1. Introduction
The term ‘stem cell’ (
stammzelle) was first introduced into the scientific community in the late 19th century by zoologists Theodor Boveri and Valentin Häcker, who proposed the existence of a universal precursor cell for both primordial germ and somatic cells [
1,
2,
3,
4]. Over the past century, our understanding of stem cells has evolved, culminating in their definition as unspecialized cells with an inherent capability for differentiation and self-renewal [
5]. Given their aptitude for regeneration, stem cells have shown tremendous promise in clinical therapy. The first use of stem cells as therapy occurred nearly 70 years ago, when the first bone marrow transplant was administered in 1956 [
6]. Since then, stem cells have been extensively applied and studied in numerous medical specialties, including Orthopaedics, where they have been a major focus of research in the context of bone healing.
It has been suggested that up to 10% of fractures that occur heal incorrectly, leading to an imperfect union [
7,
8,
9]. Extensive research has revealed the critical role of the immune microenvironment in influencing the outcomes of bone healing and regeneration processes [
10,
11]. Claes et al. performed an
in vivo study investigating differences in the cytokine environment between mice with a singular tibial fracture, multiple fractures, or multi-fracture and soft tissue injury. They found that increasing the severity of the injury increased IL-6 levels one day post-injury and halved the load to failure in mechanical tests with reduced callus volume after 28 days of healing [
12]. This experiment highlights the multiplying effect of injury and the need for optimal functioning of the tissues surrounding a fracture in order to obtain the most effective healing of fractures. Understanding the physiology and pathology of fracture healing at the molecular level may provide insight into therapeutic measures to minimize fracture healing failure. Stem cells provide a promising solution to this problem; however, creating an environment that enables stem cells to differentiate correctly and promote regeneration remains a challenge.
This manuscript aims to offer a comprehensive overview of stem cell technology in fracture healing and management, including current applications, potential risks, and complications. It categorizes various stem cells and their roles in bone repair and summarizes the current in vitro and in vivo research on bone regeneration. Additionally, it explores future directions and therapeutic applications of stem cells in enhancing fracture healing.
2. Clinical Background on Bone Healing
Fracture healing is a well-studied topic in orthopedic research. All mechanisms of bone healing involve four basic tissue layers: cortical bone, periosteum, fascial tissue surrounding the fracture, and bone marrow [
13]. Generally, there are two principal mechanisms by which a bone undergoes healing after injury: primary and secondary bone healing. Primary bone healing occurs when a fracture is managed via open reduction and internal fixation, leading to a highly stabilized fracture site [
14]. First, the edges of the bone undergo direct appositional bone growth, followed by the small gaps rapidly filling with mature lamellar bone and the large gaps slowly filling with primitive woven bone [
15]. Woven bone requires further remodeling and undergoes a process known as contact healing. During this stage, a “basic remodeling unit” forms a cutting cone, which includes peripheral osteoclasts that create a cavity across the fracture, allowing the vasculature and mesenchymal stem cells (MSC) to enter the tunnel. MSCs eventually differentiate into osteoblasts, which then restore the lamellar bone to the fracture site [
15].
In contrast, secondary healing is a more common form of bone healing and is observed when there is an increased level of mobility, leading to micromotion at the fracture site [
16]. This type of fracture is often observed in less stable fixation techniques such as splinting, external fixation, and intramedullary nailing. Secondary healing can be divided into five temporal stages. The first stage involves the formation of a hematoma, which is created from the bleeding bone [
17]. Inflammation occurs following hematoma formation, which is essential for the recruitment of the necessary molecular components for bone healing. Many important signaling molecules and their functions were summarized by Phillips in 2005, including interleukin 1 (IL-1), interleukin 6 (IL-6), transforming growth factor β (TGF β), insulin-like growth factor (IGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and bone morphogenetic proteins (BMPs) [
18]. Without these molecules, the bone cannot properly heal itself [
18]. Inflammation also leads to angiogenesis at the fracture site, which is essential for osteogenesis [
16]. Once the site is vascularized, a cartilaginous callus begins to form, and the cartilage eventually calcifies into the bone. Finally, some of the bone is degraded and remodeling occurs via osteoclasts [
18]. Sometimes, these five stages are simplified into three phases: inflammation, repair, and remodeling [
16].
Bone fractures are among the most frequent traumatic injuries encountered in the emergency department [
19,
20]. It has been estimated that there may be three million fractures in 2025 due to the increasing number of elderly patients in the United States [
21]. The orthopaedic field has taken an interest in stem cells, as they show potential benefits for bone healing and regeneration.
2.1. Stem Cell Therapy Current State and Applications
The goal of stem cell therapy is to artificially introduce highly regenerative cells into decrepit biological tissues. The benefit of these cells is that in the right conditions they are molecularly and functionally indistinguishable from standard human tissue. The use of stem cells as a therapeutic to repair damage and regenerate natural tissue has been the focus of many researchers over the past few decades and continues to be a heavily funded endeavor, with the stem cell market reaching nearly 15 billion dollars in 2023 and is expected to increase by 11.43% annually [
22]. This increase in the stem cell market largely stems from the widespread belief that stem cells are not just functional in theory, but also have a role in clinical applications.
3. Stem Cells
Stem cells are a unique type of cell that have the ability to self-renew and differentiate into a variety of cell lineages [
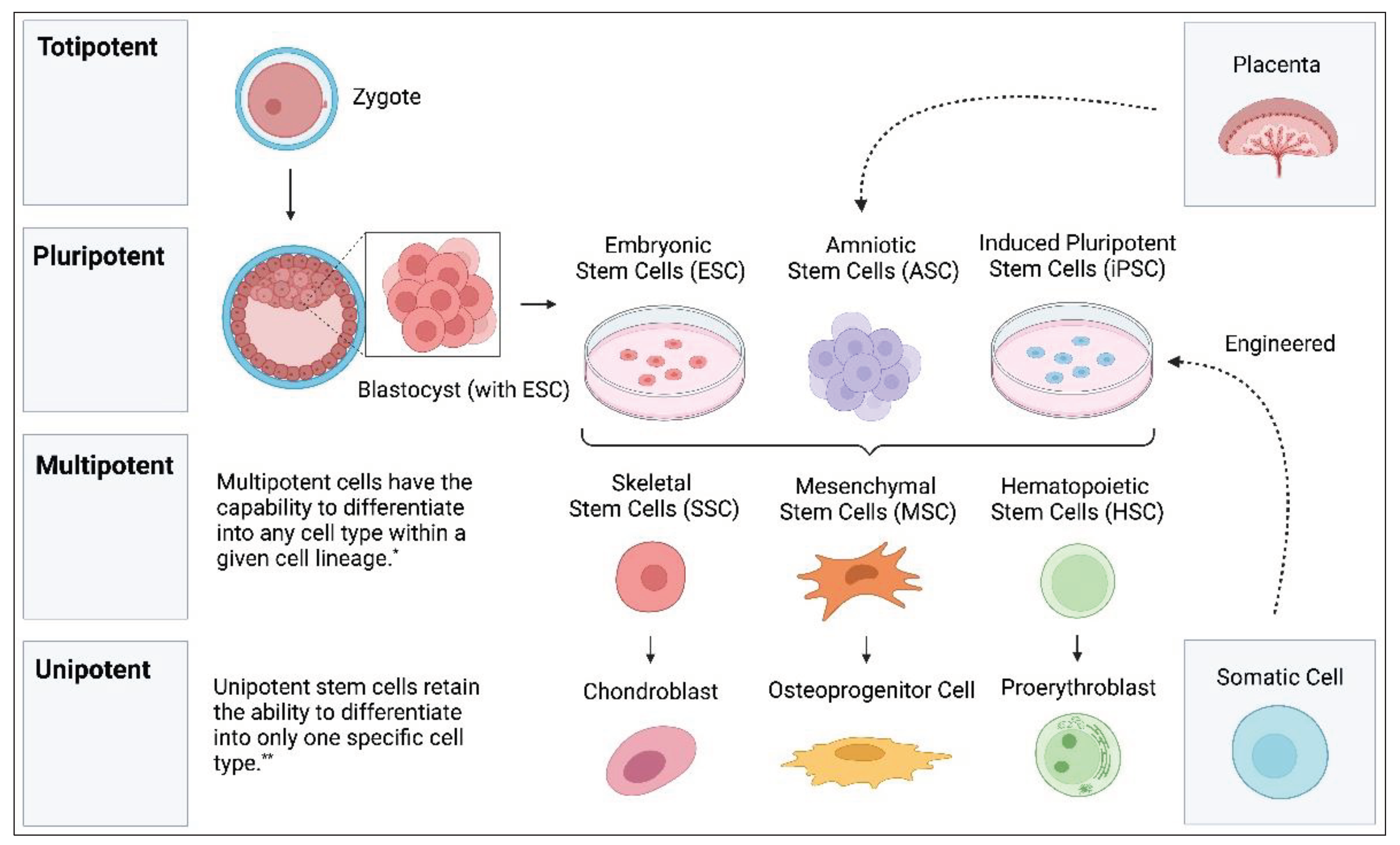
6]. The ability of stem cells to differentiate is deemed potency. Totipotent stem cells are the most versatile type of stem cells and are capable of developing into any cell type in a given organism (both extra-embryonic and embryonic tissue) [
