1. Introduction
Any solid cancer has two components: tumor cells and stroma. Neoplastic cell groups without the capacity to stimulate vessel and stroma growth will remain subclinical.
The tumor stroma consists of a matrix (basement membrane and extracellular matrix), cells (fibroblasts, immune cells) and vasculature. Tumor stroma is a modified connective tissue, which might promote growth, invasion and metastasis. Carcinoma-associated fibroblasts (CAFs) constitute a major portion of the reactive tumor stroma and play a central role in tumor progression. This transformation is driven by cytokines secreted by tumor cells. [
1]
Colorectal cancer is the third most common type of cancer in men and the second in women. [
2] Most patients in early stages have an excellent prognosis. Still, a minority of patients have more aggressive early disease. We cannot satisfactory predict which patient is at higher risk of relapse and thus needs more aggressive multimodal treatment. There is some evidence that the area covered by stroma on a microscopical cross section of the tumor might give hints about the tumor’s aggressiveness. The data regarding the different studies examining the methodology and role of the tumor-to-stroma ratio (TSR) is described in detail in the Discussion section. In general a stromal content of more than around 50% is assessed by the different authors, although different cut-off values are also used for very-high stroma tumors. Both optical and automated reading of stroma is used [
3] TSR is not yet adopted in clinical guidelines, and we consider its adoption early. In our study we tried to confirm or dismiss the hypothesis that a simple, unselective optical measurement of stroma quantity at the invasion front is an easy-to-use and strong prognostic tool.
2. Materials and Methods
