Submitted:
06 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
Polymer Samples
Surfactant Samples
Oil Samples
Rocket Samples
| number | Length(cm) | Diameter(cm) | Porosity(%) | Permeability(mD) |
|---|---|---|---|---|
| 1 | 9.899 | 2.495 | 26.116 | 100.0 |
| 2 | 9.980 | 2.504 | 27.373 | 100.0 |
Brines
2.2. Experimental Methods
Viscosity Test
Interfacial Tension Test
Emulsion Stability Test
Core Flooding Test
3. Results and Discussion
3.1. Polymer Selection
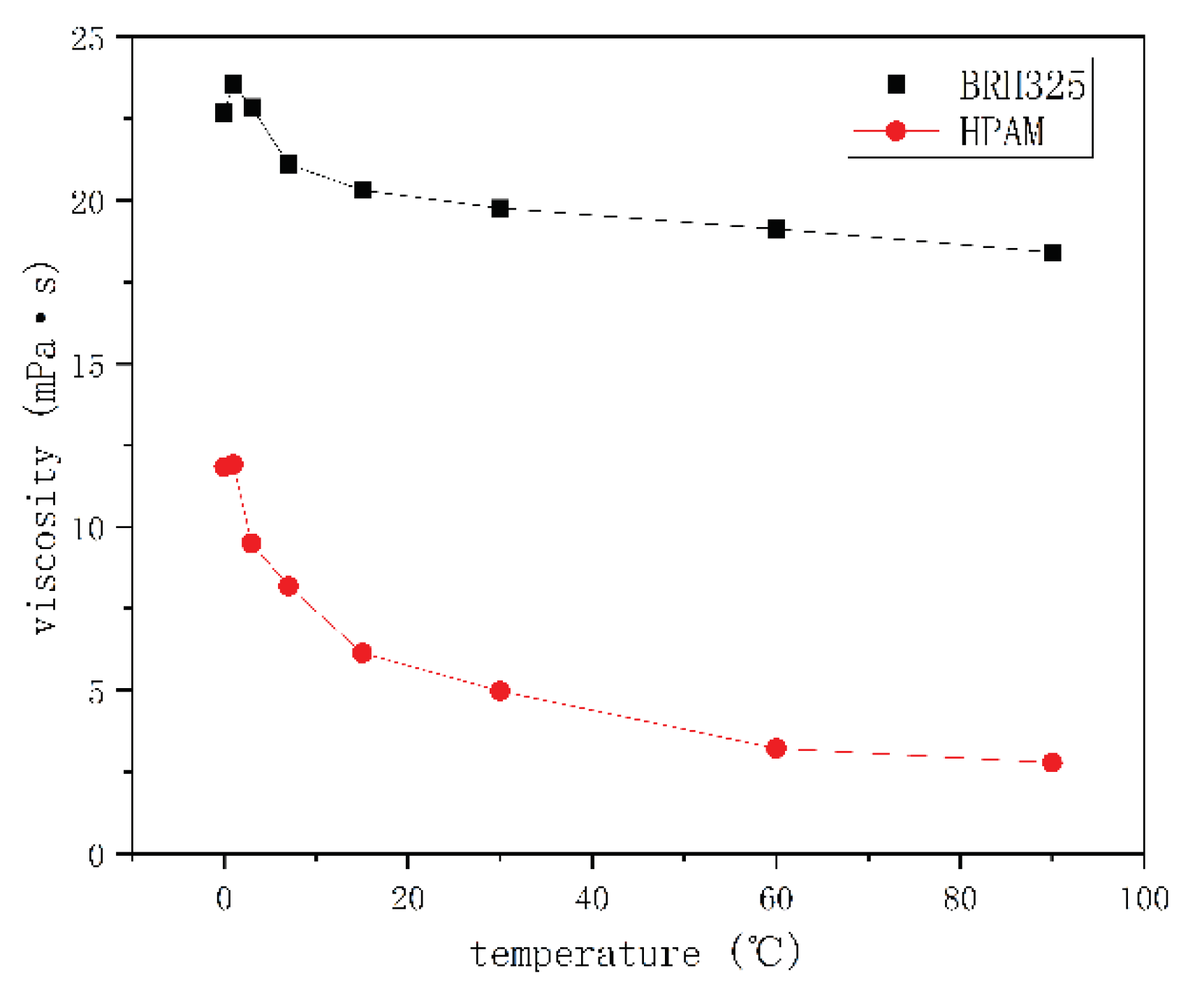
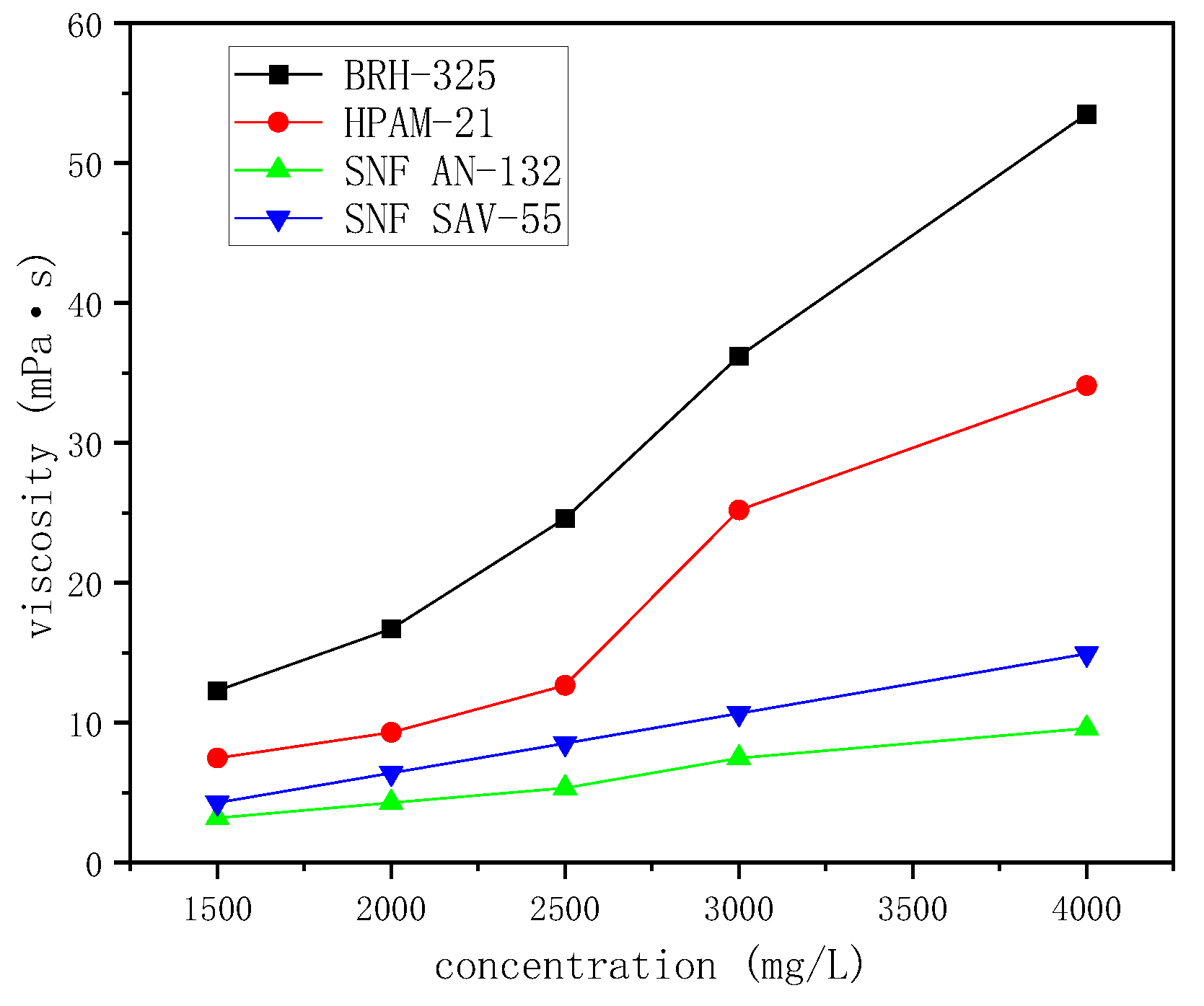
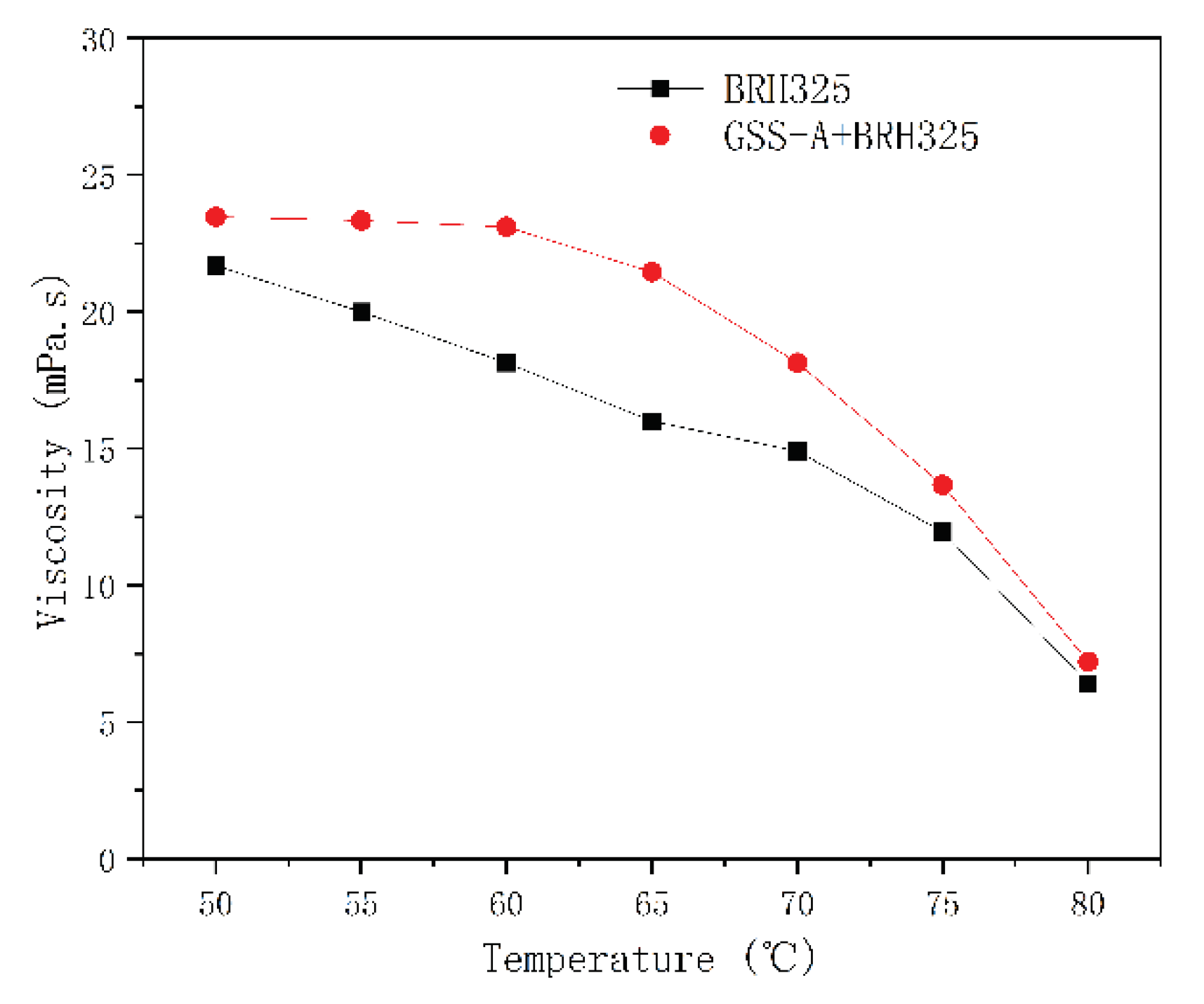
Viscosity Test
Thermal Stability