6]. Totipotent stem cells are the cells observed at the zygote stage of embryogenesis. Soon after zygote formation, these cells differentiate into an inner cell mass and trophectoderm [
23]. The inner cell mass consists of the next most potent stem cell, pluripotent stem cells. While pluripotent stem cells alone are unable to become a complete organism, they are capable of differentiating into any of the three embryonic tissue types (endoderm, mesoderm, and ectoderm) [
24]. The final three categories include multipotent, oligopotent, and unipotent stem cells, which can differentiate into specific cell lineages, various cell types, and a single cell type, respectively [
5]. Within the human body, certain stem cells are inherent to specific organs or tissues; however, these cells are not considered primitive. These include satellite cells in muscles and intestinal stem cells, which possess tissue-specific differentiation capabilities rather than pluripotency. For therapeutic applications, the focus is often shifted to pluripotent stem cells, which are capable of differentiating into various tissue types.
Figure 1 illustrates the predominant stem cells currently utilized in the field.
3.1. Human Pluripotent Stem Cells (hPSCs)
Human pluripotent stem cells are stem cells derived from human tissue that can produce any type of embryonic tissue. These cells are typically grouped into two categories: naturally derived embryonic stem cells and induced pluripotent stem cells (iPSCs).
3.1.1. Embryonic Stem Cells (ESCs)
Embryonic Stem Cells (ESCs) are pluripotent stem cells extracted from the inner cell mass of a blastula [
27]. Neonatal MSCs, on the other hand, originate from the umbilical cord, placenta, and amnion [
28,
29]. The fact that ESCs can self-renew at a high capacity, coupled with their ability to differentiate into embryonic tissues, makes them an extremely attractive cell type for potential regenerative therapies. However, major obstacles remain to the successful implementation of ESCs in clinical practice. One challenge with ESCs is being able to differentiate them into the desired cell lines [
30]. Another complication of ESCs is the risk of them differentiating into cancerous tissues [
31]. Several studies have evaluated ESCs and their risk for teratoma formation in both immunocompromised and immunocompetent mice models [
32,
33,
34,
35,
36,
37,
38,
39,
40]. An additional challenge of allogeneic and autologous ESCs is that they carry high and moderate risks of host immune rejection, respectively. This is particularly true when the cell reaches a differentiated state, as it is more likely to have a higher number of donor major histocompatibility class I (MHC I) proteins on its surface [
41]. To make matters more challenging, immune-mediated cell death of donor ESCs often occurs rapidly, preventing the cells from having time to produce their regenerative effect [
30]. Finally, and most importantly, there is an ongoing debate regarding whether the use of embryonic stem cells for research is ethical. Much of this discussion centers on what scientifically constitutes a human life and often involves a vast array of differing social, cultural, and religious ideologies [
1,
42].
Despite a shift away from the use of ESCs, ESC use is still prevalent, with 50 clinical trials on ESCs having been performed between 2011 and 2022 [
43]. However, these clinical trials have largely been limited owing to ethical and experimental concerns [
44]. Some of these limitations include the development of chromosomal abnormalities when cultured for long periods, failure of ESCs to become mature and functional cells, the aforementioned potential for ESCs to create teratomas, and continual ethical concerns [
45,
46,
47].
In Orthopaedics, ESCs have shown the potential to induce chondrogenesis and osteogenesis
in vitro and
in vivo; however, their clinical applications have been sparse. Bielby and Polak showed that osteogenic differentiation of human ESCs into bone tissue was successfully observed via the identification of osteocalcin and Runx2, an osteogenic transcription factor. Furthermore, they implanted this tissue into immunocompromised mice using a poly-D, L-lactide scaffold and found evidence of ESC-derived mineralized tissue after 35 days [
48]. In 2021, Petrigliano et al. performed xenotransplantation of human ESCs into porcine knee joints and analyzed chondrogenesis. They found that chondrocyte-differentiated ESCs led to better cartilage repair of the knee articular cartilage six months after transplantation. Additionally, they found no difference in the inflammatory infiltrates between the ESC and control groups, indicating a lack of immunogenicity [
49].
3.1.2. Induced Pluripotent Stem Cells (iPSCs)
Induced Pluripotent Stem Cells (iPSCs) are a type of engineered stem cell, similar in function to Embryonic Stem Cells (ESCs). Unlike ESCs, these cells are produced from somatic cells and are biologically induced to become pluripotent. The discovery of iPSCs came about in 2006 when it was elucidated by Takahashi and Yamanaka [
50]. They discovered that the alteration of four murine transcription factors through a retrovirus can lead to an induced stem cell with pluripotent capabilities nearly identical to those of ESCs. One year later, Yamanaka et al. successfully performed these experiments using dermal fibroblasts [
51]. Since then, the bioengineering of iPSCs has increased exponentially, and new gene cell therapies have been used to induce pluripotency, which no longer requires the use of a retrovirus. The recent discovery of CRISPR/Cas9 gene editing has made the genetic editing of iPSCs even easier and more flexible [
52].
The advantages of using iPSCs are numerous, but the two most obvious are the relatively lower level of ethical concern compared to the use of ESCs and the autologous nature of iPSC therapy. Given that iPSCs are harvested from somatic cells rather than embryonic cells, the ethical concerns are minimal compared to ESCs [
53]. In addition to ethical advantages, iPSCs also provide improvements from an immune rejection standpoint. As mentioned earlier, therapeutic ESCs are necessarily harvested from a non-self-human, which, like any allogeneic transplant, presents a risk of transplant rejection. iPSCs, on the other hand, can be harvested from the patient, engineered to pluripotency, and then reintroduced into the patient’s body. Unfortunately, some studies have found various instances of immune rejection when using iPSC transplantation [
54,
55]. Coupled with this disadvantage, iPSCs have often been shown to form teratomas [
56,
57,
58]. The teratogenicity of iPSCs warrants further research to elucidate their characteristics on safety and efficacy in clinical applications. [
56]. Finally, the induction of iPSCs into the desired cell lineage can be tremendously challenging. One study reported a success rate of less than 1% for the induction of mouse somatic cells [
59].