We included 74 consecutive patients with colorectal cancer who underwent curative abdominal surgery as the first therapeutic step in the period of January 2006 and December 2013 at our tertiary academic cancer unit. We thought it is appropriate to merge rectal and non-rectal colon cancer, since other study groups has done the same. Inclusion criteria were colon adenocarcinoma treated curatively in the above period and a standardized pathology examination. Exclusion criteria were synchronous colon or other cancer types, neoadjuvant treatment, positive resection margins, very early colon cancer (pT1/2 N0M0, since it has very good prognosis), incomplete follow-up and low-quality pathology specimens.
Neoadjuvant treatment was excluded as it can increase the percentage of stroma by tumor cell apoptosis. [
4] We have also excluded mucinous tumors, known to have a worse prognosis.
We choose to evaluate surgical specimens instead of biopsies in order to have the whole tumor analyzed. We have selected representative regions from the tumor.
In all our cases one pathology specialist with more than 20 years of experience selected the most invasive part of the resected tumor (the portion which is used to establish the T denominator of the tumor and comprises “the invasion front”, defined as the region with the deepest infiltration). [
5] From this portion, 5 µm thick sections were acquired, colored with hematoxylin-eosin (HE) staining and examined under conventional microscopy.
The invasion front was pinpointed searching with a 2.5x or 5 x objectives. This region was then examined through a 10x objective and a selection of an area with both tumor and stromal cells was performed. For the field of view (FOV) an area with tumor cells on all margins was chosen. When mucus was present the pathologists visually excluded it from the area.
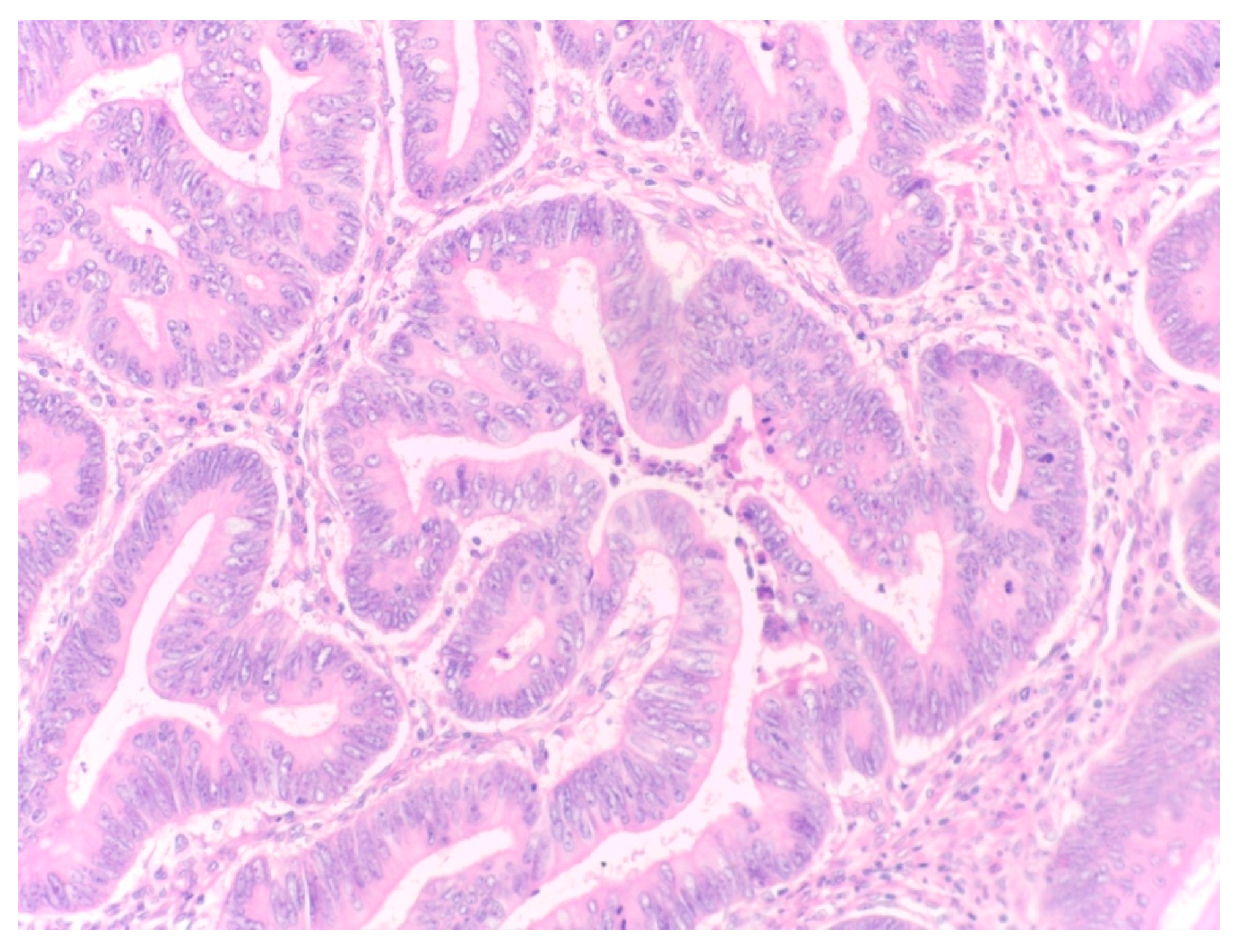
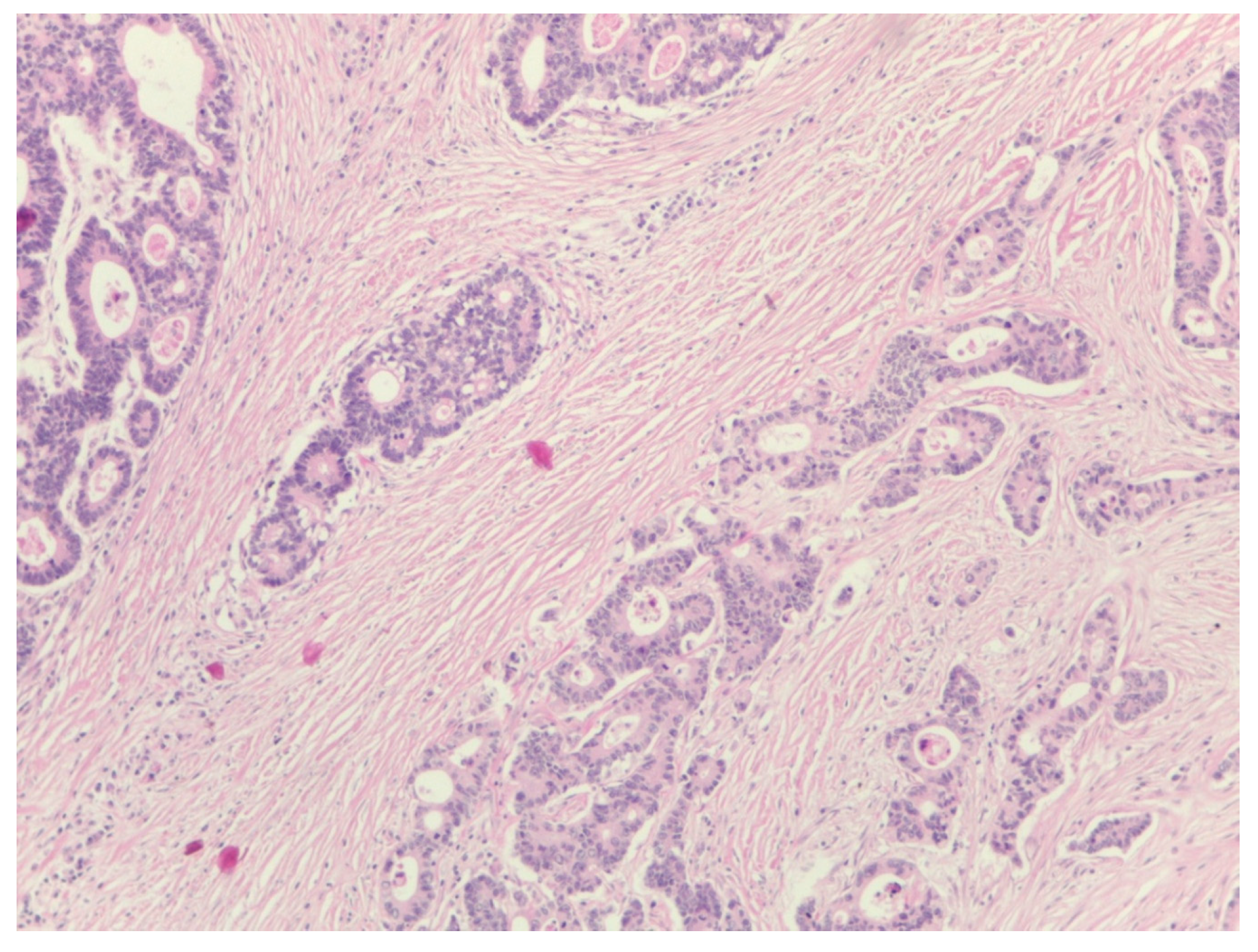
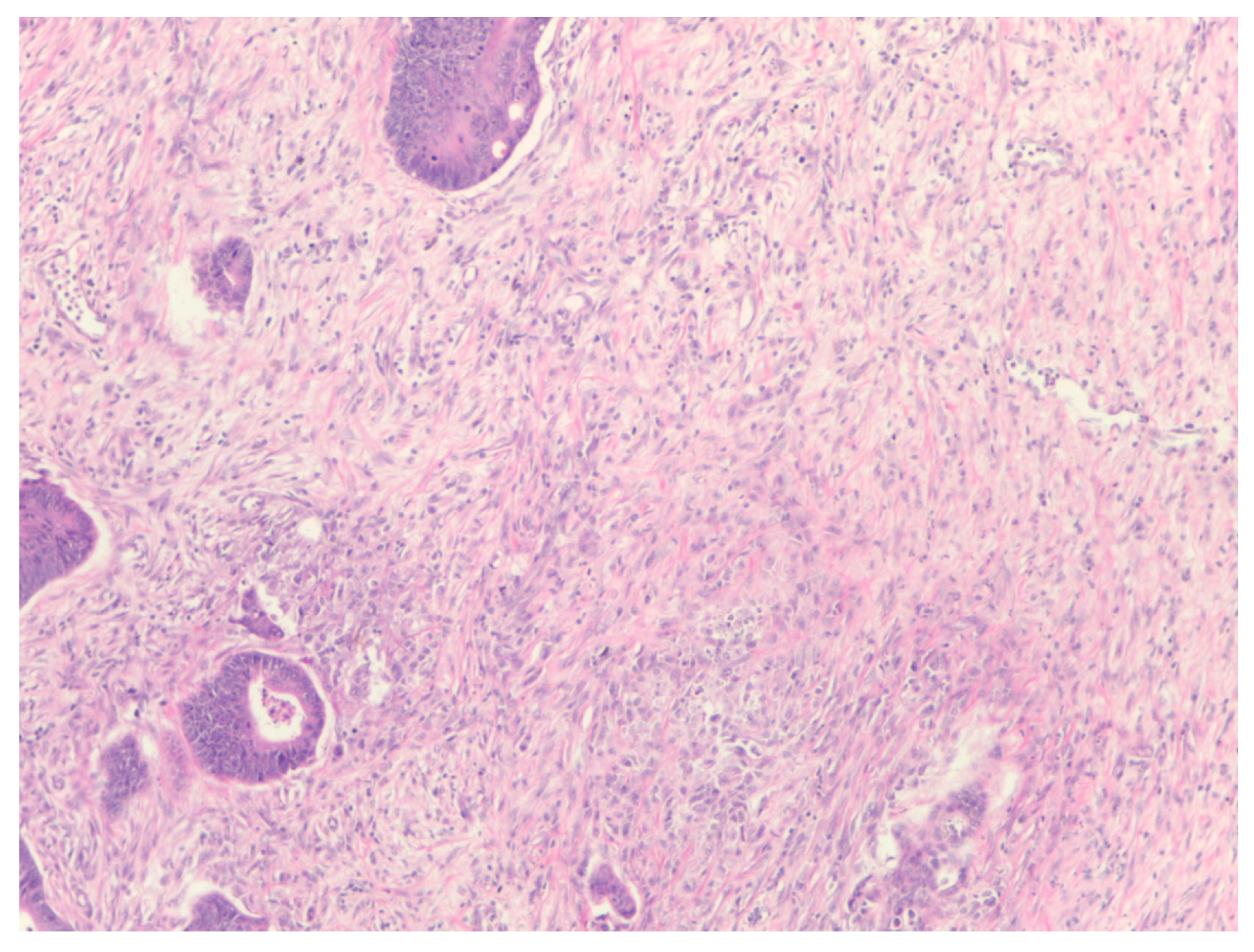
The percentage of stroma was estimated by two examiners (L.R. and A.R.). The tumors were grouped in three categories, stroma-poor (stroma <10%), medium-stroma (between 10 and 50%) and stroma rich (over 50%), based on earlier publications [
6,
7,
8]. For representative microscopic section see
Figure 1,
Figure 2 and
Figure 3.
