Submitted:
09 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods and Materials
Experimental Insects
The Effect of Isolated-Predator on the Development and Fecundity of B. dorsalis
The Effect of Predator Odor and Development and Fecundity of B. dorsalis
Data Analysis
Results
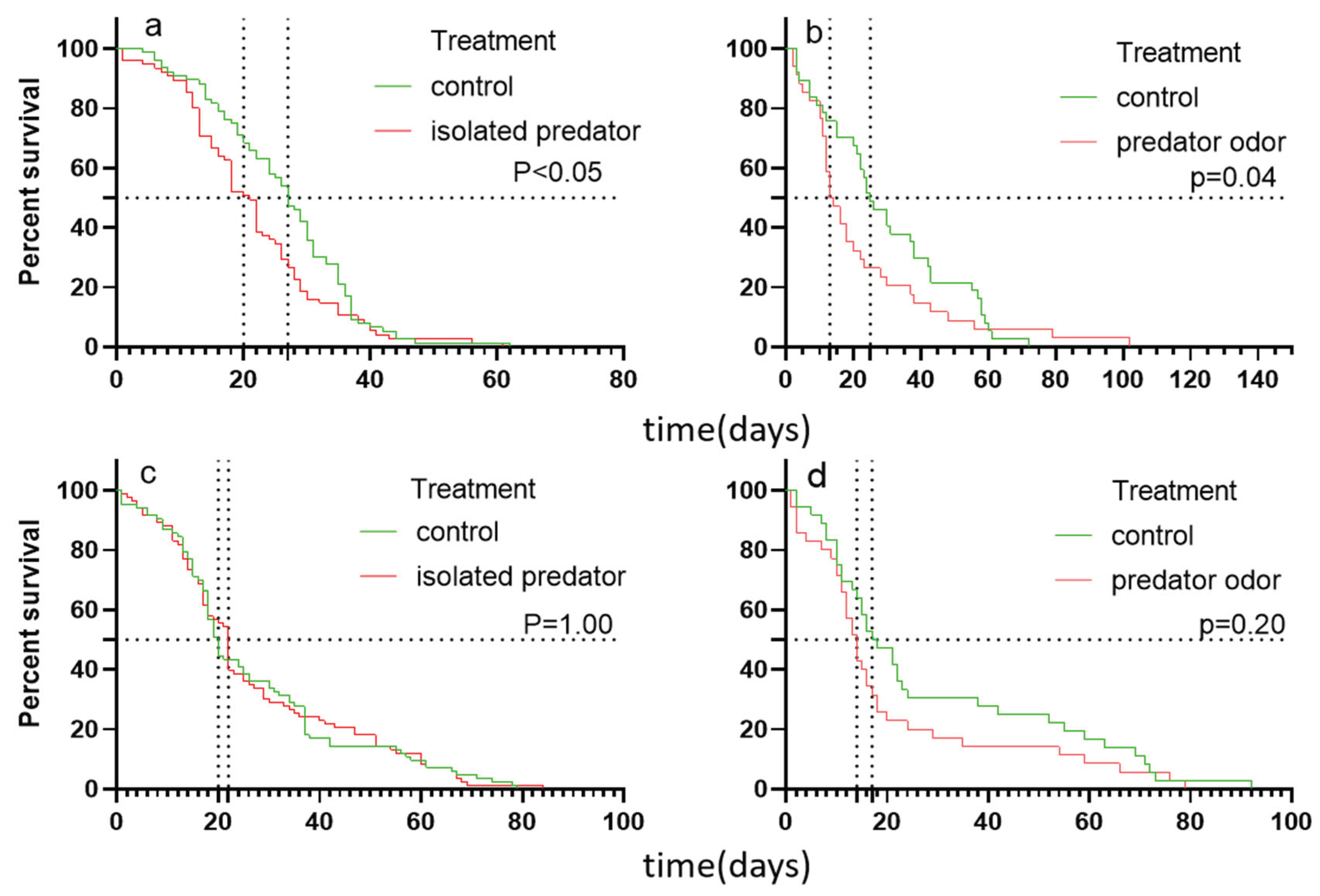
Development Time
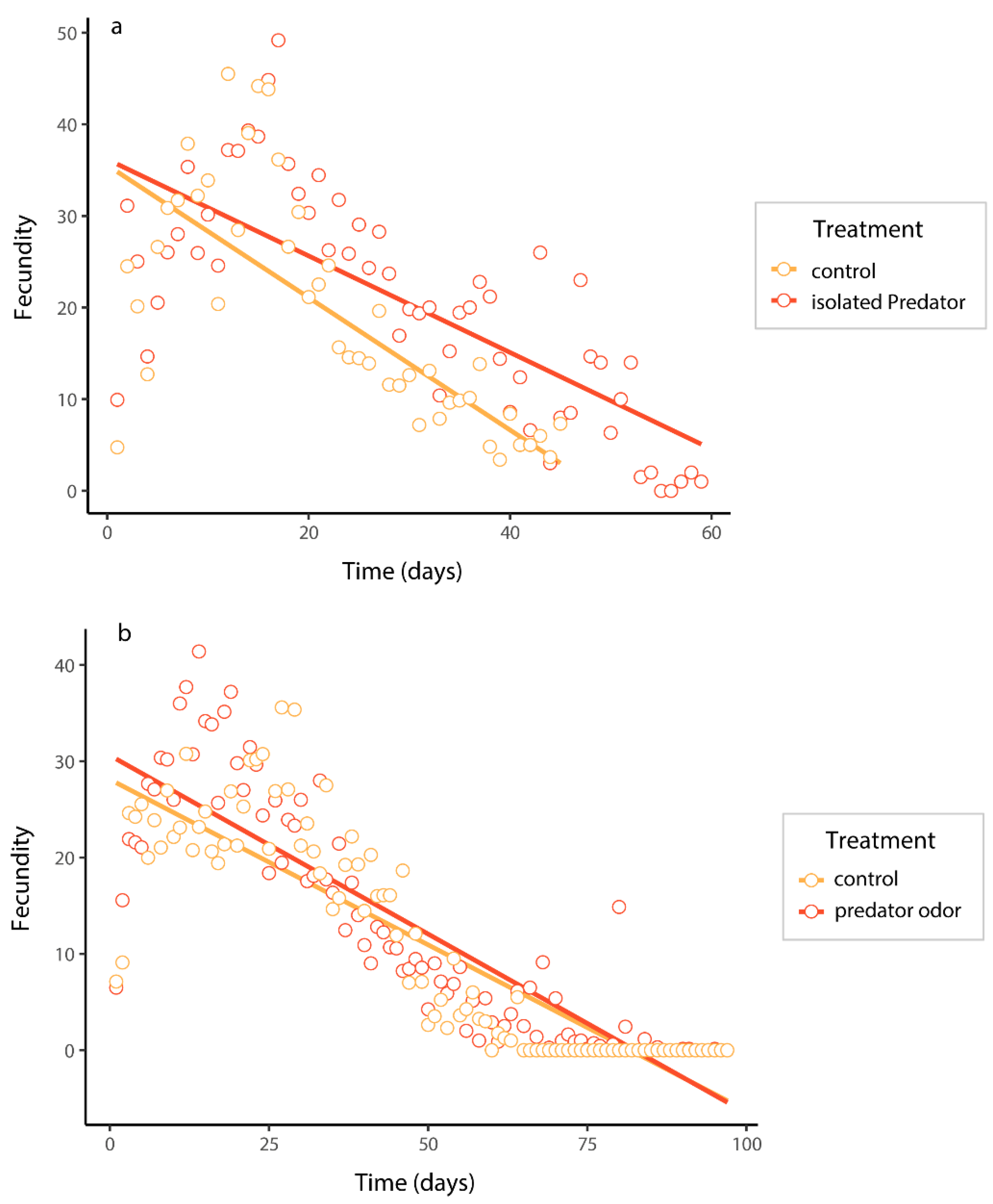
Fecundity
Body Weight at Death
Discussion
References
- Barbosa, P.; Lgnacio, C. Ecology of predator-prey interactions. Oxford University Press. 2005.