The use of iPSCs in clinical trials has increased significantly since 2016, presumably owing to their advantages over ESCs. From 2017 to 2022, 53 clinical trials involving iPSCs have been performed [
43]. Some of the clinical applications studied include cancer, cardiac, and ocular therapies [
43]. In the context of bone healing, several studies have examined the effectiveness of iPSC therapy
in vivo. In 2022, Zhou et al. isolated bone marrow mesenchymal stem cells from 16 patients with femoral head osteonecrosis and 12 patients with femoral neck fractures. These mesenchymal stem cells were reprogrammed into iPSCs and then differentiated into iPSC mesenchymal stem cells (iPSC-MSCs). When compared
in vivo to bone marrow mesenchymal stem cells (BM-MSCs), iPSC-MSCs were morphologically and immunophenotypically similar. They also showed similar DNA methylation patterns. However, the iPSC-MSCs showed a higher level of proliferation. In a pre-clinical
in vivo rat femoral head necrosis model, iPSC-MSCs showed equivalent results in comparison to BM-MSCs in regards to bone maintenance and repair, indicating the feasibility of using iPSCs in clinical bone-healing therapies [
60].
A similar study was performed by Jungbluth et al., who examined the effects of iPSC-MSCs on osteogenesis
in vivo using a mini-pig model. Following radiologic and histomorphometric analysis, they found that iPSC-MSC transplant with calcium phosphate granules (CPG) showed significantly greater amounts of new bone formation compared to transplant with CPG alone after 6 weeks [
61]. iPSC-MSCs showed comparable results to bone marrow MSC transplants and autologous bone marrow concentrate transplants [
61]. In 2018, Wu et al. observed calcified matrix growth upon the ectopic implantation of iPSC-MSCs into nude mice. Interestingly, they also found a significant increase in angiogenesis around these sites when iPSC-MSCs were co-transplanted with anti-bone morphogenic protein 2 (BMP2) antibodies. They concluded that these antibodies bind to BMP2 receptors on iPSC-MSCs, inducing angiogenesis and promoting greater osteogenesis [
62].
3.1.3. Amniotic Stem Cells (ASCs)
A third, less common form of pluripotent stem cells exists, which is derived from gestational tissues (namely the placenta) and has aptly been termed amniotic stem cells (ASCs) [
6]. Within the category of ASCs, there are a number of subtypes of cells, including human amniotic mesenchymal stromal cells (hAMSCs), human umbilical cord mesenchymal stromal cells (hUMSCs), and human amniotic epithelial stem cells (hAESCs) [
63]. During the first two weeks of embryonic development, blastocysts are created and implanted into the endometrial stroma. Following this, a primordial amniotic cavity is created. A layer of epiblast cells surrounds this cavity, which eventually differentiates into amnioblasts, from which the amniotic epithelial layer and hAESCs are derived [
6,
64]. In contrast, other amnioblasts are derived from the hypoblast layer and eventually differentiate into amniotic connective tissues. This tissue is the origin of hAMSCs [
6,
64].
ASCs have several potential advantages over ESCs, iPSCs, and mesenchymal stem cells. First, these cells are often relatively easy to harvest and are obtained from the placenta, which would otherwise be discarded [
63]. Similar to iPSCs, ASCs are derived from non-embryonic gestational tissue and thus have fewer ethical concerns [
6]. Additionally, ASCs have been shown to have very low immunogenicity, which provides clear advantages for ASCs in potential clinical therapy. The immuno-privilege that ASCs experience is due to their low expression levels of major histocompatibility class I antigen (HLA-ABC) and lack of expression of major histocompatibility class II antigen (HLA-DR), β2 microglobulin, and HLA-ABC costimulatory molecules CD40, CD80, and CD8635 [
6,
65]. In 2018, a clinical trial involving hAESC transplantation was performed in infants with bronchopulmonary dysplasia. Of the six babies that received the transplant, none showed acute immune rejection [
66].
Arguably, the most notable advantage of ASCs is their suspected lack of tumorgenicity. Numerous studies have analyzed the tumor-forming potential of ASCs
in vivo and have shown no tumor formation [
67,
68,
69,
70]. In addition, Phermthai et al. used karyotype analysis to show that ASCs display high chromosomal stability [
71]. Recently, studies have been performed to determine whether ASCs can be used for cancer treatment. Interestingly, they found that ASCs can exhibit both tumor promotion and tumor suppression effects depending on the cytokines they secrete [
72]. Another study by Meng et al. similarly examined ASCs in the context of tumor treatment and found that umbilical cord MSCs (hUCMSCs) showed great anti-tumor potential, whereas amniotic membrane MSCs (hAMSCs) showed both inhibitory and excitatory effects [
73]. Although there is some evidence that ASCs promote pre-existing tumors, they are still widely recognized as non-teratoma-forming stem cells and are thus attractive therapeutically [
74].
In 2019, Mohammed et al. compared
in vivo the healing effects of ASCs to what is by some considered the gold standard for stem cell therapy, bone marrow mesenchymal stem cells (BM-MSC). They induced a surgical defect in the lumbar spine of rats and subsequently injected ASCs or BM-MSCs at the injury site. They found that the rats that received ASC therapy not only showed osteogenesis but also showed that it occurred at a more advanced stage than that seen in BM-MSC therapy [
75]. Basile et al. examined the effects of human-ASC transplantation compared to mouse bone marrow stromal cells (mBMSCs) transplantation in mice with calvarial bone defects. ASCs were exposed to an osteogenic stem cell medium plus ascorbic acid for ten days before transplantation and then transplanted using a scaffold. Through GFP staining of host cells, they showed that ASCs promoted the incorporation of host cells into the graft. The transplant also produced bone-like tissue in the defect compared to controls. However, it was concluded that ASCs did not themselves promote osteogenic differentiation; rather, it was their recruitment of host cells that led to bone growth [
76].
3.2. Multipotent Stem Cells
Multipotent stem cells are cells that have the capability to differentiate into any cell type within a given cell lineage [
25]. These cells are naturally found throughout the human body, and are often harvested from different cell lineages. These cells can be classified into three primary categories: mesenchymal stem cells (MSC), hematopoietic stem cells (HSC), and neuronal stem cells [
77]. However, our review focuses on MSCs, HSCs, and a more recently discovered skeletal stem cell (SSC), as these are the primary cells implicated in bone healing [
78,
79].
3.2.1. Mesenchymal Stem Cells (MSCs)
Mesenchymal Stem cells (MSCs) are multipotent stem cells that are found throughout the human body. These regenerative cells are constantly working, creating new tissues to replace old or damaged cells. Adult MSCs are derived from adult tissues, such as bone marrow, adipose tissue, peripheral blood, and dental pulp. In clinical research, it has been suggested that adipose tissue MSCs (AT-MSCs) are the most frequently used MSCs [
80]. This is likely due to the relative ease of harvesting AT-MSCs, particularly when compared to other commonly used tissues, such as bone marrow MSCs.