We have included in our study all classical prognostic factors, such as gender, stage, histological subtype, grading, lymphovascular and perineural invasion, tumor size and location, treatment type and sequence. We have also included in our analysis body mass index, the presence of diabetes, the individual surgical team, distance from the anal verge and presentation in complete occlusion. All patients underwent an R0 resection. Patients were treated according to local guidelines based on current ESMO and NCCN recommendations. Microsatellite status was not evaluated at the time of the analysis.
The statistical analysis has been performed in Excel Microsoft Office. With the chi-square test for distinct values (with Yate’s correction) we have analyzed the risk factors for relapse. Survival analysis has been performed with the help Kaplan-Meier curves and log-rank test. A multivariate analysis has been performed with the Cox regression. A p value of p≤0.05 has been considered statistically significant.
Staging has been performed according to 7th edition of the American Joint Committee on Cancer Staging (AJCC) Manual (2010).
3. Results
The age of the patients ranged from 34 to 79, with a median of 59. From 74 patients 22 (29.7%) had non-rectal colon cancer and 52 (70.3%) rectal cancer; 38 (51,40%) were males. Patient characteristics can be observed in
Table 1.
Of the 60 rectal cancer patients, 36 underwent anterior resection with sphincter sparing, 1 patient anterior resection with temporary stoma and 23, abdomino-perineal resection. Of the 14 colon cancer patients, 8 patients underwent limited segmental resection and 6 patients hemicolectomy. Out of the 60 rectal cancer patients, 44 required adjuvant chemoradiation. Tumor size on the pathologic specimen measured between 1.5 and 10 cm, with a median of 5 cm.
The median follow-up was 75.8 months (range 6.2-98.7). In this period there were 15 deaths (20%), 12 (16%) due to cancer relapse and 3 (4%) from other causes.
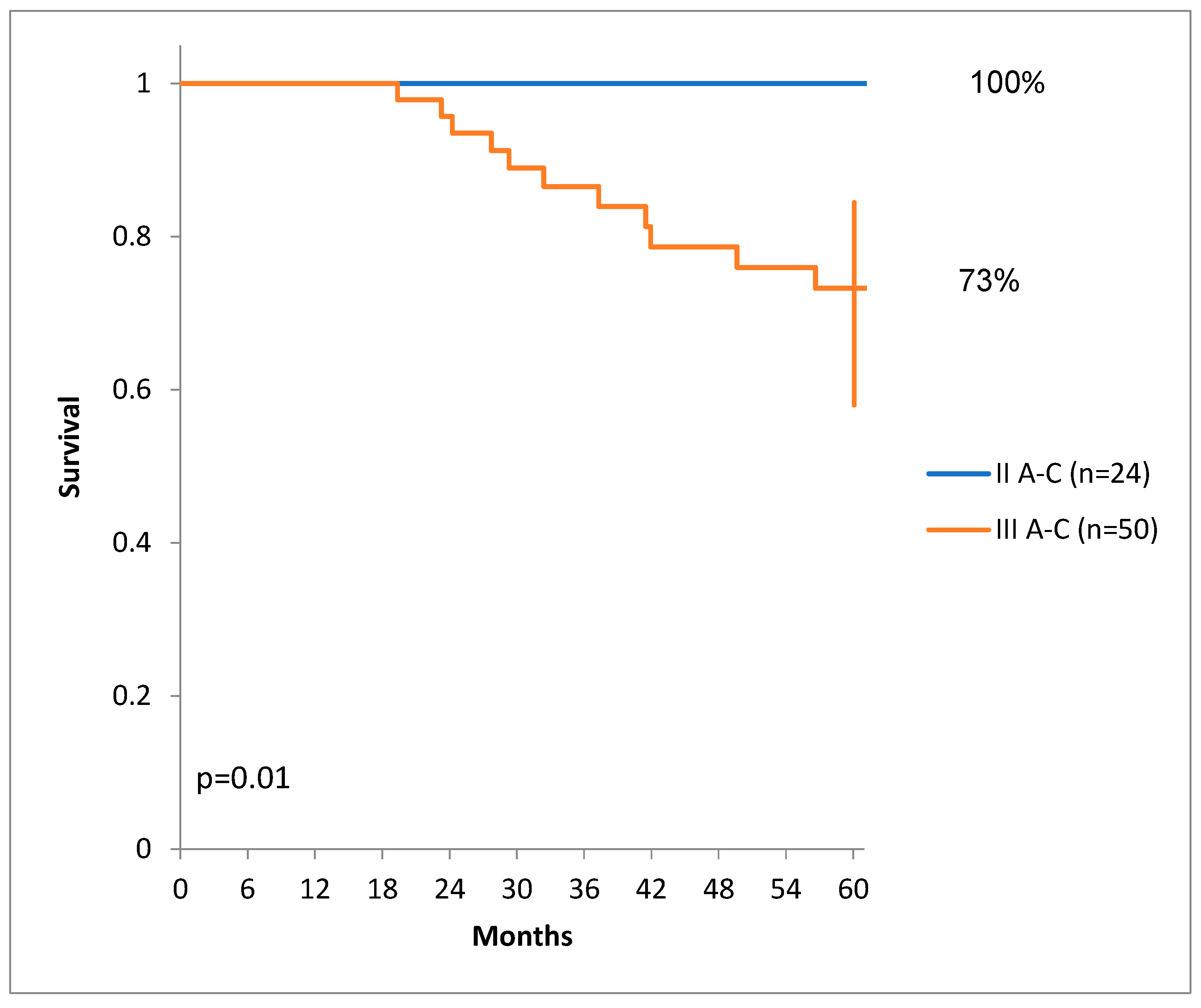
There was a statistically significant difference of DSS for patients with lower stages than patients with higher stages of colon cancer. (
Figure 4.)