3.2. Surfactant Selection
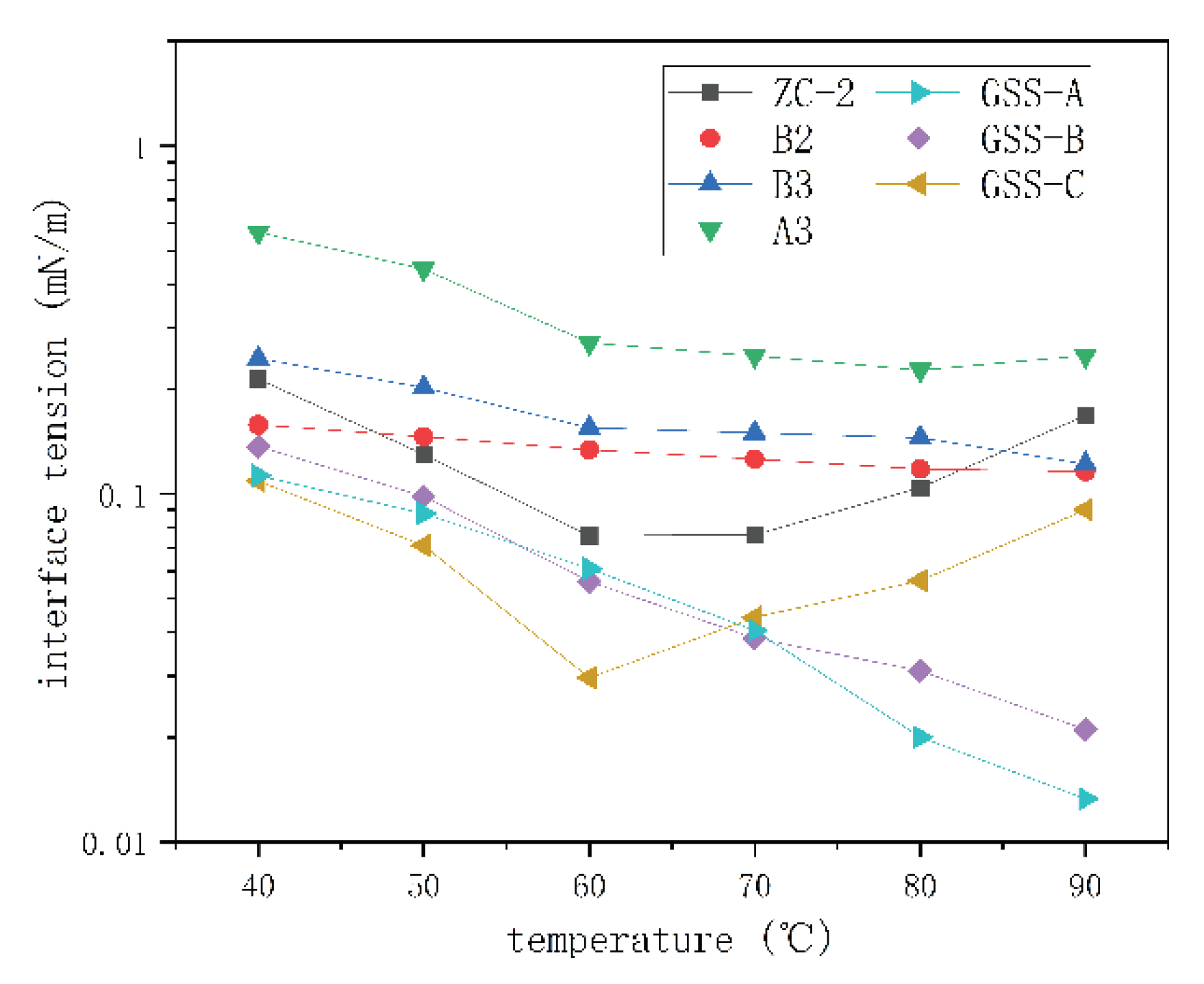
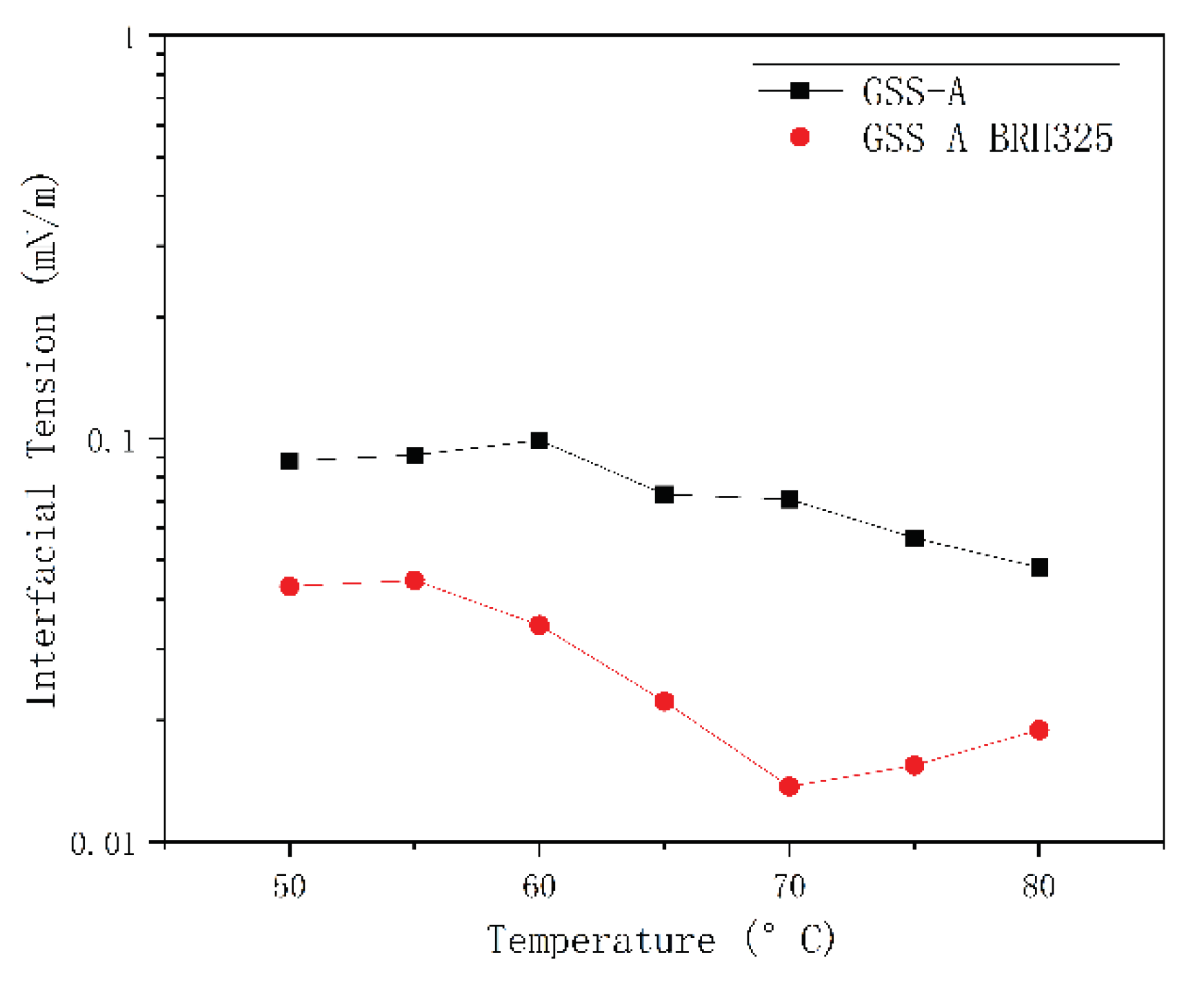
Interfacial Tension Test
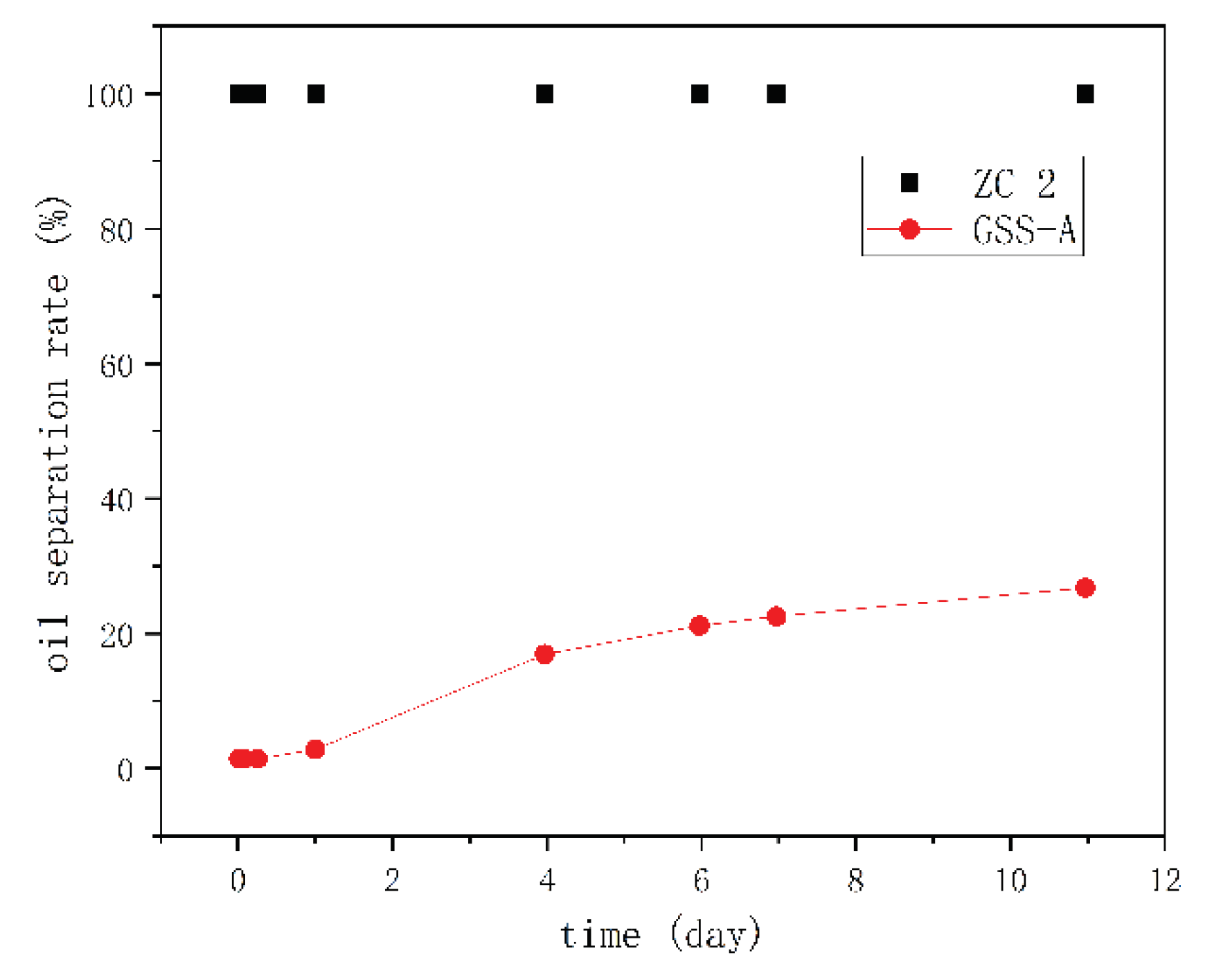
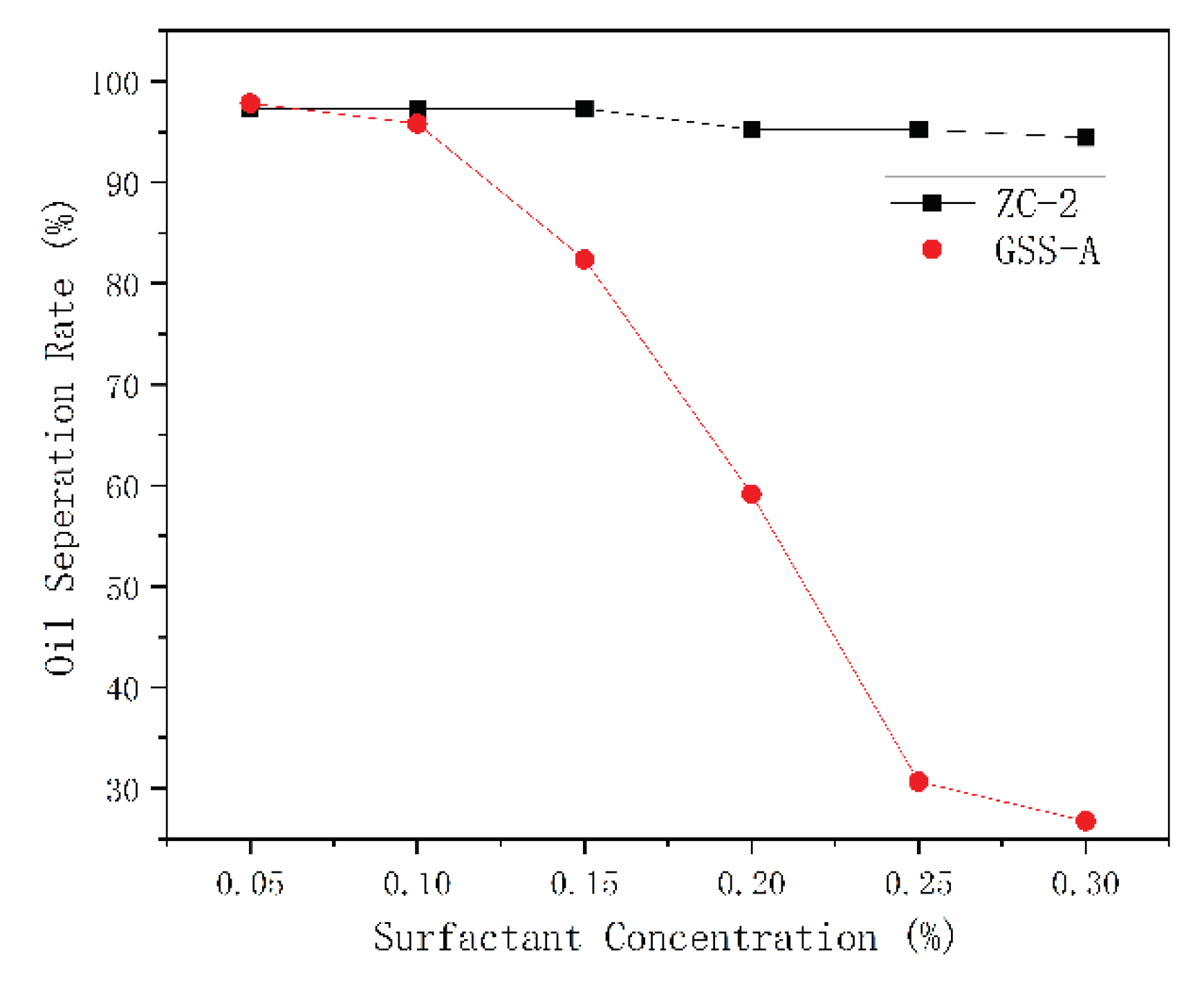
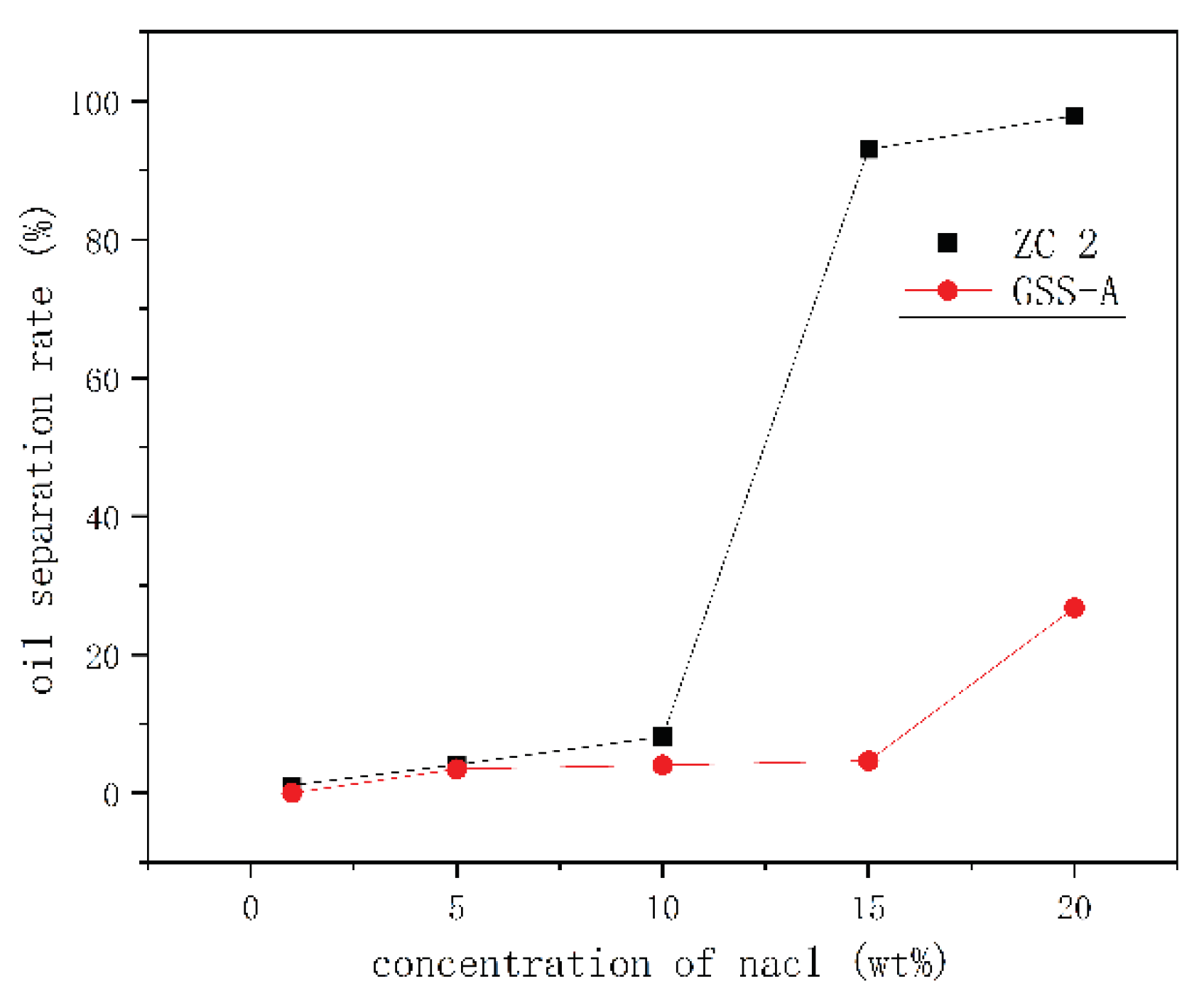
Emulsion Stability Test
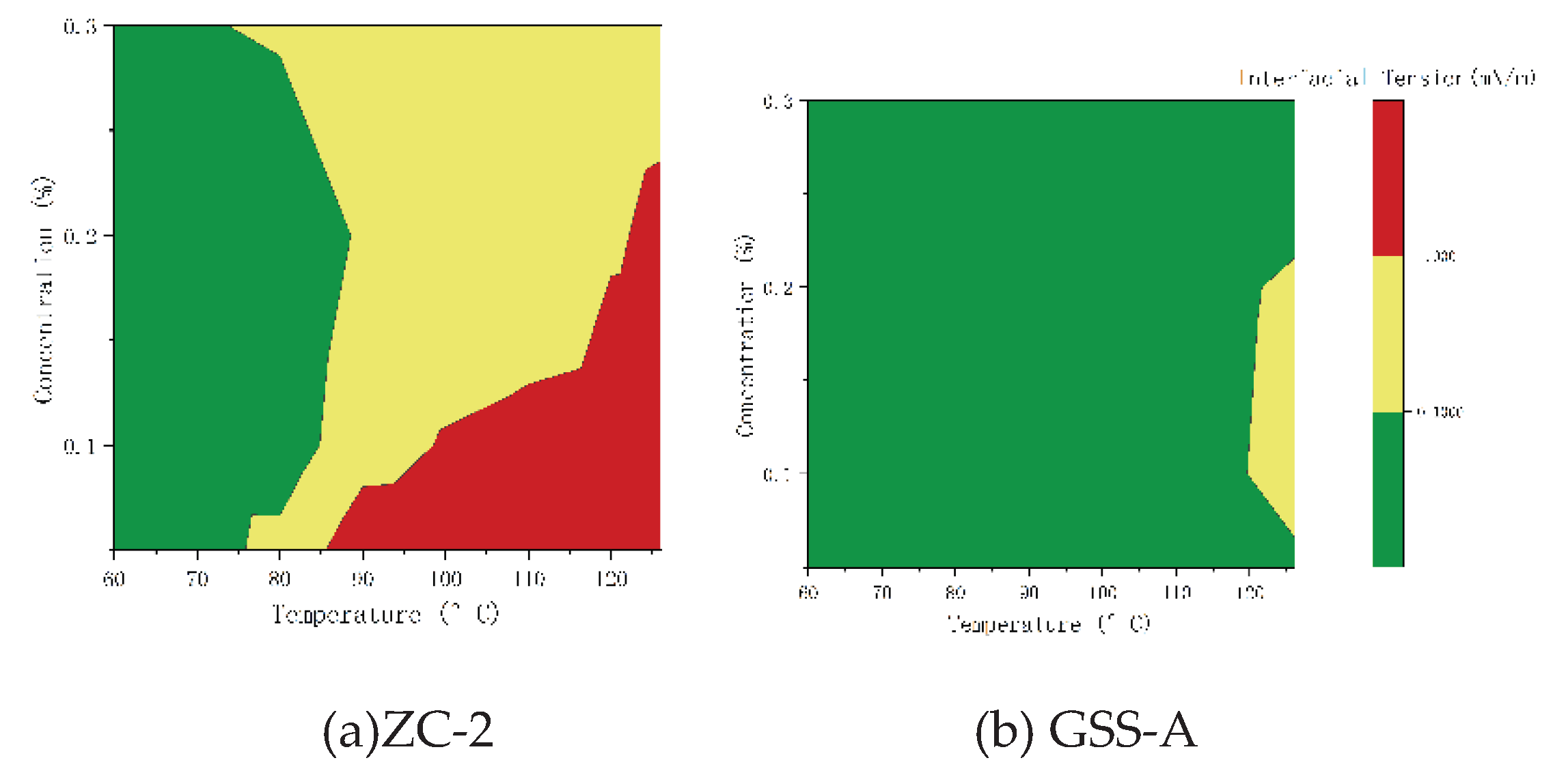
3.3. Polymer-Surfactant Compatibility Test
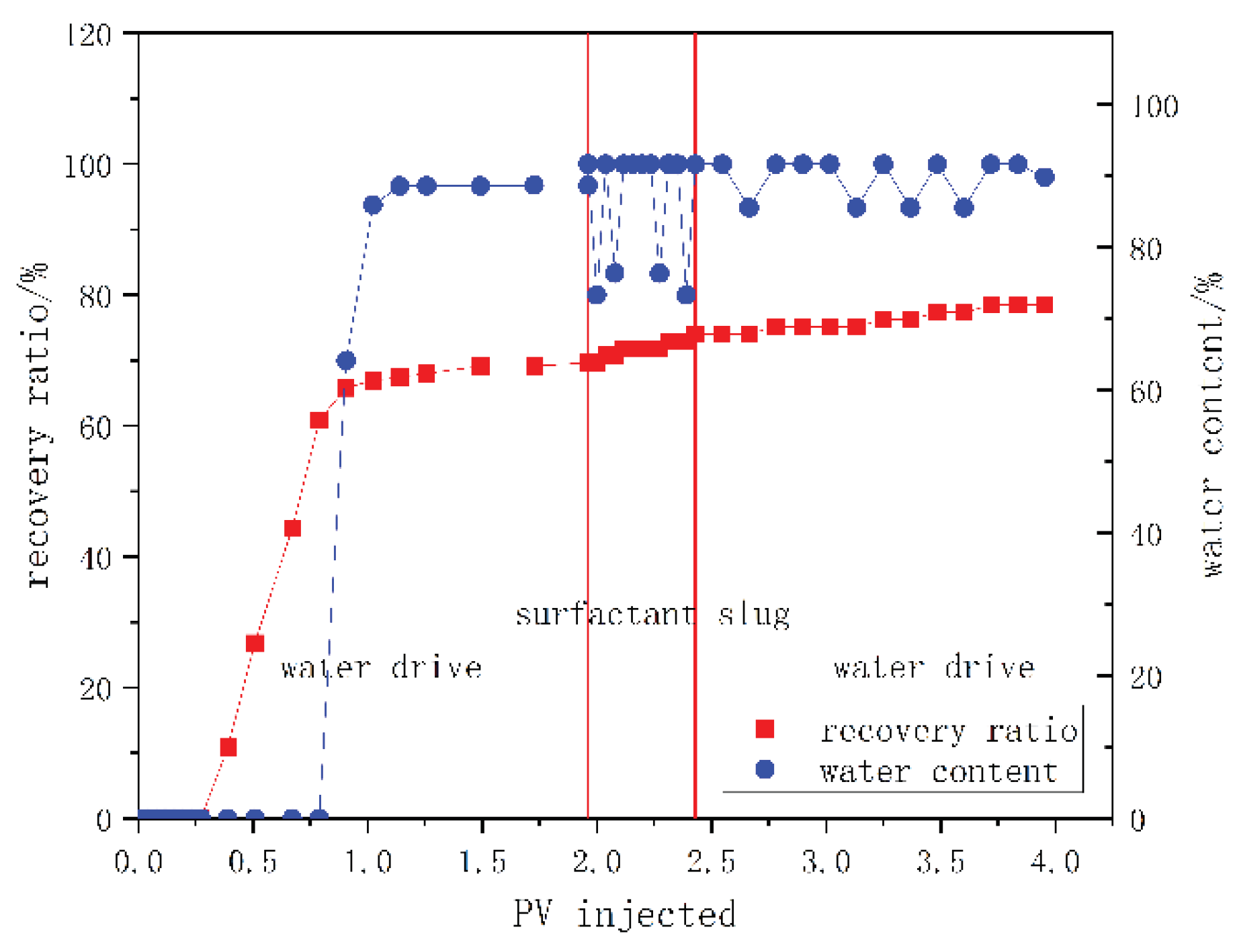
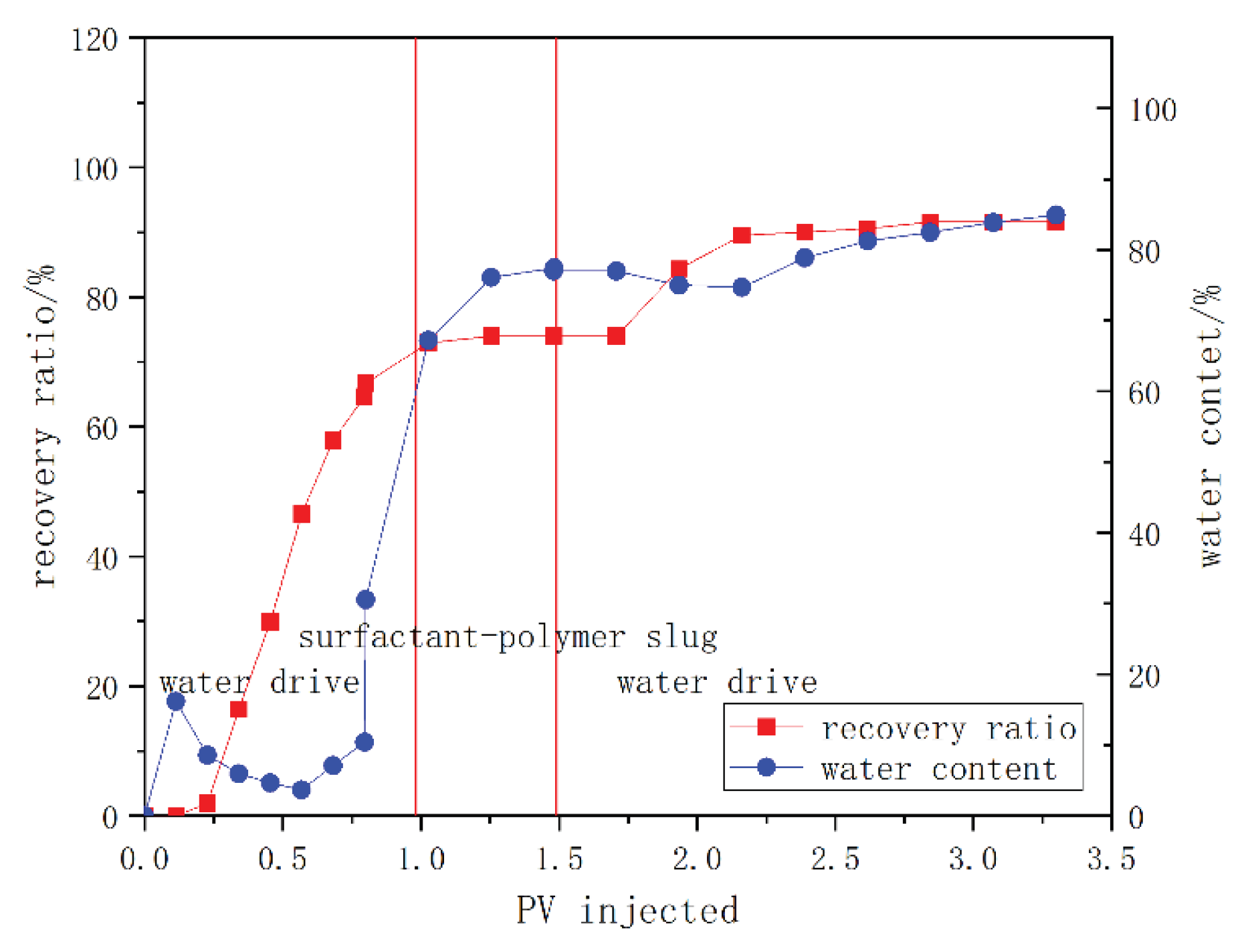
3.4. Core Flooding Test
4. Conclusions
References
- Sun, C.; Guo, H.; Li, Y.; Song, K. Recent Advances of Surfactant-Polymer (SP) Flooding Enhanced Oil Recovery Field Tests in China. Geofluids 