Numerous studies have been performed on MSCs and their capability to effectively produce and heal bone
in vitro. Much of the discussion of
in vitro MSCs study centers on the efficacy of BM-MSCs versus AT-MSCs. One study performed by Mohamed-Ahmed et al. compared human BM-MSCs and AT-MSCs from nine donors. Their results showed that AT-MSCs led to a significantly higher number of adipogenesis-related genes as well as lipid vesicle formation [
81]. In contrast, BM-MSCs showed a greater propensity for bone and cartilage regeneration as measured by increased alkaline phosphatase (ALP) activity, greater calcium deposits, and increased expression of osteogenic gene markers [
81]. These results were corroborated by another study by Im et al., who considered similar metrics when comparing the two types of MSCs. They also found increased levels of alkaline phosphatase in BM-MSCs and a higher number of mineralization nodules in BM-MSCs than in AT-MSCs when stained with Von Kossa staining [
82]. Laio and Chen wrote a review article on this topic in 2014 and determined that all seven of the articles they looked at comparing BM-MSCs to AT-MSCs found BM-MSCs to have either greater or equal osteogenic capabilities
in vitro [
83].
Although the use of AT-MSCs in bone healing does not appear as promising compared to BM-MSCs, there are other factors that may contribute to better differentiation. One study compared AT-MSCs to different types of fat cells, known as dedifferentiated fat cells (DFAT). They found that both stem cell types had greater difficulty differentiating when hydrogen peroxide was introduced to the system, mimicking oxidative stress. In contrast, catalase introduction led to a 2.5-fold increase in osteogenesis in both cell types [
84]. Another study by Wu et al. examined the bone regeneration capacity of BM-MSCs vs. AT-MSCs under different culture conditions. They found that AT-MSCs showed higher seeding efficiency and Type I collagen expression, whereas BM-MSCs showed greater osteogenic capacity under all conditions [
85]. Moreover, culture conditions also affected osteogenesis. Dynamic culturing, which consisted of stirring and perfusing the culture, enhanced both AT-MSC and BM-MSC osteogenic proliferation compared to static culture. This indicates that the process by which MSCs are cultured affects their regenerative capabilities [
85].
Ultimately there is little consensus on the type of multipotent stem cell that is most effective for bone healing based on
in vivo studies. Several studies have tested the ability of BM-MSCs and AT-MSCs to induce bone growth
in vivo and have shown success in both cell types [
86]. Similarly, one review compared BM-MSCs and AT-MSCs and found that half of the six studies reviewed showed increased osteogenesis in BM-MSCs compared to AT-MSCs, whereas half showed equal levels of bone growth [
83]. In 2013, Aykan et al. examined the effect of BM-MSC transplantation at a mandibular osteotomy site in sheep after three and six weeks. Mandibles on each side were cut and treated with BM-MSC mixed with PBS on left side and PBS alone on right side. At both extinction time periods, the left hemimandibles showed greater radiodensity and cortical bone growth than the right hemimandibles [
87].
3.2.2. Hematopoietic Stem Cells (HSCs)
Hematopoietic Stem Cells (HSCs) are a unique self-renewing cell type, from which all human blood cells are derived. These two lineages may differentiate into myeloid and lymphoid lineages. Myeloid cells include platelets, neutrophils, macrophages, and osteoclasts, as well as others. Lymphoid cells include T and B lymphocytes as well as natural killer cells [
88]. They can be found throughout the human body, including in the peripheral blood, bone marrow, and even the umbilical cord [
89]. Given the high degree of turnover observed in blood cells, nearly one trillion new blood cells are created daily [
89].
Therapeutically, HSCs have technically been used since the 1950s when the first bone marrow transplant was performed by E. Donnall Thomas [
90]. Since then, they have been used extensively and their applications have been growing. HSCs are most commonly used in hematologic and immunological disorders [
91,
92]. Some of these include chemotherapeutics and treatments for devastating immunodeficiencies, such as severe combined immunodeficiency and chronic granulomatous disease [
91]. However, the use of HSCs in osteogenic engineering has not been widely studied. HSCs switch to proliferative and differentiative states upon fracture. These cells differentiate into the cells necessary to begin the bone repair process (i.e., osteoblasts, osteoclasts, and endothelial progenitor cells) [
93]. HSCs are often characterized by cell surface protein cluster of differentiation 34 (CD34). Cells of this origin have been shown to exhibit osteogenic and angiogenic effects [
93]. In 2022, Oliveira et al. reviewed the current literature on HSC and bone healing. They provided many valuable findings, but most relevantly, HSCs repeatedly showed increased osteogenesis when transplanted
in vivo [
93].
3.2.3. Skeletal Stem Cells (SSCs)
Skeletal Stem Cells (SSCs) are another type of multipotent stem cell that can be found naturally in fetal and adult bones or engineered from iPSCs or BMP2-treated human adipose stroma. These cells can be identified by the presence of Podoplanin (PDPN), Cluster of Differentiation 73 (CD73), and Cluster of Differentiation 164 (CD164) surface cell markers, along with the absence of Cluster of Differentiation 146 (CD146) [
79]. Chan et al. performed a study in which they transplanted either injured or uninjured human phalanges into the flank of immunodeficient mice to observe bone growth and measure the proliferation of SSCs. After transplantation, the bones grew along the flank of the mice. Upon removal and digestion of the transplant, they found that phalanges with an introduced injury showed higher levels of human SSCs than uninjured samples, indicating that SSCs proliferate in response to skeletal injury, leading to regeneration [
79]. Another study examined SSCs in bone healing in diabetic mice. They introduced high serum level of tumor necrosis factor-α to inhibit Indian hedgehog (Ihh) signaling. In mice with lower expression levels of Ihh, less expansion of SSCs was observed, and consequently, impaired healing occurred. When Ihh was introduced back into the fracture via the use of a slow-release hydrogel, and SSC expansion along with bone healing was restored [
94]. Please refer to
Table 1 for a summary of stem cell applications.
4. Potential Mechanisms of Stem Cells in Fracture Healing
There are two primary mechanisms by which stem cells potentially heal bones after fractures. The original dogma was that the stem cells were incorporated into the tissue of implantation, similar to other forms of cell differentiation, stem cells receive tissue-specific cues and become bony regenerate. Recently, more research suggests another possible mechanism, that stem cells themselves are not incorporated into the site of injury; instead, they utilize paracrine signaling to attract host cells by providing the necessary regenerative components, leading to bone growth without having to become engrafted into the new bone.
4.1. Direct Osteogenic Formation
For many years, the prevailing thought has been that stem cells differentiate and incorporate directly into human bone during regeneration. This remains a possible mechanism and has been shown to be true in various studies. Geuze et al. studied the incorporation of MSCs into mouse and rat spines and observed stem cell incorporation into bone via bioluminescence imaging (BLI). They implanted MSCs both ectopically (subcutaneously) and orthotopically (within the spine), this was only performed in rat cohorts [
95]. BLI findings showed successful implantation of MSCs into bone growth in mice throughout the implantation period. This method of stem cell therapy points to the idea that stem cells, such as MSCs, differentiate and are then incorporated directly into bone growth. Interestingly, in this study, the same effect was not observed in the rat cohorts. Of the 117 rats, many showed the presence of MSCs at the two-week mark, but only one showed successful incorporation of MSCs into spinal bone formation by the end of the implantation period [
95]. Another study by Bhumiratana et al. aimed to engineer the ramus-condyle unit (RCU) using autologous stem cells in Yucatán minipigs. They infused AT-MSCs into a decellularized bone scaffold and cultured it for three weeks. After implantation, successful regrowth of the RCU was observed with the incorporation of MSCs analyzed via µCT scanning and Movat’s pentachrome staining of bone and soft tissues [
96].
