Submitted:
08 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
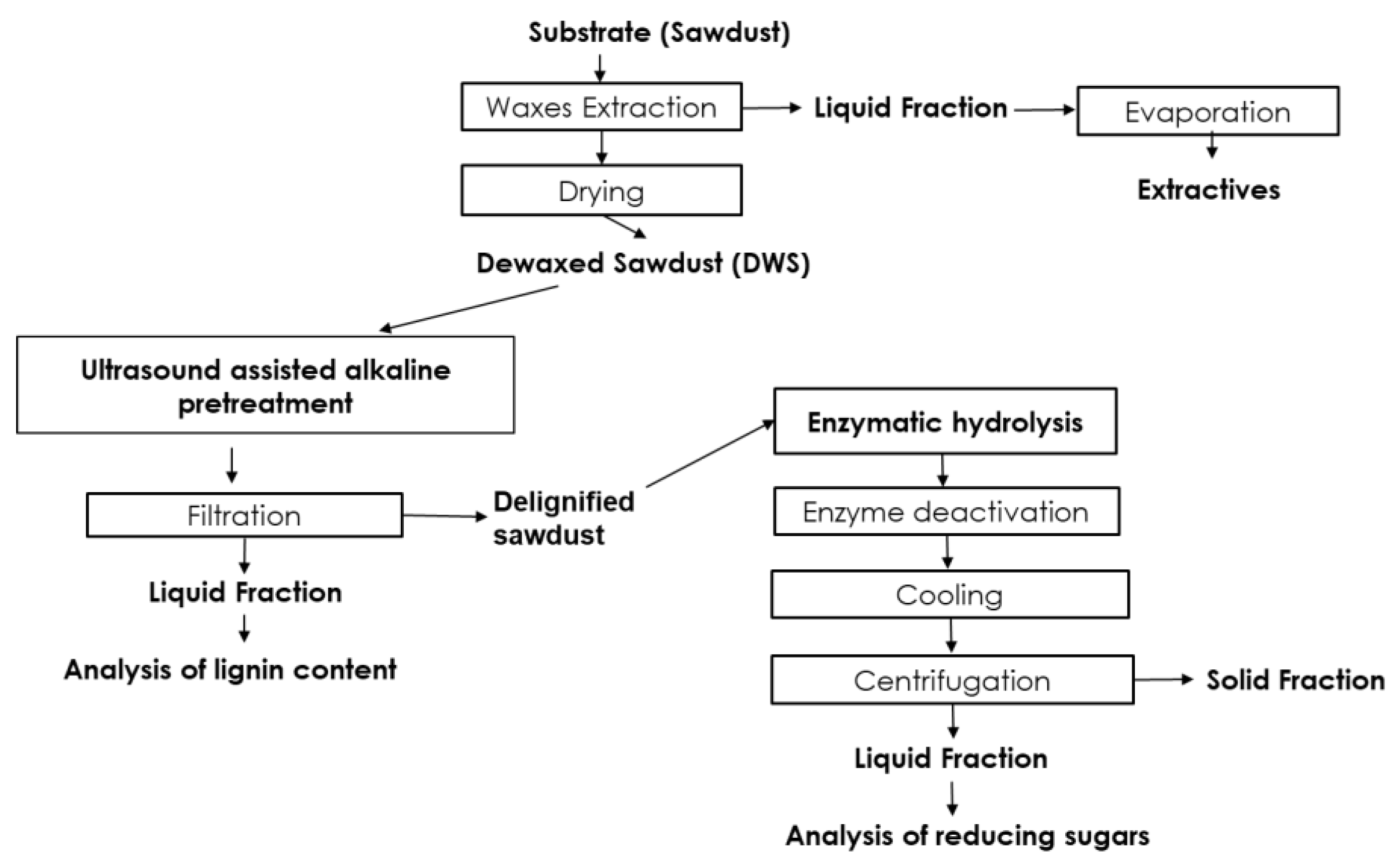
2. Materials and Methods
2.1. Materials
2.2. Procedure of Ultrasound Assisted Alkaline Pretreatment
2.3. Analysis of the Soluble Lignin Content
2.4. Enzymatic Hydrolysis Procedure
2.5. Analysis of Reducing Sugars Content
2.6. Statistical Analysis
3. Results and Disscusions
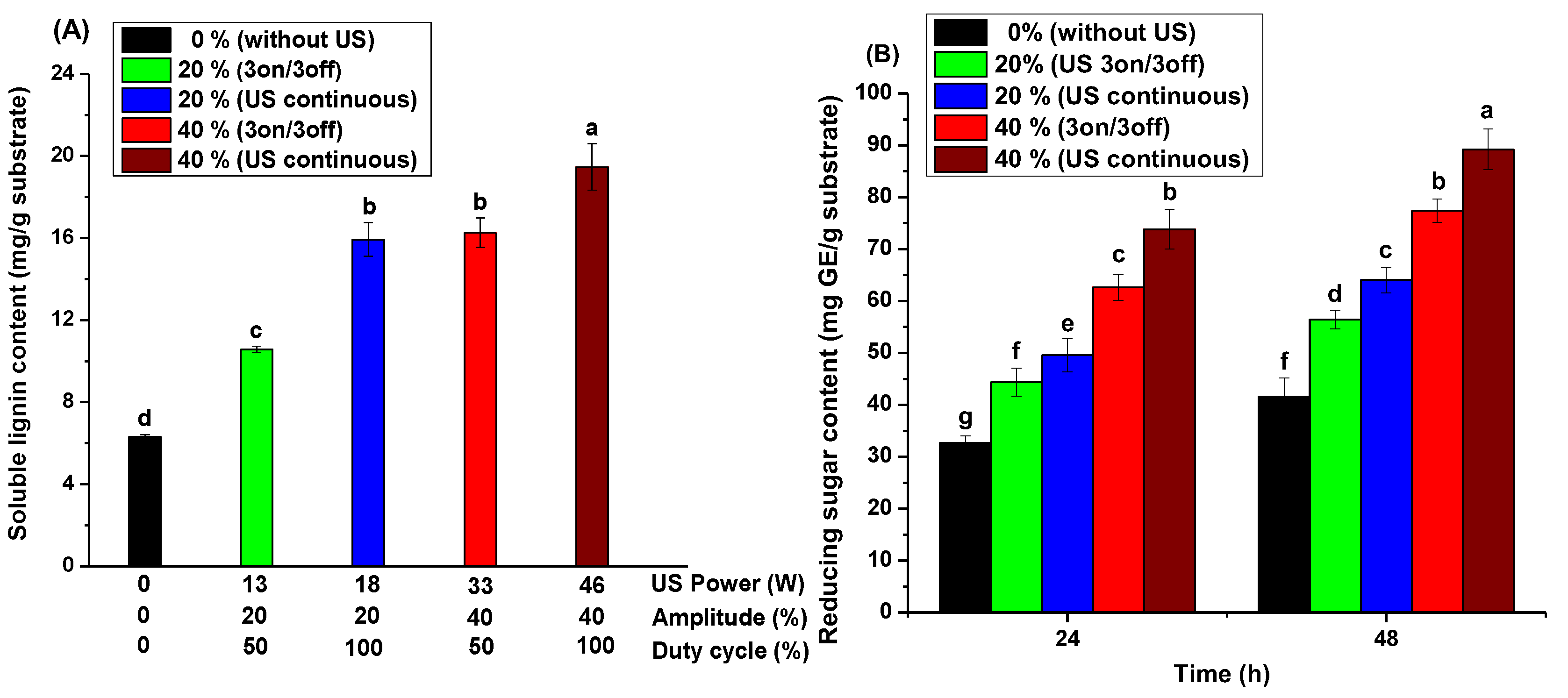
3.1. Influence of Ultrasound Equipment on the Assisted Alkaline Pretreatment
3.2. The Influence of Ultrasonic Power on the Lignin Extraction and Sugars Yield
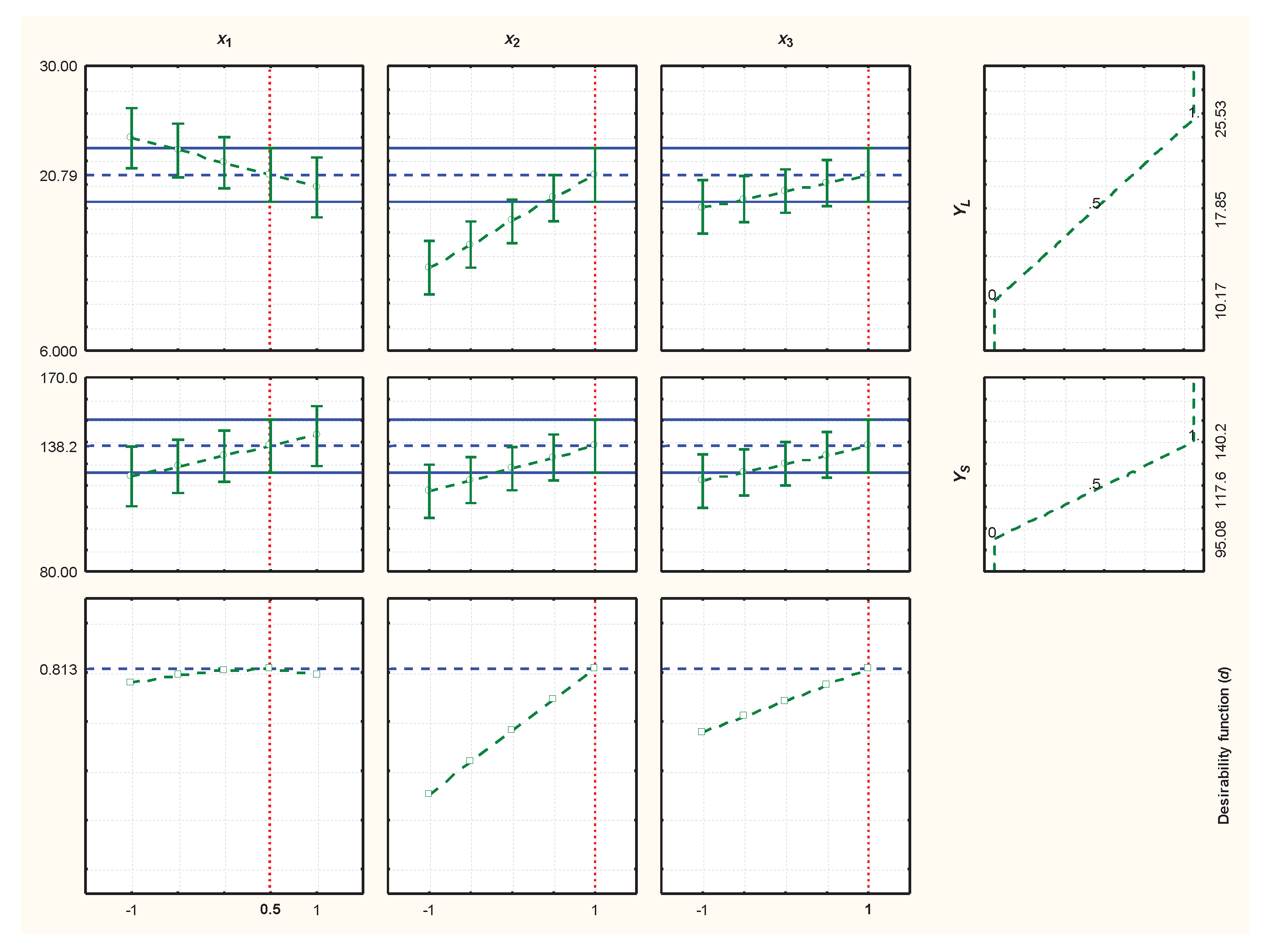
3.3. Statistical Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent advances of greener pretreatment technologies of lignocellulose. Current Research in Green and Sustainable Chemistry 2020, 3, 100035. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renewable and Sustainable Energy Reviews 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Bussemaker, M.J.; Zhang, D. Effect of Ultrasound on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Industrial & Engineering Chemistry Research 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnology Advances 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, G.; Zhang, P.; Li, F.; Fan, S.; Li, J. Thermo-chemical pretreatment and enzymatic hydrolysis for enhancing saccharification