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Fan, J. Developments of ASP/SP flooding formulations for Huabei fault block reservoir. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, 2015; pp. SPE-176117-MS. [Google Scholar]
- Barnes, J.R.; Smit, J.P.; Smit, J.R.; Shpakoff, P.G.; Raney, K.H.; Puerto, M.C. Development of surfactants for chemical flooding at difficult reservoir conditions. In Proceedings of the SPE Improved Oil Recovery Conference, 2008; pp. SPE-113313-MS.
- Huang, L.; Lips, A.; Co, C.C. Microemulsification of Triglyceride Sebum and the Role of Interfacial Structure on Bicontinuous Phase Behavior. Langmuir 2004, 20, 3559–3563. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, D.L.; Yan, W.; Puerto, M.; Hirasaki, G.J.; Miller, C.A. Favorable Attributes of Alkaline-Surfactant-Polymer Flooding. SPE J. 2008, 13, 5–16. [Google Scholar] [CrossRef]
- Yu, H. A Research On Reservoir Heterogeneity of N21 Reservoir Gasikule Oilfield of Qinghai Provence. Chengdu University of Technology, 2002.
- Jia Zhiwei, C.C. , Zhu Xiuyu, Pu Lantian, Han Yu, Hu Futang. Oil Recovery Enhancement by Composite Flooding Technology for Gasi N1-N21 Ultra-high-salinity reservoir in Qinghai Oilfield. Petroleum Drilling Techniques 2021, 49, 81–87. [Google Scholar]