Sex and age did not influence DSS in our patient group.
The proportion of tumor stroma ranged from 5% to 70% with a median of 25%.
Five patients (6.8%) had stroma-rich tumors (over 50% stroma), 3 patients (4%) stroma-poor tumors (less than 10% stroma) and 66 patients (89.2%) medium quantity of stroma.
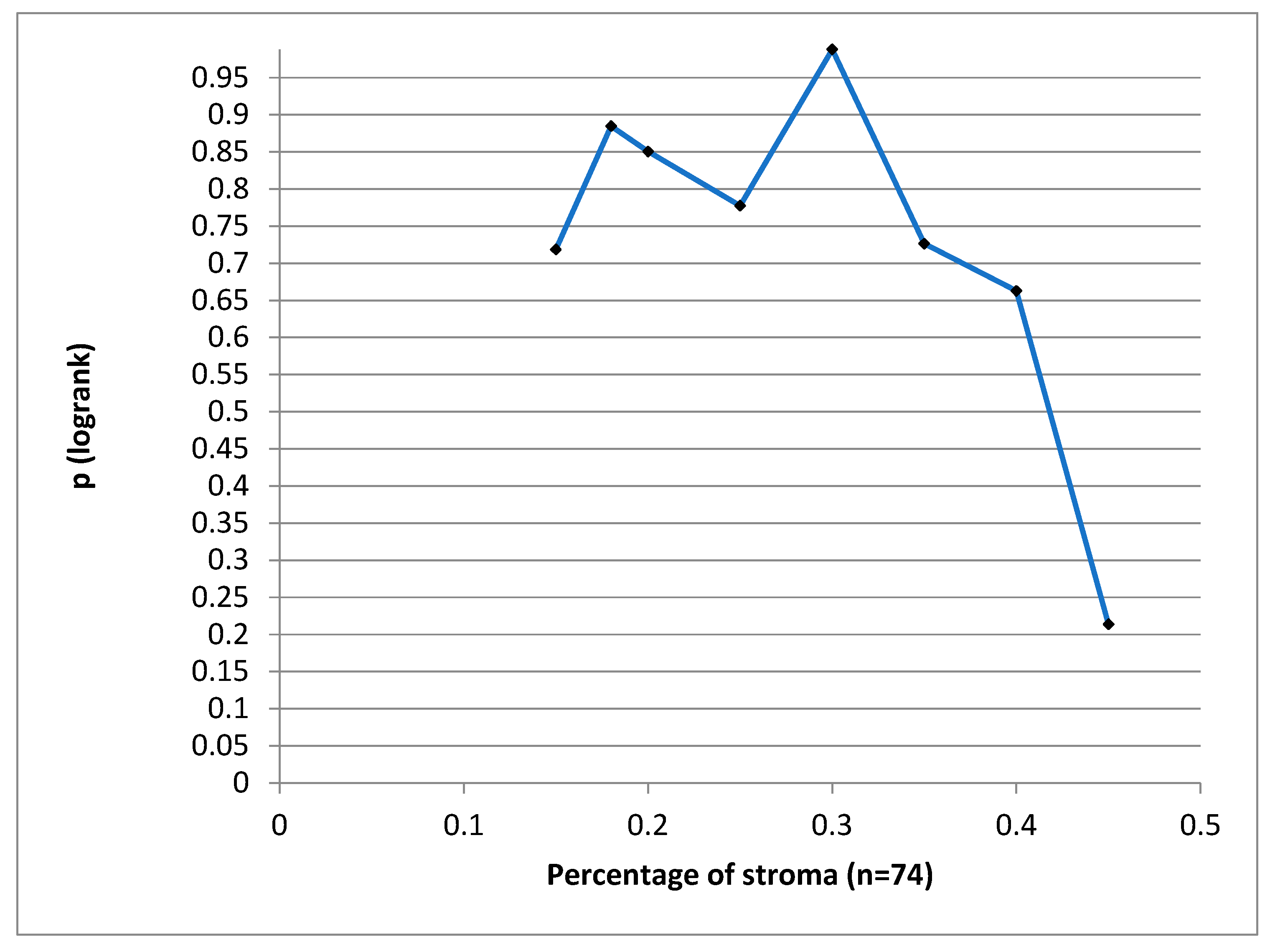
We have tried to define a cut-off value for the stroma proportion which could have statistical significance for the DSS, but we could not find such a value. (
Figure 5)
Since the median percentage of stroma is 25% for our patients, for illustration purposes we drew the DSS for a cut-off of 25% in
Figure 6.
We analyzed separately the influence of tumor stroma on local control rate and metastases, but there was no statistically significant difference. Rectal cancers were no different from other colon cancers in regards of stroma proportion.
