023. 3(6): P. 100511.
- Bassani-Sternberg, M.; et al. Mass Spectrometry of Human Leukocyte Antigen Class I Peptidomes Reveals Strong Effects of Protein Abundance and Turnover on Antigen Presentation*[S]. Molecular & Cellular Proteomics, 2015. 14(3): P. 658-673.
- Kawashima, I.; et al. Identification of gp100-derived, melanoma-specific cytotoxic T-lymphocyte epitopes restricted by HLA-A3 supertype molecules by primary in vitro immunization with peptide-pulsed dendritic cells. Int J Cancer, 1998. 78(4): P. 518-24.
- Walter, S.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med, 2012. 18(8): P. 1254-61.
- Slingluff, C.L., Jr.; et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res, 2007. 13(21): P. 6386-95.
- Aurisicchio, L.; et al. A novel minigene scaffold for therapeutic cancer vaccines. Oncoimmunology, 2014. 3(1): P. e27529.
- Kuznetsov, A.; et al. Critical Review of Existing MHC I Immunopeptidome Isolation Methods. Molecules, 2020. 25(22).
- Sturm, T.; et al. Mild Acid Elution and MHC Immunoaffinity Chromatography Reveal Similar Albeit Not Identical Profiles of the HLA Class I Immunopeptidome. J Proteome Res, 2021. 20(1): P. 289-304.
- Laugel, B.; et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem, 2007. 282(33): P. 23799-810.
- Lissina, A.; et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods, 2009. 340(1): P. 11-24.
- Dolton, G.; et al. Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells. Clin Exp Immunol, 2014. 177(1): P. 47-63.
- Tungatt, K.; et al. Antibody stabilization of peptide-MHC multimers reveals functional T cells bearing extremely low-affinity TCRs. J Immunol, 2015. 194(1): P. 463-74.
- Rasmussen, M.; et al. Pan-Specific Prediction of Peptide-MHC Class I Complex Stability, a Correlate of T Cell Immunogenicity. J Immunol, 2016. 197(4): P. 1517-24.
- Trofimov, A.; et al. Two types of human TCR differentially regulate reactivity to self and non-self antigens. iScience, 2022. 25(9): P. 104968.
- Kvedaraite, E. and F. Ginhoux, Human dendritic cells in cancer. Sci Immunol, 2022. 7(70): P. eabm9409.
- Laoui, D.; et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun, 2016. 7: P. 13720.
- Wculek, S.K.; et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol, 2020. 20(1): P. 7-24.
- Bevers, S.; et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol Ther, 2022. 30(9): P. 3078-3094.
- Gebre, M.S.; et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature, 2022. 601(7893): P. 410-414.
- Huff, A.L., E. M. Jaffee, and N. Zaidi, Messenger RNA vaccines for cancer immunotherapy: Progress promotes promise. J Clin Invest, 2022. 132(6).
- Lutz, J.; et al. Local immunotherapy with the RNA-based immune stimulator CV8102 induces substantial anti-tumor responses and enhances checkpoint inhibitor activity. Cancer Immunol Immunother, 2023. 72(5): P. 1075-1087.
- Ramos da Silva, J.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl Med, 2023. 15(686): P. eabn3464.
- Sittplangkoon, C.; et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front Immunol, 2022. 13: P. 983000.
- Sahin, U.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature, 2020. 585(7823): P. 107-112.
- Liu, J.; et al. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J Hematol Oncol, 2022. 15(1): P. 28.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