- Wang, S.; Chen, C.; Yuan, N.; Ma, Y.; Ogbonnaya, O.I.; Shiau, B.; Harwell, J.H. Design of extended surfactant-only EOR formulations for an ultrahigh salinity oil field by using hydrophilic lipophilic deviation (HLD) approach: From laboratory screening to simulation. Fuel 2019, 254, 115698. [Google Scholar] [CrossRef]
- Miñana-Perez, M.; Graciaa, A.; Lachaise, J.; Salager, J.-L. Solubilization of polar oils with extended surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1995, 100, 217–224. [Google Scholar] [CrossRef]
- Puerto, M.; Hirasaki, G.J.; Miller, C.A.; Barnes, J.R. Surfactant Systems for EOR in High-Temperature, High-Salinity Environments. SPE J. 2011, 17, 11–19. [Google Scholar] [CrossRef]
- Graciaa, A.; Lachaise, J.; Cucuphat, C.; Bourrel, M.; Salager, J.L. Improving solubilization in microemulsions with additives. 1. The lipophilic linker role. Langmuir 1993, 9, 669–672. [Google Scholar] [CrossRef]
- Edgar Acosta, H.U. , David A. Sabatini. The role of hydrophilic linkers. Surfactants and Detergents 2002, 5, 151–157. [Google Scholar]
- Vermolen, E.C.; Van Haasterecht, M.J.; Masalmeh, S.K.; Faber, M.J.; Boersma, D.M.; Gruenenfelder, M.A. Pushing the Envelope for Polymer Flooding Towards High-temperature and