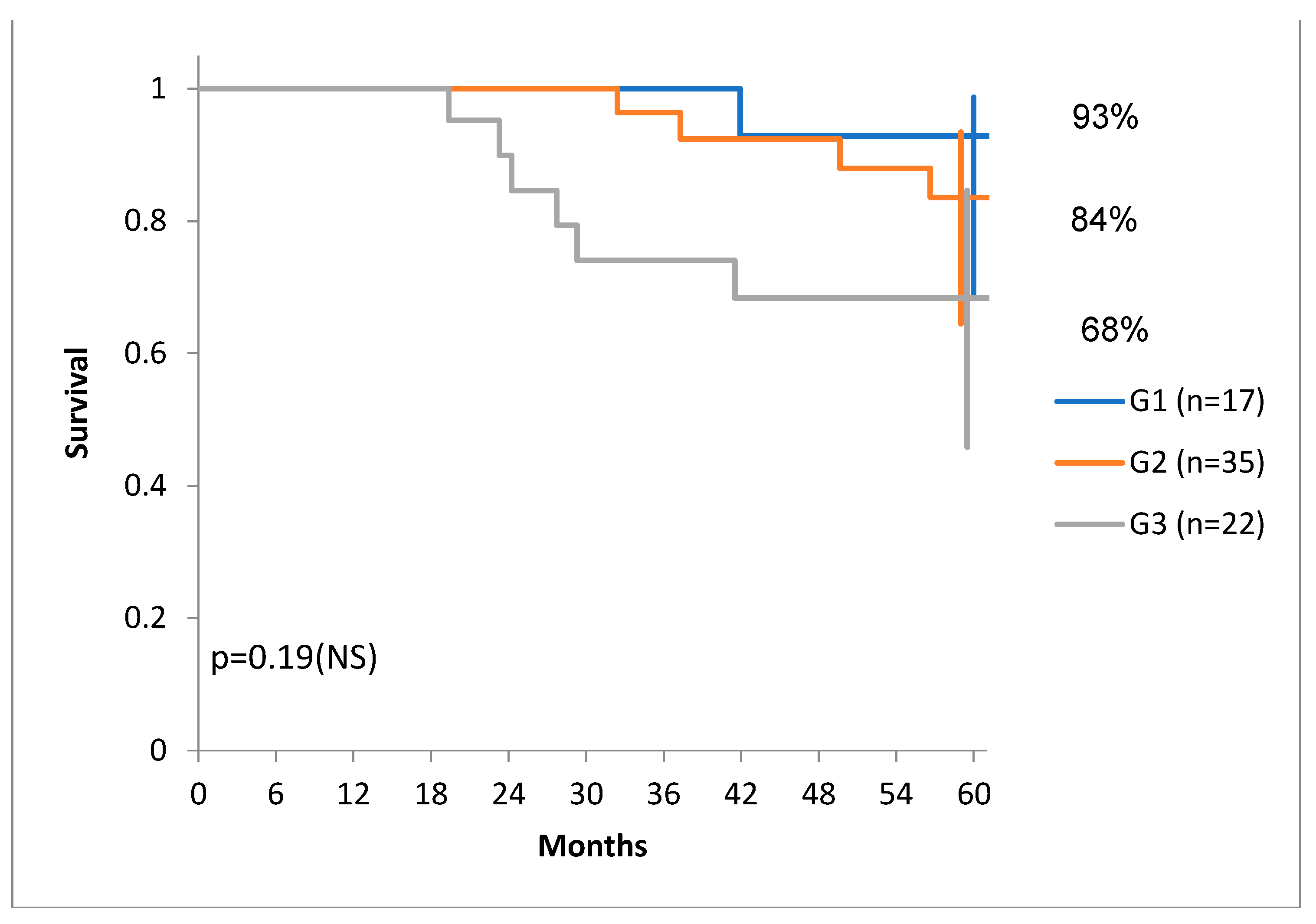
We have to note, that there was a tendency of higher DSS for G1 and G1 tumors compared to G3 tumors (
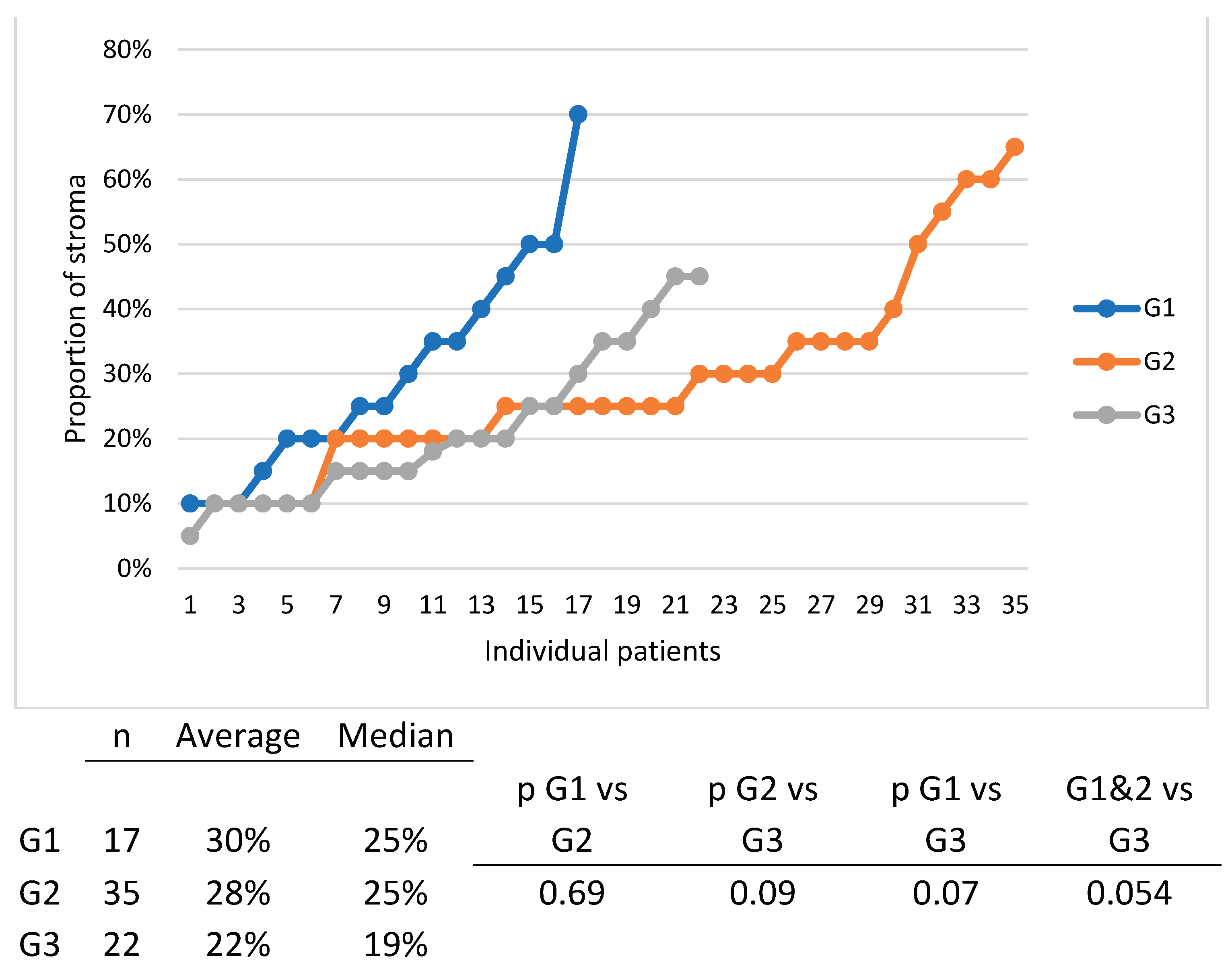
Figure 7) and there was also a tendency of distribution of tumors with more stroma in the G1 and G1 group, compared to the G3 group. (
Figure 8).