- Abrams, P. The evolution of predator-prey interactions: Theory and evidence. Annual Review of Ecology and Systematics. 2000, 31, 79–105. [Google Scholar] [CrossRef]
- Preisser, E.L.; Bolnick, D.I.; Benard, M.F. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology. 2005, 86, 501–509. [Google Scholar] [CrossRef]
- Orrock, J.L.; Grabowski, J.H.; Pantel, J.H.; Peacor, S.D.; Peckarsky, B.L.; Sih, A.; Werner, E.E. Consumptive and nonconsumptive effects of predators on metacommunities of competing prey. Ecology. 2008, 89, 2426–35. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Hawlena, D.; Schmitz, O.J. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc Natl Acad Sci U S A. 2010, 107, 15503–15507. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, C.; Lohroff, T.; Biefel, F.; Cocherell, D.E.; Carson, E.W.; Hung, T.C.; Connon, R.E.; Fangue, N.A.; Todgham, A.E. Effects of turbidity, temperature and predation cue on the stress response of juvenile delta smelt. Conserv Physiol. 2023, 11, coad036. [Google Scholar] [CrossRef]
- Nicieza, A.G. Interacting effects of predation risk and food availability on larval anuran behaviour and development. Oecologia. 2000, 123, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Culshaw-Maurer, M.; Sih, A.; Rosenheim, J.A. Bugs scaring bugs: Enemy-risk effects in biological control systems. Ecology Letters. 2020, 23, 1693–1714. [Google Scholar] [CrossRef]
- Weed, A.S.; Frank, J.H. Oviposition behavior of pheropsophus aequinoctialis L. (coleoptera: Carabidae): A natural enemy of scapteriscus mole crickets (orthoptera: Gryllotalpidae). Journal of Insect Behavior. 2005, 18, 707–723. [Google Scholar] [CrossRef]
- Cresswell, W. Non-lethal effects of predation in birds. Ibis. 2008, 150, 3–17. [Google Scholar] [CrossRef]
- Johnson, E.C.; Braco, J.T.; Whitmill, M.A. Connecting nutrient sensing and the endocrine control of metabolic allocation in insects. Insect Science. 2014, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Karez, R. Competition, coexistence and diversity on rocky shores. In Competition and coexistence, Ecological Studies. Springer, 2002; 133–163. [Google Scholar]
- Werner, E.E. Individual behavior and higher-order species interactions. The American Naturalist. 1992, 140, S5–S32. [Google Scholar] [CrossRef]
- Werner, E.E.; Anholt, B.R. Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. The American Naturalist. 1993, 142, 242–272. [Google Scholar] [CrossRef] [PubMed]
- Hermann, S.L.; Thaler, J.S. Prey perception of predation risk: Volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia. 2014, 176, 669–676. [Google Scholar] [CrossRef]
- Siepielski, A.M.; Fallon, E.; Boersma, K. Predator olfactory cues generate a foraging-predation trade-off through prey apprehension. Royal Society. 2016. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Lynch, B.; Rochette, R. Trade-off between mating and predation risk in the marine snail, littorina plena. Invertebrate Biology. 2007, 126, 257–267. [Google Scholar] [CrossRef]
- Urban, M.C. The growth-predation risk trade-off under a growing gape-limited predation threat. Ecology. 2007, 88, 2587–97. [Google Scholar] [CrossRef] [PubMed]
- Gotthard, K. Increased risk of predation as a cost of high growth rate: An experimental test in a butterfly. Journal of Animal Ecology. 2000, 69, 896–902. [Google Scholar] [CrossRef]
- Lin, X.M.; Cui, X.X.; Tang, J.H.; Zhu, J.W.; Li, J.H. Predation risk effects of lady beetle menochilus sexmaculatus (fabricius) on the melon aphid, aphis gossypii glover. insects. 2024, 15, 13. [Google Scholar] [CrossRef]
- Wei, X.; Liu, J.; Zhang, Z.-Q. Predation stress experienced as immature mites extends their lifespan. Biogerontology. 2023, 24, 67–79. [Google Scholar] [CrossRef]
- Li, Y.P.; Ge. F. Effect of prey stress from Propylea japonica on development and fecundity of Drosophila melanogaster in successive three generations. Entomological Knowledge. 2010, 47, 139–145. [Google Scholar]
- Wang, L.; Atlihan, R.; Chai, R.; Dong, Y.; Luo, C.; Hu, Z. Assessment of non-consumptive predation risk of coccinella septempunctata (coleoptera: Coccinellidae) on the population growth of sitobion miscanthi (hemiptera: Aphididae). insects. 2022, 13, 524. [Google Scholar] [CrossRef]