The successful incorporation of MSCs into bone or a similar substance may depend on the scaffold used for the graft [
97]. Generally, bone defects that are 50 mm or smaller can be repaired using autologous bone grafting procedures. However, larger lesions are more difficult to manage [
98]. The use of scaffolds in combination with stem cell engineering is a promising alternative to bone induction [
99]. Meinel et al. examined the use of BM-MSCs for healing a 4 mm bone defect in the cranium of mice using a silk fibroin-based scaffold. This scaffold is advantageous because its makeup is largely collagen, and 90% of bone consists of type I collagen. In this way, tissue-engineered and human MSC-seeded scaffolds showed new bone growth at implant-host interfaces, as well as extracellular matrix proteins sialoprotein and osteopontin. However, osteocalcin was not detected in this study, indicating that MSCs did not achieve full osteoblastic maturity [
100]. Collins et al. performed a comprehensive review of scaffolds used for bone healing and suggested that successful bone grafts will likely use a variety of different materials in concordance with the mixed composition of natural bone [
97]. Ultimately, these studies support the concept that cell integration depends on tissue scaffolds that mimic the desired tissues for stem cell integration. This highlights the challenges of cell integration, in that stem cells alone are often insufficient for tissue regeneration. Instead, a medium that represents defective tissue is required for successful incorporation of stem cells.
4.2. Paracrine Signaling
Numerous cytokines have been shown to play an important role in bone growth and regeneration. Some of the key growth factors include Transforming Growth Factor (TGF)-β, Hypoxia-Inducible Factor (HIF) 1-α, CXCR-4, Stromal Cell-Drive Factor (SDF)-1, Runx2, Osterix, Osteocalcin, Platelet-Derived Growth Factor (PDGF), Bone Morphogenic Protein (BMP), and vascular endothelial growth factor (VEGF) [
101]. The role of these growth factors in the context of bone regeneration was characterized in depth by Setiawan et al. [
16]. These growth factors are stored and released from either the stem cell secretome or extracellular vesicles (EVs) during the inflammatory phase of the fracture healing process. Importantly, MSC secretomes are cell-free injections that provide important cytokines, growth factors, and transcription factors to the injury site to promote bone healing. For example, a secretome may include angiogenic factors such as VEGF and PDGF, which recruit novel blood vessel formation at the site; osteogenic factors such as Osteopontin, Osterix, Osteocalcin, Runx2, and TGF-β, which are involved in bone formation at the site; and a myriad of interleukins that act as immunosuppressors or immunomodulators, leading to or repressing inflammation. The combination of these molecules leads to increased levels of native osteoblast differentiation, and thus, bone formation [
16].
Additionally, the function of these immune cytokines and other immune cells in bone healing was explained by Yang and Liu [
10]. Many studies have suggested that the dominant role of stem cells is the induction of these cytokines via paracrine signaling during the angiogenesis stage of the inflammatory response to fracture healing. More specifically, it is believed that paracrine signaling is the act of cell-to-cell communication where a cell produces a signal that induces changes in a nearby cell, thus altering its behavior or function. This is a local method of signaling, and not one that relays messages at great distances or hematogenously, as in endocrine signaling. Rather, paracrine signaling acts locally and is broken down rapidly such that its effect is localized.
Linero and Chaparro performed an experiment in which they attempted to induce osteogenesis in rabbit mandibles using either AT-MSCs or merely conditioned media, without stem cells that was obtained from AT-MSCs. Both the conditioned media and AT-MSCs were administered via a Human Blood Plasma Hydrogel. They found that AT-MSCs were detectable at the injection site after three days but could no longer be seen after twelve days. Additionally, their analysis showed that conditioned media showed increased levels of bone regeneration [
102]. Both the elimination of AT-MSCs and improved osteogenesis with conditioned media suggest that a paracrine signaling mechanism of bone regeneration is not only sufficient for tissue regeneration but is also superior to direct cell integration [
102]. Another study by Osugi et al. similarly examined the effect of conditioned stem cell media on calvarial bone regeneration. Using computed tomography imaging, they found that the conditioned media cohort showed a greater area of bone growth than the stem cell-treated cohort, although this cohort still showed bone growth. This suggests that the paracrine environment produced by stem cells may be more essential than the cells themselves [
103]. Similarly, a study performed by Reichert et al. explored bone regeneration capacities of BM-MSCs vs. recombinant human bone morphogenic protein 7 (rhBMP-7) for a 3 cm long resection of sheep tibias. They compared this to the gold standard surgical treatment of autologous bone grafting, which has a myriad of additional surgical complications. Their findings revealed that scaffolds cultured with both MSCs and rhBMP-7 promoted bone regeneration at the resection site, with rhBMP-7 achieving superior bone growth. This was confirmed through X-ray, CT, micro-CT scans, biomechanical tests, and histological analysis [
104].
5. Future Directions
Stem cell therapy, in the context of bone healing and regeneration, is an exciting and promising field of research. Our understanding of the cellular and biochemical environment during bone healing has grown tremendously over the past twenty years. However, there are still many unanswered questions before these stem cell technologies can be widely implemented in the clinical setting.
First, the need for characterization and standardization of these treatments are increasingly necessary. Liquid chromatography-mass spectrometry has been used in some instances to characterize acellular stem cell populations, which are notoriously the least defined of all stem cell populations [
105,
106]. Protein microarrays have also been used to define stem cell populations [
107]. Mass spectrometry and cytokine arrays have also been helpful tools to characterize the protein content of many acellular preparations of stem cell secretomes, with a variety of findings and inconsistent concentrations of protein within the various secretomes themselves [
107,
108,
109,
110]. In order to reinforce the reliability of various stem cell therapies, characterization of both the acellular and cellular components of stem cells is required with further translation of these findings in pre-clinical studies.
Secondly, the intricacies of the biochemical mechanisms of stem cell healing should be the focus of future studies. One specific area of study that requires clarification is determining which type of MSC is better for
in vivo use. There is no consensus on whether
in vivo use of BM-MSCs or AT-MSCs leads to greater osteogenesis [
111]. The current debate is whether the use of live MSC cultures (e.g. cell transplants, grafts) or acellular preparations derived from these cells (e.g. conditioned media, extracellular vesicles) for regenerative purposes. If paracrine signaling is central to regenerative therapy, the cellular content is assumed to be superfluous. More studies on acellular secretome preparations versus cellular stem cell therapies need to be compared side-by-side in both
in vitro and
in vivo studies for further verification of efficacy. Understanding the immunomodulatory effect of stem cells and their acellular counterparts may prove vital to appreciating the differences in these therapies.