Multivariate analysis with Cox regression did not yield any significant prognostic factor.
4. Discussion
We think that the current analysis, although performed on a lower patient number, is important, since it could not confirm the results of the first study teams that described that unselective TSR measurement is a prognostic factor in colorectal cancer, namely Huijbers et al [
6], West et al [
7] and Park et al [
8]. These differences could be explained by several factors. At first look, we could easily conclude, that simply, a smaller number of patients is the main causative factor, although the explanation might be more complex, as we would demonstrate below. Certainly, we have included only 74 patients, versus 710, 145 and 330. At the same time, if this pathologic feature has a measurable impact only when analyzing very large number of patients, it means that its importance as a prognostic factor is not substantial or there are more to this prognostic factor than sheer unselective measurement at the tumor invasion front.
In the study of Huijbers et al [
6] the authors have divided the tumors into ‘stroma-high’ (>50%) and ‘stroma-low’ (≤50%) as determined a priori to have maximum discriminative power. Almost 30% of the tumors were scored as stroma-high. In the stroma-high population, the 5-year OS was lower, only 69% versus 83.4% within the stroma-low population. For the DFS, the 5-year survival rates for stroma-high and stroma-low were 58.6% versus 77.3%.In our study only 6.8% of patients had rich stroma, and thus this might be a reason of the negative results.
West et al [
7] set a similarly high cut-off for stroma high tumors, i.e.,>53%. Almost half of their patients had stroma-high tumors, as compared to our group, where only 6.8% presented stroma-high cancers. Stroma proportion was an independent prognostic factor for OS in their population.
In the study published by Park et [
8] the authors chose a similar cut-off value for stroma-high tumors (>50%) and found that 24% of tumors were stroma-high. Low TSR was associated with worse prognosis and with high T and N stages.
Micke at al argued
against using TSR as a simple prognostic tool in cancer. [
9] The primary aim of their study was to provide a comprehensive and objective analysis of the tumor stroma in 16 different solid cancer types, including over 2500 patients. They used an immunofluorescent staining for epithelial markers and a machine learning image analysis and calculated the stroma fraction for each individual cancer case. Major differences in the median levels of stroma fraction, ranging from less than 25% in renal cell carcinoma to over 70% in pancreatobiliary type periampullary cancer were found, but also large variations in one histological subtype. For example, the median value for colorectal cancer was around 25% (identical to our study), and varied greatly from less than 2-3% to over 90%.
In colon cancer the OS was not influenced by the TSR. Higher stroma fraction was significant only in intestinal type periampullary cancer, with a HR of 3.59. Contrarily, in periampullary cancer pancreatobiliary type, a higher stroma fraction was associated with improved OS, HR 0.56. The same positive association was observed for ER negative breast cancer, HR 0.41.
The main characteristics of the studies involving TSR in colon cancer can be seen in
Table 2.
Rani et al showed opposite findings, namely that in head and neck cancer stroma-low tumors have a poorer prognosis. [
10]
We have used only one FOV for establishing TSR and did not use several fields to calculate an average value, as did Park et al. [
8]. Micke et al [
9], who argue against the importance of TSR, used the whole available specimen and did not select the invasion front as we did in our analysis, or Park et al did.
The Huijbers-Pelt study team has published in 2018 a guideline to rate tumor to stroma ratio. [
11] They have described their method in more detail, stating that
the region with the highest stroma proportion on the slide of the most invasive part should be decisive. Thus they direct their measurement toward the stroma rich area. Their method was cited at this by 70 scientific articles and 8 of these are original studies which tried to replicate their findings. From these 8 studies, 5 produced positive results [
12,
13,
14,
15,
16], two negative [
17,
18] and one quite the opposite in regards of prognostic value [
19]. We think that these mixed results and operator- and method-dependency has determined a low enthusiasm in adopting TSR measurement in clinical guidelines.
The results published by Martin et al were the most surprising [
19]. The authors showed that a high tumor proportion/low stromal proportion (≥ 54% of tumor cells) at the invasion front was associated with distant metastasis and worse overall survival. Additionally, a multivariate Cox regression showed that a high tumor proportion was an independent risk factor from T stage, microsatellite stability, and tumor budding grade. The hazard ratio of patients with high tumor percentage was 3.2. The authors themselves called the findings “quite surprising, because, so far, only a low TP/high SP has been linked to a worse prognosis”. On the other hand very high stroma percentage of more than 85% was also associated with a worse overall survival. Our own findings can be easily explained in the light of these mixed results: we had an insufficient number of very high stroma patients, which seems to bear the worse prognosis, and push survival data on the worse prognosis domain for high stroma tumors.
Moreover, Martin et al [
18] introduces a better prognostic feature than TSR, according to their recent publication. They introduce the term SARIFA, Stroma AReactive Invasion Front Areas. The definition of SARIFA is the “direct contact between a tumor gland/tumor cell cluster (≥5 cells) and surrounding adipose tissue in the invasion front”. TSR was not a prognostic factor in this study.
Strous et al [
12], Aboelnasr et al [
13], Smit et al [
14], Schiele et al [
15] and Kang et al [
16] published positive results. Schiele et al included only pT3 and pT4 tumors. All authors included mucinous tumors, excluded by our group.
Negative results were reported by the Dutch T1 CRC Working Group. [
17] Dang et al showed that stroma proportion is non-prognostic in T1 colon cancers, but they have not excluded, that in larger tumors it might have a prognostic value.