of catalpa sawdust. Bioresource Technology 2016, 205, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.S.; Bellesia, G.; Uppugundla, N.; da Costa Sousa, L.; Gao, D.; Cheh, A.M.; Agarwal, U.P.; Bianchetti, C.M.; Phillips, G.N., Jr.; Langan, P.; et al. Restructuring the Crystalline Cellulose Hydrogen Bond Network Enhances Its Depolymerization Rate. Journal of the American Chemical Society 2011, 133, 11163–11174. [Google Scholar] [CrossRef]
- Yu, Y.; Lou, X.; Wu, H. Some Recent Advances in Hydrolysis of Biomass in Hot-Compressed Water and Its Comparisons with Other Hydrolysis Methods. Energy & Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chemical reviews 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Z.; Smith, R.L. Ultrasound-enhanced conversion of biomass to biofuels. Progress in Energy and Combustion Science 2014, 41, 56–93. [Google Scholar] [CrossRef]
- Kininge, M.M.; Gogate, P.R. Intensification of alkaline delignification of sugarcane bagasse using ultrasound assisted approach. Ultrasonics Sonochemistry 2022, 82, 105870. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresource Technology 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends in Analytical Chemistry 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Du, R.; Su, R.; Qi, W.; He, Z. Enhanced enzymatic hydrolysis of corncob by ultrasound-assisted soaking in aqueous ammonia pretreatment. 3 Biotech 2018, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Jun-Hong, L.; Kun-Yu, W. Study on Technology Optimization of Lignin Removalin Cellulose Extraction from Wheat Bran by Combination of Ultrasound and Hydrogen Peroxide. Biotechnology(Faisalabad) 2016, 15, 135–140. [Google Scholar] [CrossRef]
- Mohammadabadi, S.I.; Javanbakht, V. Lignin extraction from barley straw using ultrasound-assisted treatment method for a lignin-based biocomposite preparation with remarkable adsorption capacity for heavy metal. International Journal of Biological Macromolecules 2020, 164, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Saharan, V.; Yadav, A.; Aggarwal, N.K. Ultrasound-assisted alkaline pretreatment of Parthenium hysterophorus for fermentable sugar production using a response surface approach. Sustainable Chemistry for Climate Action 2023, 2, 100027. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; HoBowacz, I.; !Aukajtis, R.; Glinka, M.; KamiDski, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Sluiter, A.H., B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass, NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- BioSolutions Novozymes. Available online: https://biosolutions.novozymes.com/en/juice-fruit-vegetables/products/vegetables/celluclast-1.5-l/ (accessed on 10 November 2023).