High-salinity Reservoirs with Polyacrylamide Based Ter-polymers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar] [CrossRef]
- Wu, Y.; Mahmoudkhani, A.; Watson, P.; Fenderson, T.R.; Nair, M. Development of new polymers with better performance under conditions of high temperature and high salinity. In Proceedings of the SPE EOR conference at oil and gas West Asia, 2012; pp. SPE-155653-MS.
- Zhang, H.; Feng, Y. Dependence of intrinsic viscosity and molecular size on molecular weight of partially hydrolyzed polyacrylamide. J. Appl. Polym. Sci. 2021, 138, 50850. [Google Scholar] [CrossRef]
- Choi, J.; Ka, D.; Chung, T.; Jung, J.; Koo, G.; Uhm, T.; Jung, S.H.; Park, S.; Jung, H.-T. Evaluation of highly stable ultrahigh-molecular-weight partially hydrolyzed polyacrylamide for enhanced oil recovery. Macromol. Res. 2015, 23, 518–524. [Google Scholar] [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Krebs, T.; Schroën, C.; Boom, R. Separation kinetics of an oil-in-water emulsion under enhanced gravity. Chem. Eng. Sci. 2012, 71, 118–125. [Google Scholar] [CrossRef]
- Chinese Oil and Gas Industry Standards: Analytical method of alkali-surfactant-polymer flooding system. 2001, SY/T 6424-2000.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