Zhao et al [
20] published yet the largest single cohort, of 814 patients. They employed both human and automated reading of stroma. A cut-off of 48.8%of stroma tissue was found to significant. Stroma-high status was associated with reduced OS in both the discovery (HR 1.72, 95% CI 1.24-2.37, P=0.001) and validation cohort (2.08, 1.26-3.42, 0.004). Their method to evaluate stroma is involving the whole specimen and it is difficult to reproduce.
Eriksen et al [
21] has found that stroma rich stage II colon cancer has a worse survival and it is associated with higher rate of microsatellite stability.
Rather than the TSR, the function of the stroma and thus the proteins expressed [
22] and the gene signature of the tumor-stroma complex seems to be more important in a wider number of colon tumors. [
23] For example in breast cancer the smooth muscle actin (SMA) expression on stromal cells (peritumoral myofibroblasts) was correlated with a higher histological grade. There was even a trend for reduced PFS in the group of node negative tumors with strong SMA stromal expression. [
22] In bladder cancer certain IHC features of the stroma can classify tumors by aggressiveness. [
24]
The DNA or mRNA fragments obtained from the tumor without specific microdissection can be both tumor cell and fibroblast derived. [
25] Isella et al, with our collaboration [
23] described in rectal cancer three main subtypes according to mARN profiles of the tumor-stroma complex: transit-amplifying/enterocyte, goblet/inflammatory and stem/serrated/mesenchymal (SSM). The SSM subtype has been more resistant to chemoradiation. The mARN profile of the SSM subtype has been dominated by mARNs released by stroma fibroblasts. These findings constitute indirect proof that amplified stroma function is a negative prognostic factor.
Another reason which might have cancelled the importance of stroma abundance in our study is tumor grade, since tumor grade is inversely correlated with the quantity of stroma (G1 and G2 tumors have more stroma than G3 tumors), thus canceling out each other’s influence. We propose if anyone is to analyze the contribution of stroma one should only do so after matching tumors with the same grade. This was not possible in our study since we would have needed more patients.
Lymphatic microvessel density (LMVD) is another highly debated prognostic factor with conflicting results and difficulty of reproduction. [
26]
Recently Benias et al. [
27,
28] and Cenaj et al. [
29] described in more detail the human interstitium which merits the denomination of that of a previously unrecognized organ, which is part of the submucosa and it is a fluid-filled space, draining into the lymph vessels and lymph nodes and it is supported by a complex network of thick collagen bundles. These collagen fibers are intermittently lined by fibroblast-like cells that stain with endothelial markers and vimentin. There is a body-wide network of these fluid-filled interstitial spaces. This new information refines the classical knowledge about the simplified local, lymphatic, hematogenous and intracavitary spread. The variable characteristics of the interstitium need to be further investigated in relationship with the metastatic capability of different tumors.
Liu et all [
30] in a preprint article, based on all previous data on TSR tried to define different subtypes of stroma. The study team has showed the tumors with disorganized or heterogeneous tumor stroma with neovascularization were associated with a poor survival, whereas those with aligned and organized tumor stromal "strands" were frequently among the top survival-favorable groups. There is a need to further subdivide colon cancer by microscopic subtype.
5. Conclusions
We can conclude that a simple measurement of TSR is not a robust and easily reproducible prognostic factor in colon cancer patients who underwent primary surgery. The percentage of stroma-high tumors, excluding mucinous tumors, is low in our patient group. Moreover, tumor type, stroma type, T stage, tumor front characteristics such as SARIFA and other factors might play an important role of the TSR measurement. Further international collaboration and adoption automated techniques and machine-learning is needed to standardize tumor categorization and stroma measurement. The complex nature of the stroma-tumor interaction has to be further explained.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, including the use of clinical data for scientific analysis.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient privacy.
Acknowledgments
The authors thank the personnel of the Institute of Oncology “Prof dr. Ion Chiricuta“, Cluj-Napoca for their continuous efforts in fighting cancer.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.-T. The Role of Tumor Stroma in Cancer Progression and Prognosis: Emphasis on Carcinoma-Associated Fibroblasts and Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shen, Z.; Deng, Z.; Mei, L. Impact of Tumor–Stroma Ratio on the Prognosis of Colorectal Cancer: A Systematic Review. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-B.; Wu, J.-P.; Huang, W.-B.; Zhou, H.; Xu, L.-W.; Zhao, J.-H.; Zhu, J.-G.; Su, J.-H. Intensity of stromal changes is associated with tumor relapse in clinically advanced prostate cancer after castration therapy. Asian J. Androl. 2014, 16, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Togano, S.; Yashiro, M.; Miki, Y.; Yamamato, Y.; Sera, T.; Kushitani, Y.; Sugimoto, A.; Kushiyama, S.; Nishimura, S.; Kuroda, K.; et al. Microscopic distance from tumor invasion front to serosa might be a useful predictive factor for peritoneal recurrence after curative resection of T3-gastric cancer. PLOS ONE 2020, 15, e0225958. [Google Scholar] [CrossRef]
- Huijbers, A.; Tollenaar, R.A.E.M.; Pelt, G.W.V.; Zeestraten, E.C.M.; Dutton, S.; McConkey, C.C.; Domingo, E.; Smit, V.T.H.B.M.; Midgley, R.; Warren, B.F.; et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann. Oncol. 2013, 24, 179–185. [Google Scholar] [CrossRef]