In summary, understanding the underlying mechanisms and standardizing the development and techniques for using stem cell preparations is vital for establishing a consistent response to therapy. It is difficult to measure the effectiveness of a treatment if the implementation methods are inconsistent. There is an urgent need to fully standardize or at least agree on common methods of stem cell development/technique to achieve consensus in terms of which preparation or approach is best for augmenting bone healing.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Marcel G. Brown and Xue Ma; formal analysis, Marcel G. Brown, Davis J. Brady, Kelsey M. Healy, Kaitlin A. Henry, Ayobami S. Ogunsola, Xue Ma; investigation, Marcel G. Brown, Davis J. Brady, Kelsey M. Healy, Kaitlin A. Henry, Ayobami S. Ogunsola, Xue Ma; writing—original draft preparation, Kelsey M. Healy, Davis J. Brady, Marcel G. Brown; writing—review and editing, Marcel G. Brown, Davis J. Brady, Kelsey M. Healy, Kaitlin A. Henry, Ayobami S. Ogunsola, Xue Ma; visualization, Kaitlin A. Henry, Ayobami S. Ogunsola; supervision, Marcel G. Brown, Ayobami S. Ogunsola, Xue Ma; project administration, Marcel G. Brown, Ayobami S. Ogunsola, Xue Ma. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Charitos:, I.A.; Ballini, A.; Cantore, S.; Boccellino, M.; Di Domenico, M.; Borsani, E.; Nocini, R.; Di Cosola, M.; Santacroce, L.; Bottalico, L. Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues. Stem Cells International 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hansford, S.; Huntsman, D.G. Boveri at 100: Theodor Boveri and Genetic Predisposition to Cancer. J Pathol 2014, 234, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S. Stem Cell: What’s in a Name? Nature Reports Stem Cells 2009. [Google Scholar] [CrossRef]
- Ramalho-Santos, M.; Willenbring, H. On the Origin of the Term “Stem Cell. ” Cell Stem Cell 2007, 1, 35–38. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res Ther 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem Cells: A Comprehensive Review of Origins and Emerging Clinical Roles in Medical Practice. Orthopedic Reviews 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. Bone Healing: Little Secrets. Clin Cases Miner Bone Metab 2011, 8, 17–20. [Google Scholar]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture Healing: Mechanisms and Interventions. Nat Rev Rheumatol 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. Emerging Bone Healing Therapies. Journal of Orthopaedic Trauma 2010, 24, S4–S8. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. International Journal of Medical Sciences 2021, 18, 3697. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Claes, L. The Effect of Both a Thoracic Trauma and a Soft-Tissue Trauma on Fracture Healing in a Rat Model. Acta Orthopaedica 2011, 82, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The Science of Fracture Healing. Journal of Orthopaedic Trauma 2005, 19, S4. [Google Scholar] [CrossRef] [PubMed]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin Plast Surg 2021, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- McKinley, T. Principles of Fracture Healing. Surgery (Oxford 2003, 21, 209–212. [Google Scholar] [CrossRef]
- Setiawan, F.; Wahjuningrum, D.A.; Utomo, D.N. The Property of Mesenchymal Stem Cells (MSCs) Secretome as a Bone Stimulator Candidate in Regeneration of Injured Bone. 2021.
- McKibbin, B. The Biology of Fracture Healing in Long Bones. The Journal of Bone & Joint Surgery British. [CrossRef]
- Phillips, A.M. Overview of the Fracture Healing Cascade. Injury 2005, 36, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Bais, M.; McLean, J.; Sebastiani, P.; Young, M.; Wigner, N.; Smith, T.; Kotton, D.N.; Einhorn, T.A.; Gerstenfeld, L.C. Transcriptional Analysis of Fracture Healing and the Induction of Embryonic Stem Cell–Related Genes. PLoS ONE 2009, 4, e5393. [Google Scholar] [CrossRef] [PubMed]
- Röding, F.; Lindkvist, M.; Bergström, U.; Lysholm, J. Epidemiologic Patterns of Injuries Treated at the Emergency Department of a Swedish Medical Center. Inj Epidemiol 2015, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton III, L.J. Trends in Fracture Incidence: A Population-Based Study Over 20 Years. Journal of Bone and Mineral Research 2014, 29, 581–589. [Google Scholar] [CrossRef]
- Stem Cells Market Size, Share And Growth Report, 2030 Available online:. Available online: https://www.grandviewresearch.com/industry-analysis/stem-cells-market (accessed on 27 February 2024).
- Mitalipov, S.; Wolf, D. Totipotency, Pluripotency and Nuclear Reprogramming. In Engineering of Stem Cells; Martin, U., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2009; ISBN 978-3-540-88805-5. [Google Scholar]
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef]
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Med Iran 2017, 55, 6–23. [Google Scholar] [PubMed]
- Singh, V.K.; Saini, A.; Kalsan, M.; Kumar, N.; Chandra, R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In Silico Diagnostics. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Research National Research Council (US) and Institute of Medicine (US) Committee on the Biological and Biomedical Applications of Stem Cell. “Embryonic Stem Cells. In Stem Cells and the Future of Regenerative Medicine, National Academies Press (US; 2002.
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Archives of Medical Research 2021, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Díez Villanueva, P.; Sanz-Ruiz, R.; Núñez García, A.; Fernández Santos, M.E.; Sánchez, P.L.; Fernández-Avilés, F. Functional Multipotency of Stem Cells: What Do We Need from Them in the Heart? Stem Cells Int 2012, 2012, 817364. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, A. The Benefits and Risks of Stem Cell Technology. Oral Diseases 2012, 18, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Wuputra, K.; Ku, C.-C.; Wu, D.-C.; Lin, Y.-C.; Saito, S.; Yokoyama, K.K. Prevention of Tumor Risk Associated with the Reprogramming of Human Pluripotent Stem Cells. J Exp Clin Cancer Res 2020, 39, 100. [Google Scholar] [CrossRef] [PubMed]
- Aldahmash, A.; Atteya, M.; Elsafadi, M.; Al-Nbaheen, M.; Al-Mubarak, H.A.; Vishnubalaji, R.; Al-Roalle, A.; Al-Harbi, S.; Manikandan, M.; Matthaei, K.I.; et al. Teratoma Formation in Immunocompetent Mice After Syngeneic and Allogeneic Implantation of Germline Capable Mouse Embryonic Stem Cells. Asian Pacific Journal of Cancer Prevention 2013, 14, 5705–5711. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S. Embryonic Stem Cells Injected into the Mouse Knee Joint Form Teratomas and Subsequently Destroy the Joint. Rheumatology 2003, 42, 162–165. [Google Scholar] [CrossRef]
- Baharvand, H.; Matthaei, A. Culture Condition Difference for Establishment of New Embryonic Stem Cell Lines from the C57BL/6 and BALB/c Mouse Strains.
- Przyborski, S.A. Differentiation of Human Embryonic Stem Cells After Transplantation in Immune-Deficient Mice. STEM CELLS 2005, 23, 1242–1250. [Google Scholar] [CrossRef]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.I.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of Undifferentiated Murine Embryonic Stem Cells in the Heart: Teratoma Formation and Immune Response. FASEB j. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Dressel, R.; Schindehütte, J.; Kuhlmann, T.; Elsner, L.; Novota, P.; Baier, P.C.; Schillert, A.; Bickeböller, H.; Herrmann, T.; Trenkwalder, C.; et al. The Tumorigenicity of Mouse Embryonic Stem Cells and In Vitro Differentiated Neuronal Cells Is Controlled by the Recipients’ Immune Response. PLoS ONE 2008, 3, e2622. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, T.A.; Harkness, L.M.; Frandsen, U.; Ditzel, N.; Schrøder, H.D.; Burns, J.S.; Kassem, M. Teratoma Formation by Human Embryonic Stem Cells Is Site Dependent and Enhanced by the Presence of Matrigel. Stem Cells Dev 2009, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, Z.; Geng, W. Engineering Vascularized Bone Graft with Osteogenic and Angiogenic Lineage Differentiated Bone Marrow Mesenchymal Stem Cells. Artificial Organs 2012, 36, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Y.; Meng, X.; Kwok, K.Y.; Huang, X.; Choy, K.W.; Wang, C.C.; Lan, H.; Yuan, P. Suppression of Malignancy by Smad3 in Mouse Embryonic Stem Cell Formed Teratoma. Stem Cell Rev Rep 2013, 9, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Haworth, R.; Sharpe, M. Accept or Reject: The Role of Immune Tolerance in the Development of Stem Cell Therapies and Possible Future Approaches. Toxicol Pathol 2021, 49, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.C.; Martinez Arias, A.; Pera, M.F.; Moris, N.; M’hamdi, H.I. An Ethical Framework for Human Embryology with Embryo Models. Cell 2023, 186, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Kobold, S.; Bultjer, N.; Stacey, G.; Mueller, S.C.; Kurtz, A.; Mah, N. History and Current Status of Clinical Studies Using Human Pluripotent Stem Cells. Stem Cell Reports 2023, 18, 1592. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Chatziparasidou, A.; Ranjbarvan, P.; Niknam, Z.; Ardeshirylajimi, A. Embryonic Stem Cells in Clinical Trials: Current Overview of Developments and Challenges. In Cell Biology and Translational Medicine, Volume 11: Stem Cell Therapy - Potential and Challenges; Turksen, K., Ed.; Springer International Publishing: Cham, 2021; ISBN 978-3-030-71925-8. [Google Scholar]
- Choumerianou, D.M.; Dimitriou, H.; Kalmanti, M. Stem Cells: Promises Versus Limitations. Tissue Engineering Part B: Reviews 2008, 14, 53–60. [Google Scholar] [CrossRef]
- Khan, F.A.; Almohazey, D.; Alomari, M.; Almofty, S.A. Isolation, Culture, and Functional Characterization of Human Embryonic Stem Cells: Current Trends and Challenges. Stem Cells International 2018, 2018, e1429351. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Bolli, R. A Realistic Appraisal of the Use of Embryonic Stem Cell-Based Therapies for Cardiac Repair. European Heart Journal 2020, 41, 2397–2404. [Google Scholar] [CrossRef]
- Bielby, R.C.; Polak, J.M. In Vitro Differentiation and In Vivo Mineralization of Osteogenic Cells Derived from Human Embryonic Stem Cells.