- Kempraj, V.; Park, S.J.; Taylor, P.W. Forewarned is forearmed: Queensland fruit flies detect olfactory cues from predators and respond with predator-specific behaviour. Scientific Reports. 2020, 10, 7297. [Google Scholar] [CrossRef]
- Fortuna, R.; Covas, R.; D'Amelio, P.B.; Silva, L.R.; Parenteau, C.; Bliard, L.; Rybak, F.; Doutrelant, C.; Paquet, M. Interplay of cooperative breeding and predation risk on egg allocation and reproductive output. Behavioral Ecology. 2024, 35. [Google Scholar] [CrossRef] [PubMed]
- Kral, K. Visually guided search behavior during walking in insects with different habitat utilization strategies. Journal of Insect Behavior. 2019, 32, 290–305. [Google Scholar] [CrossRef]
- Zanuzzo, F.S.; de, C.B.A.L.; Pereira, R.T.; Valença-Silva, G.; Barcellos, L.J.G.; Barreto, R.E. Innate response based on visual cues of sympatric and allopatric predators in nile tilapia. Behavioural Processes. 2019, 109–114. [Google Scholar] [CrossRef]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Écoscience. 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Grubb, T.C., Jr. Antipredator defenses in birds and mammals. The Auk 2006, 123, 601–605. [Google Scholar] [CrossRef]
- Munoz, N.E.; Blumstein, D.T. Multisensory perception in uncertain environments. Behavioral Ecology. 2012, 23, 457–462. [Google Scholar] [CrossRef]
- Hettena, A.M.; Munoz, N.; Blumstein, D.T. Prey responses to predator's sounds: A review and empirical study. Ethology. 2014, 120, 427–452. [Google Scholar] [CrossRef]
- Brown, G.E.; Magnavacca, G. Predator inspection behaviour in a characin fish: An interaction between chemical and visual information? Ethology. 2003, 109, 739–750. [Google Scholar] [CrossRef]
- Binz, H.; Bucher, R.; Entling, M.H.; Menzel, F. Knowing the risk: Crickets distinguish between spider predators of different size and commonness. Ethology. 2014, 120, 99–110. [Google Scholar] [CrossRef]
- Schmitz, O.A.O. Predator and prey functional traits: Understanding the adaptive machinery driving predator-prey interactions. F1000Researc, 2017; 6, 1767. [Google Scholar]
- Poulin, R.X.; Lavoie, S.; Siegel, K.; Gaul, D.A.; Weissburg, M.J.; Kubanek, J. Chemical encoding of risk perception and predator detection among estuarine invertebrates. Proc Natl Acad Sci U S A. 2018, 115, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wu, J.; Zhao, D.X. Research progress on Hierodula patellifera serville. Journal of Southern Agriculture. 2014; 45, 53–57. [Google Scholar]
- Wang, S.J.; Wu, J.; Zhao, Y.A.; Li, R.X.; Zhao, D.X. Functional response of adult Hierodula patellifera (Serville, 1839) (Mantodea: Mantidae) to Tessaratoma papillosa (Drury) (Hemiptera:Tessaratomidae). International Journal of Tropical Insect Science. 2020, 4, 1053–1058. [Google Scholar] [CrossRef]
- Lin, J.T.; Zeng, L.; Lu, Y.Y.; Liang, G.W.; Xu, Y.J. Research Advances in biology and control of Bactrocera (Bactrocera) dorsalis (Hendel). Joumal of ZhongKai Agrotechnical College. 2004, 60–67. [Google Scholar]
- Huang, J.F.; Zhang, Y.J. Research progress of oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tetriphitidae). Deciduous Fruits, 2023; 55, 68-71+3. [Google Scholar]
- Jin, Y.X.; Zhang, D.M.; Xie, C.F.; Li, M.M.; Meng, L.L.; Shang, M.Q.; Zhou, H.-X. Research advance on green prevention and control technology of Bactrocera dorsalis H. Plant Quarantine, 2022; 36, 1–6. [Google Scholar]
- Zhu, Y.F.; Shang, M.Q.; Teng. Z.W.; Tan, X.M; et al. Analysis of Invasion, Distribution and Spreding Trend of Bactrocera dorsalis. Shandong Agricultiral Sciences. 2020, 52, 141–149. [Google Scholar]
- Zhu, X.S.; Liu, Y.; Dai, S.Z.; Luo, R.; Jia, H.S.; He, P.; Zhao, L. The control experiment of three kinds of potion on bactrocera dorsalis in apple orchard. Yunnan Agricultural Science and Technology. 2021, 03, 9–10. [Google Scholar]
- Quan, J.C.; Chen, G.F.; Jiang, Y.H. Damage Investigation and Field Control Test of Bactrocera dorsalis in Guangxi. South China Fruits. 2019, 48, 86–91. [Google Scholar]
- Yuan, R.L.; Zheng, C.W.; Feng, F.D. Study on new feeding method of bactrocera dorsalis. Agricultural Technolofg Service. 2020, 37, 27–30. [Google Scholar]