- ULTRA-MINT Technologies. Available online: http://ultramint.chimie.upb.ro/en/index.php (accessed on 10 November 2023).
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Analytical Chemistry 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass and Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Applied Sciences 2019, 9, 766. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Synergistic Effect of Ultrasound and Three Phase Partitioning for the Extraction of Curcuminoids from Curcuma longa and its Bioactivity Profile. Process Biochemistry 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Gogate, P.R.; Kabadi, A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochemical Engineering Journal 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Dobre, T.; Egri, D.; Moț, A.; Popa, V.; Crăciun, M.E. Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor. Processes 2021, 10, 37. [Google Scholar] [CrossRef]
- Draghici-Popa, A.M.; Boscornea, A.C.; Brezoiu, A.M.; Tomas, S.T.; Parvulescu, O.C.; Stan, R. Effects of Extraction Process Factors on the Composition and Antioxidant Activity of Blackthorn (Prunus spinosa L.) Fruit Extracts. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]

| Run |
RSL (g/100 mL) |
A (%) |
t (°C) |
x1 | x2 | x3 |
YL (mg/g) |
YS (mg GE/g) |
YL,pred (mg/g) |
YS,pred (mg GE/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 20 | 30 | -1 | -1 | -1 | 11.55 | 95.08 | 13.42 | 87.04 |
| 2 | 1.5 | 20 | 30 | 1 | -1 | -1 | 10.17 | 97.47 | 9.27 | 105.70 |
| 3 | 0.5 | 60 | 30 | -1 | 1 | -1 | 22.57 | 101.79 | 21.21 | 107.97 |
| 4 | 1.5 | 60 | 30 | 1 | 1 | -1 | 17.17 | 132.58 | 17.07 | 126.64 |
| 5 | 0.5 | 20 | 50 | -1 | -1 | 1 | 15.47 | 98.47 | 16.10 | 103.22 |
| 6 | 1.5 | 20 | 50 | 1 | -1 | 1 | 14.04 | 126.39 | 11.96 | 121.88 |
| 7 | 0.5 | 60 | 50 | -1 | 1 | 1 | 25.53 | 126.61 | 23.90 | 124.15 |
| 8 | 1.5 | 60 | 50 | 1 | 1 | 1 | 17.17 | 140.17 | 19.75 | 142.82 |
| 9 | 1 | 40 | 40 | 0 | 0 | 0 | 15.96 | 106.31 | 16.58 | 114.93 |
| 10 | 1 | 40 | 40 | 0 | 0 | 0 | 16.15 | 129.30 | 16.58 | 114.93 |
| 11 | 1 | 40 | 40 | 0 | 0 | 0 | 16.29 | 118.66 | 16.58 | 114.93 |
| 12 | 1 | 40 | 40 | 0 | 0 | 0 | 16.94 | 106.31 | 16.58 | 114.93 |
| 13 | 1.25 | 60 | 50 | 0.5 | 1 | 1 | 18.73 | 131.56 | 20.79 | 138.15 |
| 14 | 1.25 | 60 | 50 | 0.5 | 1 | 1 | 20.16 | 136.84 | 20.79 | 138.15 |
| 15 | 1.25 | 60 | 50 | 0.5 | 1 | 1 | 22.45 | 140.18 | 20.79 | 138.15 |
| Regressor | k | βk1 | SEk1 | tk1 | pk1 | βk2 | SEk2 | tk2 | pk2 |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 1 | 16.58 | 0.468 | 35.46 | 0.0000 | 114.9 | 2.564 | 44.82 | 0.0000 |
| x1 | 2 | -2.073 | 0.573 | -3.619 | 0.0068 | 9.332 | 3.140 | 2.972 | 0.0178 |
| x2 | 3 | 3.899 | 0.573 | 6.806 | 0.0001 | 10.47 | 3.140 | 3.333 | 0.0103 |
| x3 | 4 | 1.343 | 0.573 | 2.345 | 0.0471 | 8.090 | 3.140 | 2.576 | 0.0328 |
| R2 | 0.890 | 0.769 | |||||||
| R2adj | 0.849 | 0.682 | |||||||
| SEE | 0.044 | 8.882 | |||||||
| F | 21.64 | 8.859 | |||||||
| p (significance F) | 3.4E-04 | 6.4E-03 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