- Petrigliano, F.A.; Liu, N.Q.; Lee, S.; Tassey, J.; Sarkar, A.; Lin, Y.; Li, L.; Yu, Y.; Geng, D.; Zhang, J.; et al. Long-Term Repair of Porcine Articular Cartilage Using Cryopreservable, Clinically Compatible Human Embryonic Stem Cell-Derived Chondrocytes. npj Regen Med 2021, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced Pluripotent Stem Cell Technology: A Decade of Progress. Nat Rev Drug Discov 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr Rev 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, X.; Lu, X.; Zhang, C.; Yu, H.; Zhao, T. Cells Derived from iPSC Can Be Immunogenic — Yes or No? Protein Cell 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Wada, H.; Murata, T.; Seino, K. Immune Reaction and Regulation in Transplantation Based on Pluripotent Stem Cell Technology. Inflammation and Regeneration 2020, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Wanczyk, H.; Jensen, T.; Finck, C. Assessment of iPSC Teratogenicity throughout Directed Differentiation toward an Alveolar-like Phenotype. Differentiation 2019, 105, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Worringer, K.A.; Rand, T.A.; Hayashi, Y. The Let-7/LIN-41 Pathway Regulates Reprogramming to Human Induced Pluripotent Stem Cells by Controlling Expression of Prodifferentiation Genes. Cell Stem Cell 2014, 14, 40–52. [Google Scholar] [CrossRef]
- Kumazaki, T.; Kurata, S.; Matsuo, T.; Mitsui, Y.; Takahashi, T. Establishment of Human Induced Pluripotent Stem Cell Lines from Normal Fibroblast TIG-1. Hum Cell 2011, 24, 96–103. [Google Scholar] [CrossRef]
- Péault, B. Stem Cell Technology for Bone Regeneration: Current Status and Potential Applications. Stem Cells and Cloning: Advances and Applications, Feb. [CrossRef]
- Zhou, M.; Xi, J.; Cheng, Y.; Sun, D.; Shu, P.; Chi, S.; Tian, S.; Ye, S. Reprogrammed Mesenchymal Stem Cells Derived from iPSCs Promote Bone Repair in Steroid-Associated Osteonecrosis of the Femoral Head. Stem Cell Research & Therapy 2021, 12, 175. [Google Scholar] [CrossRef]
- Jungbluth, P.; Spitzhorn, L.-S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, M.S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human iPSC-Derived iMSCs Improve Bone Regeneration in Mini-Pigs. Bone Res 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, B.; Cao, C.; Hu, K.; Wang, P.; Man, Y. Therapeutic Antibody Directed Osteogenic Differentiation of Induced Pluripotent Stem Cell Derived MSCs. Acta Biomaterialia 2018, 74, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Ge, Z.; Cui, W.; Yu, L.; Li, J. Human Amniotic Epithelial Stem Cells: A Promising Seed Cell for Clinical Applications. IJMS 2020, 21, 7730. [Google Scholar] [CrossRef] [PubMed]
- Miki, T. Amnion-Derived Stem Cells: In Quest of Clinical Applications. Stem Cell Res Ther 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-W.; Huang, Q.-M.; Wu, H.-Y.; Zuo, G.-S.-L.; Gu, H.-C.; Deng, K.-Y.; Xin, H.-B. Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells. Int J Mol Sci 2021, 22, 970. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants With Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Translational Medicine 2018, 7, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Huang, X.; Huang, F.; Liu, X. [Preliminary study on transdifferentiation of human amniotic epithelial cells and its intrasplenic transplantation]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2011, 25, 144–148. [Google Scholar] [PubMed]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of Amniotic Stem Cell Lines with Potential for Therapy. Nat Biotechnol 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Dobreva, M.P.; Pereira, P.N.G.; Deprest, J.; Zwijsen, A. On the Origin of Amniotic Stem Cells: Of Mice and Men. Int. J. Dev. Biol. 2010, 54, 761–777. [Google Scholar] [CrossRef]
- Kang, N.-H.; Hwang, K.-A.; Kim, S.U.; Kim, Y.-B.; Hyun, S.-H.; Jeung, E.-B.; Choi, K.-C. Potential Antitumor Therapeutic Strategies of Human Amniotic Membrane and Amniotic Fluid-Derived Stem Cells. Cancer Gene Ther 2012, 19, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Phermthai, T.; Odglun, Y.; Julavijitphong, S.; Titapant, V.; Chuenwattana, P.; Vantanasiri, C.; Pattanapanyasat, K. A Novel Method to Derive Amniotic Fluid Stem Cells for Therapeutic Purposes. BMC Cell Biol 2010, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Rezaei-Tavirani, M.; Farhadihosseinabadi, B.; Zali, H.; Niknejad, H. Human Amniotic Mesenchymal Stem Cells to Promote/Suppress Cancer: Two Sides of the Same Coin. Stem Cell Res Ther 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.-Y.; Li, L.; Wang, W.-J.; Liu, F.-F.; Song, J.; Yang, S.-L.; Tan, J.; Gao, H.; Zhao, Y.-Y.; Tang, W.-W.; et al. Assessment of Tumor Promoting Effects of Amniotic and Umbilical Cord Mesenchymal Stem Cells in Vitro and in Vivo. J Cancer Res Clin Oncol 2019, 145, 1133–1146. [Google Scholar] [CrossRef]
- Dziadosz, M.; Basch, R.S.; Young, B.K. Human Amniotic Fluid: A Source of Stem Cells for Possible Therapeutic Use. American Journal of Obstetrics and Gynecology 2016, 214, 321–327. [Google Scholar] [CrossRef]
- Mohammed, E.E.A.; El-Zawahry, M.; Farrag, A.R.H.; Aziz1, N.N.A.; Sharaf-ElDin, W.; Abu-Shahba, N.; Mahmoud, M.; Gaber, K.; Ismail, T.; Mossaad, M.M.; et al. Osteogenic Differentiation Potential of Human Bone Marrow and Amniotic Fluid-Derived Mesenchymal Stem Cells in Vitro & in Vivo. Open Access Maced J Med Sci 2019, 7, 507–515. [Google Scholar] [CrossRef]
- Basile, M.; Marchegiani, F.; Novak, S.; Kalajzic, I.; Di Pietro, R. Human Amniotic Fluid Stem Cells Attract Osteoprogenitor Cells in Bone Healing. Journal Cellular Physiology 2020, 235, 4643–4654. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific An Overview of Pluripotent and Multipotent Stem Cell Targets - US Available online:. Available online: https://www.thermofisher.com/us/en/home/life-science/antibodies/antibodies-learning-center/antibodies-resource-library/antibody-methods/pluripotent-multipotent-stem-cell-targets.html (accessed on 26 February 2024).