- Duong, T.M.; McCauley, S.J. Predation risk increases immune response in a larval dragonfly (leucorrhinia intacta). Ecology. 2016, 97, 1605–1610. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-immunity trade-offs in insects. Annu Rev Entomol. 2016, 61, 239–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, Z.Q. Level-dependent effects of predation stress on prey development, lifespan and reproduction in mites. Biogerontology. 2022, 23, 515–527. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Wang, Z.; Lin, T.; Feng, J.; Jiang, T. Effects of predation risks of bats on the growth, development, reproduction, and hormone levels of spodoptera litura. Front. Ecol. Evol. 2023, 11. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhang, Z.Q. Development, lifespan and reproduction of spider mites exposed to predator-0induced stress across generations. Biogerontology. 2019, 20, 871–882. [Google Scholar] [CrossRef]
- Chandrasegaran, K.; Kandregula, S.R.; Quader, S.; Juliano, S.A. Context-dependent interactive effects of non-lethal predation on larvae impact adult longevity and body composition. PLoS ONE. 2018, 13, e0192104. [Google Scholar] [CrossRef] [PubMed]
- Segev, O.; Verster, R.; Weldon, C. Testing the link between perceived and actual risk of predation: Mosquito oviposition site selection and egg predation by native and introduced fish. J Appl Ecol. 2017, 54, 854–861. [Google Scholar] [CrossRef]
- Dumont, F.; Lucas, É.; Alomar, O. Oviposition behavior of the mirid Macrolophus pygmaeus under risk of intraguild predation and cannibalism. Insect Science. 2021, 28, 224–230. [Google Scholar] [CrossRef]
- Ninkovic, V.; Feng, Y.; Olsson, U.L.; Pettersson, J. Ladybird footprints induce aphid avoidance behavior. Biological Control. 2013, 65, 63–71. [Google Scholar] [CrossRef]
- Chamberlain, J.D.; Clifton, I.T.; Gifford, M.E. Influence of prey size on reproduction among populations of Diamond-backed Watersnakes (Nerodia rhombifer). Canadian Journal of Zoology. 2017, 95, 929–935. [Google Scholar] [CrossRef]
- Mills, N.J. Satiation and the functional response: A test of a new model. Ecological Entomology. 1982, 7, 305–315. [Google Scholar] [CrossRef]
- Xiong, X.; Michaud, J.P.; Li, Z.; Wu, P.; Chu, Y.; Zhang, Q.; Liu, X. Chronic, predator-induced stress alters development and reproductive performance of the cotton bollworm, helicoverpa armigera. BioControl. 2015, 60, 827–837. [Google Scholar] [CrossRef]
- Mikolajewski, D.J; Brodin, T.; Johansson, F.; Joop, G. ; Phenotypic plasticity in gender specifc life-history: Efects of food availability and predation. Oikos. 2005, 110, 91–100. [Google Scholar] [CrossRef]

| Response variables | Predictor variables | Coef | Exp (coef ) | Se (coef) | Z | Pr (>|z|) |
| Development time (isolated predator experiments) | treatments | 0.349 | 1.417 | 0.164 | 2.132 | 0.033 |
| sexes | -0.107 | 0.898 | 0.163 | -0.656 | 0.512 | |
| Treatments: sexes | -0.346 | 0.708 | 0.226 | -1.529 | 0.126 | |
| Development time break//(predator odor experiments) | treatments | 0.395 | 1.485 | 0.172 | 2.304 | 0.021 |
| sexes | -0.025 | 0.975 | 0.172 | -0.145 | 0.885 |
| Response variables | Predictor variables | df | chisq | Pr (>F) |
| Fecundity (isolated predator experiments) | treatment | 1 | 33.10 | <0.001 |
| time | 1 | 83.48 | <0.001 | |
| treatment: time | 1 | 8.33 | 0.004 | |
| Fecundity (predator odor experiments) | treatment | 1 | 3.95 | <0.001 |
| time | 1 | 359.95 | 0.047 | |
| treatment: time | 1 | 1.76 | 0.183 |
| Response variables | Predictor variables | df | chisq | Pr (>F) |
| (a) Body weight at death (isolated predator experiments) | time | 1 | 2.599 | 0.107 |
| treatment | 1 | 1.214 | 0.271 | |
| sex | 1 | 13.437 | <0.001 | |
| time:treatment | 1 | 0.171 | 0.679 | |
| time:sex | 1 | 0.818 | 0.366 | |
| treatment:sex | 1 | 0.487 | 0.485 | |
| time:treatment:sex | 1 | 0.471 | 0.493 | |
| (b) Body weight at death (predator odor experiments) | time | 1 | 19.753 | <0.001 |
| treatment | 1 | 2.012 | 0.156 | |
| sex | 1 | 9.781 | 0.002 | |
| time:treatment | 1 | 1.440 | 0.230 | |
| time:sex | 1 | 1.313 | 0.252 | |
| treatment:sex | 1 | 0.280 | 0.597 | |
| time:treatment:sex | 1 | 1.574 | 0.210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