- West, N.P.; Dattani, M.; McShane, P.; Hutchins, G.; Grabsch, J.; Mueller, W.; Treanor, D.; Quirke, P.; Grabsch, H. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br. J. Cancer 2010, 102, 1519–1523. [Google Scholar] [CrossRef]
- Park, J.; Richards, C.; McMillan, D.; Horgan, P.; Roxburgh, C. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann. Oncol. 2014, 25, 644–651. [Google Scholar] [CrossRef]
- Micke, P.; Strell, C.; Mattsson, J.; Martín-Bernabé, A.; Brunnström, H.; Huvila, J.; Sund, M.; Wärnberg, F.; Ponten, F.; Glimelius, B.; et al. The prognostic impact of the tumour stroma fraction: A machine learning-based analysis in 16 human solid tumour types. EBioMedicine 2021, 65, 103269. [Google Scholar] [CrossRef]
- Rani, P.; Gupta, A.J.; Mehrol, C.; Singh, M.; Khurana, N.; Passey, J.C. Clinicopathological correlation of tumor-stroma ratio and inflammatory cell infiltrate with tumor grade and lymph node metastasis in squamous cell carcinoma of buccal mucosa and tongue in 41 cases with review of literature. J. Cancer Res. Ther. 2020, 16, 445–451. [Google Scholar] [CrossRef]
- Van Pelt, G.W.; Kjær-Frifeldt, S.; van Krieken, J.H.J.M.; Al Dieri, R.; Morreau, H.; Tollenaar, R.A.E.M.; Sørensen, F.B.; Mesker, W.E. Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. 2018, 473, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.T.A.; van der Linden, R.L.A.; Gubbels, A.L.H.M.; Faes, T.K.E.; Bosscha, K.; Bronkhorst, C.M.; Janssen-Heijnen, M.L.G.; de Bruïne, A.P.; Vogelaar, F.J. Node-negative colon cancer: histological, molecular, and stromal features predicting disease recurrence. Mol. Med. 2023, 29, 1–10. [Google Scholar] [CrossRef]
- Aboelnasr, L.S.; El-Rebey, H.S.; Mohamed, A.; Abdou, A.G. The prognostic impact of tumor border configuration, tumor budding and tumor stroma ratio in colorectal carcinoma. Turk. J. Pathol. 2022, 39, 83–93. [Google Scholar] [CrossRef]
- Smit, M.A.; van Pelt, G.W.; Terpstra, V.; Putter, H.; Tollenaar, R.A.E.M.; Mesker, W.E.; van Krieken, J.H.J.M. Tumour-stroma ratio outperforms tumour budding as biomarker in colon cancer: a cohort study. Int. J. Color. Dis. 2021, 36, 2729–2737. [Google Scholar] [CrossRef]
- Schiele, S.; Arndt, T.T.; Martin, B.; Miller, S.; Bauer, S.; Banner, B.M.; Brendel, E.-M.; Schenkirsch, G.; Anthuber, M.; Huss, R.; et al. Deep Learning Prediction of Metastasis in Locally Advanced Colon Cancer Using Binary Histologic Tumor Images. Cancers 2021, 13, 2074. [Google Scholar] [CrossRef]
- Kang, G.; Pyo, J.-S.; Kim, N.-Y.; Kang, D.-W. Clinicopathological Significances of Tumor–Stroma Ratio (TSR) in Colorectal Cancers: Prognostic Implication of TSR Compared to Hypoxia-Inducible Factor-1α Expression and Microvessel Density. Curr. Oncol. 2021, 28, 1314–1324. [Google Scholar] [CrossRef]
- Dang, H.; van Pelt, G.W.; Haasnoot, K.J.C.; Backes, Y.; Elias, S.G.; Seerden, T.C.J.; Schwartz, M.P.; Spanier, B.W.M.; Cappel, W.H.d.V.T.N.; van Bergeijk, J.D.; et al. Tumour-stroma ratio has poor prognostic value in nonpedunculated T1 colorectal cancer: A multicentre case-cohort study. United Eur. Gastroenterol. J. 2020, 9, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Grosser, B.; Kempkens, L.; Miller, S.; Bauer, S.; Dhillon, C.; Banner, B.M.; Brendel, E.-M.; Sipos. ; Vlasenko, D.; et al. Stroma AReactive Invasion Front Areas (SARIFA)—A New Easily to Determine Biomarker in Colon Cancer—Results of a Retrospective Study. Cancers 2021, 13, 4880. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Banner, B.M.; Schäfer, E.-M.; Mayr, P.; Anthuber, M.; Schenkirsch, G.; Märkl, B. Tumor proportion in colon cancer: results from a semiautomatic image analysis approach. Virchows Arch. 2020, 477, 185–193. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Z.; Yao, S.; Wang, Y.; Wu, X.; Xu, Z.; Wu, L.; Huang, Y.; Liang, C.; Liu, Z. Artificial intelligence quantified tumour-stroma ratio is an independent predictor for overall survival in resectable colorectal cancer. EBioMedicine 2020, 61, 103054. [Google Scholar] [CrossRef]
- Eriksen, A.C.; Sørensen, F.B.; Lindebjerg, J.; Hager, H.; Christensen, R.D.; Kjær-Frifeldt, S.; Hansen, T.F. The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. A nationwide population-based study. Int. J. Color. Dis. 2018, 33, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Catteau, X.; Simon, P.; Jondet, M.; Vanhaeverbeek, M.; Noël, J.-C. Quantification of stromal reaction in breast carcinoma and its correlation with tumor grade and free progression survival. PLOS ONE 2019, 14, e0210263. [Google Scholar] [CrossRef] [PubMed]
- Isella, C.; Terrasi, A.; Bellomo, S.E.; Petti, C.; Galatola, G.; Muratore, A.; Mellano, A.; Senetta, R.; Cassenti, A.; Sonetto, C.; et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 2015, 47, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mezheyeuski, A.; Segersten, U.; Leiss, L.W.; Malmström, P.-U.; Hatina, J.; Östman, A.; Strell, C. Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Berral-González, A.; López-Cade, I.; Galindo-Pumariño, C.; Bueno-Fortes, S.; Martín-Merino, M.; Carrato, A.; Ocaña, A.; De La Pinta, C.; López-Alfonso, A.; et al. Cancer-associated fibroblast-derived gene signatures determine prognosis in colon cancer patients. Mol. Cancer 2021, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Y. Lymphangiogenesis and colorectal cancer. SciVee 2017, 38, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; Klavan, H.; Reidy, J.; Buonocore, D.; Miranda, M.; Kornacki, S.; Wayne, M.; Carr-Locke, D.L.; et al. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; Klavan, H.; Reidy, J.; Buonocore, D.; Miranda, M.; Kornacki, S.; Wayne, M.; Carr-Locke, D.L.; et al. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Cenaj, O.; Allison, D.H.R.; Imam, R.; Zeck, B.; Drohan, L.M.; Chiriboga, L.; Llewellyn, J.; Liu, C.Z.; Park, Y.N.; Wells, R.G.; et al. Evidence for continuity of interstitial spaces across tissue and organ boundaries in humans. Commun. Biol. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Liu B, Polack M, Coudray N et all. Self-Supervised Learning Reveals Clinically Relevant Histomorphological Patterns for Therapeutic Strategies in Colon Cancer. bioRxiv [Preprint]. 2024 Mar 21:2024.02.26.582106. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).