- Xu, G.-P. Current and Future Uses of Skeletal Stem Cells for Bone Regeneration. World Journal of Stem Cells 2020, 12, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front Cell Dev Biol 2019, 7, 268. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-Derived and Bone Marrow Mesenchymal Stem Cells: A Donor-Matched Comparison. Stem Cell Res Ther 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I.; Shin, Y.W.; Lee, K.B. Do Adipose Tissue-Derived Mesenchymal Stem Cells Have the Same Osteogenic and Chondrogenic Potential as Bone Marrow-Derived Cells? Osteoarthritis Cartilage 2005, 13, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-T. Osteogenic Potential: Comparison between Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. WJSC 2014, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Sons, H.C.; Schiefer, J.L.; Fuchs, P.C.; Windolf, J.; Suschek, C.V. Comparative Study of the Osteogenic Differentiation Potential of Adipose Tissue-Derived Stromal Cells and Dedifferentiated Adipose Cells of the Same Tissue Origin under Pro and Antioxidant Conditions. Biomedicines 2022, 10, 3071. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Le, A.V.; Mendez, J.J.; Chang, J.; Niklason, L.E.; Steinbacher, D.M. Osteogenic Performance of Donor-Matched Human Adipose and Bone Marrow Mesenchymal Cells Under Dynamic Culture. Tissue Engineering Part A 2015, 21, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone Marrow Mesenchymal Stem Cells: Aging and Tissue Engineering Applications to Enhance Bone Healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Aykan, A.; Ozturk, S.; Sahin, I.; Gurses, S.; Ural, A.U.; Oren, N.C.; Isik, S. Biomechanical Analysis of the Effect of Mesenchymal Stem Cells on Mandibular Distraction Osteogenesis. Journal of Craniofacial Surgery 2013, 24, e169. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr Osteoporos Rep 2018, 16, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, S.-H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int J Stem Cells 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Henig, I.; Zuckerman, T. Hematopoietic Stem Cell Transplantation—50 Years of Evolution and Future Perspectives. Rambam Maimonides Med J 2014, 5, e0028. [Google Scholar] [CrossRef]
- Chabannon, C.; Kuball, J.; Bondanza, A.; Dazzi, F.; Pedrazzoli, P.; Toubert, A.; Ruggeri, A.; Fleischhauer, K.; Bonini, C. Hematopoietic Stem Cell Transplantation in Its 60s: A Platform for Cellular Therapies. Science Translational Medicine 2018, 10, eaap9630. [Google Scholar] [CrossRef] [PubMed]
- Koniali, L.; Lederer, C.W.; Kleanthous, M. Therapy Development by Genome Editing of Hematopoietic Stem Cells. Cells 2021, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Carreira, M.; Correia, C.R.; Mano, J.F. The Therapeutic Potential of Hematopoietic Stem Cells in Bone Regeneration. Tissue Engineering Part B: Reviews 2022, 28, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.; Seo, E.Y.; Marecic, O.; McArdle, A.; Tong, X.; Zimdahl, B.; Malkovskiy, A.; Sinha, R.; Gulati, G.; Li, X.; et al. Pharmacological Rescue of Diabetic Skeletal Stem Cell Niches. Science Translational Medicine 2017, 9, eaag2809. [Google Scholar] [CrossRef]
- Geuze, R.E.; Prins, H.-J.; Öner, F.C.; Van Der Helm, Y.J.M.; Schuijff, L.S.; Martens, A.C.; Kruyt, M.C.; Alblas, J.; Dhert, W.J.A. Luciferase Labeling for Multipotent Stromal Cell Tracking in Spinal Fusion Versus Ectopic Bone Tissue Engineering in Mice and Rats. Tissue Engineering Part A 2010, 16, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-Engineered Autologous Grafts for Facial Bone Reconstruction. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Advanced Functional Materials 2021, 31, 2010609. [Google Scholar] [CrossRef]
- 9Dumic-Cule, I.; Pecina, M.; Jelic, M.; Jankolija, M.; Popek, I.; Grgurevic, L. Biological aspects of segmental bone defects management. Int. Orthop 2015, 39, 1005–1011. [Google Scholar] [CrossRef]
- Decambron, A.; Fournet, A.; Bensidhoum, M.; Manassero, M.; Sailhan, F.; Petite, H. Low-Dose BMP-2 and MSC Dual Delivery onto Coral Scaffold for Critical-Size Bone Defect Regeneration in Sheep. J. Orthop. Res 2017, 35, 2637–2645. [Google Scholar] [CrossRef]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B.; Vunjak-Novakovic, G.; Kaplan, D. Silk Implants for the Healing of Critical Size Bone Defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef]
- Garg, P.; Mazur, M.M.; Buck, A.C.; Wandtke, M.E.; Liu, J.; Ebraheim, N.A. Prospective Review of Mesenchymal Stem Cells Differentiation into Osteoblasts. Orthop Surg 2017, 9, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine Effect of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Bone Regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Engineering. Part A 2012, 18, 1479. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A Tissue Engineering Solution for Segmental Defect Regeneration in Load-Bearing Long Bones. Science Translational Medicine 2012, 4, 141ra93–141ra93. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhu, Y.; Lu, L.; Gu, F.; Chen, H. The Applications and Features of Liquid Chromatography-Mass Spectrometry in the Analysis of Traditional Chinese Medicine. Evid Based Complement Alternat Med 2016, 2016, 3837270. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, M.; Cabooter, D. Current Developments in LC-MS for Pharmaceutical Analysis. Analyst 2020, 145, 1129–1157. [Google Scholar] [CrossRef] [PubMed]
- Sutandy, F.X.R.; Qian, J.; Chen, C.-S.; Zhu, H. Overview of Protein Microarrays. Curr Protoc Protein Sci, 27. [CrossRef]
- Chen, Z.; Dodig-Crnković, T.; Schwenk, J.M.; Tao, S. Current Applications of Antibody Microarrays. Clinical Proteomics 2018, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Poetz, O.; Ostendorp, R.; Brocks, B.; Schwenk, J.M.; Stoll, D.; Joos, T.O.; Templin, M.F. Protein Microarrays for Antibody Profiling: Specificity and Affinity Determination on a Chip. Proteomics 2005, 5, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Venkatarame Gowda Saralamma, V.; Vetrivel, P.; Kim, S.M.; Ha, S.E.; Lee, H.J.; Lee, S.J.; Kim, Y.S.; Pak, J.E.; Lee, H.J.; Heo, J.D.; et al. Proteome Profiling of Membrane-Free Stem Cell Components by Nano-LS/MS Analysis and Its Anti-Inflammatory Activity. Evidence-Based Complementary and Alternative Medicine 2019, 2019, e4683272. [Google Scholar] [CrossRef]
- Luby, A.O.; Ranganathan, K.; Lynn, J.V.; Nelson, N.S.; Donneys, A.; Buchman, S.R. Stem Cells for Bone Regeneration: Current State and Future Directions. Journal of Craniofacial Surgery 2019, 30, 730. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).